Abstract
Calcium and phosphorus homeostasis is crucial for the performance and bone health of laying hens. The calcium and phosphorus transporters play an important role in calcium and phosphorus absorption, reabsorption, and excretion. In the present study, Hy-Line Brown layers were sampled at brooding period (1, 4, 6 wk), growing and developing period (12, 18 wk) and laying period (20, 28, 80 wk) respectively, and the calcium transporters CaBP-D28k and PMCA1b and phosphorus transporters NPt2a and NPt2b were respectively measured in duodenum, jejunum, ileum and kidney. The result showed that serum calcium increased (P < 0.0001) and phosphorus level fluctuated (P = 0.0019), while alkaline phosphatase activity decreased with age (P < 0.0001). The mRNA and protein expressions of CaBP-D28k in small intestine elevated after maturity (P ≤ 0.0001). In contrast, the PMCA1b mRNA showed a trend to increase with age in jejunum (P = 0.0059) and ileum (P = 0.0825) whereas there was a decrease for PMCA1b protein in 12-18 wk (P ≤ 0.0009). The peak of NPt2b mRNA were observed at 28 wk in duodenum (P = 0.0001) and jejunum (P = 0.0622) and 1 wk in ileum (P < 0.0001). The NPt2b protein expression reached the top point at 4 or 6 wk and 20 wk and decreased to the lowest point around 12 wk (P ≤ 0.0850). In kidney, CaBP-D28k mRNA was not influenced by age (P = 0.4999), while PMCA1b highly expressed in 6-12 wk (P = 0.0003). The protein expressions of CaBP-D28k (P = 0.0148) and PMCA1b (P = 0.0003) decreased with age and lowly expressed in 12-18 wk and increased thereafter. In contrast, NPt2a expression increased steadily with age and decreased at 80 wk (P < 0.0001). In conclusion, the expressions of intestinal calcium and phosphorus transporters were changed by age, intestinal CaBP-D28k and renal NPt2a showed a dramatic increase after maturity, which coincide with the increased calcium and phosphorus requirement for egg production.
Key words: calcium transporter, phosphorus transporter, laying hens
INTRODUCTION
Calcium (Ca) and phosphorus (P) are the most abundant elements and play a critical role in a wide range of biological processes. In Hy-Line Brown layers, dietary Ca and P levels are essential for the formation of eggshell. In laying hens, the homeostasis of Ca and P is sustained by the absorption from small intestine, reabsorption from kidney, mobilization from bone, and loss via eggshell formation. In layer pullets, the effective supply of Ca and P has detrimental influence on bone health at laying period (Eusebio-Balcazar et al., 2018). Hence, the Ca and P metabolism is crucial for the layer-type chickens. During laying period, the sufficient supply of Ca and P is important to the maintenance of laying performance, egg quality, and bone health (Fleming et al., 1998, 2003; Jiang et al., 2015). The progressive loss of Ca and P from the structural bone increases the fragility of the bones makes them more susceptible to fractures and is involved in the development of osteoporosis (Whitehead, 2004).
Calbindin 28k (CaBP-D28k), an intracellular Ca2+-binding protein that transports Ca from the apical to the basolateral membrane, is involved in Ca2+ transport in intestine, kidney, and eggshell gland in birds (Wasserman and Taylor, 1966; Corradino et al., 1976; Bar and Hurwitz, 1979, 1984; Pasteels et al., 1987). Plasma membrane calcium ATPase (PMCA), mediating Ca2+ extrusion from cells, participates in the translocation of Ca (Carafoli, 1991; Zylińska et al., 2002; Fleet and Schoch, 2010). PMCA1b is the primary isomer expressed in small intestine, kidney, and uterus of birds (Melancon and DeLuca, 1970; Davis et al., 1987; Qin and Klandorf, 1993; Quinn et al., 2007; Parker et al., 2008). The phosphorus transporters type II-B Na/P co-transporter (NPt2b) is the major P transporter in small intestine that primary expressed at the brush-border membranes of epithelium (Hilfiker et al., 1998). Recently, our result indicates that duodenum has the highest expression level of NPt2b and is influenced by dietary P level (Li et al., 2018a). However, the expression profiles of the Ca and P transporters with age remain to be elucidated.
In the present study, the expression of genes CaBP-D28k, PMCA1b, NPt2a, and NPt2b in intestine and kidney were investigated in Hy-Line Brown layers at rearing stage and laying period, respectively. The present result provides basic information about the expression profiles of Ca and P transporters in intestinal tract and kidney.
MATERIALS AND METHODS
All procedures used in this study were approved by the Animal Care Committee of Shandong Agricultural University (Taian, China) and were carried out in accordance with the guidelines for experimental animals of the Ministry of Science and Technology (Beijing, China).
Animals and Diets
Two thousand 1-day-old Hy-Line Brown pullets were obtained from a local farm. The chickens were reared in an environmentally controlled house locating at 117° E, 36° N. The chickens were reared in brooder battery cages (Big Herdsman, Shandong, China) of 20 pullets/cage and 536 cm2/bird, and thereafter moved to layer cages (Big Herdsman) of 3 layers/cage and 552 cm2/bird at 12 wk of age. The pullet and layer cage respectively provided 5 nipple drinker and 150-cm-length feeder trough and one nipple drinker and a 36-cm-length feeder trough. During the whole experimental period, the chickens had free access to feed and water. The chickens were fed with a starter, grower and developer, layer diet successively (Table 1). The lighting program and vaccination schedule followed the management procedures of Hy-Line Brown laying hens (Tables 2 and 3).
Table 1.
The composition of experimental diets.
| Composition | Starter (0 to 6 wk) | Grower and developer (7 to18 wk) | Layer (19 to 80 wk) |
|---|---|---|---|
| Corn | 64.82 | 61.08 | 60.72 |
| Soybean meal | 31.01 | 22.13 | 25.39 |
| Wheat bran | - | 6.38 | - |
| Limestone | 1.40 | 1.31 | 8.76 |
| CaHPO4 | 1.41 | 1.20 | 1.62 |
| Soybean oil | 0.35 | 1.61 | 1.43 |
| Maifan stone 1 | - | 5.20 | 1.05 |
| NaCl | 0.32 | 0.32 | 0.32 |
| Lysine | 0.09 | 0.14 | 0.23 |
| Methionine | 0.09 | 0.09 | 0.14 |
| Threonine | - | 0.11 | - |
| Choline chloride | 0.26 | 0.18 | 0.09 |
| Mineral premix 2 | 0.20 | 0.20 | 0.20 |
| Vitamin premix 3 | 0.05 | 0.05 | 0.05 |
| Nutrient levels | |||
| Crude protein, % | 19.00 | 16.00 | 16.50 |
| AME, Kcal/kg | 2850 | 2750 | 2700 |
| Calcium, % | 0.90 | 0.80 | 3.50 |
| Measured calcium, % | 1.31 | 1.16 | 3.76 |
| Available phosphorus, % | 0.40 | 0.35 | 0.41 |
| Measured phosphorus, % | 0.78 | 0.52 | 0.73 |
| Lysine, % | 1.00 | 0.86 | 0.95 |
| Methionine, % | 0.40 | 0.35 | 0.41 |
| Met+Cys, % | 0.73 | 0.63 | 0.70 |
Maifan stone without calcium and phosphorus; the phytase addition rate of dietary deducted from Maifan stone.
