INTRODUCTION: NEAR INFRARED SCIENCE
Surgery, by its very nature, relies on the ability of the surgeon to visualize and distinguish healthy and non-healthy tissue or structures. Without advanced technology, surgeons can visualize only that which can be seen with the naked eye or using white-light imaging. Critical information on tissues, anatomical structures and physiological processes remain hidden and difficult to discern.
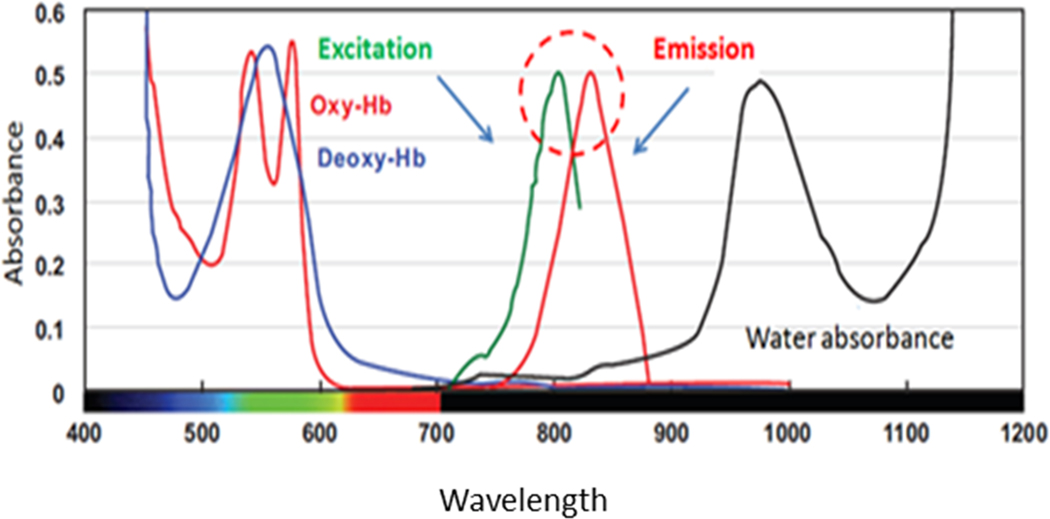
Fluorescence imaging augments the basic surgical information. This form of imaging entails injecting a contrast or fluorescence agent (fluorophore) that is then illuminated by the appropriate wavelength of light required to excite the fluorophore. The excited fluorophore emits light of a slightly longer wavelength that is selectively imaged to produce a fluorescence image. The first fluorescent agent used in surgery was an intravenous injection of fluorescein, where it was used to enhance intracranial neoplasms. [1] One particular imaging agent, Indocyanine Green (ICG), has been a significant driver of adoption of fluorescence imaging. ICG was first developed in 1955 by Kodak Research Laboratories [2] and was approved by the FDA in 1959 for retinal angiography. Since that time, this fluorophore has been used for a variety of surgical applications due to its unique properties: relative non-toxicity, depth of visualization through tissue and remaining confined to intravascular and lymphatic spaces due to binding predominately to lipoproteins. The large depth of visualization results from the fluorescence properties of bound ICG which is optimally excited with 805 nm light and emits over an approximate wavelength range from 810 nm to 875 nm. These near-infrared (NIR) wavelengths, invisible to the naked eye, pass through tissue particularly well due to the low adsorption of light by the various structures of tissue such as hemoglobin and water. As a result, the tissue is relatively transparent to this light and images of structures as much as 5 mm below the tissue surface can be formed. By comparison, fluorescence imaging with fluorescein images only 2–3 mm below the tissue surface [3]; thus, subsurface structures cannot be imaged using visible fluorophores (Figure 1).
Figure 1.
Near-infrared (NIR) Wavelengths
Supplemental surgical information from fluorescence imaging can be divided into two categories: anatomical imaging and physiologic imaging. The former entails fluorescence imaging highlighting critical anatomical structures while the latter reveals how the patient’s physiology is performing. Using applications of ICG imaging as examples, visualization of the biliary anatomy during laparoscopic cholecystectomy or performing lymphatic mapping both use ICG to identify specific anatomical structures that are hidden and hard to discern with the naked eye alone. ICG perfusing imaging during colorectal surgery provides the surgeon the advantage of understanding where blood is flowing and where it is not; the physiologic process is revealed and, thus, may be directly correlated to healing mechanisms.
Processing and display of the fluorescence image must be done with great care. When the image is acquired by modern image sensors, the signal varies linearly with the brightness of the fluorescence. As a result, when displayed on a screen, the user perceives an image which is easily interpreted. However, simple forms of processing, such as contrast enhancement or introduction of a brightness offset, result in the displayed image no longer varying linearly with fluorescence brightness. During anatomical imaging, such as ICG fluorescence mapping of lymph nodes, the fluorescence signal merely needs to be present and visible in the areas containing ICG within the field of view with little background noise. However, when assessing physiologic processes, the brightness of the fluorescence and its rate of change provide surgical information to distinguish the health of the tissue. Therefore, applying a non-linear adjustment to the image such as adjusting contrast, has the potential to distort the surgical information, possibly to the point of compromising surgical decisions relying on the images.
As the technology for fluorescence imaging continues to improve, surgeons ask the technology to provide even more information forcing the equipment to become even more precise. For example, because ICG imaging provides visualization of physiologic processes, the natural progress of this visualization is to use the ICG image to measure the process or to quantify the fluorescence to better gauge the chance of healing. To be useful, such measurements must produce the same result regardless of how the imaging device is positioned relative to the tissue being imaged. When the distance between a camera and its subject is doubled, the amount of light the camera collects to form the image decreases by the square of the distance or to ¼ that of the closer distance. However, if the imaging device reports a fluorescence quantification change by a factor of 4 when the distance doubles, users become confused and quantification fails to provide value. Therefore, to provide the advanced information desired by the surgical visualization, fluorescence imaging equipment intended to provide measurement of the amount of fluorescence must do so independent of the distance to the tissue and, by extension, independent of the location in the field of view.
While the equipment must continue to improve to meet increasingly complex demands, the future of fluorescence guided surgery will also likely entail the use of a much broader range of fluorescent agents. Today, surgeons routinely use ICG, but many other imaging agents are in development by multiple commercial and academic groups. Some of these imaging agents will be intended for identification of critical anatomical structures such as ureters and nerves allowing surgeons to improve surgical safety. Targeted imaging agents, intended to preferentially migrate to cancer cells or tumors, may allow surgeons to intraoperatively ensure clear resection margins and reduce post-operative morbidity associated with inaccurate dissection of adjacent tissues. The more sophisticated fluorescence imaging becomes, the more demand will be placed on the surgeons to be informed about the many nuances of the myriad of agents and limitations of the technology. At the same time, the equipment must continue to advance to allow imaging of these agents simple and routine while not impacting surgical time, improving on existing consistency, remaining cost effective and most importantly continuing to improve the quality of care for the patient.
Critical information on tissues, anatomical structures and physiological processes remain hidden and difficult to discern.
With the adoption of commercially available, intraoperative fluorescence imaging tools across a variety of surgical specialties gains more traction, inclusive of devices, imaging agents and imaging probes, visualization of once hidden or difficult to discern tissues, anatomical structures and physiological processes will become the intraoperative standard of care. This image guided surgery will lead to improved clinical and economic outcomes across the health care continuum and allow surgeons the opportunity to offer “personalized” or “precision” surgical treatment based on imaging that goes far beyond what can be visualized with the naked eye or white light.
LYMPHATIC SYSTEM AND LYMPH NODE MAPPING
Uterine Corpus: Indocyanine Green and Near Infrared Technology in Sentinel Lymph Node Mapping for Uterine Cancer
The use of indocyanine green dye (ICG) and near-infrared imaging (NIR) technology in sentinel lymph node mapping has revolutionized the surgical management and treatment of endometrial cancer. After injecting ICG dye into the cervix, NIR technology enables the surgeon to easily identify the sentinel lymph nodes. ICG dye highlights the lymphatic routes that identifies the primary sentinel lymph nodes. While this user-friendly system has increased detection rates of sentinel lymph nodes, it has also decreased the total number of lymph nodes removed at the time of staging surgery. This, in turn, may lessen the burden of postoperative morbidity experienced by the patient. Thus, the use of the Memorial Sloan Kettering sentinel lymph node mapping algorithm has become common practice in the surgical treatment of endometrial cancer.
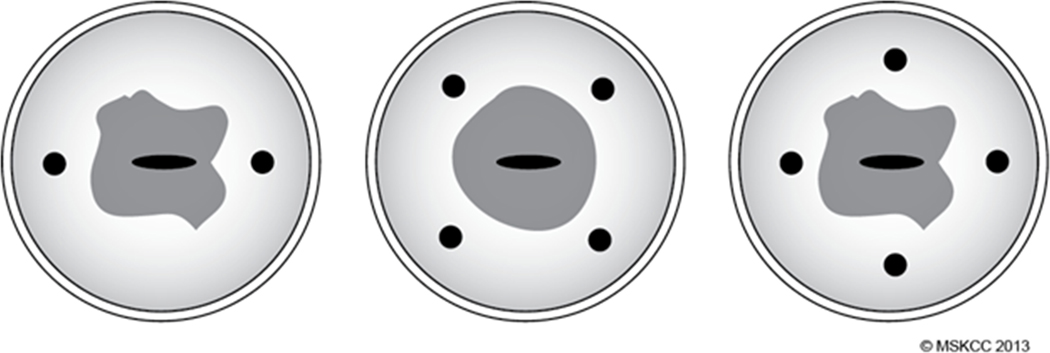
Using a 25-gauge spinal needle, 1 cc of ICG dye is injected superficially (1–3 mm deep) and deep (1–2 cm deep) into the cervical stroma at the 3 o’clock and 9 o’clock positions (Figure 2), for a total of 4 cc of dye. If it is not possible to inject at these two points, a 4-point injection site (Figure 2) is acceptable. The option of injecting ICG only superficially at 3 o’clock and 9 o’clock is acceptable, but 4 cc total volume is recommended in any case.
Figure 2.
Cervical injection sites, 3 o’clock and 9 o’clock positions preferred.
