Abstract
Dietary soy protein isolate (SPI) and the isoflavones daidzein and genistein have been shown to provide neuroprotection from stroke. However, the mechanisms remain uncertain. We sought to determine whether the addition of isoflavones to a diet containing caseinate (CAS) as the protein source would induce behavioral neuroprotection similar to that seen previously in rats fed SPI. Furthermore, we aimed to characterize the baseline and poststroke expression of mRNAs involved in pathways previously published as perhaps mediating soy-based neuroprotection from stroke and other markers of neuronal plasticity, oxidative stress, and inflammation. Adult male rats were fed a semipurified diet containing (1) sodium caseinate (CAS), (2) CAS plus daidzein and genistein (CAS+ISO), or (3) SPI for 2 weeks. A subset of rats was euthanized, and tissue was collected for quantitative real-time PCR (qPCR). Remaining rats underwent a middle cerebral artery occlusion to induce a stroke. Samples for qPCR were collected on day 3 poststroke. Rats fed SPI made fewer errors on the skilled ladder rung walking task after stroke compared to rats fed CAS (P < .05). Rats fed CAS+ISO were not different from rats fed CAS or SPI. Significant effects of diet were found at day 0 for Syp, Pparg, and Ywhae and at day 3 for Rtn4 expression. We concluded that the benefits of SPI are not solely attributable to daidzein and genistein.
Keywords: behavior, diet, isoflavone, MCAO, neuroprotection
INTRODUCTION
An occlusive stroke occurs when the flow of blood to the brain is blocked either temporarily or permanently.1 Permanent blockage results in the death of the affected brain tissue, which in turn results in cognitive, motor, and/or sensory dysfunction.2 A complete return of these functions after stroke is uncommon, but some functional recovery may occur spontaneously, although it is often incomplete.2,3
Bioactive dietary compounds are emerging as potential therapies aimed at reducing the severity of peri- and poststroke behavioral deficits.4 Previous studies by our laboratory and others have demonstrated that administering soy compounds (i.e., soy protein isolate [SPI], purified isoflavones, and so on) can result in a significant reduction in poststroke behavioral deficits when provided before stroke as part of a diet, administered through oral gavage, or even subcutaneously at the time of stroke.5–8 Of the >100 bioactive compounds in soybeans, the most plentiful and best characterized are the isoflavones daidzein and genistein, which are thought to at least partially mediate the neuroprotective benefits of soy-based diets.6,9–13 Isoflavones are considered “phytoestrogens” which, like estradiol, have the ability to cross the blood–brain barrier to bind and activate estrogen receptors (ERs) in the brain.6,7,11 Although others have demonstrated that ERs likely contribute to soy-mediated neuroprotection from stroke in ovariectomized female rats,11 the mechanisms mediating the benefits of diets containing soy are not fully understood, especially in male rodents, where brain ER expression after stroke is lower.14,15
In the present study, we sought to determine whether the addition of isoflavones to a diet containing caseinate (CAS) as the protein source was sufficient to induce behavioral neuroprotection similar to that seen previously in rats fed SPI.5 We also performed quantitative real-time PCR (qPCR) to compare the relative influence of the study diets on baseline and poststroke expression of mRNAs involved in pathways previously identified as possible mediators of soy-based neuroprotection from stroke, as well as mRNA expression of several markers of neuronal plasticity, oxidative stress, and inflammation.11,13,16–22 Therefore, data were collected for the expression of 10 mRNAs: Arg1 (arginase 1), Gap43 (growth associated protein 43), Pparg (peroxisome proliferator-activated receptor gamma), Sirt1 (sirtuin 1), Sod1 (superoxide dismutase 1), Syp (synaptophysin), Ywhae (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon), Bclxl (Bcl2-like 1), Bcl2 (BCL2, apoptosis regulator), and Rtn4 (reticulon 4).
MATERIALS AND METHODS
Diets
The semipurified study diets were based on those we utilized previously5 and were prepared to order for us by Research Diets, Inc. (New Brunswick, NJ). As in our previous study, the protein source of the diets was different between the sodium caseinate (CAS) diet and the SPI diet, with all other nutrients held constant and based on AIN-93 guidelines. For the third diet group (CAS+ISO), sodium caseinate was the protein source, as for the CAS group, but purified isoflavones daidzein (LC Laboratories, Woburn, MA) and genistein (LC Laboratories) were added to the diet during formulation to approximate the levels of these compounds contained endogenously in the SPI diet (daidzein and genistein are produced by soybeans as glycosides and are found in SPI). Isoflavone content of the raw materials used to prepare the study diets was determined by a third party (NP Analytical Laboratories, St. Louis, MO) using HPLC Mass Spectrometry, and these values were used to develop the final diets. All three diets were isocaloric (Table 1).
