Abstract
Anti-phospholipase A2 receptor autoantibody (PLA2R-Ab) plays a critical role in the pathogenesis of primary membranous nephropathy (PMN), an autoimmune kidney disease characterized by immune deposits in the glomerular subepithelial spaces and proteinuria. However, the mechanism of how PLA2R-Abs interact with the conformational epitope(s) of PLA2R has remained elusive. PLA2R is a single transmembrane helix receptor containing ten extracellular domains that begin with a CysR domain followed by a FnII and eight CTLD domains. Here, we examined the interactions of PLA2R-Ab with the full PLA2R protein, N-terminal domain truncations, and C-terminal domain deletions under either denaturing or physiological conditions. Our data demonstrate that the PLA2R-Abs against the dominant epitope (the N-terminal CysR-CTLD1 triple domain) possess weak cross-reactivities to the C-terminal domains beyond CTLD1. Moreover, both the CysR and CTLD1 domains are required to form a conformational epitope for PLA2R-Ab interaction, with FnII serving as a linker domain. Upon close examination, we also observed that patients with newly diagnosed PMN carry two populations of PLA2R-Abs in sera that react to the denatured CysR-CTLD3 (the PLA2R-Ab1) and denatured CysR-CTLD1 (the PLA2R-Ab2) domain complexes on Western blots, respectively. Furthermore, the PLA2R-Ab1 appeared at an earlier time point than PLA2R-Ab2 in patients, whereas the increased levels of PLA2R-Ab2 coincided with the worsening of proteinuria. In summary, our data support that an integrated folding of the three PLA2R N-terminal domains, CysR, FnII, and CTLD1, is a prerequisite to forming the PLA2R conformational epitope and that the dominant epitope-reactive PLA2R-Ab2 plays a critical role in PMN clinical progression.
Keywords: PLA2R, conformational epitope, PLA2R autoantibody, primary membranous nephropathy, proteinuria
Abbreviations: CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; EGFP, enhanced green fluorescent protein; FnII, fibronectin type II domain; HRP, horseradish peroxidase; PLA2R, phospholipase A2 receptor; PLA2R-Ab, anti-phospholipase A2 receptor autoantibody; PLA2R-Ab1, anti-denatured CysR-CTLD3 autoantibody; PLA2R-Ab2, anti-denatured CysR-CTLD1 autoantibody; PMN, primary membranous nephropathy; TBST, Tris-buffered saline/Tween-20; 1D4-Ab, mouse anti-1D4 monoclonal antibody
Primary membranous nephropathy (PMN) is a kidney-specific autoimmune glomerular disease (1, 2, 3, 4). The dominant antigen, phospholipase A2 receptor (PLA2R), accounts for ∼80% of clinical cases (5). The circulating anti-PLA2R autoantibodies (PLA2R-Ab) bind to PLA2R expressed on the basal surface of the glomerular epithelial cells, where they form in situ immune complexes. The shedding and deposition of the immune complexes in glomerular subepithelial spaces impair glomerular filtration function causing massive leakage of plasma proteins in urine.
Phospholipase A2 receptor is a type-I transmembrane receptor that belongs to the mannose receptor family consisting of five members: the mannose receptor, Endo180, DEC205, PLA2R, and the avian FcRY receptor (6). All family members share a common structural feature: a large extracellular region that begins with a cysteine-rich domain (CysR), followed by a fibronectin type II domain (FnII), and 8 to 10 C-type lectin-like domains (CTLDs); a single transmembrane helix and a short cytoplasmic tail. The 3D reconstructed EM structure of the mannose receptor (7), Endo180 (8), DEC205 (9), and FcRY (10) are available, which show that all have a folded conformation at the acidic pH and an extended conformation at the basic pH. In addition, a transmission-EM (11) and a cryo-EM (12) of the PLA2R extracellular region at a low resolution have also been reported. Although the 3D reconstructed structures from the two studies differ, both support that the extracellular region of PLA2R is compactedly folded, with some domains more accessible to antibody interaction than others at physiological conditions.
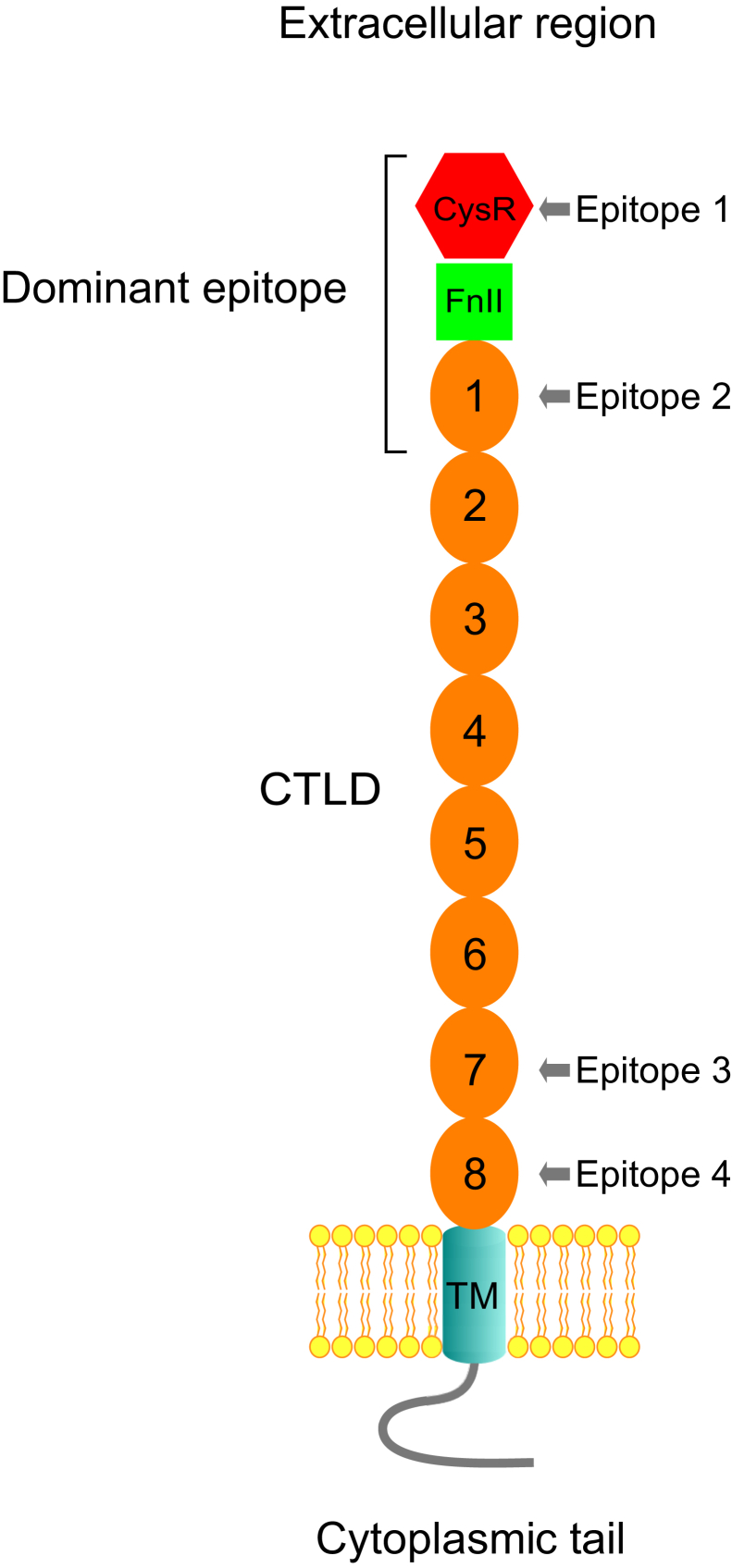
Anti-phospholipase A2 receptor autoantibody was identified using human glomerular extract (5). It reacts to the denatured and nonreduced PLA2R on Western blot, indicating that the reactive epitope is conformational (5). Previous studies determined that the dominant conformational epitope is at the N-terminal end of PLA2R encompassing the CysR-CTLD1 region (13). It was also reported that four single-domain epitopes (CysR, CTLD1, CTLD7, and CTLD8) exist in the large PLA2R extracellular region (11, 14, 15), and that epitope spreading occurs among these epitopes associated with disease progression (14, 16) (Fig. 1). However, the latter finding was contradicted by an intense analysis of 150 biopsy-proven PMN patients, which showed that the reported epitope spreading had no apparent clinical association (15).
Figure 1.
Topological location of the proposed conformational epitopes in PLA2R. Phospholipase A2 receptor consists of a large extracellular region that begins with a CysR domain (Hexagonal), followed by a FnII domain (square) and eight CTLD domains (oval), a single transmembrane helix (TM), and a short cytoplasmic tail. The dominant epitope resides at the extreme N-terminus encompassing the CysR, FnII, and CTLD1 domains. The four proposed single-domain epitopes reside in the CysR, CTLD1, CTLD7, and CTLD8 domains, respectively. CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; FnII, fibronectin type II domain; PLA2R, phospholipase A2 receptor.
Patients who are seropositive for PLA2R-Abs are reported to have a more severe disease course than seronegative patients (17, 18), yet ∼30% of seropositive patients may enter spontaneous remission with conservative management (no immunosuppression) (19, 20). In general, the levels of circulating PLA2R-Abs correlate positively with the degree of proteinuria and negatively with the treatment outcome (21, 22, 23, 24, 25). However, there is some reported discordance in these findings such that some patients with a high level of circulating PLA2R-Ab had no/or mild proteinuria, whereas some patients with a lower level of PLA2R-Ab had severe proteinuria, and some patients with high levels of baseline PLA2R-Ab at diagnosis entered spontaneous remission (25, 26, 27, 28, 29, 30). The mechanism that underlies these conflicting clinical observations has remained unclear.