The mineral premixes provided the following per kilogram of diet: Fe 55 mg, Mn 88 mg, Cu 5.5 mg, Zn 88 mg, I 1.7 mg, Se 0. 3mg.
The vitamin premixes provided the following per kilogram of diet: VA 8,800 IU, VD3 3,300 IU, VE 16.15 IU, VK 2.2 mg, VB1 1.7 mg, VB2 5.5 mg, VB3 28 mg, VB5 6.6 mg, VB6 3.3 mg, VB7 0.1 mg, VB9 0.6 mg, VB12 0.05 mg.
Table 2.
The lighting program of Hy-Line Brown layers.
| Weeks of age | Light (h)/Dark (h) | Light intensity (Lux) |
|---|---|---|
| 1 | 22/2 | 30 |
| 2 | 20/4 | 30 |
| 3 | 18/6 | 25 |
| 4 | 16/8 | 25 |
| 5 | 14/10 | 15 |
| 6 | 12/12 | 15 |
| 7–18 | 10/14 | 5 |
| 19 | 11/13 | 15 |
| 20 | 12/12 | 20 |
| 21 | 13/11 | 25 |
| 22 | 14/10 | 30 |
| 23 | 15/9 | 35 |
| 24–80 | 16/8 | 40 |
Table 3.
The vaccination schedule of Hy-Line Brown layers.
| Days of age | Vaccine | Dose | Pattern |
|---|---|---|---|
| 1 | Combined Newcastle disease and infectious bronchitis vaccine (live) | 1 plume | Eye drops |
| 8 | Combined Newcastle disease, avian influenza, bursa of Fabry-Peri and adenovirus vaccine (inactivated) | 0.4 mL | Subcutaneous injection |
| Combined Newcastle disease and infectious bronchitis vaccine (live) | 1 plume | Eye drops | |
| 18 | Mycoplasma Synoviae vaccine (inactivated) | 0.4 mL | Subcutaneous injection |
| Avian pox vaccine (live) | 1.5 plume | Wing-web puncture | |
| 21 | Newcastle disease vaccine (live) | 2 plume | Eye drops |
| 25 | Reassortant avian influenza virus (H5+H7) trivalent vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| 34 | Combined Newcastle disease and infectious bronchitis vaccine (live) | 2 plume | Eye drops, nose drops |
| 35 | Newcastle disease, infectious bronchitis and avian influenza (H9) vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| 40 | Infectious coryza (serotype A) vaccine (inactivated) | 0.5 mL | Subcutaneous injection |
| 45 | Infectious laryngotracheitis vaccine (live) | 1 plume | Eye drops |
| 64 | Newcastle disease vaccine (live) | 2 plume | Drinking |
| 65 | Reassortant avian influenza virus (H5+H7) trivalent vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| 75 | Newcastle disease, avian influenza virus (H9), fowl adenovirus vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| Infectious laryngotracheitis vaccine (live) | 1 plume | Eye drops | |
| 80 | Combined Newcastle disease and infectious bronchitis vaccine (live) | 2 plume | Drinking |
| 90 | Infectious coryza (A+B+C) vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| 100 | Combined avian encephalomyelitis and avian pox vaccine (live) | 1 plume | Wing-web puncture |
| 110 | Newcastle disease vaccine (live) | 3 plume | Intramuscular injection |
| 120 | Newcastle disease, infectious bronchitis, egg drop syndrome and avian influenza (H9) vaccine (inactivated) | 0.5 mL | Intramuscular injection |
| 129 | Combined Newcastle disease and infectious bronchitis vaccine (live) | 2 plume | Drinking |
| 130 | Reassortant avian influenza virus (H5+H7) trivalent vaccine (inactivated) | 0.5 mL | Intramuscular injection |
During brooding period (the end of 1, 4, and 6 wk of age), growing and developing period (the end of 12 and 18 wk of age) and laying period (the end of 20, 28, and 80 wk of age), eight birds were randomly selected for sampling, to observe the effect of age on levels of serum parameters and calcium and phosphorus transporters.
Tissue Sampling
At the end of 1, 4, 6, 12, 18, 20, 28, and 80 wk of age, 8 birds were randomly selected. After overnight fasting, a blood sample was drawn from the left wing vein using 5-mL heparinized syringe (needle length: 3 cm) from each hen and collected with ice-cold tube. Serum sample was obtained after centrifugation at 3,000 g for 10 min at 4°C and stored at −20°C for further analysis. Thereafter, the birds were euthanized by cervical dislocation and the duodenum, jejunum, ileum, and kidney were sampled. Tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80°C for further analysis.
Serum Calcium, Phosphorus, and Alkaline Phosphatase Analysis
Serum Ca and P levels and alkaline phosphatase (ALP) activity were measured with commercial kits (Maccura Biotechnology Co., Ltd., Sichuan, China) by automatic biochemistry analyzer (7020 Clinical Analyzer; Hitachi High-Tech GLOBAL, Japan).
Real-Time PCR Analysis
The mRNA expressions of NPt2a, NPt2b, CaBP-D28k, and PMCA1b were measured. Total RNA was extracted from intestine and kidney using TransZol Up (TransGen Biotech, Beijing, China). Then the concentration of the RNA was measured by spectrophotometry (Eppendorf, Hamburg, Germany), and the RNA purity was verified by calculating the ratio between the absorbance values at 260 and 280 nm (A260/280 ≈ 1.75∼2.01). Next, reverse transcription was performed using total RNA (1 μg) for first-strand cDNA synthesis with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). The cDNA was amplified in a 20-μL PCR reaction system containing 0.2 μmol/L of each specific primer (Sangon, Shanghai, China) and the SYBR Green master mix (Roche) according to the manufacturer's instruction. Real-time PCR was performed at the ABI QuantStudio 5 PCR machine (Applied biosystems Inc., Carlsbad, CA). Primers against β-actin were used as internal controls, and all of the mRNA values were normalized the differences between individual samples. Primers used in this study were designed using Primer 5.0 software and synthesized by Sangon Biotech (Shanghai, China, Table 4). The specificity of the amplification product was verified by the standard curve and dissolution curve.
