Abstract
Rationale
There is an association between body mass index (BMI) and mortality in chronic obstructive pulmonary disease (COPD), with underweight individuals having higher mortality risk. Mortality and exacerbation risks among individuals with higher BMI are unclear.
Objectives
To examine the relationship between BMI and adverse outcomes in COPD.
Methods
This post hoc analysis included data from TIOSPIR (Tiotropium Safety and Performance in Respimat) (N = 17,116) and tiotropium-treated patients in UPLIFT (Understanding Potential Long-term Impacts on Function with Tiotropium) (N = 2,986). BMI classes (underweight [BMI < 20 kg/m2], normal weight [BMI 20 to <25 kg/m2], overweight [BMI 25 to <30 kg/m2], obesity class I [BMI 30 to <35 kg/m2], obesity class II [BMI 35 to <40 kg/m2], and obesity class III [BMI ⩾ 40 kg/m2]) were examined for adjusted associations with mortality, exacerbation, and nonfatal cardiovascular event risk using over 50,000 patient-years of cumulative follow-up data. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox regression models.
Results
In TIOSPIR, obesity prevalence was 22%, overweight 32%, and underweight 12%. The proportion of females was highest in obesity classes II and III. Overweight and obese participants had better baseline lung function versus other BMI classes; underweight participants were more likely to be current smokers. Underweight participants had a significantly higher risk of death (HR, 1.88; 95% CI, 1.62–2.20; P < 0.0001) and severe exacerbations (HR, 1.31; 95% CI, 1.16–1.47; P < 0.0001) versus normal-weight participants; however, overweight and obese participants were at lower to no additional risk. Results from UPLIFT were similar to TIOSPIR.
Conclusions
These results suggest that there is a strong association between body weight, COPD events, and risk of death. A holistic management approach taking into account respiratory and cardiovascular risk factors and nutritional status is needed to improve the general well-being of patients with COPD.
Keywords: body mass index, COPD, exacerbation, mortality, obesity
The global prevalence of obesity has nearly tripled since 1975 (1) and has been a rising public health concern in many countries (1, 2). A concomitant rise in obesity has been observed in specific patient populations, including patients with chronic obstructive pulmonary disease (COPD) (3, 4). Although the prevalence of obesity in COPD likely varies by disease severity, some studies report that it is higher than in the general population, even after matching for age, sex, and other demographic factors (4).
Body mass index (BMI) is a measure commonly used to categorize a person’s weight. Variations in BMI may play a role in the pathophysiology of COPD and influence patient outcomes (2). For example, individuals with COPD and obesity experience higher rates of dyspnea, increased use of inhaled medication, poorer quality of life (QoL), and an increased prevalence of other respiratory diseases, including asthma (5–7). A meta-analysis of over 200 studies including healthy nonsmokers in four continents found a strong association between being overweight or obese and increased all-cause mortality across all regions (8). Being underweight is also associated with higher mortality risk relative to normal-weight patients (9). However, contradictory evidence suggests a “protective” effect associated with being overweight. A systematic review and meta-analysis of studies in the general population indicated an association between being overweight (BMI 25 to <30 kg/m2) and lower all-cause mortality relative to those of normal weight, whereas obesity (BMI ⩾ 30 kg/m2) was associated with higher all-cause mortality (10). This phenomenon, known as the “obesity paradox,” has also been demonstrated in some COPD studies, indicating that excess body mass may have a protective effect against mortality (11, 12). However, this protective effect may depend on COPD disease severity and be more prevalent in patients with severe airflow limitation (9).
The impact of obesity on mortality and exacerbation risk in COPD has not yet been assessed in a large, well-characterized cohort from multiple continents with longitudinal follow-up. Furthermore, there is a paucity of studies including very obese people. The TIOSPIR (Tiotropium Safety and Performance in Respimat) and the UPLIFT (Understanding Potential Long-term Impacts on Function with Tiotropium) trials are among the largest and longest COPD trials. TIOSPIR was specifically designed with adequate power to analyze all-cause mortality and time to first COPD exacerbation in a large patient population, with broad inclusion criteria that closely reflect the real-world population of patients with COPD (13). These results enabled further analysis of specific patient sub-populations (14, 15). This study aims to determine whether BMI is an important susceptibility factor for adverse outcomes in patients with COPD (who are receiving maintenance bronchodilator treatment), focusing on data from the large-scale TIOSPIR trial and supported by the UPLIFT trial.
Elements of the data have been previously presented at international conferences (16, 17).
Methods
Study Design and Population
The TIOSPIR trial (NCT01126437) was a large (N = 17,116), 2–3-year, randomized, double-blind, parallel-group, double-dummy, event-driven trial comparing the efficacy and safety of once-daily tiotropium Respimat 5 μg and 2.5 μg versus once-daily tiotropium HandiHaler 18 μg in moderate to very severe COPD. The UPLIFT trial (NCT00144339) was a 4-year, randomized, double-blind, placebo-controlled, parallel-group trial evaluating the effect of tiotropium HandiHaler 18 μg once-daily versus placebo on lung function, QoL, exacerbations, and mortality in patients (N = 5,993) with moderate to very severe COPD. Detailed methods have been published previously for TIOSPIR (13, 18) and UPLIFT (19). In both trials, participants were aged 40 years or more, had confirmed diagnosis of COPD (70% or less predicted postbronchodilator forced expiratory volume in 1 second [FEV1], and FEV1/forced vital capacity [FVC] ratio of 0.70 or less) and at least 10 pack-years of smoking history. Details of inclusion–exclusion criteria have been published (18, 19). There were no inclusion–exclusion criteria related to BMI in either trial.
Assessments
Participants from TIOSPIR and UPLIFT were categorized into six BMI classes at baseline: underweight (BMI < 20 kg/m2), normal weight (BMI 20 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), obesity class I (BMI 30 to <35 kg/m2), obesity class II (BMI 35 to <40 kg/m2), and obesity class III (BMI ⩾ 40 kg/m2) (20).