In this study, we performed an in-depth analysis of the interactions between PLA2R-Abs and the PLA2R epitopes under denaturing and physiological conditions. Our data support that the anti-PLA2R dominant epitope antibody plays a critical role in PMN clinical progression.
Results
Surface expression of PLA2R N-terminal domain truncations in HEK 293 cells
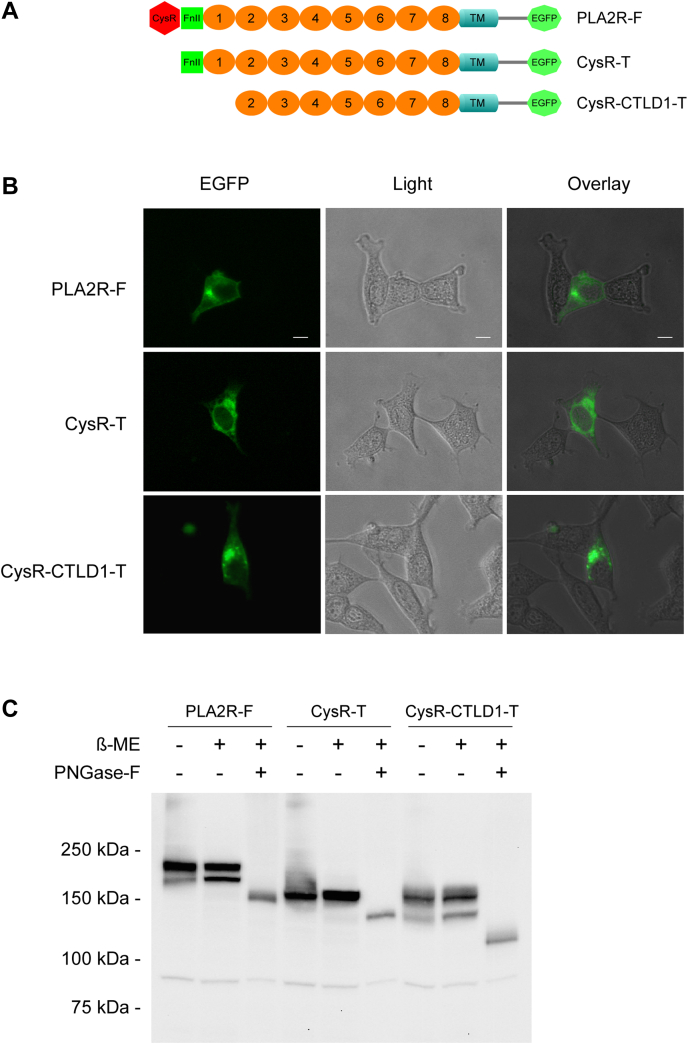
The PLA2R N-terminal domain truncations were used as antigens to identify the domain-specific PLA2R-Abs in patients (14). However, truncations at the N-terminal region of a transmembrane protein pose a high risk of triggering protein misfolding and intracellular retention and subsequently preventing its cell surface expression (31, 32). To determine if the N-terminal domain truncations affect PLA2R cell surface processing, we selectively truncated the CysR or the CysR-CTLD1 triple domain from an enhanced green fluorescent protein (EGFP)-fused PLA2R (Fig. 2A). The amino acid sequence of the truncated domains precisely followed the previous report (14). The constructs were transiently expressed in HEK 293 cells and fixed with 4% paraformaldehyde for fluorescence microscopy. The results showed that the full-length PLA2R formed a clear line along the cell edge, indicating its cell surface expression. In contrast, the two N-terminal domain truncations were concentrated in regions around the nucleus with no detectable distribution along the cell edge, indicating their intracellular retention (Fig. 2B). We then assessed the glycosylation status of the N-terminal domain truncations. Figure 2C shows, although both N-terminal domain truncations migrated similarly on SDS-PAGE under nonreducing and reduced conditions, peptide N-glycosidase F treatment caused a much more significant shift of the CysR-CTLD1 truncation compared to that of the CysR truncation, indicating that it has additional glycosylation consensus sites being modified.
Figure 2.
Surface expression and deglycosylation study of PLA2R N-terminal domain truncations.A, schematic illustration of the EGFP-fused PLA2R constructs. B, HEK 293 cells expressing PLA2R full-length, CysR, or CysR-CTLD1 domain truncations were plated at a low density on polylysine coated coverslips, fixed with 4% paraformaldehyde (pH 7.4) and imaged under a fluorescence microscope. The scale bar indicates 10 μm. C, cell lysates from transfected HEK 293 cells were treated with PNGase-F following the manufacturer’s instruction. Protein samples were resolved by 7.5% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a rabbit anti-human PLA2R polyclonal antibody. Each experiment was performed 3 to 5 times. -, without β-ME or PNGase-F; +, with β-ME or PNGase-F; β-ME, β-Mercaptoethanol; Anti-PLA2R-Ab, rabbit anti-human PLA2R polyclonal antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; CysR-CTLD1-T, CysR-CTLD1 triple domain truncation; CysR-T, CysR domain truncation; EGFP, enhanced green fluorescent protein; PLA2R, phospholipase A2 receptor; PLA2R-F, PLA2R full-length; PNGase-F, peptide N-glycosidase F.
Reactivity of PLA2R-Ab positive serum to PLA2R N-terminal domain truncations
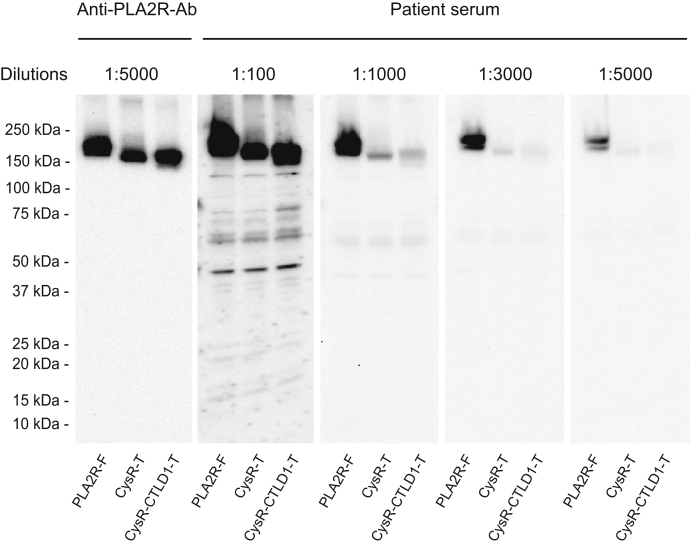
The above study demonstrated that the PLA2R N-terminal domain truncations are intracellularly retained. To assess if these constructs react to patient serum as reported (14, 15), we serially diluted seven patient sera with high levels of PLA2R-Ab (13) and then individually applied each on Western blot (nonreducing conditions were used for all serum studies otherwise indicated). Figure 3 shows that both PLA2R full-length and the two N-terminal domain truncations reacted to a representative serum (used throughout the study otherwise indicated) at 1:100 dilutions, indicating that the serum contains autoantibodies against the PLA2R C-terminal domains beyond the CysR and CTLD1 domain. However, on further serum dilution, we observed that the reactivity of PLA2R full-length dropped steadily but remained to be detectable at 1:5000, whereas the reactivity of the two N-terminal domain truncations dropped sharply at 1:1000 and became barely detectable at 1:3000. This observation directly contrasts with the PLA2R dominant-epitope (CysR-CTLD1), which showed similar reactivities as PLA2R full-length to PLA2R-Ab at 1:10,000 serum dilutions (13). Of note, all the seven serum samples had the same reactive pattern to the N-terminal domain truncations on Western blot (data not shown), consistent with the previous reports (14, 15). This data indicates that the PLA2R N-terminal domain truncations react to PLA2R-Ab differently from the PLA2R full-length or the dominant-epitope, requiring a much higher serum concentration.
Figure 3.
Representative data of PLA2R-Ab reactivity to the PLA2R N-terminal domain truncations on Western blot. Protein samples prepared from the transfected HEK 293 cells were resolved by 4 to 20% SDS-PAGE under nonreducing conditions, transferred to a nitrocellulose membrane, and probed with the Anti-PLA2R-Ab or the PLA2R-Ab positive serum at various dilutions. The serum probed blots were lined up and exposed simultaneously to a film with an equal exposure time for the best comparison of band intensities. The experiment was performed three times. 1D4-Ab, mouse anti-1D4 monoclonal antibody; Anti-PLA2R-Ab, rabbit anti-human PLA2R polyclonal antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; CysR-CTLD1-T, CysR-CTLD1 triple domain truncation; CysR-T, CysR domain truncation; PLA2R, phospholipase A2 receptor; PLA2R-F, PLA2R full length.