Table 4.
Gene-specific primer of related genes.
| Gene | Sequences (5’→3’) | Accession NO. | Product size (bp) |
|---|---|---|---|
| CaBP-D28k | F: TGTTATGGAGTGCAGGATGG | NM_205513 | 131 |
| R: TAGAGCGAACAAGCAGGTGA | |||
| PMCA1b | F: TTCAGGTACTCATGTGATGGAAGG | XM_015277056 | 98 |
| R: CAGCCCCAAGCAAGGTAAAG | |||
| NPt2a | F: CCAAACTGCACGGCTTCT | XM_015293846 | 249 |
| R: TGGGAGGTCAGT GTTGATGA | |||
| NPt2b | F: ACTGGCTTGCTGTGTTTGC | NM_204474 | 113 |
| R: AGGGGCATCTTCACCACTTT | |||
| β-actin | F: CTGGCACCTAGCACAATGAA | NM_205518 | 123 |
| R: CTGCTTGCTGATCCACATCT |
Abbreviations: CaBP-D28k, calbindin 28k; NPt2a, type II-A Na/P co-transporter; NPt2b, type II-B Na/P co-transporter; PMCA1b, plasma membrane calcium ATPase 1b.
Western Blot Analyses
The protein expressions of NPt2b, CaBP-D28k, and PMCA1b were determined. The tissue samples were homogenized in 1 mL of lysis buffer (Beyotime, Shanghai, China). After centrifuged at 12,000 g for 10 min at 4°C, the supernatant was collected and then quantified for protein by the method of BCA protein assay kit (Beyotime) according to the manufacturer's protocol. An equal amount of proteins (18 μg) were separated by 7.5 to 10% SDS polyacrylamide gels (Bio-Rad, Hercules, CA) and the proteins were transferred onto polyvinylidene fluoride membrane (Millipore, Bilrica, MA) at 200 mA for 2 h in a Tris-glycine buffer with 20% anhydrous ethanol at 4°C. Then membrane was blocked with western blocking buffer (Beyotime) for 1 h at room temperature. The membrane was incubated with specific primary antibodies at 4°C with gentle shaking overnight. The primary antibodies used were anti-NPt2b (GenScript, Nanjing, China), anti-CaBP-D28k (Sigma, St Louis, MO), anti-PMCA1b (ThermoFisher Scientific, Waltham, MA) and anti-β-actin (Beyotime). The membrane was washed with Tris-buffered saline/Tween buffer for 10 min 3 times, and then was incubated with secondary antibodies (HRP-conjugated anti-rabbit or anti-mouse IgG, 1:1000; Beyotime) for 4 h at 4°C. After being washed as before, membrane was then visualized by exposure to Hyperfilm ECL (Beyotime). Western blots were developed and quantified using BioSpectrum 810 with VisionWorksLS 7.1 software (UVP LLC, Upland, CA). The band intensity was normalized to the β-actin band in the same sample.
Statistical Analysis
All the data were analyzed with one-way ANOVA mathematical model (Y, result; μ, intercept; α, the age effect; e, random error; i = 1, 4, 6, 12, 18, 20, 28, 80; j = 1, 2, 3, 4, 5, 6, 7, 8) by using the Statistical Analysis Software (SAS version 8e, SAS Institute, Cary, NC) to estimate the main effect of age. Comparisons between means used Duncan's Multiple Range test. P ≤ 0.05 was considered statistically significant. The data are presented as means ± SE (n = 8).
RESULTS
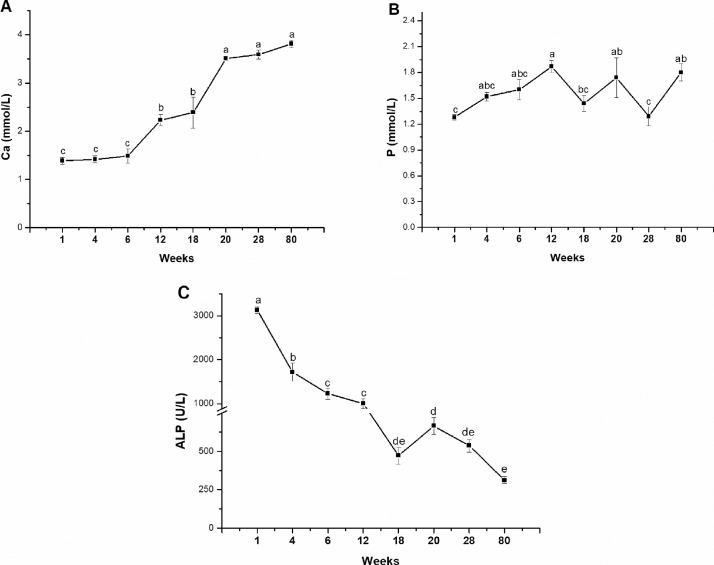
Serum Ca level increased with age and showed the highest level at laying period and the lowest level at starter stage (P < 0.0001, Figure 1A). Serum P level, however, showed fluctuate change with age and the highest level was observed at developer stage whereas the lowest level was respectively detected at starter and peak-laying periods (P = 0.0019, Figure 1B). Serum ALP activity gradually dropped with age and reached the lowest level at 80 wk of age (P < 0.0001, Figure 1C).
Figure 1.
The expression profiles of serum calcium (A), phosphorus (B), and alkaline phosphatase (ALP, C) in laying-type chickens at different ages. Data were presented as means ± SE (n = 8); a-e Means with different superscript letter differ significantly (P < 0.05).
