Abstract
Nasal surveillance cultures were performed for 54 patients exhibiting ≥103 CFU of methicillin-resistant coagulase-negative staphylococci per ml in central venous catheter (CVC) rinse cultures over a 6-month period. Forty-two of the nasal cultures yielded growth of methicillin-resistant coagulase-negative staphylococci, and 33 of the 42 cultures contained organisms that belonged to the same species as the CVC isolates. Of the 33 same-species isolates, 20 appeared to be identical strains by pulsed-field gel electrophoresis analysis. These data suggest that measures should be taken to reduce cross-contamination between the respiratory tract and intravascular devices. However, the potential interest in detecting methicillin-resistant coagulase-negative staphylococcus carriage in high-risk patients is hampered by the lack of sensitivity of nasal surveillance cultures.
Coagulase-negative staphylococci are the most frequently reported pathogens in nosocomial bloodstream infections (20, 21, 27). During the 1980s, the incidence of bloodstream infections due to coagulase-negative staphylococci increased to the point that coagulase-negative staphylococci now cause more than 25% of nosocomial bloodstream infections (2, 17, 21). Patients with indwelling or implanted foreign polymer bodies represent the most important group of patients susceptible to coagulase-negative staphylococcal infections. In fact, although coagulase-negative staphylococcus isolates are frequently considered to be contaminants from normal skin and mucous flora, intravascular catheters represent a major point of entry for coagulase-negative staphylococcal septicemia (11, 13, 15, 21, 25). A large proportion of nosocomial isolates of coagulase-negative staphylococci are resistant to multiple antibiotics, including penicillinase-resistant penicillins (2). Multiple drug resistance has been documented more often in disease-causing strains of Staphylococcus epidermidis than in skin-colonizing strains (1).
In our hospital, 65% of the bacteria colonizing intravascular devices are coagulase-negative staphylococci, and 72% of them are methicillin-resistant coagulase-negative staphylococci. The endemic spread of multiresistant coagulase-negative staphylococci may be of concern, since the emergence of strains with decreased susceptibility to vancomycin and, especially, teicoplanin has been reported in several studies (2, 28, 32). Skin colonization with antibiotic-resistant coagulase-negative staphylococci constitutes a reservoir for antibiotic resistance genes, which is promoted by systemic antibiotic administration (1, 2, 18). Whereas catheter seeding by microorganisms present in the bloodstream is an uncommon route to gain access to the catheter, the insertion site of the catheter is considered the major portal of microbial access leading to catheter-related sepsis (26). Moreover, the catheter hub has been implicated as an additional entry point leading to catheter-related sepsis, justifying local use of antibiotics in preventive control measures (16). However, the role of skin and mucous microbiota in coagulase-negative staphylococcal infections remains unclear.
Controversial data have been published concerning the role of the tracheal colonization in bacteremic neonates (22, 29, 30). In adults, coagulase-negative staphylococcus colonization remains stable over many years (14), and coagulase-negative staphylococci causing postoperative infections have the same resistance profiles as colonizing strains (1). However, other studies showed evidence for the changing nature of microbial skin colonization in neonates (3, 6, 26), concluding that surveillance cultures of superficial sites (3) or the catheter hub (26) are a poor reflection of catheter tip colonization and thus cannot be used to predict the development of catheter-related sepsis.