Table 1.
Composition of Experimental Diets
| Ingredient | Diets |
||
|---|---|---|---|
| CAS |
SPI |
CAS+ISO |
|
| g/kg/diet | |||
| Caseinate, Sodiuma | 200 | 0 | 200 |
| SPIb | 0 | 200 | 0 |
| dl-Methionine | 3 | 3 | 3 |
| Corn starch | 440 | 440 | 440 |
| Maltodextrin 10 | 100 | 100 | 100 |
| Sucrose | 100 | 100 | 100 |
| Cellulose, BW200 | 50 | 50 | 50 |
| Cocoa butter | 12.5 | 12.5 | 12.5 |
| Linseed oil | 1.5 | 1.5 | 1.5 |
| Palm oil | 17.5 | 17.5 | 17.5 |
| Safflower oil | 9.5 | 9.5 | 9.5 |
| Sunflower oil, Trisun Extra | 9 | 9 | 9 |
| Salts, S10026 | 10 | 10 | 10 |
| DiCalcium phosphate | 13 | 13 | 13 |
| Calcium carbonate | 5.5 | 5.5 | 5.5 |
| Potassium citrate, 1 H2O | 16.5 | 16.5 | 16.5 |
| Vitamins, V13401 | 10 | 10 | 10 |
| Vitamin E acetate, 50% | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 2 | 2 | 2 |
| TBHQ | 0.03 | 0.03 | 0.03 |
| Total genisteinc | Not detected | 0.21 | 0.32 |
| Total daidzeinc | Not detected | 0.12 | 0.17 |
| Protein kcal% | 21 | 21 | 21 |
| Carbohydrate kcal% | 67 | 67 | 67 |
| Fat kcal% | 12 | 12 | 12 |
| Total kcal% | 100 | 100 | 100 |
Alanate 180, New Zealand Milk Products, Inc.
SUPRO® Isolated Soy Protein, DuPont Nutrition and Biosciences.
Expressed as aglycone equivalents.
SPI, soy protein isolate.
At the beginning of the study (day-14), adult (6-month-old) male Long Evans Hooded rats (Charles River, Wilmington, MA) were randomly assigned to one of the three study diets. Rats had ad lib access to their assigned diet and water. All rats remained on their assigned diet until the end of the study. Rats were maintained on a 12-h light/12-h dark cycle in the vivarium of Southern Illinois University Carbondale for the duration of the study, according to the institutional guidelines for the care and use of laboratory animals.
Skilled ladder rung walking
We utilized the skilled ladder rung task23 to gauge motor function in the contralesional forelimb before and after stroke in rats, as performed previously.8 For this, rats were exposed to the apparatus and allowed to cross the ladder three times each on two consecutive days before their baseline session. On day 0, rats were video recorded while performing the task to establish their baseline performance before middle cerebral artery occlusion (MCAO) surgery. Rats were video recorded again on day +3. On both testing days, rats were placed on the ladder and allowed to cross to reach their home cage. Three trials were recorded on both testing days.
After the end of the study, the videos were viewed and scored by an observer who was blind to the treatment group for each rat. Performance was recorded for the contralesional impaired forelimb, and errors on the task were scored as either slight or deep slips using criteria reported previously,8,23,24 which were noted separately. Rats with >10 total slips on day 0 were excluded from the behavioral analysis. To control for repeated measures, the number of total slips committed at baseline was subtracted from the total number of day 3 slips (i.e., post-pre) for each animal. The resulting data were then analyzed using a one-way ANOVA with Tukey's post hoc for multiple comparisons between groups. A one-way ANOVA was used to compare baseline (day 0) performance between groups. Animal weights were compared across the study using two-way ANOVA with repeated measures. P values <.05 were considered significant. All statistics were performed with Prism 7.04 for Windows (GraphPad Software, La Jolla, CA, USA).