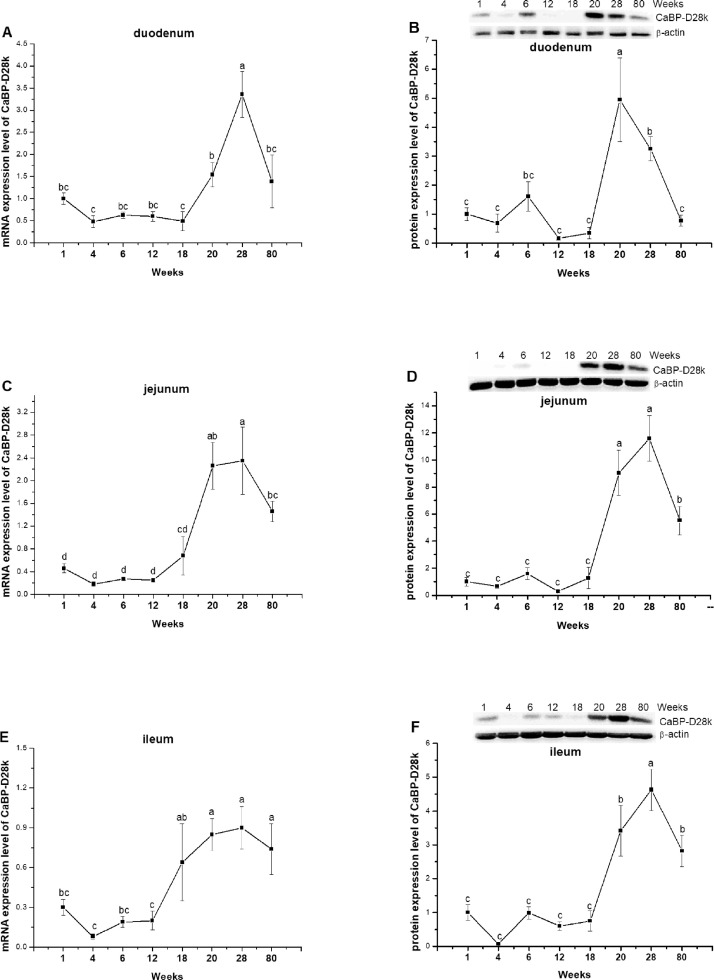
The mRNA level of CaBP-D28k was highly expressed in duodenum, then jejunum, and lowly expressed in ileum (Figures 2A, 2C, 2E). In duodenum and jejunum, the expression level of CaBP-D28k was not obviously changed before 18 wk of age (P = 0.0784 for duodenum, P = 0.1799 for jejunum), reached the peak point at 28 wk of age, and thereafter decreased significantly (P < 0.0001, Figures 2A, 2C). In ileum, the expression level of CaBP-D28k began to increase at 18 wk of age (P = 0.0001) and was not significantly changed during laying period (P = 0.8074, Figure 2E). The protein level of CaBP-D28k showed similar trend in duodenum, jejunum, and ileum, increasing dramatically after laying and peaked at 20 wk of age and then gradually decreased with age (P < 0.0001, Figures 2B, 2D, 2F).
Figure 2.
The mRNA and protein expression levels of calbindin-D28k (CaBP-D28k) in duodenum (A, B), jejunum (C, D), and ileum (E, F) of laying-type chickens. Data were presented as means ± SE (n = 8); a-d Means with different superscript letter differ significantly (P < 0.05).
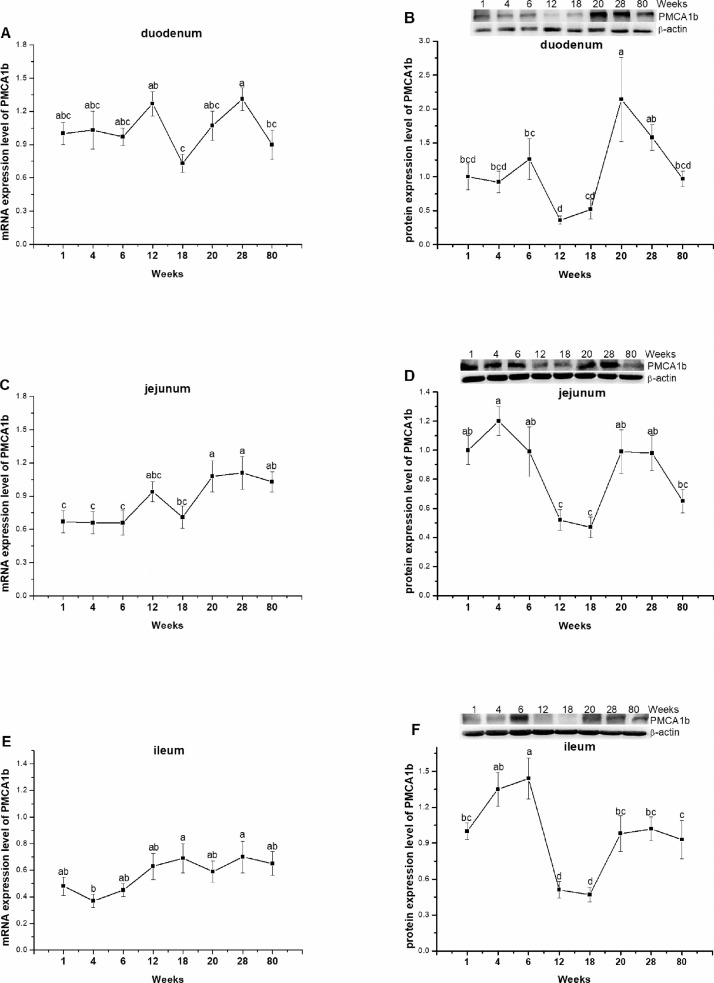
In duodenum, the lowest and highest mRNA level of PMCA1b was respectively observed at 18 and 28 wk of age (P = 0.0208, Figure 3A). In jejunum, the expression of PMCA1b had no significant change (P = 0.2444) until 18 wk age and significantly increased (P = 0.0059) after 20 wk of age (Figure 3C). In ileum, however, PMCA1b expression tended to increase gradually and reach the peak point at 18 wk of age (P = 0.0825, Figure 3E). The protein level of PMCA1b was lower during the period of 12 to 18 wk of age in the three segments of intestine. The peak level, however, was observed during laying period in duodenum (P = 0.0009) but detected at grower stage in jejunum and ileum (P < 0.0001), respectively (Figures 3B, 3D, 3F).
Figure 3.
The mRNA and protein expression levels of plasma membrane calcium ATPase 1b (PMCA1b) in duodenum (A, B), jejunum (C, D), and ileum (E, F) of laying-type chickens. Data were presented as means ± SE (n = 8); a-d Means with different superscript letter differ significantly (P < 0.05).