Adverse events, including exacerbations and deaths, were reviewed by independent data and safety monitoring committees; deaths were additionally adjudicated by a mortality adjudication committee blinded to study group assignments. COPD exacerbations were defined as moderate (i.e., treatment with antibiotics or systemic steroids without hospitalization) or severe (i.e., involving hospitalization or causing death). Exacerbations included in this analysis were recorded from treatment commencement to last dose of study drug in both trials. Mortality was recorded to the study end. Serious, nonfatal cardiovascular (CV) events were counted from treatment commencement to 30 days after the last dose of study drug. Pharmacovigilance endpoints used to define respiratory death, cardiac death, and nonfatal CV events are described in Tables E1 and E2 (see online supplement).
Data from the TIOSPIR trial were pooled, as primary results showed no differences in outcomes between the three tiotropium treatment arms in other post hoc analyses (13, 15). UPLIFT was used as a validating cohort for the TIOSPIR findings. In this analysis, only tiotropium-treated patients from UPLIFT were included, because tiotropium reduced mortality and exacerbations versus placebo in this trial.
Statistical Analysis
In this post hoc analysis, statistical significance and all presented P values are considered nominal. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for time to all-cause death, respiratory death, CV death, first serious nonfatal CV event, and first moderate and/or severe exacerbation using Cox regression models (however, the study was not designed and powered to assess the statistical significance of correlations between BMI class and adverse events). Kaplan–Meier analysis was used to show the risk of an event occurring over time. The main analysis adjusted for several covariates considered a priori confounders: age, sex, region, smoking status, smoking history (pack-years), Global Initiative for Chronic Obstructive Lung Disease stage, medication use (long-acting β2-agonist only, inhaled corticosteroid only, both inhaled corticosteroid and long-acting β2-agonist, or neither), and cardiac history. BMI was coded as a disjoint categorical variable with all classes included simultaneously. Participants with missing covariate data were excluded (TIOSPIR: 48 patients; UPLIFT: 9 patients). SAS was used for all analyses.
Results
Baseline Characteristics
Baseline characteristics for the 17,116 participants from TIOSPIR are presented in Table 1. Mean follow-up time for all-cause mortality was 838.2 days (TIOSPIR) and 1,359.5 days (UPLIFT). In TIOSPIR, 12.1% of participants were underweight, 33.4% normal weight, 32.2% overweight, and 22.3% obese (15.3% obesity class I; 4.9% obesity class II; and 2.1% obesity class III). Similar proportions were observed in UPLIFT (Table E3). In TIOSPIR, age did not vary greatly between the different groups, although obesity class III participants tended to be younger (mean 60.4 yr vs. 65.5 yr in the normal-weight group). The proportion of females and participants from North America increased with increasing BMI; there was a higher proportion of underweight and normal-weight participants from Asia (36.0% and 19.9% among underweight and normal weight, respectively, vs. 7.3% overweight, 2.6% obesity class I, 1.2% obesity class II, and 0% obesity class III) (Table 1). Number of pack-years increased with increasing BMI; however, underweight participants were more likely to be current smokers than overweight participants (43.7% of underweight participants were current smokers vs. 35.9% of overweight and 39.9% of normal-weight participants) (Table 1). Similar patterns in baseline characteristics were found in UPLIFT (Table E3).
Table 1.
Patient baseline characteristics by BMI class in TIOSPIR
| Underweight (BMI < 20)* n = 2,063 |
Normal Weight† (BMI 20 to <25)* n = 5,721 |
Overweight and Obese |
||||
|---|---|---|---|---|---|---|
| Overweight (BMI 25 to <30)* n = 5,509 |
Obesity Class I (BMI 30 to <35)* n = 2,624 |
Obesity Class II (BMI 35 to <40)* n = 841 |
Obesity Class III (BMI ⩾ 40)* n = 358 |
|||
| Gender, male, n (%) | 1,449 (70.2) | 4,169 (72.9) | 4,037 (73.3) | 1,851 (70.5) | 547 (65.0) | 184 (51.4) |
| Age, years, mean (SD) | 64.6 (9.3) | 65.5 (9.2) | 65.4 (9.0) | 64.6 (8.7) | 63.4 (8.6) | 60.4 (8.1) |
| Race, n (%) | ||||||
| White | 1,206 (58.5) | 4,324 (75.6) | 4,868 (88.4) | 2,432 (92.7) | 795 (94.5) | 338 (94.4) |
| Black | 44 (2.1) | 80 (1.4) | 58 (1.1) | 45 (1.7) | 15 (1.8) | 14 (3.9) |
| Asian | 756 (36.6) | 1,165 (20.4) | 421 (7.6) | 74 (2.8) | 11 (1.3) | 1 (0.3) |
| Region, n (%) | ||||||