The purpose of the present study was to investigate the nasal flora of patients presenting colonization of intravascular catheters by methicillin-resistant coagulase-negative staphylococci. From February to July 1997, a nasal swab was requested for 54 patients exhibiting colonization by methicillin-resistant coagulase-negative staphylococci of a central venous catheter (CVC). Quantitative cultures of CVC were performed by rinsing the distal 6-cm segment of the catheter with 1 ml of broth and inoculating 100 μl of the broth on blood agar (7, 9). The cultures were considered significant when the bacterial count was ≥103 CFU/ml (7). Initial identification of coagulase-negative staphylococci was based on colony morphology, Gram stain characteristics, coagulase reactions, and the results of the Pastorex Staph Plus test (Sanofi Diagnostics Pasteur, Marnes la Coquette, France). As soon as the methicillin resistance of a coagulase-negative staphylococcus isolate was documented, which was within 48 to 72 h after the laboratory had received the catheter, the hospital ward of the patient was contacted and a nasal swab was required. Therefore, although the study was not prospective, the nasal swabs were promptly collected, and they closely reflected the nasal colonization at the time of CVC colonization. To select for methicillin-resistant strains, the nasal swabs were inoculated onto oxacillin agar plates, containing Mueller-Hinton agar supplemented with 4% NaCl and 6 μg of oxacillin per ml. The plates were then incubated for 48 h at 35°C. When the culture exhibited a unique morphotype, the antibiogram was initially performed with a mixture of 10 to 12 independent colonies, in order to detect the expression of different antibiotypes. When different resistance profiles appeared, the discriminant markers were used for the subsequent isolation of the corresponding strains. Further investigations were performed with the subculture of independent colonies, each one exhibiting a unique morphotype and/or antibiotype. Complete identification of the strains was achieved by the APISTAPH system (bioMérieux, La Balme Les Grottes, France), according to the manufacturer’s recommendations. The methicillin resistance of the strains was confirmed by the oxacillin agar screen test, as directed in the National Committee for Clinical Laboratory Standards guidelines (24). The susceptibility of the strains to 10 antibiotics (Table 1) was determined by the disk diffusion assay on Mueller-Hinton plates (Becton Dickinson, Cockeysville, Md.) and interpreted in accordance with the French recommendations (10). Finally, pulsed-field gel electrophoresis (PFGE) analysis, which has proved to be the most powerful marker for coagulase-negative staphylococci (8, 12, 19, 21), was performed when strains from the catheter and nares belonged to the same species. Isolates were embedded in agarose plugs, digested with the SmaI restriction enzyme, and separated on agarose gel with the Bio-Rad GenePath system (Bio-Rad, Hercules, Calif.) according to the manufacturer’s recommendations. The S. epidermidis reference strain ATCC 14990 and the Bio-Rad Staphylococcus aureus control strain were included in each run. DNA fingerprints were interpreted according to the criteria of Tenover et al. (31), without computerized assistance.
TABLE 1.
Properties of methicillin-resistant coagulase-negative staphylococcus isolates found in both catheters and nares
| Patient | Warda | Isolate | Siteb | Species identification | Resistance profilec | PFGE type |
|---|---|---|---|---|---|---|
| 2 | CS | 6654 | CVC | S. epidermidis | Kar Genr Cfr Rfr Fur For Tmr Cmr | A |
| 8424 | N | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr Cmr | A | ||
| 5 | CS | 6382 | CVC | S. epidermidis | Kar Genr Emr Cfr Fur Tmr | D |
| 7361 | N | S. epidermidis | Kar Genr Emr Cfr Fur Tmr | D | ||
| 12 | SICU | 1122 | CVC | S. epidermidis | Cfr | B |
| 0348 | N | S. epidermidis | Cfr | B | ||
| 13 | MICU | 1723 | CVC | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr Cmr | A |
| 2957 | N | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr Cmr | A | ||
| 15 | SICU | 4333 | CVC | S. epidermidis | Kar Emr Fur | E |
| 4527 | N | S. epidermidis | Kar Emr Fur | E | ||
| 19 | PICU | 0869 | CVC | S. epidermidis | Kar Genr Rfr For Tmr | C |
