Key Points
Question
Is nonalcoholic fatty liver disease (NAFLD) associated with severe hypoglycemia in individuals with type 2 diabetes?
Findings
In cohort study of more than 1.9 million individuals, participants with type 2 diabetes and NAFLD without cirrhosis had an approximately 26% increased risk of severe hypoglycemia after adjustment for multiple clinical covariates.
Meaning
Knowledge of the association of NAFLD with severe hypoglycemia in adults with type 2 diabetes, independent of obesity status, could help to inform management.
This cohort study explores the association of nonalcoholic fatty liver disease (NAFLD) with severe hypoglycemia among patients with type 2 diabetes.
Abstract
Importance
Previous studies have indicated that liver cirrhosis is associated with hypoglycemia, but there have been no studies investigating the association between nonalcoholic fatty liver disease (NAFLD) and hypoglycemia in noncirrhotic populations with type 2 diabetes.
Objective
To explore the association of NAFLD with severe hypoglycemia among patients with type 2 diabetes.
Design, Setting, and Participants
This nationwide population-based retrospective cohort study using the National Health Insurance System of South Korea included individuals aged 20 years or older who had undergone a medical health examination between January 1, 2009, and December 31, 2012, and were diagnosed with type 2 diabetes. Participants were followed up until December 31, 2015. Data analyses were performed between January 1, 2019, and February 2, 2021.
Exposures
The baseline fatty liver index (FLI) was used as a surrogate marker for NAFLD.
Main Outcomes and Measures
The outcome of interest, severe hypoglycemia, was measured using hospital admission and emergency department visit records with a primary diagnosis of hypoglycemia.
Results
Among 1 946 581 individuals with type 2 diabetes, 1 125 187 (57.8%) were male. During a median (IQR) follow-up of 5.2 (4.1-6.1) years, 45 135 (2.3%) experienced 1 or more severe hypoglycemia events. Participants with severe hypoglycemia, vs those without severe hypoglycemia, were older (mean [SD] age, 67.9 [9.9] years vs 57.2 [12.3] years; P < .001) and had lower mean (SD) body mass index (24.2 [3.43] vs 25.1 [3.4]; P < .001). Patients with NAFLD tended to have less severe hypoglycemia without consideration of obesity status. However, after adjustment of multiple clinical covariates, including body mass index, there was a J-shaped association between FLI and severe hypoglycemia (5th decile: adjusted hazard ratio [aHR], 0.86; 95% CI, 0.83-0.90; 9th decile: aHR, 1.02; 95% CI, 0.96-1.08; 10th decile: aHR, 1.29; 95% CI, 1.22-1.37), and the estimated risk of hypoglycemia was higher in participants with NAFLD (aHR, 1.26; 95% CI, 1.22-1.30). The association was more prominent in female participants (aHR, 1.29; 95% CI, 1.23-1.36) and those with underweight (aHR, 1.71; 95% CI, 1.02-2.88).
Conclusions and Relevance
In this study, NAFLD was associated with a higher risk of severe hypoglycemia in patients with type 2 diabetes independent of obesity status. Presence of NAFLD should be considered when evaluating vulnerability to hypoglycemia in patients with type 2 diabetes.
Introduction
Hypoglycemia is the most commonly reported adverse effect in the management of diabetes.1 Severe hypoglycemia, defined as any hypoglycemia event requiring external assistance for recovery,2 is often accompanied by emergency department (ED) visit or hospitalization.3 In previous meta-analyses, the pooled prevalence of hypoglycemia is approximately 45% for minor events and 6% for severe events in patients with type 2 diabetes.4 Severe hypoglycemia is associated with falls and driving accidents,5 dementia,6 cardiovascular events, and mortality.7 A hypoglycemia experience also increases patients’ fear and distress and lowers psychological health quality.8 Considerable costs are associated with its management, ranging from $12 to $1850 per episode.9,10 Therefore, the ability to identify individuals at high risk of hypoglycemia is sorely needed.
Older age, kidney insufficiency, and insulin therapy are well known risk factors for hypoglycemia in patients with type 2 diabetes, and Karter et al3 recently developed a hypoglycemia risk stratification tool using 6 inputs (age, chronic kidney disease [CKD], insulin use, sulfonylurea use, prior hypoglycemia-related utilization, and prior year ED visit).3 Regarding the association between obesity and hypoglycemia, the Action to Control Cardiovascular Risk in Diabetes Study reported that body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]) of 30 or greater was associated with a lower risk of severe hypoglycemia compared with BMI less than 25 (hazard ratio [HR], 0.65; 95% CI, 0.50-0.85).11 Additionally, a recent study observed an inverse, J-shaped association between BMI and the development of severe hypoglycemia.12
Nonalcoholic fatty liver disease (NAFLD) is a major metabolic liver disease worldwide, and its prevalence, estimated as 25%,13 is expected to increase rapidly due to increased prevalence of obesity and aging populations. Because of its association with obesity and insulin resistance, the overall prevalence of NAFLD in patients with type 2 diabetes is reported to be 55.5%.14,15 Moreover, NAFLD is an emerging risk factor for various complications, including metabolic syndrome, cardiovascular and kidney diseases, cancers, and overall mortality.16,17,18,19,20 However, its association with the development of severe hypoglycemia in patients with type 2 diabetes remains unclear. Therefore, we investigated the association between NAFLD and severe hypoglycemia in patients with type 2 diabetes using a nationwide population-based cohort study.