| Europe/Africa/Aus/NZ | 821 (39.8) | 3,118 (54.5) | 3,452 (62.7) | 1,632 (62.2) | 472 (56.1) | 144 (40.2) |
| Latin America | 115 (5.6) | 379 (6.6) | 321 (5.8) | 144 (5.5) | 30 (3.6) | 11 (3.1) |
| North America | 385 (18.7) | 1,087 (19.0) | 1,336 (24.3) | 781 (29.8) | 329 (39.1) | 203 (56.7) |
| Asia | 742 (36.0) | 1,137 (19.9) | 400 (7.3) | 67 (2.6) | 10 (1.2) | 0 (0.0) |
| Current smokers, n (%) | 902 (43.7) | 2,281 (39.9) | 1,977 (35.9) | 912 (34.8) | 304 (36.1) | 143 (39.9) |
| Smoking history, pack-years, mean (SD) | 41.0 (21.9) | 42.8 (23.7) | 44.2 (25.0) | 45.3 (26.2) | 47.6 (28.0) | 49.4 (31.0) |
| Postbronchodilator pulmonary function data | ||||||
| FEV1, L, mean (SD) | 1.1 (0.4) | 1.3 (0.5) | 1.4 (0.5) | 1.5 (0.5) | 1.5 (0.5) | 1.4 (0.5) |
| FEV1/FVC ratio, mean (SD) | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| FVC, L, mean (SD) | 2.5 (0.8) | 2.7 (0.9) | 2.8 (0.9) | 2.7 (0.8) | 2.6 (0.8) | 2.5 (0.8) |
| FEV1% predicted, mean (SD) | 41.7 (14.8) | 47.3 (14.5) | 51.0 (13.9) | 52.2 (13.5) | 53.1 (13.8) | 53.5 (13.9) |
| FVC% predicted, mean (SD) | 74.6 (20.9) | 79.5 (20.4) | 80.1 (19.2) | 78.1 (17.9) | 77.5 (19.8) | 75.6 (17.5) |
| Duration of COPD, years, mean (SD) | 7.1 (6.2) | 7.3 (6.2) | 7.7 (6.3) | 7.5 (6.2) | 7.4 (5.8) | 6.6 (5.5) |
| mMRC dyspnea scale number, n (%) | ||||||
| 0 | 159 (7.7) | 473 (8.3) | 513 (9.3) | 187 (7.1) | 57 (6.8) | 16 (4.5) |
| 1 | 703 (34.1) | 2,237 (39.1) | 2,128 (38.6) | 941 (35.9) | 266 (31.6) | 99 (27.7) |
| 2 | 715 (34.7) | 2,044 (35.7) | 2,013 (36.5) | 1,051 (40.1) | 336 (40.0) | 111 (31.0) |
| 3 | 436 (21.1) | 860 (15.0) | 771 (14.0) | 405 (15.4) | 159 (18.9) | 107 (29.9) |
| 4 | 50 (2.4) | 94 (1.6) | 80 (1.5) | 37 (1.4) | 22 (2.6) | 24 (6.7) |
| Sputum-producing cough, n (%) | 1,284 (62.2) | 3,673 (64.2) | 3,520 (63.9) | 1,688 (64.3) | 518 (61.6) | 203 (56.7) |
| GOLD stages, n (%) | ||||||
| I | 9 (0.4) | 26 (0.5) | 32 (0.6) | 13 (0.5) | 12 (1.4) | 7 (2.0) |
| II | 600 (29.1) | 2,451 (42.8) | 2,933 (53.2) | 1,489 (56.7) | 473 (56.2) | 198 (55.3) |
| III | 957 (46.4) | 2,414 (42.2) | 2,045 (37.1) | 896 (34.1) | 292 (34.7) | 129 (36.0) |
| IV | 489 (23.7) | 770 (13.5) | 421 (7.6) | 170 (6.5) | 39 (4.6) | 15 (4.2) |
| CV disease history‡, n (%) | 359 (17.4) | 1,242 (21.7) | 1,587 (28.8) | 858 (32.7) | 298 (35.4) | 125 (34.9) |
| MI, n (%) | 77 (3.7) | 266 (4.6) | 387 (7.0) | 199 (7.6) | 75 (8.9) | 22 (6.1) |
| Cardiac arrhythmia, n (%) | 152 (7.4) | 523 (9.1) | 621 (11.3) | 357 (13.6) | 123 (14.6) | 49 (13.7) |
| IHD/CAD, n (%) | 173 (8.4) | 677 (11.8) | 993 (18.0) | 508 (19.4) | 170 (20.2) | 73 (20.4) |
| Stroke, n (%) | 30 (1.5) | 129 (2.3) | 137 (2.5) | 66 (2.5) | 22 (2.6) | 4 (1.1) |
| Use of CV medication, n (%) | 608 (29.5) | 2,372 (41.5) | 3,178 (57.7) | 1,723 (65.7) | 601 (71.5) | 271 (75.7) |
| Number of patients with moderate/severe exacerbations in previous year, n (%) | ||||||
| 0 | 990 (48.0) | 2,885 (50.4) | 2,855 (51.8) | 1,407 (53.6) | 478 (56.8) | 204 (57.0) |
| 1 | 615 (29.8) | 1,646 (28.8) | 1,583 (28.7) | 714 (27.2) | 221 (26.3) | 97 (27.1) |
| 2 | 260 (12.6) | 736 (12.9) | 640 (11.6) | 305 (11.6) | 93 (11.1) | 44 (12.3) |
| 3 | 111 (5.4) | 279 (4.9) | 266 (4.8) | 118 (4.5) | 33 (3.9) | 10 (2.8) |
| 4 | 41 (2.0) | 96 (1.7) | 110 (2.0) | 44 (1.7) | 10 (1.2) | 1 (0.3) |
| ⩾5 | 46 (2.2) | 73 (1.3) | 53 (1.0) | 33 (1.3) | 5 (0.6) | 2 (0.6) |
Definition of abbreviations: Aus = Australia; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CV = cardiovascular; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; GOLD = Global Initiative for Chronic Obstructive Lung Disease; IHD = ischemic heart disease; MI = myocardial infarction; mMRC = modified Medical Research Council; NZ = New Zealand; SD = standard deviation; TIOSPIR = TIOtropium Safety and Performance In Respimat.
All BMI expressed as kg/m2.
Highlighted normal weight data are the reference dataset for subsequent comparisons.
Cardiovascular history is defined as a history of MI, IHD/CAD, cardiac arrhythmia, or heart failure.
Baseline postbronchodilator FEV1, FEV1% predicted, and FEV1/FVC ratio were higher in overweight and obese participants versus underweight and normal-weight participants in TIOSPIR (Table 1). The FEV1/FVC ratio was 0.45 in underweight participants and 0.58 in obesity class III; the FVC% predicted was lower in participants at either end of the BMI spectrum (i.e., underweight and obesity class III) versus normal weight (Table 1). In UPLIFT, FVC% predicted was lower in obesity class III versus other BMI classes (Table E3).
In TIOSPIR, generally a higher proportion of overweight/obese participants had a history of CV disease and were using CV medications at baseline versus underweight/normal-weight participants (Table 1). This was also observed in UPLIFT (Table E3).
