Summary
Microbial inoculations contribute to reducing agricultural systems' environmental footprint by supporting sustainable production and regulating climate change. However, the indirect and cascading effects of microbial inoculants through the reshaping of soil microbiome are largely overlooked. By discussing the underlying mechanisms of plant- and soil-based microbial inoculants, we suggest that a key challenge in microbial inoculation is to understand their legacy on indigenous microbial communities and the corresponding impacts on agroecosystem functions and services relevant to climate change. We explain how these legacy effects on the soil microbiome can be understood by building on the mechanisms driving microbial invasions and placing inoculation into the context of ecological succession and community assembly. Overall, we advocate that generalizing field trials to systematically test inoculants' effectiveness and developing knowledge anchored in the scientific field of biological/microbial invasion are two essential requirements for applying microbial inoculants in agricultural ecosystems to tackle climate change challenges.
Subject areas: Global change, Biogeoscience, Microbiology, Agricultural techniques, Agricultural soil science
Graphical abstract
Global change; Biogeoscience; Microbiology; Agricultural techniques; Agricultural soil science
Introduction
Increasing or even maintaining crop yields is becoming more difficult with the need to decrease the environmental footprint of agricultural practices and their potential effects on climate change (Brisson et al., 2010; Pe'er et al., 2014; Smit and Skinner, 2002). With a faster-growing global market compared to agrochemicals (Batista and Singh, 2021), microbial inoculants, (i.e., beneficial microorganisms or mixtures of microorganisms applied to either the soil or the plant to improve soil quality and crop productivity; hereafter called soil inoculants and plant-based inoculants, respectively), are gaining importance in enhancing the sustainability of agroecosystems. For instance, plant-based inoculants contribute to higher plant growth, yield, resistance to abiotic (e.g., higher plant resistance to drought), and biotic (e.g., soil-borne pathogens) stresses (reviewed in (Bashan, 1998; Vejan et al., 2016)). Microbial inoculation thus potentially offers nature-based solutions (Eggermont et al., 2015) to climate change through their influence on plant growth and different relevant agroecosystem functions and services.
Despite the benefits of plant growth, inoculation practices can potentially lead to changes in soil microbial communities, which are often neglected (Trabelsi and Mhamdi, 2013). Recent reports have indicated that microbial inoculation, even when not successful, triggers significant changes at the level of indigenous soil microbial communities, following a framework linked to microbial invasions (see Glossary). Specifically, microbial invaders (here inoculants) favor or outcompete native microbial populations, leading to changes in species diversity and community composition (Bannar-Martin et al., 2018). As a consequence, the invaders can also reshape the functionality of resident soil communities, such as carbon resources utilization and CO2 emission (Amor et al., 2020; Mallon et al., 2018; Xing et al., 2020a, 2020b). Such an impact can propagate to the ecosystem level, depending on the strength and duration of the invasion. Microbial inoculations can thus have widespread and unexpected influences on soil microbial communities by triggering secondary succession (Horn, 1974) with cascading effects on the functions and services provided by agroecosystems. Therefore, climate change regulation or the adaptation of agroecosystems to climate change could be influenced by reshaping the soil microbiome in response to inoculant applications.
Glossary
Deterministic processes: predictable process governing community assembly with a determinable outcome such as selection.
Ecological secondary succession: one of two main ecological successions, referring to the process of community changes which started by disturbances.
Functional redundancy: the ecological phenomena that different species performed a similar or the same function in a microbial community.
Microbial community assembly: rules shaping the microbial community diversity and its distribution, functions, succession, and biogeography, including four basic processes (diversification, dispersal, selection, and drift).
Microbial invasion: the process by which alien microorganisms enter and affect the resident community.
Microbial keystone taxa: highly connected taxa significantly affect microbiome structure and functioning relative to their abundance across space and time.
Plant growth-promoting rhizobacteria (PGPR): are microbes that colonize the rhizosphere and directly or indirectly benefit plant growth and development.
Resilience: the capacity for a system to recover in response to disturbances.
Resistance: the ability of a system to remain unchanged when subjected to disturbances.
Stochastic processes: ecological processes that control community assembly in a random manner, such as ecological drift.
Tipping point: the critical threshold where a system shifts abruptly into a different state.
Broadly, microbial inoculants can cause legacy effects on agroecosystem functions relevant to climate change through three main pathways: direct effects of inoculants carrying specific functions relevant to climate change; indirect effects through modified plant growth and development; and indirect effects through the reshaping of soil microbial community (Table 1). Although our current understanding of the potential of microbial inoculations to tackle climate change challenges largely derives from studies on plant-based inoculants, such as plant growth-promoting rhizobacteria (PGPR), the three pathways mentioned above are often inseparable and work together with the introduction of inoculants to influence soil functions, although their effects may be different (Trabelsi and Mhamdi, 2013).
Table 1.
