Dear Editor,
Since the outbreak of Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, numerous SARS-CoV-2 variants of concern (VOCs), such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2), have emerged. Recently, the newly identified SARS-CoV-2 VOC, Omicron (B.1.1.529), has rapidly spread in many countries1 and is expected to replace Delta as the dominant variant circulating in the world. Compared to other VOCs, Omicron contains at least 32 mutations in spike (S) protein, including 15 mutations in the receptor-binding domain (RBD), and these mutations are known to confer resistance to neutralizing antibodies (nAbs) and sera of convalescent patients and people who have received COVID-19 vaccines.2–5 Therefore, vaccines able to induce potent and durable neutralizing antibodies against Omicron and other VOCs are urgently needed.
We recently developed a pan-sarbecovirus vaccine (CF501/RBD-Fc) consisting of human IgG Fc fragment-conjugated RBD (RBD-Fc) of the original SARS-CoV-2 WA1 strain as the immunogen and a novel small-molecule non-nucleotide STING agonist (CF501) (Fig. 1a) as the adjuvant.6,7 This CF501/RBD-Fc vaccine could elicit extremely potent nAb responses against SARS-CoV-2 WA1 strain and its 9 variants (Alpha, Beta, Gamma, Delta, Epsilon, Zeta, Eta, Iota and Kappa), SARS-CoV and SARS-related coronaviruses (SARSr-CoVs) from bats.7 Here, we investigated the ability of CF501/RBD-Fc vaccine to elicit nAbs in non-human primates (NHPs) and potently neutralize Omicron infection.
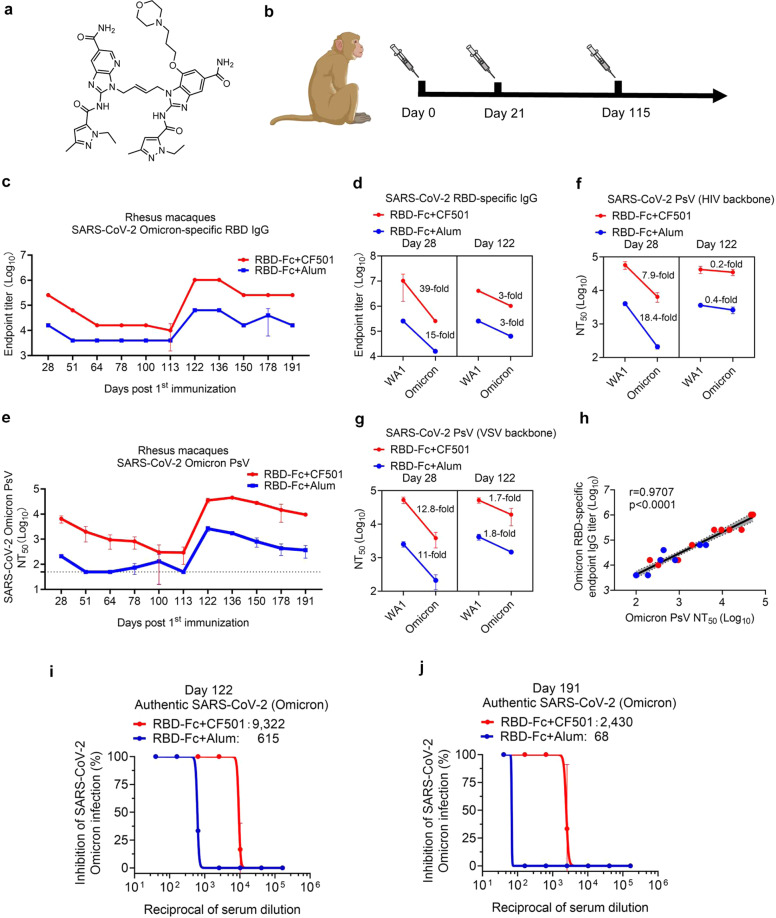
Fig. 1. CF501/RBD-Fc induced potent and durable binding and neutralizing antibodies against Omicron in rhesus macaques.