Mortality Risk by BMI Class
Risk of all-cause death
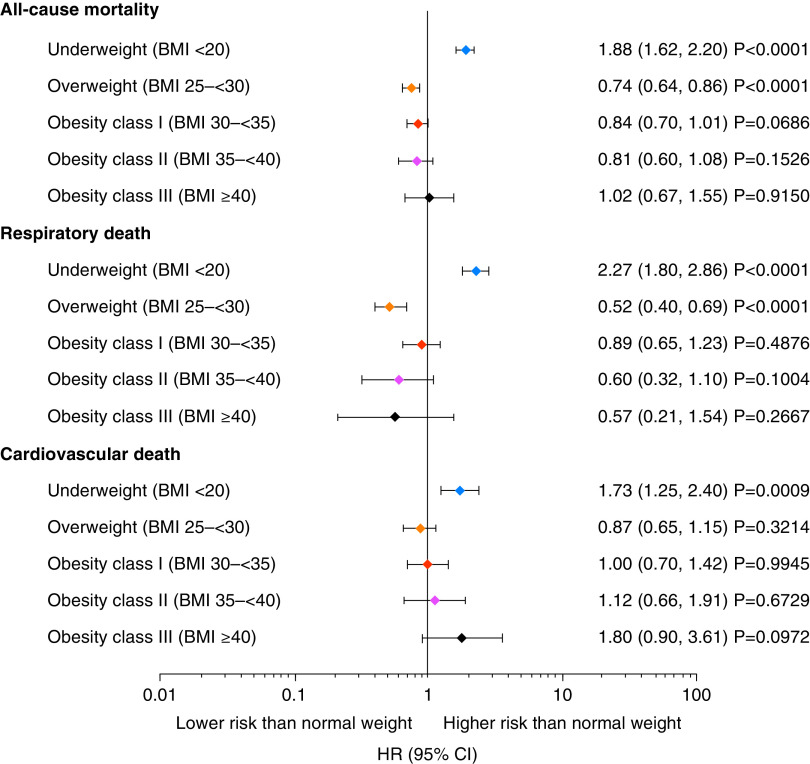
Underweight participants in TIOSPIR had a significantly higher risk of all-cause mortality versus normal-weight participants (HR, 1.88; 95% CI, 1.62–2.20) (Figures 1 and 2A). Conversely, overweight participants had a significantly lower risk of all-cause mortality versus normal-weight participants (HR, 0.74; 95% CI, 0.64–0.86) (Figure 1). The protective effect of being overweight decreased with increasing obesity class in TIOSPIR, with obesity class III at the same risk as normal-weight participants (HR, 1.02; 95% CI, 0.67–1.55). Consistent with TIOSPIR, underweight participants in UPLIFT had a significantly higher risk of all-cause mortality versus normal-weight participants, and overweight participants had a significantly lower risk (Figures E1 and E2A). Similarly, the protective effect of being overweight decreased with increasing BMI; obesity class III participants had a significantly higher risk of all-cause mortality versus overweight participants (HR, 2.56; 95% CI, 1.11–5.90).
Figure 1.
Relative risk of death (including vital status follow-up) for participants in TIOSPIR by body mass index (BMI) class versus normal-weight (BMI 20 to <25 kg/m2), set as hazard ratio (HR) = 1. All BMI expressed as kg/m2. Cox regression with “BMI class” as factor and adjusted for other covariates: age class, sex, region, smoking status, smoking history (pack-years), Global Initiative for Chronic Obstructive Lung Disease stages, medication variable (long-acting β2-agonist only, inhaled corticosteroid only, both inhaled corticosteroid and long-acting β2-agonist, or neither), and cardiac history. CI = confidence interval; TIOSPIR = TIOtropium Safety and Performance In Respimat.
Figure 2.
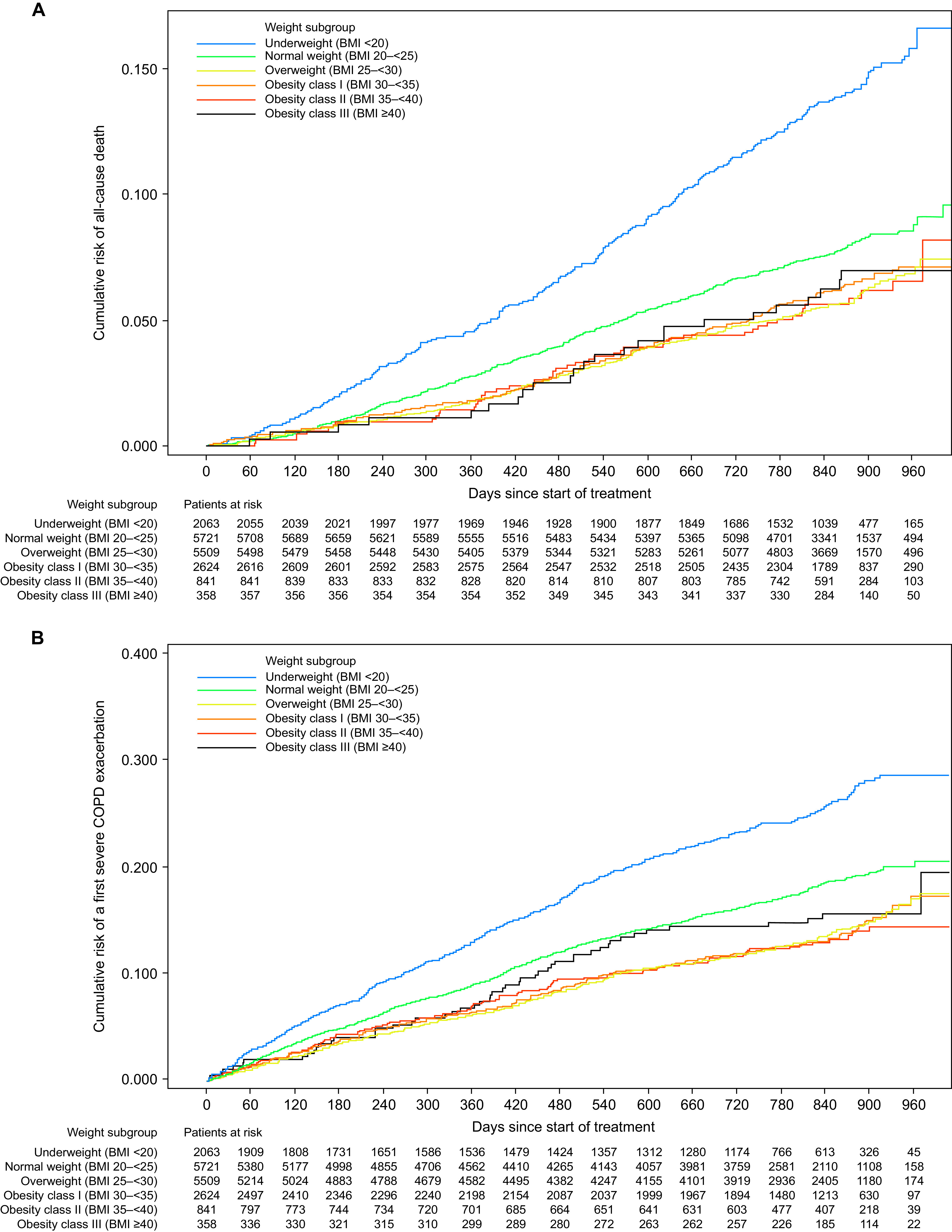
Kaplan–Meier curves by body mass index (BMI) in TIOSPIR for (A) all-cause mortality and (B) severe COPD exacerbation. All BMI expressed as kg/m2. COPD = chronic obstructive pulmonary disease; TIOSPIR = TIOtropium Safety and Performance In Respimat.