Methods
Data Source
The National Health Insurance Service (NHIS) of South Korea consists of 2 main programs: National Health Insurance for employees and self-employed individuals (97% of the population) and Medical Aid for individuals with low income (the remaining 3% of the population). As a mandatory single insurance organization, the NHIS covers the entire South Korean population of more than 50 million individuals.21,22 NHIS records contain demographic characteristics (eg, age, sex, income, residential area), a claims database (consultations, diagnoses by the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10], and prescriptions), and health check-up data, including anthropometric, laboratory, and questionnaire data (medical history and health behaviors, such as smoking, alcohol drinking, and physical activity).22,23 NHIS enrollees are recommended to undergo a general health examination biennially; the participation rate was 74.8% in 2014.23
This study was approved by the institutional review board of the Severance Hospital, Yonsei University College of Medicine, and informed consent from study participants was waived due to the retrospective nature of the study. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Population
We evaluated the records of participants aged 20 years or older who had undergone the medical health examination between January 1, 2009, and December 31, 2012. Among them, participants with prevalent type 2 diabetes at baseline and those who developed type 2 diabetes during follow-up were selected. Type 2 diabetes diagnosis was based on either prescription of antidiabetic agents with ICD-10 diagnosis (E11-E14) in the claims database or fasting plasma glucose level of at least 126 mg/dL on health examination data (to convert glucose to millimoles per liter, multiply by 0.0555). Exclusion criteria were as follows: heavy alcohol consumption (>210 g/week of alcohol consumption for men and >140 g/week for women); hepatitis B or C carrier; a diagnosis of liver cirrhosis (ICD-10 code K74),24 acute or chronic pancreatitis (ICD-10 codes K85, K86.0, and K86.1), and other diseases of the pancreas (ICD-10 codes K86.2, K86.3, K86.8, and K86.9); prior diagnosis of pancreas, liver, or biliary tract cancers (ICD-10 codes C25 and C22) to exclude hypoglycemia by liver and pancreatic disease other than NAFLD; and participants with missing data. A total of 1 946 581 participants with type 2 diabetes were enrolled and followed up until December 31, 2015, for a median (IQR) of 5.2 (4.1-6.1) years (eFigure 1 in the Supplement).
Clinical and Laboratory Measurements
Demographic and anthropometric data including age, sex, BMI, and waist circumference (WC) were abstracted. Smoking habits were classified as noncurrent or current; alcohol drinking was identified as less than 30 g/day or 30 g/day or greater (alcohol drinker); exercise habit was categorized as less than 3 times/week or 3 times/week or more of vigorous exercise for at least 20 minutes (physically active). Low socioeconomic status was defined as participants with the lowest 20% income status. Blood samples, collected after overnight fasting, included serum glucose, total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, liver panel, and γ-glutamyl transferase (GGT) levels as well as kidney function (creatinine level and estimated glomerular filtration rate [eGFR]).
Hypertension was defined as ICD-10 codes I10-I13 and I15, with antihypertensive drug treatment or systolic/diastolic blood pressure (BP) of 140/90 mm Hg or greater. Hyperlipidemia was identified as ICD-10 code E78, with lipid-lowering agent prescription or serum total cholesterol level of at least 240 mg/dL (to convert total cholesterol to millimoles per liter, multiply by 0.0259). CKD was considered an eGFR of less than 60 mL/min/1.73 m2 using the 4-variable modification of diet in kidney disease formula.25 Cardiovascular disease (CVD) was considered as previous heart disease and/or ischemic stroke, which was defined by a combination of ICD-10 codes (I21-I22, I50 and I63-I64, G458-G459, respectively) and medical histories. Use of antidiabetic drugs (insulin, metformin, sulfonylurea, glinide, thiazolidinedione, and dipeptidyl-dipeptidase 4 [DPP4] inhibitor) was obtained through claims data.
Definition of NAFLD and Severe Hypoglycemia
The fatty liver index (FLI) was used as a surrogate marker for NAFLD. FLI was calculated using the following formula26:
| FLI = ex / (1 + ex) × 100, where |
| x = 0.953 × ln(TG) + 0.139 × BMI + 0.718 × ln(GGT) + 0.053 × WC – 15.745, |
with triglycerides (TG) measured in millimoles per liter, GGT in U/L, and WC in cm. The index value ranged from 0 to 100. According to a previous study, an FLI score of less than 30 can be used to estimate the absence of fatty liver (sensitivity 87%) and FLI of at least 60 to estimate the presence of fatty liver (specificity 86%).26 In this study, participants were classified into 3 groups according to FLI: low FLI (<30); intermediate FLI (30-59); and high FLI (≥60).
The outcome of interest was measured using hospital admission and ED visit records with a primary diagnosis of hypoglycemia (ICD-10 codes E16.0, E16.1, E16.2, E11.63, E13.63, and E14.63). Previous epidemiologic studies have used hospital admission records to identify severe hypoglycemia events using NHIS data.12,27
Statistical Analysis
Baseline characteristics are presented as either mean (SD) or number (proportion). The HR and 95% CI of severe hypoglycemia events according to FLI was estimated using Cox proportional hazard regression model. Model 1 was unadjusted; model 2 was adjusted for potential confounding factors, such as age, sex, smoking and alcohol habits, exercise, and BMI; model 3 was further adjusted for severe hypoglycemia within the previous 3 years; insulin, sulfonylurea, and/or glinide use; and history of hypertension, CKD, and CVD, which were shown to be associated with the development of severe hypoglycemia (eTable 1 in the Supplement). For example, insulin use was associated with 3.07-fold increased risk of severe hypoglycemia (95% CI, 3.00-3.14). We further analyzed using the Fine-Gray subdistribution hazard regression to account for death as competing risk for severe hypoglycemia.
