Abstract
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a CD30-positive, anaplastic lymphoma kinase-negative T-cell lymphoma. Where implant history is known, all confirmed cases to date have occurred in patients with exposure to textured implants. The etiopathogenesis of BIA-ALCL is likely to be multifactorial, with current evidence-based theories recognising the combination of chronic infection in setting of textured implants, gram-negative biofilm formation, chronic inflammation, host genetics (e.g. JAK/STAT, p53) and time in tumorigenesis. Proposed triggers for the development of malignancy are mechanical friction, silicone implant shell particulates, silicone leachables and bacteria. Of these, the bacterial hypothesis has received significant attention, supported by a plausible biological model. In this model, bacteria form an adherent biofilm in the favourable environment of the textured implant surface, producing a bacterial load that elicits a chronic inflammatory response. Bacterial antigens, primarily of gram-negative origin, may trigger innate immunity and induce T-cell proliferation with subsequent malignant transformation in genetically susceptible individuals. Future research, investigating BIA-ALCL genetic mutations and immunological modulation with Gram-negative biofilm in BIA-ALCL models is warranted to establish a unifying theory for the aetiology of BIA-ALCL.
Keywords: Antigens, Bacterial, Breast implants, Lymphoma, T-cells
Abbreviations: BIA-ALCL, Breast implant-associated anaplastic large cell lymphoma
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a recently recognised and distinct malignancy of T lymphocytes exclusively associated with textured breast implants used for both aesthetic and reconstructive surgery.1 There is a spectrum of disease presentation, with the most commonly occurring as a seroma with an indolent course. A less common presentation occurs as locally advanced mass disease or rarely as metastatic disease. The pathognomic histological response to breast implant insertion is benign capsule formation. Smooth surface implants are associated with higher rates of benign capsular contracture,2,3 because they predispose to a planar arrangement of fibroblasts with organised collagen deposition around implants. By contrast, textured implants typically have grooves larger than the diameter of a fibroblast,4,5 disrupting the planar arrangement of cells and reducing the risk of capsular contracture. Surface texturing on the external silicone shell of breast implants may involve salt loss, gas diffusion, imprinting and other recent proprietary “nano” texturing techniques.6,7
The exact pathogenic mechanisms surrounding BIA-ALCL are unclear. Prevailing theories recognise the role of the breast microbiome and textured implants, which potentially trigger malignant transformation in the milieu of sustained antigen-driven inflammation. Despite the reduction of capsular contracture rates with implant texturisation,2,3 their greater surface area and rough interface enhances bacterial adhesion and biofilm burden.8,9
Anaplastic large cell lymphoma
Primary breast lymphomas are rare, accounting for 0.04%–0.5% of all breast cancers.10,11 The vast majority of these lymphomas are derived from B-lymphocytes and less than 10% are of T-cell origin.10, 11, 12 Anaplastic Large Cell Lymphoma (ALCL) is a subset of T-cell lymphoma that includes systemic, primary cutaneous and breast implant-associated subtypes. Systemic ALCL is further subcategorised according to the expression or absence of the anaplastic lymphoma kinase (ALK) protein; ALK-positive disease is most commonly the result of 2p23/ALK aberrations, including the classic t(2;5)(p23;q35)/NPM1-ALK translocation. Systemic ALCL with negative ALK expression confers a poor prognosis with a reported 5-year estimated survival of 40%–60%.13 Cutaneous ALCL falls with the group of so-called primary cutaneous CD30-positive lymphoproliferative diseases that include the very indolent condition of lymphomatoid papulosis which manifests as spontaneous waxing and waning skin papules that self-resolve, to the potentially more aggressive primary cutaneous ALCL. Importantly, and unlike its systemic counterpart, primary cutaneous ALCL is typically ALK-negative yet has a comparably very favourable 10-year survival rate of 90%.14 BIA-ALCL is a CD30+, ALK-negative lymphoma with a typically indolent progression.15 In summary, although the spectrum of ALCL has a similar histological appearance, their clinical behaviour across the various types varies widely.
Immunology
A cogent synthesis of BIA-ALCL pathobiology warrants a brief overview of the relevant T cell immunology.