| 2082 | N | S. epidermidis | Kar Genr For Tmr | C | ||
| 20 | NN | 1945 | CVC | S. epidermidis | Kar Genr Tcr Emr Rfr | C |
| 3672 | N | S. epidermidis | Kar Genr Tcr Emr Rfr Tmr | C | ||
| 21 | CS | 2398 | CVC | S. epidermidis | Kar Emr Fur Tmr | H |
| 3263 | N | S. epidermidis | Kar Emr Fur Tmr | H | ||
| 23 | PS | 2925 | CVC | S. epidermidis | Kar Genr Emr Rfr For Tmr | C |
| 4204 | N | S. epidermidis | Kar Genr Emr Rfr For Tmr | C | ||
| 25 | CS | 4779 | CVC | S. epidermidis | Cfr | B |
| 6278 | N | S. epidermidis | Cfr | B | ||
| 28 | DS | 6521 | CVC | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr Cmr | I |
| 7638 | N | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr Cmr | I | ||
| 29 | PN | 9038 | CVC | S. epidermidis | Kar Genr Emr Cfr Rfr Fur Cmr | B |
| 0553 | N | S. epidermidis | Cfr | B | ||
| 30 | PICU | 0167 | CVC | S. haemolyticus | Kar Genr Emr Cfr Rfr For Tmr | G |
| 1995 | N | S. haemolyticus | Kar Genr Cfr For Tmr | G | ||
| 31 | US | 1374 | CVC | S. epidermidis | Kar Genr Cfr Rfr Fur For Tmr Cmr | A |
| 2673 | N | S. epidermidis | Kar Genr Cfr Rfr Fur For Tmr Cmr | A | ||
| 32 | PICU | 2832 | CVC | S. epidermidis | Kar Genr Emr Rfr Fur For Tmr | C |
| 4905 | N | S. epidermidis | Kar Genr Emr Rfr For Tmr Cmr | C | ||
| 33 | CS | 4389 | CVC | S. haemolyticus | Kar Genr Cfr For Tmr | J |
| 4840 | N | S. haemolyticus | Kar Genr Cfr For Tmr | J | ||
| 34 | CS | 3168 | CVC | S. epidermidis | Emr Cfr Rfr | B |
| 4841 | N | S. epidermidis | Cfr | B | ||
| 39 | MW | 5631 | CVC | S. epidermidis | Kar Genr Emr Cfr Fur Tmr | B |
| 7234 | N | S. epidermidis | Cfr | B | ||
| 40 | CS | 6502 | CVC | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr | A |
| 7061 | N | S. epidermidis | Kar Genr Emr Cfr Rfr Fur For Tmr | A | ||
| 44 | NN | 8712 | CVC | S. epidermidis | Kar Genr Tcr Rfr For Tmr | C |
| 1677 | N | S. epidermidis | Kar Genr Tcr Rfr For Tmr | C |
CS, cardiac surgery; DS, digestive surgery; MICU, medical intensive care unit; MW, medical ward; NN, neonatalogy; NS, neurosurgery; PICU, pediatric intensive care unit; PN, pneumology; PS, pediatric surgery; SICU, surgical intensive care unit; US, urogenital surgery.
N, nares.
Ka, kanamycin; Gen, gentamicin; Em, erythromycin; Cf, ciprofloxacin; Rf, rifampin; Fu, fucidin; Fo, fosfomycin; Tm, trimethoprim; Cm, chloramphenicol.
Forty-two (77.7%) of the 54 patients exhibited a nasal methicillin-resistant coagulase-negative staphylococcus culture. The APISTAPH and susceptibility patterns revealed that 31 patients most likely harbored a unique isolate, while 11 patients harbored 2 different isolates. Out of the 97 isolates (47 from catheters, 50 from nasal swabs), 80 (82.5%) were S. epidermidis. The other species were Staphylococcus haemolyticus (12 isolates), Staphylococcus warneri (2 isolates), Staphylococcus hominis (1 isolate), Staphylococcus capitis (1 isolate), and Staphylococcus simulans (1 isolate). Thirty-three patients had nasal and catheter isolates belonging to the same species. In 20 of these patients, nasal and catheter strains exhibited identical PFGE patterns. The properties of these strains are presented in Table 1, and representative PFGE patterns are displayed in Fig. 1. The resistance profiles of these identical strains were strictly similar in 13 cases, differed by one or two markers in 5 cases, and differed by five markers in 2 cases (Table 1). While 29 different DNA fingerprints were generated by the 97 isolates, three epidemic strains, called A, B, and C (Fig. 1), were found in nine, seven, and seven patients, respectively. Whereas strain C was only isolated in pediatric and neonatal patients, strains A and B were found in the cardiac surgery ward and in unrelated wards. The persistence of distinct strains of coagulase-negative staphylococci, as evidence of nosocomial transmission, has previously been shown to occur in cardiac surgery and neonatal intensive care units and implicates hands as a route of transmission (5, 8, 12, 13, 19, 23). Interestingly in our study, 14 of the 20 patients who had nasal and catheter cross-contamination harbored epidemic strains. These data suggest that these epidemic strains, within a heterogeneous population of methicillin-resistant coagulase-negative staphylococci, are potentially more virulent than the sporadic strains.