The number (multiplied by 100 times) of severe hypoglycemia per person during the follow-up period according to FLI was estimated using a generalized linear model adjusted for confounders (as in model 3) and follow-up duration and was reported as the least-square mean (SE). We also examined the association between FLI and severe hypoglycemia events according to age subgroups (<60 or ≥60 years), sex (male or female), BMI (<18.5, 18.5-22.9, 23-24.9, and ≥25), CKD status (yes or no), CVD status (yes or no), insulin use (yes or no), and sulfonylurea and/or glinide use (yes or no). All statistical analyses were conducted using SAS version 9.2 (SAS Institute) and R version 4.1.0 (R Project for Statistical Computing). Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
Baseline Characteristics of Study Population
Among 1 946 581 individuals with type 2 diabetes, 1 125 187 (57.8%) were men. A total of 45 135 participants (2.3%) experienced at least 1 episode of severe hypoglycemia during a median (IQR) follow-up period of 5.2 (4.1-6.1) years. Participants with severe hypoglycemia were older (mean [SD] age, 57.2 [12.3] years vs 67.9 [9.9] years; P < .001) and had lower mean (SD) BMI (25.1 [3.4] vs 24.2 [3.43]; P < .001) than participants without severe hypoglycemia. They had a higher rate of comorbidities, such as hypertension, CKD, and CVD, and the proportion of those using insulin, sulfonylurea, and glinides were higher compared with participants without severe hypoglycemia. Baseline characteristics of the study population according to incidence of severe hypoglycemia are summarized in Table 1.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Participants, No. (%) | P value | |
|---|---|---|---|
| No hypoglycemia (n = 1 901 446) | Severe hypoglycemia (n = 45 135) | ||
| Demographic parameters | |||
| Age, mean (SD), y | 57.2 (12.3) | 67.9 (9.9) | <.001 |
| Men | 1 104 968 (58.1) | 20 219 (44.8) | <.001 |
| Women | 796 478 (41.9) | 24 916 (55.2) | |
| Height, mean (SD), cm | 162.1 (9.2) | 157.5 (9.1) | <.001 |
| Weight, mean (SD), kg | 66.2 (11.7) | 60.1 (10.4) | <.001 |
| BMI, mean (SD) | 25.1 (3.4) | 24.2 (3.5) | <.001 |
| Waist circumference, mean (SD), cm | 85.2 (8.6) | 84.8 (8.9) | <.001 |
| BP, mean (SD), mmHg | |||
| Systolic | 128.9 (15.7) | 130.9 (17.3) | <.001 |
| Diastolic | 79.0 (10.1) | 77.7 (10.5) | <.001 |
| Current smoking | 465 293 (24.5) | 7236 (16.0) | <.001 |
| Current alcohol use | 758 761 (39.9) | 9543 (21.1) | <.001 |
| Physically active | 916 430 (48.2) | 15 406 (34.1) | <.001 |
| Low socioeconomic status | 515 189 (27.1) | 12 841 (28.5) | <.001 |
| Laboratory parameters, mean (SD) | |||
| Fasting glucose, mg/dL | 143.5 (43.0) | 141.0 (56.7) | <.001 |
| Total cholesterol, mg/dL | 197.8 (41.9) | 189.8 (43.8) | <.001 |
| Triglycerides, mg/dL | 174.5 (117.5) | 163.0 (104.1) | <.001 |
| HDL-C, mg/dL | 51.3 (16.6) | 50.4 (20.0) | <.001 |
| LDL-C, mg/dL | 113.0 (37.6) | 107.8 (38.3) | <.001 |
| AST, IU/L | 27.9 (17.3) | 26.1 (17.5) | <.001 |
| ALT, IU/L | 30.5 (23.2) | 24.5 (18.2) | <.001 |
| GGT, IU/L | 48.5 (64.0) | 42.2 (72.7) | <.001 |
| Creatinine, mg/dL | 1.0 (0.7) | 1.1 (0.8) | <.001 |
| eGFR, mL/min/1.73 m2 | 85.1 (35.3) | 73.1 (35.8) | <.001 |
| Comorbidities | |||
| Hypertension | 1 054 443 (55.5) | 34 507 (76.5) | <.001 |
| Dyslipidemia | 798 915 (42.0) | 21 626 (47.9) | <.001 |
| Chronic kidney disease | 204 376 (10.8) | 15 359 (34.0) | <.001 |
| Cardiovascular disease | 89 642 (6.1) | 4395 (10.9) | <.001 |
| Antidiabetic drugs | |||
| Insulin | 132 442 (7.0) | 12 683 (28.1) | <.001 |
| Metformin | 850 459 (44.7) | 29 900 (66.3) | <.001 |
| Sulfonylurea | 764 336 (40.2) | 33 923 (75.2) | <.001 |
| Glinides | 38 432 (2.0) | 2742 (6.1) | <.001 |
| Thiazolidinedione | 116 254 (6.1) | 4809 (10.7) | <.001 |
| DPP4 inhibitor | 172 405 (9.1) | 4177 (9.3) | .17 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DPP4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: To convert ALT, AST, and GGT to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; HDL-C, LDL-C, and total cholesterol to millimoles per liter, multiply by 0.0259; and triglycerides to millimoles per liter, multiply by 0.0113.
