Abstract
Introduction:
The efficacy and safety profile of ustekinumab (UST) in Crohn’s disease (CD) is favorable, however, data in elderly patients is lacking. We aimed to assess the safety and efficacy of UST in elderly CD.
Methods:
We performed a retrospective cohort study of CD patients classified as elderly (age≥65 years at UST initiation) or nonelderly (<65 years) treated at a large, tertiary referral center. Outcomes assessed were clinical (measured by physician global assessment [PGA]) and steroid-free response, remission, adverse events, and post-surgical complications were compared by age category. Multivariable regression modeling and survival analysis was also performed.
Results:
117 patients (elderly n=39, nonelderly n=78) were included in the study. Elderly patients had predominantly moderate disease (87.2%), while nonelderly had a higher proportion of severe disease activity (44.9%) (p=0.001), though no differences in baseline endoscopic activity, prior biologic use, or steroid or immunomodulator use at baseline existed (p>0.05 all). While nearly 90% patients in both groups experienced clinical response to UST, compared to nonelderly, elderly patients were less likely to achieve complete clinical remission (28.2% vs. 52.6%, p=0.01). On regression modeling, age was not associated with clinical outcomes (p>0.05 all). Mucosal healing was achieved in 26% elderly and 30% nonelderly patients (p=0.74). There were no significant differences in infusion reactions (2.6% vs. 6.4%, p=0.77), infection (5.2% vs. 7.7%, p=0.7) or post-surgical complications (p=0.99) by age category.
Conclusion:
UST is safe and effective in elderly CD. Although limited by sample size and retrospective design, such real-world data can inform biologic positioning in this IBD population.
Keywords: Ustekinumab, Crohn’s disease, Elderly, Inflammatory Bowel Disease, IBD, comparative effectiveness, real-world
INTRODUCTION
Crohn’s disease (CD) is chronic idiopathic inflammatory disease of the gastrointestinal tract that requires medical therapy to control symptoms, minimize inflammation, and reduce the risk of long-term complications. CD affects all spectrum of ages. It is estimated that 25% to 30% of inflammatory bowel disease (IBD) patients are 60 years or older.1 In a population-based study of 24,834 patients, almost 23% of patients were aged 60 years or more, with mean age of 69 years.2 Furthermore, the proportion of elderly IBD patients is rising. In a Canadian study, the prevalence of elderly IBD patients increased by 5.8% as compared to 3.9% in nonelderly group between 1999 and 2008.3 As populations of developed countries continue to age, the proportion of elderly IBD patients will steadily increase. Elderly IBD patients are often considered a challenging IBD subpopulation due to higher prevalence of comorbidities, polypharmacy, malnutrition, frailty, hospitalization, and infection risk with immune modulation.4 Consequently, there is an underutilization of biologic agents and steroid-sparing agents in elderly patients.5–7 Real-world comparative effectiveness studies are needed to assess the safety concerns in elderly IBD patients, particularly as new agents come to market.
Ustekinumab (UST) is a fully human monoclonal antibody that binds the p40 subunit of interleukin (IL)-12 and IL-23 and inhibits downstream signaling.8 It has demonstrated favorable efficacy and safety in clinical trials for treatment of CD and UC and was approved by US Food and Drug Administration in 2016 for CD and 2019 for UC.9, 10 Ongoing data from long-term extension arms of the clinical trial programs in both CD and UC have suggested favorable safety profiles for UST comparable to placebo-treated participants.11, 12 Recently, several real-world studies have confirmed effectiveness and adverse events ranging from 40%−60% and 6–12%, respectively of UST for treatment of CD, but few studies include or examine elderly cohorts.13, 14 Given the recent UST approval for the treatment of ulcerative colitis in the U.S., its application in IBD will continue to expand.15 Unfortunately, elderly patients are often underrepresented in clinical trials and efficacy evaluations by age is challenging due to limited sample size. Thus, targeted real-world comparative effectiveness studies of this underrepresented population represent a critical knowledge gap. Thus, we aimed to assess the real-world effectiveness and safety of UST in elderly patients with CD.
MATERIAL AND METHODS
Study Design
We identified all adult patients (>18 years) in the Cleveland Clinic health system who received UST for treatment of CD from September 2016 to September 2019. We performed a retrospective chart review to abstract data elements. The decision to start UST was individualized based on standard of care in our center after patient and physician discussion. Patients were included if they had prior confirmed diagnosis of CD, received at least one infusion of UST, and had at least one follow up (either clinical or endoscopic) after first infusion. We excluded pediatric patients, any patient with diagnosis of ulcerative colitis, indeterminate colitis, a non-IBD primary indication for UST (e.g., psoriasis), or missing follow up data. Patients were then divided by age at first UST infusion into elderly (≥ 65 years) or nonelderly (≥ 18 and < 65 years) groups. We arbitrarily used age 65 due to multiple cutoffs reported in the literature. In addition, European Crohn’s and Colitis Organization review on IBD in elderly7 cites 60–65 years so we chose higher age cutoff to maximize potential differences in the groups. Pre-specified coding and standardized data collection form was used to collect data from October 2019 to May 2020.