Risk of respiratory death
In TIOSPIR, underweight participants had a more than twofold higher risk of respiratory death (HR, 2.27; 95% CI, 1.80–2.86) compared with normal-weight participants, and overweight participants had a 48% lower risk of respiratory death (HR, 0.52; 95% CI, 0.40–0.69) (Figures 1 and E3A), with both results significant (similar significant results were found in UPLIFT) (Figures E1 and E2B). Overall, results in the obese categories indicated no clear association between increasing BMI and the risk of respiratory death.
Risk of CV death
Underweight participants in TIOSPIR had a significantly higher risk of CV death versus normal-weight participants (HR, 1.73; 95% CI, 1.25–2.40), and overweight participants had a lower, though nonsignificant, risk (HR, 0.87; 95% CI, 0.65–1.15). There was an increasing risk of CV death with increasing BMI; obesity class III participants had a significantly higher risk of CV death versus overweight participants (HR, 2.08; 95% CI, 1.04–4.17). Consistent with TIOSPIR, underweight participants in UPLIFT had a significantly higher risk of CV death versus normal-weight participants, and overweight participants had a lower, though nonsignificant, risk (Figures E1 and E2C). The risk of CV death versus normal weight in UPLIFT increased from overweight to obesity class III, reaching significance for obesity classes II and III (Figure E1).
Risk of severe and moderate exacerbations
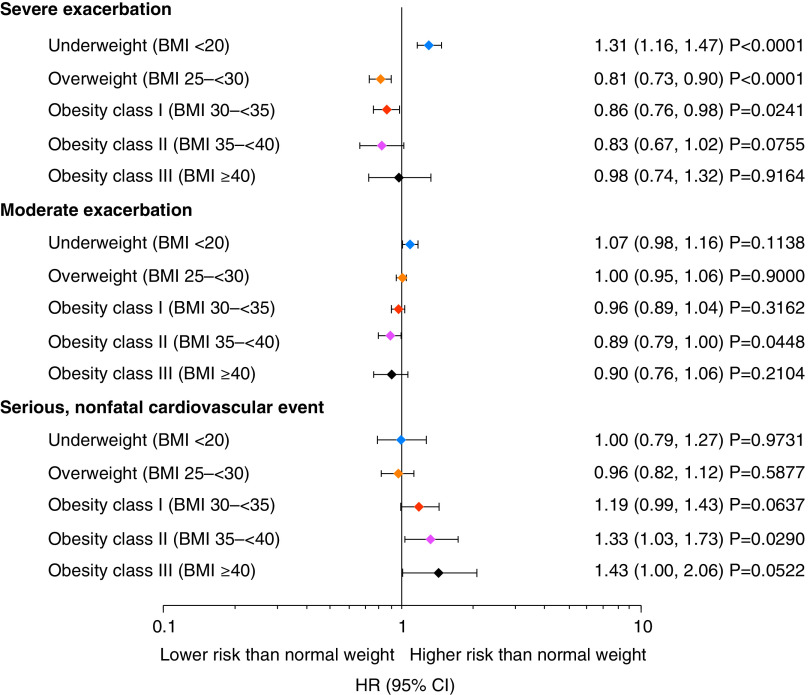
In TIOSPIR, underweight participants had a significantly higher risk of severe exacerbations than normal-weight participants (HR, 1.31; 95% CI, 1.16–1.47) (Figures 2B and 3), and this was also observed in UPLIFT (Figures E2D and E4). In TIOSPIR, the risk of severe exacerbations was significantly lower in overweight participants (HR, 0.81; 95% CI, 0.73–0.90). There was no clear association between BMI and the risk of moderate exacerbations in obese participants in either trial (Figures 3 and E4).
Figure 3.
Relative risk of COPD exacerbations and nonfatal cardiovascular events for participants in TIOSPIR by body mass index (BMI) class versus normal-weight (BMI 20 to <25 kg/m2), set as hazard ratio (HR) = 1. All BMI expressed as kg/m2. Cox regression with “BMI class” as factor and adjusted for other covariates: age class, sex, region, smoking status, smoking history (pack-years), Global Initiative for Chronic Obstructive Lung Disease stages, medication variable (long-acting β2-agonist only, inhaled corticosteroid only, both inhaled corticosteroid and long-acting β2-agonist, or neither), and cardiac history. CI = confidence interval; COPD = chronic obstructive pulmonary disease; TIOSPIR = TIOtropium Safety and Performance In Respimat.
Risk of a serious nonfatal CV event in TIOSPIR and UPLIFT
In TIOSPIR, the risk of serious nonfatal CV events was similar in underweight (HR, 1.00; 95% CI, 0.79–1.27) and overweight (HR, 0.96; 95% CI, 0.82–1.12) versus normal-weight participants. As seen for fatal CV events, the risk of nonfatal CV events increased with increasing BMI, with obesity class II reaching significance versus normal weight (HR, 1.33; 95% CI, 1.03–1.73; Figures 3 and E3C). Comparison of obese versus overweight participants showed a significantly higher risk of nonfatal CV events in all obesity classes (I–III) versus overweight participants (obesity class I HR, 1.24; 95% CI, 1.04–1.48; obesity class II HR, 1.39 95% CI 1.08–1.79; obesity class III HR, 1.50; 95% CI, 1.05–2.15 versus overweight). A similar pattern was present in UPLIFT (Figure E4).