Risk of Severe Hypoglycemia According to FLI
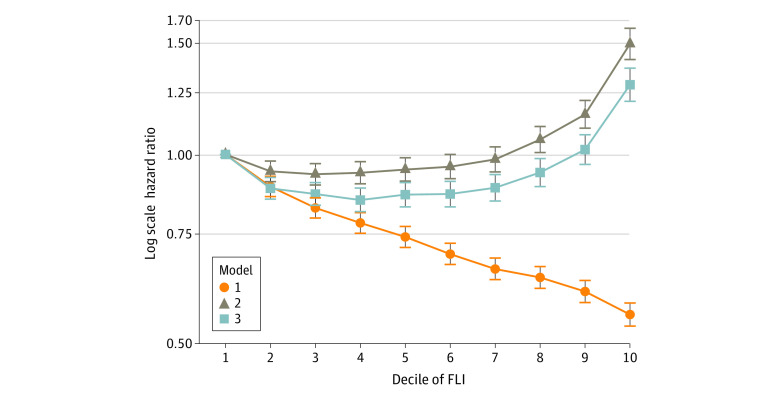
To assess the association between NAFLD and severe hypoglycemia, HRs for severe hypoglycemia were determined according to FLI deciles (Figure 1). In the unadjusted model (model 1), the risk of severe hypoglycemia gradually decreased with increasing FLI. However, after adjustment for age, sex, smoking and alcohol habits, exercise, and BMI in model 2, the pattern reversed, and there was a J-shaped association between FLI and severe hypoglycemia. This remained after further adjustment for other variables, including severe hypoglycemia within the previous 3 years; insulin, sulfonylurea, and glinide use; and history of hypertension, CKD, and CVD (model 3). The risk of severe hypoglycemia gradually increased from the 5th decile (aHR, 0.86; 95% CI, 0.83-0.90), then sharply increased from the 9th decile (aHR, 1.02; 95% CI, 0.96-1.08) to the 10th decile (aHR, 1.29; 95% CI, 1.22-1.37). The cutoff value of the ninth decile was 83.5 in male individuals and 70.4 in female individuals (eTable 2 in the Supplement).
Figure 1. Hazard Ratios for Severe Hypoglycemia According to Fatty Liver Index (FLI) Deciles.
Model 1 was unadjusted. Model 2 was adjusted for age, sex, smoking and alcohol habits, exercise, and body mass index. Model 3 was further adjusted for severe hypoglycemia within previous 3 years; insulin, sulfonylurea, or glinides use; and history of hypertension, chronic kidney disease, and cardiovascular disease. Error bars indicate 95% CIs.
Although the duration of diabetes may be associated with severe hypoglycemia,28,29 the duration of diabetes was not available in this data set, so further analysis was performed only in patients with newly diagnosed type 2 diabetes. Participants with FLI of 60 or greater showed an 88% increased risk of severe hypoglycemia compared with those with FLI of less than 30 (95% CI, 1.67-2.11) (eTable 3 in the Supplement), suggesting the strong association between NAFLD and severe hypoglycemia in newly diagnosed type 2 diabetes.
Next, participants were classified into 3 groups according to FLI: absence of NAFLD (<30), intermediate FLI (30-59), or presence of NAFLD (≥60). The crude incidence rates of severe hypoglycemic events were significantly lower in participants with FLI 30 to 59 and FLI of 60 or greater compared with those with FLI<30 (Table 2). However, in the fully adjusted model, participants with FLI 30 to 59 showed a similar incidence of severe hypoglycemia compared with those with FLI of less than 30 (adjusted HR [aHR], 0.99; 95% CI, 0.97-1.02), whereas participants with FLI of 60 or greater showed a 26% increased risk of severe hypoglycemia compared with those with FLI of less than 30 (aHR, 1.26; 95% CI, 1.22-1.30). The association between higher FLI and severe hypoglycemia was more prominent in women than men (women: aHR, 1.29; 95% CI, 1.23-1.36; men: aHR, 1.17; 95% CI, 1.12-1.23). The Fine-Gray subdistribution HR for severe hypoglycemia for participants with FLI of 60 or greater compared with those with FLI of less than 30 was 1.16 (95% CI, 1.12-1.20) (eTable 4 in the Supplement).
Table 2. Association Between Fatty Liver Index and Incident Severe Hypoglycemia Events.
| Fatty liver index score | Participants, No. | Incident rate per 1000 person-years | HR (95% CI)a | |||
|---|---|---|---|---|---|---|
| Incident cases | Person-years | Model 1 | Model 2 | Model 3 | ||
| Overall | ||||||
| <30 | 22 213 | 3 880 165 | 5.7 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30-59 | 14 632 | 3 310 049 | 4.4 | 0.77 (0.76-0.79) | 1.03 (1.01-1.06) | 0.99 (0.97-1.02) |
| ≥60 | 8290 | 2 572 210 | 3.2 | 0.56 (0.55-0.58) | 1.25 (1.21-1.29) | 1.26 (1.22-1.30) |
| Male participants | ||||||
| <30 | 9424 | 1 775 198 | 5.3 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30-59 | 6613 | 1 958 450 | 3.4 | 0.64 (0.62-0.66) | 0.97 (0.94-1.01) | 0.97 (0.94-1.01) |
| ≥60 | 4182 | 1 870 224 | 2.2 | 0.42 (0.41-0.44) | 1.14 (1.08-1.19) | 1.17 (1.12-1.23) |
| Female participants | ||||||
| <30 | 12 789 | 2 104 967 | 6.1 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30-59 | 8019 | 1 351 598 | 5.9 | 0.98 (0.95-1.00) | 1.10 (1.06-1.13) | 1.02 (0.99-1.06) |
| ≥60 | 4108 | 701 985 | 5.9 | 0.96 (0.93-1.00) | 1.47 (1.40-1.54) | 1.29 (1.23-1.36) |
Abbreviation: HR, hazard ratio.