Demographic and baseline data were collected for the following variables: age at UST initiation, disease duration, gender, smoking history, prior hospitalizations, prior surgeries, extra-intestinal complications, prior treatment history including corticosteroids, immunomodulators and biologics. Data on concomitant immunomodulator and steroid use at the initiation of UST was also collected. Disease phenotype was classified based on Montreal classification. Baseline disease severity was classified according to clinical assessment of treating physician. Similarly, endoscopic severity was graded based the most recent endoscopy report within the 12 weeks of UST initiation.
Outcomes
Our study aim was to assess real-world effectiveness and safety of UST in elderly CD patients as compared to younger patients. For effectiveness, outcomes of interest were proportion of patients achieving clinical response or remission, steroid-free response or remission, and mucosal healing. Clinical response or remission was classified based on PGA, where response was defined as partial if <50% or significant if ≥ 50 % reduction in CD related symptoms. Complete clinical remission was defined as complete resolution of CD related symptoms. We used objective scale such as Harvey Bradhsaw Index or Crohn’s Disease Activity index if available. Additionally, it is standard practice at our center to routinely record variables to be used for PRO-2 calculation such as number of bowel movements. These could then be compared data prior to UST initiation for percentage calculations. If on the rare occasion no such data was included, we interpreted the clinical documentation describing patient subjective response using superlative language such as most, many, majority, or similar language to indicate >50% response. In contrast, language such as few, a little, some, or similar descriptors denoted <50% improvement. We used the criteria per the protocol of previous large multicenter consortium such as VICTORY.16 Steroid-free response or remission was only assessed in patients who were using prednisone or budesonide at the time of UST initiation. Steroid-free response or remission was defined as remission if steroids were completely tapered off along with clinical remission, and as response if steroids were being tapered below baseline dose and patient achieved partial or significant clinical response. Mucosal healing was defined based on clinically indicated endoscopic assessment as absence of ulcers or erosions as previous reported in VICTORY consortium16 and the fact that endoscopic scores are always not available in endoscopic reports. These outcomes and definitions were similar to those utilized in other real-world comparative effectiveness studies of newer biologic agents.16–18 Any discrepancy or uncertainty was resolved through consensus between the investigators (RG and MA). Dose escalation was performed as clinically indicated and was defined as any increase in frequency of UST injection from standard every 8 weeks dosing or reinduction with intravenous UST. Patients were followed until UST discontinuation or last observed follow up clinic visit or endoscopy. Survival analysis by age group was run for time to remission, time to response, and time to steroid-free state only for those patients with baseline steroid treatment.
Safety outcomes assessed included infusion or injection site reactions, infections or serious adverse events. Infusion or injection reactions were further categorized into mild if patients were able to continue UST or serious if the reaction resulted in stopping therapy. Infections were further classified if they required antibiotics, hospitalization, UST discontinuation or resulted in death. For those patients who underwent CD-related intra-abdominal surgery during the observation period, data on any post-operative complications such as infections, intra-abdominal sepsis or reoperation were also collected.
Statistical Analysis
Categorical variables were presented as proportions and compared by age group via chi square or Fisher exact testing where appropriate. Continuous variables were presented as mean and standard deviation and compared with one-way ANOVA. Multivariable logistic regression models were constructed for overall PGA remission and steroid-free state. The methods of variance inflation and condition indices were used to identify the terms that could be used in a multivariable model. For each response, backwards elimination logistic regression was used to generate reduced models (models containing only those terms with P-values < .05) for each of the responses of interest. Kaplan-Meier analysis with stratification by age group was run for time to remission, time to response, and time to steroid-free state only for those patients with baseline steroid treatment. Log-rank and Wilcoxon testing was used to assess for differences in survival outcomes by age group. The analysis was done using SAS software version 9.4.
Ethical Considerations
Cleveland Clinic Institutional Review Board (Study number 19–1271) approved this study.
RESULTS
Demographics and baseline factors
A total of 117 (39 elderly and 78 nonelderly) patients were included in our study. We selected all elderly patients as study group and cohort on non-elderly patients as controls. Demographically, the elderly group had significantly longer disease duration (20.6 ± 10.7 vs 13.3 ± 7.9 years, p=0.01) and fewer active smokers (0% vs. 15.4%, p=0.005) than the nonelderly group (Table 1). Regarding CD characteristics, elderly CD was more often colonic (28.2% vs. 3.8%, p= <0.001), penetrating (43.6% vs. 19.2%, p=0.014) and later onset of CD diagnosis (64.1% elderly diagnosed onset at >40 vs. 12.8% nonelderly, p<0.001). Elderly patients had significantly less rates of perianal involvement (10.3% vs. 33.3%, p=0.007). Rates of prior CD surgery (73.7 elderly, 72.4% nonelderly, p=0.26) and prior biologic use (94.9% elderly, 98.7% nonelderly, p= 0.26) were not significantly different by age group.