T-cell development and activation
The T lymphocyte coordinates the adaptive immune response and together with the B lymphocyte, is responsible for generating immunologic memory against pathogens. T-cell programming is controlled by the T-cell receptor (TCR), which is generated by recombination of genomic DNA sequences in the thymus.16 Thymic progenitors rearrange the TCR genes in a temporal and location-specific manner.17 These naïve cells migrate from the thymus to secondary lymphoid tissues (e.g. spleen, lymph nodes) and are then activated by the coordinated interactions between an antigen-presenting cell, which binds an antigen to a major histocompatibility (MHC) class I or II molecule.16 The TCR (composed of two chains, α and β) associates with a complex of membrane glycoproteins proteins, the cluster differentiation (CD) family, to stabilise the antigen and MHC complex into an immunological synapse. Other co-stimulatory signals are required for complete T-cell activation.16
T-cell differentiation
The initial differentiation from a naïve T cell either into a T helper (CD4+) or T cytotoxic (CD8+). Once activated, the CD4+ cells can further differentiate into a number of subsets including Th1 (intracellular viral and bacterial pathogens), Th2 (large extracellular pathogens and allergic response), Th9 (parasitic infections), Th17 (mucosal immunity and autoimmune disorders), Th22 (inflammatory skin disorders), TFH (follicular helper T cells regulate B cell activity) and Treg (inhibit pro-inflammatory T cells).16 Although T-cell lymphomas have been associated with viral infection such as EBV infection (Nasal NK lymphoma, Hydroavaccineforme-like lymphoma) and HTLV-I (Adult T-cell lymphoma/leukaemia), no viral elements (i.e. EBER) have been found in BIA-ALCL to date.
Pathogenesis
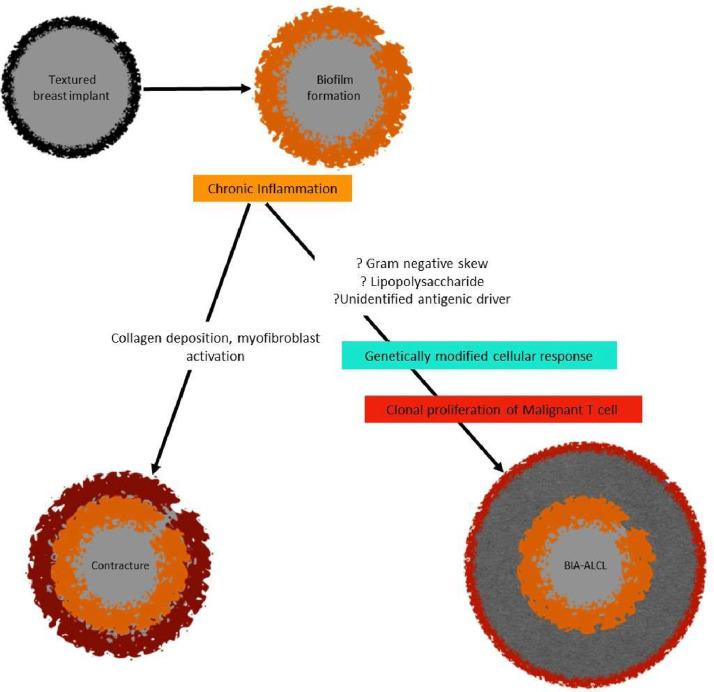
The prevailing hypothesis for BIA-ALCL formation unifies biofilm, implant texturing, chronic inflammation, and genetics in tumourigenesis (Fig. 1). Chronic antigenic stimulation is a key initiator and driver of lymphogenesis.18
Fig. 1.
A unifying hypothesis for BIA-ALCL, recognising the potential role of biofilm, chronic inflammation, textured implants and genetics.