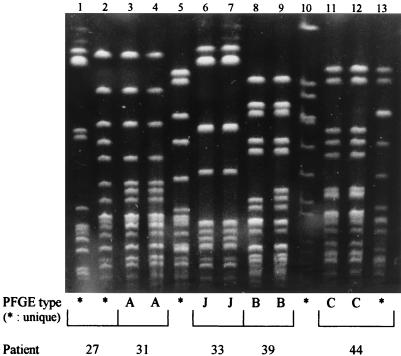
FIG. 1.
PFGE patterns of representative methicillin-resistant coagulase-negative staphylococcus isolates. Lanes: 1 and 2, unique patterns (patient 27, nares and catheter); 3 and 4, pattern A (patient 31, nares and catheter); 5, unique pattern (reference coagulase-negative staphylococcus strain); 6 and 7, pattern J (patient 33, nares and catheter); 8 and 9, pattern B (patient 39, nares and catheter); 10, unique pattern, (Bio-Rad S. aureus control strain); 11 and 12, pattern C (patient 44, catheter and first nasal isolate); 13, unique pattern (patient 44, second nasal isolate).
There have been few data showing the cross-contamination between the sites of carriage and the sites of colonization or infection by coagulase-negative staphylococci, and none was focused on methicillin-resistant coagulase-negative staphylococci. It is known that mucosal damage of the alimentary tract and concurrent colonization of mucous membranes are risk factors for S. epidermidis infections (13, 17). By studying the reservoirs of coagulase-negative staphylococci in preterm infants, Eastick et al. found a certain degree of correlation between bacterial counts found at “source” and “destination” sites (13). Other investigators isolated identical S. epidermidis strains from blood and tracheal aspirates of preterm newborns, but their study was restricted to a small number of patients (4). In the present work, PFGE comparison analysis of CVC and nasal isolates revealed strain identity for 60.6% of the patients who exhibited colonization of both sites. The presence in the nares of methicillin-resistant coagulase-negative staphylococci potentially infecting CVC is in agreement with results from a previous study reporting that coagulase-negative staphylococci causing bacteremia can be cultured from the nares before the blood culture is obtained (17). However, of the 54 patients at risk of methicillin-resistant coagulase-negative staphylococcal catheter-related sepsis, only 20 (37%) were nasally colonized with the same strain, which suggests that nasal surveillance is not a sensitive indicator of patients at risk of sepsis. Similarly, other investigators found less than 30% of the strains were colonizing catheters in the skin and catheter hub cultured after catheter withdrawal (3). Two drawbacks may explain the lack of sensitivity of the methicillin-resistant coagulase-negative staphylococcus detection reported in our study. First, the detection was based on a single nasal swab, and the sensitivity would likely increase if the swabbings were repeated and extended to additional and preferable sites, such as axilla (18). Second, the molecular analysis was focused on unique isolates, and it is possible that methicillin-resistant coagulase-negative staphylococcus cultures, although phenotypically identical and exhibiting the same resistance pattern, are genetically polymicrobial. However, even if the sensitivity of methicillin-resistant coagulase-negative staphylococcus detection was underestimated, prospective studies and more longitudinal data are needed before a cause-and-effect relationship between methicillin-resistant coagulase-negative staphylococcus carriage and sepsis can be proposed. Methicillin-resistant coagulase-negative staphylococcus detection may also be hampered by a lack of specificity, reflecting the high rate of methicillin-resistant coagulase-negative staphylococcus colonization in certain hospital wards. For example, coagulase-negative staphylococci isolated from surveillance blood cultures in surgical intensive care units are known to be more likely contaminants than indicators of bloodstream infection (17). Other wards, such as neonatal wards in our hospital, exhibit a lower rate of colonization by methicillin-resistant coagulase-negative staphylococci and may gain more benefit from methicillin-resistant coagulase-negative staphylococcus carriage surveillance.
