Abstract
Rationale
Individuals with chronic obstructive pulmonary disease (COPD) have a high prevalence of depression, which is associated with increased COPD hospitalizations and readmissions.
Objectives
Examine the impact of depressive symptoms compared with FEV1% on COPD morbidity.
Methods
Using longitudinal data from individuals with COPD in the Subpopulations and Intermediate Outcome Measures in COPD Study, longitudinal growth analysis was performed to assess COPD morbidity by assessing differences in baseline 6-minute walk distance and patient reported outcomes (PROs) and their rate of change over time explained by depressive symptoms or lung function, as measured by Hospital Anxiety and Depression Scale or FEV1% respectively. PROs consisted of in-person completion of St. George's Respiratory Questionnaire, COPD Assessment Test, Functional Assessment of Chronic Illness Therapy Fatigue, and Modified Medical Research Council Dyspnea Scale measures.
Results
Of the individuals analyzed (n = 1,830), 43% were female, 81% Caucasian with mean ± SD age of 65.1 ± 8.1, and 52.7 ± 27.5 pack-years smoking. Mean ± SD FEV1% was 60.9 ± 23.0% and 20% had clinically significant depressive symptoms. Adjusted models showed higher Hospital Anxiety and Depression Scale scores and lower FEV1% each were associated with worse PROs at baseline (P ⩽ 0.001). Depression accounted for more baseline variance in St. George's Respiratory Questionnaire, COPD Assessment Test, and Functional Assessment of Chronic Illness Therapy Fatigue than FEV1%, explaining 30–67% of heterogeneity. FEV1% accounted for more baseline variance in Modified Medical Research Council Dyspnea Scale and 6-minute walk distance than depression, explaining 16–32% of heterogeneity. Depressive symptoms accounted for 3–17% variance in change over time in PROs. In contrast, FEV1% accounted for 1–4% variance over time in PROs.
Conclusions
Depression is more strongly associated with many PROs at baseline and their change over time compared with FEV1%. Recognizing and incorporating the impact of depressive symptoms into individualized care may improve COPD outcomes.
Keywords: depression, COPD, patient reported outcome measures
Individuals with chronic obstructive pulmonary disease (COPD) have comorbid depression at a prevalence of 23–40% (1–3) compared with 8–17% in the general population (2, 4, 5). This prevalence is also higher compared with those with other chronic diseases (6). Furthermore, depressive symptoms are associated with greater healthcare utilization in patients with COPD (3, 7–12). Specifically, depression has been shown to be associated with increased risk for severe COPD exacerbations requiring hospitalizations, a longer hospital length of stay for COPD exacerbations, and increased risk for readmissions (8, 9, 13). Individuals with depression have shown greater variability in symptom recall in other diseases but the association of depression with reported symptom burden has not been evaluated in pulmonary disease (14).
The updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines emphasize patient reported outcomes as a key component of medical management decisions and assessment of disease control rather than lung function alone (15–18). This shift in approach reflects the increasing amount of literature demonstrating that lung function alone does not fully predict risk of exacerbations or capture disease impact on functional status and patient reported outcomes such as quality of life (19, 20). Therefore, we hypothesize that depressive symptoms will contribute more to the differences between participants in COPD patient reported outcomes than does post-bronchodilator percentage of predicted forced expiratory volume in one second (FEV1%).
Furthermore, previous studies examining the association of depression and COPD morbidity have looked at their association at a single point in time, lacking the ability to postulate how depression may affect changes in COPD morbidity over time (7, 8). To more thoroughly understand the association between depression and changes in COPD morbidity over time, we investigated longitudinal data from the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study).
Methods
Participants and Study Design
We conducted a secondary data analysis from the SPIROMICS cohort. Data in this analysis were collected from 2010 to 2014 from 12 sites located in cities across the United States and include 2,978 individuals. SPIROMICS inclusion criteria required participants to be 40–80 years old and have either <1 pack-year smoking history (nonsmoking controls) or >20 pack-year smoking history. Participants with body mass index ⩾40 kg/m2, diagnosis of unstable cardiac disease, and history of intolerance or hypersensitivity to bronchodilators were excluded. Data were gathered during in-person visits at baseline and up to three annual follow-up visits. Assessment visits collected measures of patient reported outcomes (PROs), completed spirometry, and 6-minute walk distances (6MWD) according to ATS guidelines (21, 22). Participants were also contacted quarterly by phone to assess exacerbation occurrence and health status. Further details of the SPIROMICS protocol have been published elsewhere (23).