Chronic inflammation
Although the exact pathogenesis of BIA-ALCL has not been delineated, chronic inflammation has been hypothesised as a precursor to tumourigenesis. A sustained antigenic stimulus promotes a chronic inflammatory milieu, associated with the induction of reactive oxygen and nitrogen species, microRNA instability and epigenetic changes, promoting genetic instability19 (Fig. 1). There is evidence that cutaneous T-cell lymphomas are preceded by chronic inflammation.20 Similarly, the biological peptide, gluten, in combination with the intestinal microbiome, has been shown to drive T-cell receptor changes in patients with coeliac disease towards T-cell lymphoma transformation.21,22
Supporting the chronic inflammation hypothesis, Lechner et al.23 detected high production of T-cell-associated cytokines IL-6 and IL-10 in their BIA-ALCL model. Autocrine IL-6 production has also been identified as a driver of tumourigenesis in some diffuse large B-cell lymphomas, as well as solid tumours including breast, lung and ovarian carcinomas.24,25 Moreover, Kadin et al.26 confirmed a Th17/Th1 phenotype of BIA-ALCL tumour lymphocytes, supporting the potential role of antigenic stimulation and chronic inflammation. Furthermore, Wolfram et al.27 showed intracapsular T cells producing IL-17, IFN-ϒ, IL-6, IL-8 and TGF-β, suggesting a Th17/Th1 weighted local immune response in silicone implants with capsular fibrosis. Nonetheless, it is clear that more research is required to evaluate the cytokine milieu and cellular phenotype of this condition.
Biofilm, implant texturing and the ALCL microbiome
The observation of chronic inflammation and the Th17/Th1 phenotype of BIA-ALCL lymphocytes supports the potential association between bacterial biofilms and tumourigenesis.17 Other potential drivers of chronic inflammation include silicone particles,27,28 friction29 and heavy metals.30 With regard to silicone, there is no data to show that silicone particles or implant surfaces alone can initiate an immune response, sufficient to drive a T cell to malignancy.31 Regarding biofilms specifically, microbial adhesion to implant surfaces is facilitated by mechanical factors (pili/ flagellae) and bacterial secretion of extra-cellular polysaccharide (EPS) matrix. Following attachment, bacterial proliferation and EPS matrix synthesis occurs, resulting in the formation of highly structured microcolonies.3,9,32 Dispersion of biofilm cells through active or passive detachment may contribute to local extension over the implant surface. The biofilm architecture contributes to host-resistance and survival advantages.3,9,20,32 The recruitment of macrophages and myofibroblasts, within the chronic inflammatory milieu, contributes to fibrous capsule formation around the implant.33
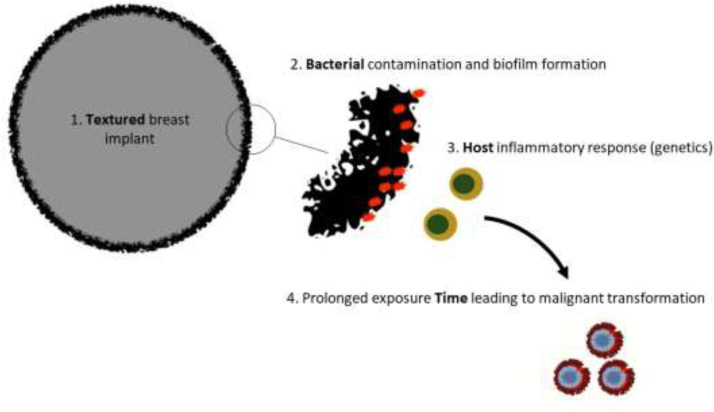
A hypothesis involving the role of bacteria in the aetiology of BIA-ALCL has been proposed34,35 (Fig. 2). In this model, colonisation by bacteria of textured implants having a high surface area produces a biofilm, which, when the bacterial load exceeds a certain threshold value, leads to chronic antigen stimulation in genetically susceptible individuals. Eventually transformation occurs, leading to the emergence and proliferation of monoclonal CD30-positive ALK-negative T cells and the development of BIA-ALCL. Dysregulation of the JAK1/STAT3 pathway in affected cells is likely involved, and the observed process is a slow one, typically requiring 8–12 years between implantation and BIA-ALCL diagnosis.34,35
Fig. 2.
Proposed hypothesis for the genesis of BIA-ALCL.