a The structure of CF501. b The immunization strategy. Rhesus macaques were intramuscularly immunized with the SARS-CoV-2 WA1 RBD-Fc formulated with CF501 or Alum at days 0, 21 and 115. c Evaluation of the Omicron RBD-specific IgG endpoint titers in the pooled sera from the immunized macaques at the indicated time points. Data are shown as means ± SD. d The fold reduction of Omicron RBD-specific IgG endpoint titer in the pooled sera from the immunized macaques at days 28 and 122 post 1st immunization compared to the WA1 RBD-specific IgG endpoint titer. Data are shown as means ± SD. e Detection of the nAb titer against the Omicron PsV (HIV backbone) at the indicated time points in Huh-7 cells. Data are shown as means ± SD. f, g Fold reduction of nAb titer against Omicron PsV for HIV backbone (f) and VSV backbone (g) in the pooled sera from the immunized macaques at days 28 and 122, compared to the titer of nAbs against SARS-CoV-2 WA1 PsV. Data are shown as means ± SD. h Correlation between the Omicron RBD-specific IgG endpoint titer and the nAb titer against Omicron PsV. Spearman rank test was used to perform the correlation analysis. i, j Titers of nAbs in the pooled sera at day 122 (i) and 191 (j) in the CF501/RBD-Fc and Alum/RBD-Fc groups against authentic Omicron in Vero-E6-TMPRSS2 cells. Data are shown as means ± SD.
As we previously described,7 rhesus macaques were assigned into two groups and were immunized with CF501/RBD-Fc (n = 3) or Alum/RBD-Fc (n = 3), respectively, at days 0, 21 and 115 (Fig. 1b). First, we evaluated the titer of Omicron or WA1 RBD-specific IgG in sera of these immunized NHPs. After two immunizations, the Omicron RBD-specific IgG endpoint titer reached to 256,000 in the sera from the CF501/RBD-Fc group at day 28, about 15-fold higher than that of the Alum/RBD-Fc group (16,000) (Fig. 1c). The Omicron RBD-binding endpoint titers in the sera of CF501/RBD-Fc and Alum/RBD-Fc groups were about 39- and 15-fold lower than the WA1 RBD-binding endpoint titers (Fig. 1d). To monitor the antibody dynamics, we further tested the Omicron RBD-binding IgG endpoint titers at days 51, 64, 78, 100, and 113 post 1st immunization, respectively. We found that the Omicron RBD-binding IgG endpoint titers in the sera from both the CF501/RBD-Fc and Alum/RBD-Fc groups had gradually decreased (Fig. 1c). However, during this period, IgG titers in the CF501/RBD-Fc group remained consistently higher than those in Alum/RBD-Fc group (Fig. 1c). Surprisingly, after the third immunization, the Omicron RBD-binding endpoint IgG titers surged to 1,024,000 (CF501/RBD-Fc) and 64,000 (Alum/RBD-Fc), respectively, at day 122 post 1st immunization. The Omicron RBD-binding endpoint titers in sera of both CF501/RBD-Fc and Alum/RBD-Fc groups were about 3-fold lower than the WA1 RBD-binding endpoint titers (Fig. 1d). We then further evaluated the Omicron RBD-specific IgG titer at days 136, 150, 178 and 191 post 1st immunization, respectively, and found that the Omicron RBD-binding IgG endpoint titer in sera of the CF501/RBD-Fc group continued to maintain high levels after the 3rd immunizations (Fig. 1c). Therefore, even though the Omicron RBD-binding IgG endpoint titers in sera of both CF501/RBD-Fc and Alum/RBD-Fc groups were lower than the WA1 RBD-binding IgG endpoint titer, the Omicron RBD-binding IgG endpoint titers in sera of the CF501/RBD-Fc group were consistently and significantly higher than those in Alum/RBD-Fc group. The 3rd immunization could indeed make a quantitatively significant improvement in the immunogenicity of the pan-sarbecovirus vaccine to elicit high titers of RBD-specific IgG.