For the purposes of this longitudinal analysis, we included current and former smokers with COPD (defined as ⩾20 pack-years smoking history, FEV1/FVC < 0.7, no evidence of non-COPD obstructive lung disease), decreasing available participants from 2,978 to 1,830. Of these 1,830 individuals with COPD, there were missing observations at annual assessments, which were addressed using an imputation analysis. We also ran a sensitivity analysis limited to 593 participants with at least three annual assessments with no missing observations on any variables (see online supplement). All participants were enrolled with informed consent in accordance with and under the approval of the Institutional Review Board of participating sites.
Measures
Depressive symptoms were measured by the Hospital Anxiety and Depression Scale (HADS), which is a validated screening tool used to assess symptom presence for anxiety and depression and was measured at baseline and yearly follow-up. HADS depression sub-scores range from 0–21 with 1.5 indicating a minimal clinically importance difference and a score of ⩾8 meets an accepted cutoff for clinically significant symptom burden (24–26). Lung function was measured by spirometry at baseline and yearly follow-up. Our analysis focused on post-bronchodilator FEV1% (21).
Patient reported outcome measures were completed at in-person study visits. St. George’s Respiratory Questionnaire (SGRQ) (range 0–100) is a 14-item questionnaire that measures COPD health status (quality of life) (27). The COPD Assessment Test (CAT) (range 0–40) is an eight-item questionnaire to assess symptom control (28). The modified Medical Research Council Dyspnea Scale (mMRC) (range 0–4) is one question assessment with responses scored 0–4 depending on severity of dyspnea related to physical activities (29). The Functional Assessment of Chronic Illness Therapy Fatigue Total (FACIT-F) (range 0–160) measures health related quality of life related to functional and emotional well-being (30). A higher score indicates more impairment for the SGRQ, CAT, and mMRC (27–29).
6MWD was conducted during yearly in-person visits. Participants were monitored and walked for 6 minutes while the distance in meters was recorded (31). Together, PROs and 6MWD assessments are referred to as measures of COPD morbidity.
Measured covariates were obtained at baseline and included age, sex (self-reported as male or female), smoking pack-year history, body mass index comorbidity count (one point for each: stroke, coronary heart disease, congestive heart failure, diabetes, hypertension, anemia, gastroesophageal reflux disease, asthma, sleep apnea) (32), and socioeconomic status (dichotomized as education level higher than a high school diploma or not).
Statistical Analysis
Baseline descriptive statistics were obtained. Participant characteristics were compared between those with (HADS ⩾ 8) and without (HADS < 8) significant symptoms of depression using Chi-squared test for categorical and unpaired t tests for continuous variables.
To assess the association between depression (or FEV1%) and baseline COPD morbidity and its change over time, linear mixed model regression of COPD morbidity on time, depression (or FEV1%), and time × depression (or FEV1%) interaction was run, adjusted by covariates and specified with random effects for intercept and slope for time (see online supplement). Time (visit = 1, 2, 3, and 4) was modeled continuously; depression and FEV1% were each operationalized time-invariant, represented by its within-person average across visits. To assess the contribution of depression (or FEV1%) on the variations in baseline COPD morbidity and its change over time, we evaluated the proportional reduction in random effect components across the models with and without inclusion of depression (or FEV1%), adjusted by covariates (see online supplement). A secondary analysis explored the contribution of depression or FEV1% when including both in the same model. Because there were missing observations across visits, multiple imputation analysis was performed using all analytic variables as predictors in the imputation model. The number of imputation was set at 30, and Rubin’s method was used to combine the parameter estimates across the imputed dataset (see online supplement) (33). As sensitivity, we ran a complete case analysis with the participants who had three or more annual assessments with no missing data. An additional sensitivity analysis was run using δ-method, a controlled MI under pattern-mixture modeling framework, to assess robustness of the results under missing-not-at-random assumption (see online supplement) (34).
Statistical significance was defined as P < 0.05. All analyses were conducted using STATA 15.1.
Results
Participants had a mean ± SD age of 65.1 ± 8.1 years and were 43% female (Table 1). Participants had a mean smoking history of 52.7 ± 27.5 pack years and mean comorbidities of 2.0 ± 1.4. The mean FEV1% predicted was 60.9% ± 23.0, with the within-person average of 60.3% ± 22.9. Those with complete data had fewer reported symptoms than those without complete data (Table E1).
Table 1.