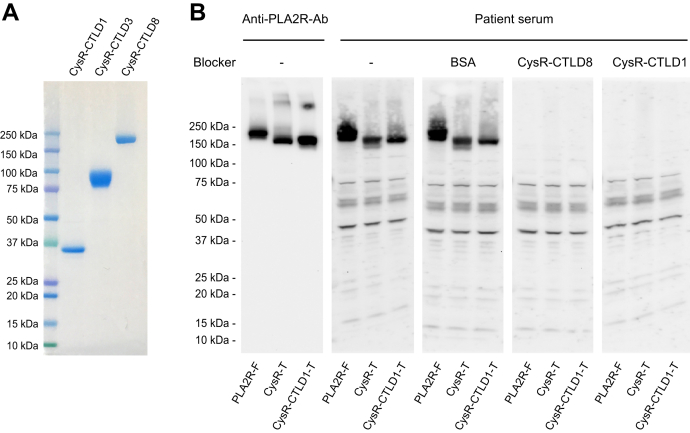
We next performed an epitope competition study using the affinity-purified His-tagged PLA2R domain fragments. Figure 4A shows that all the three PLA2R domain fragments were in high purity with minimal contamination. The patient serum was then mixed with either CysR-CTLD8 (PLA2R full-domain) or CysR-CTLD1 domain fragment and incubated for 2 h at room temperature, and subsequently, the mixture was applied on Western blot at 1:100 dilutions. The result shows that bovine serum albumin (negative control) did not affect serum reactivity to the PLA2R full-length and the two N-terminal domain truncations, whereas the PLA2R full-domain completely blocked the serum reactivity to each of the three constructs (Fig. 4B). Surprisingly, the PLA2R dominant-epitope, which only contains three N-terminal domains, CysR-FnII-CTLD1, also fully blocked the serum reactivity to all three constructs (Fig. 4B). This data demonstrates that the intracellularly retained PLA2R N-terminal truncations react to PLA2R-Abs; however, the reactive PLA2R-Abs are not specific to the remaining PLA2R C-terminal domains. Instead, they react to the N-terminal dominant-epitope.
Figure 4.
Epitope competition study.A, the Ni-NTA affinity-purified His-tagged CysR-CTLD1, CysR-CTLD3, and CysR-CTLD8 domain fragments were heated for 5 min at 100 degrees in SDS-sample buffer (no β-ME) and resolved by 4 to 20% SDS-PAGE. The gel was stained with Coomassie blue overnight. B, the PLA2R-Ab positive sera (20 μl each) were individually mixed with 30 μg BSA, 30 μg CysR-CTLD8, or 30 μg CysR-CTLD1 fragments in 100 μl TBS (pH 7.4) and incubated on a rotating shaker for 2 h at room temperature. The mixture was then applied on the membrane blocked in 2 ml TBSTM buffer and incubated for 2 h at room temperature. The level of each protein sample on the blot was verified by the Anti-PLA2R-Ab. Each experiment was performed at least three times. -, no blocking reagents; Anti-PLA2R-Ab, rabbit anti-human PLA2R polyclonal antibody; BSA, bovine serum albumin; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; CysR-CTLD1-T, CysR-CTLD1 triple domain truncation; CysR-T, CysR domain truncation; PLA2R, phospholipase A2 receptor; PLA2R-F, PLA2R full-length.
Reactivity of PLA2R-Ab positive serum to PLA2R domain fragments under physiological conditions
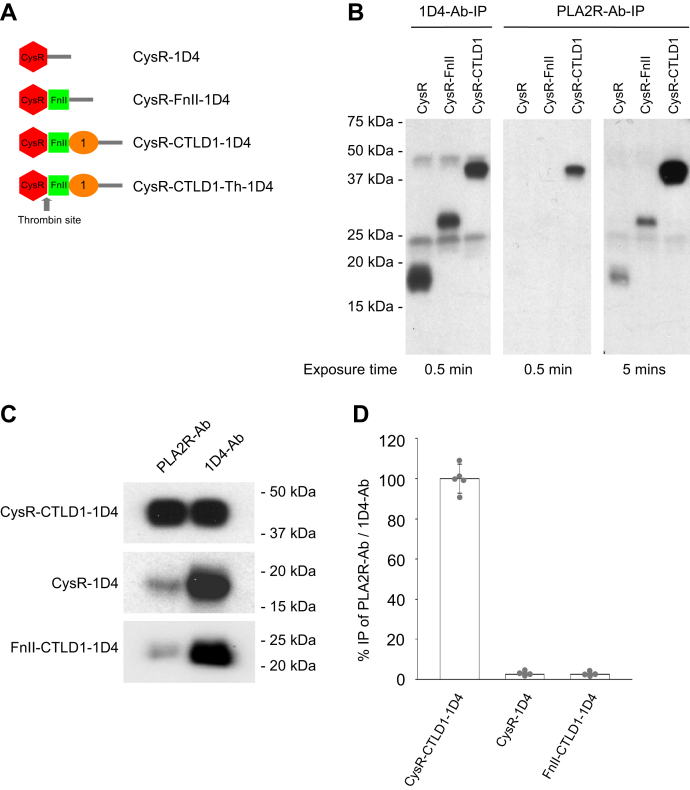
Our previous study showed that the denatured and nonreduced CysR domain does not react to PLA2R-Ab (13). Studies using different experimental approaches suggested that the CysR domain reacts to PLA2R-Ab only under nondenaturing conditions (11). To investigate these experimental discrepancies, we compared the reactivity of CysR, CysR-FnII, and CysR-CTLD1 domain fragments to PLA2R-Ab under physiological conditions (Fig. 5A). An equal amount of 1D4-tagged (TETSQVAPA from Rhodopsin) PLA2R domain fragments were individually mixed with the patient serum or a mouse anti-1D4 monoclonal antibody (1D4-Ab) in tris-buffered saline (TBS) buffer (pH 7.4). After 2-h incubation at room temperature, the immune complexes were immunoprecipitated by the protein G-sepharose resin. Results showed that the 1D4-Ab immunoprecipitated all the three PLA2R domain fragments at a similar level (Fig. 5B, left panel). However, the patient serum only strongly immunoprecipitated the CysR-CTLD1 fragment (Fig. 5B, middle panel), and detection of the immunoprecipitated CysR and CysR-FnII fragments required extended film exposure (Fig. 5B, right panel). We then tested PLA2R-Ab immunoprecipitation of the CTLD1 domain using a protease-released FnII-CTLD1 domain fragment from the CysR-CTLD1-Th construct (a 1D4-tagged CysR-CTLD1 fragment containing a thrombin cleavage site between CysR and FnII domain) (13). Figure 5, C and D show that compared to 1D4-Ab mediated immunoprecipitation, the serum immunoprecipitated almost 100% of the CysR-CTLD1 fragment but less than 3% of the CysR or the FnII-CTLD1 fragment. This data suggests that an integrated folding of the CysR, FnII, and CTLD1 domain is required to form a conformational epitope for strong PLA2R-Ab interaction.
Figure 5.
Characterization of PLA2R-Ab interaction with PLA2R domain fragments under physiological conditions.A, schematic illustration of the 1D4-tagged PLA2R extracellular domain fragments. B, phospholipase A2 receptor N-terminal 1D4-tagged domain fragments, CysR, CysR-FnII, and CysR-CTLD1 were individually mixed with either the 1D4-Ab or the PLA2R-Ab positive serum in 500 μl TBS (pH 7.4) and incubated for 2 h at room temperature followed with protein G-sepharose resin immunoprecipitation. The immunoprecipitated proteins were eluted with SDS-sample buffer containing β-ME. The protein samples were resolved by 4 to 20% SDS-PAGE, transferred to nitrocellulose membrane, and probed by the 1D4-Ab. The film was exposed for 0.5 min and 5 min to detect the immunoprecipitated protein fragments. The experiment was performed three times. C, the 1D4-tagged CysR-CTLD1, CysR, and FnII-CTLD1 domain fragments (released from CysR-CTLD1-Th by thrombin digestion) were individually immunoprecipitated with the patient serum or 1D4-Ab in TBS buffer (pH 7.4) and further processed as described above. D, the blots were scanned and quantified using densitometry. The error bars represent mean ± S.D. (n = 5). 1D4-Ab-IP, immunoprecipitation with the anti-1D4 antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; FnII, fibronectin type II domain; PLA2R, phospholipase A2 receptor; PLA2R-Ab-IP, immunoprecipitation with patient serum.
Differential reactivity of PLA2R-Abs to PLA2R full-length and PLA2R dominant-epitope
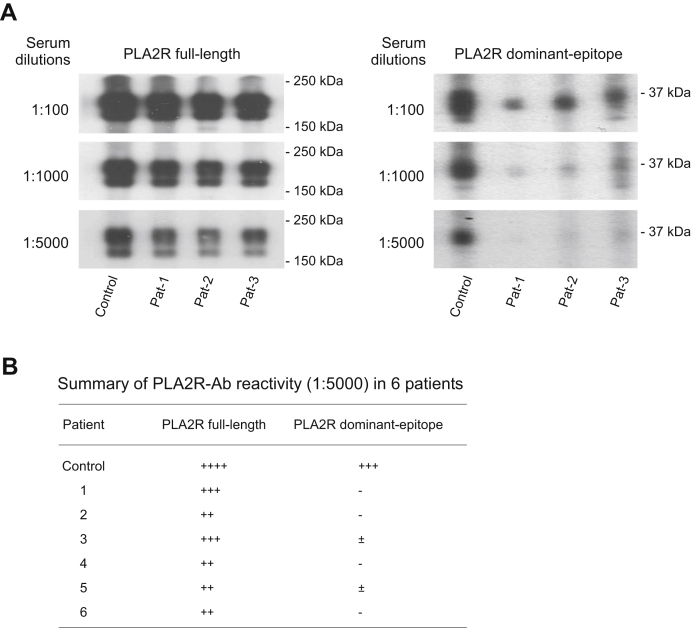
On analysis of the reactivity of 48 PLA2R-Ab positive sera to the PLA2R dominant-epitope, we observed unexpectedly that six sera reacted significantly weaker to the PLA2R dominant-epitope than to the PLA2R full-length (Table S1). To examine this phenomenon, we diluted all the six sera at 1:100, 1000, and 5000, respectively, and then applied each on Western blot. The data shows, compared to the control serum, the six sera reacted strongly to the PLA2R full-length but weakly to the dominant-epitope at all three dilutions (Fig. 6, A and B), suggesting that these patients possess different populations of circulating PLA2R-Abs: a large population (the PLA2R-Ab1) that reacts to a region (or conformation) in the denatured PLA2R other than the dominant-epitope and a small population (the PLA2R-Ab2) that reacts to the denatured dominant-epitope (CysR-CTLD1 domain complex).