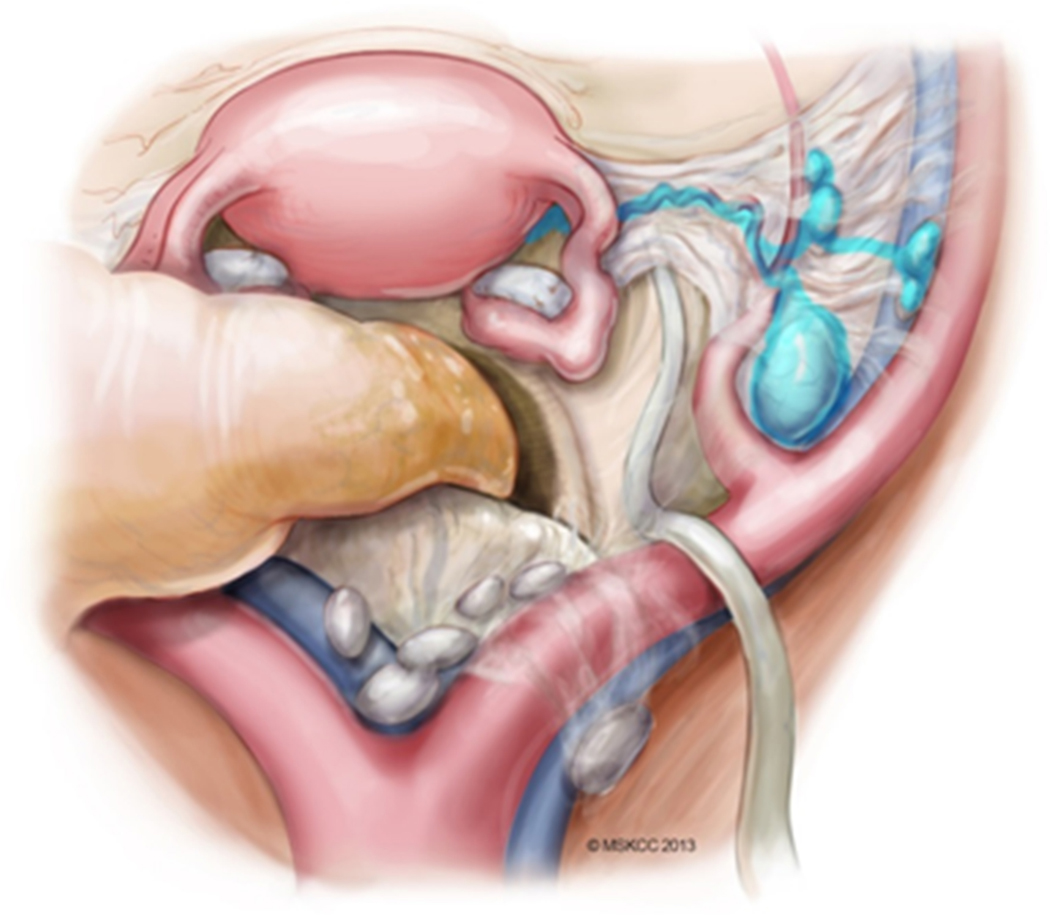
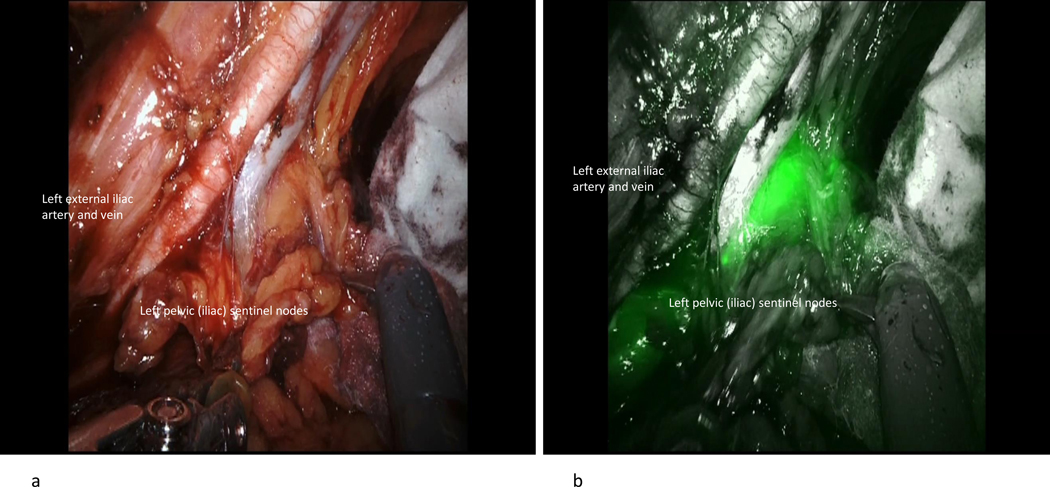
In order to identify the correct sentinel lymph node, it is vital to delineate the course of ICG dye after injection. There are two potential drainage patterns following a cervical injection. The first, and most common, is the anterior paracervical pathway (Figure 3). After injection into the cervix, ICG dye condenses in the paracervix; the lymphatic channels course anteriorly through the parametria, crossing over the obliterated umbilical ligament, then drain into lymph nodes that are located medial to the external iliac vessels, ventral to the hypogastric vessels, and in the superior part of the obturator space. The most proximal colored nodes from the main paracervix lymphatic trunks are the sentinel nodes. Once mapped SLNs are removed. It is important to investigate any suspicious or grossly enlarged lymph nodes, and these should also be removed regardless of the mapping. Caution is needed not to remove the first green nodes that may be located more obviously (such as anterior to the external iliac vessels) when the operation begins, as these maybe secondary or tertiary echelons, It is essential that the obliterated umbilical ligament is identified and traced cranially to find the lymphatic trunks crossing from medial to lateral over the umbilical to help localize the first draining nodes which would be the sentinel nodes.
Figure 3.
Anterior paracervical pathway of lymphatic spread. Adapted from Abu-Rustum.117
When the course of the dye has been confirmed, dissection begins by dividing the round ligament. Once cut, an incision is made medial and parallel to the round ligament to facilitate identification of the caudal part of the obliterated umbilical vessel. The obliterated umbilical ligament is an important landmark denoting the lymphatic channels in the anterior paracervical pathway. The lateral part of the paravesical space is also developed to identify the obturator space structures. The surgeon should follow the umbilical ligament cranially to identify the lymphatics crossing from medial to lateral over the obliterated umbilical ligament, to the nodal basins in the iliac and obturator regions. The lymph node draining immediately to the channels is the sentinel lymph node and should be excised. Secondary sentinel lymph nodes--those that take up dye but are cranial to the sentinel lymph node--may also be removed (not mandatory); however, these should not be labeled as sentinel lymph nodes.
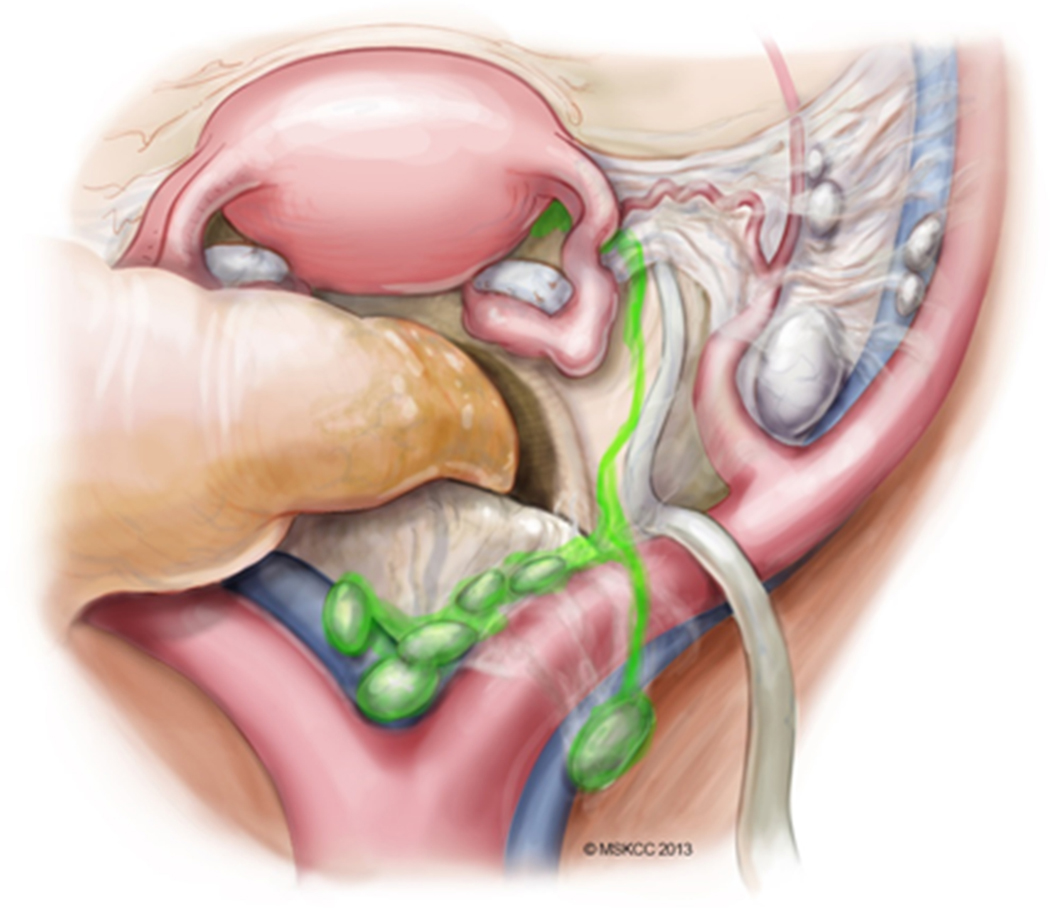
The second (and less common) drainage pattern is the posterior paracervical pathway (Figure 4). After injection into the cervix, ICG dye courses cranially in the mesoureter, draining into the nodal basin located in the presacral region or the common iliac vessels region.
Figure 4.
Posterior paracervical pathway of lymphatic spread. Adapted from Abu-Rustum.117
The FIRES trial (Fluorescence Imaging for Robotic and Endometrial Sentinel Lymph Node Biopsy) investigated the sensitivity and negative predictive value of SLN mapping compared to traditional lymphadenectomy in clinically diagnosed stage I endometrial cancers. [1] In this trial, the sensitivity of SLN mapping technique with ICG dye reached 97% (95% confidence interval [CI], 85–100) with a negative predictive value of 99.6% (95% CI, 97.9–100).
While the detection rates of using ICG dye are encouraging, these must be compared with the detection rates of traditional isosulfan blue dye. The FILM trial (Fluorescence Imaging for Lymphatic Mapping) was designed as a non-inferiority prospective randomized trial to determine the safety and efficacy of ICG dye compared with isosulfan blue dye [2]. The trial demonstrated that 97% of all lymph nodes were detected with ICG dye, whereas only 47% were detected with blue dye. Furthermore, ICG dye identified one or more SLNs in 96% of patients; isosulfan blue detected SLNs in 74% of patients. ICG dye also proved to be more accurate than isosulfan-blue dye in identifying bilateral SLNs (78% vs, 31%, respectively). The FILM study validated the superiority of ICG dye versus isosulfan blue dye, and ICG is now the dye of preference for SLN detection where the technology is available.
Although ICG and NIR technology have demonstrated impressive detection rates and reassuring negative predictive value, this innovative technology poses some challenges. It is important to distinguish between a true sentinel lymph node and a ‘secondary lymph node’. Understanding the two potential “pitfalls” associated with ICG mapping failures is essential. Not every green node is SLN; moreover, sometimes a dilated lymphatic channel may appear as a node and on final pathology no nodal tissue is identified, so the surgeon must use caution and precision to ensure that the removed SLN is a true node and not just adipose or lymphatic channels [3]; likewise, secondary and tertiary echelons maybe removed but should not be called sentinel. Although studies with ICG’s revealed high SLN detection rates, increased sensitivity and negative predictive value have confirmed that it is a useful and vital tool in the modern surgical staging of endometrial cancer.
It is also important to add that in the event of a mapping failure on one or both pelvic sides, a side-specific lymph node dissection must be performed as per the MSK algorithm. Likewise, macroscopic or suspicious nodes must be removed regardless of the mapping.
UTERINE CERVIX
Cervical Lymphatic / drainage mapping
The spread of cervical carcinoma mainly involves the parametria, upper vagina and uterus, and pelvic lymph nodes. [1,2] The incidence of lymph node metastasis increases in relation to clinical FIGO stage. According to the literature, pelvic node metastasis in stage Ib, stage IIa, and stage IIb cervical cancer is approximately 12%– 22%, 10%–27%, and 34%–43%, respectively. [3] Nodal metastasis is related to clinical stage, deep cervical stromal invasion, lymph-vascular space invasion, corpus and parametrial invasion. Para-aortic node metastasis is generally secondary to pelvic node involvement, and the risk of aortic spread rises to 25% if positive pelvic nodes are identified. [4]
Lymph node involvement is one of the most important prognostic factors in cervical cancer. The reported survival rates for women with stage I cervical cancer are between 80% and 98%. However, the 5-year survival of these patients decreases dramatically—to as low as 50%--if positive lymph nodes are encountered. [5,6] To date, studies evaluating the patterns of lymphatic spread in cervical cancer have focused mainly on detection of solid parametrial and pelvic lymph nodes. Benedetti-Panici and colleagues microscopically examined 109 giant section specimens of patients with early-stage and locally advanced cervical cancer. [1] They demonstrated the presence of metastatic and non-metastatic parametrial lymph nodes in the superficial and deep layers of the vesico-uterine ligament (VUL), the uterosacral ligament (USL) and the distal part of the lateral parametrium. Although this study confirmed that paracervical tissue forms a major route for lymphatic spread, it did not reveal any specific organ-draining lymphatic pathways. Hence, it failed to resolve the clinical question regarding the required extent of parametrectomy.