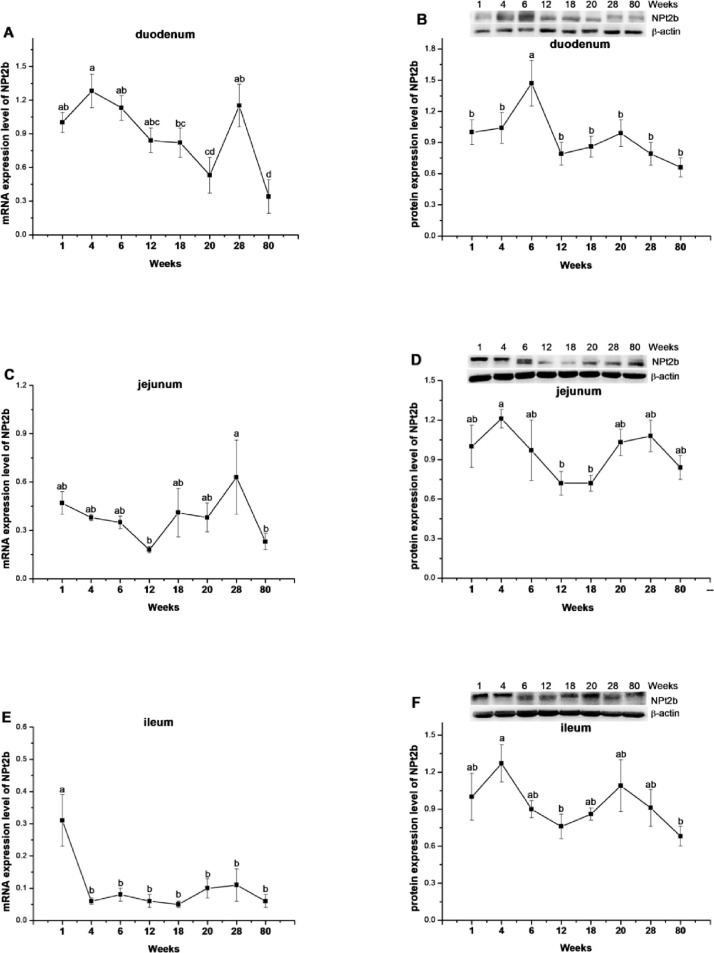
In duodenum and jejunum, the mRNA expression of NPt2b gradually decreased until 20- and 12-wk age, then dramatically increased at 28 wk age, and then decreased at 80 wk age (P = 0.0001 for duodenum, P = 0.0622 for jejunum, Figures 4A, 4C). In ileum, the mRNA expression of NPt2b decreased at 4 wk of age (P < 0.0001) and maintained as such till 80 wk of age (P = 0.4538; Figure 4E). In duodenum, the protein level of NPt2b was higher at 6-wk age (P = 0.0055) compared with all the other time points (Figure 4B). In jejunum and ileum, the NPt2b level showed a trend of bimodal expression profile, the highest point was respectively observed at 4 wk age and 20- or 28-wk age, and the lowest level was detected at 12 wk age (P = 0.0848 for jejunum, P = 0.0850 for ileum, Figures 4D, 4F).
Figure 4.
The mRNA and protein expression levels of type II-B Na/P co-transporter (NPt2b) in duodenum (A, B), jejunum (C, D), and ileum (E, F) of laying-type chickens. Data were presented as means ± SE (n = 8);a-d Means with different superscript letter differ significantly (P < 0.05).
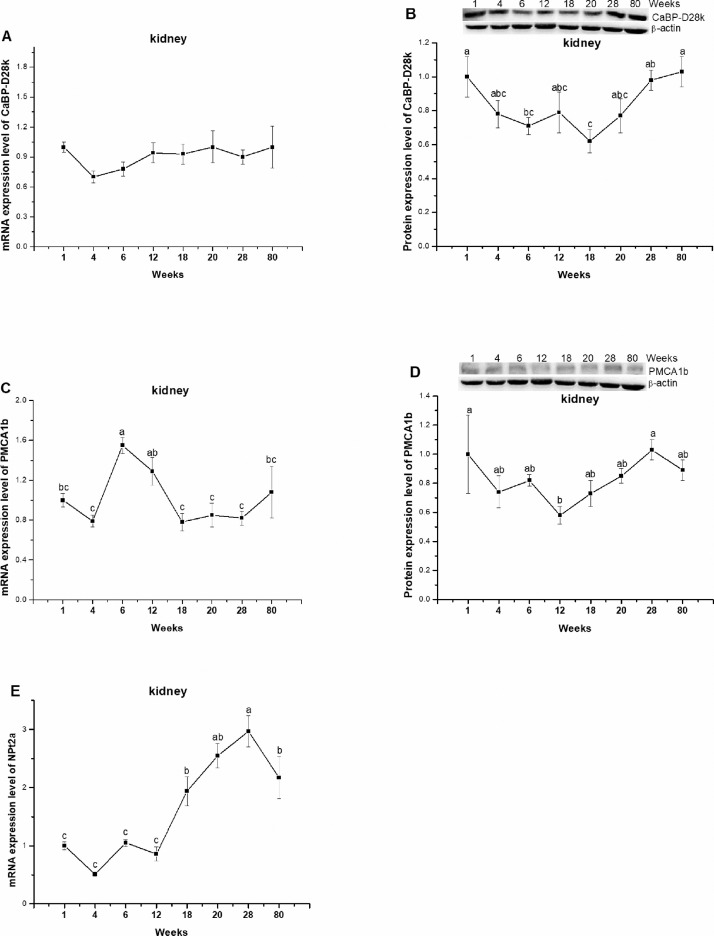
In kidney, the mRNA level of CaBP-D28k was not changed by age (P = 0.4999, Figure 5A). The protein level of CaBP-D28k was highly expressed at 1 wk of age, then was decreased gradually and reached the lowest level at 18 wk age, and thereafter was increased steadily and approach the highest level at 80 wk of age (P = 0.0148, Figure 5B). In contrast, the highest PMCA1b mRNA level was observed at 6- and 12 wk age, compared to the other time points (P = 0.0003, Figure 5C). PMCA1b protein was highly expressed at 1- and 28 wk age respectively and the lowly expressed at 12 wk age (P = 0.0003, Figure 5D). The NPt2a mRNA level increased gradually and the highest expression level was detected at 28 wk of age (P < 0.0001, Figure 5E).
Figure 5.
The mRNA and protein expression levels of Calbindin-D28k (CaBP-D28k) (A, B) and plasma membrane calcium ATPase 1b (PMCA1b) (C, D) and the mRNA expression level of type II-A Na/P co-transporter (NPt2a) (E) in kidney of laying-type chickens. Data were presented as means ± SE (n = 8); a-c Means with different superscript letter differ significantly (P < 0.05).