Table 1:
Baseline demographics, disease characteristics, and treatment history.
| Patient Age Groups | |||
|---|---|---|---|
| Factor | < 65 yrs (N=78) | ≥ 65 yrs (N=39) | p-value |
| Age biologic | 37.6±13.5 | 69.6±3.5 | <0.001 |
| Disease duration | 13.3±7.9 | 20.6±10.7 | 0.011 |
| Female | 41(52.6) | 17(43.6) | 0.36 |
| Smoker | 0.005 | ||
| Never | 55(70.5) | 26(66.7) | |
| Current | 12(15.4) | 0(0.0) | |
| Former | 11(14.1) | 13(33.3) | |
| Hospitalization within 1 year | 44(56.4) | 14(35.9) | 0.037 |
| Age, Montreal | <0.001 | ||
| <16 year | 25(32.1) | 3(7.7) | |
| 17–40 year | 43(55.1) | 11(28.2) | |
| >40 year | 10(12.8) | 25(64.1) | |
| Location, Montreal | <0.001 | ||
| Ileal | 7(9.0) | 2(5.1) | |
| Colonic | 3(3.8) | 11(28.2) | |
| Ileo-colonic | 68(87.2) | 26(66.7) | |
| Behaviour, Montreal | 0.014 | ||
| Non-stricturing, non-penetrating | 21(26.9) | 5(12.8) | |
| Stricturing | 42(53.8) | 17(43.6) | |
| Penetrating | 15(19.2) | 17(43.6) | |
| Perianal Involvement | 26(33.3) | 4(10.3) | 0.007 |
| Disease severity per PGA* | 0.001 | ||
| Mild | 2(2.6) | 0(0.0) | |
| Moderate | 41(52.6) | 34(87.2) | |
| Severe | 35(44.9) | 5(12.8) | |
| Endoscopic severity | 0.25 | ||
| Mild | 3(4.6) | 4(13.8) | |
| Moderate | 33(50.8) | 15(51.7) | |
| Severe | 29(44.6) | 10(34.5) | |
| Prior surgery | 0.26 | ||
| Never | 21(27.6) | 10(26.3) | |
| Within 1year | 26(34.2) | 8(21.1) | |
| >1 year | 29(38.2) | 20(52.6) | |
| Type of surgery | 0.12 | ||
| Colectomy | 5(8.2) | 6(21.4) | |
| Ileo-colonic resection | 13(21.3) | 8(28.6) | |
| Segment small bowel resect | 4(6.6) | 4(14.3) | |
| Abscess drain | 3(4.9) | 0(0.0) | |
| Multiple | 31(50.8) | 10(35.7) | |
| Baseline Steroids | 0.31 | ||
| Never Used | 6(7.7) | 1(2.6) | |
| Former Use | 37(47.4) | 22(56.4) | |
| Steroid responsive | 30(38.5) | 16(41.0) | |
| Steroid-refractory | 5(6.4) | 0(0.0) | |
| Baseline Thiopurine | 0.36 | ||
| Never Used | 26(33.3) | 18(46.2) | |
| Current Use | 11(14.1) | 2(5.1) | |
| Former - 6-MP or AZA use | 41(52.6) | 19(48.7) | |
| Prior Biologic use | 77(98.7) | 37(94.9) | 0.26 |
| Biologic type | <0. 001 | ||
| Infliximab | 27 (33.3) | 9 (23.1) | |
| Adalimumab | 27(33.3) | 11 (28.2) | |
| Golimumab | 8 (9.9) | 12 (30.8) | |
| Certolizumab | 12 (14.8) | 4 (10.3) | |
| Vedolizumab | 7 (8.6) | 0 (0) | |
| Total number of biologics/patient | 2.3±1.00 | 2.1±1.4 | 0.23 |
| Baseline Steroids | 37(49.3) | 20(51.3) | 0.84 |
| Steroid dose (mg)1 | 22.6±14.8 | 26.5±11.8 | 0.30 |
PGA= Physician global assessment Statistics presented as Mean ± SD, Median [P25, P75], Median (min, max) or N (column %).
Bold indicates significant p-value <0.05. 1: Prednisone equivalent
Based on physician impression at UST initiation, elderly patients were more often categorized as having moderate clinical disease severity (87.2% vs. 52.6%) and less likely to have severe clinical disease activity (12.8% vs. 44.9%) (p<0.001); however, endoscopies prior to UST initiation did not show significant differences in endoscopic severity (34.5% elderly severe vs. 44.6% nonelderly severe, p=0.25). At UST initiation, nearly half of elderly (51.3%) and nonelderly (49.3%) patients were receiving corticosteroids (p=0.84). There was no significant difference in mean steroid dose in prednisone equivalent (elderly 26.5 ± 11.8 vs. nonelderly 22.6 ± 14.8, p= 0.30).