The present data show the possible contamination from the nasopharyngeal tract to the intravascular device and suggest that more precautions should be taken to avoid contamination from one site to another. It might be possible to eradicate methicillin-resistant coagulase-negative staphylococci at the site of catheter implantation by using antiseptics and to prevent ingress of coagulase-negative staphylococci by a combination of occlusive dressings and careful handling of catheters and insertion sites with gloved hands. The detection of methicillin-resistant coagulase-negative staphylococcus carriage in high-risk patients, such as cardiac surgery patients, preterm newborns, or immunocompromised patients, may represent a substantial help for the early treatment of coagulase-negative staphylococcal sepsis. However, such detection needs to be more sensitive and should probably be restricted to hospital wards exhibiting a low rate of methicillin-resistant coagulase-negative staphylococcus colonization.
REFERENCES
- 1.Archer G L. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev Infect Dis. 1991;13(Suppl. 10):805–809. doi: 10.1093/clinids/13.supplement_10.s805. [DOI] [PubMed] [Google Scholar]
- 2.Archer G L, Climo M W. Antimicrobial susceptibility of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1994;38:2231–2237. doi: 10.1128/aac.38.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atela I, Coll P, Rello J, Quintana E, Barrio J, March F, Sanchez F, Barraquer P, Ballus J, Cotura A, Prats G. Serial surveillance cultures of skin and catheter hub specimens from critically ill patients with central venous catheters: molecular epidemiology of infection and implications for clinical management and research. J Clin Microbiol. 1997;35:1784–1790. doi: 10.1128/jcm.35.7.1784-1790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bétrémieux P, Donnio P Y, Pladys P. Use of ribotyping to investigate tracheal colonization by Staphylococcus epidermidis as a source of bacteremia in ventilated newborns. Eur J Clin Microbiol Infect Dis. 1995;14:342–346. doi: 10.1007/BF02116529. [DOI] [PubMed] [Google Scholar]
- 5.Boyce J M, Potter-Bynoe G, Opal S M, Dziobek L, Medeiros A A. A common-source outbreak of Staphylococcus epidermidis infections among patients undergoing cardiac surgery. J Infect Dis. 1990;161:493–499. doi: 10.1093/infdis/161.3.493. [DOI] [PubMed] [Google Scholar]
- 6.Brown E, Wenzel R P, Hendley J O. Exploration of microbial anatomy of normal human skin by using plasmid profiles of coagulase-negative staphylococci: search for the reservoir of resident skin flora. J Infect Dis. 1989;160:644–650. doi: 10.1093/infdis/160.4.644. [DOI] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med. 1987;147:873–877. [PubMed] [Google Scholar]
- 8.Burnie J P, Naderi-Nasab M, Loudon K W, Matthews R C. An epidemiological study of blood culture isolates of coagulase-negative staphylococci demonstrating hospital-acquired infection. J Clin Microbiol. 1997;35:1746–1750. doi: 10.1128/jcm.35.7.1746-1750.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleri D J, Corrado M L, Seligman S J. Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis. 1980;141:781–786. doi: 10.1093/infdis/141.6.781. [DOI] [PubMed] [Google Scholar]
- 10.Comité de l’Antibiogramme de la Société Française de Microbiologie. Valeurs critiques pour l’antibiogramme. Pathol Biol. 1997;45:1–12. [Google Scholar]
- 11.de Cicco M, Panarello G, Chiaradia V, Fracasso A, Veronesi A, Testa V, Santini G, Tesio F. Source and route of microbial colonization of parenteral nutrition catheters. Lancet. 1989;ii:1258–1261. doi: 10.1016/s0140-6736(89)91861-8. [DOI] [PubMed] [Google Scholar]