Model 1 was unadjusted. Model 2 was adjusted for age, sex, smoking and alcohol habits, exercise, and body mass index. Model 3 was further adjusted for severe hypoglycemia within previous 3 years; insulin, sulfonylurea, or glinides use; and history of hypertension, chronic kidney disease, and cardiovascular disease. Values with statistical significance are those for which the 95% CI does not cross 1.
Additionally, the number of severe hypoglycemia episodes according to FLI was examined. Among 1 946 581 participants, 32 652 (1.7%) had 1, 7613 (0.4%) had 2, and 4870 (0.3%) had 3 or more events of severe hypoglycemia. The number (multiplied by 100 times) of severe hypoglycemia per person during follow-up in the group with FLI of 60 or greater was 4.16, significantly higher than 3.57 in the group with FLI of less than 30 and 3.52 in the group with FLI of 30 to 59 group (P < .001) (eFigure 2 in the Supplement).
We further examined risk of severe hypoglycemia according to FLI components (eTable 5 in the Supplement). The highest quartiles of GGT and WC were significantly associated with increased risk (GGT: aHR, 1.05; 95% CI, 1.02-1.08; WC: aHR, 1.10; 95% CI, 1.05-1.14). A significantly increased risk of hypoglycemic events was observed in participants with underweight (BMI <18.5) compared with those in the reference range (BMI 18.5-22.9), whereas lower risk was observed in participants with overweight and obesity. There was no significant association between hypertriglyceridemia and risk of severe hypoglycemia.
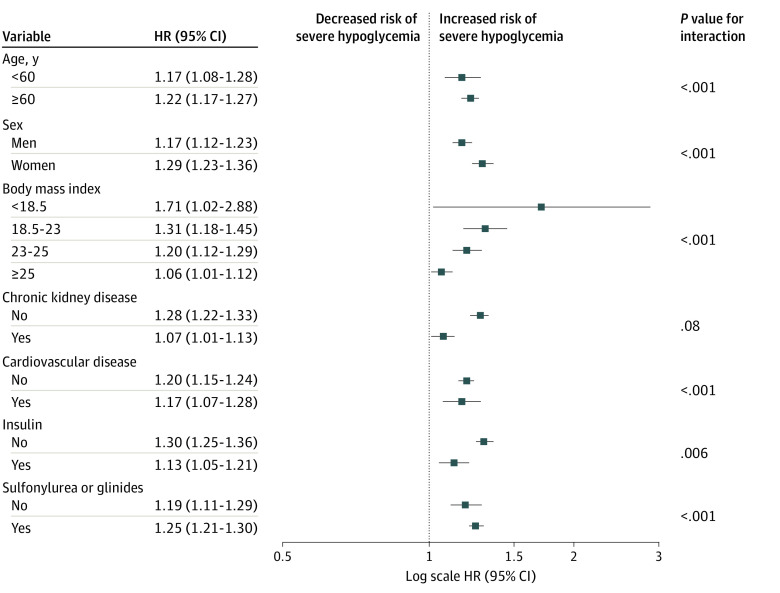
Differential Associations of NAFLD With the Risk of Severe Hypoglycemia in Various Subgroups
The association of NAFLD with severe hypoglycemia was assessed in detailed subgroups by calculating the relative HR in the group with FLI of 60 or greater compared with the group with FLI of less than 30 in a fully adjusted model (Figure 2; eTable 6 in the Supplement). There were significant subgroup differences according to age, sex, BMI, and the use of insulin, sulfonylurea, glinide (eg, participants <60 years: aHR, 1.17; 95% CI, 1.08-1.28; participants ≥60 years: aHR, 1.22; 95% CI, 1.17-1.27; P for interaction < .001). The associations between NAFLD and severe hypoglycemia were stronger among participants aged 60 years and older, women, and those who used sulfonylurea or glinide. The associations were most prominent in the lowest BMI (ie, underweight) group (aHR, 1.71; 95% CI, 1.02-2.88). In addition, t he association of degree of NAFLD with the incidence of severe hypoglycemia was lower in those who used insulin compared with those who did not. There was no significant interaction between the subgroup of CKD.
Figure 2. Adjusted Hazard Ratios (HRs) for Severe Hypoglycemia in the Group With Fatty Liver Indices of 60 or Greater vs Those with Fatty Liver Indices of Less Than 0, by Subgroup.
Cox proportional hazard regression models were used to estimate HRs and 95% CIs. Models were adjusted for age; sex; smoking and alcohol habits; exercise; body mass index (calculated as weight in kilograms divided by height in meters squared); severe hypoglycemia within previous 3 years; insulin, sulfonylurea, or glinides use; and history of hypertension, chronic kidney disease, and cardiovascular disease. Error bars indicate 95% CIs.
In addition, we performed further analysis using the aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio (cutoff value, 0.8)30 as a surrogate marker for liver fibrosis. Participants were divided into 6 groups according to AST/ALT ratio and FLI, and Cox regression analysis for severe hypoglycemia was performed with the reference group set as those with an AST/ALT ratio of less than 0.8 and an FLI of less than 30. Participants with an AST/ALT ratio of 0.8 or greater had increased risk of severe hypoglycemia compared with the reference group. There was a significant association between NAFLD and severe hypoglycemia risk in groups with fibrosis (eTable 7 in the Supplement). In the fully adjusted model, participants with FLI of 60 or greater and an AST/ALT ratio of 0.8 or greater showed a 38% increased risk of severe hypoglycemia compared with those with an FLI of less than 30 and an AST/ALT ratio of less than 0.8 (aHR, 1.38; 95% CI, 1.31-1.45). This suggests a higher risk of developing severe hypoglycemia in patients with advanced NAFLD.