Baseline participant characteristics stratified by depressive symptoms*
| All, N = 1,830† | Not Depressed, n = 1,431‡ | Depressed, n = 366‡ | |
|---|---|---|---|
| Female, n (%) | 780 (42.6) | 584 (40.8) | 180 (49.2) |
| Age | 65.1 (8.1) | 66.10 (7.7) | 61.22 (8.1) |
| BMI | 27.4 (5.3) | 27.38 (5.2) | 27.30 (5.7) |
| Race | |||
| Caucasian, n (%) | 1,484 (81.1) | 1,170 (81.8) | 295 (80.6) |
| Black, n (%) | 280 (15.3) | 210 (14.7) | 56 (15.3) |
| Asian, n (%) | 22 (1.2) | 17 (1.2) | 5 (1.4) |
| American Indian, n (%) | 8 (0.4) | 4 (0.3) | 4 (1.1) |
| Multiracial, n (%) | 27 (1.5) | 23 (1.6) | 4 (1.1) |
| Missing, n (%) | 9 (0.5) | 7 (0.5) | 2 (0.6) |
| HS grad or below, n (%) | 712 (39.0) | 497 (34.8) | 191 (52.3) |
| Smoking pack-years | 52.7 (27.5) | 52.2 (25.7) | 54.6 (33.5) |
| Comorbidity count | 2.0 (1.4) | 2.0 (1.4) | 2.2 (1.5) |
| Antidepressant therapy | 876 (47.9) | 664 (46.4) | 197 (53.8) |
| FEV1% predicted | 60.9 (23.0) | 63.0 (23.0) | 53.2 (21.5) |
Definition of abbreviations: BMI = body mass index; FEV1 = forced expiratory volume in 1 second; HADS = hospital anxiety and depression scale; HS grad = high school graduate; SD = standard deviation.
Reported as mean (SD) unless otherwise noted as n (%)
Variables with N < 1,830 are HS grad or below (n = 1,826), comorbidity count (n = 1,725), smoking pack-years (n = 1,236), and FEV1% predicted (n = 1,827).
All dichotomized rows do not add up to 1,830 because the initial total for all variables is not 1,830 as designated above. Additionally, 33 individuals do not have any HADS scores, as such these were imputed in analysis.
366 out of 1,830 participants (20%) reported clinically significant symptoms of depression (HADS ⩾8) at baseline. The baseline mean ± SD HADS was 4.9 ± 3.6, with the within-person average of 4.7 ± 3.3. Those who reported clinically significant symptoms of depression were more likely to be younger, males, with lower educational status and a higher number of comorbid diseases than those with low symptom burden from depression (Table 1). There was a high prevalence of antidepressant use in participants overall. The rate of reported use of antidepressant medication was higher in those with significant depressive symptoms than those without depressive symptoms at study entry (Table 1). Indication for use of antidepressants was not available.
Depressive Symptoms and FEV1% Association with Baseline Morbidity and Change Over Time
Models specified random intercept and random slope based on statistically significant differences in participants’ baseline morbidity and its change over time (Tables E2 and E3). Adjusting for covariates, higher depressive symptoms were adversely associated with baseline morbidity and its change over time (Table 2). A one-point increase in HADS was associated with worse baseline SGRQ (β = 3.13, P < 0.001), CAT (β = 1.07, P < 0.001), FACIT-F (β = −5.64, P < 0.001), and mMRC (β = 0.11, P < 0.001); as well as lower baseline 6MWD (β = −9.17, P < 0.001). In addition, a one-point increase in HADS was associated with a higher rate of change over time in SGRQ (β = 0.17, P = 0.001), CAT (β = 0.10, P < 0.001), and FACIT-F (β = −0.42, P < 0.001). Depressive symptoms were not significantly associated with the rate of change in mMRC or 6MWD.
Table 2.