All reported cases of BIA-ALCL have exclusively occurred in textured implants.36 Despite the benefits of tissue incorporation with texturisation, several in vitro studies have reported higher rates of biofilm formation in textured implants compared to smooth implants.4,37, 38, 39 This is attributed to their greater surface area and the enhanced bacterial adhesion on rough surfaces. Hu et al.8 reported that contamination of textured implants supports 30 times more biofilm bacteria than contaminated smooth implants. The authors also showed a linear correlation between the bacterial load in implant capsules and the number of activated lymphocytes and bacterial burden. The correlation was strongest for CD4+ T cells, the same phenotype as BIA-ALCL tumour cells.8
Despite the antigenic stimulus and incumbent T cell proliferation, not all implant-associated biofilms and their capsules develop a malignant progeny (Fig. 1). In a study evaluating the microbiome of BIA-ALCL and non-tumour capsule samples, Hu et al.9 reported a gram-negative shift in ALCL specimens, with significantly greater proportions of Ralstonia spp. observed. Ralstonia spp., which are non-fermenting gram-negative bacilli found in soil and water, have been reported in nosocomial infections resulting from contamination of medical solutions.9,40 Contrastingly, Staphylococcus epidermidis, a normal constituent of skin and endogenous breast microflora, is the most frequently identified organism on benign breast capsules.41, 42, 43, 44, 45 Reflecting these contrasting microbiomes, Hu et al.9 hypothesised two pathways of inflammation associated with breast implants: i) Gram-positive biofilm favouring fibrosis and capsular contracture formation and ii) Gram-negative biofilm favouring potential T-cell oncogene activation and malignant transformation (BIA-ALCL)
Helicobacter pylori: an exemplum of microbiome induced malignancy
The association between Ralstonia spp. and BIA-ALCL parallels the link between H. pylori and lymphomas of gastric mucosa-associated lymphoid tissue (MALT). Like the Ralstonia spp., H. pylori is also a non-fermenting gram-negative bacillus.40 Chronic inflammation induced by H. pylori, which is mediated by direct activation of T cells, drives B cell mutations in MALT tissue leading to the emergence of a neoplastic B cell clone.8,46 H. pylori virulence factors such as cytotoxin-associated gene A protein deregulate intra-cellular signalling pathways, promoting tumourigenesis.46,47 However, unlike BIA-ALCL, lymphoma arising from H. pylori infection is of B-cell origin. This cellular difference possibly reflects the preferential activation of T-cell oncogenes in breast capsules in BIA-ALCL.
Bacterial super-antigens and lipopolysaccharide
Several hypotheses have recently been proposed to help explain the association between bacterial antigens and BIA-ALCL tumourigenesis (Fig. 1). The lipopolysaccharide coat of gram-negative bacteria, which is linked to other infectious and autoimmune diseases, has been proposed as a potential malignant trigger.48 Bacterial superantigens may also play a role in tumourigenesis. Superantigens bind directly to the outer leaflet of the MHC Class II and the variable β chain (Vβ) of the T-cell receptor, causing direct activation and hyperstimulation of T cells of all subtypes.49 Unlike conventional antigens that activate less than 0.01% of T cells, superantigens activate around 20% of quiescent T cells causing a massive induction of cytokine release and T cell differentiation through a preferential Th1 pathway.49 Importantly, bacterial superantigens have been shown to be the only means to restrict the expansion of polyconal T cells in the Vβ region.50 Kadin et al.51 showed an expanded CD30+ TCRVβ T cell population in a case of BIA-ALCL, potentially implicating a role for a yet undefined, bacterial superantigen. No such antigen has been identified for Ralstonia spp. or related species to date.
Despite the associations, the observation of Ralstonia spp. in the microbiome does not prove causation of BIA-ALCL. Critics suggest that the Ralstonia spp. predominance in BIA-ALCL samples may represent an “opportunistic” infection drawn into the peri‑tumoural region by chemotaxis or complex signal transduction.52,53 Research investigating host-bacteria interaction and virulence is warranted.
Genetic mutations
BIA-ALCL is likely a multifactorial disease of at-risk patients and a genetic predisposition for the development of BIA-ALCL continues to be explored.