Discussion
This secondary analysis of two large international COPD studies of participants from multiple continents, including a large number of morbidly obese individuals (BMI ⩾40 kg/m2), adds to the current literature by demonstrating that BMI can affect mortality and exacerbation risk in patients with COPD (21–23). An increased risk of CV-related mortality was observed for participants at either end of the BMI scale (BMI < 20 kg/m2 and BMI ⩾ 35 kg/m2) compared with normal-weight participants (though not reaching significance in TIOSPIR for BMI ⩾ 35 kg/m2). Underweight participants had a significantly increased risk of all-cause mortality, respiratory-related mortality, CV-related mortality, and severe exacerbations compared with normal-weight participants. Conversely, overweight and obese (BMI ⩾ 25 kg/m2) participants had lower or no additional risk of all-cause death, respiratory-related death, or severe exacerbations versus normal-weight participants. It is of note that the protective effect of being overweight or obese against all-cause mortality in these studies was reduced in morbidly obese patients. Overall, these data suggest that underweight patients are at increased risk for COPD-related events (exacerbations or respiratory-related death) and CV-related mortality; patients with higher BMI may be at higher risk of fatal and nonfatal CV events, but they appear to have no increased risk of respiratory-related events (exacerbations or respiratory-related deaths).
The finding that underweight participants with COPD had a significantly higher risk of death and exacerbations versus normal-weight participants is consistent with previous studies (9, 11). However, underweight status may not be the sole driver of such adverse outcomes. In this study, underweight participants had poorer lung function and were more likely to be current smokers. Although adjustments were made to account for these differences in the models, residual confounding was possible. Nonetheless, the effect size and consistency of these results between studies suggests underlying mechanisms for this association should be considered. For example, underweight individuals may have disease phenotypes that are more commonly associated with adverse outcomes. Previous cluster analyses from a Spanish and a French cohort, and the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) study, demonstrated a higher risk of death and exacerbations among individuals with a more “severe respiratory disease” phenotype; this subgroup had lower BMIs than other phenotypic clusters (24–26). Whether the impact of respiratory disease causes low BMI and cachexia is unclear. Work by Celli and colleagues described a phenotype of individuals with COPD having “extrapulmonary tissue loss,” which included individuals similar to those described here, having lower BMI, lower FEV1, higher exacerbation risk and mortality, but a higher burden of emphysema on computed tomography imaging, and certain biomarkers indicating higher burden of tissue damage and repair (27). All individuals in this subgroup showed a higher risk of mortality. Such studies provide important insight into the potential mechanisms leading to low BMI in this subtype and demonstrate how differences in comorbidities among different BMI groups may influence outcomes. A recent multicenter observational study with over 1,600 patients showed that different BMI categories are associated with diverse clinical expressions of COPD and comorbidity patterns (28). Patients who were underweight (BMI ⩽ 21 kg/m2) had the highest rate of respiratory-related mortality despite having among the lowest total number of comorbidities compared with other BMI categories (28). Nutritional support leading to weight gain can lead to improved COPD outcomes for underweight patients (29). However, weight gain leading to ectopic fat accumulation could be detrimental to cardiovascular and metabolic health in COPD, and development of lifestyle modification programs focusing on both nutritional quality and physical activity/exercise should be considered (30, 31).
Analysis of nonfatal CV events showed higher risk among obesity class II in TIOSPIR and UPLIFT. These results are consistent with previous studies, including a recent multicenter observational study that reported a higher prevalence of cardiac and metabolic comorbidities in patients with BMI ⩾ 35 kg/m2 (28). In addition, cluster analyses in patients with COPD from both Spanish and French cohorts identified a cluster of individuals with obesity and a higher risk of CV endpoints, as well as more systemic manifestations of disease (24, 25). These findings are unsurprising given the magnitude of evidence reporting an increased risk of CV disease with obesity; obesity influences various CV risk factors, has adverse effects on CV structure and function, and is a major risk factor for hypertension, heart failure, and coronary heart disease (32–34). In obese patients, purposeful weight reduction is supported for prevention and treatment of CV diseases (33). Long-term dietary interventions in obese individuals have been shown to promote and sustain long-term weight loss and reduce CV risk (35), while in obese COPD patients, dietary energy restriction coupled with resistance exercise training demonstrated significant improvements in BMI, exercise tolerance, and health status (36).
Suggestions as to why overweight or obese individuals show this paradoxical reduction in respiratory mortality and morbidity include earlier presentation of symptoms, greater likelihood of receiving optimal treatment, the cardioprotective metabolic effects of increased body fat, and benefits from greater metabolic reserves (9–12). In TIOSPIR and UPLIFT, the duration of COPD was relatively balanced between different BMI groups; these results are therefore not confounded by earlier presentation, although other explanations are possible.
Obesity has been suggested to provide benefits through a mechanism akin to chest wall strapping to improve airflow (37). In COPD, obesity is associated with lower lung volumes and increased airway conductance. This potential “chest wall strapping-like” effect of obesity could protect small airways and lower the risk of progression to emphysema or death (37). However, lung function measures can be affected by obesity; the FEV1/FVC ratio, a parameter for airflow obstruction, is influenced by the lung volume-reducing effect of increasing weight (38). O’Donnell and colleagues note that mild and moderate obesity have minimal impact on dyspnea, symptom-limited oxygen uptake, and respiratory mechanics during cardiopulmonary exercise testing, and that physiologic changes during exercise for individuals with COPD and obesity should raise questions about other contributing factors, including chronic comorbidities (39). Here, a clinically meaningful difference in FEV1/FVC is shown, with ratios increasing with increased BMI. As an FEV1/FVC ratio of less than 0.70 is required for diagnosis, having a higher ratio might obscure diagnosis of airflow obstruction in obese patients and affect rates of emphysema diagnosis (40). In underweight patients, potential contributors to the lower FEV1/FVC ratio include air-trapping, hyperinflation, emphysema, diaphragmatic weakness from sarcopenia, and loss of the normal curvature of the diaphragm (41, 42).