DISCUSSION
In the present study, the serum Ca and P levels showed different trends with age and the Ca concentration increased with age. The increased blood Ca level after lay should be ascribed to the enhance Ca metabolism in laying hens. This result was line with the previous work by Parsons and Combs (1981), which reported that blood Ca level was high in laying hens while P was similar to that of layer pullets. The blood ionized Ca was monitored in layer-type chickens between 4 and 110 wk of age, presenting that pullets (4–15 wk) have the lowest level and the first cycle laying hens (20–68 wk) have the highest one (Schaal et al., 2016). The increased blood Ca level with age is observed at the onset of egg laying, related to the regulation of reproductive hormones. It is well known that estrogen regulates the Ca homeostasis in hens (Beck and Hansen, 2004). Exogenous estrogen injection stimulate 25-OH-D3-l-hydroxylase activity and in turn the production of 1,25-(OH)2D3 (Baksi and Kenny, 1980). In the letrozole induced low estrogen model, the expression of Ca transporters in duodenum is significantly suppressed (Li et al., 2018b).
In the present study, the serum P fluctuated in the whole rearing period from 1 to 80 wk of age with the lowest level at 1 and 28 wk of age. This result could be attributed to the dietary P level and the laying state. In laying hens, P is related to dietary P level (Miles et al., 1983). The blood P concentration reflects dietary P content (Boorman and Gunaratne, 2001; Jing et al., 2018; Wang et al., 2018). Serum P in non-laying hens is lower than that of laying hens (Garlich et al., 1984). During eggshell calcification, the breakdown of hydroxyapatite in medullary bone contributes to the elevated blood concentration of P (Etches, 1987).
Intestinal Expression Profiles of Calcium Transporters
In chickens, Ca balance is maintained through the absorption in the small intestine, reabsorption in the kidney, mobilization from the bone, and deposition in eggshell (Bar, 2008). The CaBP-D28k and PMCA1 are transporters that involved in the transepithelial Ca transport (Craviso et al., 1987; Carafoli, 1991). Therefore, we determined the expressions of the CaBP-D28k and PMCA1b in the intestinal tract and kidney. The result indicated that CaBP-D28k mRNA was highly expressed in the duodenum and followed by jejunum and ileum, in line with the previous works (Ebeid et al., 2012; Li et al., 2018a), suggesting that duodenum is the primary intestinal segment for calcium absorption. The expression of intestinal CaBP-D28k is regulated by 1,25(OH)2D3, dietary Ca and/or P levels, and sex hormones (Bar, 2009). It is suggested that CaBP-D28k in the duodenum is the primary target for estrogen regulation in order to control Ca homeostasis in hens (Li et al., 2018b). The protein expression of CaBP-D28k was significantly elevated after the onset of egg production, which is favorable for Ca absorption and Ca requirement during laying period. This result was line with the previous works (Reviewed by Bar, 2008, 2009). Our previous study also reported that dietary NaHCO3 supplementation increased duodenal protein expression of CaBP-D28k, contributing to higher Ca retention and balance (Jiang et al., 2015).
In chicken, PMCA1b is the predominant isomer expressed in the intestine (Davis et al., 1987) and kidney (Qin and Klandorf, 1993). The intestinal expression of PMCA1b was, however, different from that of CaBP-D28k, in the present result. There was a small increase in PMCA1b mRNA level in jejunum and ileum whereas the elevated PMCA1b protein level was detected in duodenum after maturity. The result indicated that maturity has an influence on the expression of PMCA1b mainly in the duodenum in chicken, increasing after maturity and decreasing at the post-peak period. This result is line with the result of Li et al. (2018b), which reported that duodenum PMCA1b expression is reduced in low-estrogen layers. At 80 wk of age, however, the PMCA1b protein level showed a decline in duodenum, this may be related to the decreased sex hormones level, the decreased egg production, and the decreased calcium requirements at the post-peak period. This result was not in accordance with the work by Wistedt et al. (2019), which reported that the staining intensity of PMCA1b in duodenum is not influenced by the age of layer from 21- to 70 wk of age. Moreover, there was a decline in the PMCA1b from 12 to 18 wk of age, the underlying mechanism needs to be investigated further. According to our knowledge, this is the first report about the expression profile of intestinal PMCA1b in chicken.
Intestinal Expression Profile of Phosphorus Transporters
In small intestine, P absorption is primary adjusted by NPt2b (Werner and Kinne, 2001; Yan et al., 2007; Sabbagh et al., 2009; Marks et al., 2006; Ikuta et al., 2017) and the expression of gene NPt2b in intestinal tract mainly locates at the duodenum (Yan et al., 2007; Li et al., 2018a). In the growing period, the NPt2b protein reached the top point at 4 or 6 wk of age and decreased to the lowest point around 12 wk of age, this NPt2b expression changes with age was associated with the dietary P level, which was lowest around 12 wk of age. In the laying period, though the mRNA level of NPt2b reached the peak point at 28 wk of age and decreased at 80 wk of age in duodenum and jejunum, the protein level of NPt2b was not changed by age, suggesting that NPt2b is stably expressed in intestine. This result was supported by our previous report that the mRNA expression of NPt2b is not affected by diet available P levels from 0.15 to 0.45% in duodenum and jejunum of laying hens (Jing et al., 2018). Collectively, the intestinal expression of NPt2b was flexible with age in growing period while stabile in laying period.
Kidney Expression Profiles of Calcium and Phosphorus Transporters
Ca and P are reabsorbed and excreted in the kidney. In the present study, the unobvious changed protein levels of CaBP-D28k and PMCA1b with age after 20 wk of age indicated that Ca reabsorption is not influenced with age during laying period. As CaBP-D28k and PMCA1 are vitamin D-dependent Ca transporters (Johnson and Kumar, 1994), we thus speculate that their expression in kidney was not changed after maturity when vitamin D is sufficiently provided in the diet. This result was line with previous report that the renal CaBP-D28k gene and protein expressions were not changed in low estrogen layers (Li et al., 2018b). Our previous study also showed that the mRNA and protein levels of CaBP-D28k were not changed by dietary available P level from 0.15 to 0.82% (Li et al., 2018a). In vitamin D depleted rabbits, however, Ca2+ transport by both the luminal and the basolateral membranes of the distal tubule was significantly diminished (Bouhtiauy et al., 1993).