There have been recent reports regarding the somatic genetic lesions (Fig. 1) associated with BIA-ALCL.4,23,26,54, 55, 56, 57, 58 Moreover, some have postulated that germline mutations may predispose to this condition.54,59,60 Future BIA-ALCL genomic studies, investigating potential germline and somatic tumour mutations would be beneficial.
Janus kinase and signal transducer and activator of transcription (JAK-STAT)
JAK-STAT pathway has been shown to mediate inflammation-associated cancers and been reported to have a key role in BIA-ALCL.61 The JAK-STAT pathway regulates embryonic development and signalling and is implicated in cell proliferation, differentiation and apoptosis.54,56 The pathway is deregulated across various types of T-cell lymphomas; however, the extent of deregulation is significantly higher in BIA-ALCL,26,54,56,57 present in 60% of cases according to a recent larger study.61
The exact role of JAK/STAT signalling of oncogenesis in myoproliferative neoplasms is not understood. The mutations cause a hereditary thrombocytosis, but haematopoiesis is polyclonal and individuals do not develop haematological malignancies or solid tumours, suggesting that JAK/STAT activation alone does not drive malignant disease but are thought to promote survival of malignant cells.62 The consistent activation of the JAK-STAT3 pathway also suggests potential therapeutic options for BIA-ALCLs.
Several factors are likely to contribute to dysregulation of JAK-STAT. A loss of function SOCS1 mutation, causing constitutive activation of the JAK-STAT pathway, has been associated with BIA-ALCL.56 IL-2 and IL-6 overexpression has also been implicated in autocrine activation of JAK-STAT pathway in-vitro studies.23 Kadin et al.26 reported overexpression of SOC2 and SOC3 in primary cutaneous and BIA-ALCL cases.
Other
A recent comprehensive genetic analysis of 29 patients found that Genome-wide chromosomal copy number aberrations (CNAs) were detected in 94% of BIA-ALCLs, with losses at chromosome 20q13.13 in 66% of the samples supporting previous studies.58,61,63,64 Loss of 20q13.13 was reported to be characteristic of BIA-ALCL compared to other classes of ALCL.
Germline mutations have been recognised in patients with BIA-ALCL including activating mutations of TP5359,60) as well as mutations of JAK3.54 TP53 mutations are associated with hereditary cancer syndromes and confer an increased risk to a variety of solid organ malignancies but the relevance of germline mutations as predisposing to this disease requires further exploration. One study involving 13 patients found the human leucocyte antigen allele A*26 to be significantly (p < 0.001) less often compared with the general population.64 Larger studies are needed to better understand the role of patient genetics in the development of BIA-ALCL.
Conclusion
The etiopathogenesis of BIA-ALCL is likely to be multifactorial, with current evidence-based theories recognising the combination of chronic infection (skewed toward Gram-negative organisms), biofilm formation, implant texturisation, chronic inflammation, host genetics and time in tumorigenesis, providing the preconditions for T-cell malignant transformation. Although silicone bleeds and particles can theoretically provide the antigenic stimulus for chronic inflammation and potential malignant transformation, this premise has yet to be scientifically proven. Additional research is required to establish a unifying theory for the aetiology of BIA-ALCL. Future directions for research should include the identification of specific bacterial antigens or other antigenic stimuli, differential studies of benign and malignant seromas (cytokines, inflammatory, and CD30+ count), and additional genetic sequencing studies in BIA-ALCL cases. Current thinking will continue to evolve as more robust scientific evidence accrues, potentially facilitating better surveillance and treatment.
Declaration of Competing Interest
Prof. Deva is a consultant, research coordinator, educator to Allergan, Mentor (Johnson & Johnson), Sientra, Motiva and Acelity (KCI.) He has previously coordinated industry-sponsored research for these companies relating to both biofilms and breast prostheses.
Dr S. Lajevardi, Dr P. Rastogi and Dr D. Isacson have no affiliations or financial interests to disclose.
Acknowledgments
Funding
None.
Ethical approval
N/A.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2021.11.006.