Surgical procedures
On day 0, a subset of rats from each diet group was randomly chosen to undergo a permanent MCAO procedure to produce a stroke lesion, as described previously.5,8,25 Briefly, rats in the MCAO groups were anesthetized with isoflurane (5% in oxygen) in an induction chamber. Rats were then placed in a stereotaxic device with an integrated anesthesia port, where they were maintained on isoflurane (1% to 2.5% in oxygen) for the duration of the MCAO procedure. First, the left common carotid artery (CCA) was visualized and permanently ligated. Next, a craniotomy was made to visualize the left middle cerebral artery (MCA) proximal to the rhinal fissure and ligate it. Rats then underwent a temporary occlusion of the right (contralateral) CCA. At the end of the procedure, rats were removed from anesthesia, allowed to recover, and returned to their home cages. All surgical and animal care procedures were approved by the SIUC Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
Rats that were not assigned to the MCAO groups were euthanized on day 0 to determine mRNA expression in rats in each diet group (i.e., CAS, CAS+ISO, and SPI) at that time point.
qPCR protocol and data analysis
For the qPCR study, day 0 (N = 4/group) or day 3 poststroke (N = 6/group) rats were euthanized, and brains were removed for isolation of RNA. Brain tissue was sliced into 1 mm coronal sections, and tissue samples (∼2 × 2 × 1 mm per side) were taken at the rostrocaudal midpoint of the lesion from both ipsilateral peri-infarct cortex (damaged) and contralateral (opposite) homotopic cerebral cortex using a scalpel blade. Care was taken to avoid including the underlying white matter. For day 0 rats (which did not receive an MCAO), samples were taken from both left and right sides of the cerebral cortex. Tissue samples were immediately placed into Trizol™, homogenized, and stored at −80 until RNA extraction. RNA was extracted using the Trizol Reagent protocol (Life Technologies, Carlsbad, CA) and quantified using a NanoDrop™ 2000 UV spectrophotometer (Thermo Scientific). Total RNA was reverse transcribed using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA) as per the manufacturer's instructions using a Bio-Rad iCycler Thermocycler (Hercules, CA). All qPCRs were performed in triplicate, utilizing SYBR green incorporation (Life Technologies) for quantification of the PCR products (see Table 2 for primer sequences). Calibration cDNA and water control wells were included on each 96 well plate. The resulting mRNA expression values were normalized to the cerebral cortex mean of day 0 (uninjured control) CAS-fed rats using the 2ΔΔCt method,26 as appropriate. Day 0 qPCR data were analyzed using one-way ANOVA with Tukey's post hoc test for multiple comparisons between diet groups. Day 3 qPCR data were analyzed using two-way ANOVA with Tukey's post hoc test for multiple comparisons between groups and/or hemispheres. Significance was set at P < .05 for all analyses.
Table 2.
Primers Used for Quantitative Real-Time Polymerase Chain Reaction
| Gene | Primer sequence |
|---|---|
| Arg1 | Forward-ACAAGACAGGGCTACTTTCAGG Reverse-TTTGCGGTGATGCCCCAGAT |
| Gap43 | Forward-GCCAAGGAGGAGCCTAAACA Reverse-GTCGGGCACTTTCCTTAGGT |
| Pparg | Forward-GAAGGGGCCTGGACCTCTGCT Reverse-CCGAAGTTGGTGGGCCAGAATGG |
| Sirt1 | Forward-CCAGTAGCACTAATTCCAAGTTC Reverse-GCATACTCGCCACCTAAC |
| Sod1 | Forward-TGTGCGTGCTGAAGGGCG Reverse-CTTCATCCGCTGGACCGCCA |
| Syp | Forward-GGTCTTTGCCATCTTCGCCT Reverse-ACGAGGAGTAGTCCCCAACC |
| Ywhae | Forward-ACGCGGAGCGAGAAGCTGAG Reverse-TCAGCTCCACGTCCATTCCTGC |
| Bclxl | Forward-AGCTTCGCAATTCCTCTGGC Reverse-TCCAAAGCCAAGATAGGGTTATTC |
| Bcl2 | Forward-ATTGTGGCCTTCTTTGAGTTCG Reverse-GTTCCACAAAGGCATCCCAG |
| Rtn4 | Forward-ATGAAGGCCACCCATTCA Reverse-AGACCATTGAACAAGGCACCA |
| Gapdh | Forward-GAGAAGGCTGGGGCTCAC Reverse-CCCTTCCACGATGCCAAAGT |
RESULTS
Skilled ladder rung walking
Rats in the CAS, CAS+ISO, and SPI groups demonstrated an equivalent number of errors on day 0 (P = .433). After stroke, rats fed SPI exhibited a significantly smaller increase in the total number of forelimb slips committed while crossing the bar walk apparatus compared to CAS-fed rats (Fig. 1; P = .037). The poststroke increase in the total number of slips committed by rats in the CAS+ISO group was not significantly different from slips committed by rats in either the CAS (P = .372) or the SPI groups (P = .443). No difference in body weight was detected between groups across the study (P > .05; data not shown).