Subsequently, we assessed the titers of nAbs in these sera against Omicron and WA1 pseudoviruses (PsVs). After two immunizations, CF501/RBD-Fc elicited potent nAbs against Omicron PsV (HIV backbone) with 50% neutralization titer (NT50) of 6469 at day 28 post 1st immunization, about 30-fold higher than that of Alum/RBD-Fc (Fig. 1e). The titers of nAbs elicited by CF501/RBD-Fc and Alum/RBD-Fc against Omicron PsV were about 7.9- and 18.4-fold lower than those against SARS-CoV-2 WA1 PsV (Fig. 1f). We noted no detectable nAbs against Omicron PsV in the Alum/RBD-Fc group at day 51 post 1st immunization (Fig. 1e). Consistent with the results of Omicron RBD-binding IgG, the nAbs after 2nd immunization against Omicron PsV in the CF501/RBD-Fc group had also gradually decreased. However, the CF501/RBD-Fc group still maintained a relatively high titer of nAbs against Omicron PsV during days 28–113 (NT50 of 293 at day 113) (Fig. 1e). Again, however, after the 3rd immunization, the nAbs against Omicron PsV in sera from the CF501/RBD-Fc group surged to an extremely high level (NT50: 35,066), a level of nAbs similar to that against SARS-CoV-2 WA1 PsV and about 12-fold higher than that observed in the Alum/RBD-Fc group (NT50: 2620) (Fig. 1e). As a control, the HIV neutralizing mAb N68 could effectively neutralize the HIV-1 Bal.1 PsV, while the sera from the immunized macaques could not inhibit the entry of HIV Bal.01 PsV which contains the same backbone as the SARS-CoV-2 WA1 and Omicron PsVs, confirming that the neutralizing activity of the sera against SARS-CoV-2 WA1 and Omicron is specific (Supplementary information, Fig. S1). To confirm the results, we further used VSV backbone-based Omicron and WA1 PsVs to test these sera. Results from the neutralization assay using HIV backbone-based PsV (Fig. 1f) were consistent with those from the neutralization assay using the VSV backbone-based PsV (Fig. 1g). Most importantly, sera from the CF501/RBD-Fc group maintained extremely high levels of nAbs (NT50: 9520–45,323) against the Omicron PsV during days 122–191 post 1st immunization, following the third immunization (Fig. 1e). It is worth noting that the nAb titers after the 3rd immunization decreased slower compared to that after the 2nd immunization, suggesting that the second boost (i.e., the 3rd immunization) plays a more important role in extending the durability of nAb against Omicron. We also found a strong correlation (r = 0.9707, P < 0.0001) between Omicron-binding endpoint IgG titer and Omicron-neutralizing antibody titer (Fig. 1h). When we tested the titers of nAbs in sera for activity against SARS-CoV-2 Mu (B.1.621), a variant of interest (VOI), we found that nAbs elicited by CF501/RBD-Fc could also potently neutralize PsV of Mu variant with NT50 of 21,826 and 35,697 for sera collected from NHPs one week after the 2nd and 3rd immunizations, respectively (28 and 122 days after the 1st immunization) (Supplementary information, Fig. S2). In addition, the titers of nAbs elicited by CF501/RBD-Fc against Mu PsV were about 1.6- and 0.1-fold lower than those against WA1 PsV after the 2nd and 3rd immunizations, respectively (Supplementary information, Fig. S2).
Next, we assessed the titer of nAbs in macaque sera against the authentic Omicron variant (hCoV-19/Hong Kong/HKU-344/2021). Again, the sera from CF501/RBD-Fc group could neutralize authentic SARS-CoV-2 Omicron with NT50 of 9322 at day 122 (Fig. 1i), about 14-fold higher than that in sera from the Alum/RBD-Fc group (NT50: 615) and only about 1.3-fold lower than that against the authentic SARS-CoV-2 (HKU-001a) reference strain (Supplementary Information, Fig. S3). Most importantly, CF501/RBD-Fc elicited nAb titer against the authentic Omicron at day 191 could attain 2430 (Fig. 1j), suggesting that the nAb responses against Omicron induced by the 2nd booster are more potent and durable compared with that by the 1st booster (i.e., the 2nd immunization).