Incremental change in morbidity accounted for by depressive symptoms compared to FEV1% in generalized linear models*
| Baseline |
Trajectory |
|||||||
|---|---|---|---|---|---|---|---|---|
| β | Std Error | P Value | % Variance Explained | β | Std Error | P Value | % Variance Explained | |
| SGRQ | ||||||||
| Depression | 3.13 | 0.11 | <0.001 | 35.3% | 0.17 | 0.05 | 0.001 | 4.6% |
| FEV1% | −0.40 | 0.02 | <0.001 | 29.3% | −0.02 | 0.01 | 0.006 | 3.8% |
| CAT | ||||||||
| Depression | 1.07 | 0.05 | <0.001 | 30.6% | 0.10 | 0.03 | <0.001 | 8.1% |
| FEV1% | −0.12 | 0.01 | <0.001 | 18.9% | −0.01 | 0.00 | 0.082 | 2.6% |
| FACIT-F† | ||||||||
| Depression | −5.64 | 0.13 | <0.001 | 67.8% | −0.42 | 0.08 | <0.001 | 17.4% |
| FEV1% | 0.23 | 0.02 | <0.001 | 5.9% | 0.01 | 0.01 | 0.304 | 1.2% |
| mMRC | ||||||||
| Depression | 0.11 | 0.01 | <0.001 | 22.7% | 0.01 | 0.00 | 0.184 | 2.8% |
| FEV1% | −0.02 | 0.00 | <0.001 | 31.7% | 0.00 | 0.00 | 0.501 | 1.4% |
| 6MWD† | ||||||||
| Depression | −9.17 | 0.87 | <0.001 | 8.0% | −0.61 | 0.43 | 0.157 | 0.9% |
| FEV1% | 1.77 | 0.12 | <0.001 | 15.5% | 0.28 | 0.06 | <0.001 | 7.8% |
Definition of abbreviations: 6MWD = 6-minute walk distance (meters); CAT = Chronic Obstructive Pulmonary Disease Assessment Test; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue; FEV1 = forced expiratory volume in 1 second; HADS = Hospital Anxiety and Depression Scale; mMRC = modified Medical Research Council Dyspnea Scale; SGRQ = St. George's Respiratory Questionnaire; Std = standard.
Models were adjusted for age, sex, comorbidity count, body mass index, smoking pack-year history, and highest education attained. All analytic variables had some observations imputed except for age and sex and included a total of 7,320 individual data points to achieve three time points of data per individual.
FACIT-F, 6MWD, FEV1% all decline with worse disease. SGRQ, CAT, and mMRC all increase with worse disease.
For “Baseline,” “β” represents the estimated mean difference in the baseline outcome for 1-point increase in HADS depression score or 1-unit increase in FEV1%; “% Variance Explained” represents the proportional reduction in the between-person variance estimate for intercept (see Table E2) attributed to depression or FEV1%.
For “Rate of Change,” “β” represents the estimated mean difference in the slope of time (or rate of change) for 1-point increase in HADS depression score or 1-unit increase in FEV1%; “% Variance Explained” represents the proportional reduction in the between-person variance estimate of the slope of time (or rate of change) (see Table E2) attributed to depression or FEV1%.
A lower FEV1% was associated with worse baseline morbidity for all measures and worse rate of change for SGRQ and 6MWD (Table 2). Adjusting for covariates, one percentage point lower FEV1% was associated with worse baseline measures (e.g., lower for 6MWD and higher for SGRQ, CAT and MMRC) evidenced by SGRQ (β = −0.40, P < 0.001), CAT (β = −0.12, P < 0.001), FACIT-F (β = 0.23, P < 0.001), mMRC (β = −0.02, P < 0.001), and 6MWD (β = 1.77, P < 0.001). For clinical context, this portends to a 10% decline in FEV1% being associated with a 4-point increase in SGRQ, 1.2-point increase in CAT, 2.3-point decline in FACIT-F, 0.2 increase in mMRC, and 17.7 m decline in 6MWD. Each drop in percent predicted for FEV1% was associated with a worse/steeper rate of decline in SGRQ over time (β = −0.02, P = 0.006) and 6MWD over time (β = 0.28, P < 0.001). FEV1% was not significantly associated with steeper rate of change in CAT FACIT-F, or mMRC.