Effectiveness
The mean duration of follow up was 1.3 ± 1.5 years in elderly and 1.3 ± 1.1 years in nonelderly group (p=0.95). Dose escalation was performed in 17.9% in elderly group and 25.6% in the nonelderly group (p=0.35). The rates of complete clinical remission, significant response, partial response and no response was 28.2%, 46.2%, 17.9% and 7.7% in elderly group and 52.6%, 23.1%, 12.8% and 11.5% in nonelderly group, respectively (Table 2 and Figure 1). Elderly patients experienced significantly lower rates of complete remission (p=0.03) but were able to achieve significant response in nearly half (46%) of patients. Similarly, of those on baseline steroids, 50% elderly patients were able to achieve steroid-free response and 30% steroid-free remission compared to 32% and 54% in nonelderly patients, respectively (p=0.22 for both). Follow up data on mucosal healing was available in 71 patients. The rate of mucosal healing on subsequent endoscopy (from UST intitation to last follow up) was not significantly different in elderly compared to nonelderly (25.9 % vs. 29.5 %, p=0.96). A total of 114 (71 nonelderly, 33 elderly) patients were on UST at the end of study period
Table 2:
Disease-related outcomes and adverse events during ustekinumab treatment by age group
| Patient Age Groups | |||
|---|---|---|---|
| Factor | < 65 yrs (N=78) | ≥ 65 yrs (N=39) | p-value |
| Overall PGA response achieved | 0.032 | ||
| No response | 9(11.5) | 3(7.7) | |
| Partial response | 10(12.8) | 7(17.9) | |
| Significant response | 18(23.1) | 18(46.2) | |
| Complete response | 41(52.6) | 11(28.2) | |
| Time to remission (years) | 0.42±0.51 | 0.47±0.25 | 0.72 |
| Steroid free response (based on baseline steroid use) | 0.22 | ||
| No response | 5(13.5) | 4(20.0) | |
| Steroid-free response | 12(32.4) | 10(50.0) | |
| Steroid-free remission | 20(54.1) | 6(30.0) | |
| Time to Steroid free response (years) | 0.51 ± 0.22 | 0.57 ± 0.28 | 0.73 |
| Dose escalation | 0.35 | ||
| No | 58(74.4) | 32(82.1) | |
| Yes | 20(25.6) | 7(17.9) | |
| Mucosal Healing Achieved (N=71)* | 0.74 | ||
| No | 31(70.5) | 20(74.1) | |
| Yes | 13(29.5) | 7(25.9) | |
| Adverse infusion reaction | 0.77 | ||
| No | 73(93.6) | 38(97.4) | |
| Yes - continue infusion | 4(5.1) | 1(2.6) | |
| Stop infusion/therapy | 1(1.3) | 0(0.0) | |
| Adverse infection | 0.70 | ||
| No | 72(92.3) | 37(94.9) | |
| Yes-antibiotic | 5(6.4) | 1(2.6) | |
| Yes-Need hospitalization | 1(1.3) | 1(2.6) | |
| Surgery complication (N=17)* | 0.99 | ||
| No | 14(93.3) | 2(100.0) | |
| Yes | 1(6.7) | 0(0.0) | |
| Total follow up (years) | 1.3±1.1 | 1.3±1.5 | 0.95 |
Data not available for all subjects. Number of missing values: Mucosal healing = 46, Surgery complications = 100.
PGA: Physician global assessment Statistics presented as Mean ± SD, Median [P25, P75], Median (min, max) or N (column %).