Synthetic view on the potential of microbial inoculants to steer biogeochemical processes in agroecosystems to tackle climate change (CC) challenges
| Type of inoculant | Object | Effect | Modified function(s) | Service regarding climate change (CC) | Demonstration of effectiveness | Examples of ref |
|---|---|---|---|---|---|---|
| N2O-reducing bacteria | Soil | Increased N2O reduction(A) | Decreased soil N2O emissions | CC mitigation through reduced GHG emissions | In soil microcosms and the field: N2O emissions diminished by 28%–189% | (Akiyama et al., 2016; Domeignoz-Horta et al., 2016) |
| Methanotrophs | Soil | Increased biological CH4 oxidation(A) | Decreased soil CH4 emissions and removal of CH4 from the atmosphere | CC mitigation through reduced GHG emissions | In paddy field: CH4 emissions diminished by 6.9%–12% | (Rani et al., 2021) |
| (Engineered) CO2-fixing microorganisms | Soil | Promoted microbial CO2 sequestration(A) | Reduced soil CO2 emissions | CC mitigation through reduced GHG emissions | In culture medium: the CO2 fixation rates achieved were comparable to the capacity of the autotrophic microbes | (Gong et al., 2015) |
| Microorganisms producing EPS-like compounds | Soil | The input of organic compounds like extracellular polymeric (EPS) substances into the soil(A) | Better soil aggregates formation and water-holding capacity | Better crop adaptation to drought/salinity and CC mitigation via better carbon (C) sequestration | In planted soil pots: dry matter yield of roots and shoots increased by 149%–527 and 85%–281% under drought stress | (Ashraf et al., 2004; Sandhya and Ali, 2015) |
| Plant Growth Promoting Rhizobacteria (PGPR) - general | Plant | Stimulated root growth and development(B) | Better water uptake by roots from deep soil layers and enhanced physiological traits of seedlings | Better crop adaptation to drought/salinity | In planted soil pots and the field: plant biomass increased vary from 11% to 87% | (Chandra et al., 2019; Silambarasan et al., 2019; Zhang et al., 2020) |
| PGPR - general | Plant | Increased whole plant biomass production(B) | Better plant carbon sequestration | CC mitigation via better carbon sequestration (if plant C is well managed) | In planted soil pots: plant growth and plant-derived C inputs to soil increased by an average of 42 and 91% under elevated CO2 | (Nie et al., 2015) |
| PGPR producing VOCs | Plant | Production of volatile organic compounds (VOCs)(B) | Better germination, higher plant activities of antioxidant defense enzymes | Better crop adaptation to drought/salinity | In planted soil pots: plant phytohormones increased by 49%–255%; the activities of antioxidant defense enzymes increased by 9%–70% | (Yasmin et al., 2020) |
| PGPR producing IAA | Plant | Production of phytohormone indole acetic acid (IAA)(B) | Adjustment of the timing of plant flowering | Better crop adaptation to CC via modulation of plant phenology | In planted soil pots: plant flowering time delayed by ∼3 days | (Lu et al., 2018) |
| Plant-nodulating rhizobia influencing interactions within the rhizosphere microbiome | Plant | Reshaped community interaction networks (though the same composition)(C) | Modified interactions between microbial populations change their ability to express the genes required to help plants tolerate stresses | Better crop adaptation to drought/salinity | In planted soil pots: the salt stress-induced loss of plant shoot weight diminished by 50% | (Benidire et al., 2020) |
| PGPR Azospirillum lipoferum | Plant | Increased nitrite reducer abundance (up to 60–90%) but only moderately increased abundances of N2O-reducers in sites with high C limitation; decreased nirS-denitrifier abundance (0 to -20%) and N2O reducer abundance (down to -20%) in sites with low C limitation(C) | Increased gross (up to +113%) and net (+37%) N2O production in sites with high C limitation; decreased gross and net N2O productions (-15 and -40%, respectively) in sites with low C limitation | Modification of CC mitigation through GHG emissions (on soils with a high C content, GHG emissions at the regional level can be increased by 2–5%) | In planted soil mesocosms and the field: variable outcomes in situ, from -6% to +25% | (Bounaffaa et al., 2018; Florio et al., 2017, 2019) |
We distinguish the effect directly linked to the inoculant (A) and cascading effect through plants (B) or native soil community (C).
In this opinion article, we first provide an overview of the potential benefits of microbial inoculants regarding climate change mitigation and adaptation and to what extent these have been demonstrated to be effective under field conditions. We then present the three main pathways mentioned above and discuss the potential effects of inoculants by placing them in a framework of ecological successions. We argue that grasping the legacy of microbial inoculations is essential to promote the more widespread and effective use of inoculants in the face of climate change.
Potential of microbial inoculants to steer agroecosystem functions relevant to climate change
Microbial inoculants provide several opportunities for mitigating the negative consequences of climate change in agroecosystems (Table 1). Later in discussion we discuss these opportunities by placing them into three categories: direct effects, indirect effects acting through plants, and indirect effects on soil communities.
Direct effects of inoculants
The direct effects of microbial inoculants that help to tackle the climate change challenge depend on the specific function they bring in. For instance, the inoculation of soil N2O-reducing bacteria that cannot produce N2O (such as Dyadobacter fermentans) is expected to reduce N2O emissions (Domeignoz-Horta et al., 2016). Indeed, the inoculation of these bacteria increases the N2O reducers-to-N2O producers' abundance ratio of soil, which is negatively associated with the net soil N2O production (Assémien et al., 2019; Florio et al., 2019). Other examples include the inoculation of methanotrophs or (possibly engineered) CO2-fixing microorganisms (Table 1). Similarly, the inoculation of microorganisms that produce extracellular polymeric substances has been proven to promote the formation and stability of soil aggregates and increase water-holding capacity, hence improving plant adaptation to drought and salinity (Ashraf et al., 2004; Sandhya and Ali, 2015). Soil aggregates also play an essential role in carbon (C) sequestration and thus climate change mitigation through protecting soil organic matter from degradation within a stable structure (Ahmed et al., 2019). Altogether, these soil microbial inoculants can influence important functions for climate change mitigation (i.e., reduction of greenhouse gas (GHG) emissions; increase in soil C sequestration) or plant adaptation to climate change (increase in soil water-holding capacity). However, adequate demonstration of their effectiveness at the agroecosystem scale is still needed (Table 1).
Indirect effects of inoculants through plants
The cascading effect on plants is one of the primary mechanisms through which microbial inoculants modify biogeochemical cycles and ecosystem functions and services relevant for mitigation of/adaptation to climate change (Table 1). In the case of plant-based microbial inoculants, i.e., inoculants selected for their capacity to interact with plants and steer plant functioning and development, many studies have analyzed the mechanisms underlying the effects on agroecosystem functions and services. For instance, the inoculation of PGPR that produces substances like 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole acetic acid (IAA) promotes the development of the root system, allowing plants to access a larger soil volume and deeper soil layers. This contributes to crop adaptation to drought stress (Lu et al., 2018; Silambarasan et al., 2019; Zhang et al., 2020), which can be crucial in regions where climate change is associated with more frequent and/or more intense drought events. In addition, the increased carbon pool that derives from increased plant biomass production can be sequestered either through litter inputs and root exudation or through adequate management (e.g., the burial of plant residues and promotion of the stabilization of part of these residues). This might be an important asset for sequestering C in agroecosystems and mitigating climate change (Nie et al., 2015). Overall, there is already ample knowledge on the mechanisms through which PGPR promote crop growth and development (Backer et al., 2018), and we could expect this knowledge, coupled with knowledge on C cycling and sequestration in agroecosystems, to now be turned into actions contributing to initiatives that can make agroecosystems important for tackling climate change, like the 4 per mille initiative (Minasny et al., 2017). However, as stated above, the actual outcomes of these plant-based inoculants remain variable between studies/conditions, even when using the same microbial strain and plant variety. This is often attributed to subtle changes in plant ecophysiological responses depending on environmental conditions (Compant et al., 2019). Still, the promotion of crop growth and development by the inoculants is possibly only one facet of their effects, and the indirect effects through the reshaping of the soil microbiome also matter (which has been demonstrated, e.g., for the PGPR Azospirillum lipoferum CRT1 (Florio et al., 2017)). Lack of mechanistic understanding underlying the effects of plant-based inoculants on the soil/rhizosphere microbiome can thus still be considered a deterrent to their deployment at a large scale.