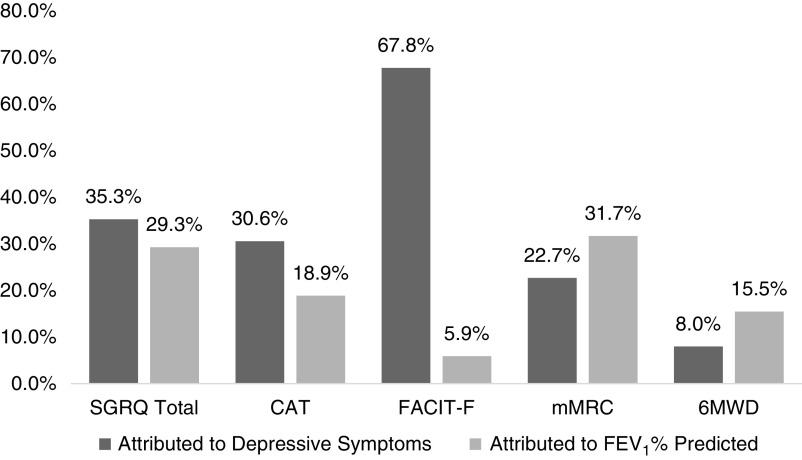
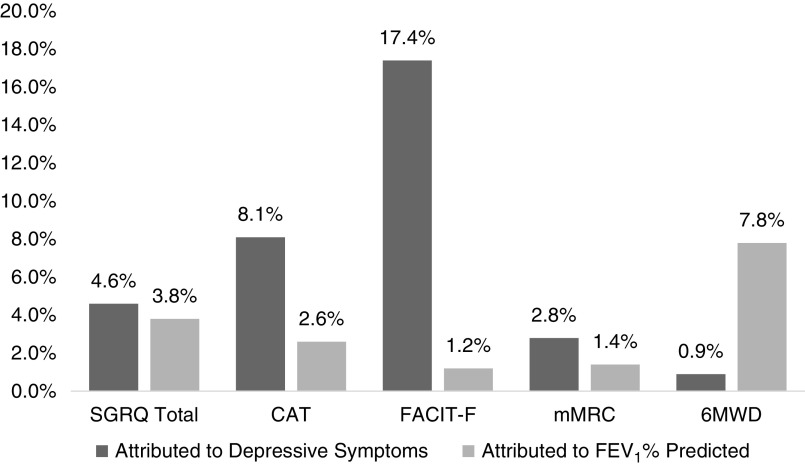
When looking at heterogeneity in baseline morbidity and subsequent change over time, adjusting for covariates, depressive symptoms explained the differences in participants’ baseline SGRQ by 35.3%, CAT by 30.6%, FACIT-F by 67.8%, mMRC by 22.7%, and 6MWD by 8.0%. In comparison, FEV1% explained the differences in participants’ baseline SGRQ by 29.3%, CAT by 18.9%, FACIT-F by 5.9%, mMRC by 31.7%, and 6MWD by 15.5% (Table 2 and Figure 1). Similarly, when adjusting for covariates, depressive symptoms explained the heterogeneity in SGRQ’s rate of change by 4.6%, CAT’s by 8.1%, FACIT-F by 17.4%, and mMRC by 2.8%. Depressive symptoms did not explain the participant differences in 6MWD’s rate of change (0.9%). In comparison, FEV1% explained 7.8% of such difference in 6MWD and 3.8% in SGRQ; but did not explain any other outcomes’ rate of change (Table 2 and Figure 2). In the secondary analysis, the above results remained intact when adjusting for either FEV1% or depression by including both in the same model (Table E4).
Figure 1.
Proportion of variance accounted for in baseline morbidity by depressive symptoms compared with FEV1% predicted. 6MWD = 6-minute walk distance (meters); CAT = Chronic Obstructive Pulmonary Disease Assessment Test; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue; FEV1 = forced expiratory volume in 1 second; mMRC = modified Medical Research Council Dyspnea Scale; SGRQ = St. George's Respiratory Questionnaire.
Figure 2.
Proportion of variance accounted for in change in morbidity over time by average depressive symptoms compared with FEV1% predicted. 6MWD = 6-minute walk distance (meters); CAT = Chronic Obstructive Pulmonary Disease Assessment Test; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue; FEV1 = forced expiratory volume in 1 second; mMRC = modified Medical Research Council Dyspnea Scale; SGRQ = St. George's Respiratory Questionnaire.
Although the statistical significances of the differences between depressive symptoms and FEV1% could not be formally tested—because the two models were not nested—AIC suggests that these differences are likely to be meaningful based on the magnitude of the differences that exceeded two (Table E5), an often-used benchmark for indicating significant model fit improvement (35).
Given that multiple imputation analysis was used due to missing data, we conducted a sensitivity analysis using only participants with complete data for three or more time points (Table E6). The percentage of explained variance from depressive symptoms and FEV1% was similar to those from the multiple imputation analysis (Table E6). The sensitivity analysis under missing-not-at-random assumption also returned similar results (Tables E7–E9).
Discussion
Our results demonstrated that individuals with COPD and depressive symptoms had worse patient reported outcomes, including worse quality of life and respiratory symptoms; and worse functional status, compared with their counterparts without depressive symptoms. Importantly, depressive symptoms explained 8–68% of heterogeneity in baseline patient-reported outcomes, and were a larger contributor to patient reported morbidity than FEV1%. Depressive symptoms were also a greater contributor than FEV1% to change over time in PROS such as CAT, SGRQ, and FACIT-F.