A recent analysis of 3,631 patients from the COPDGene (Genetic Epidemiology of COPD) study showed that obesity (BMI ⩾ 30 kg/m2) was associated with worse COPD-related outcomes versus normal-weight and overweight patients (BMI 18.5 to 29.9 kg/m2), including QoL, dyspnea, and incidence of severe exacerbations (43). Baseline characteristics of the current study indicate that patients with obesity class III have a higher burden of dyspnea and sputum-producing cough versus normal-weight and overweight individuals. In contrast to COPDGene, the current study found in TIOSPIR that overweight and slightly obese participants (BMI 25 to <35 kg/m2) were at a lower risk of severe exacerbations versus normal-weight participants. This could be due to the controlled conditions and treatment with tiotropium in this study, which could result in fewer exacerbations in overweight and slightly obese patients, compared with what is reported in the COPDGene study. Adherence to medications is also likely to be higher under the trial conditions of this study, compared with the observational COPDGene study, and this could potentially have an influence on the results. Findings reported in this study are, however, in line with two recent analyses, which showed that a higher BMI is associated with fewer COPD exacerbations in the study population, although different BMI cutoffs were selected (22, 44).
Overall, these studies emphasize the need to closely monitor individuals with COPD at either extreme of the BMI spectrum. In addition, these data suggest that BMI and obesity should be considered in relation to other important phenotypic characteristics and comorbidities that could affect treatment outcomes.
BMI is a simple measure that can easily be incorporated into clinical decision-making. However, it does not give a complete picture of fat/lean mass distribution throughout the body (2, 20). Previous studies suggest that patients with COPD may have a different fat/lean mass distribution versus the general population (45). Measures of fat distribution, such as waist/hip ratio and waist circumference, have been linked with lung function in adults, suggesting that the location of fat may be important (20, 45). For CV outcomes, measurement of fat distribution is particularly important, as abdominal obesity is a better risk indicator of CV diseases than BMI (30). Other measurements of weight distribution, such as dual-energy X-ray absorptiometry, have shown that changes in body composition, including limb and trunk lean mass and distribution of fat deposits, are associated with COPD severity (45). Whether these techniques provide better prognostic information than BMI alone in a population such as ours remains to be determined.
Strengths and Limitations
One limitation of our study is that it was post hoc rather than a predefined subgroup analysis. Whether the outcomes we observed would apply to other populations who had been selected differently in a global clinical trial is uncertain. However, as the largest long-term, randomized study in a broad range of patients with COPD performed to date, TIOSPIR provided a substantial dataset for investigation of our hypothesis, supported by the dataset from UPLIFT. Another possible limitation is that the BMI cutoffs in this study may not account for differences in race in the patient population; it has been suggested that different populations (e.g., Asian people) should use lower cutoffs for overweight/obesity BMI categories (46). Indeed, it was noted that Asian patients in this study tended to be underweight. Additionally, detailed body composition assessment, including fat distribution, was not performed in this study. As BMI was measured at baseline and not longitudinally in TIOSPIR, we could not analyze BMI as a time-varying exposure, such as reported in the recent study by Peralta and colleagues (47). This limits any conclusions on the association of weight changes over time with outcomes, especially whether there was an accumulation of patients with “wasting syndrome” in the lower BMI classes. Lastly, data may be confounded as they were not adjusted for exercise capacity and lung volumes (hyperinflation), which can affect mortality and are themselves affected by the patient’s weight.
This dataset is unique because of the high number of patients, including those who were underweight and morbidly obese (predominantly from TIOSPIR), who were observed under strictly controlled study conditions for 2–4 years; such a dataset has not been previously studied in COPD. Furthermore, the large, well-powered dataset and relatively open inclusion criteria allowed for the inclusion of comorbid CV disease, a common comorbidity in COPD.
Conclusions
BMI is a commonly used and recorded measurement both in clinical trials and general practice. In this analysis, after we adjusted for confounding factors such as age, sex, ethnicity, and smoking status, we demonstrated a strong association between body weight, COPD events, and risk of death. These data underscore the need for the holistic management of COPD patients that considers not just the management of their respiratory symptoms but also CV risk factors, nutritional status, and lifestyle modification to improve their general well-being.
Acknowledgments
Acknowledgment
The authors thank Helen Moore, Ph.D., at MediTech Media for providing editorial assistance in the development of this manuscript.
Footnotes
Supported by Boehringer Ingelheim.
Author Contributions: N.P., A.R.A., P.M.A.C., B.R.C., D.P.T., N.M., A.M., and R.A.W. confirm involvement in the conception, hypotheses delineation, and design of the study; acquisition, analysis, or interpretation of the data; and writing or substantial involvement in the article’s revision prior to submission.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. 2016. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis . 2014;9:723–733. doi: 10.2147/COPD.S50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J . 2012;19:e18–e24. doi: 10.1155/2012/732618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García-Rio F, Soriano JB, Miravitlles M, Muñoz L, Duran-Tauleria E, Sánchez G, et al. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One . 2014;9:e105220. doi: 10.1371/journal.pone.0105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mancuso P. Obesity and lung inflammation. J Appl Physiol (1985) . 2010;108:722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cecere LM, Littman AJ, Slatore CG, Udris EM, Bryson CL, Boyko EJ, et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD . 2011;8:275–284. doi: 10.3109/15412555.2011.586660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med . 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 8. Global BMI Mortality Collaboration. Di Angelantonio E, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet . 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 10. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA . 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One . 2012;7:e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zapatero A, Barba R, Ruiz J, Losa JE, Plaza S, Canora J, et al. Malnutrition and obesity: influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J Hum Nutr Diet . 2013;26:16–22. doi: 10.1111/jhn.12088. [DOI] [PubMed] [Google Scholar]