Indirect effects of inoculants through the reshaping of soil microbiome
Plant-based and soil microbial inoculants can directly interact with the indigenous soil microbial community (Mawarda et al., 2020), or indirectly by, e.g., adjusting plant root exudates (Cesari et al., 2019; Shcherbakova et al., 2017). These interactions may largely determine whether the inoculant can survive and how they function, especially in steering agroecosystems to tackle climate change challenges. For instance, competition with native microbial species can affect the survival of inoculants, thus their functioning in the soil and/or the host plant. Indeed, the short survival time and weak functioning of inoculants in complex soil environments have largely constrained their application and performance in the field (Sammauria et al., 2020). This also encouraged the development of microbial consortia as inoculants. Pieces of evidence showed that promoting the positive interactions related to the inoculant helped in plant growth-promoting abilities (Emami et al., 2020; Olanrewaju and Babalola, 2019).
Soil microbial communities are intrinsically associated with the C and nutrient cycles through their role in soil organic matter mineralization, stabilization and sequestration, and GHG emission regulation (production and consumption) (Conrad, 2009; Kallenbach et al., 2016), and may exert great function in sustaining crop productivity (Chen et al., 2021). The reshaping of soil microbiome caused by inoculants could thus influence the potential to steer agroecosystem functions relevant to climate change adaptation and mitigation (Table 1). For example, recent evidence showed that the beneficial effects of microbial inoculation (Pseudomonas strains) on plant growth were best explained by changes in the indigenous community diversity, composition, and the relative abundance of initially rare taxa, rather than direct effects on plant (Hu et al., 2021). Besides, some studies have demonstrated how a PGPR (Azospirillum lipoferum CRT1), used to promote maize root growth and development, also generated cascading effects on the soil denitrifying community (Florio et al., 2017, 2019). Specifically, in sites with high C limitation, inoculation induced substantial increases in the abundance of nitrite reducers involved in N2O production, by up to 60–90%, but only moderate increases in the abundance of N2O-reducers; which together led to an increase in the gross (up to +113%) and net (+37%) N2O production by cropland soil (Florio et al., 2017, 2019). In this case, the cascading effect of the inoculant generated a disservice through increased risk of higher N2O emissions. In sites with low C limitation, however, inoculation decreased the abundances of nitrite reducers and N2O reducers (down to -20% for both), reducing gross and net N2O productions by -15% to -40% (Florio et al., 2017, 2019). These results represent a clear example of how inoculation effects on functions linked to climate change regulation can be context-dependent and even counterproductive, which urges for more (multidisciplinary) research in this area.
Some examples show the risk of ignoring the cascading effects of microbial inoculants that may aggravate climate change from some perspectives, such as contributing to GHG emissions. Theoretically, the inoculations of N2-fixing bacteria were expected to increase N2O emission with the help of native microorganisms that can sequentially promote the conversion of ammonium to nitrate through nitrification and the reduction of nitrate and nitrite to NO and N2O through denitrification (Pajares and Bohannan, 2016). Recent laboratory studies reported that the inoculation of the model bacterium Escherichia coli in soil microcosms could significantly increase soil CO2 efflux by changing the pattern of C sources utilization of native communities (Xing et al., 2020a, 2020b). As shown by a field experiment, microbial inoculants with the capacity to degrade highly recalcitrant plant biomass substrate (e.g., lignocellulose) can accelerate soil C turnover and promote GHG emission (Liu et al., 2015). In this case, soil seasonal CH4 emission increased by 6–13% after applying microbial inoculants and rice straw compared to straw input only owing to the better formation of substrates that methanogens can use during the straw decomposition (Liu et al., 2015). Recently, a suite of studies based on experiments in mesocosms, field trials, as well as agronomic and economic modeling, demonstrated that the inoculation of maize by A. lipoferum CRT1 in a French region with soils having a high C content could increase GHG emissions by 2–5%, even when accounting to the reduced use of fertilizers allowed by the inoculation practice (Bounaffaa et al., 2018). This is the only example of an attempt to evaluate the possible effect of a microbial inoculant (here plant-based) on climate change mitigation at a regional scale. Overall, it is essential to grasp the cascading effects of inoculants on native soil microbiome when considering the potential of inoculants for influencing agroecosystem functions and services linked to climate change mitigation and adaptation.
Quantifying inoculation effects on soil and agro-ecosystem
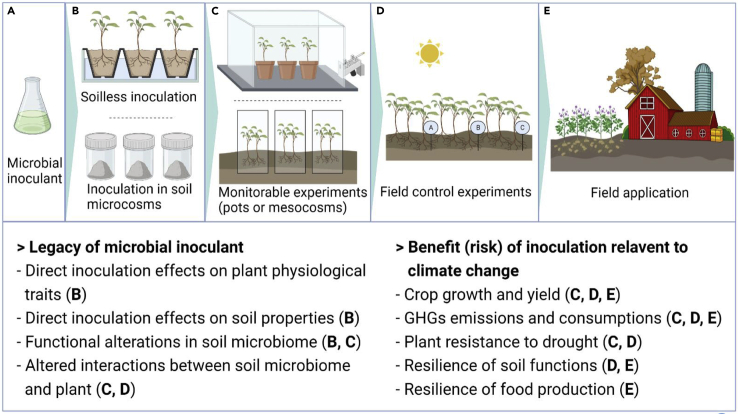
The approaches used for evaluating microbial inoculation effects on agroecosystem in the climate change context range from microcosms and mesocosms studies to field control experiments and large-scale field application (Figure 1); the most appropriate choice depends on the question being asked. For example, the mechanistic understanding of the legacy of microbial inoculants in agroecosystems is better achieved through microcosms and mesocosms studies (Figure 1, panels B, C). Quantifying the actual benefits of inoculants to climate change mitigation and adaptation require field control experiments and large-scale field application (Figure 1, panels D, E). For many inoculants, studies focused on the effects of inoculants mainly in planted soil pots/mesocosms (56% of the papers analyzed in Table 1). In contrast, evaluation of the effectiveness of the inoculants under field conditions remains limited (for only 31% of the papers and 40% of the inoculants analyzed in Table 1). Even for microbial inoculants that have been tested in the field, the effect sizes are often inconsistent depending on the study or the location or year. For instance, Bounaffaa et al. reviewed the effects of maize inoculation by the PGPR A. lipoferum CRT1 (Bounaffaa et al., 2018). For most published studies, the change in maize yield owing to inoculation was not statistically significant or not consistent between situations; when an increase in yield was observed, it ranged from +3 to +25% (Bounaffaa et al., 2018). Overall, it should be recognized that many benefits of microbial inoculants regarding climate change mitigation or adaptation have not been demonstrated to function effectively in the field, or at least not in a reliable manner across local conditions and years (hence including soil and climate). This highlights that the design and use of these inoculants remain too often empirical and that we need to reinforce the science-based knowledge of their effects.