In sum, after two immunizations, the titer of nAbs against Omicron PsV in the CF501/RBD-Fc group was significantly higher than that in the Alum/RBD-Fc group, confirming the superior adjuvanticity of CF501 over Alum. Notably, the titer of nAbs against Omicron PsV induced by the 2nd immunization with CF501/RBD-Fc was much lower than that against SARS-CoV-2 WA1 PsV. However, after the 3rd immunization, the titer of nAbs against Omicron PsV induced by 3rd immunization with CF501/RBD-Fc immediately jumped to an extremely high level, nearly as high as that against the WA1 strain. A similar result was also obtained from the neutralization assay using the authentic SARS-CoV Omicron variant. Collectively, these results support the hypothesis that an additional boost immunization is necessary to increase nAb responses against both WA1 and variants of SARS-CoV-2 and suggest that this CF501/RBD-Fc-based vaccine could be used to prevent infection by the current SARS-CoV-2 variants, including Omicron, and future emerging sarbecoviruses.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92169112 to S.J.; 82041036 to L.L.); the Health and Medical Research Fund (COVID1903010 – Project 7 to J.F.-W.C.), The Government of the Hong Kong Special Administrative Region; Health@InnoHK, Innovation and Technology Commission, The Government of the Hong Kong Special Administrative Region (to K.-Y.Y.); the National Key Research and Development Programme on Public Security Risk Prevention and Control Emergency Project (to K.-Y.Y.); the National Program on Key Research Project of China (2020YFA0707500 and 2020YFA0707504 to J.F.-W.C; 2021YFC2300703 to L.L.); the National Key R&D Program of China (2021YFC2300703 to L.L.); Program of Shanghai Academic/Technology Research Leader (20XD1420300 to L.L.). and China Postdoctoral Science Foundation (2021M700840 to Z.L.).
Author contributions
L.L. and S.J. conceived the idea; L.L., S.J., K.-Y.Y., and Y.W. supervised the project; Z.L., J.F.-W.C., J.Z., M.W., G.Z. and K.K.-H. C. performed the experiments; Y.Z. provided CF501; Z.L. J.F.-W.C., Q.W., and W.X. analyzed the data; Z.L., L.L., S.J. wrote the manuscript; K.-Y.Y., J.F.-W.C., Y.W. and Y.Z. revised the manuscript.
Competing interests
L.L., S.J., Z.L, J.Z., W.X., Q.W. and Y.Z. filed a patent application for the STING agonist of CF501. J.F.-W.C. has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited and was an invited speaker for Gilead Sciences Hong Kong Limited and Luminex Corporation. Other authors declare that they have no competing interests.
Footnotes
These authors contributed equally: Zezhong Liu, Jasper Fuk-Woo Chan, Jie Zhou, Meiyu Wang.
Contributor Information
Youchun Wang, Email: wangyc@nifdc.org.cn.
Kwok-Yung Yuen, Email: kyyuen@hku.hk.
Lu Lu, Email: lul@fudan.edu.cn.
Shibo Jiang, Email: shibojiang@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41422-022-00631-z.
References
- 1.WHO. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (2021).
- 2.Carreño, J. M. et al. Nature10.1038/d41586-021-03846-z (2022).
- 3.Cao, Y. et al. Nature10.1038/d41586-021-03796-6 (2022).
- 4.Wang Y, et al. Emerg. Microbes Infect. 2022;11:1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejnirattisai W, et al. Cell. 2022;185:467–484. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, et al. Signal. Transduct. Target Ther. 2022;27:282. [Google Scholar]
- 7.Liu, Z. et al. Cell Res. 10.1038/s41422-022-00612-2 (2022).
- 8.Huang J, et al. Immunity. 2016;45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.