Our results show that 20% of individuals with COPD in the SPIROMICS cohort had significant depressive symptoms, which is similar to previous estimates of 15–20% (3, 36) and higher than the general U.S. population (4). This patient cohort has a similar mean burden of depressive symptoms over time to the baseline burden of depression in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (9). Our study adds to literature previously reported from ECLIPSE in that we demonstrate that depressive symptoms are an important driver of COPD morbidity compared with spirometry (9). The utility of lung function as a solitary measure for disease progression has previously been challenged and may not be the best marker to follow for disease burden (18, 19). Current GOLD guidelines emphasize a multifaceted approach to COPD management incorporating spirometry, patient symptoms, comorbidities, and exacerbation data (15). In particular, the CAT and the mMRC are patient reported outcomes that are used to determine GOLD Disease Classification and guide therapeutic management (28, 29). As these guidelines direct clinical practice, our data suggest that it is of particular importance to remain cognizant of patients’ depressive symptoms when interpreting CAT and mMRC. Data presented here supports that depressive symptoms are more strongly associated with CAT scores than FEV1% at baseline and more strongly associated over time with CAT and mMRC scores, highlighting a need for better understanding of what COPD disease control means to patients and providers.
Depression accounted for 30.6% of heterogeneity in CAT at baseline and 8.1% in its change over time while depression accounted for 22.7% of heterogeneity in mMRC at baseline and 2.8% in its change over time. The difference in the amount of heterogeneity explained at baseline and the measure of its change over time was larger for the CAT as compared with the mMRC. This larger difference may reflect the items in CAT focusing on a range of symptoms and impact on life, such as fatigue and energy level, compared with the mMRC with a singular focus on breathlessness. This can be helpful when thinking about how best to utilize the GOLD disease classifications. Presence of depressive symptoms has a stronger association with CAT’s change over time than does FEV1%, while mMRC’s change over time was not significantly associated with either depression or FEV1%. The 6MWD was the only outcome where FEV1% accounted for a greater proportion of variance in the outcome’s change over time than did depressive symptoms. The distance individuals can walk is often discordant with other measures of COPD severity and varies independently of subjective dyspnea. This difference may in part explain why depression explained more variance in patient reported outcomes than 6MWD (37).
Furthermore, our current study included longitudinal analysis of depressive symptoms and associations with SGRQ, CAT, FACIT-F, and mMRC. When depressive symptoms go unrecognized, patients may be experiencing a worse disease trajectory over time. This could translate into increased health care utilization and increased prescribing of inhaled medications with little symptomatic benefit in the absence of addressing underlying depression. It has previously been noted that this lack of recognition stems from patient and provider factors and increased awareness of depressive symptoms in patients with COPD may help advance attention to mental health in this patient population and target comprehensive patient centered care (38). Together these results support the need to identify psychological health across all aspects of care and create opportunities for multidisciplinary approaches to COPD care in pulmonary and primary care clinics.
Although there are no widely adopted integrated programs to address depression in COPD, interventions in heart failure have shown success. Interventions in COPD designed to address depressive symptoms and psychosocial aspects of disease have included multidisciplinary approaches to care (39, 40). Attending pulmonary rehabilitation programs has shown some benefit in depressive symptoms in individuals with COPD (41). There is also growing information and interest in developing early palliative care interventions to address psychosocial well-being and symptom control in patients with COPD (42). Weekly and monthly nurse follow-up phone calls to assess social support and health, mood, and well-being in addition to cardiac symptom burden showed improvement in New York Heart Failure Classification and in physical well-being (40). These together speak to the multidimensional approach needed to address COPD and its common comorbidities in attempt to improve quality of life and health outcomes. Given the high prevalence of depression, clinicians may need to consider efficacious behavioral treatments for the COPD population given the dearth of evidence supporting pharmacotherapy for depression (43).
A major strength of our study is the robust cohort from which this data is drawn. While there were individuals on treatment for depression, it is unclear if symptoms were appropriately controlled on medications, if the medications were in a state of ongoing adjustment, or if the antidepressants were prescribed for an indication other than depression. This is reflected by the absence of a statistically significant difference in use of antidepressant medication in those with significant depressive symptoms compared with those without depressive symptoms.
This study did have some limitations to acknowledge. This study used the HADS for assessment of depressive symptoms. The HADS is a screening tool and not a complete psychiatric interview for diagnosis of depression or anxiety; however, this is a validated and widely used tool to detect the presence of depressive symptoms (25, 44). The FACIT-F includes mood, energy, and motivation in its assessment among functional daily activity questions, and thus has some overlap with depressive symptoms analysis. This study presents a multiple imputation analysis of longitudinal data and includes a supplemental analysis including only individuals with complete data points over three time visits. The supplemental analysis had a relatively low number of individuals with three complete visits in part due to loss to follow up but also because slow enrollment resulted in many participants not completing three years of follow-up during the funding period. The missing mechanism was unlikely to be missing-completely-at-random because participants in the supplemental analysis showed better health outcomes than those without complete data. Therefore, we presented multiple imputation analysis under missing-at-random as our primary analysis and the missing-completely-at-random analysis with complete data over three time points as supplemental analysis. The results of these two analyses were similar. An additional sensitivity analysis using δ-method, a type of controlled multiple imputation approach under the situation where missing-at-random is violated, also showed similar results to our primary analysis, suggesting robustness of our conclusions across different missing data assumptions (34).