Figure 6.
Representative data of patient sera reactivity to PLA2R full-length and PLA2R dominant-epitope on Western blot.A, nitrocellulose membranes blotted with the 1D4-tagged PLA2R full-length or PLA2R dominant-epitope were assembled into a Bio-Rad multiscreen apparatus and probed with patient sera at dilutions of 100, 1000, or 5000 in TBSTM buffer for 2 h at room temperature. A patient serum that reacts to both PLA2R full-length and dominant-epitope was used as a positive control. B, summary of the selected six sera reactivity to PLA2R full-length and dominant-epitope. The experiment was performed at least three times. Pat, patient; PLA2R, phospholipase A2 receptor.
Identification of additional autoantibody reactive region in PLA2R
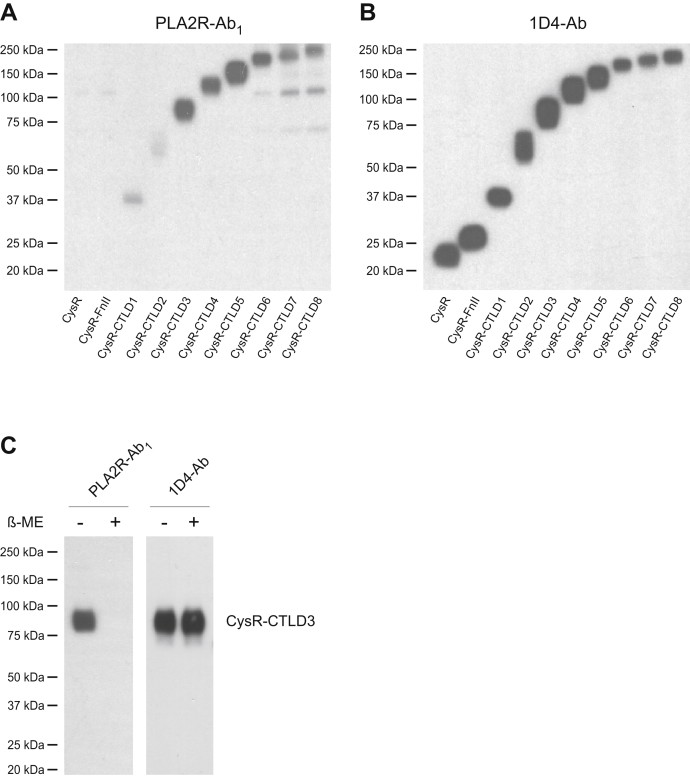
We next identified this additional antibody reactive region using the PLA2R C-terminal domain deletions reported previously (13). Briefly, the 1D4-tagged PLA2R extracellular domain fragments ranging from the CysR, CysR-FnII, and up to CysR-CTLD8 were resolved on SDS-PAGE, transferred to a nitrocellulose membrane, and probed with one of the PLA2R-Ab1 dominant sera (patient sample 1, used throughout the following study otherwise indicated) at 1:5000 dilutions. Figure 7, A and B show a weak reaction to the CysR-CTLD1 and CysR-CTLD2 fragments, whereas a strong reaction to the CysR-CTLD3 fragment and above, suggesting that PLA2R-Ab1 reacts to a larger denatured PLA2R fragment ranging from the CysR to CTLD3 domain. Treatment of the CysR-CTLD3 fragment with β-mercaptoethanol completely abolished the PLA2R-Ab1 reactivity (Fig. 7C), indicating that this epitope is conformational. A similar reactive pattern was detected in all the five remaining sera (Fig. S1), indicating the commonality of this epitope among PMN patients.
Figure 7.
Identification of additional autoantibody reactive region in PLA2R.A, the 1D4-tagged PLA2R extracellular domain fragments were resolved by 4 to 20% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with the PLA2R-Ab1 dominant serum (from patient 1) at 1:5000 dilutions in TBSTM. B, the membrane was stripped and reprobed with the 1D4-Ab. C, in separate experiments, the 1D4-tagged CysR-CTLD3 fragment was resolved under nonreducing and reduced conditions and probed by the patient serum or 1D4-Ab. Each experiment was performed 3 to 5 times. 1D4-Ab, mouse anti-1D4 monoclonal antibody; PLA2R, phospholipase A2 receptor.
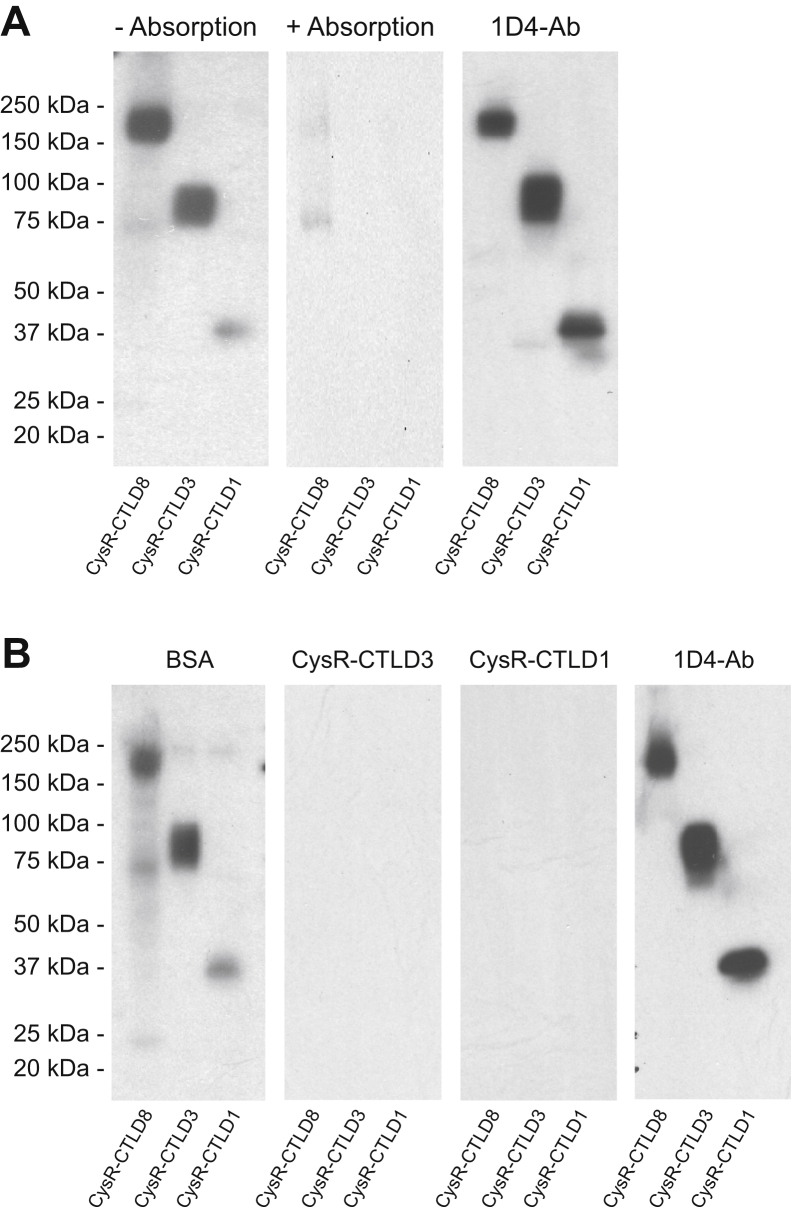
To characterize how PLA2R-Ab1 interacts with the CysR-CTLD3 fragment, we attempted to isolate the PLA2R-Ab1 by absorbing PLA2R-Ab2 out of the serum using a column coated with the purified CysR-CTLD1 fragment. Figure 8A shows, the serum without absorption reacted strongly to the CysR-CTLD3 yet weakly to the CysR-CTLD1 fragment, whereas the absorbed serum completely lost its reactivity to either of the fragments, suggesting that both PLA2R-Ab1 and PLA2R-Ab2 react to the native CysR-CTLD1 fragment leading to their depletion from the serum. We next individually incubated the serum with the purified CysR-CTLD3 or CysR-CTLD1 fragment (Fig. 4A) and then applied the mixture on Western blot. As shown in Figure 8B, like the CysR-CTLD3 fragment, the CysR-CTLD1 fragment completely blocked the serum reactivity to the CysR-CTLD3 fragment and the PLA2R full-domain, confirming that PLA2R-Ab1 reacts to the native CysR-CTLD1 fragment.
Figure 8.
Antibody absorption and epitope competition study of the PLA2R-Ab1dominant serum.A, PLA2R-Ab1 dominant serum (from patient 1) was absorbed through gravity columns either with or without precoated CysR-CTLD1 fragments. The flow-throughs were collected and concentrated using Amicon spin columns. The unabsorbed (left) and the postabsorbed (middle) sera were applied on Western blot at 1:1000 dilutions. The level of each protein fragment was verified by the 1D4-Ab (right). B, the PLA2R-Ab1 dominant serum (20 μl) preincubated with 30 μg BSA (left-1), 30 μg His-tagged CysR-CTLD3 (left-2), or 30 μg CysR-CTLD1 (left-3) fragment were applied on Western blot at 1:5000 dilutions. The level of each protein fragment on the blot was verified by the 1D4-Ab (right). Each experiment was performed at least three times. 1D4-Ab, mouse anti-1D4 monoclonal antibody; BSA, bovine serum albumin; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; PLA2R, phospholipase A2 receptor.