Figure 1.
Diversity of approaches used for evaluating microbial inoculation effects on soil and agro-ecosystem in the climate change context
Characterization and mechanistic understanding of the legacy of microbial inoculants are better achieved through microcosms and mesocosms studies (Panels B, C) while quantifying the actual benefits of inoculants to climate change mitigation and adaptation –including possible risks– requires field control experiments and large-scale field application (Panels D, E).
We suggest combining multiple approaches to test the effectiveness of microbial inoculants and the mechanisms determining the inoculation effects (Figure 1). In brief, the direct and indirect effects of inoculants on soil microbiome, plants, and changes in their interactions can be adequately studied on simplified systems before the widespread application of microbial inoculants. Such monitoring and research should be based on the validation of different types of experiments (e.g., including but not limited to indoor culture experiments, monitorable pot or mesocosm experiments, and field control experiments). This should also be based on long-term studies (e.g., spanning several crop growing seasons or even the post-crop harvest period). Notably, the benefits and potential risks to climate change after inoculation should be measured to strike a balance between the two. Only by addressing this balance and possible effects, particularly on the soil microbiome, can we make inoculation a more effective and reliable practice. In the following section, we explain how placing the inoculants into the context of microbial invasion, and secondary succession can provide a framework to understand better the inoculation effect on the soil microbiome and agroecosystem functions associated with climate change regulation in terms of temporal scale and mechanisms.
Merging knowledge on microbial invasions and ecological succession to understand inoculation effect on soil microbiome
The knowledge on microbial invasion, which represents a biotic disturbance of the local microbial community that begins with dispersal (Vellend, 2010) provides a framework for understanding the impacts of microbial inoculants on native communities. The inoculation effect on native communities depends on the capacity of the inocula (i.e., invaders) to maintain themselves into the soil native community they arrive in (or the matrix they are placed in). In general, the longer the inoculum survives in the soil, the more significant its impact will be (Xing et al., 2020a). Both abiotic and biotic factors can influence inoculum survival (Mallon et al., 2015; Trexler and Bell, 2019; Wei et al., 2015). Among them, abiotic factors such as pH and temperature may exert selective pressure on invaders. In contrast, biotic factors refer to interactions between invaders and native microorganisms, such as competition and mutualism. For instance, microbial communities with higher phylogenetic diversity are more resistant to inoculations (Mallon et al., 2015; van Elsas et al., 2012). Importantly, understanding inoculant survival goes beyond the universal diversity-invasion relationship, as poor survivors might also leave a footprint on native communities that lasts long after their eradication (Xing et al., 2020a), a concept recently identified in larger organisms (Reynolds et al., 2017).
To address the potential legacy effect of inoculants on agroecosystem functions, it is crucial to take a temporal approach by unifying the mechanisms of microbial invasion and the subsequent trajectory of the microbial community. Specifically, introduced microorganisms trigger secondary succession in the native community (Mawarda et al., 2020). They can disturb the resident community by reshaping interspecies interactions (e.g., competition, predation, cooperation, or antagonism) and environmental conditions. Upon inoculations, community secondary succession can be summarized into three models – facilitation, tolerance, and inhibition – based on whether microbial invaders are suitable to the new community (Connell and Slatyer, 1977) (Figure 2).
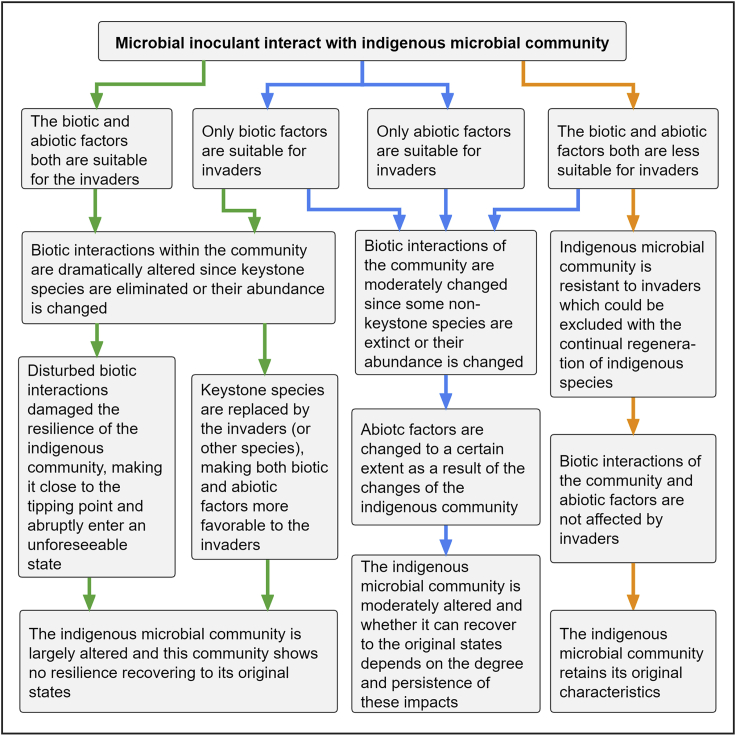
Figure 2.
Secondary succession patterns for understanding the consequences of microbial inoculants for the indigenous microbial community
Arrows represent different succession processes on inoculation, where trajectories facilitate (green), tolerate (blue), or inhibit (orange) inoculant establishment. Given that more prolonged inoculant survival has a larger impact on soil community structure, native communities that follow the facilitation models would be most impacted by inoculation, potentially reaching alternative stable states. In contrast, those following the inhibition model would be resistant to the invasion
The facilitation model (Figure 2, green arrows) assumes that invaders have a relatively high chance of colonizing the resident community when facilitated by biotic and abiotic factors. In this scenario, microbial keystone taxa that have a crucial role in microbial communities (in other words, exercising a set of critical functions while other functions might be redundant across other taxa) can be damaged through direct negative interactions with invaders— leading to the disorder of biological interactions and changes of community structure (Banerjee et al., 2018). As the keystone taxa are replaced by invaders or other species, the biotic interactions and environmental conditions of the entire community may be reshaped in the process of succession. Otherwise, the disrupted interactions will damage community stability (resulting in lower resilience and resistance) (Herren, 2020). Such instability may bring the native community close to tipping points and quickly shift into another state with modified ecological functions (Amor et al., 2020; Scheffer et al., 2009), particularly key functions linked to climate change mitigation/adaptation. The tolerance model explains that the community can be changed to a moderate degree (Figure 2, blue arrows), where invaders are subjected to less facilitation than in the previous model. In this model, microbiome structure and environmental factors may gradually change as the invaders grow while non-keystone species are mainly affected. The inhibition model represents the preservation of the original states of the soil community because invaders cannot survive in the new environment (Figure 2, orange arrows). Note that even if the indigenous community encounters transient invaders, the impacts on the community structure and functions can be expected (Amor et al., 2020; Mallon et al., 2018). This suggests that when studying the impact of microbial inoculation, it is essential to pay attention to the changes of community states over time rather than only observe the survival rate of invaders.