Previously it has been shown individuals with a higher burden of respiratory symptoms have a higher degree of depression and anxiety (10). It is likely that psychological symptoms may influence a patient’s perception of disease control in addition to possibly being related to behaviors affecting disease, thus having a bidirectional association with patient-reported outcome measures (11). As symptoms worsen over time, the impact of such disease may also contribute to worse psychological symptoms. However, results presented in this paper demonstrate an association of pre-existing depressive symptoms with increased burden of disease overtime.
Conclusions
Depressive symptoms explain a greater proportion of patient reported outcomes than FEV1% in individuals with COPD and emphasizes the importance of multidisciplinary programs for COPD management. Further analyses of how depression as a major comorbidity of COPD impacts patient reported outcomes, specifically CAT, SGRQ, and mMRC will help the development of more comprehensive approaches to care.
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org.
Current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, Wayne H. Anderson, Mehrdad Arjomandi, Igor Barjaktarevic, R. Graham Barr, Lori A. Bateman, Surya P. Bhatt, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper; Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Brad Drummond, Christine M. Freeman, Craig Galban, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Yvonne Huang, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell Jr., Robert Paine III, Laura Paulin, Stephen P. Peters, Cheryl Pirozzi, Nirupama Putcha, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J Michael Wells, Robert A. Wise, and Prescott G. Woodruff.
Project officers from the Lung Division of the National Heart, Lung, and Blood Institute: Lisa Postow and Lisa Viviano.
Footnotes
A complete list of the SPIROMICS Investigators may be found before the beginning of the REFERENCES.
Supported by grants from the National Heart, Lung, and Blood Institute (National Institutes of Health [NIH] R01 HL128620-02, NIH F32 HL143864-01, NIH 5 T32 HL 7534-35, NIH K23HL123594), and the National Institute of Environmental Health Sciences (R01ES023500).
Author Contributions: J.O’T., N.P., N.N.H., and M.N.E. were involved in study design. J.O’T., H.W., and M.N.E. were involved in data analysis. N.P., C.B.C., P.W., R.E.K., R.P., R.P.B., A.C., K.F.H., J.A.K., M.H., M.D., A.S.I., D.C., S.P.P., G.C., V.K., R.G.B., F.J.M., and N.N.H. were involved in SPIROMICS (SubPopulations and InteRmediate Outcome Measures In COPD Study) study design, cohort recruitment or data acquisition. J.O’T. and M.N.E. drafted the manuscript with critical revision received by all listed authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
the SPIROMICS Investigators:
Neil E. Alexis, Wayne H. Anderson, Mehrdad Arjomandi, Igor Barjaktarevic, R. Graham Barr, Lori A. Bateman, Surya P. Bhatt, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Brad Drummond, Christine M. Freeman, Craig Galban, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Yvonne Huang, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Jr., Robert Paine, III, Laura Paulin, Stephen P. Peters, Cheryl Pirozzi, Nirupama Putcha, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J Michael Wells, Robert A. Wise, and Prescott G. Woodruff
References
- 1. Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med . 2008;23:1757–1762. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider C, Jick SS, Bothner U, Meier CR. COPD and the risk of depression. Chest . 2010;137:341–347. doi: 10.1378/chest.09-0614. [DOI] [PubMed] [Google Scholar]
- 3. Iyer AS, Holm KE, Bhatt SP, Kim V, Kinney GL, Wamboldt FS, et al. COPDGene Investigators Symptoms of anxiety and depression and use of anxiolytic-hypnotics and antidepressants in current and former smokers with and without COPD - A cross sectional analysis of the COPDGene cohort. J Psychosom Res . 2019;118:18–26. doi: 10.1016/j.jpsychores.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pratt L, Brody D.Depression in the US household population, 2009–2012. 2014. [PubMed]
- 5. Hanania NA, Müllerova H, Locantore NW, Vestbo J, Watkins ML, Wouters EF, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med . 2011;183:604–611. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 6. Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med . 2006;32:1826–1831. doi: 10.1007/s00134-006-0333-0. [DOI] [PubMed] [Google Scholar]
- 7. Ng T-P, Niti M, Fones C, Yap KB, Tan W-C. Co-morbid association of depression and COPD: a population-based study. Respir Med . 2009;103:895–901. doi: 10.1016/j.rmed.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 8. Iyer AS, Bhatt SP, Garner JJ, Wells JM, Trevor JL, Patel NM, et al. Depression is associated with readmission for acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2016;13:197–203. doi: 10.1513/AnnalsATS.201507-439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yohannes AM, Mülerová H, Lavoie K, Vestbo J, Rennard SI, Wouters E, et al. The association of depressive symptoms with rates of acute exacerbations in patients with COPD: Results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc . 2017;18:955–959.e6. doi: 10.1016/j.jamda.