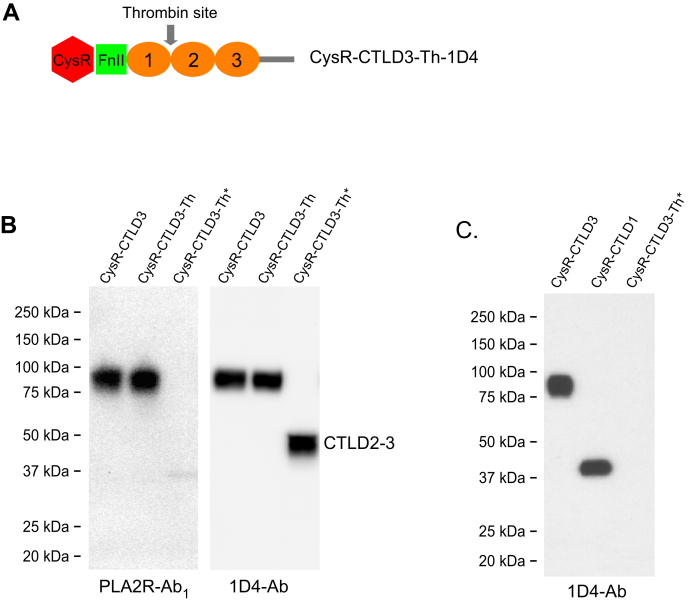
To test if the CTLD2-3 domain harbors potential interactive sites for PLA2R-Ab1, we introduced a protease (thrombin) site into the linker region between CTLD1 and CTLD2 in the 1D4-tagged CysR-CTLD3 fragment (CysR-CTLD3-Th) (Fig. 9A). Before the study, we confirmed that the thrombin used had no nonspecific proteolytic effects on PLA2R domain fragments (Fig. S2). Figure 9B shows, the CysR-CTLD3-Th had a similar reactivity to the patient serum as the CysR-CTLD3 fragment had on the Western-blot (1:5000 dilutions). However, upon protease cleavage, the fragmented CysR-CTLD3-Th lost its reactivity to the serum, suggesting that neither the remaining CysR-CTLD1 fragment (a weak reaction was detected because of residual PLA2R-Ab2) nor the released CTLD2-3 domain (denatured form) could react to PLA2R-Ab1. To test if the native CTLD2-3 domain reacts to PLA2R-Ab1, we mixed the thrombin-treated CysR-CTLD3-Th with the serum in TBS buffer (pH 7.4) and subsequently immunoprecipitated with protein G-sepharose resin. As shown in Figure 9C, both 1D4-tagged CysR-CTLD3 and CysR-CTLD1 fragments were immunoprecipitated, but not the thrombin released 1D4-tagged CTLD2-3 domain, suggesting that the CTLD2-3 domain either has a very low reactivity or does not directly interact with PLA2R-Ab1. This data, however, does not rule out that an epitope might be present in the linker region between the CTLD1 and CTLD2 domain which was disrupted by thrombin cleavage.
Figure 9.
Reactivity of PLA2R-Ab1to CTLD2-3 fragment.A, schematic illustration of the introduced thrombin cleavage site in CysR-CTLD3 fragment (CysR-CTLD3-Th-1D4). B, the CysR-CTLD3-Th-1D4 fragment without or with thrombin treatment was resolved on 4 to 20% SDS-PAGE and probed by the PLA2R-Ab1 dominant serum (left panel, 1:5000) or 1D4-Ab (right panel). The CysR-CTLD3-1D4 fragment was included as a control. C, the 1D4-tagged CysR-CTLD3, CysR-CTLD1, and the thrombin-treated CysR-CTLD3-Th-1D4 were individually mixed with the PLA2R-Ab1 dominant serum (in TBS buffer, pH 7.4) and incubated for 2 h at room temperature followed with protein G-sepharose resin immunoprecipitation. The protein samples were resolved by 4 to 20% SDS-PAGE and probed with the 1D4-Ab. Each experiment was performed 3 to 5 times. Asterisks indicate thrombin-treated protein samples. 1D4-Ab, mouse anti-1D4 monoclonal antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; PLA2R, phospholipase A2 receptor.
Clinical relevance of different PLA2R-Ab populations
To assess the potential clinical relevance of the two antibody populations, we compared the disease course of the six patients dominated with PLA2R-Ab1 to the patients possessing high levels of PLA2R-Ab2. These six patients were enrolled in a clinical trial (Table 1; Patient 1–6) with PLA2R-Ab associated PMN and were under conservative treatment, and the sera were collected right before the initiation of immunosuppressive therapy with a combined regimen of Rituximab plus Cyclosporine (33). Interestingly, these patients had a rapid response to immunosuppression with all achieving complete antibody depletion by 3 months, five achieved partial remission of proteinuria by 3 months, and all achieved partial or complete remission by 6 months (Table 1; complete remission was defined as proteinuria ≤0.3 g per 24 h, partial remission as proteinuria ≤3.5 g per 24 h, and a >50% reduction from baseline proteinuria). In contrast, three patients enrolled in the same trial (Patient 11, 17, and 26) who had a high level of PLA2R-Ab2 had a delayed treatment response with a slower reduction in proteinuria that required at least 6 months to achieve partial remission and antibody depletion. For the rest of the patients with high levels of PLA2R-Ab2, seven had recurrent membranous nephropathy following kidney transplant, and 32 had PMN for a longer duration (4∼7 years) and/or had previous exposure to immunosuppression and/or had a relapsing/remitting course (Table S1). This analysis indicates that PLA2R-Ab1 is dominant at the early diagnosis, whereas PLA2R-Ab2 increases significantly at the late stage of PMN, and that patients dominated with PLA2R-Ab1 are likely to have a better response to immunosuppression with rapid remission of proteinuria.
Table 1.
Clinical relevance of the two PLA2R-Ab populations
| Patient ID | Dominant PLA2R-Ab population | Time to partial remission (month) | Proteinuria at enrollment (g/24 h) | Proteinuria 3 months post treatment (g/24 h) | Proteinuria 6 months post treatment (g/24 h) | PLA2R-Ab titer at enrollment (RU/ml) | PLA2R-Ab titer 3 months post treatment (RU/ml) | PLA2R-Ab titer 6 months post treatment (RU/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ab1 | 3 | 9.6 | 1.23 | 0.22 | 260.5 | 0.88 | 1.01 |
| 2 | Ab1 | 3 | 10.5 | 1.1 | 0.20 | 162.7 | 2.4 | 1.45 |
| 3 | Ab1 | 3 | 15.9 | 2.9 | 1.2 | 576.1 | 0.7 | 0.63 |
| 4 | Ab1 | 3 | 7.1 | 0.3 | 0.2 | 142.6 | 1.4 | 1.5 |
| 5 | Ab1 | 3 | 13.7 | 1.1 | 1.1 | 78.5 | 1.8 | 2.4 |
| 6 | Ab1 | 6 | 8.3 | 4.0 | 0.44 | 137.7 | 1.1 | 0.84 |
| 11 | Ab2 | 18 | 10.6 | 10.9 | 10.8 | 3260 | 621 | 70 |
| 17 | Ab2 | 6 | 14.1 | 11.3 | 3.4 | 730.9 | 46.07 | 3.6 |
| 26 | Ab2 | 9 | 9.8 | 7.2 | 5.9 | 295.6 | 5.4 | 19 |
The treatment response of nine patients participated in the study to an immunosuppressive regimen (with combined Rituximab and Cyclosporine) are summarized. These patients were followed-up for 24 months in a clinical trial. Patients dominated with PLA2R-Ab1 showed a quick response to the treatment regimen with a rapid decline in proteinuria in 3 months, whereas patients possessed a high level of PLA2R-Ab2 had a delayed treatment response with a slowed reduction in proteinuria. PLA2R-Ab titers were determined using enzyme-linked immunosorbent assay kit from EUROIMMUN.