In the kidney, NPt2a is the main transport for the reabsorption of P. As antibody against chicken NPt2a is not available, only the mRNA expression of NPt2a in kidney was measured in the present study, which indicated that NPt2a expressed at a relative low level from 1 to 12 wk of age and increased thereafter, suggesting that renal P reabsorption capacity is enhanced with age. This result was in accordance with the NPt2b mRNA expression in jejunum. We speculate that hens showed a negative balance of P during laying period. This speculation was supported by the work by Neijat et al. (2011). Moreover, the expression of renal NPt2a is dependent of dietary P intake. Increased dietary P intake suppresses inorganic P transport capacity and NPt2a and NPt2b expression in laying hens (Huber et al., 2006; Li et al., 2018a). In contrast, reducing layer diet available P from 0.45% up to 0.15% has no detectable influence on mRNA expression of NPt2a (Jing et al., 2018). Besides, many factors are involved in the regulation of NPt2a in kidney, for example, parathyroid hormone, glucocorticoids, vitamin D3, estrogen, and thyroid hormone (Blaine et al., 2011).
CONCLUSIONS
In conclusion, the present study indicated that the expression of intestinal Ca and P transporters were changed by age. The intestinal expression of CaBP-D28k and renal NPt2a showed a dramatic increase after maturity, which coincides with the increased Ca and P requirement for egg production.
ACKNOWLEDGMENTS
This research was funded by the National Natural Science Foundation of China, grant number 32172787, the National Key Research and Development Program of China, grant number 2018YFE0128200, the Modern Agro-industry Technology Research System, grant number CARS-40-K09, the Taishan Scholars Program, grant number 201511023, and the Funds of Shandong “Double Tops” Program (2019).
DISCLOSURES
The authors have declared no conflicts of interest.
REFERENCES
- Baksi S.N., Kenny A.D. Estradiol-induced stimulation of 25-hydroxyvitamin D3-1-hydroxylase in vitamin D-deficient Japanese quail. Pharmacology. 1980;20:298–303. doi: 10.1159/000137384. [DOI] [PubMed] [Google Scholar]
- Bar A. Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;151:477–490. doi: 10.1016/j.cbpa.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009;152:447–469. doi: 10.1016/j.cbpa.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bar A., Hurwitz S. The interaction between dietary calcium and gonadal hormones in their effect on plasma calcium, bone, 25-hydroxycholecalciferol-1-hydroxylase, and duodenal calcium-binding protein, measured by a radioimmunoassay in chicks. Endocrinology. 1979;104:1455–1460. doi: 10.1210/endo-104-5-1455. [DOI] [PubMed] [Google Scholar]
- Bar A., Hurwitz S. Egg shell quality, medullary bone ash, intestinal calcium and phosphorus absorption, and calcium-binding protein in phosphorus-deficient hens. Poult. Sci. 1984;63:1975–1979. doi: 10.3382/ps.0631975. [DOI] [PubMed] [Google Scholar]
- Beck M.M., Hansen K.K. Role of estrogen in avian osteoporosis. Poult. Sci. 2004;83:200–206. doi: 10.1093/ps/83.2.200. [DOI] [PubMed] [Google Scholar]
- Blaine J., Weinman E.J., Cunningham R. The regulation of renal phosphate transport. Adv. Chronic Kidney. Dis. 2011;18:77–84. doi: 10.1053/j.ackd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Boorman K.N., Gunaratne S.P. Dietary phosphorus supply, egg-shell deposition and plasma inorganic phosphorus in laying hens. Br. Poult. Sci. 2001;42:81–91. doi: 10.1080/713655018. [DOI] [PubMed] [Google Scholar]
- Bouhtiauy I., Lajeunesse D., Brunette M.G. Effect of vitamin D depletion on calcium transport by the luminal and basolateral membranes of the proximal and distal nephrons. Endocrinology. 1993;132:115–120. doi: 10.1210/endo.132.1.8419116. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The calcium pumping ATPase of the plasma membrane. Annu. Rev. Physiol. 1991;53:531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- Corradino R.A., Fullmer C.S., Wasserman R.H. Embryonic chick intestine in organ culture: stimulation of calcium transport by exogenous vitamin D-induced calcium-binding protein. Arch. Biochem. Biophys. 1976;174:738–743. doi: 10.1016/0003-9861(76)90404-5. [DOI] [PubMed] [Google Scholar]
- Craviso G.L., Garrett K.P., Clemens T.L. 1,25-Dihydroxyvitamin D3 induces the synthesis of vitamin D-dependent calcium-binding protein in cultured chick kidney cells. Endocrinology. 1987;120:894–902. doi: 10.1210/endo-120-3-894. [DOI] [PubMed] [Google Scholar]
- Davis W.L., Jones R.G., Farmer G.R., Matthews J.L., Martin J.H., Bridges G. Electron microscopic cytochemical localization of a basolateral calcium adenosine triphosphatase in vitamin D replete chick enterocytes. Anat. Rec. 1987;219:384–393. doi: 10.1002/ar.1092190409. [DOI] [PubMed] [Google Scholar]
- Ebeid T.A., Suzuki T., Sugiyama T. High ambient temperature influences eggshell quality and calbindin-D28k localization of eggshell gland and all intestinal segments of laying hens. Poult. Sci. 2012;91:2282–2287. doi: 10.3382/ps.2011-01898. [DOI] [PubMed] [Google Scholar]
- Etches J.R. Calcium logistics in the laying hen. J. Nutr. 1987;117:619–628. doi: 10.1093/jn/117.3.619. [DOI] [PubMed] [Google Scholar]
- Eusebio-Balcazar P.E., Purdum S., Hanford K., Beck M.M. Limestone particle size fed to pullets influences subsequent bone integrity of hens. Poult. Sci. 2018;97:1471–1483. doi: 10.3382/ps/pex412. [DOI] [PubMed] [Google Scholar]
- Fleet J.C., Schoch R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010;47:181–195. doi: 10.3109/10408363.2010.536429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Effects of dietary particulate limestone, vitamin K3 and fluoride and photostimulation on skeletal morphology and osteoporosis in laying hens. Br. Poult. Sci. 2003;44:683–689. doi: 10.1080/00071660310001643688. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., Mccormack H.A., Whitehead C.C. Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. Br. Poult. Sci. 1998;39:434–440. doi: 10.1080/00071669889024. [DOI] [PubMed] [Google Scholar]