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 10. Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res . 2014;15:122. doi: 10.1186/s12931-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest . 2013;144:766–777. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- 12. Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, et al. National Emphysema Treatment Trial (NETT) Research Group Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med . 2007;167:2345–2353. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 13. von Siemens SM, Jörres RA, Behr J, Alter P, Lutter J, Lucke T, et al. COSYCONET study group Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res . 2019;20:30. doi: 10.1186/s12931-019-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-Zeev D, Young MA. Accuracy of hospitalized depressed patients’ and healthy controls’ retrospective symptom reports: an experience sampling study. J Nerv Ment Dis . 2010;198:280–285. doi: 10.1097/NMD.0b013e3181d6141f. [DOI] [PubMed] [Google Scholar]
- 15.European Respiratory Society. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report. 2019. https://erj.ersjournals.com/content/53/5/1900164.long [DOI] [PubMed]
- 16. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med . 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 18. Han MK, Muellerova H, Curran-Everett D, Dransfield M, Washko G, Regan et al. Implications of the GOLD 2011 Disease Severity Classification in the COPDGene Cohort. Lancet Respir Med . 2013;1(EA):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators Characterisation of COPD heterogeneity in the ECLIPSE cohort Respir Res 2010. 11 122 20831787 [Google Scholar]
- 20. Bowler RP, Kim V, Regan E, Williams AAA, Santorico SA, Make BJ, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest . 2014;146:941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22. Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. . Eur Respir J . 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 23. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax . 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes . 2008;6 doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res . 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 26. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis . 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 28. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J . 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 29. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax . 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-shair K, Muellerova H, Yorke J, Rennard SI, Wouters EF, Hanania NA, et al. ECLIPSE investigators Examining fatigue in COPD: development, validity and reliability of a modified version of FACIT-F scale. Health Qual Life Outcomes . 2012;10:100. doi: 10.1186/1477-7525-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J . 2011;37:784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One . 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 34. Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide. Stat Med . 2020;39:2815–2842. doi: 10.1002/sim.8569. [DOI] [PubMed] [Google Scholar]
- 35.Hilbe J. Negative Binomial Regression . 2nd ed. New York, NY: Cambridge University Press; 2011. [Google Scholar]
- 36. Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care . 2013;58:858–866. doi: 10.4187/respcare.01862. [DOI] [PubMed] [Google Scholar]
- 37. Pierobon A, Sini Bottelli E, Ranzini L, Bruschi C, Maestri R, Bertolotti G, et al. COPD patients’ self-reported adherence, psychosocial factors and mild cognitive impairment in pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis . 2017;12:2059–2067. doi: 10.2147/COPD.S133586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev . 2014;23:345–349. doi: 10.1183/09059180.00007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Angermann CE, Ertl G. Depression, anxiety, and cognitive impairment: comorbid mental health disorders in heart failure. Curr Heart Fail Rep . 2018;15:398–410. doi: 10.1007/s11897-018-0414-8. [DOI] [PubMed] [Google Scholar]
- 40. Angermann CE, Störk S, Gelbrich G, Faller H, Jahns R, Frantz S, et al. Competence Network Heart Failure Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail . 2012;5:25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 41. Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest . 2019;156:80–91. doi: 10.1016/j.chest.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 42. Iyer AS, Dionne-Odom JN, Ford SM, Crump Tims SL, Sockwell ED, Ivankova NV, et al. A formative evaluation of patient and family caregiver perspectives on early palliative care in chronic obstructive pulmonary disease across disease severity. Ann Am Thorac Soc . 2019;16:1024–1033. doi: 10.1513/AnnalsATS.201902-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Machmutow K, Meister R, Jansen A, Kriston L, Watzke B, Härter MC, et al. Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults. Cochrane Database Syst Rev . 2019;5:CD012855. doi: 10.1002/14651858.CD012855.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jutte JE, Needham DM, Pfoh ER, Bienvenu OJ. Psychometric evaluation of the Hospital Anxiety and Depression Scale 3 months after acute lung injury. J Crit Care . 2015;30:793–798. doi: 10.1016/j.jcrc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]