The infectious complications in elderly patients were one patient with recurrent bladder infections and one developed Mycobacterium avium-intracellulare infection. In nonelderly cohort, the infectious complications were 3 cases of sinopulmonary infections (1 recurrent sinus infections, 1 recurrent pharyngitis and 1 multiple pneumonias), 1 case of each scrotal abscess, herpes zoster, and recurrent impetigo.
Figure 1:
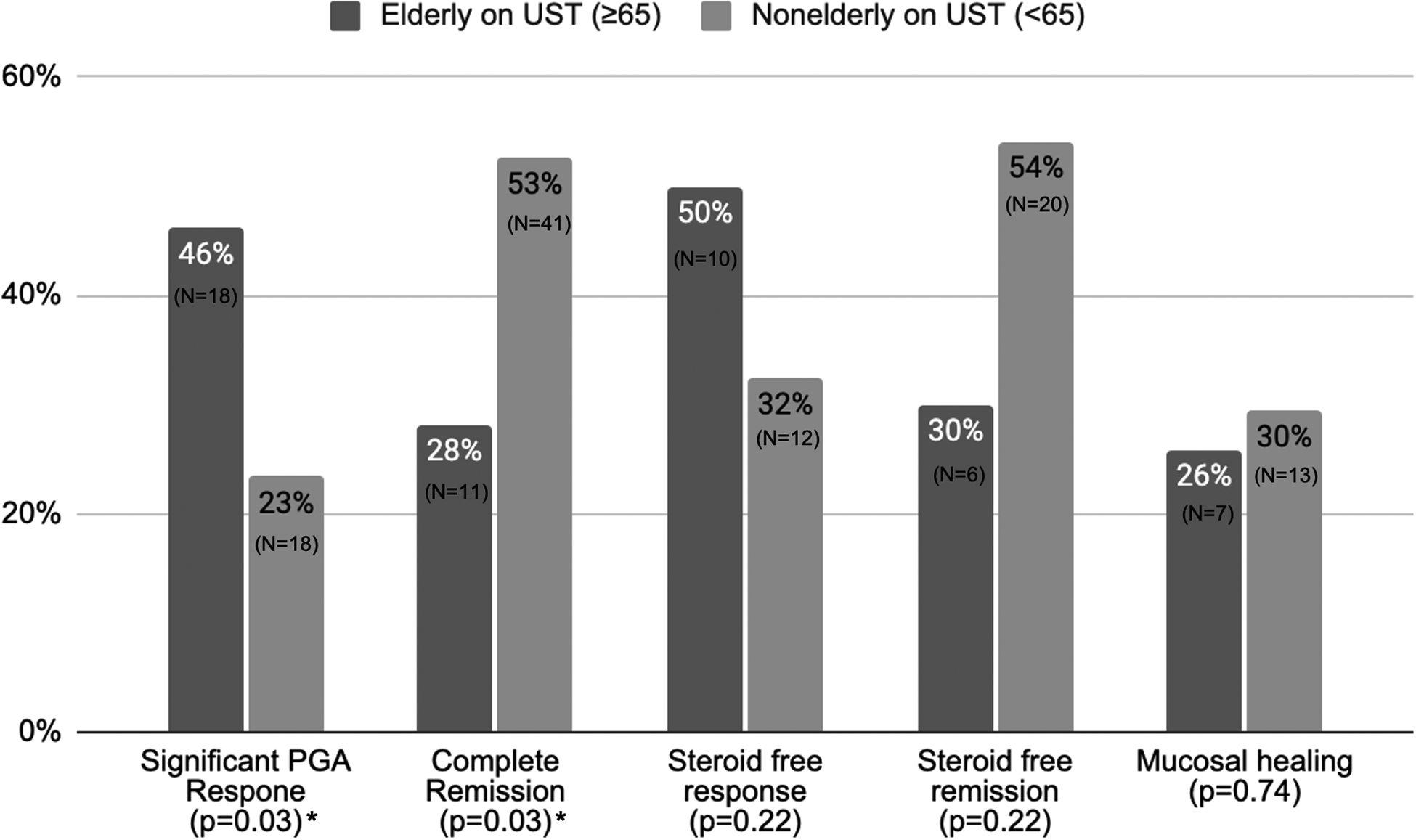
Clinical outcomes comparing elderly and nonelderly patients treated with ustekinumab. (* indicates significant p-value <0.05)
On multivariable analysis after adjusting for confounders, age group, disease duration, steroid use, number of prior biologics and immunomodulator use were not significantly associated with clinical remission (Table 3). Dose escalation of UST with odds ratio (OR) of 0.14 (95% confidence interval (CI) 0.04 – 0.53, p=0.004) was the only associated factor with clinical remission. Similarly, there was a signal towards significant lower rates of steroid-free remission (30% vs. 54.1%) and higher response rates (50% vs. 32.4%), in elderly patients as compared to nonelderly patients, respectively, but it did not reach statistical significance (p=0.22). On multivariable analysis, dose escalation was again the only independently associated factor with steroid-free state (response or remission) with OR of 0.11 (95% CI 0.02 – 0.46, p=0.003) whereas age, disease duration, concurrent steroid use and prior biologic number were not associated.
Table 3:
Multivariate logistic regression analysis for clinical remission and steroid-free state
| Overall PGA remission | Steroid-Free State | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age | 0.99 (0.96 −1.03) | 0.72 | 1.03 (0.96 – 1.04) | 0.88 |
| Disease Duration | 1.02 (0.93 – 1.07) | 0.95 | 0.95 (0.89 – 1.03) | 0.26 |
| Baseline Steroid use | 1.48 (0.53 – 4.15) | 0.45 | 1.38 (0.46 – 4.13) | 0.56 |
| Dose Escalation | 0.14 (0.04 – 0.53) | 0.004 | 0.11 (0.02 – 0.46) | 0.003 |
| Number of Prior Biologics | 0.83 (0.49 – 1.40) | 0.48 | 0.88 (0.51 – 1.51) | 0.65 |
| Immunomodulator use | 0.65 (0.20 – 2.14) | 0.48 | - | - |
OR: Odds Ratio, CI: Confidence Interval, PGA: physician global assessment
Time to achieve remission or steroid-free state
The mean time to achieve clinical remission was not significantly different by age group (0.47 ± 0.25 years elderly vs. 0.42 ± 0.51 years nonelderly, p=0.72). On Kaplan-Meier analysis, there was no statistically significant difference between both groups for clinical remission (Log rank p = 0.58, Wilcoxon p = 0.21) (Figure 2a) or response (Log rank p = 0.21, Wilcoxon P = 0.25) (Figure 2b). The time to achieve steroid-free state (response or remission) was 0.57 ± 0.28 years in elderly and 0.51 ± 0.22 years in nonelderly group (p=0.73). On time to steroid-free state analysis, there was no significant difference (Log rank p = 0.15, Wilcoxon p = 0.21) between both elderly and nonelderly group (Figure 2c).