Appendix. Supplementary materials
References
- 1.Rastogi P., Riordan E., Moon D., Deva A.K. Theories of Etiopathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg. 2019;143:23s–29s. doi: 10.1097/PRS.0000000000005566. [DOI] [PubMed] [Google Scholar]
- 2.Barnsley G.P., Sigurdson L.J., Barnsley S.E. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 3.Wong C.H., Samuel M., Tan B.K., Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118:1224–1236. doi: 10.1097/01.prs.0000237013.50283.d2. [DOI] [PubMed] [Google Scholar]
- 4.Ajdic D., Zoghbi Y., Gerth D., Panthaki Z.J., Thaller S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016;36:297–309. doi: 10.1093/asj/sjv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Braber E.T., de Ruijter J.E., Smits H.T., et al. Effect of parallel surface microgrooves and surface energy on cell growth. J Biomed Mater Res. 1995;29:511–518. doi: 10.1002/jbm.820290411. [DOI] [PubMed] [Google Scholar]
- 6.Henderson P.W., Nash D., Laskowski M., Grant R.T. Objective Comparison of Commercially Available Breast Implant Devices. Aesthetic Plast Surg. 2015;39:724–732. doi: 10.1007/s00266-015-0537-1. [DOI] [PubMed] [Google Scholar]
- 7.Sforza M., Zaccheddu R., Alleruzzo A., et al. Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: a Single-Center Experience With 5813 Consecutive Breast Augmentation Cases. Aesthet Surg J. 2018;38:S62–S73. doi: 10.1093/asj/sjx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H., Jacombs A., Vickery K., et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg. 2015;135:319–329. doi: 10.1097/PRS.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 9.Hu H., Johani K., Almatroudi A., et al. Bacterial Biofilm Infection Detected in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. doi: 10.1097/PRS.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 10.Shahriari N., Ferenczi K., Heald P.W. Breast implant-associated anaplastic large cell lymphoma: a review and assessment of cutaneous manifestations. Int J Womens Dermatol. 2017;3:140–144. doi: 10.1016/j.ijwd.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talwalkar S.S., Miranda R.N., Valbuena J.R., et al. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 12.Blombery P., Prince H.M., Seymour J.F. Primary Breast Lymphoma-Population-Level Insights into an Infrequent but Increasingly Recognized Subtype of Lymphoma. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djx010. [DOI] [PubMed] [Google Scholar]
- 13.Hapgood G., Savage K.J. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17–25. doi: 10.1182/blood-2014-10-567461. [DOI] [PubMed] [Google Scholar]
- 14.Savage K.J., Harris N.L., Vose J.M., et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 15.Clemens M.W., Medeiros L.J., Butler C.E., et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol. 2016;34:160–168. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennock N.D., White J.T., Cross E.W., et al. T cell responses: naive to memory and everything in between. Adv Physiol Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner S. An exploration into the orgins and pathogenesis of anaplastic large cell lymphoma, anaplastic lymphoma kinase (ALK)-positive Cancers 2017: 9.