Ercoli and colleagues examined the paracervical lymphatic pathways by injecting Lipiodol dye into 18 cadaveric cervices. They identified a supra-ureteral, infra-ureteral, and neural pathway in 96%, 22% and 7% of cases, respectively. [7]
Subsequent validation studies eliminated some of the shortcomings in the AGO study, and more recent literature has shown that the sentinel lymph node mapping procedure is highly reliable in detecting lymph node metastases. The SENTICOL study (International validation study of sentinel node biopsy in early cervical cancer) was performed at multiple sites in France by experienced surgeons who utilized both radioisotope and blue dye in patients with tumors < 4 cm in size. [8,9] They reported a sentinel node detection rate of 98%. When a sentinel node was detected in a hemi-pelvis the sensitivity of the procedure was reportedly 96%, with a negative predictive value of 98%. In the largest retrospective validation study of the sentinel lymph node mapping concept in cervical cancer patients, [10] also reported a sensitivity of 96%. Both these studies highlighted the importance of following a strict sentinel node algorithm, consisting of: 1) removing any suspicious nodes, regardless of mapping; 2) completing a side-specific lymphadenectomy in unmapped hemi-pelvises; 3) performing ultrastaging and immunohistochemistry on all sentinel node specimens found to be negative on H & E. (These three steps comprise the Memorial Sloan Kettering Cancer Center SLN Algorithm.) [11] In an analysis of 43 sentinel lymph node studies in cervical cancer patients, [12] Tax et al. found that, when the Memorial Sloan Kettering Cancer Center SLN Algorithm was utilized in the setting of tumors < 4 cm in size, the pooled sensitivity of the procedure was 99% (95% confidence interval [CI] 98–100%). Based on the strong data in prospective and retrospective studies, reviews, and meta-analyses, the National Comprehensive Cancer Network (NCCN) now includes lymphatic mapping and sentinel node biopsy as an option for assessing lymph nodes in women with clinical stage I cervical cancer whose tumors measure < 4 cm in size.
The aims of the ongoing SENTICOL-3 trial will be to standardize the SLN mapping technique as well to provide solid prospective randomized data regarding the oncologic safety of the SLN mapping alone.
As rates of sentinel node detection increase, the need to perform complete lymphadenectomies in unmapped hemi-pelvises will decrease. This, in turn, has the potential to improve patients’ postoperative quality of life in both the short- and long-term, lessening the risk of debilitating morbidities such as wound problems and lower extremity lymphedema. Mapping with either blue dye or radioisotope detects at least 1 sentinel node in 85–90% of patients, with bilateral detection rates in 55% of patients. [12] Combining blue dye and radioisotope improves the detection rate to 94% and the bilateral detection rate to 72%. [12] It is important to note that despite utilization of combined blue dye and radiocolloid, 28% of women with cervical cancer will still require complete lymphadenectomies in one or both hemipelves. Therefore, improved detection rates--particularly bilateral detection rates--remains a major goal for investigators.
One promising mapping technique for increasing sentinel node detection is indocyanine green (ICG) and near infrared imaging (NIR) cameras. In the FILM trial (Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers), a phase III randomized study comparing ICG with blue dye in the detection of sentinel nodes in women with cervical and uterine cancers, ICG dye identified sentinel nodes in 96% of patients, with bilateral detection rates of 78%. [13] This is an improvement over the rates reported for combined blue dye and radioisotope and obviates the need for radioactive compounds. However, cervical cancer is a rare disease, and few studies have focused on cervical cancer alone when assessing ICG as a mapping substance. Thus, women with cervical cancer accounted for only 4% of patients enrolled in the FILM study. In other studies that included both cervical and uterine cancer patients, only 5–10% of the participants were patients with cervical cancer. [13–15]
Other retrospective studies have evaluated ICG as a mapping substance in women undergoing surgery (both standard laparoscopic and robotic) for cervical cancer. In one study evaluating ICG and near infrared imaging (NIR) using standard laparoscopic equipment, Buda et al. found at least one sentinel node in 100% of patients, and bilateral sentinel nodes in 95% of patients with stages IA1-IB2 cervical cancers. [16] In the same study, Buda and colleagues also evaluated the ability of ICG and NIR to detect sentinel nodes in women with cervical lesions > 2 cm in size. In this subgroup, the sentinel node detection rate was 100% and the bilateral detection rate was 92%. [16] Utilization of ICG and near infrared imaging with the robotic surgical system also shows high detection rates in women with early stage cervical cancer [17] found at least 1 sentinel node in 100% of these patients and bilateral sentinel nodes in 87%.
All studies reporting on ICG and NIR for the detection of sentinel nodes in women with cervical cancer have utilized minimally invasive (“closed”) systems (either standard laparoscopic or robotic systems). However, many gynecologic oncologists are now performing surgery for cervical cancer via laparotomy (“open”), due to oncologic concerns with minimally invasive surgery recently reported large prospective and retrospective studies. [18,19] This creates a conundrum, as ICG and NIR have demonstrated superiority to other modalities (radiocolloid and/or blue dye) in detecting sentinel nodes, but the technique has been approved for use only in the laparoscopic system. Some practitioners have attempted to repurpose the laparoscopic camera for use in open surgery as a way to incorporate the technology into laparotomy procedures. [20] However, as noted above, the technology was designed for and trialed only in the setting of minimally invasive surgery, so its performance in the open surgical setting is unknown. The unmet need for surgeons seeking to perform radical hysterectomy and sentinel lymph node biopsy during laparotomy, while including ICG and NIR in the procedure, is an FDA-approved handheld device that can be used during open surgery. This would require a prospective clinical trial (and FDA submission) for one of the handheld NIR systems. As the detection of sentinel nodes is typically performed prior to hysterectomy and requires visualization deep into the obturator space, such a hand-held system should ideally be small enough to fit into a narrow female pelvis. The available systems become smaller in size with each new model; however, a smaller handheld device would enable the surgeon to comfortably map SLNs in all women regardless of pelvic width and uterine size.
VULVA
The Use of Near Infrared Imaging for the Detection of Inguinofemoral Sentinel Lymph Nodes in Patients with Vulvar Cancer
Primary surgical management of vulvar carcinoma includes resection of the tumor, and dissection of the inguinofemoral lymph nodes to evaluate and remove metastatic disease. The classic “longhorn” incision was the traditional surgical approach, in which the vulva and tumor were excised en bloc with the inguinofemoral lymphatics. However, the morbidity entailed by this approach was considerable, and efforts were made to reduce the radicality of the operation without jeopardizing oncologic outcomes. As such, “triple incision” approaches, in which separate bilateral inguinofemoral incisions as well as a single incision with a 1 cm margin at the primary tumor site, were adopted. [1–3] This considerably reduced the morbidity of the procedure, particularly that associated with resection of the primary tumor. However, morbidity related to lymph node dissection—including wound infection, wound seroma, and chronic debilitating lower extremity lymphedema—remained. [4–6] For this reason, sentinel lymph node mapping and sampling became more widely adopted. Sentinel inguinofemoral lymph node sampling was found to result in considerably less short- and long-term morbidity, with significant reductions in chronic lymphedema. The oncologic efficacy of this approach was solidified by two prospective trials: the GOG-173 and the GROINSS-V. These studies reported a false negative predictive value of 3.7% and a groin failure rate of approximately 2% in appropriately selected patients undergoing inguinofemoral lymph node dissection. [7, 8] Based on this prospective data, as well as mounting retrospective evidence, sentinel lymph node biopsy for patients with vulvar cancer has become the standard of care.
As SLN mapping has been more widely adopted, the procedure has evolved. Initial efforts utilizing blue dye and radiocolloid lymphoscintigraphy have given way to more precise and reliable methods such as near infrared imaging (NIR) with indocyanine green (ICG). In this section, we will review the lymphatic drainage of the vulva, discuss the evolution of techniques for identifying the sentinel inguinofemoral lymph node, and examine the use of NIR for this indication.
Vulvar lymphatic drainage
The first descriptions of the lymphatic anatomy of the vulva were made by Sappey in 1874. He observed that the vulva drained mainly to the ipsilateral inguinofemoral lymph nodes. [9] In 1929, Rentschler further characterized vulvar lymphatic drainage based on his knowledge of the spread of carcinoma, distinguishing the anterior one-third of the vulva where collecting trunks run directly to the mons, from the posterior two-thirds of the vulva where collecting trunks proceed directly to the terminal lymph nodes. [10] In 1948, Stanley Way described a classification system for lymphatic drainage based on his observations performing radical en bloc resections in patients with vulvar carcinoma. [11,12] Way’s classification system consisted of five groups: 1) the superficial inguinal nodes; 2) the deep inguinal nodes; 3) the sub-inguinal or superficial femoral nodes; 4) the deep femoral nodes; 5) the external iliac nodes. These categories were further corroborated by his contemporary, Taussig, who described his own observations performing en bloc resections. [13] Based on these reports, the typical drainage of the vulva is considered to originate at the location of the primary tumor. Tumors located in the anterior one-third of the vulva tend to drain through the mons and to the superficial inguinal nodes, whereas drainage from the posterior two-thirds of the vulva tends to course laterally and, subsequently, to the superficial or deep inguinal nodes. Clitoral lesions may drain to the deep or superficial nodes, depending on dominant channels leading either through the clitoral circulation or through the surrounding tissue to the superficial inguinal chains.
SLN identification techniques
Initially, the use of blue dyes and radiocolloid lymphoscintigraphy dominated sentinel lymph node mapping. In the GOG-173, all patients were required to undergo mapping with blue dye, with optional inclusion of radiocolloid lymphoscintigraphy. However, two years after the study was opened, retrospective evidence demonstrated that preoperative lymphoscintigraphy improved SLN detection rates. Consequently, preoperative lymphoscintigraphy and intraoperative radiolocalization were required. The study also permitted utilization of other blue dyes such as methylene blue (in 2007) due to a nationwide shortage of isosulfan blue. None of the patients in GOG-173 underwent localization with near infrared imaging. In this study, 92.5% of patients had at least one SLN identified at surgery. Sixty-one percent of patients had nodes which were both blue and “hot” (identified using intraoperative radiolocalization); 24% of patients had nodes that were blue only; 15% had nodes that were identified with radiolocalization only. False negative rates were 7.8% for radiocolloid alone, 2.0% for blue dye alone, and 1.6% for radiocolloid plus blue dye. [7] A meta-analysis in 2014 by Meads et al. reviewed mapping techniques by radiocolloid lymphoscintigraphy, as well as by blue dye. They reported SLN detection rates of 94.0% (95% confidence interval [CI], 90%−96%) for radiocolloid lymphoscintigraphy alone and 68.7% (95% CI, 63–74%) for blue dye alone. [14] While detection rates were quite high with the combined technique, the approach causes considerable dissatisfaction for both patients and clinicians because it requires a second procedure for completion. In the case of preoperative lymphoscintigraphy with intraoperative radiolocalization, this procedure may need to be performed a day in advance. Additionally, intraoperative detection is cumbersome and relies on auditory rather than visual cues. This requires multiple disruptions of the dissection in order to detect the radiolabeled lymph node. While the blue dye does allow visual localization of the lymph node, the localization is useful only when the lymphatics and lymph node are identified. Therefore, clinicians sought alternative detection methods that would eliminate some of these challenges.