FIG. 1.
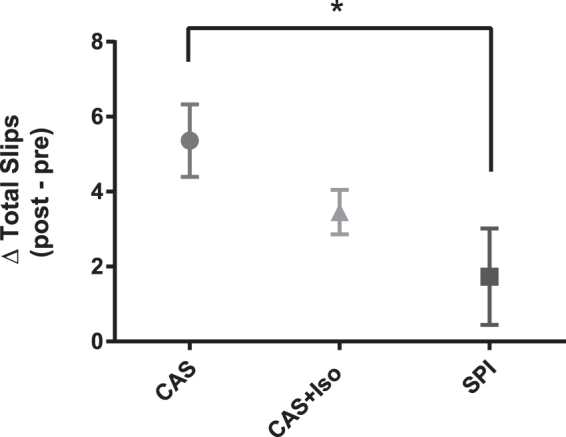
Skilled ladder rung walking on day 3 poststroke. Rats fed SPI (N = 11) demonstrated fewer total forelimb slips than rats fed CAS (N = 11; P = .037). Rats fed CAS+ISO (N = 11) demonstrated intermediate performance and were not significantly different in their performance from either CAS (P = .372) or SPI rats (P = .443). *P < .05. SPI, soy protein isolate.
Effects of diet on relative mRNA expression at day 0
We quantified expression of the mRNAs of interest at day 0 in rats fed CAS, CAS+ISO, and SPI diets, using the mean expression of each mRNA in the CAS group for normalization and expression of fold differences between groups for statistical comparison as described in the Materials and Methods section. This was done to compare the effects of the study diets on gene expression without the influence of a stroke lesion.
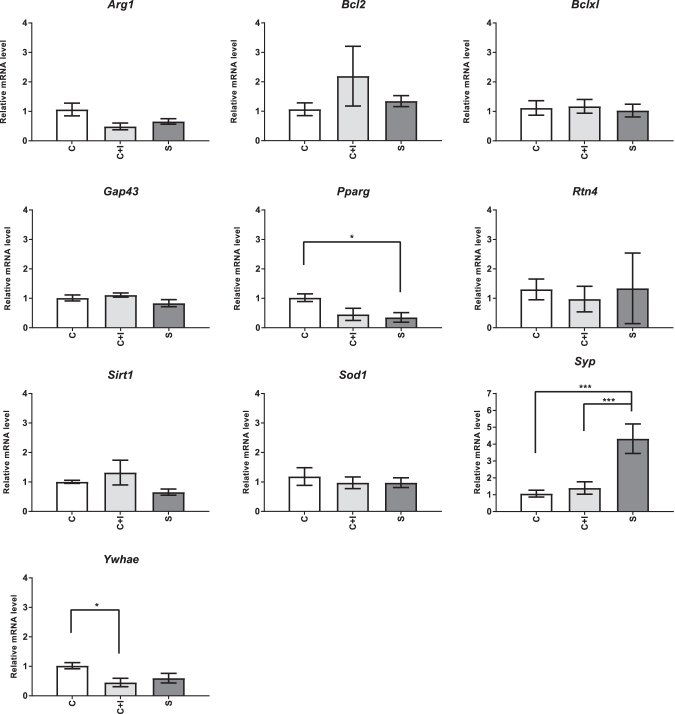
We found that relative mRNA expression of Pparg was significantly higher in the CAS diet group than in the SPI diet group (P = .024; Fig. 2) at day 0. Expression of Pparg in the CAS+ISO diet group was not significantly different than either the CAS (P = .069) or SPI diet (P = .99) groups.
FIG. 2.
Relative mRNA expression at day 0. Baseline (day 0) expression of genes of interest in adult male Long Evans Hooded rats (N = 4/group) fed a diet containing caseinate (CAS), SPI, or caseinate with added isoflavones (CAS+ISO). Relative expression of the indicated genes in total cellular RNA was determined by qPCR. *P < .05, and ***P < .001. qPCR, quantitative real-time PCR.