- 18.Malcolm T.I., Hodson D.J., Macintyre E.A., Turner S.D. Challenging perspectives on the cellular origins of lymphoma. Open Biol. 2016:6. doi: 10.1098/rsob.160232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe D.B., Storkus W.J. Chronic inflammation and immunologic-based constraints in malignant disease. Immunotherapy. 2011;3:1265–1274. doi: 10.2217/imt.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burg G., Kempf W., Haeffner A., et al. From inflammation to neoplasia: new concepts in the pathogenesis of cutaneous lymphomas. Recent Results Cancer Res. 2002;160:271–280. doi: 10.1007/978-3-642-59410-6_32. [DOI] [PubMed] [Google Scholar]
- 21.Han A., Newell E.W., Glanville J., et al. Dietary gluten triggers concomitant activation of CD4+ and CD8+ alphabeta T cells and gammadelta T cells in celiac disease. Proc Natl Acad Sci U S A. 2013;110:13073–13078. doi: 10.1073/pnas.1311861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy M.Y., Tye-Din J.A. Coeliac disease: a unique model for investigating broken tolerance in autoimmunity. Clin Transl Immunology. 2016;5:e112. doi: 10.1038/cti.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechner M.G., Megiel C., Church C.H., et al. Survival signals and targets for therapy in breast implant-associated ALK–anaplastic large cell lymphoma. Clin Cancer Res. 2012;18:4549–4559. doi: 10.1158/1078-0432.CCR-12-0101. [DOI] [PubMed] [Google Scholar]
- 24.Sansone P., Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao S.P., Mark K.G., Leslie K., et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadin M.E., Deva A., Xu H., et al. Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2016;36:773–781. doi: 10.1093/asj/sjw023. [DOI] [PubMed] [Google Scholar]
- 27.Wolfram D., Rabensteiner E., Grundtman C., et al. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast Reconstr Surg. 2012;129:327e–337e. doi: 10.1097/PRS.0b013e31823aeacf. [DOI] [PubMed] [Google Scholar]
- 28.Fleury E.F., Rego M.M., Ramalho L.C., et al. Silicone-induced granuloma of breast implant capsule (SIGBIC): similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer (Dove Med Press) 2017;9:133–140. doi: 10.2147/BCTT.S126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzocchi M., Dessy L.A., Corrias F., Scuderi N. A clinical study of late seroma in breast implantation surgery. Aesthetic Plast Surg. 2012;36:97–104. doi: 10.1007/s00266-011-9755-3. [DOI] [PubMed] [Google Scholar]
- 30.Ravi-Kumar S., Sanaei O., Vasef M., Rabinowitz I., Fekrazad M.H. Anaplastic large cell lymphoma associated with breast implants. World J Plast Surg. 2012;1:30–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Cappellano G., Ploner C., Lobenwein S., et al. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mladick R.A. Significance of Staphylococcus epidermidis causing subclinical infection. Plast Reconstr Surg. 2005;115:1426–1427. doi: 10.1097/01.prs.0000157604.65522.be. author reply 27-8. [DOI] [PubMed] [Google Scholar]
- 33.Clinton A., Carter T. Chronic Wound Biofilms: pathogenesis and Potential Therapies. Lab Med. 2015;46:277–284. doi: 10.1309/LMBNSWKUI4JPN7SO. [DOI] [PubMed] [Google Scholar]
- 34.Loch-Wilkinson A., Beath K.J., Knight R.J.W., et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-Surface-Area Textured Implants Are Associated with Increased Risk. Plast. Reconstr. Surg. 2017;140:645–654. doi: 10.1097/PRS.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 35.Deva A.K. Response to "breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): why the search for an infectious etiology may be irrelevant". Aesthetic Surgery Journal. 2017;37:NP122–NNP28. doi: 10.1093/asj/sjx133. [DOI] [PubMed] [Google Scholar]
- 36.Loch-Wilkinson A., Beath K.J., Knight R.J.W., et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: high-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg. 2017;140:645–654. doi: 10.1097/PRS.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 37.Myint A.A., Lee W., Mun S., et al. Influence of membrane surface properties on the behavior of initial bacterial adhesion and biofilm development onto nanofiltration membranes. Biofouling. 2010;26:313–321. doi: 10.1080/08927010903576389. [DOI] [PubMed] [Google Scholar]
- 38.Teughels W., Van Assche N., Sliepen I., Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17(Suppl 2):68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 39.Jacombs A., Tahir S., Hu H., et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133:471e–480e. doi: 10.1097/PRS.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 40.Ryan M.P., Adley C.C. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 2014;33:291–304. doi: 10.1007/s10096-013-1975-9. [DOI] [PubMed] [Google Scholar]