- Garlich J., Brake J., Parkhurst C.R., Thaxton J.P., Morgan G.W. Physiological profile of caged layers during one production year, molt, and postmolt: egg production, egg shell quality, liver, femur, and blood parameters. Poult. Sci. 1984;63:339–343. doi: 10.3382/ps.0630339. [DOI] [PubMed] [Google Scholar]
- Hilfiker H., Hattenhauer O., Traebert M., Forster I., Biber M.J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K., Hempel R., Rodehutscord M. Adaptation of epithelial sodium-dependent phosphate transport in jejunum and kidney of hens to variations in dietary phosphorus intake. Poult. Sci. 2006;85:1980–1986. doi: 10.1093/ps/85.11.1980. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Segawa H., Sasaki S., Hanazaki A., Fujii T., Kushi A., Kawabata Y., Kirino R., Sasaki S., Noguchi M., Kaneko I., Tatsumi S., Ueda O., Wada N.A., Tateishi H., Kakefuda M., Kawase Y., Ohtomo S., Ichida Y., Maeda A., Jishage K-I., Horiba N., Miyamoto K-I. Effect of Npt2b deletion on intestinal and renal inorganic phosphate (Pi) handling. Clin. Exp. Nephrol. 2017;22:517–528. doi: 10.1007/s10157-017-1497-3. [DOI] [PubMed] [Google Scholar]
- Jiang M.J., Zhao J.P., Jiao H.C., Wang X.J., Zhang Q., Lin H. Dietary supplementation with sodium bicarbonate improves calcium absorption and eggshell quality of laying hens during peak production. Br. Poult. Sci. 2015;56:740–747. doi: 10.1080/00071668.2015.1113499. [DOI] [PubMed] [Google Scholar]
- Jing M., Zhao S., Rogiewicz A., Slominski B.A., House J.D. Assessment of the minimal available phosphorus needs of laying hens: Implications for phosphorus management strategies. Poult. Sci. 2018;97:2400–2410. doi: 10.3382/ps/pey057. [DOI] [PubMed] [Google Scholar]
- Johnson J.A., Kumar R. Renal and intestinal calcium transport: roles of vitamin D and vitamin D- dependent calcium binding proteins. Semin. Nephrol. 1994;14:119–128. [PubMed] [Google Scholar]
- Li P., Wang R., Jiao H., Wang X., Zhao J., Hai L. Effects of dietary phosphorus level on the expression of calcium and phosphorus transporters in laying hens. Front. Physiol. 2018;9:627. doi: 10.3389/fphys.2018.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhao X., Wang S., Zhou Z. Letrozole induced low estrogen levels affected the expressions of duodenal and renal calcium-processing gene in laying hens. Gen. Comp. Endocrinol. 2018;255:49–55. doi: 10.1016/j.ygcen.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Marks J., Srai S.K., Biber J., Murer H., Unwin R.J., Debnam E.S. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp. Physiol. 2006;91:531–537. doi: 10.1113/expphysiol.2005.032516. [DOI] [PubMed] [Google Scholar]
- Melancon M.J., De Luca H.F. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry. 1970;9:1658–1664. doi: 10.1021/bi00810a002. [DOI] [PubMed] [Google Scholar]
- Miles R.D., Costa P.T., Harms R.H. The influence of dietary phosphorus level on laying hen performance, egg shell quality, and various blood parameters. Poult. Sci. 1983;62:1033–1037. doi: 10.3382/ps.0621033. [DOI] [PubMed] [Google Scholar]
- Neijat M., House J.D., Guenter W., Kebreab E. Calcium and phosphorus dynamics in commercial laying hens housed in conventional or enriched cage systems. Poult. Sci. 2011;90:2383–2396. doi: 10.3382/ps.2011-01401. [DOI] [PubMed] [Google Scholar]
- Parker S.L., Lindsay L.A., Herbert J.F., Murphy C.R., Thompson M.B. Expression and localization of Ca2+-ATPase in the uterus during the reproductive cycle of king quail (Coturnix chinensis) and zebra finch (Poephila guttata) Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;149:30–35. doi: 10.1016/j.cbpa.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Parsons A.H., Combs G.F. Blood ionized calcium cycles in the chicken. Poult. Sci. 1981;60:1520–1524. doi: 10.3382/ps.0601520. [DOI] [PubMed] [Google Scholar]
- Pasteels B., Parmentier M., Lawson E.M., Verstappen A., Pochet R. Calcium binding protein immunoreactivity in pigeon retina. Invest. Ophthalmol. Vis. Sci. 1987;28:658–664. [PubMed] [Google Scholar]
- Qin X., Klandorf H. Effect of estrogen in relation to dietary vitamin D3 and calcium on activity of intestinal alkaline phosphatase and Ca-ATPase in immature chicks. Gen. Comp. Endocrinol. 1993;90:318–327. doi: 10.1006/gcen.1993.1087. [DOI] [PubMed] [Google Scholar]
- Quinn S.J., Kifor O., Kifor I., Butters R.R., Brown E.M. Role of the cytoskeleton in extracellular calcium-regulated PTH release. Biochem. Biophys. Res. Commun. 2007;354:8–13. doi: 10.1016/j.bbrc.2006.12.160. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y., O'Brien S.P., Song W., Boulanger J.H., Stockmann A., Arbeeny C., Schiavi S.C. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009;20:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal T.P., Arango J., Wolc A., Brady J.V., O'Sullivan N.P. Commercial Hy-Line W-36 pullet and laying hen venous blood gas and chemistry profiles utilizing the portable i-STAT®1 analyzer. Poult. Sci. 2016;95:466–471. doi: 10.3382/ps/pev350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.M., Zhao J.P., Wang X.J., Jiao H.C., Wu J.M., Lin H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poult. Sci. 2018;97:2258–2266. doi: 10.3382/ps/pey092. [DOI] [PubMed] [Google Scholar]
- Wasserman R.H., Taylor A.N. Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966;152:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Werner A., Kinne R.K. Evolution of the Na-P(i) cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R301–R312. doi: 10.1152/ajpregu.2001.280.2.R301. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Wistedt A., Ridderstråle Y., Wall H., Holm L. Age-related changes in the shell gland and duodenum in relation to shell quality and bone strength in commercial laying hen hybrids. Acta. Vet. Scand. 2019;61:14. doi: 10.1186/s13028-019-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Angel R., Ashwell C.M. Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult. Sci. 2007;86:67–76. doi: 10.1093/ps/86.1.67. [DOI] [PubMed] [Google Scholar]
- Zylińska L., Kawecka I., Lachowicz L., Szemraj J. The isoform- and location-dependence of the functioning of the plasma membrane calcium pump. Cell. Mol. Biol. Lett. 2002;7:1037–1045. [PubMed] [Google Scholar]