Expression of Syp was significantly affected by diet (P = .0005) at day 0. Rats fed SPI expressed Syp mRNA at a level ∼4-fold greater than rats fed CAS (P = .0004) or CAS+ISO (P = .0013), which were not different from one another (P > .05; Fig. 2).
An effect of diet was detected on expression of Ywhae mRNA at day 0 (P = .044). Expression of Ywhae was significantly lower in the CAS+ISO group than in CAS (P = .043). SPI-fed rats demonstrated an intermediate level of expression which was not different from either CAS+ISO (P = .743) or CAS (P = .135).
No significant effects of diet were observed at day 0 on the relative expression of Arg1, Bcl2, Bclxl, Gap43, Rtn4, Sirt1, or Sod1 (Fig. 2).
Effects of diet and hemisphere on relative mRNA expression at day 3 poststroke
Fold changes of the mRNAs of interest at day 3 poststroke in rats fed CAS, CAS+ISO, and SPI diets were again determined by normalizing to the mean expression of each mRNA in the day 0 CAS group, including for the day 3 CAS group.
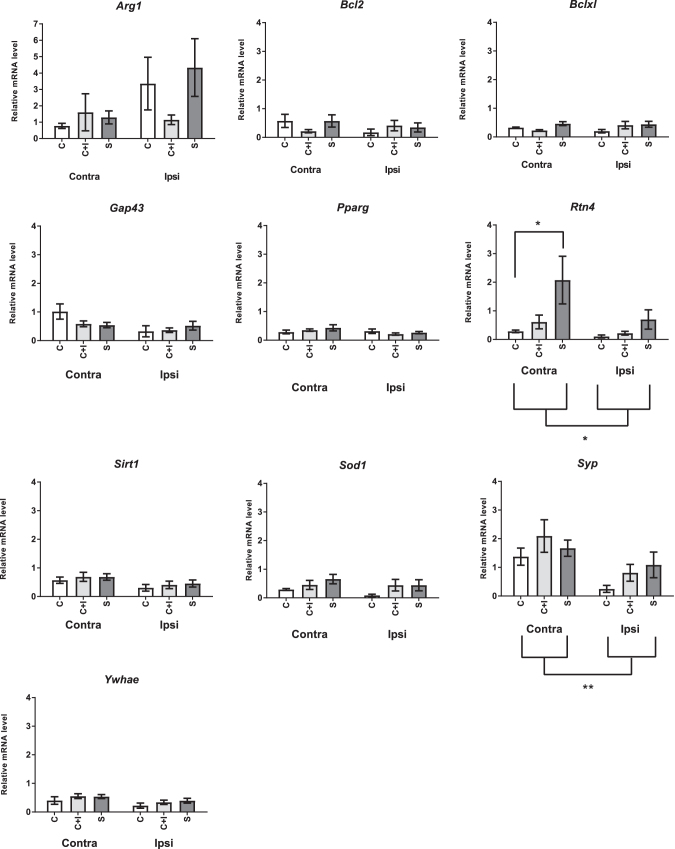
We found that no effects of hemisphere or diet were found for Arg1, Bcl2, Bclxl, Gap43, Pparg, Sirt, or Sod1 mRNA expression (P > .05; Fig. 3) on day 3 poststroke.
FIG. 3.
Relative mRNA expression at day 3 poststroke. Expression of genes of interest on day 3 poststroke in rats (N = 6/group) fed a diet containing caseinate (CAS), SPI, or caseinate with added isoflavones (CAS+ISO). Relative expression of the indicated genes in total cellular RNA was determined by qPCR. Relative expression for all aged groups was normalized to expression at day 0 in rats fed CAS. *P < .05, and **P < .01. Contra, Ipsi = gene expression in hemispheres contralateral or ipsilateral to stroke injury, respectively.
Relative expression of Rtn4 was affected by both hemisphere (P = .0375; Fig. 3) and diet (P = .0073). Overall expression was significantly higher in the hemisphere contralateral to the stroke. Within the contralateral hemisphere, the Rtn4 mRNA expression in the SPI group was significantly higher than in the CAS group (P = .022; Fig. 3), but was not different from the CAS+ISO group (P = .0731).
At day 3, relative mRNA expression of Syp was slightly but significantly higher in the contralateral hemisphere than the ipsilateral hemisphere (P = .0028; Fig. 3). However, no effects of diet were found either within or between hemispheres (P > .05).