Discussion
In this large, population-based longitudinal study, we found that participants with type 2 diabetes and NAFLD had an approximately 26% increased risk of severe hypoglycemia after adjustment for multiple clinical covariates. As lower BMI is known as an independent risk factor for severe hypoglycemia,11,12,31 patients with NAFLD seemed to have less risk of hypoglycemia without consideration of BMI. However, after adjusting for BMI, the risk of severe hypoglycemia was significantly increased among participants with NAFLD in a dose-dependent manner. The association of NAFLD with severe hypoglycemia was more prominent in women and in individuals with underweight. These results suggest that clinicians should be aware of the potential for patients with type 2 diabetes and NAFLD to develop severe hypoglycemia.
The liver plays an essential role in glucose production through glycogenolysis and gluconeogenesis, which also serve as defense mechanisms if plasma glucose levels fall below the physiologic range.32 In the presence of chronic liver disease, such as liver cirrhosis, glucose metabolism can be dysregulated; previous studies have reported that approximately 30% of patients with liver cirrhosis may have diabetes33 and 12% to 16% of patients with both diabetes and cirrhosis had hypoglycemia.34,35 However, to our knowledge, there have been no studies investigating the association between NAFLD and hypoglycemia in populations with cirrhosis. We rigorously excluded individuals with liver cirrhosis, those with heavy alcohol use, and hepato-pancreatico-biliary cancers, all of which may affect the occurrence of hypoglycemia. In particular, although we did not diagnose NAFLD by histological or imaging evaluation, the present findings show that patients with an FLI of 60 or greater had significantly higher incidence of severe hypoglycemia than those with an FLI of 30 to 59, indicating a clear dose-dependent association between severity of hepatic steatosis and incidence of severe hypoglycemia in patients with type 2 diabetes.
In our subgroup analyses, participants with NAFLD and a low or even reference range BMI were at greater risk of severe hypoglycemia compared with participants with obesity and NAFLD. Although NAFLD typically occurs in individuals with obesity, a smaller but significant proportion of people develop NAFLD despite a reference range BMI (ie, 25), which is called nonobese NAFLD.36,37 The prevalence of nonobese NAFLD is reported as 10% to 20%,37 but histologic severity and clinical outcomes compared with patients with obesity and NAFLD are conflicting.36,37,38 In this study, we first demonstrated a significant association between nonobese NAFLD and severe hypoglycemia in participants with type 2 diabetes. As lower BMI can reflect malnutrition and coexisting chronic disease,39 individuals with a lower BMI might be susceptible to the development of hypoglycemia.
Previous studies have found that older age, prior episodes of hypoglycemia, use of insulin and sulfonylurea, and comorbidities, such as kidney impairment and CVD, are established risk factors for hypoglycemia.3,40 Our study results agree with previous results regarding traditional hypoglycemia risk factors (eTable 1 in the Supplement). Also, the present study found that the presence of NAFLD was associated with increased risk of severe hypoglycemia by 1.3-fold, a relatively modest association compared with established risk factors such as insulin or sulfonylurea use and CKD. However, considering the high prevalence of NAFLD in patients with type 2 diabetes, the contribution of NAFLD to hypoglycemia risk is not negligible.
Regarding sex differences in development of hypoglycemia, the previous results are inconsistent. Some studies have reported significantly greater prevalence in women by 1.5- to 1.8-fold,41,42 whereas others have not.31,40 Our subgroup analyses revealed that female participants with NAFLD were more vulnerable to the development of severe hypoglycemia than their male counterparts. The exact reason is unknown, but hepatic estrogen receptor might have a role in sex differences in hepatic metabolism.43 In contrast, those who use insulin were less affected by the presence of NAFLD. As insulin use itself was associated with risk of severe hypoglycemia (aHR, 3.07; 95% CI, 3.00-3.14) (eTable 1 in the Supplement), NAFLD appears to have less additive association.
Possible mechanisms explaining the association of NAFLD with severe hypoglycemia include altered glucose metabolism in NAFLD.44,45 Glucagon level is found to be increased in the presence of NAFLD,46 and hyperglucagonemia might induce downregulation of hepatic glucagon receptor or blunt the counter-regulatory response to hypoglycemic events in hepatic glucose production.47 Also, NAFLD may be associated with glycemic variability through increased oxidative stress,48,49 which is an important determinant of hypoglycemia.50
Limitations
This study has limitations. First, we used a previously validated FLI index to define NAFLD26 because liver biopsy or imaging was not available in our data set. In the previous study,51 FLI showed acceptable accuracy in estimating the presence of steatosis (any histological steatosis ≥5%) in patients with NAFLD. Second, for the outcome of severe hypoglycemia, we were not able to capture events that were asymptomatic or occurred outside the ED or hospital. Additionally, we defined severe hypoglycemia using ICD codes but anthropometric or laboratory measurements of diabetes parameters (diabetes duration, serum glucose, glycated hemoglobin) at the time of the event were not available. Third, comorbidities were identified using medical claims data from the NHIS, and coding errors are present in these data sets. Fourth, we were unable to consider the possibility that some patients may progress to more serious disease during the observation period52 owing to limited access to the database. Fifth, although, cirrhosis was excluded using the ICD-10 code, patients with undiagnosed cirrhosis were likely to have been included in the analysis, as NHIS records do not have histological or imaging data of liver. To avoid overestimation and improve diagnostic accuracy, we collected both ICD-10 codes and prescription or medical questionnaire data. Despite these limitations, this nationwide epidemiologic study found an association between NAFLD and severe hypoglycemia in patients with type 2 diabetes. We analyzed a large historical population cohort of more than 1.9 million patients, which strengthens statistical power and the reliability of results. Also, given that hypoglycemia is common in those with severe alcohol use disorder53 and patients with liver disease54 because of impairment in gluconeogenesis and glycolysis, we strictly excluded participants with alcohol- or viral-related liver disease and chronic hepato-pancreatic disease.