The models differ markedly on the expectation that native communities would be able or not to rapidly return to pre-perturbation states. The resident community shows no resilience in the facilitation model, high resilience under certain conditions in the tolerance model, or high resistance in the inhibition model. Understanding the processes that regulate the assembly of the native community on inoculation could indicate whether these changes are permanent.
A conceptual model for predicting ecological consequences of microbial inoculation for soil microbiome
To gain further insight into the community succession processes after inoculation, we propose a conceptual model (Figure 3) where the change in the community assembly processes is linked to the secondary succession models presented in Figure 2. Specifically, stochastic and deterministic processes regulate microbial community assembly in response to disturbances, with significant consequences for microbial community dynamics and functionalities (Dini-Andreote et al., 2015). Within this conceptual model, the relative change of the community assembly process following a disturbance mainly depends on the balance between the biotic selection pressure and the environmental selection pressure within the community. The reduction or extinction of keystone species caused by invaders will weaken the biotic selective pressure on other microorganisms in the scenario of the facilitation model. For example, some fungal and bacterial species linked to organic matter decomposition or N2 fixation have been identified as keystone species (Banerjee et al., 2016). Thus, the community's trophic interactions and assemblage can be severely damaged if these taxa are destroyed following inoculation (Gralka et al., 2020). Considering the tolerance model, when keystone species are not undermined, the entry of invaders will increase the biotic selective pressure of the community owing to participation in the biotic interactions (whether competition or cooperation), thus promoting deterministic processes. Inoculations may also change environments to increase or decrease the abiotic selective pressure on the community. For instance, microbial inoculants for bioremediations may help release abiotic selective pressures by reducing the concentration of toxic pollutants (e.g., polycyclic aromatic hydrocarbons, pesticides, and heavy metals) from contaminated lands (Ahmad et al., 2018).
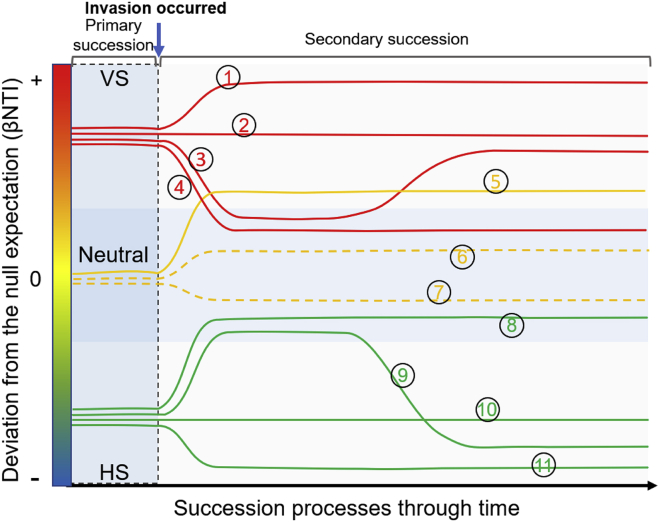
Figure 3.
Hypothesized conceptual model linking microbial succession and assembly processes for predicting the impacts of microbial inoculation (invasion)
Community assembly processes can be measured by the β-nearest taxon index (βNTI) (Stegen et al., 2013). Ecological selection is weak in the center of the vertical axis and is stronger toward both extremes. Different lines with a number represent various trends of the community assembly process during succession through time. Depending on βNTI values, the assemblage of the resident community in a primary succession is inferred to be dominated by variable selection (VS, red lines), homogeneous selection (HS, green lines), or stochastic processes (Neutral, yellow lines). Both in the communities driven by VS (red lines) and HS (green lines), the strong selective pressure can be removed in the facilitation model as a result of damaged biotic interactions or abiotic pressure, such as the extinction of keystone microbial taxa, leading to stochasticity (lines 3, 4, 8, and 9), and these trends can be reversed when invaders impose strong selection and/or shape a more selective environment (lines 3 and 9). In the tolerance model, the increase of biotic selection and/or abiotic pressure triggered by invaders could lead to the intensive variable selection (lines 1 and 5); a constant selective pressure (lines 2 and 10), or an increase of homogeneous selection (line 11) following an invasion event is expected if the resulting environment does show a weak selection. Lines 2 and 10 can also correspond to microbial invasions having no impacts on the primary selective pressure in the inhibition model. The effects of invasions on ecologically neutral communities are difficult to predict theoretically (show as dash lines 6 and 7).
Based on the above mechanisms, examples of the post-inoculation (i.e., post-invasion) trend of the community assembly process along successional processes can be hypothesized. When the invaded microorganism significantly changes the succession trajectory of the soil microbiome, following the facilitation model and the tolerance model, it may reshape the role of the soil microbiome in the agroecosystem and affect how the agroecosystem is restructured and its functions. Thus, exploring fluctuations of the community assembly process, especially in combination with quantitative methods (Stegen et al., 2013), will be crucial to understanding the successional mechanisms of native microbial communities. For instance, if the deterministic processes of a community that is driven by variable selection increase after inoculation (e.g., line 1 in Figure 3), we can judge that inoculants did not cause significant disturbance to keystone species. This indicates that the changes in community structure or function are mainly related to the invaders' selection pressure. Similarly, a constant selective pressure indicates that no impact occurred or a counteraction between biotic and abiotic selections (e.g., lines 2 and 10 in Figure 3). The changes of these communities in the future succession process have relatively high predictability owing to assemblage governed by deterministic process, contrary to communities whose trajectory becomes governed by neutral processes owing to invasions (e.g., lines 4 and 8 in Figure 3). A clear picture of changes in soil physicochemical properties, microbial community structure, and functionality (e.g., using multi-omics approaches (Subramanian et al., 2020)) can help analyze how the inoculant affects the indigenous microbial community.