Relative mRNA expression of Ywhae was slightly higher at day 3 in the contralateral hemisphere compared to the ipsilateral hemisphere (P = .0224; Fig. 3) but did not reach the threshold of a twofold difference, so was not considered biologically significant. No effect of diet was detected either within or across hemispheres (P > .05).
DISCUSSION
Skilled ladder rung walking
In a previous experiment, we demonstrated that prefeeding adult male rats a semipurified diet containing SPI significantly protected poststroke performance on the skilled forelimb reaching task compared to rats prefed a control diet containing CAS as the protein source.5 Based on these findings, we conducted the current studies to determine whether daidzein and genistein are the primary neuroprotective components mediating the relative neuroprotective effects of SPI over CAS. Therefore, we utilized SPI and CAS diets as we did previously, but we also included a group that received a modified diet containing CAS plus the isoflavones daidzein and genistein added to approximate the concentrations found in the SPI diet (CAS+ISO). The inclusion of the CAS+ISO diet group allowed us to evaluate the relative contribution of the isoflavones daidzein and genistein to the demonstrated neuroprotective properties of the SPI diet.
Herein, we report several important findings. First, we have replicated the key finding from our previous article5: adult male rats fed a semipurified diet containing SPI as the sole protein source performed significantly better on a behavioral task requiring skilled forelimb use after stroke than rats fed CAS.
Second, we determined that the isoflavones daidzein and genistein are not solely sufficient for mediating the observed benefits of the SPI diet compared to CAS in adult male rats. Interestingly, the skilled ladder rung walking results support the conclusion that the addition of isoflavones to the CAS diet provided some behavioral neuroprotection in adult rats, as skilled ladder performance by adult rats fed CAS+ISO was intermediate between groups fed CAS and SPI, but was not statistically different from either. This is an important finding, as it supports the hypothesis that isoflavones themselves probably provide at least some protection from stroke in young adult rats. Furthermore, this finding is consistent with previous studies in ovariectomized female rats7,11,21,27 and our previous study in which we infused daidzein using a subcutaneous osmotic mini pump.8
ERs have been shown to play a role in dietary isoflavone-mediated neuroprotection from stroke in ovariectomized young adult female rats.9 However, others have demonstrated that ER activation is not always necessary for neuroprotection by daidzein,13 but this has not yet been examined in a model of stroke. Further research is needed to more completely understand the role of ER in isoflavone-mediated neuroprotection in male rats.
mRNA expression at day 0
The expression of mRNA associated with inflammatory, apoptotic, and neuroplasticity pathways has been documented by others before and after stroke in a variety of models.11,13,16–22 However, few studies of the effects of isoflavones and/or SPI on poststroke gene expression have been published to date.21
In the current study, we chose to examine 10 individual mRNA of interest from several of these established pathways at day 0 (i.e., in the absence of stroke) to elucidate whether expression of these mRNAs was differentially induced in CAS, CAS+ISO, and SPI diet groups and for comparison to poststroke levels.
We found that diet affected the expression of Syp, Pparg, and Ywhae mRNA at day 0. Adult rats in both groups that received diets that contained isoflavones (CAS+ISO and SPI) were lower in Pparg expression than the CAS group, although only the difference between SPI and CAS reached statistical significance. Furthermore, the expression pattern of Pparg at the day 0 time point mirrors the behavioral outcome we found at day 3 poststroke: CAS and SPI groups were significantly different from one another, and CAS+SPI was not different from either SPI or CAS.
Modulation of the expression or activity of PPARg represents a candidate mechanism which could contribute to the neuroprotective benefits observed in rats fed diets containing SPI.10,28,29 Like daidzein and genistein,10 antidiabetic medications in the glitazone family are known activators for PPARg,30–32 and their administration has been shown to be protective after brain injury.33–35 However, the mechanism mediating the baseline reduction in Pparg mRNA by the CAS+ISO and SPI diets in the current study is unknown, as is the relationship of this reduction, if any, to the observed poststroke outcomes. Therefore, more research is needed to address this question.
Lower expression of Ywhae mRNA at day 0 in rats fed CAS+ISO and the intermediate effect in rats fed SPI is likely related to the reduced expression of Pparg in these groups, as Ywhae is downstream of Pparg.