Role of different PLA2R-Ab populations in PMN clinical progression
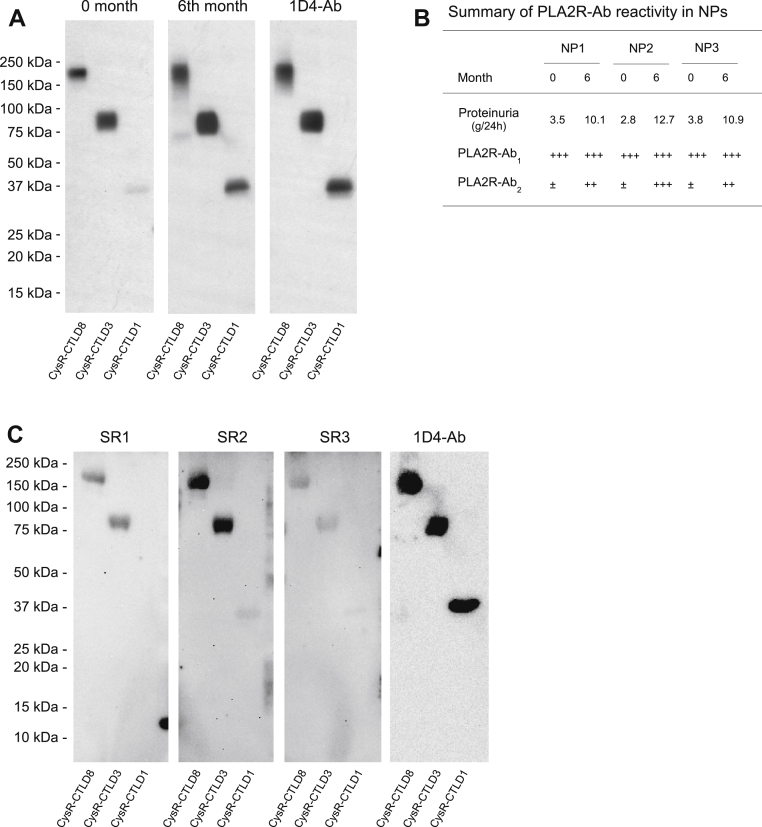
To determine the potential interrelationships between PLA2R-Ab1 and Ab2 in PMN, we obtained three PLA2R-Ab positive sera from newly diagnosed patients. The urinary protein excretion in these patients was low initially (∼3.4 g/24 h) but increased substantially (∼11 g/24 h) during the 6 months of conservative management. Western blot analyses showed that, as proteinuria worsens, the composition of PLA2R-Ab in patient sera changed significantly, with PLA2R-Ab1 dominant early at diagnosis whereas PLA2R-Ab2 significantly increased 6 months post conservative management (Fig. 10, A and B). This observation suggests that PLA2R-Ab1 is likely to be developed before PLA2R-Ab2 in patients, and an increased level of PLA2R-Ab2 is associated with PMN clinical progression.
Figure 10.
Representative data of PLA2R-Ab reactivity to the 1D4-tagged PLA2R domain fragments in patients with worsened proteinuria or spontaneous remission.A, serum samples collected from the first new patient at initial diagnosis (0 months, left panel) and 6 months post conservative management (6 months, middle panel) were applied on Western blot at 1:5000 dilutions in TBSTM buffer. The level of each protein fragment was verified by the 1D4-Ab (right panel). B, summary of PLA2R-Ab reactivity to PLA2R domain fragments in all three newly diagnosed patients with worsened proteinuria. ±, undetermined; +, detected; and +++, strongly detected. C, serum samples from patients with spontaneous remission were applied on Western blot at 1:1000 dilutions in TBSTM buffer. The level of each protein fragment was verified by the 1D4-Ab (right panel). Each experiment was performed at least three times. 1D4-Ab, mouse anti-1D4 monoclonal antibody; NP, new patient; PLA2R, phospholipase A2 receptor; SR, spontaneous remission.
Spontaneous remission occurs in ∼30% of PMN patients. After screening sera collected from 14 PMN patients who had spontaneous remission, we detected three patients having PLA2R-Ab associated PMN, and subsequently, their sera were subjected to the PLA2R epitope analysis. Figure 10C shows that all three sera reacted to the PLA2R full-domain and CysR-CTLD3 fragment, but minimally to the CysR-CTLD1 fragment, indicating the dominance of PLA2R-Ab1 in these serum samples. Of note, these three patients had very low levels of serum PLA2R-Ab, which required a high serum concentration (at 1:1000 dilutions) for Western blot assay, and a prolonged film exposure to detect the bound PLA2R-Abs.
Discussion
In this study, we analyzed the interactions between PLA2R-Ab and PLA2R conformational epitopes under denaturing and physiological conditions. Moreover, we detected two populations of PLA2R-Abs in patients, with one that reacts to the denatured CysR-CTLD3 domain complex (the PLA2R-Ab1) dominant at early diagnosis and the other that reacts to the denatured CysR-CTLD1 (the PLA2R-Ab2) increasing significantly as the disease progresses. Our data support that an integrated folding of the CysR, FnII, and CTLD1 domains is a prerequisite to forming a conformational epitope for strong PLA2R interaction, and that PLA2R-Ab2, the anti-PLA2R dominant-epitope autoantibody, plays a critical role in PMN clinical progression.
We previously located the PLA2R dominant conformational epitope at the CysR-CTLD1 region using sequential C-terminal domain deletions on Western blot (13). Although N-terminal domain truncations have led to the detection of four single-domain epitopes in PLA2R (14, 15), the present study demonstrated that both CysR and CysR-CTLD1 domain truncations are intracellularly retained, and their reaction to PLA2R-Ab requires a high serum concentration. Moreover, the reactive autoantibodies are not specific to the PLA2R C-terminal domains beyond CTLD1 but rather to the CysR-CTLD1 region. Likely, the N-terminal domain truncations are partially folded in a conformation that weakly interacts with PLA2R-Ab, which explains why the phenomenon of “epitope spreading” is directly proportionate to the level of PLA2R-Ab in patient sera (15, 34). We propose that the detected antibody reactivity against the PLA2R C-terminal domains beyond CTLD1 is because of the cross-reactivity of PLA2R-Abs against the dominant-epitope, the CysR-CTLD1 region. Indeed, protein sequence alignment indicates that PLA2R CTLD1 shares significant sequence similarity with other CTLD domains except for CTLD3 (Tables 2 and S2; Fig. S3), and further, the recent crystal structure of PLA2R CTLD7 domain showed a typical CTLD fold with high sequence similarity to the CTLD1 domain (35).
Table 2.
Amino acid sequence similarities between PLA2R CTLD1(238–355) and other CTLDs
| Domains | Identities (%) | Positives (%) | Gaps (%) |
|---|---|---|---|
| CTLD1 (238–355) | 100 | 100 | 0 |
| CTLD2 (395–502) | 28 | 45 | 3 |
| CTLD3 (522–643) | - | - | - |
| CTLD4 (673–797) | 36 | 53 | 9 |
| CTLD5 (819–938) | 30 | 45 | 8 |
| CTLD6 (965–1096) | 39 | 56 | 4 |
| CTLD7 (1121–1232) | 29 | 45 | 4 |
| CTLD8 (1257–11,378) | 31 | 47 | 9 |
The PLA2R domains were determined using the UnitProt program and aligned using the Blast program. -, no alignment.
The PLA2R extracellular region adopts a compact dual-ring-shaped conformation, as shown in the cryo-EM structure (12). The CysR and CTLD1 domain are part of the smaller ring and stay on the top of the molecule, indicating their open access to antibody interaction; whereas the CTLD7 and CTLD8 domains hide beneath the double-ring-shaped head adjacent to the lipid bilayer, indicating their restricted antibody access due to local steric hindrance. We predict that PLA2R-Ab is unlikely able to react to the CTLD7 or CTLD8 domain on the living cell surface, producing any meaningful pathological effect, as suggested by the recent patient study (15).
Our data demonstrate that neither the CysR nor the CTLD1 domain alone could serve as a single-domain epitope. Although a weak reactivity of PLA2R-Ab to the CysR or CTLD1 single domain is detectable under physiological conditions, such reactivity is negligible compared to that of the CysR-CTLD1 domain complex, as shown in the immunoprecipitation study. This data complements our previous study and supports that all the three N-terminal domains are required to form a conformational epitope for PLA2R-Ab interaction (13). Technically, we transfer protein samples from SDS-PAGE gels to nitrocellulose membranes using the Bio-Rad tank blotting system, which had no loss of CysR domain fragments binding to the membranes. However, we did observe a poor binding of CysR domain fragments to membranes when semi-dry conditions were used. Therefore, we feel it unlikely that the CysR domain is not hydrophobic enough for its transfer to polyvinylidene difluoride membranes but rather due to the Western blot conditions used in the previous study (14).
The interaction between a conformational epitope and the corresponding antibody has been extensively analyzed and is well understood in the organ transplant research field (36, 37). The donor-specific antibodies recognize and bind to the conformational epitopes on the surface of human leukocyte antigen (HLA) class-I or class-II molecules that cover an area of ∼15 Å radius (36). The conformational epitopes of HLA class-I are formed by two adjacent alpha 1 and alpha 2 domains of the heavy chain, whereas the conformational epitopes of HLA class-II are formed either by the alpha or beta chain alone or both. Therefore, by inference, it is conceivable that a tightly folded CysR-FnII-CTLD1 domain complex forms the PLA2R conformational epitope.
Patients with PLA2R-associated PMN have unpredictable disease courses, with some being more responsive to immunosuppressive treatment than others. Our study indicates that a population of PLA2R-Ab1 reacting to a large denatured CysR-CTLD3 fragment exists in patients at early diagnosis. Surprisingly, PLA2R-Ab1 reacts to the native CysR-CTLD1 domain complex under physiological conditions, suggesting that the CysR-CTLD1 region contributes significantly to forming this conformational epitope. We predict that PLA2R-Ab1 may have a different binding affinity to the nondenaturing CysR-CTLD1 and the denatured CysR-CTLD3 domain complex. The detailed mechanism that underlies this phenomenon requires further investigation.
Our study supports that PLA2R-Ab2 is the final mature form of the pathogenic autoantibody. We observed that an increase of PLA2R-Ab2 in patients initially dominated with PLA2R-Ab1 is closely associated with the worsening of proteinuria, and that PLA2R-Ab2 was at high levels in all patients who had PMN for years with repeated relapses, and patients who had recurrent PLA2R-Ab associated membranous nephropathy after kidney transplantation. It is known that during clonal selection, only the B cell clones producing high-affinity antibodies will be retained for antibody production, whereas the clones producing low-affinity antibodies will be annihilated (38). We predict that antibody affinity maturation plays a critical role in PMN clinical progression. Our data do not rule out the possibility that there exist PLA2R-Abs recognizing various domains of PLA2R generated during the course of PMN, given the fact that every domain of PLA2R could be processed and presented by the antigen-presenting cells in patients. However, these antibodies are likely to have a low affinity for PLA2R (15), and they may not be pathogenic if their corresponding epitopes are buried in the folded PLA2R protein complex. These antibodies potentially contribute to the research observation that PLA2R-Abs are detectable in patients months to years before their documented clinical manifestation (39), and the clinical observation that certain patients possess high levels of circulating PLA2R-Abs yet have low/no proteinuria (26, 27).