Conclusions
In this study, the presence of NAFLD was associated with a 26% increased risk of severe hypoglycemia among participants with type 2 diabetes participants, independent of obesity status. The association was stronger in women and in participants with underweight. This result provides clinicians with additional information about which patients might have a high risk of hypoglycemia to hopefully reduce its incidence and ultimately improve patient safety via individualized therapy. Further validation studies in other racial and ethnic populations and to evaluate causality and mechanisms regarding NAFLD and hypoglycemia risk are warranted.
eFigure 1. Study Flowchart With Data From the Database of National Health Insurance Service
eFigure 2. Number (Multiplied by 100 ) of Cases of Severe Hypoglycemia per Person During the Follow-up Period According To Fatty Liver Index
eTable 1. Association Between Traditional Risk Factors and Severe Hypoglycemia Events
eTable 2. Cutoff Values of Fatty Liver Index Deciles
eTable 3. Association Between Fatty Liver Index and Incident Severe Hypoglycemia Events in Patients With Newly Diagnosed Type 2 Diabetes
eTable 4. Fine-Gray Competing Risk Model for Estimating the Subdistribution Hazard Ratio
eTable 5. Risk of Severe Hypoglycemia by Fatty Liver Index Components
eTable 6. Adjusted Hazard Ratios for Severe Hypoglycemia in Groups With FLI 60 or Greater and Between 30 and 60 vs Group With FLI Less Than 30, in Detailed Subgroups
eTable 7. Association Between Fatty Liver Index, AST/ALT Ratio, and Incident Severe Hypoglycemia Events
References
- 1.Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116-1124. doi: 10.1001/jamainternmed.2014.1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395. doi: 10.2337/dc12-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karter AJ, Warton EM, Lipska KJ, et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med. 2017;177(10):1461-1470. doi: 10.1001/jamainternmed.2017.3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edridge CL, Dunkley AJ, Bodicoat DH, et al. Prevalence and incidence of hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PLoS One. 2015;10(6):e0126427. doi: 10.1371/journal.pone.0126427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Signorovitch JE, Macaulay D, Diener M, et al. Hypoglycaemia and accident risk in people with type 2 diabetes mellitus treated with non-insulin antidiabetes drugs. Diabetes Obes Metab. 2013;15(4):335-341. doi: 10.1111/dom.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565-1572. doi: 10.1001/jama.2009.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018;41(1):104-111. doi: 10.2337/dc17-1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolucci A, Pintaudi B, Rossi MC, et al. The social burden of hypoglycemia in the elderly. Acta Diabetol. 2015;52(4):677-685. doi: 10.1007/s00592-015-0717-0 [DOI] [PubMed] [Google Scholar]
- 9.Foos V, Varol N, Curtis BH, et al. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Econ. 2015;18(6):420-432. doi: 10.3111/13696998.2015.1006730 [DOI] [PubMed] [Google Scholar]
- 10.Kim G, Lee YH, Han MH, et al. economic burden of hypoglycemia in patients with type 2 diabetes mellitus from Korea. PLoS One. 2016;11(3):e0151282. doi: 10.1371/journal.pone.0151282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller ME, Bonds DE, Gerstein HC, et al. ; ACCORD Investigators . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun JS, Park YM, Han K, Cha SA, Ahn YB, Ko SH. Association between BMI and risk of severe hypoglycaemia in type 2 diabetes. Diabetes Metab. 2019;45(1):19-25. doi: 10.1016/j.diabet.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Choi YJ, Huh BW, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab. 2014;99(5):1879-1884. doi: 10.1210/jc.2013-4133 [DOI] [PubMed] [Google Scholar]
- 15.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793-801. doi: 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 16.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330-344. doi: 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 17.Cheng YL, Wang YJ, Lan KH, et al. Fatty liver index and lipid accumulation product can predict metabolic syndrome in subjects without fatty liver disease. Gastroenterol Res Pract. 2017;2017:9279836. doi: 10.1155/2017/9279836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145-152. doi: 10.1002/hep.24356 [DOI] [PubMed] [Google Scholar]
- 19.Han E, Lee YH. Non-alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes Metab J. 2017;41(6):430-437. doi: 10.4093/dmj.2017.41.6.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YH, Kim KJ, Yoo ME, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68(4):764-772. doi: 10.1016/j.jhep.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 21.Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014;38(5):395-403. doi: 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YH, Han K, Ko SH, Ko KS, Lee KU; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association . Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016;40(1):79-82. doi: 10.4093/dmj.2016.40.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheol Seong S, Kim YY, Khang YH, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799-800. doi: 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive predictive value of International Classification of Diseases, 10th Revision, codes for cirrhosis and its related complications. Clin Gastroenterol Hepatol. 2018;16(10):1677-1678. doi: 10.1016/j.cgh.2018.01.042 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 26.Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko SH, Park YM, Yun JS, et al. Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J Diabetes Complications. 2018;32(2):157-163. doi: 10.1016/j.jdiacomp.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med. 2014;174(2):251-258. doi: 10.1001/jamainternmed.2013.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda M, Doi K, Sugawara M, Naka Y, Mochizuki K. Survey of hypoglycemia in elderly people with type 2 diabetes mellitus in Japan. J Clin Med Res. 2015;7(12):967-978. doi: 10.14740/jocmr2340w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265-1269. doi: 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 31.Zoungas S, Patel A, Chalmers J, et al. ; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410-1418. doi: 10.1056/NEJMoa1003795 [DOI] [PubMed] [Google Scholar]