Figure 2:
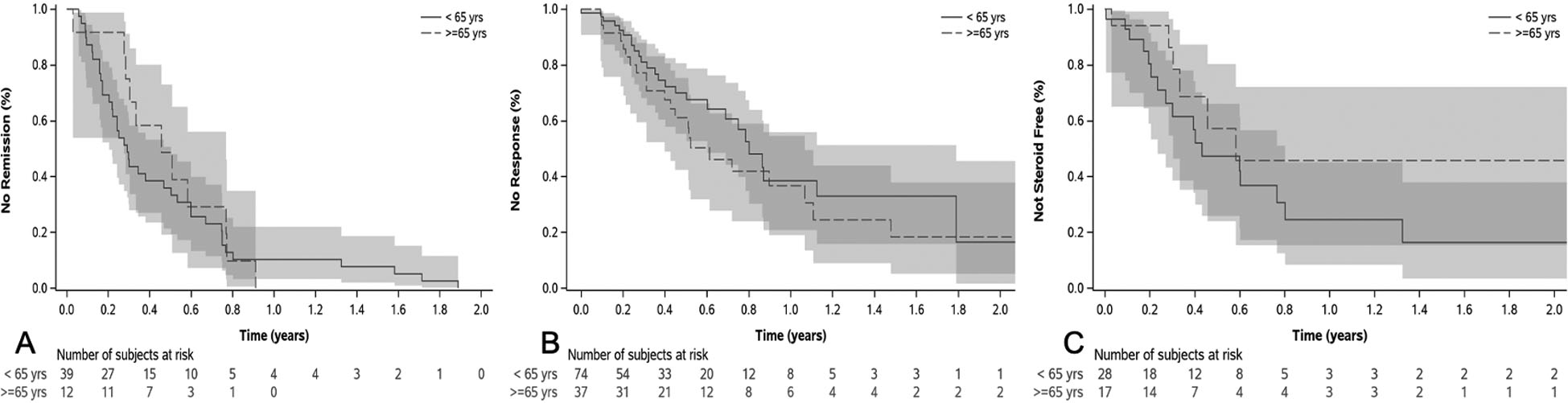
Kaplan-Meier curve for time to clinical remission in remitters (A), clinical response in responders (B) and steroid-free state in those receiving steroids at baseline (C) by age group. There were no significant differences in times to remission (Log Rank P = 0.58), response (Log Rank P = 0.21), or steroid-free state (Log Rank P = 0.15).
Safety
There were total of six infusion or injection-site reactions (5 nonelderly, 1 elderly), only one of which required discontinuation of UST therapy in nonelderly group (Table 2). The one drug reaction that required discontinuation was a severe allergic reaction to UST second subcutaneous dose. The patient developed severe knees and ankles swelling and was unable to bend his knees and ankles. The patient was given oral methylprednisolone and symptoms resolved over the next 3 days. His UST was stopped, and he was switched to Vedolizumab (VDZ). There was no significant difference in incidence of infusion or injection reactions between both groups (p=0.77). Two patients in elderly group and 6 patients nonelderly group developed infectious complications (p=0.70). The infectious complications in elderly patients were one patient with recurrent cystitis and one developed Mycobacterium avium-intracellulare infection. In nonelderly cohort, the infectious complications were 3 cases of sinopulmonary infections (1 recurrent sinus infections, 1 recurrent pharyngitis and 1 multiple pneumonias), 1 case each of scrotal abscess, herpes zoster, and recurrent impetigo. There were no deaths in either group attributed to UST infectious complications. Similarly, there was no difference in post-operative complications between both groups in patients who underwent surgery (n=16) on UST therapy (p=0.99).
DISCUSSION
In this study, we found UST generally has a comparable effectiveness and safety profile in elderly patients as compared to nonelderly patients for treatment of CD. Though elderly patients were less likely to achieve complete clinical and steroid-free remission as compared to nonelderly patients, the majority experienced a significant clinical response, were off steroids by one year, and mucosal healing rates in those assessed were similar. On regression modeling, age was not significantly associated with clinical outcomes (p>0.05 all). The rate of infusion reaction, infectious complications and post-operative complications were not significantly greater in elderly CD patients. This data suggests that UST is an effective and safe biologic option for elderly CD.
In this study, elderly CD patients were more likely to achieve significant response, but not complete remission compared to nonelderly counterparts, while rates of mucosal healing in the subset with endoscopy outcome data were similar. However, on multivariate analysis, age was not significantly associated with clinical remission or steroid free response. The rate of clinical response and remission after UST in clinical trials range from 33 to 70% and 20% to 40%, respectively.8 The clinical (28.2%) and steroid-free remission (27.8%) rates observed in elderly patients is lower than existing literature suggesting 12 week response and 24 week remission rates of 60% and 39%, respectively.8 19 Reassuringly, our nonelderly clinical remission (50%) was similar to these data suggesting external comparability. One potential explanation for the differences in the elderly vs nonelderly observed in the current analysis may lie in the PGA metric used to assess this outcome in the current study. With aging patients, there is increased likelihood of musculoskeletal complaints (e.g. arthralgias, osteoarthritis), gastrointestinal comorbidities (e.g. lactose intolerance), or other contributing comorbidity factors that could contribute to a patient overreporting symptoms attributed to IBD and consequently a physician under-interpreting clinical response or remission.20, 21 Furthermore, corticosteroid use in the elderly (nearly 50% at baseline in current study) has been shown to associate with higher rates of depression and anxiety, factors which can influence symptomatology.22 As a result, we see an approximately equal proportion experience benefit of either response or remission with similar time to benefit, and no difference in steroid-free state or objective outcomes such as mucosal healing. Additionally, the mucosal healing rates observed in the elderly cohort are in line with other real-world studies describing rates between 9% to 38%, suggesting our objective findings are comparable.11 Together, this data suggests UST is an effective option in elderly CD.
In our cohort, dose escalation above every 8 weeks maintenance was performed in 18% elderly and 26% nonelderly patients. On multivariate modeling of factors association with clinical outcomes, dose escalation was negatively associated with clinical remission and steroid-free state. While data has suggested clinical benefit to dose escalation (either interval reduction or intravenous reinduction), this is typically performed for steroid dependence, loss of response, or refractory disease and thus biases results towards worse clinical outcomes, with nearly half to 2/3 of patients not responding to dose intensification in small studies.23, 24 This may explain the negative association in the current study.