Our findings of the two antibody populations may provide important new clues to help identify the group of patients who would benefit from immediate immunosuppressant intervention. Our data showed that PLA2R-Ab2 is closely associated with disease progression and that when patients were dominated with PLA2R-Ab1, they responded promptly to the immunosuppressive therapy with a sharp decrease in proteinuria in ∼3 months; whereas patients who had a high level of PLA2R-Ab2 experienced a significantly delayed (>6 months) treatment response. Considering the severe potential side effects of immunosuppressive therapy, PMN patients could benefit from assessing the levels of PLA2R-Ab1 and Ab2 at the time of initial diagnosis and during follow-up along with clinical parameters (proteinuria, renal function) to determine the best time for the initiation of immunosuppressive therapy.
Experimental procedures
Patient sera
Deidentified patient sera were collected at the Clinical Research Center, National Institute of Diabetes and Digestive and Kidney Diseases/Kidney Disease Section according to the Institutional Review Board and abided by the Declaration of Helsinki principles.
Molecular cloning
A full-length human PLA2R either fused with an EGFP or a 1D4 tag (TETSQVAPA from Rhodopsin) at the C-terminus was constructed using PCR cloning. Based on the PLA2R-EGFP and PLA2R-1D4 clones, a CysR domain (amino acid Q36-D165) and a CysR-FnII-CTLD1 triple domain (amino acid Q36-Y357) truncation were generated following the previous report (14). The 1D4-tagged PLA2R C-terminal domain deletions were constructed following reference 13 (except the CysR-CTLD8 fragment ends at amino acid A1386). A thrombin cleavage site (LVPRGS) was introduced into the 1D4-tagged CysR-CTLD1 fragment at the amino acid position of L166HTIKG171 between the CysR and FnII domain and the 1D4-tagged CysR-CTLD3 fragment at the amino acid position of I365VEKDA370 between the CTLD1 and CTLD2 domain using QuikChange II site-directed mutagenesis kit (Agilent). In separate experiments, a Histidine tag (six histidines) was individually introduced to the C-terminal end of the CysR-CTLD1, CysR-CTLD3, and CysR-CTLD8 followed with a stop codon. The complete sequence of each construct was verified by DNA sequencing.
Fluorescence microscopy
HEK 293 cells transfected with the EGFP-fused PLA2R constructs were cultured in DMEM media containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (all reagents were from ThermoFisher Scientific) at 37 °C in 5% CO2. Eighteen hours posttransfection, the cells were harvested using trypsin and replated at a low density on the polylysine (MilliporeSigma) coated coverslips in 60-mm tissue culture dishes. Twenty hours later, the cells were washed twice with ice-cold PBS (140 mM NaCl, 3 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) and fixed in ice-cold 4% paraformaldehyde (dissolved in PBS, pH 7.4) for 30 min. The cells were then washed three times with PBS, mounted on a glass slide, and imaged under a fluorescence microscope (Olympus) at excitation wavelength 488 nm and emission wavelength 510 nm.
Deglycosylation study
The protein deglycosylation study was performed using peptide N-glycosidase F (New England BioLabs) following the manufacturer’s instruction. Briefly, HEK 293 cells (60 mm dish) expressing 1D4-tagged PLA2R full-length, CysR, or CysR-CTLD1 domain truncations were collected and lysed in 200 μl protein lysis buffer (1% (v/v) Igepal, 0.5% (w/v) sodium deoxycholate, 5 mM EDTA, 137 mM NaCl, 20 mM Tris-HCl, pH 7.4), containing the complete protease inhibitor cocktail (MilliporeSigma) for 30 min on ice. The cell lysate was centrifuged for 10 min at full speed on an Eppendorf bench centrifuge at 4 °C, and 9 μl supernatant was collected and proceeded for deglycosylation study under the denaturing reaction conditions.
Protein expression and sample preparation
The 1D4-tagged PLA2R C-terminal domain deletions were transiently expressed in HEK 293 cells, as described previously (13). Briefly, HEK 293 cells cultured in Gibco FreeStyle 293 expression medium were transiently transfected with various PLA2R C-terminal domain deletion constructs using TurboFect reagent (ThermoFisher Scientific) following the manufacturer’s instruction. The culture medium was collected 48 h posttransfection.
The collected culture media were centrifuged at 4000 rpm for 10 min at 4 °C to pellet cell debris. The supernatant was filtered (0.22 μm) and loaded onto Amicon Ultra-15 Centrifugal Filter Unit (MilliporeSigma) and centrifuged at 4000 rpm for 30 min at 4 °C. The concentrated protein samples were buffer exchanged into TBS buffer (137 mM NaCl, 20 mM Tris, pH 7.4). In separate experiments, the culture medium containing His-tagged PLA2R fragment was loaded onto a Ni-NTA Superflow (Qiagen) packed gravity column (Bio-Rad) following the manufacturer’s instruction. The protein concentration of each sample was determined by the bicinchoninic acid assay method. The purity of the eluted protein samples was analyzed on SDS-PAGE followed by Coomassie blue staining.
Protease digestion
Protein samples were mixed with human thrombin (restriction grade, Novagen, MilliporeSigma) at 1 unit/10 μl in TBS buffer (containing 2.5 mM CaCl2) and incubated for 6 h on a rotating shaker at room temperature. The reaction was stopped by adding the complete protease inhibitor cocktail (MilliporeSigma). The efficiency of thrombin cleavage was assessed using Western-blot.
Immunoprecipitation
Phospholipase A2 receptor C-terminal domain deletions were individually mixed with 5 μl of patient serum or 2 μg of a mouse anti-1D4 monoclonal antibody (1D4-Ab, from Flintbox) in 500 μl TBS buffer and incubated on a rotating shaker for 2 h at room temperature. Fifty microliters of protein G-Sepharose resin (50% (v/v) in TBS, MilliporeSigma) was then added into the mixture and further incubated for 2 h. The resin was collected by centrifugation and washed three times with the protein lysis buffer. The resin was mixed with 2× SDS-sample buffer containing 2% (v/v) β-mercaptoethanol and heated at 100 °C for 5 min. Protein samples were cooled to room temperature and centrifuged at 9000 rpm for 1 min to sediment insoluble material. The protein samples were resolved by 4 to 20% SDS-PAGE.
Western blot analysis
Protein samples mixed with 2× SDS-sample buffer (without or with 2% β-Mercaptoethanol) were heated at 100 °C for 5 min. The samples were then electrophoresed on 7.5% or 4 to 20% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad), and probed by a rabbit anti-human PLA2R polyclonal antibody (MilliporeSigma), 1D4-Ab or patient sera in TBSTM buffer (Tris-buffered saline/Tween-20 (TBST); 0.1% (v/v) Tween-20, 137 mM NaCl, and 20 mM Tris (pH 7.4) containing 5% (w/v) nonfat dry milk) for 2 h at room temperature. The membranes were washed three times with TBST buffer and probed with horseradish peroxidase (HRP)-conjugated goat anti-mouse, goat anti-rabbit, or goat anti-human IgG (Jackson ImmunoResearch) in TBSTM buffer for 1 h and subsequently processed with ECL reagent (Cytiva) and exposed to Amersham Highperfilm ECL (Cytiva).
Antibody absorption and epitope competition assay
A Poly-Prep chromatography column (Bio-Rad) packed with 500 μl of 50% (v/v) Ni-NTA agarose slurry (Qiagen) was washed extensively with TBS buffer. Three times excessive amounts of His-tagged CysR-CTLD1 fragment (calculated based on the binding capacity of Ni-NTA agarose) diluted in 2 ml TBS buffer were then loaded onto the column. The flow-through was collected and reloaded three times to maximize the binding of CysR-CTLD1 fragments to the resin. After five column volume washes with TBS, the resin was ready for the absorption study. Serum from patient 1 (100 μl) diluted in 2 ml TBS was loaded onto the column, and the flow-through was collected and reloaded three times for maximal absorption of PLA2R-Abs. The column was then rinsed three times with 2 ml TBS for any residual serum. All the flow-throughs were collected, combined, and concentrated using an Amicon spin column to a final volume of 100 μl. In control experiments, an equal volume of patient serum went through an uncoated Ni-NTA agarose column as described above. The absorbed sera were applied at 1:1000 dilutions in TBSTM buffer on Western blot.
Epitope competition assay was performed as previously described with modification (13). Briefly, patient sera (20 μl) mixed either with 30 μg bovine serum albumin, 30 μg CysR-CTLD8, 30 μg CysR-CTLD3, or 30 μg CysR-CTLD1 fragments (in 100 μl TBS) were placed on a rotating shaker and incubated for 2 h at room temperature. The mixture was then applied onto nitrocellulose membranes preblocked with TBSTM buffer either at 1:100 or 1:5000 dilutions for 2 h at room temperature and further processed as described above.