- 41.Pajkos A., Deva A.K., Vickery K., et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 42.Rieger U.M., Mesina J., Kalbermatten D.F., et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100:768–774. doi: 10.1002/bjs.9084. [DOI] [PubMed] [Google Scholar]
- 43.Schreml S., Heine N., Eisenmann-Klein M., Prantl L. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Ann Plast Surg. 2007;59:126–130. doi: 10.1097/01.sap.0000252714.72161.4a. [DOI] [PubMed] [Google Scholar]
- 44.Virden C.P., Dobke M.K., Stein P., Parsons C.L., Frank D.H. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg. 1992;16:173–179. doi: 10.1007/BF00450610. [DOI] [PubMed] [Google Scholar]
- 45.Galdiero M., Larocca F., Iovene M.R., et al. Microbial Evaluation in Capsular Contracture of Breast Implants. Plast Reconstr Surg. 2018;141:23–30. doi: 10.1097/PRS.0000000000003915. [DOI] [PubMed] [Google Scholar]
- 46.Wang F., Meng W., Wang B., Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Wang M.Y., Liu X.F., Gao X.Z. Helicobacter pylori virulence factors in development of gastric carcinoma. Future Microbiol. 2015;10:1505–1516. doi: 10.2217/fmb.15.72. [DOI] [PubMed] [Google Scholar]
- 48.Munford R.S. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect Immun. 2008;76:454–465. doi: 10.1128/IAI.00939-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jappe U. Superantigens and their association with dermatological inflammatory disease: facts and hypotheses. Acta Derm Venereol. 2000;20:321–328. doi: 10.1080/000155500459231. [DOI] [PubMed] [Google Scholar]
- 50.Irwin M.J., Hudson K.R., Ames K.T., Fraser J.D., Gascoigne N.R. T-cell receptor beta-chain binding to enterotoxin superantigens. Immunol Rev. 1993;131:61–78. doi: 10.1111/j.1600-065x.1993.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 51.Kadin M.E., Morgan J., Xu H., Glicksman C.A. CD30+ T Cells in Late Seroma May Not Be Diagnostic of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J. 2017;37:771–775. doi: 10.1093/asj/sjw286. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey D., O'Sullivan G.C., Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther. 2010;10:3–14. doi: 10.2174/156652310790945575. [DOI] [PubMed] [Google Scholar]
- 53.Myckatyn T.M., Parikh R.P. Discussion: breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: high-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg. 2017;140:655–658. doi: 10.1097/PRS.0000000000003712. [DOI] [PubMed] [Google Scholar]
- 54.Blombery P., Thompson E.R., Jones K., et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101:e387–e390. doi: 10.3324/haematol.2016.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crescenzo R., Abate F., Lasorsa E., et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Napoli A., Jain P., Duranti E., et al. Targeted next generation sequencing of breast implant-associated anaplastic large cell lymphoma reveals mutations in JAK/STAT signalling pathway genes, TP53 and DNMT3A. Br J Haematol. 2018;180:741–744. doi: 10.1111/bjh.14431. [DOI] [PubMed] [Google Scholar]
- 57.Letourneau A., Maerevoet M., Milowich D., et al. Dual JAK1 and STAT3 mutations in a breast implant-associated anaplastic large cell lymphoma. Virchows Arch. 2018 doi: 10.1007/s00428-018-2352-y. [DOI] [PubMed] [Google Scholar]
- 58.Oishi N., Brody G.S., Ketterling R.P., et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018 doi: 10.1182/blood-2017-12-821868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastorello R.G., D'Almeida Costa F., Osorio C., et al. Breast implant-associated anaplastic large cell lymphoma in a Li-FRAUMENI patient: a case report. Diagn Pathol. 2018;13:10. doi: 10.1186/s13000-018-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y.S., Filie A., Arthur D., Fojo A.T., Jaffe E.S. Breast implant-associated anaplastic large cell lymphoma in a patient with Li-Fraumeni syndrome. Histopathology. 2015;67:925–927. doi: 10.1111/his.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurent C., Nicolae A., Laurent C., et al. Gene alterations in epigenetic modifiers and JAK-STAT signaling are frequent in breast implant-associated ALCL. Blood. 2020;135:360–370. doi: 10.1182/blood.2019001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flex E., Petrangeli V., Stella L., et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blombery P., Thompson E., Ryland G.L., et al. Frequent activating STAT3 mutations and novel recurrent genomic abnormalities detected in breast implant-associated anaplastic large cell lymphoma. Oncotarget. 2018;9:36126–36136. doi: 10.18632/oncotarget.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Los-de Vries G.T., de Boer M., van Dijk E., et al. Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood. 2020;136:2927–2932. doi: 10.1182/blood.2020005372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.