Patients with prior biologic refractory disease tends to have lower therapeutic efficacy of subsequent biologics. Information on subsequent biologic positioning in biologic exposed populations is needed to determine optimal treatment algorithms. In our study, the rate of prior biologic exposure was similar in both groups and number of prior biologics was not a significant predictor of clinical response on multivariate logistic regression. In a recent study comparing effectiveness of UST and VDZ in anti-TNF refractory CD, patients treated with UST were more likely to achieve steroid‐free clinical remission (odds ratio [OR]: 2.58, 95% CI: 1.36‐4.90, P = 0.004), biochemical remission (OR: 2.34, 95% CI: 1.10‐4.96, P = 0.027), and combined steroid‐free clinical and biochemical remission (OR: 2.74, 95% CI: 1.23‐6.09, P = 0.014) as compared to VDZ group.25 This suggests that UST may be preferred after anti-TNF failure and these findings may possibly be extrapolated to elderly CD patients as well, albeit VDZ was not directly assessed in the current study.
There were significant demographic and clinical characteristic differences between the elderly and nonelderly cohorts that may have influenced the observed outcomes. Not surprisingly, elderly patients had longer disease duration and fewer active smokers (more elderly had quit). Longer disease duration has been shown to associate with less robust response to multiple biologics including anti-TNFs and VDZ.26–28 Similarly, active smoking, while associated with worse CD outcomes, also provides another potential intervention to improve response to biologics. Despite more moderate disease severity as assessed by physician in elderly patients, the baseline disease activity (endoscopically and baseline steroid use) was similar between groups. One would have expected these differences in severity to favor improved response or remission rates in the elderly compared to nonelderly; however, the opposite was observed. This may suggest some degree of differential response by age stratified by severity. Due to limited sample size, a meaningful subgroup analysis was not possible to investigate this consideration.
Safety of biologics in elderly population is a significant issue with clinical importance due to higher incidence of adverse events and advanced comorbidities. We found that UST use in elderly is not associated with higher rate of infusion reaction, infections or postoperative complications as compared to nonelderly patients. In psoriasis, UST safety in elderly populations reported no increased risk of adverse events.29, 30 It should be noted, however, that UST dosing in psoriasis (45 mg subcutaneous induction followed 4 weeks later by 45 mg subcutaneously every 12 weeks) is different than IBD dosing and may not be generalizable, highlighting the importance of the current study4 Previous studies in IBD have shown that age is an independent risk factor for infections and malignancy with other biologic medications including anti-tumor necrosis factor (TNF) α agents and tofacitinib.31, 32 In contrast, VDZ has also been reported to have similar safety in elderly patients when compared to anti-TNFα agents.33 A post hoc analysis of GEMINI trial across different age groups reported similar rates of malignancy and infections in elderly age group (age ≥ 55 years) compared to younger groups with VDZ.34 Few data exist on UST in elderly cohorts. However, in an overall adult population UST was not found to associated with higher risk of adverse events as compared to placebo suggesting a favorable safety signal and our data support this.35 Recent pooled data from large clinical trials reported similar adverse events rates per 100-patient-years of follow up with UST (118.32, 95% CI, 113.25–123.55) as compared to placebo (165.99, 95% CI, 155.81–176.67).36 Our data extrapolates on these findings and does not suggest any additional safety signal in elderly compared to the general adult population. Together, this suggests that UST could potentially preferred in the elderly population with prior a-TNF exposure.
There are several important limitations in our study. First, it was a retrospective study based on chart review that introduces multiple potential biases including confounding by indication. We attempted to mitigate this impact performing multiple logistic regression controlling for significant confounders; however, this approach does not address all potential confounders. Second, all patients were treated at tertiary center that limits generalizability of our results, but we expect the safety to be similar in community setting or perhaps even more favorable given the milder severity of community populations. Similarly, our patient population was rather refractory with almost all patients with prior anti-TNF exposure and half requiring corticosteroids at baseline. Whether the results translate to first-line utilization of UST is unknown. There is potential of selection bias in our study given variable follow up. We attempted to assess this impact by performing survival analysis and censoring by follow up. In addition, relatively small sample size of elderly CD patients limits conclusions of this study. We were also unable to do compare UST outcomes based on CD onset (older onset vs. younger) due to small sample size of elderly patients. We did not assess histologic remission due to unclear role in CD. We did not collect data on stool or serum biomarkers and immunogenicity. Finally, the use of physician global assessment as the primary metric for response and remission has many shortcomings but has been previously utilized for such real-world analyses and retrospective studies with corroboration by objective data when available.16, 18 Nevertheless, this is the first study to report safety and effectiveness of UST in elderly CD patients.
In conclusion, UST is safe in elderly and achieved clinical response and mucosal healing comparable to nonelderly patients. This data is reassuring given the increasing anti-TNF exposed elderly CD population. Future studies to validate current findings, evaluate outcomes in UC, and comparative studies against other agents are needed.
Disclosures:
RG, MA, RB, JPA, BL and TQ have nothing to disclose.