- 13. Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. TIOSPIR Investigators Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med . 2013;369:1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 14. Calverley PMA, Anzueto AR, Dusser D, Mueller A, Metzdorf N, Wise RA. Treatment of exacerbations as a predictor of subsequent outcomes in patients with COPD. Int J Chron Obstruct Pulmon Dis . 2018;13:1297–1308. doi: 10.2147/COPD.S153631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calverley PM, Tetzlaff K, Dusser D, Wise RA, Mueller A, Metzdorf N, et al. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int J Chron Obstruct Pulmon Dis . 2017;12:3391–3405. doi: 10.2147/COPD.S145814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Putcha N, Dusser D, Anzueto A, Metzdorf N, Mueller A, Wise RA. Mortality and exacerbation risk by body mass index in the (TIOSPIR) trial. Am J Respir Crit Care Med . 2016;193:A3530. [Google Scholar]
- 17. Tashkin DP, Anzueto A, Metzdorf N, Mueller A, Hinkel U, Celli B. Secondary analysis of the UPLIFT trial by body mass index. Eur Respir J . 2016;48:4049. [Google Scholar]
- 18. Wise RA, Anzueto A, Calverley P, Dahl R, Dusser D, Pledger G, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationale. Respir Res . 2013;14:40. doi: 10.1186/1465-9921-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 20. Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today . 2015;50:117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeLapp DA, Glick C, Furmanek S, Ramirez JA, Cavallazzi R. Patients with obesity have better long-term outcomes after hospitalization for COPD exacerbation. COPD . 2020;17:373–377. doi: 10.1080/15412555.2020.1781805. [DOI] [PubMed] [Google Scholar]
- 22. Smulders L, van der Aalst A, Neuhaus EDET, Polman S, Franssen FME, van Vliet M, et al. Decreased risk of COPD exacerbations in obese patients. COPD . 2020;17:485–491. doi: 10.1080/15412555.2020.1799963. [DOI] [PubMed] [Google Scholar]
- 23. Ji Z, de Miguel-Díez J, Castro-Riera CR, Bellón-Cano JM, Gallo-González V, Girón-Matute WI, et al. Differences in the outcome of patients with COPD according to body mass index. J Clin Med . 2020;9:710. doi: 10.3390/jcm9030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burgel PR, Paillasseur JL, Caillaud D, Tillie-Leblond I, Chanez P, Escamilla R, et al. Initiatives BPCO Scientific Committee Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J . 2010;36:531–539. doi: 10.1183/09031936.00175109. [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Aymerich J, Gómez FP, Benet M, Farrero E, Basagaña X, Gayete À, et al. PAC-COPD Study Group Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax . 2011;66:430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 26. Rennard SI, Locantore N, Delafont B, Tal-Singer R, Silverman EK, Vestbo J, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc . 2015;12:303–312. doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 27. Celli BR, Locantore N, Tal-Singer R, Riley J, Miller B, Vestbo J, et al. ECLIPSE Study Investigators Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur Respir J . 2018;51:1702146. doi: 10.1183/13993003.02146-2017. [DOI] [PubMed] [Google Scholar]
- 28. Divo MJ, Cabrera C, Casanova C, Marin JM, Pinto-Plata VM, de-Torres JP, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis (Miami) . 2014;1:229–238. doi: 10.15326/jcopdf.1.2.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J . 2014;44:1504–1520. doi: 10.1183/09031936.00070914. [DOI] [PubMed] [Google Scholar]
- 30. Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation . 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 31. Martin M, Almeras N, Després J-P, Coxson HO, Washko GR, Vivodtzev I, et al. Ectopic fat accumulation in patients with COPD: an ECLIPSE substudy. Int J Chron Obstruct Pulmon Dis . 2017;12:451–460. doi: 10.2147/COPD.S124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol . 2008;52:605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol . 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 34.Pérez Pérez A, Ybarra Muñoz J, Blay Cortés V, de Pablos Velasco P. Obesity and cardiovascular disease. Public Health Nutr. 2007;10:1156–1163. doi: 10.1017/S1368980007000651. [DOI] [PubMed] [Google Scholar]
- 35. Metz JA, Stern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med . 2000;160:2150–2158. doi: 10.1001/archinte.160.14.2150. [DOI] [PubMed] [Google Scholar]
- 36. McDonald VM, Gibson PG, Scott HA, Baines PJ, Hensley MJ, Pretto JJ, et al. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology . 2016;21:875–882. doi: 10.1111/resp.12746. [DOI] [PubMed] [Google Scholar]
- 37. Eberlein M, Schmidt GA, Brower RG. Chest wall strapping. An old physiology experiment with new relevance to small airways diseases. Ann Am Thorac Soc . 2014;11:1258–1266. doi: 10.1513/AnnalsATS.201312-465OI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Donnell DE, Deesomchok A, Lam YM, Guenette JA, Amornputtisathaporn N, Forkert L, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest . 2011;140:461–468. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- 39. O’Donnell DE, Ciavaglia CE, Neder JA. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc . 2014;11:635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 40. Lutchmedial SM, Creed WG, Moore AJ, Walsh RR, Gentchos GE, Kaminsky DA. How common is airflow limitation in patients with emphysema on CT scan of the chest? Chest . 2015;148:176–184. doi: 10.1378/chest.14-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith BM, Hoffman EA, Basner RC, Kawut SM, Kalhan R, Barr RG. Not all measures of hyperinflation are created equal: lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD study. Chest . 2014;145:1305–1315. doi: 10.1378/chest.13-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boriek AM, Lopez MA, Velasco C, Bakir AA, Frolov A, Wynd S, et al. Obesity modulates diaphragm curvature in subjects with and without COPD. Am J Physiol Regul Integr Comp Physiol . 2017;313:R620–R629. doi: 10.1152/ajpregu.00173.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. COPDGene Investigators Obesity is associated with increased morbidity in moderate to severe COPD. Chest . 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei YF, Tsai YH, Wang CC, Kuo PH. Impact of overweight and obesity on acute exacerbations of COPD - subgroup analysis of the Taiwan Obstructive Lung Disease cohort. Int J Chron Obstruct Pulmon Dis . 2017;12:2723–2729. doi: 10.2147/COPD.S138571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee LW, Lin CM, Li HC, Hsiao PL, Chung AC, Hsieh CJ, et al. Body composition changes in male patients with chronic obstructive pulmonary disease: aging or disease process? PLoS One . 2017;12:e0180928. doi: 10.1371/journal.pone.0180928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet . 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 47. Peralta GP, Marcon A, Carsin AE, Abramson MJ, Accordini S, Amaral AF, et al. Body mass index and weight change are associated with adult lung function trajectories: the prospective ECRHS study. Thorax . 2020;75:313–320. doi: 10.1136/thoraxjnl-2019-213880. [DOI] [PMC free article] [PubMed] [Google Scholar]