- 12.Degener J E, Heck M E O C, van Leeuwen W J, Heemskerk C, Crielaard A, Joosten P, Caesar P. Nosocomial infection by Staphylococcus haemolyticus and typing methods for epidemiological study. J Clin Microbiol. 1994;32:2260–2265. doi: 10.1128/jcm.32.9.2260-2265.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastick K, Leeming J P, Bennett D, Millar M R. Reservoirs of coagulase-negative staphylococci in preterm infants. Arch Dis Child. 1996;74:F99–F104. doi: 10.1136/fn.74.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans C A, Strom M S. Eight year persistence of individual differences in the bacterial flora of the forehead. J Investig Dermatol. 1982;79:51–52. doi: 10.1111/1523-1747.ep12510587. [DOI] [PubMed] [Google Scholar]
- 15.Freeman J, Epstein M F, Smith N E, Platt R, Sidebottom D G, Goldmann D A. Extra hospital stay and antibiotic usage with nosocomial coagulase-negative staphylococci bacteremia in two neonatal intensive care unit populations. Am J Dis Child. 1990;144:324–329. doi: 10.1001/archpedi.1990.02150270074029. [DOI] [PubMed] [Google Scholar]
- 16.Gaillard J L, Merlino R, Pajot N, Goulet O, Fauchere J L, Ricour C, Veron M. Conventional and nonconventional modes of vancomycin administration to decontaminate the internal surface of the catheters colonized with coagulase-negative staphylococci. J Parenter Enteral Nutr. 1990;14:593–597. doi: 10.1177/0148607190014006593. [DOI] [PubMed] [Google Scholar]
- 17.Herwaldt L A, Hollis R J, Boyken L D, Pfaller M A. Molecular epidemiology of coagulase-negative staphylococci isolated from immunocompromised patients. Infect Control Hosp Epidemiol. 1992;13:86–92. doi: 10.1086/646478. [DOI] [PubMed] [Google Scholar]
- 18.Høiby N, Jarløv J O, Kemp M, Tvede M, Bangsborg J M, Kjerulf A, Pers C, Hansen H. Excretion of ciprofloxacin in sweat and multiresistant Staphylococcus epidermidis. Lancet. 1997;349:167–169. doi: 10.1016/S0140-6736(96)09229-X. [DOI] [PubMed] [Google Scholar]
- 19.Huebner J, Pier G B, Maslow J N, Muller E, Shiro H, Parent M, Kropec A, Arbeit R D, Goldmann D A. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J Infect Dis. 1994;169:526–531. doi: 10.1093/infdis/169.3.526. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis W R, Martone W J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29(Suppl. A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 21.Kloos W E, Bannerman T L. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau Y L, Hey E. Sensitivity and specificity of daily tracheal aspirate cultures in predicting organisms causing bacteremia in ventilated neonates. Pediatr Infect Dis. 1991;10:290–294. doi: 10.1097/00006454-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lyytikaïnen O, Saxen H, Ryhänen R, Vaara M, Vuopio-Varkila J. Persistence of a multiresistant clone of Staphylococcus epidermidis in a neonatal intensive-care unit for a four-year period. Clin Infect Dis. 1995;20:24–29. doi: 10.1093/clinids/20.1.24. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 25.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 26.Salzman M B, Isenberg H D, Shapiro J F, Lipsitz P J, Rubin L G. A prospective study of the catheter hub as the portal of entry for microorganisms causing catheter-related sepsis in neonates. J Infect Dis. 1993;167:487–490. doi: 10.1093/infdis/167.2.487. [DOI] [PubMed] [Google Scholar]
- 27.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 28.Schwalbe R S, Stapleton J T, Gilligan P H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987;316:927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- 29.Sherman M P, Chance K H, Goetzman B W. Gram’s stains of tracheal secretions predict neonatal bacteremia. Am J Dis Child. 1984;138:848–850. doi: 10.1001/archpedi.1984.02140470048015. [DOI] [PubMed] [Google Scholar]
- 30.Storm W. Transient bacteremia following endotracheal suctioning in ventilated newborns. Pediatrics. 1980;65:487–490. [PubMed] [Google Scholar]
- 31.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veach L A, Pfaller M A, Barrett M, Koontz F P, Wenzel R P. Vancomycin resistance in Staphylococcus haemolyticus causing colonization and bloodstream infection. J Clin Microbiol. 1990;28:2064–2068. doi: 10.1128/jcm.28.9.2064-2068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]