Jessica Philpott- Abbvie speaker bureau
Benjamin L. Cohen has served as a speaker, a consultant, or an advisory board member for AbbVie, Alfasigma, Allergan, Celltrion, Ferring, Grifols, Janssen, and Sublimity Therapeutics. Andres Yarur has received consulting fees from Takeda Pharmaceuticals and Prometheus Laboratories; and has served on the Speakers Bureau for AbbVie, Takeda Pharmaceuticals, and Prometheus Laboratories
Florian Rieder- Consulting or Advisory Board: Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB. Funding: NIH, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, Pfizer, Boehringer Ingelheim, Morphic, Kenneth Rainin Foundation.
Miguel Regueiro- Research Support from Abbvie, Janssen, Takeda, Pfizer. Unrestricted Educational Grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead. Advisory Boards and Consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A. CME Companies: CME Outfitters, Imedex, GI Health Foundation (GiHF), Cornerstones, Remedy, MJH life sciences. Royalties: Wolters Kluwer Health as Author/Editor of UpToDate.
Benjamin Click - Consulting and speakers bureau - Takeda; consulting - TARGET-RWE, consulting - MedEd
REFERENCES
- 1.Jeuring SF, van den Heuvel TR, Zeegers MP, et al. Epidemiology and Long-term Outcome of Inflammatory Bowel Disease Diagnosed at Elderly Age-An Increasing Distinct Entity? Inflamm Bowel Dis 2016;22:1425–34. [DOI] [PubMed] [Google Scholar]
- 2.Everhov AH, Halfvarson J, Myrelid P, et al. Incidence and Treatment of Patients Diagnosed With Inflammatory Bowel Diseases at 60 Years or Older in Sweden. Gastroenterology 2018;154:518–528 e15. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20:1761–9. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc JF, Wiseman D, Lakatos PL, et al. Elderly patients with inflammatory bowel disease: Updated review of the therapeutic landscape. World J Gastroenterol 2019;25:4158–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 6.Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci 2012;57:2408–15. [DOI] [PubMed] [Google Scholar]
- 7.Sturm A, Maaser C, Mendall M, et al. European Crohn’s and Colitis Organisation Topical Review on IBD in the Elderly. J Crohns Colitis 2017;11:263–273. [DOI] [PubMed] [Google Scholar]
- 8.Deepak P, Sandborn WJ. Ustekinumab and Anti-Interleukin-23 Agents in Crohn’s Disease. Gastroenterol Clin North Am 2017;46:603–626. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008;135:1130–41. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367:1519–28. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J Crohns Colitis 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 12.Panaccione R, Danese S, Sandborn WJ, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther 2020;52:1658–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis 2020;14:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iborra M, Beltran B, Fernandez-Clotet A, et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther 2019;50:278–288. [DOI] [PubMed] [Google Scholar]
- 15.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 16.Dulai PS, Singh S, Jiang X, et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol 2016;111:1147–55. [DOI] [PubMed] [Google Scholar]
- 17.Bohm M, Xu R, Zhang Y, et al. Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn’s disease. Aliment Pharmacol Ther 2020;52:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narula N, Peerani F, Meserve J, et al. Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium. Am J Gastroenterol 2018;113:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel T, Yung DE, Ma C, et al. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis 2019;51:1232–1240. [DOI] [PubMed] [Google Scholar]
- 20.Boring MA, Hootman JM, Liu Y, et al. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation by Urban-Rural County Classification - United States, 2015. MMWR. Morbidity and mortality weekly report 2017;66:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Stefano M, Veneto G, Malservisi S, et al. Lactose malabsorption and intolerance in the elderly. Scand J Gastroenterol 2001;36:1274–8. [DOI] [PubMed] [Google Scholar]
- 22.Geisz M, Ha C, Kappelman MD, et al. Medication Utilization and the Impact of Continued Corticosteroid Use on Patient-reported Outcomes in Older Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2016;22:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ollech JE, Normatov I, Peleg N, et al. Effectiveness of Ustekinumab Dose Escalation in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedano R, Guizzetti L, McDonald C, et al. Intravenous Ustekinumab Reinduction Is Effective in Prior Biologic Failure Crohn’s Disease Patients Already on Every-4-Week Dosing. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 25.Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020;52:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faleck DM, Winters A, Chablaney S, et al. Shorter Disease Duration Is Associated With Higher Rates of Response to Vedolizumab in Patients With Crohn’s Disease But Not Ulcerative Colitis. Clin Gastroenterol Hepatol 2019;17:2497–2505.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010;105:1574–82. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis 2013;7:213–21. [DOI] [PubMed] [Google Scholar]
- 29.Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther 2018;18:897–903. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Umezawa Y, Fukuchi O, et al. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J Dermatol 2014;41:974–80. [DOI] [PubMed] [Google Scholar]
- 31.Borren NZ, Ananthakrishnan AN. Safety of Biologic Therapy in Older Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019;17:1736–1743 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Panés J, D’Haens GR, et al. Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin Gastroenterol Hepatol 2019;17:1541–1550. [DOI] [PubMed] [Google Scholar]
- 33.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor alpha antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther 2019;49:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yajnik V, Khan N, Dubinsky M, et al. Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn’s Disease Patients Stratified by Age. Adv Ther 2017;34:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolston VS, Kimmel J, Popov V, et al. Ustekinumab Does Not Increase Risk of Adverse Events: A Meta-Analysis of Randomized Controlled Trials. Dig Dis Sci 2020. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Feagan BG, Danese S, et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm Bowel Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