The Era of Near Infrared Imaging
In 2010, Crane and colleagues described their experience using a custom-built near infrared light source and camera for intraoperative detection of ICG-labeled sentinel inguinofemoral lymph nodes in patients with vulvar carcinoma. The authors presciently concluded that this technique might eventually replace the conventional use of blue dye and radiocolloid injection in gynecologic cancers, breast cancer, and melanoma. [15] In a publication the following year, the authors reported their results of NIR imaging in in evaluating 16 groins from 10 patients. In these patients, a total of 29 SLNs were identified by radiocolloid, 26 of which were detected with near infrared imaging and 21 with blue dye. The authors also noted that transcutaneous mapping was possible in 5 of 16 groins. [16] Over the next two years, three other groups published small retrospective experiences utilizing near infrared imaging with ICG-labeled inguinofemoral SLNs. In vivo SLN detection rates varied from 95.7–100% in these studies, compared to in vivo detection rates with of 64.9–78.6% using blue dye alone. [17–19]
In 2017 Soergel and colleagues published their findings comparing detection modalities, including radiocolloid, ICG, and blue dye. In their series of 27 patients, representing 52 at-risk groins, 91 SLNs were detected and all were positive for ICG. Furthermore, 8 SLNs that were not detected by intraoperative radiolocalization or blue dye were identified by ICG alone. [20]
Future of Near Infrared Imaging in Vulvar Cancer
The utilization of NIR for detection of inguinofemoral SLNs in patients with vulvar cancer has increased steadily over time. As more evidence emerges that NIR localization of SLNs in vulvar cancer is as effective (or more effective), than conventional techniques, this method will continue to replace the use of radiocolloid and blue dye. NIR cameras are now more appropriately tailored to this procedure and can easily be used in real time, during surgery. This allows for constant reference and improvement in surgical precision using this technique. However, definitive studies evaluating the comparative efficacy of NIR imaging versus radiocolloid or blue dye in the detection of inguinofemoral SLNs are needed (and are currently ongoing) before the use of radiocolloid or blue dye can be eliminated from practice.
OVARY
Lymphatic drainage pathways of the ovaries and their detection by near infrared technology using indocyanine green in ovarian cancer
In this section, we review the anatomic structures pertinent to lymphatic drainage of the ovary, and discuss the use of indocyanine green and near infrared technology in detecting sentinel lymph nodes in ovarian cancer.
Epithelial ovarian cancer can metastasize intraperitoneally (in the peritoneal cavity), lymphatically and hematogenously. Lymphatic metastases of epithelial ovarian cancer occur mainly in the paraaortic and paracaval lymph nodes. An excellent grasp of lymphatic anatomy, including lymphatic channel routes and the location of lymph nodes, is fundamental to understanding the dissemination of retroperitoneal nodes and, consequently, of targeted lymphadenectomy in ovarian cancer.
Ovarian lymphatic system
The lymphatic system of the female genitalia was first described by Reiffenstuhl in 1964, and subsequently by Plentl and Freedman in 1971. [1,2] They described the lymphatic vessels of the ovary converging upon the hilus to form the sub-ovarian lymphatic plexus. From this plexus are three different routes of lymphatic drainage: 1) the trunks that course along the ovarian blood vessels and terminate in the aortic nodes (the aortic nodes draining the right ovary are located where the right ovarian vein enters the vena cava; the nodes draining the left ovary are located below the left renal vein at the crossing with the ovarian vein); 2) the trunks that course within the broad ligaments towards the lateral pelvic wall and terminate in the external iliac and interiliac nodes (from there, lymph reaches the common iliac nodes and then the aortic region); 3) the third route, which is less frequently involved, courses along the round ligament and drains into the external iliac and inguinal lymph nodes.
In a recent study, Kleppe et al. [3] examined the lymphatic drainage pathways of the ovaries by immunohistochemical analysis, from a microscopic point of view. They confirmed the presence of two major pathways and one minor pathway. The first major pathway is the abdominal pathway, running via the infundibulopelvic ligament to the para-aortic (left side)/paracaval (right side) regions; the second major pathway is the pelvic pathway, draining via the lateral parametrium and supra-ureteral pathway to the internal iliac artery and obturator fossa; the minor pathway is the inguinal pathway, draining via the round ligament of the uterus to the inguinal regions.
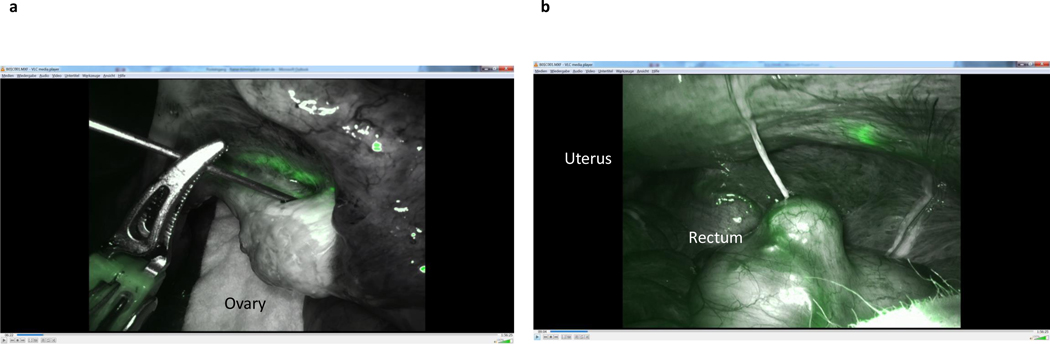
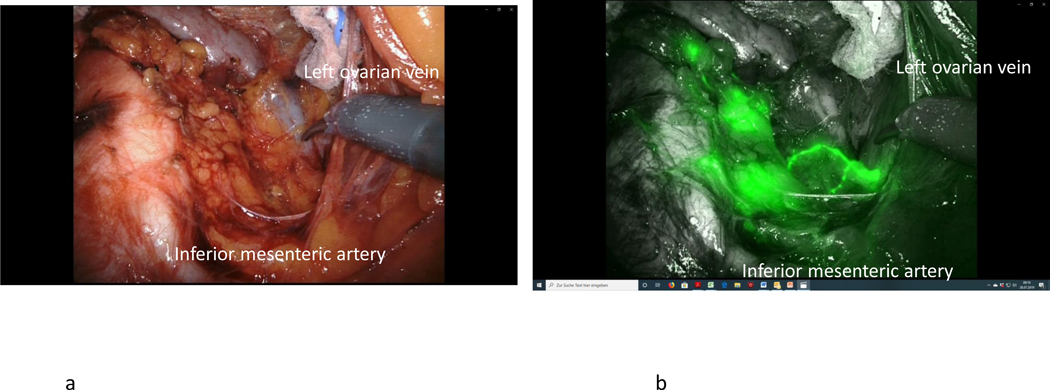
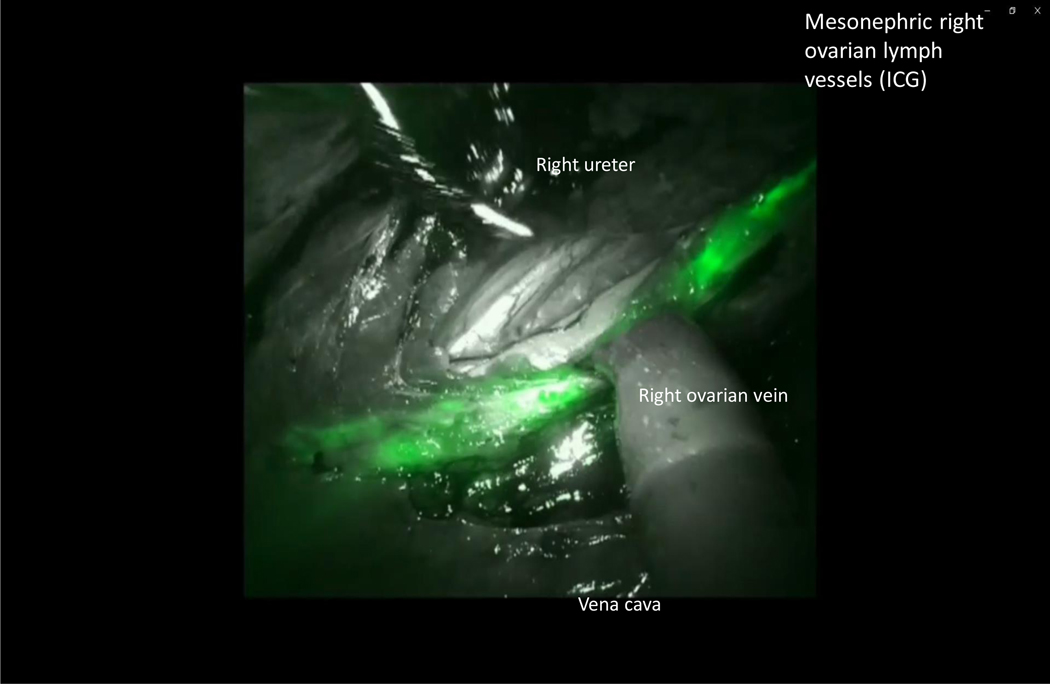
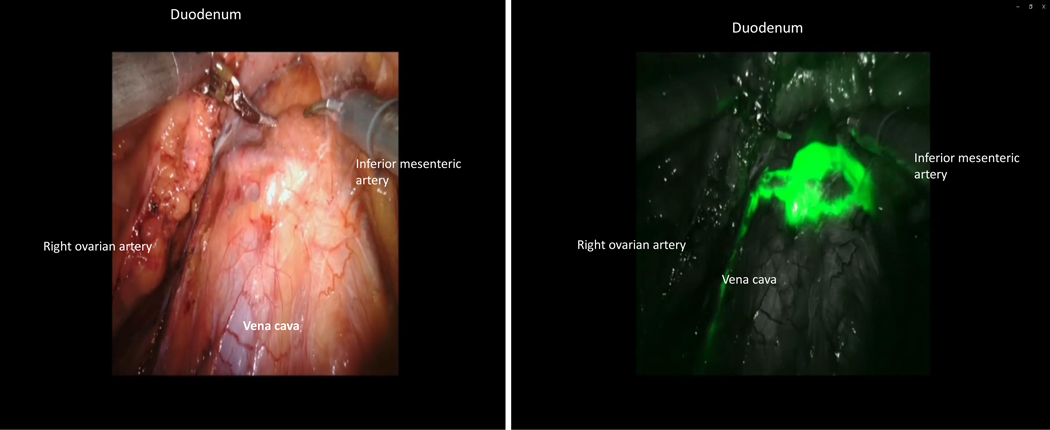
After ICG injection, functional investigations can be performed with video documentation of lymphatic drainage, dependent on the site of injection. It has been shown that the lymphatic drainage of the ovary is identical to that of the uterine corpus, using the same lymphatic pathways along the ovarian vessels to the right and left infrarenal, paraaortic region, and the pelvic pathway along the uterine artery to the interiliac region. The pelvic pathway can be demonstrated following injection of ICG dye to the mesovary (Figure 5), indicating the continuous nature of the lymphatic system in the utero-ovarian lymphatic network. With respect to the intraoperative dynamics of ICG drainage, the two main routes along the Müllerian (uterine) and the mesonephric (ovarian) pathways can be demonstrated reproducibly; however, drainage along the round ligaments to the inguinal region has never been observed, indicating either the presence of protective valves or obliteration of these vessels in the adult female. [4–7] Representative illustrations show the right and left ovarian drainage to the aortic region. On the right side, the regional lymph nodes are located ventrally in the interaortacaval region and along the right aortic wall, about 1–2 cm above the infra-mesenteric artery (Figure 6); drainage to the right paracaval region has never been observed along this pathway. On the left side, the lymphatic vessels connect to the nodes about 2 cm higher, and exclusively on the left infrarenal paraortic area (Figure 7). The pelvic connections and first fluorescent nodes are similar to the drainage of uterine corpus cranioventrally, the uterine artery to the interiliac nodes medially, and the external iliac vessels caudally (Figure 8, Figure 9).