- 32.Sherwin RS. Role of the liver in glucose homeostasis. Diabetes Care. 1980;3(2):261-265. doi: 10.2337/diacare.3.2.261 [DOI] [PubMed] [Google Scholar]
- 33.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120(10):829-834. doi: 10.1016/j.amjmed.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 34.Gundling F, Seidl H, Strassen I, et al. Clinical manifestations and treatment options in patients with cirrhosis and diabetes mellitus. Digestion. 2013;87(2):75-84. doi: 10.1159/000343458 [DOI] [PubMed] [Google Scholar]
- 35.Pfortmueller CA, Wiemann C, Funk GC, et al. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care. 2014;29(2):316.e7-316.e12. doi: 10.1016/j.jcrc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54-64. doi: 10.1002/hep.28697 [DOI] [PubMed] [Google Scholar]
- 37.Wei JL, Leung JC, Loong TC, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015;110(9):1306-1314. doi: 10.1038/ajg.2015.235 [DOI] [PubMed] [Google Scholar]
- 38.Dela Cruz AC, Bugianesi E, George J, et al. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(5):S909. doi: 10.1016/S0016-5085(14)63307-2 [DOI] [Google Scholar]
- 39.Dixon JB, Lambert GW. The obesity paradox—a reality that requires explanation and clinical interpretation. Atherosclerosis. 2013;226(1):47-48. doi: 10.1016/j.atherosclerosis.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 40.Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2017;40(12):1661-1667. doi: 10.2337/dc17-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kautzky-Willer A, Kosi L, Lin J, Mihaljevic R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes Obes Metab. 2015;17(6):533-540. doi: 10.1111/dom.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misra-Hebert AD, Pantalone KM, Ji X, et al. Patient characteristics associated with severe hypoglycemia in a type 2 diabetes cohort in a large, integrated health care system from 2006 to 2015. Diabetes Care. 2018;41(6):1164-1171. doi: 10.2337/dc17-1834 [DOI] [PubMed] [Google Scholar]
- 43.Della Torre S, Mitro N, Meda C, et al. Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab. 2018;28(2):256-267.e5. doi: 10.1016/j.cmet.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14(6):804-810. doi: 10.1016/j.cmet.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charbonneau A, Unson CG, Lavoie JM. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol. 2007;579(Pt 1):255-267. doi: 10.1113/jphysiol.2006.121954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Junker AE, Gluud L, Holst JJ, Knop FK, Vilsbøll T. Diabetic and nondiabetic patients with nonalcoholic fatty liver disease have an impaired incretin effect and fasting hyperglucagonaemia. J Intern Med. 2016;279(5):485-493. doi: 10.1111/joim.12462 [DOI] [PubMed] [Google Scholar]
- 47.Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomark Med. 2016;10(11):1141-1151. doi: 10.2217/bmm-2016-0090 [DOI] [PubMed] [Google Scholar]
- 48.Hashiba M, Ono M, Hyogo H, et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PLoS One. 2013;8(11):e76161. doi: 10.1371/journal.pone.0076161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochi T, Kawaguchi T, Nakahara T, et al. Differences in characteristics of glucose intolerance between patients with NAFLD and chronic hepatitis C as determined by CGMS. Sci Rep. 2017;7(1):10146. doi: 10.1038/s41598-017-09256-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsch IB. Glycemic variability and diabetes complications: does it matter? of course it does! Diabetes Care. 2015;38(8):1610-1614. doi: 10.2337/dc14-2898 [DOI] [PubMed] [Google Scholar]
- 51.Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V; LIDO Study Group . Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209-1222. doi: 10.1111/apt.12963 [DOI] [PubMed] [Google Scholar]
- 52.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59(7):969-974. doi: 10.1136/gut.2009.205088 [DOI] [PubMed] [Google Scholar]
- 53.Krebs HA, Freedland RA, Hems R, Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969;112(1):117-124. doi: 10.1042/bj1120117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desimone ME, Weinstock RS. Non-diabetic hypoglycemia. In: De Groot LJ, Chrousos G, Dungan K, et al. , eds. Endotext. MDText.com; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Flowchart With Data From the Database of National Health Insurance Service
eFigure 2. Number (Multiplied by 100 ) of Cases of Severe Hypoglycemia per Person During the Follow-up Period According To Fatty Liver Index
eTable 1. Association Between Traditional Risk Factors and Severe Hypoglycemia Events
eTable 2. Cutoff Values of Fatty Liver Index Deciles
eTable 3. Association Between Fatty Liver Index and Incident Severe Hypoglycemia Events in Patients With Newly Diagnosed Type 2 Diabetes
eTable 4. Fine-Gray Competing Risk Model for Estimating the Subdistribution Hazard Ratio
eTable 5. Risk of Severe Hypoglycemia by Fatty Liver Index Components
eTable 6. Adjusted Hazard Ratios for Severe Hypoglycemia in Groups With FLI 60 or Greater and Between 30 and 60 vs Group With FLI Less Than 30, in Detailed Subgroups
eTable 7. Association Between Fatty Liver Index, AST/ALT Ratio, and Incident Severe Hypoglycemia Events