Moreover, this conceptual model can assist in screening microbial inoculants in the context of practical applications. For example, inoculants that can survive longer and cause more significant effects in a specific soil environment may lead to the increased selective pressure in the soil microbiome after inoculation (e.g., the cases represented by lines 1 and 11 in Figure 3). In contrast, the alteration of community resilience after inoculation might indicate loss of soil microbiome functions (e.g., the cases represented by lines 4 and 8 in Figure 3). However, some limitations, e.g., microbial community structure and function decoupling caused by functional redundancy, might limit the accuracy of the predictions derived from this conceptual model (Box 1). Despite this, the model represents the first step toward quantitative analysis of microbial inoculations as microbial invasions and the community successions they induce, which overcomes the shortcomings of simply monitoring changes in community structure. More empirical evidence is needed to verify the extent to which this conceptual model can support tracking the impact of inoculation on agroecosystems in a climate change context.
Box 1. Potential limitations in applying the conceptual model.
Some limitations lie in using the proposed conceptual model presented in Figure 3. Firstly, it may be less reliable to reflect changes in microbial community functions when functional redundancy widely exists in target microbial communities (Wertz et al., 2007). More specifically, the reliability of the outputs of the model depends on the choice of functions concerned. Broadly distributed metabolic processes such as respiration and biomass production often seem decoupled from particular taxonomic assemblages. In contrast, narrow functions such as the degradation of specific compounds can strongly correlate with taxonomic community composition (Louca et al., 2018). In addition, the effectiveness of the statistical method for analyzing the assembly process may vary with the complexity and assemblage of the focal community, spatial scales examined, sampling errors, null model algorithms, and so forth (Zhou and Ning, 2017). For example, the relative importance of the deterministic process for governing community structure will increase at a relatively small range or under harsh conditions (Chase and Myers, 2011). Considering these limitations, more empirical evidence is needed to trace the mechanisms driving microbial community succession following inoculant applications.
Concluding remarks
Microbial inoculations can influence agroecosystem functions in multiple ways and show great opportunities to increase services such as climate change mitigation and adaptation to climate change in agroecosystems. This potential is related to the direct effects of inoculants when they harbor relevant specific functions, as well as indirect, cascading effects through plants and/or native microbial communities. However, whereas the mechanisms underlying plant-inoculant interactions are well studied, how microbial inoculants cause changes in native microbial communities and their functioning is rarely studied. Understanding these cascading effects may help design new approaches to tackle climate change, as envisaged, for example, part of the Moonshot project “Cool Earth via Microbes in Agriculture” promoted by the New Energy and Industrial Technology Development Organization (NEDO) in Japan. Conversely, neglecting these cascading effects may lead to unintended disservices regarding climate change regulation in agroecosystems (Bounaffaa et al., 2018). It remains particularly challenging to evaluate how the inoculation-induced effects change over time (Estoup et al., 2016) and how they might go beyond the intended purpose owing to, for example, the long-term persistence of microbial inoculants in soil or legacy effects of inoculants on the soil microbiome (Narożna et al., 2015). Understanding inoculant effects should be based on a framework of microbial invasion explicating the ecological successions of microbial populations induced by inoculation. We thus proposed a conceptual model that links microbial successional mechanisms and assembly processes to guide the analyses of the ecological consequences of microbial inoculations. We anticipate that there will be increasing empirical evidence to apply and test this conceptual model to improve our ability to understand the role of microbial inoculations (and, more generally, invasions) in soil ecosystems over space and time. In doing so, an increasing number of strategies based on microbial inoculants will likely be developed to steer agroecosystem functions and particularly to tackle the climate change challenge, and their actual benefits will have to be more systematically tested under natural field conditions. Applied at a large scale, inoculations could then contribute to pursuing agricultural sustainability in the Anthropocene.
Resource availability
Lead contact
Further information and requests should be directed to the Lead contact, Joana Falcão Salles (J.falcao.salles@rug.nl).
Materials availability
This study did not generate new unique reagents.
Outstanding questions
To what extent microbial inoculants can steer soil functions and services, in particular, to favor climate change regulation and adaptation of agroecosystems to climate change under field conditions?
How to systematically evaluate whether a microbial inoculant is helpful to climate change mitigation, and what functions/indicators should be considered?
What role does microbial invasion (inoculation) play in community/ecosystem resilience and resistance?
How do the impacts of microbial invasion (inoculation) on communities propagate to the ecosystem level?
Is there a conflict between the use of microbial inoculants for climate change mitigation and the stabilization (preservation) of some ecosystem functions? How to balance the potential opportunities and risks when steering inoculants to tackle climate change?
Acknowledgments
X.L. was supported by a scholarship from the China Scholarship Council. X.L.R. and J.F.S. were funded through the ANR Project IMMINENT (ANR-20-CE02-0014-01), J.F.S was financed by ERA-NET Cofund SusCrop project potatoMETAbiome (Grant No 771134), supported by EU Horizon 2020 research and innovation program and NWO, and part of the Joint Programming Initiative on Agriculture, Food Security and Climate Change (FACCE-JPI). X.L.R. was also funded through the European Joint Program on Soil (EJP Soil).
Author contributions
All authors equally contributed to the work conception and article writing.
Conflicts of interest
The authors declare no competing interests.
Data and code availability
There are no data and no code associated with this article.