Figure 5.
Injection of indocyanine green in the right mesovary (A) and consecutive visualization of uterine Müllerian lymphatic system broad ligament (B).
Figure 6.
(A) Native image of the targeted infrarenal sentinel lymph nodes of the left ovary. (B) Indocyanine green fluorescence of the targeted infrarenal sentinel lymph nodes of the left ovary.
Figure 7.
Indocyanine green (ICG) fluorescence of the targeted infrarenal sentinel lymph nodes of the right ovary.
Figure 8.
(A) Native image of the targeted infrarenal sentinel lymph nodes of the right ovary. (B) Indocyanine green fluorescence of the targeted infrarenal sentinel lymph nodes of the right ovary.
Figure 9.
(A) Native image of the targeted pelvic sentinel lymph nodes of the left ovary/uterine corpus. (B) Indocyanine green fluorescence of the targeted pelvic sentinel lymph nodes of the left ovary/uterine corpus.
Positive nodes are found in 10–15% of patients with disease apparently confined to the ovary; this rate increases to 64–67% when disease has spread to the abdomen. [8]
The most common locations of lymph node metastases in patients with ovarian cancer are: solely in the high paraaortic/paracaval area (50%), solely in the pelvic area (20%), and in both the paraaortic/paracaval and pelvic areas (30%). [3] Contralateral metastases have also been identified in patients with ovarian cancer, although these are very rare. [9] In vivo microscopic studies have not detected any connection between the right and left lymphatic ovarian drainage pathways. A possible explanation of contralateral metastases could be retrograde metastasis from one side to the other at the uterine level. [3]
Sentinel lymph node detection in ovarian cancer guided by ICG
Sentinel lymph node mapping has become a widely accepted procedure in early-stage cervical and endometrial cancer and has been integrated into the NCCN and ESGO guidelines. [10–12] In ovarian cancer, the principle of sentinel lymph node mapping would seem ideal in the setting of early disease, and its feasibility has been reported in a few preliminary studies. The first description of sentinel lymph node identification from the ovary was reported by Vanneuville et al. in 2004. [13–15]
In all, 10 studies including 145 patients with ovarian cancer who underwent sentinel lymph node mapping, have been published. [13,16–24] These studies are characterized by small numbers of patients and a broad range of technical approaches.
As described in the literature, the sites of injection have varied. A majority of studies published to date have used the proper ovarian ligament and the infundibulopelvic ligament as the sites for injection. [20–24] Usually, tracer injections were given on the dorsal and ventral side of the proper ovarian ligament and the infundibulopelvic ligament, close to the ovary and just underneath the peritoneum. In one study the injection was given in the remnants of both ligaments after the involved adnexa had already been resected (n=10), either during the same surgical procedure or during a second operation. [24]
A variety of tracers have been reported in the literature. In a majority of patients radiocolloid technetium-99 (Tc99) was the most commonly used tracer, either alone [13,20] or in combination with patent blue. [17,18,20,21,23] One study used a charcoal solution (1 ml), composed of 10 mg of carbon particles, 20 nm in diameter, and 4 mg of polyvinyl pyrolidone with a concentration of 0.05–0.2 ml. [8] Near-infrared detection using fluorescent indocyanine green dye was used in three studies. [11,14,16] One of the benefits of the ICG tracer is that it eliminates the need for a radioactive tracer; in both open and minimally invasive procedures the dye is injected directly, in the operating room, after induction of general anesthesia. In two recent studies ICG was injected alone. [11,14] In another, earlier study ICG was used in combination with Tc99 radiocolloid. [16] All investigators injected ICG at a concentration of 1.25 mg/Ml (25-mg vial with ICG powder was diluted in 20 mL of sterile aqueous water), and 0.5 to 1 ml of this solution were injected. The detection rate was 95.6% when a combination of radiocolloid with blue dye was used. When combining radiocolloid with ICG, the detection rate was 100%. [16] When ICG alone was used, the detection rate was 95%. [11,14]
Sentinel lymph nodes were found in 131 of 145 patients, for an overall detection rate of 90.3%. In 81 (61.8%) of these 131 patients, the sentinel nodes were located in the para-aortic region only; in 30 (22.9%) of the 131 patients, the sentinel nodes were found in the pelvic region only; and in the remaining 20 (15.3%) patients, the sentinel nodes were found in both the para-aortic and pelvic regions. Two studies have reported sentinel lymph nodes located above or below the level of the inferior mesenteric artery. [10,14]
The ESGO guidelines for ovarian cancer surgery recommended the (midline) laparotomy as the standard of care in early-stage disease, [15] although this recommendation was never proven in prospective randomized studies. Applying these guidelines to sentinel lymph node mapping, laparotomy should also be the first choice, particularly in the presence of large ovarian masses. A laparoscopic procedure for sentinel lymph node mapping should be considered only when a second surgery is necessary to determine stage of disease, or in the presence of small, suspicious ovarian nodules.
The studies published to date have confirmed the feasibility of sentinel lymph node identification in the ovary. All these studies demonstrated detection rates ranging from 40% to 100%. However, our ability to draw definitive conclusions is limited by the fact that these studies differed with respect to the exact location, the number of injections, and the tracer(s) used.
Targeted compartmental lymphadenectomy is an interesting approach that may prove to be a feasible modification of pure sentinel node excision. The concept of the targeted compartmental lymphadenectomy approach [6,7,25] includes removal of embryologically defined compartments of locoregional tumor spread. [26,27] This entails complete removal of the local lymphatic network, together with the first regional (sentinel) nodes in each lymphatic channel. Compared with traditional sentinel node biopsy, the targeted compartmental approach could potentially enhance diagnostic safety without compromising morbidity. Considering the large variability of the procedure and the different injection sites and dyes used, there is a need to standardize this procedure before it can be tested in large multicenter studies. However, the necessity of systematic pelvic and paraaortic lymphadenectomy for diagnostic purposes should undoubtedly be questioned. In fact, it may be replaced by a less radical, less morbid sentinel lymph node mapping approach in the future. Although its utility must be confirmed in adequate prospective trials, it appears to be a safe and feasible diagnostic procedure in this setting. NIR technology using ICG as a tracer will provide further insight into the structure of lymphatic drainage and functional dynamics in the individual patient.
BOWEL RESECTION
Near-infrared angiography during rectosigmoid resection and re-anastomosis performed during debulking surgeries for gynecologic malignancies
Surgery is integral to the treatment of advanced epithelial ovarian cancer. The surgeon’s objective is to achieve no gross residual disease, given that this is one of the most important prognostic factors. [1,2] This often entails upper abdominal surgery as well as some form of bowel resection.
Surgery on the intestinal tract is frequently necessary in patients with gynecologic cancers. Indications include not only the resection of disease, but bowel obstructions and other disease-or treatment-related complications as well. Mastery of the appropriate surgical techniques is vital. An excellent knowledge of anatomy, development of surgical planes respecting the blood supply and innervation patterns, maintenance of hemostasis, and gentle tissue handling form the basis of successful intestinal surgery.
Complications from intestinal surgery are devastating and unforgiving. An anastomotic leak after bowel resection is a known serious complication, associated with significant morbidity and mortality. The incidence of anastomotic leaks reported in the literature is between 5% to 15%, with a mortality rate of 3% to 21% (mostly secondary to sepsis with generalized peritonitis). [3] Anastomotic leaks reportedly occur at a rate of 7% in patients undergoing debulking surgery for ovarian cancer requiring bowel resection. [4] Poor oxygenation due to diminished blood supply is believed to play a major role in leakage and failed anastomosis. Some risks factors have been identified; these include low anastomosis, preoperative radiation, intraoperative adverse events, poor nutrition, incomplete donuts, active Crohn’s disease or ulcerative colitis, active chemotherapy, or high-dose steroids. Specifically, rectosigmoid resections seem to be associated with the highest rate of anastomotic leaks. Reducing the anastomotic leak rates associated with rectosigmoid resections is therefore of great importance. Continuous efforts have been made to identify accurate methods for assessing intestinal viability and perfusion before or during the surgical procedure. Some of these include visible light spectrophotometry and laser Doppler flowmetry (LDF). [3,5] One potential intervention is the use of near-infrared angiography (NIR) via proctoscopy. This technique offers the surgeon a way to assess anastomotic perfusion at the time of rectosigmoid resection and anastomosis. Evaluation of perfusion offers the opportunity to act on abnormal findings intraoperatively. Possible scenarios include the creation of a diverting ileostomy or revision of the anastomosis if abnormal perfusion is detected on NIR. At the same time, demonstration of a well-perfused anastomosis offers reassurance and can prevent the use of an unnecessary diverting ileostomy. However, a quantification of anastomotic perfusion (normal versus abnormal) is lacking, and the clinical value of NIR has not been established, leading to a concern that use of NIR intraoperatively could result in unnecessary interventions, prolonged OR time and increased costs.
NIR has previously been reported as a safe intervention with promising results in identifying at-risk anastomoses, decreasing leakage rates and improving outcomes. [5–10] However, the literature investigating this technique to date has occurred within the context of colorectal cancer. PILLAR II was a prospective observational study of patients with benign or malignant colorectal pathology who underwent evaluation of colon anastomoses by intraoperative fluorescence angiography (IFA). [8] This study demonstrated that, with the use of IFA, the anastomotic leak rate was 1.4%. The PILLAR III study (ClinicalTrials.gov registry identifier NCT02205307) is a multicenter randomized controlled trial investigating the use of IFA in colorectal cancer patients. Accrual has ended, but the results are still pending. [11] IntAct is an ongoing European prospective, multicenter, unblinded, randomized controlled trial comparing surgery with IFA against standard of care (i.e., surgery with no IFA) in patients undergoing resection for rectal cancer. [7]
We performed a retrospective cohort study at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1st, 2013 and December 31st, 2018 of cases requiring low anterior resection at the time of gynecologic cancer surgery. [12] A total of 410 patients were identified as having undergone a rectosigmoid resection during surgical debulking for ovarian or uterine carcinoma during this time. NIR via proctoscopy to assess anastomotic perfusion at the time of rectosigmoid resection and anastomosis was used in 134 (32.7%) of these cases (the NIR cohort). The median procedure times did not differ significantly between the NIR and non-NIR cohorts, suggesting that the use of NIR technology did not prolong surgery significantly. All anastomoses were performed using a stapler; all patients underwent an air-leak test and were reported as tension-free. The data did demonstrate a significantly fewer number of diverting ostomies among the NIR cohort (9/134, 6.7%) compared with the non-NIR cohort (53/276, 19%) (p<0.001). The anastomotic leak rate was 2/134 (1.2%) in the NIR cohort compared with 12/276 (4.4%) in the non-NIR cohort (p=0.10). Postoperative pelvic abscesses occurred in 4/134 (6.0%) patients in the NIR cohort and 44/276 (15.9%) in the non-NIR cohort (p=0.004). Patients in the NIR cohort had significantly fewer postoperative interventional procedures (12/134, 9.0% NIR vs. 55/276, 20.0% non-NIR, p=0.01) and significantly fewer 30-day readmissions (15/134, 11.2% NIR vs. 60/276, 21.7% non-NIR, p=0.01). This work has prompted us to open a randomized controlled trial at MSKCC to evaluate the true implications of NIR proctoscopy in patients with ovarian cancer undergoing rectal resection as part of their cytoreductive surgery.