In contrast to Pparg and Ywhae, Syp mRNA expression was fourfold higher in the SPI group than in the CAS and CAS+ISO groups. This increase likely reflects an increase in synapse density in the cerebral cortex of adult rats fed SPI.36 As this did not occur in the CAS+ISO group, this increase is not likely to be due to the isoflavones in the SPI but rather some other factor and possibly contributes to the enhanced skilled ladder rung walking performance observed in SPI-fed rats on day 3 poststroke. This hypothesis requires further investigation. Although the relative importance of the observed differences in expression of Pparg, Ywhae, and Syp at day 0 is not clear, the different baseline expression of one or more of these mRNA could possibly account for the differential effects of dietary daidzein and genistein on poststroke skilled ladder rung walking performance observed herein. Further study is needed to assess this hypothesis.
mRNA expression at day 3 poststroke
We examined whether mRNA was differentially expressed at day 3 after stroke in the injured (ipsilateral) or intact (contralateral) hemisphere and whether this expression was altered by diet.
Both Rtn4 and Syp mRNA were expressed at a higher level in the contralateral hemisphere than in the ipsilateral hemisphere at day 3 poststroke. However, only expression of Rtn4 was affected by diet: rats fed SPI exhibited expression that was fourfold greater than expression in the CAS group. Importantly, Rtn4 mRNA expression in the CAS+ISO was not significantly different than either CAS or SPI, which is again consistent with the skilled ladder rung walking findings in adult rats. This difference may reflect an increase in the activation of sprouting-related genes in the contralateral hemisphere in SPI-fed rats, which in turn resulted in an increase in the expression of Rtn4 mRNA to limit this plasticity.
Other researchers evaluating soy diets found Bclxl mRNA to be significantly increased in the ischemic hemisphere, while Bcl2 mRNA was significantly decreased.6,21 Our failure to detect these effects at day 3 poststroke differs from a previous study conducted in ovariectomized adult female rats. Lovekamp-Swan et al. found that feeding a high soy diet for a total of 3 weeks before stroke resulted in an increase in Bclxl mRNA at 22.5 h poststroke.21 This discrepancy may be due to a difference in the sampling time point or other methodology differences. Alternatively, it is possible that the pathways mediating the neuroprotective effects of soy are different between female and male rats. As noted above, male rats do not demonstrate an increase in ER expression after stroke, in contrast to females.15 Further studies are needed to understand the different effects of isoflavones in male and female rats.
Taken together, our results support the hypothesis that the neuroprotective effects of SPI are not due solely to the isoflavone content of this dietary component. This is evident in the observed intermediate poststroke behavioral neuroprotection of the CAS+ISO diet-fed rats. It is likely, therefore, that one or more additional bioactive compounds in SPI is acting either centrally on the brain or in peripheral structures to mediate the behavioral protection observed in this and other studies. Further research should be aimed at identifying this compound or compounds and defining the mechanism(s) through which it works to provide neuroprotection from stroke.
ACKNOWLEDGMENTS
The authors also thank Dr. Steve Verhulst and the SIU School of Medicine Division of Statistics and Research Consulting for their help with the analyses and Jason Gumbel and Tanner Rehnberg for their contributions to qPCR data collection.
AUTHOR DISCLOSURE STATEMENT
At the time of this study, D.N.B. was used by DuPont Nutrition & Biosciences. No other authors have conflicts of interest to declare.
FUNDING INFORMATION
This work was supported by the National Institutes of Health [grant number 1R15AT006593]. Study diets were provided as a gift in kind by DuPont Nutrition & Biosciences, St. Louis, MO.