References
- Ahmad M., Pataczek L., Hilger T.H., Zahir Z.A., Hussain A., Rasche F., Schafleitner R., Solberg S.Ø. Perspectives of microbial inoculation for sustainable development and environmental management. Front. Microbiol. 2018;9:2992. doi: 10.3389/fmicb.2018.02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Ahmed Q., Abraham O., Babalola O. Handbook of Climate Change Resilence. Climate Change Management Series. Springer; 2019. Microbial inoculants for improving carbon sequestration in agroecosystems to mitigate climate change; pp. 111–119. [Google Scholar]
- Akiyama H., Hoshino Y.T., Itakura M., Shimomura Y., Wang Y., Yamamoto A., Tago K., Nakajima Y., Minamisawa K., Hayatsu M. Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep32869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor D.R., Ratzke C., Gore J. Transient invaders can induce shifts between alternative stable states of microbial communities. Sci. Adv. 2020;6:8. doi: 10.1126/sciadv.aay8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Hasnain S., Berge O., Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils. 2004;40:157–162. [Google Scholar]
- Assémien F.L., Cantarel A.A., Florio A., Lerondelle C., Pommier T., Gonnety J.T., Le Roux X. Different groups of nitrite-reducers and N2O-reducers have distinct ecological niches and functional roles in West African cultivated soils. Soil Biol. Biochem. 2019;129:39–47. [Google Scholar]
- Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Kirkby C.A., Schmutter D., Bissett A., Kirkegaard J.A., Richardson A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016;97:188–198. [Google Scholar]
- Banerjee S., Schlaeppi K., van der Heijden M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018;16:567–576. doi: 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- Bannar-Martin K.H., Kremer C.T., Ernest S.K.M., Leibold M.A., Auge H., Chase J., Declerck S.A.J., Eisenhauer N., Harpole S., Hillebrand H., et al. Integrating community assembly and biodiversity to better understand ecosystem function: the Community Assembly and the Functioning of Ecosystems (CAFE) approach. Ecol. Lett. 2018;21:167–180. doi: 10.1111/ele.12895. [DOI] [PubMed] [Google Scholar]
- Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998;16:729–770. [Google Scholar]
- Batista B.D., Singh B.K. Realities and hopes in the application of microbial tools in agriculture. Microb. Biotechnol. 2021;14:1258–1268. doi: 10.1111/1751-7915.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benidire L., El Khalloufi F., Oufdou K., Barakat M., Tulumello J., Ortet P., Heulin T., Achouak W. Phytobeneficial bacteria improve saline stress tolerance in Vicia faba and modulate microbial interaction network. Sci. Total Environ. 2020;729:139020. doi: 10.1016/j.scitotenv.2020.139020. [DOI] [PubMed] [Google Scholar]
- Bounaffaa M., Florio A., Le Roux X., Jayet P.-A. Economic and environmental analysis of maize inoculation by plant growth promoting rhizobacteria in the French Rhône-Alpes region. Ecol. Econ. 2018;146:334–346. [Google Scholar]
- Brisson N., Gate P., Gouache D., Charmet G., Oury F.-X., Huard F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crops Res. 2010;119:201–212. [Google Scholar]
- Cesari A., Paulucci N., Lopez-Gomez M., Hidalgo-Castellanos J., Pla C.L., Dardanelli M.S. Restrictive water condition modifies the root exudates composition during peanut-PGPR interaction and conditions early events, reversing the negative effects on plant growth. Plant Physiol. Biochem. 2019;142:519–527. doi: 10.1016/j.plaphy.2019.08.015. [DOI] [PubMed] [Google Scholar]
- Chandra D., Srivastava R., Gupta V.V.S.R., Franco C.M.M., Paasricha N., Saifi S.K., Tuteja N., Sharma A.K. Field performance of bacterial inoculants to alleviate water stress effects in wheat (Triticum aestivum L.) Plant Soil. 2019;441:261–281. [Google Scholar]
- Chase J.M., Myers J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.-L., Hu H.-W., He Z.-Y., Cui L., Zhu Y.-G., He J.-Z. Potential of indigenous crop microbiomes for sustainable agriculture. Nat. Food. 2021;2:233–240. doi: 10.1038/s43016-021-00253-5. [DOI] [PubMed] [Google Scholar]
- Compant S., Samad A., Faist H., Sessitsch A. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019;19:29–37. doi: 10.1016/j.jare.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell J.H., Slatyer R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977;111:1119–1144. [Google Scholar]
- Conrad R. The global methane cycle: recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009;1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- Dini-Andreote F., Stegen J.C., van Elsas J.D., Salles J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. U S A. 2015;112:E1326–E1332. doi: 10.1073/pnas.1414261112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeignoz-Horta L.A., Putz M., Spor A., Bru D., Breuil M.-C., Hallin S., Philippot L. Non-denitrifying nitrous oxide-reducing bacteria-An effective N2O sink in soil. Soil Biol. Biochem. 2016;103:376–379. [Google Scholar]
- Eggermont H., Balian E., Azevedo J.M.N., Beumer V., Brodin T., Claudet J., Fady B., Grube M., Keune H., Lamarque P. Nature-based solutions: new influence for environmental management and research in Europe. GAIA-Ecological Perspect. Sci. Soc. 2015;24:243–248. [Google Scholar]
- Emami S., Alikhani H.A., Pourbabaee A.A., Etesami H., Motasharezadeh B., Sarmadian F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere. 2020;14:100196. [Google Scholar]
- Estoup A., Ravigné V., Hufbauer R., Vitalis R., Gautier M., Facon B. Is there a genetic paradox of biological invasion? Annu. Rev. Ecol. Evol. Syst. 2016;47:51–72. [Google Scholar]
- Florio A., Bréfort C., Gervaix J., Bérard A., Le Roux X. The responses of NO2−- and N2O-reducing bacteria to maize inoculation by the PGPR Azospirillum lipoferum CRT1 depend on carbon availability and determine soil gross and net N2O production. Soil Biol. Biochem. 2019;136:107524. [Google Scholar]
- Florio A., Pommier T., Gervaix J., Bérard A., Le Roux X. Soil C and N statuses determine the effect of maize inoculation by plant growth-promoting rhizobacteria on nitrifying and denitrifying communities. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-08589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F., Liu G., Zhai X., Zhou J., Cai Z., Li Y. Quantitative analysis of an engineered CO2-fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation. Biotechnol. Biofuels. 2015;8:1–10. doi: 10.1186/s13068-015-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralka M., Szabo R., Stocker R., Cordero O.X. Trophic interactions and the drivers of microbial community assembly. Curr. Biol. 2020;30:R1176–R1188. doi: 10.1016/j.cub.2020.08.007. [DOI] [PubMed] [Google Scholar]
- Herren C.M. Disruption of cross-feeding interactions by invading taxa can cause invasional meltdown in microbial communities. Proc. R. Soc. B: Biol. Sci. 2020;287:20192945. doi: 10.1098/rspb.2019.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H.S. The ecology of secondary succession. Annu. Rev. Ecol. Syst. 1974;5:25–37. [Google Scholar]