VASCULARIZED LYMPH NODE TRANSFER FOR LYMPHEDEMA
Introduction
Vascularized lymph node transfer (VLNT) is a procedure that has evolved tremendously over the past 10 years. The concept is straightforward: replace lymph nodes that have been surgically removed. The idea seems almost too simple, and initially drew skepticism, as well as concern that VLNT could cause iatrogenic lymphedema. [1–3] However, a number of recent innovations have dramatically reduced and even eliminated the risk of donor site lymphedema and have improved the reliability of VLNT. [4–8] New insights into pathophysiology, improved patient selection, and lymphatic imaging have resulted in VLNT becoming one of the most commonly performed surgical treatments for lymphedema today.
The role of VLNT in the context of other surgical strategies such as lymphovenous bypass (LVB) or liposuction has evolved as we have gained a better understanding of lymphedema itself. Lymphedema is commonly misunderstood. It is more than just a plumbing problem related to removal of lymph nodes, leading to a proximal obstruction of lymph; there is also a subsequent response to this surgical injury by the immune system, which leads to progressive scarring of the delicate lymphatic vessels distal to the injury. [9–11] This leads to further lymph stasis and consequently, fat hypertrophy, which has been observed in most patients to some degree. Patients with lymphedema dominated by fat hypertrophy (as opposed to pitting edema) are potential candidates for liposuction. [12–14] In contrast, patients with a fluid dominant limb who have early stage lymphedema with a few patent lymphatics may be amenable to LVB. VLNT is also indicated for fluid dominant patients and has the potential additional benefit of replacing immunologic organ and soft tissue if needed.
What is vascularized lymph node transfer?
VLNT involves transplanting lymph nodes from one part of the body to the affected limb using microsurgical technique. Typically, an arterial and venous anastomosis are performed, without a lymphatic anastomosis. Lymph nodes contain VEGF-C, the protein responsible for inducing lymphangiogenesis from the lymphedematous limb into the transplanted nodes. [9,15–18] This has been observed in both animal models and in the clinical experience using postoperative lymphoscintigraphy. [19,20]
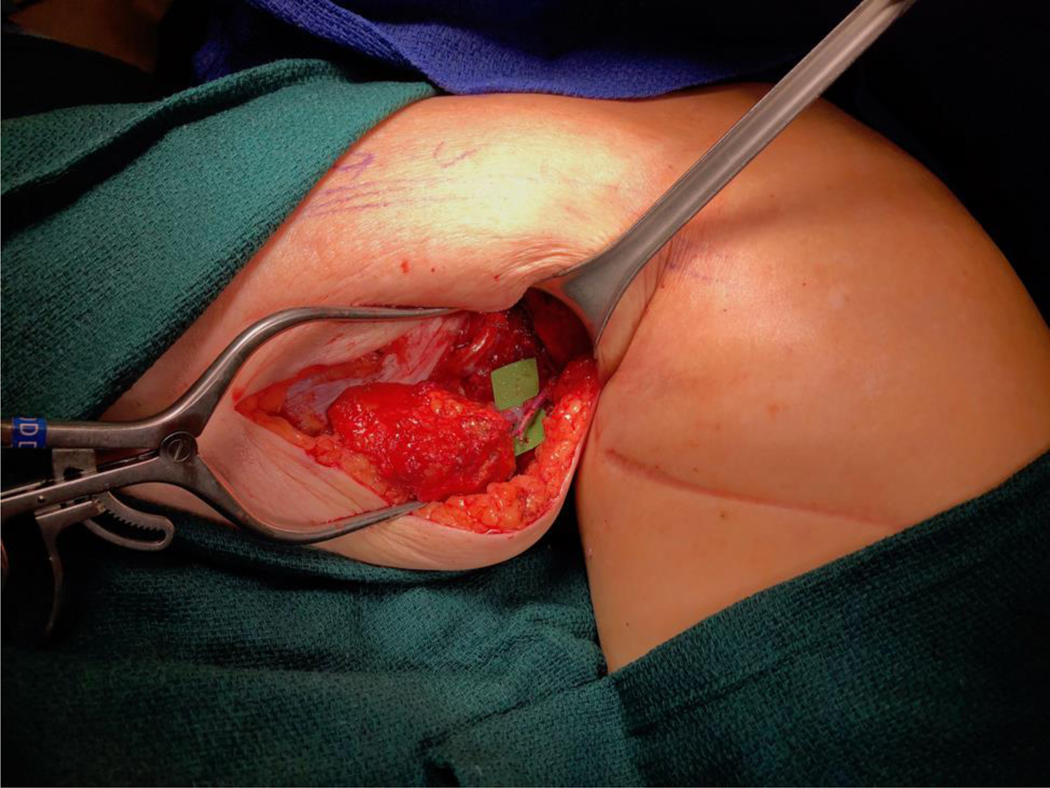
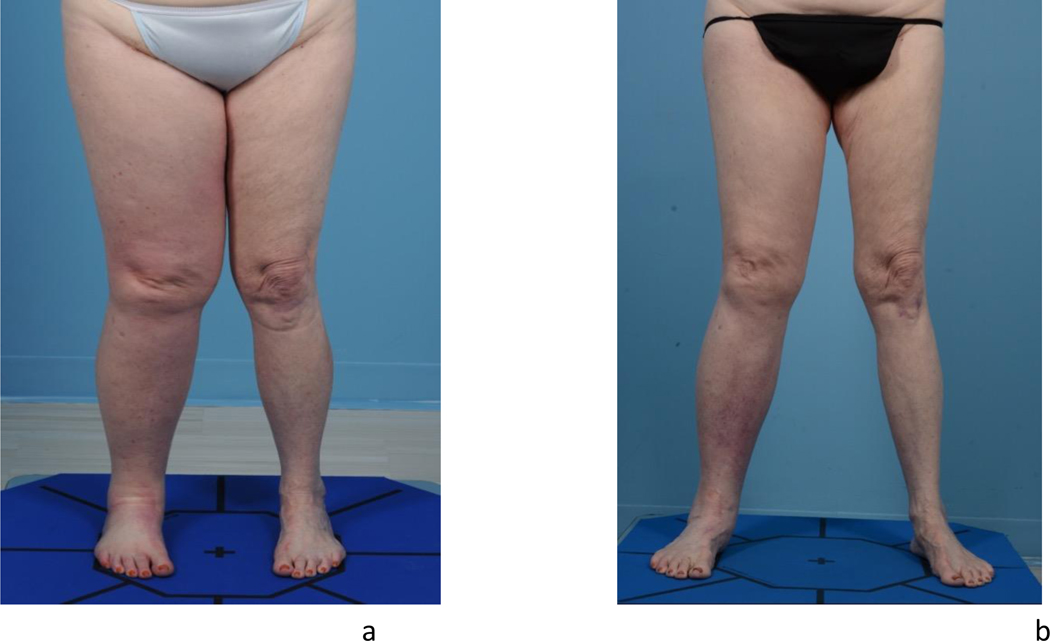
There is an evolving debate regarding exact placement of the lymph node flap. If the patient suffers from vein compression, range of motion limitation, or pain at the site of the lymphadenectomy, orthotopic transfer is generally preferred because these secondary issues can also be addressed. Lymph nodes placed at the lymphadenectomy site provide the potential to restore lymphatic continuity. In contrast, if most of the swelling is distal, then it may be preferable to place the nodes at that location. Orthotopic transfer in gynecologic cancer-related lymphedema can be challenging if the patient has had a pelvic lymphadenectomy. In this scenario lymph nodes are more commonly placed in the ankle, calf, or groin (Figure 10 and 11). [21] In these heterotopic transfers, it is hypothesized that lymph is shunted into the venous system through interconnections between the lymphatic sinuses and venules at the level of the lymph node. [22]
Figure 10.
Vascularized lymph node transfer to the medial sural vessels of the calf.
Figure 11.
Preoperative clinical photograph of a patient with right lower extremity lymphedema (A) and her 4year postoperative result following lymph node transfer to the calf (B).
Donor Site Lymphedema in VLNT
Initial approaches to VLNT involved transfer of groin or axillary lymph nodes based purely on anatomic landmarks. However, reports of the dreaded complication of iatrogenic lymphedema subsequently surfaced. [1–3] It became clear that while landmarks are a useful guide, they are not a guarantee because lymphatic collectors are all but invisible without a tracer. We previously described the technique of reverse lymphatic mapping to safely harvest lymph nodes for VLNT. [4] Briefly, two different tracers are used to distinguish the lymph nodes draining the trunk from the extremity. Technetium is injected into the limb and indocyanine green dye (ICG) is injected into the trunk. Using a near-infrared camera, the target lymph nodes draining the trunk are harvested. The lymph nodes draining the adjacent limb are avoided with the guidance of a gamma probe, preserving lymphatic drainage of the limb. In the author’s experience, there is a 6% incidence of aborting this procedure when the lymphatic drainage of the limb is shared by the targeted nodes draining the trunk. Axillary or groin lymph node transfer does provide the option of including a skin paddle, which can replace heavily radiated skin if needed.
Alternative sources of lymph nodes which eliminate the risk of donor site lymphedema have since been described. These include the gastroepiploic nodes with submental nodes, supraclavicular nodes, mesenteric nodes, and gastroepiploic nodes/omentum. [5–7,23–26] Each of these options has its own set of pros and cons in terms of number of lymph nodes, tissue bulk, and donor site morbidity. Potential complications related to each of these donor sites include: chyle leak for supraclavicular nodes, marginal mandibular nerve palsy for submental nodes, and small bowel ischemia for mesenteric nodes. Currently the author’s most common donor site is the gastroepiploic lymph node chain with omentum. This donor site provides abundant tissue and consistent anatomy. Relative contraindications include significant prior abdominal surgery and history of ovarian cancer, in which case the omentum may have been removed or potentially seeded with tumor.
Indications for VLNT
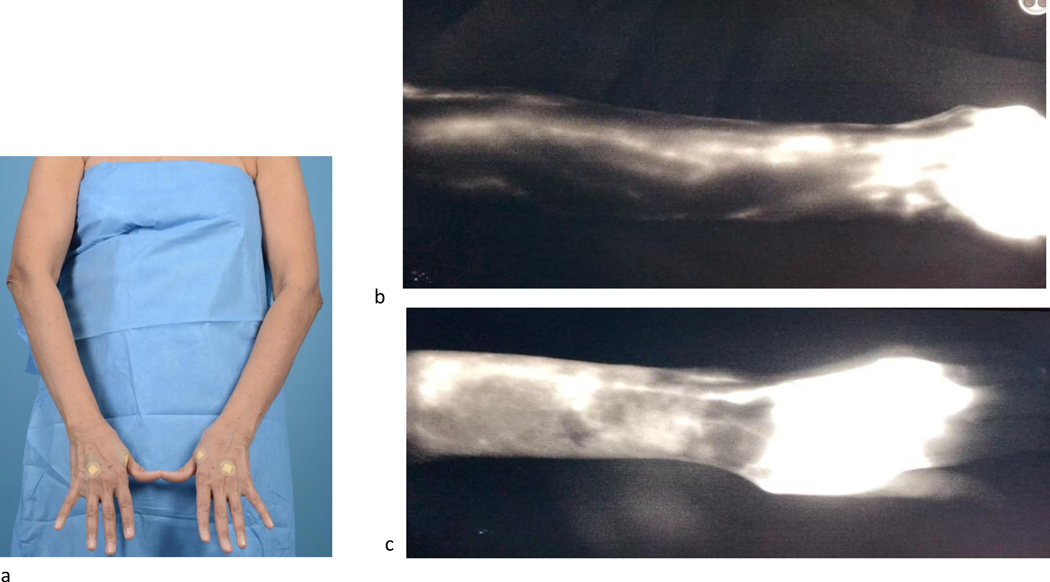
Most microsurgeons would consider the ideal candidate for VLNT to be relatively thin and healthy, with earlier stage lymphedema. Most centers have a BMI limit of 30 or 35 because there is a direct correlation between increased BMI and impaired lymphatic function, which may lead to a high failure rate. [27,28] Limb volumes and validated patient-reported outcome questionnaires are collected in addition to an imaging work-up. There are a variety of approaches, but in general this includes evaluation of the superficial lymphatic system with ICG lymphangiography in the office (Figure 12), evaluation of the deep lymphatic system using lymphoscintigraphy, and an MRA to assess the fluid/fat composition of the limb and rule out vein compression or tumor recurrence. Careful assessment is important, as there are different manifestations of lymphedema: fluid dominant limbs with pitting edema, fat dominant limbs without pitting but with significant fibrofatty hypertrophy, and a spectrum in between. Patients who have limited range of motion, pain, or vein compression due to prior lymphadenectomy or radiation may be better suited for VLNT as opposed to lymphovenous bypass, because VLNT allows placement of healthy tissue in the affected region. The general consensus among surgeons performing LVB or VLNT is to operate on earlier stage lymphedema with fluid dominance.