PLA2R-Ab screen
Patient sera were screened for PLA2R-Ab as previously reported using the Mini-PROTEAN II Multiscreen Apparatus (Bio-Rad) (13). Briefly, nitrocellulose membranes blotted with 1D4-tagged PLA2R full-length or PLA2R CysR-CTLD1 triple domain (dominant-epitope) were assembled into the multiscreen apparatus after 30 min of blocking with TBSTM buffer. Each patient serum was applied at 1:100 dilutions (in TBSTM) on the membranes and incubated for 2 h at room temperature. The membranes were then washed three times with TBST buffer and incubated with HRP-conjugated goat anti-human IgG antibodies in TBSTM buffer at a dilution of 1:5000. The 1D4-Ab was included as a positive control, detected by a secondary HRP-conjugated goat anti-mouse IgG. The membranes were then incubated with ECL reagent for 1 min and exposed to Amersham Highperfilm ECL. The exposure times were 1 min for positive bands and up to 5 min for weak or negative bands. In separate experiments, patient sera were applied at 1:100, 1:1000, or 1:5000 dilutions onto the membranes, and the films were exposed for 5 to 8 min for the positive bands.
Image and data analyses
Films from immunoblots were scanned with a CanoScan LiDE. Scanned images were quantified with GelQuant.NET software (Biochemlabsolutions.com, University of California).
Statistical analyses
Means ± SD was calculated using SigmaPlot 10 software. Statistical analysis was performed using SigmaPlot 10 software.
Data availability
All data pertaining to this article are contained within this article and supplemental data.
Supporting information
This article contains supporting information.
Conflict of interest
H. T. holds a patent to use the findings for developing diagnostic tests to monitor PMN clinical progress and prognosis, predict patient response to treatment, and developing new therapies for patient treatment. R. Z. is a student intern at ImmunoWork.
Acknowledgments
This study was funded by ImmunoWork. We express our gratitude to all the patients for their willingness to participate in the study.
Author contributions
H. T. and Q. Z. conceptualization; H. T. and Q. Z. methodology; H. T., R. Z., M. W., and Q. Z. investigation; H. T. and Q. Z. formal analysis; H. T. and Q. Z. writing–original draft; H. T., R. Z., M. W., and Q. Z. writing–review and editing.
Edited by Peter Cresswell
Supporting information
References
- 1.Couser W.G. Primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco P., Debiec H. Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 3.van de Logt A.-E., Fresquet M., Wetzels J.F., Brenchley P. The anti-PLA2R antibody in membranous nephropathy: What we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292–1302. doi: 10.1016/j.kint.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q. Anti-phospholipase A2 receptor autoantibody: A new biomarker for primary membranous nephropathy. Immunol. Endocr. Metab. Agents Med. Chem. 2016;16:4–17. doi: 10.2174/1871522215666150910205702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck L.H., Bonegio R.G.B., Lambeau G., Beck D.M., Powell D.W., Cummins T.D., Klein J.B., Salant D.J. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llorca O. Extended and bent conformations of the mannose receptor family. Cell. Mol. Life Sci. 2008;65:1302–1310. doi: 10.1007/s00018-007-7497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boskovic J., Arnold J.N., Stilion R., Gordon S., Sim R.B., Rivera-Calzada A., Wienke D., Isacke C.M., Martinez-Pomares L., Llorca O. Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J. Biol. Chem. 2006;281:8780–8787. doi: 10.1074/jbc.M513277200. [DOI] [PubMed] [Google Scholar]
- 8.Rivera-Calzada A., Robertson D., MacFadyen J.R., Boskovic J., Isacke C.M., Llorca O. Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180. EMBO Rep. 2003;4:807–812. doi: 10.1038/sj.embor.embor898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao L., Shi X., Chang H., Zhang Q., He Y. pH-dependent recognition of apoptotic and necrotic cells by the human dendritic cell receptor DEC205. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7237–7242. doi: 10.1073/pnas.1505924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., Bjorkman P.J. Structure of FcRY, an avian immunoglobulin receptor related to mammalian mannose receptors, and its complex with IgY. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12431–12436. doi: 10.1073/pnas.1106925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fresquet M., Jowitt T.A., Gummadova J., Collins R., O'Cualain R., McKenzie E.A., Lennon R., Brenchley P.E. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J. Am. Soc. Nephrol. 2015;26:302–313. doi: 10.1681/ASN.2014050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y., Cao L., Tang H., Shi X., He Y. Structure of human M-type phospholipase A2 receptor revealed by cryo-electron microscopy. J. Mol. Biol. 2017;429:3825–3835. doi: 10.1016/j.jmb.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Kao L., Lam V., Waldman M., Glassock R.J., Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2015;26:291–301. doi: 10.1681/ASN.2013121315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seitz-Polski B., Dolla G., Payré C., Girard C.A., Polidori J., Zorzi K., Birgy-Barelli E., Jullien P., Courivaud C., Krummel T., Benzaken S., Bernard G., Burtey S., Mariat C., Esnault V.L.M., et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J. Am. Soc. Nephrol. 2016;27:1517–1533. doi: 10.1681/ASN.2014111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhard L., Zahner G., Menzel S., Koch-Nolte F., Stahl R.A.K., Hoxha E. Clinical relevance of domain-specific phospholipase A2 receptor 1 antibody levels in patients with membranous nephropathy. J. Am. Soc. Nephrol. 2020;31:197–207. doi: 10.1681/ASN.2019030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seitz-Polski B., Debiec H., Rousseau A., Dahan K., Zaghrini C., Payré C., Esnault V.L.M., Lambeau G., Ronco P. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J. Am. Soc. Nephrol. 2018;29:401–408. doi: 10.1681/ASN.2017070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoxha E., Harendza S., Pinnschmidt H., Panzer U., Stahl R.A.K. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoxha E., Harendza S., Pinnschmidt H.O., Tomas N.M., Helmchen U., Panzer U., Stahl R.A.K. Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol. Dial. Transplant. 2015;30:1862–1869. doi: 10.1093/ndt/gfv228. [DOI] [PubMed] [Google Scholar]
- 19.Timmermans S.A.M.E.G., Abdul Hamid M.A., Cohen Tervaert J.W., Damoiseaux J.G.M.C., van Paassen P. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am. J. Nephrol. 2015;42:70–77. doi: 10.1159/000437236. [DOI] [PubMed] [Google Scholar]
- 20.Jullien P., Seitz Polski B., Maillard N., Thibaudin D., Laurent B., Ollier E., Alamartine E., Lambeau G., Mariat C. Anti-phospholipase A2 receptor antibody levels at diagnosis predicts spontaneous remission of idiopathic membranous nephropathy. Clin. Kidney J. 2017;10:209–214. doi: 10.1093/ckj/sfw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofstra J.M., Beck L.H., Beck D.M., Wetzels J.F., Salant D.J. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstra J.M., Debiec H., Short C.D., Pellé T., Kleta R., Mathieson P.W., Ronco P., Brenchley P.E., Wetzels J.F. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanigicherla D., Gummadova J., McKenzie E.A., Roberts S.A., Harris S., Nikam M., Poulton K., McWilliam L., Short C.D., Venning M., Brenchley P.E. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 24.Hoxha E., Thiele I., Zahner G., Panzer U., Harendza S., Stahl R.A.K. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J. Am. Soc. Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruggenenti P., Debiec H., Ruggiero B., Chianca A., Pellé T., Gaspari F., Suardi F., Gagliardini E., Orisio S., Benigni A., Ronco P., Remuzzi G. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debiec H., Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N. Engl. J. Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 27.Debiec H., Martin L., Jouanneau C., Dautin G., Mesnard L., Rondeau E., Mousson C., Ronco P. Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. Am. J. Transplant. 2011;11:2144–2152. doi: 10.1111/j.1600-6143.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoxha E., Kneißler U., Stege G., Zahner G., Thiele I., Panzer U., Harendza S., Helmchen U.M., Stahl R.A.K. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 29.Oh Y.J., Yang S.H., Kim D.K., Kang S.-W., Kim Y.S. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bech A.P., Hofstra J.M., Brenchley P.E., Wetzels J.F.M. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2014;9:1386–1392. doi: 10.2215/CJN.10471013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymann J.A.W., Subramaniam S. Expression, stability, and membrane integration of truncation mutants of bovine rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4966–4971. doi: 10.1073/pnas.94.10.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S., Sham Y.Y., Tsai C.J., Nussinov R. Protein folding and function: The N-terminal fragment in adenylate kinase. Biophys. J. 2001;80:2439–2454. doi: 10.1016/S0006-3495(01)76213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldman M., Beck L.H., Braun M., Wilkins K., Balow J.E., Austin H.A. Membranous nephropathy: Pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int. Rep. 2016;1:73–84. doi: 10.1016/j.ekir.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Logt A.-E., Wetzels J. Anti-PLA2R1 antibodies as prognostic biomarker in membranous nephropathy. Kidney Int. Rep. 2021;6:1677–1686. doi: 10.1016/j.ekir.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu B., Hu Z., Kong D., Cheng C., He Y. Crystal structure of the CTLD7 domain of human M-type phospholipase A2 receptor. J. Struct. Biol. 2019;207:295–300. doi: 10.1016/j.jsb.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Duquesnoy R.J. Reflections on HLA epitope-based matching for transplantation. Front. Immunol. 2016;7:469. doi: 10.3389/fimmu.2016.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tambur A.R., Rosati J., Roitberg S., Glotz D., Friedewald J.J., Leventhal J.R. Epitope analysis of HLA-DQ antigens. Transplantation. 2014;98:157–166. doi: 10.1097/TP.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 38.Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 39.Burbelo P.D., Joshi M., Chaturvedi A., Little D.J., Thurlow J.S., Waldman M., Olson S.W. Detection of PLA2R autoantibodies before the diagnosis of membranous nephropathy. J. Am. Soc. Nephrol. 2020;31:208–217. doi: 10.1681/ASN.2019050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data pertaining to this article are contained within this article and supplemental data.