- Hu J., Yang T., Friman V.P., Kowalchuk G.A., Hautier Y., Li M., Wei Z., Xu Y.C., Shen Q.R., Jousset A. Introduction of probiotic bacterial consortia promotes plant growth via impacts on the resident rhizosphere microbiome. Proc. Biol. Sci. 2021;288:20211396. doi: 10.1098/rspb.2021.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach C.M., Frey S.D., Grandy A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016;7:13630. doi: 10.1038/ncomms13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yu H.Y., Ma J., Xu H., Wu Q.Y., Yang J.H., Zhuang Y.Q. Effects of straw incorporation along with microbial inoculant on methane and nitrous oxide emissions from rice fields. Sci. Total Environ. 2015;518:209–216. doi: 10.1016/j.scitotenv.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Louca S., Polz M.F., Mazel F., Albright M.B.N., Huber J.A., O'Connor M.I., Ackermann M., Hahn A.S., Srivastava D.S., Crowe S.A., et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018;2:936–943. doi: 10.1038/s41559-018-0519-1. [DOI] [PubMed] [Google Scholar]
- Lu T., Ke M., Lavoie M., Jin Y., Fan X., Zhang Z., Fu Z., Sun L., Gillings M., Peñuelas J. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome. 2018;6:1–12. doi: 10.1186/s40168-018-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon C.A., Le Roux X., van Doorn G.S., Dini-Andreote F., Poly F., Salles J.F. The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader's niche. ISME J. 2018;12:728–741. doi: 10.1038/s41396-017-0003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon C.A., Poly F., Le Roux X., Marring I., van Elsas J.D., Salles J.F. Resource pulses can alleviate the biodiversity-invasion relationship in soil microbial communities. Ecology. 2015;96:915–926. doi: 10.1890/14-1001.1. [DOI] [PubMed] [Google Scholar]
- Mawarda P.C., Le Roux X., Dirk van Elsas J., Salles J.F. Deliberate introduction of invisible invaders: a critical appraisal of the impact of microbial inoculants on soil microbial communities. Soil Biol. Biochem. 2020;148:107874. [Google Scholar]
- Minasny B., Malone B.P., McBratney A.B., Angers D.A., Arrouays D., Chambers A., Chaplot V., Chen Z.-S., Cheng K., Das B.S., et al. Soil carbon 4 per mille. Geoderma. 2017;292:59–86. [Google Scholar]
- Narożna D., Pudełko K., Króliczak J., Golińska B., Sugawara M., Mądrzak C.J., Sadowsky M.J. Survival and competitiveness of Bradyrhizobium japonicum strains 20 Years after introduction into field locations in Poland. Appl. Environ. Microbiol. 2015;81:5552–5559. doi: 10.1128/AEM.01399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie M., Bell C., Wallenstein M.D., Pendall E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci. Rep. 2015;5:1–6. doi: 10.1038/srep09212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju O.S., Babalola O.O. Bacterial consortium for improved maize (Zea mays L.) production. Microorganisms. 2019;7:519. doi: 10.3390/microorganisms7110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajares S., Bohannan B.J.M. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016;7:1045. doi: 10.3389/fmicb.2016.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'er G., Dicks L.V., Visconti P., Arlettaz R., Báldi A., Benton T.G., Collins S., Dieterich M., Gregory R.D., Hartig F. EU agricultural reform fails on biodiversity. Science. 2014;344:1090–1092. doi: 10.1126/science.1253425. [DOI] [PubMed] [Google Scholar]
- Rani V., Bhatia A., Kaushik R. Inoculation of plant growth promoting-methane utilizing bacteria in different N-fertilizer regime influences methane emission and crop growth of flooded paddy. Sci. Total Environ. 2021;775:145826. doi: 10.1016/j.scitotenv.2021.145826. [DOI] [PubMed] [Google Scholar]
- Reynolds P.L., Glanz J., Yang S., Hann C., Couture J., Grosholz E. Ghost of invasion past: legacy effects on community disassembly following eradication of an invasive ecosystem engineer. Ecosphere. 2017;8:e01711. [Google Scholar]
- Sammauria R., Kumawat S., Kumawat P., Singh J., Jatwa T.K. Microbial inoculants: potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020;202:677–693. doi: 10.1007/s00203-019-01795-w. [DOI] [PubMed] [Google Scholar]
- Sandhya V., Ali S.Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation. Microbiology. 2015;84:512–519. [Google Scholar]
- Scheffer M., Bascompte J., Brock W.A., Brovkin V., Carpenter S.R., Dakos V., Held H., Van Nes E.H., Rietkerk M., Sugihara G. Early-warning signals for critical transitions. Nature. 2009;461:53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- Shcherbakova E.N., Shcherbakov A.V., Andronov E.E., Gonchar L.N., Kalenskaya S.M., Chebotar V.K. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis. 2017;73:57–69. [Google Scholar]
- Silambarasan S., Logeswari P., Cornejo P., Kannan V.R. Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 2019;26:27647–27659. doi: 10.1007/s11356-019-05939-9. [DOI] [PubMed] [Google Scholar]
- Smit B., Skinner M.W. Adaptation options in agriculture to climate change: a typology. Mitig. Adapt. Strategies Glob. Change. 2002;7:85–114. [Google Scholar]
- Stegen J.C., Lin X.J., Fredrickson J.K., Chen X.Y., Kennedy D.W., Murray C.J., Rockhold M.L., Konopka A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013;7:2069–2079. doi: 10.1038/ismej.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian I., Verma S., Kumar S., Jere A., Anamika K. Multi-omics data integration, interpretation, and its application. Bioinf. Biol. Insights. 2020;14 doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi D., Mhamdi R. Microbial inoculants and their impact on soil microbial communities: a review. Biomed. Res. Int. 2013;2013:863240. doi: 10.1155/2013/863240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler R.V., Bell T.H. Testing sustained soil-to-soil contact as an approach for limiting the abiotic influence of source soils during experimental microbiome transfer. FEMS Microbiol. Lett. 2019;366:9. doi: 10.1093/femsle/fnz228. [DOI] [PubMed] [Google Scholar]
- van Elsas J.D., Chiurazzi M., Mallon C.A., Elhottova D., Kristufek V., Salles J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. U S A. 2012;109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejan P., Abdullah R., Khadiran T., Ismail S., Nasrulhaq Boyce A. Role of plant growth promoting rhizobacteria in agricultural sustainability-A review. Molecules. 2016;21:573. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellend M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- Wei Z., Yang T., Friman V.-P., Xu Y., Shen Q., Jousset A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015;6 doi: 10.1038/ncomms9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz S., Degrange V., Prosser J., Poly F., Commeaux C., Guillaumaud N., Roux X. Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ. Microbiol. 2007;9:2211–2219. doi: 10.1111/j.1462-2920.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- Xing J., Jia X., Wang H., Ma B., Salles J., xu J. The legacy of bacterial invasions on soil native communities. Environ. Microbiol. 2020;23:669–681. doi: 10.1111/1462-2920.15086. [DOI] [PubMed] [Google Scholar]
- Xing J., Sun S., Wang H., Brookes P., xu J. Response of soil native microbial community to Eschericia coli O157:H7 invasion. Environ. Pollut. 2020;261:114225. doi: 10.1016/j.envpol.2020.114225. [DOI] [PubMed] [Google Scholar]
- Yasmin H., Rashid U., Hassan M.N., Nosheen A., Naz R., Ilyas N., Sajjad M., Azmat A., Alyemeni M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defence system in Maize (Zea mays L.). Physiol. Plant. 2020;172:896–911. doi: 10.1111/ppl.13304. [DOI] [PubMed] [Google Scholar]
- Zhang M., Yang L., Hao R., Bai X., Wang Y., Yu X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil. 2020;452:423–440. [Google Scholar]
- Zhou J.Z., Ning D.L. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017;81:e00002–e00017. doi: 10.1128/MMBR.00002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data and no code associated with this article.