Figure 12.
(A) Patient with barely noticeable left upper extremity lymphedema. (B) Indocyanine green lymphangiography of the normal right upper limb, demonstrating normal linear lymphatic collectors. (C) Despite minimal limb volume difference, indocyanine green lymphangiography reveals dramatic lymphatic abnormalities with loss of most of the normal linear lymphatics and abundant dermal reflux.
Efficacy of VLNT
It is important to state that VLNT is not a cure for lymphedema, and that most patients will still require the use of a garment. Not every patient responds, and some may continue to worsen over time. Published reports to date, however, do demonstrate improvement in quality of life and a significant reduction in limb volume. [29–33] These benefits are greatly valued by patients with a chronic and often progressive disease that dominates their lifestyle. While limb volume is the outcome most focused on, it is a flawed metric. Limb volume changes throughout the day and can be dramatically affected by the amount of lymphedema therapy and compression a patient receives. Additionally, patients with even minimal volume difference have a significant reduction in quality of life. For these reasons, validated patient-reported outcome questionnaires such as the LLIS and LYMQOL are particularly important metrics. The authors are currently conducting a prospective controlled study on lymphatic surgery using both clinical and histologic outcome measures. In summary, VLNT has become a safe and effective option for patients with chronic lymphedema. Achieving optimal patient selection and predicting the efficacy of the procedure remain a challenge, as there are many unknowns in the disease itself. There may also be a role for prophylactic VLNT in select cases of very high-risk individuals undergoing lymphadenectomy. In the future the best solution may be a combination of both lymphatic surgery and a drug to counter the pathologic immune response leading to fibrosis itself. Drug trials for lymphedema have recently become a reality, and we are doing investigational work at MSKCC. [34] This is a promising and exciting development. While these drugs represent the earliest attempts at a medical solution, they reflect a very real interest by research and pharmaceutical circles that will likely bring us closer to a cure.
References
- 1.Nagaya T, Nakamura YA, Choyke PL, et al. Fluorescence-guided surgery. Front Oncol 2017;7:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol 2013;58:R37–61. [DOI] [PubMed] [Google Scholar]
- 4.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017;18:384–92. [DOI] [PubMed] [Google Scholar]
- 5.Frumovitz M, Plante M, Lee PS, et al. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, noninferiority trial. Lancet Oncol 2018;19:1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Body N, Grégoire J, Renaud M-C, et al. Tips and tricks to improve sentinel lymph node mapping with indocyanin green in endometrial cancer. Gynecol Oncol 2018;150:267–73. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti-Panici P, Maneschi F, D’Andrea G, et al. Early cervical carcinoma: the natural history of lymph node involvement redefined on the basis of thorough parametrectomy and giant section study. Cancer 2000;88:2267–74. [PubMed] [Google Scholar]
- 8.Benedetti-Panici P, Maneschi F, Scambia G, et al. Lymphatic spread of cervical cancer: an anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol Oncol 1996;62:19–24. [DOI] [PubMed] [Google Scholar]
- 9.Sakuragi N. Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int J Clin Oncol 2007;12:165–75. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti Panici P, Basile S, Angioli R. Pelvic and aortic lymphadenectomy in cervical cancer: the standardization of surgical procedure and its clinical impact. Gynecol Oncol 2009;113:284–90. [DOI] [PubMed] [Google Scholar]
- 11.Sahraoui S, Bouras N, Acharki A, et al. [Adenocarcinoma of the cervix uteri: a retrospective study of 83 cases]. Gynecol Obstet Fertil 2002;30:291–8. [Article in French]. [DOI] [PubMed] [Google Scholar]
- 12.Richard SD, Krivak TC, Castleberry A, et al. Survival for stage IB cervical cancer with positive lymph node involvement: a comparison of completed vs. abandoned radical hysterectomy. Gynecol Oncol 2008;109:43–8. [DOI] [PubMed] [Google Scholar]
- 13.Ercoli A, Delmas V, Iannone V, et al. The lymphatic drainage of the uterine cervix in adult fresh cadavers: anatomy and surgical implications. Eur J Surg Oncol 2010;36:298–303. [DOI] [PubMed] [Google Scholar]
- 14.Lécuru F, Mathevet P, Querleu D, et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: results of the SENTICOL study. J Clin Oncol 2011;29:1686–91. [DOI] [PubMed] [Google Scholar]
- 15.Lecuru FR, McCormack M, Hillemanns P, et al. SENTICOL III: an international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int J Gynecol Cancer 2019;29:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvo G, Ramirez PT, Levenback CF, et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol Oncol 2017;145:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormier B, Diaz JP, Shih K, et al. Establishing a sentinel lymph node mapping algorithm for the treatment of early cervical cancer. Gynecol Oncol 2011;122:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tax C, Rovers MM, de Graaf C, et al. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol Oncol 2015;139:559–67. [DOI] [PubMed] [Google Scholar]
- 19.Rocha A, Domínguez AM, Lécuru F, et al. Indocyanine green and infrared fluorescence in detection of sentinel lymph nodes in endometrial and cervical cancer staging - a systematic review. Eur J Obstet Gynecol Reprod Biol 2016;206:213–9. [DOI] [PubMed] [Google Scholar]
- 20.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinellymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol 2014;133:274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buda A, Papadia A, Di Martino G, et al. Real-time fluorescent sentinel lymph node mapping with indocyanine green in women with previous conization undergoing laparoscopic surgery for early invasive cervical cancer: comparison with radiotracer±blue dye. J Minim Invasive Gynecol 2018;25:455–60. [DOI] [PubMed] [Google Scholar]
- 22.Beavis AL, Salazar-Marioni S, Sinno AK, et al. Sentinel lymph node detection rates using indocyanine green in women with early-stage cervical cancer. Gynecol Oncol 2016;143:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–904. [DOI] [PubMed] [Google Scholar]
- 24.Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med 2018;379:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rychlik A, Marin S, De Santiago J, et al. Utility of laparoscopic indocyanine green-guided sentinel node biopsy in open cervical cancer surgery. Int J Gynecol Cancer 2016;26:1288–9. [DOI] [PubMed] [Google Scholar]
- 26.DiSaia PJ, Creasman WT, Rich WM. An alternate approach to early cancer of the vulva. Am J Obstet Gynecol 1979;133:825–32. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Sugiyama V, Pham H, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol 2007;104:636–41. [DOI] [PubMed] [Google Scholar]
- 28.Preti M, Rouzier R, Mariani L, et al. Superficially invasive carcinoma of the vulva: diagnosis and treatment. Clin Obstet Gynecol 2005;48:862–8. [DOI] [PubMed] [Google Scholar]
- 29.Gould N, Kamelle S, Tillmanns T, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329–32. [DOI] [PubMed] [Google Scholar]
- 30.Rouzier R, Haddad B, Dubernard G, et al. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg 2003;196:442–50. [DOI] [PubMed] [Google Scholar]
- 31.Barton DPJ. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:683–701. [DOI] [PubMed] [Google Scholar]
- 32.Levenback CF, Ali S, Coleman RL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a Gynecologic Oncology Group Study. J Clin Oncol 2012;30:3786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oonk MH, van Hemel BM, Hollema H, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 2010;11:646–52. [DOI] [PubMed] [Google Scholar]
- 34.Sappey MPC. Anatomie, physiologie, pathologie des vaisseaux lymphatiques considérés CheZ l’homme et les vertébrés 1874. [Article in French]. [Google Scholar]
- 35.Rentschler CB. Primary epithelioma of the vulva: an analysis of seventy-one cases. Ann Surg 1929;89:709–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Way S. The anatomy of the lymphatic drainage of the vulva and its influence on the radical operation for carcinoma. Ann R Coll Surg Engl 1948;3:187–209. [PMC free article] [PubMed] [Google Scholar]
- 37.Way S. Carcinoma of the vulva. Am J Obstet Gynecol 1960;79:692–7. [DOI] [PubMed] [Google Scholar]
- 38.Taussig FJ. Cancer of the vulva: an analysis of 155 cases (1911–1940). Am J Obstet Gynecol 1940;40:764–79. [Google Scholar]
- 39.Meads C, Sutton AJ, Rosenthal AN, et al. Sentinel lymph node biopsy in vulval cancer: systematic review and meta-analysis. Br J Cancer 2014;110:2837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crane LMA, Themelis G, Buddingh KT, et al. Multispectral realtime fluorescence imaging for intraoperative detection of the sentinel lymph node in gynecologic oncology. J Vis Exp 2010. doi: 10.3791/2225. [Epub ahead of print: 20 Oct 2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane LMA, Themelis G, Arts HJG, et al. Intraoperative nearinfrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: first clinical results. Gynecol Oncol 2011;120:291–5. [DOI] [PubMed] [Google Scholar]
- 42.Schaafsma BE, Verbeek FPR, Peters AAW, et al. Nearinfrared fluorescence sentinel lymph node biopsy in vulvar cancer: a randomised comparison of lymphatic tracers. BJOG 2013;120:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathéron HM, van den Berg NS, Brouwer OR, et al. Multimodal surgical guidance towards the sentinel node in vulvar cancer. Gynecol Oncol 2013;131:720–5. [DOI] [PubMed] [Google Scholar]
- 44.Hutteman M, van der Vorst JR, Gaarenstroom KN, et al. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am J Obstet Gynecol 2012;206:89. e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soergel P, Hertel H, Nacke AK, et al. Sentinel lymphadenectomy in vulvar cancer using near-infrared fluorescence from indocyanine green compared with technetium 99m nanocolloid. Int J Gynecol Cancer 2017;27:805–12. [DOI] [PubMed] [Google Scholar]
- 46.Reiffenstuhl G. The lymphatics of female genital organs. Philadelphia: Lippincott, 1964. [Google Scholar]
- 47.Plentl AA, Friedman EA. Lymphatic system of the female genitalia. Philadelphia: WB Saunders, 1971. [PubMed] [Google Scholar]
- 48.Kleppe M, Kraima AC, Kruitwagen RFPM, et al. Understanding lymphatic drainage pathways of the ovaries to predict sites for sentinel nodes in ovarian cancer. Int J Gynecol Cancer 2015;25:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimmig R, Aktas B, Buderath P, et al. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine-green (ICG). J Surg Oncol 2016;113:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimmig R, Rusch P, Buderath P, et al. Aortic utero-ovarian sentinel nodes and left infrarenal aortic lymph node dissection by ICG supported navigation. Gynecol Oncol Rep 2017;20:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimmig R, Buderath P, Rusch P, et al. Early ovarian cancer surgery with indocyanine-green-guided targeted compartmentallymphadenectomy (TCL, pelvic part). J Gynecol Oncol 2017;28:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimmig R, Buderath P, Mach P, et al. Surgical treatment of early ovarian cancer with compartmental resection of regional lymphatic network and indocyanine-green-guided targeted compartmental lymphadenectomy (TCL, paraaortic part). J Gynecol Oncol 2017;28:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panici PB, Angioli R. Role of lymphadenectomy in ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2002;16:529–51. [DOI] [PubMed] [Google Scholar]