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. : Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheatwood JL, Emerick AJ, Kartje GL: Neuronal plasticity and functional recovery after ischemic stroke. Top Stroke Rehabil 2008;15:42–50. [DOI] [PubMed] [Google Scholar]
- 3. Cassidy JM, Cramer SC: Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res 2017;8:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherzai A, Heim LT, Boothby C, Sherzai AD: Stroke, food groups, and dietary patterns: A systematic review. Nutr Rev 2012;70:423–435. [DOI] [PubMed] [Google Scholar]
- 5. Cheatwood JL, Burnet D, Butteiger DN, Banz WJ: Soy protein diet increases skilled forelimb reaching function after stroke in rats. Behav Brain Res 2011;216:681–684. [DOI] [PubMed] [Google Scholar]
- 6. Lovekamp-Swan T, Glendenning ML, Schreihofer DA: A high soy diet enhances neurotropin receptor and Bcl-XL gene expression in the brains of ovariectomized female rats. Brain Res 2007;1159:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schreihofer DA, Do KD, Schreihofer AM: High-soy diet decreases infarct size after permanent middle cerebral artery occlusion in female rats. Am J Physiol 2005;289:R103–R108. [DOI] [PubMed] [Google Scholar]
- 8. Stout JM, Knapp AN, Banz WJ, Wallace DG, Cheatwood JL: Subcutaneous daidzein administration enhances recovery of skilled ladder rung walking performance following stroke in rats. Behav Brain Res 2013;256:428–431. [DOI] [PubMed] [Google Scholar]
- 9. Schreihofer DA: Phytoestrogens as neuroprotectants. Drugs Today (Barc) 2009;45:609–627. [DOI] [PubMed] [Google Scholar]
- 10. Hurtado O, Ballesteros I, Cuartero MI, et al. : Daidzein has neuroprotective effects through ligand-binding-independent PPARgamma activation. Neurochem Int 2012;61:119–127. [DOI] [PubMed] [Google Scholar]
- 11. Ma Y, Sullivan JC, Schreihofer DA: Dietary genistein and equol (4’, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am J Physiol 2010;299:R871–R877. [DOI] [PubMed] [Google Scholar]
- 12. Gu L, Laly M, Chang HC, et al. : Isoflavone conjugates are underestimated in tissues using enzymatic hydrolysis. J Agric Food Chem 2005;53:6858–6863. [DOI] [PubMed] [Google Scholar]
- 13. Ma TC, Campana A, Lange PS, et al. : A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J Neurosci 2010;30:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson ME, Westberry JM, Trout AL: Estrogen receptor-alpha gene expression in the cortex: Sex differences during development and in adulthood. Hormones Behav 2011;59:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westberry JM, Prewitt AK, Wilson ME: Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience 2008;152:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carmichael ST, Chesselet MF: Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci 2002;22:6062–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li S, Carmichael ST: Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis 2006;23:362–373. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez-Jimenez M, Hurtado O, Cuartero MI, et al. : Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013;44:2333–2337. [DOI] [PubMed] [Google Scholar]
- 19. Lively S, Schlichter LC: Age-related comparisons of evolution of the inflammatory response after intracerebral hemorrhage in rats. Transl Stroke Res 2012;3(Suppl 1):132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai XJ, Ye SQ, Zheng L, et al. : Selective 14-3-3gamma induction quenches p-beta-catenin Ser37/Bax-enhanced cell death in cerebral cortical neurons during ischemia. Cell Death Dis 2014;5:e1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lovekamp-Swan T, Glendenning M, Schreihofer DA: A high soy diet reduces programmed cell death and enhances bcl-xL expression in experimental stroke. Neuroscience 2007;148:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stroemer RP, Kent TA, Hulsebosch CE: Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 1998;29:2381–2393; discussion 2393–2385. [DOI] [PubMed] [Google Scholar]
- 23. Metz GA, Whishaw IQ: Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods 2002;115:169–179. [DOI] [PubMed] [Google Scholar]
- 24. Wallace DG, Winter SS, Metz GA: Serial pattern learning during skilled walking. J Integr Neurosci 2012;11:17–32. [DOI] [PubMed] [Google Scholar]
- 25. Cheatwood JL, Emerick AJ, Schwab ME, Kartje GL: Nogo-A expression after focal ischemic stroke in the adult rat. Stroke 2008;39:2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 27. Schreihofer DA, Deutsch C, Lovekamp-Swan T, Sullivan JC, Dorrance AM: Effect of high soy diet on the cerebrovasculature and endothelial nitric oxide synthase in the ovariectomized rat. Vascul Pharmacol 2010;52:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collino M, Patel NS, Thiemermann C: PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Ther Adv Cardiovasc Dis 2008;2:179–197. [DOI] [PubMed] [Google Scholar]
- 29. Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN: Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol 2010;41:180–186. [DOI] [PubMed] [Google Scholar]
- 30. Shay NF, Banz WJ: Regulation of gene transcription by botanicals: Novel regulatory mechanisms. Annu Rev Nutr 2005;25:297–315. [DOI] [PubMed] [Google Scholar]
- 31. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA: An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995;270:12953–12956. [DOI] [PubMed] [Google Scholar]
- 32. Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N: Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr 2003;133:1238–1243. [DOI] [PubMed] [Google Scholar]
- 33. Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R: PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 2008;1244:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu JS, Cheung WM, Tsai YS, et al. : Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation 2009;119:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A: Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab 2009;29:1683–1694. [DOI] [PubMed] [Google Scholar]
- 36. Tarsa L, Goda Y: Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA 2002;99:1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]