- 54.Kleppe M, Wang T, Van Gorp T, et al. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol 2011;123:610–4. [DOI] [PubMed] [Google Scholar]
- 55.Koh W-J, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:64–84. [DOI] [PubMed] [Google Scholar]
- 56.Koh W-J, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:170–99. [DOI] [PubMed] [Google Scholar]
- 57.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 2016;27:16–41. [DOI] [PubMed] [Google Scholar]
- 58.Vanneuville G, Mestas D, Le Bouedec G, et al. The lymphatic drainage of the human ovary in vivo investigated by isotopic lymphography before and after the menopause. Surg Radiol Anat 1991;13:221–6. [DOI] [PubMed] [Google Scholar]
- 59.European Society of Gynaecological Oncology. ESGO guidelines: ovarian cancer surgery guidelines, 2017. Available: http://ebooks.esgo.org/ovarian-surgery-guidelines/mobile/index.html#p=1 [DOI] [PubMed]
- 60.Negishi H, Takeda M, Fujimoto T, et al. Lymphatic mapping and sentinel node identification as related to the primary sites of lymph node metastasis in early stage ovarian cancer. Gynecol Oncol 2004;94:161–6. [DOI] [PubMed] [Google Scholar]
- 61.Nyberg RH, Korkola P, Mäenpää JU. Sentinel node and ovarian tumors: a series of 20 patients. Int J Gynecol Cancer 2017;27:684–9. [DOI] [PubMed] [Google Scholar]
- 62.Nyberg RH, Korkola P, Mäenpää J. Ovarian sentinel node: is it feasible? Int J Gynecol Cancer 2011;21:568–72. [DOI] [PubMed] [Google Scholar]
- 63.Angelucci M, Corrado G, Vizza E. Laparoscopic indocyanine green sentinel lymph node mapping in early ovarian cancer. A pilot study and review of the literature. ltal J Gynaecol Obstet 2016;28:23–8. [Google Scholar]
- 64.Hassanzadeh M, Hosseini Farahabadi E, Yousefi Z, et al. Lymphatic mapping and sentinel node biopsy in ovarian tumors: a study using intra-operative Tc-99m-phytate and lymphoscintigraphy imaging. J Ovarian Res 2016;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleppe M, Brans B, Van Gorp T, et al. The detection of sentinel nodes in ovarian cancer: a feasibility study. J Nucl Med 2014;55:1799–804. [DOI] [PubMed] [Google Scholar]
- 66.Buda A, Passoni P, Corrado G, et al. Near-infrared fluorescenceguided sentinel node mapping of the ovary with indocyanine green in a minimally invasive setting: a feasible study. J Minim Invasive Gynecol 2017;24:165–70. [DOI] [PubMed] [Google Scholar]
- 67.Speth SCJM, Kruitwagen RFPM, Kleppe M, et al. Comparison of intraoperative γ-probe imaging and postoperative SPECT/CT in detection of sentinel nodes related to the ovary. J Nucl Med 2017;58:243–5. [DOI] [PubMed] [Google Scholar]
- 68.Lago V, Bello P, Montero B, et al. Clinical application of the sentinel lymph node technique in early ovarian cancer: a pilot study. Int J Gynecol Cancer 2019;29:377–81. [DOI] [PubMed] [Google Scholar]
- 69.Kimmig R, Buderath P, Rusch P, et al. Technique of ICG-guided targeted compartmental pelvic lymphadenectomy (TCL) combined with pelvic peritoneal mesometrial resection (PMMR) for locoregional control of endometrial cancer - a proposal. Gynecol Oncol Rep 2017;20:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höckel M. Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol 2015;16:e148–51. [DOI] [PubMed] [Google Scholar]
- 71.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248–59. [DOI] [PubMed] [Google Scholar]
- 72.Rajkumar S, Nath R, Lane G, et al. Advanced stage (IIIC/IV) endometrial cancer: role of cytoreduction and determinants of survival. Eur J Obstet Gynecol Reprod Biol 2019;234:26–31. [DOI] [PubMed] [Google Scholar]
- 73.Blumetti J, Chaudhry V, Cintron JR, et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg 2014;38:985–91. [DOI] [PubMed] [Google Scholar]
- 74.Grimm C, Harter P, Alesina PF, et al. The impact of type and number of bowel resections on anastomotic leakage risk in advanced ovarian cancer surgery. Gynecol Oncol 2017;146:498–503. [DOI] [PubMed] [Google Scholar]
- 75.Urbanavičius L, Pattyn P, de Putte DV, et al. How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 2011;3:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ris F, Hompes R, Cunningham C, et al. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc 2014;28:2221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong G, Croft J, Corrigan N, et al. IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis 2018;20:O226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jafari MD, Wexner SD, Martz JE, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multiinstitutional study. J Am Coll Surg 2015;220:e81:82–92. [DOI] [PubMed] [Google Scholar]
- 79.Kudszus S, Roesel C, Schachtrupp A, et al. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 2010;395:1025–30. [DOI] [PubMed] [Google Scholar]
- 80.Jafari MD, Lee KH, Halabi WJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 2013;27:3003–8. [DOI] [PubMed] [Google Scholar]
- 81.National Library of Medicine (NIH). A study assessing perfusion outcomes with PINPOINT® near infrared fluorescence imaging in low anterior resection (Pillar III); 2014. https://clinicaltrials.gov/ct2/show/results/NCT0220
- 82.Moukarzel LA LS, Wu M, Byrne ME, et al. The impact of using near-infrared angiography during rectosigmoid resection and anastomosis in patients undergoing gynecologic cancer surgery. Int Gynecol Cancer Society 2019;29:A16.1. [Abstract]. [Google Scholar]
- 83.Liu H-L, Pang S-Y, Lee C-C. Donor limb assessment after vascularized groin lymph node transfer for the treatment of breast cancer-related lymphedema: clinical and lymphoscintigraphy findings. J Plast Reconstr Aesthet Surg 2019;72:216–24. [DOI] [PubMed] [Google Scholar]
- 84.Demiri E, Dionyssiou D, Tsimponis A, et al. Donor-site lymphedema following lymph node transfer for breast cancer-related lymphedema: a systematic review of the literature. Lymphat Res Biol 2018;16:2–8. [DOI] [PubMed] [Google Scholar]
- 85.Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516–20. [DOI] [PubMed] [Google Scholar]
- 86.Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277–85. [DOI] [PubMed] [Google Scholar]
- 87.Kenworthy EO, Nelson JA, Verma R, et al. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J Surg Oncol 2018;117:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dayan JH, Voineskos S, Verma R, et al. Managing venous hypertension in vascularized omentum lymphatic transplant: restoring bidirectional venous drainage. Plast Reconstr Surg 2018;141:326e–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen AT, Suami H. Laparoscopic free omental lymphatic flap for the treatment of lymphedema. Plast Reconstr Surg 2015;136:114–8. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen AT, Suami H, Hanasono MM, et al. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017;115:84–9. [DOI] [PubMed] [Google Scholar]
- 91.Dayan JH, Ly CL, Kataru RP, et al. Lymphedema: pathogenesis and novel therapies. Annu Rev Med 2018;69:263–76. [DOI] [PubMed] [Google Scholar]
- 92.Kataru RP, Wiser I, Baik JE, et al. Fibrosis and secondary lymphedema: chicken or egg? Transl Res 2019;209:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kataru RP, Baik JE, Park HJ, et al. Regulation of immune function by the lymphatic system in lymphedema. Front Immunol 2019;10:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg 2016;32:56–65. [DOI] [PubMed] [Google Scholar]
- 95.Brorson H. From lymph to fat: liposuction as a treatment forcomplete reduction of lymphedema. Int J Low Extrem Wounds 2012;11:10–19. [DOI] [PubMed] [Google Scholar]
- 96.Peled AW, Slavin SA, Brorson H. Long-term outcome after surgical treatment of lipedema. Ann Plast Surg 2012;68:303–7. [DOI] [PubMed] [Google Scholar]
- 97.Saaristo A, Tammela T, Timonen J, et al. Vascular endothelial growth factor-C gene therapy restores lymphatic flow across incision wounds. Faseb J 2004;18:1707–9. [DOI] [PubMed] [Google Scholar]
- 98.Lohela M, Saaristo A, Veikkola T, et al. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost 2003;90:167–84. [DOI] [PubMed] [Google Scholar]
- 99.Gould DJ, Mehrara BJ, Neligan P, et al. Lymph node transplantation for the treatment of lymphedema. J Surg Oncol 2018;118:736–42. [DOI] [PubMed] [Google Scholar]
- 100.Huang J-J, Gardenier JC, Hespe GE, et al. Lymph node transplantation decreases swelling and restores immune responses in a transgenic model of lymphedema. PLoS One 2016;11:e0168259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobbia D, Semple J, Baker A, et al. Experimental assessment of autologous lymph node transplantation as treatment of postsurgical lymphedema. Plast Reconstr Surg 2009;124:777–86. [DOI] [PubMed] [Google Scholar]
- 102.Tobbia D, Semple J, Baker A, et al. Lymphedema development and lymphatic function following lymph node excision in sheep. J Vasc Res 2009;46:426–34. [DOI] [PubMed] [Google Scholar]
- 103.Smith ML, Molina BJ, Dayan E, et al. Heterotopic vascularized lymph node transfer to the medial calf without a skin paddle for restoration of lymphatic function: proof of concept. J Surg Oncol 2017;115:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng M-H, Huang J-J, Wu C-W, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192e–8. [DOI] [PubMed] [Google Scholar]
- 105.Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: a prospective study of 100 consecutive cases. J Surg Oncol 2017;115:68–71. [DOI] [PubMed] [Google Scholar]
- 106.Poccia I, Lin C-Y, Cheng M-H. Platysma-sparing vascularized submental lymph node flap transfer for extremity lymphedema. J Surg Oncol 2017;115:48–53. [DOI] [PubMed] [Google Scholar]
- 107.Coriddi M, Kenworthy E, Weinstein A, et al. The importance of indocyanine green near-infrared fluorescence angiography in perfusion assessment in vascularized omentum lymphatic transplant. J Surg Oncol 2018;118:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coriddi M, Wee C, Meyerson J, et al. Vascularized jejunal mesenteric lymph node transfer: a novel surgical treatment for extremity lymphedema. J Am Coll Surg 2017;225:650–7. [DOI] [PubMed] [Google Scholar]
- 109.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg 2014;134:154e–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savetsky IL, Torrisi JS, Cuzzone DA, et al. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 2014;307:H165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng M-H, Chen S-C, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286–98. [DOI] [PubMed] [Google Scholar]
- 112.Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73–9. [DOI] [PubMed] [Google Scholar]
- 113.Ozturk CN, Ozturk C, Glasgow M, et al. Free vascularized lymph node transfer for treatment of lymphedema: a systematic evidence based review. J Plast Reconstr Aesthet Surg 2016;69:1234–47. [DOI] [PubMed] [Google Scholar]
- 114.Ho OA, Chu S-Y, Huang Y-L, et al. Effectiveness of vascularized lymph node transfer for extremity lymphedema using volumetric and circumferential differences. Plast Reconstr Surg Glob Open 2019;7:e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Engel H, Lin C-Y, Huang J-J, et al. Outcomes of lymphedema microsurgery for breast cancer-related lymphedema with or without microvascular breast reconstruction. Ann Surg 2018;268:1076–83. [DOI] [PubMed] [Google Scholar]
- 116.Gardenier JC, Kataru RP, Hespe GE, et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun 2017;8:14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging. J Natl Compr Canc Netw 2014;12:288–97. [DOI] [PubMed] [Google Scholar]