Abstract
Rationale
The development of novel therapies for idiopathic pulmonary fibrosis (IPF) has brought increased attention to the population burden of disease. However, little is known about the epidemiology of IPF among U.S. Veterans.
Objectives
This study examines temporal trends in incidence and prevalence, patient characteristics, and risk factors associated with IPF among a national cohort of U.S. Veterans.
Methods
We used data from the Veterans Health Administration (VHA) electronic health record system to describe the incidence, prevalence, and geographic distribution of IPF between January 1, 2010, and December 31, 2019. We evaluated patient characteristics associated with IPF using multivariate logistic regression.
Results
Among 10.7 million Veterans who received care from the VHA between 2010 and 2019, 139,116 (1.26%) were diagnosed with IPF. Using a narrow case definition of IPF, the prevalence increased from 276 cases per 100,000 in 2010 to 725 cases per 100,000 in 2019. The annual incidence increased from 73 cases per 100,000 person-years in 2010 to 210 cases per 100,000 person-years in 2019. Higher absolute incidence and prevalence rates were noted when a broader case definition of IPF was used. Risk factors associated with IPF among Veterans included older age, White race, tobacco use, and rural residence. After accounting for interactions, the average marginal difference in IPF prevalence between males and females was small. There was significant geographic heterogeneity of disease across the United States.
Conclusions
This study is the first comprehensive epidemiologic analysis of IPF among the U.S. Veteran population. The incidence and prevalence of IPF among Veterans has increased over the past decade. The effect of sex on risk of IPF was attenuated once accounting for other risk factors. The geographic distribution of disease is heterogeneous across the United States with rural residence associated with higher odds of IPF. The reasons for these trends deserve further study.
Keywords: epidemiology, Veterans, interstitial lung disease, idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrosing interstitial lung disease that affects older adults (1). Without therapy, median survival time ranges from 2 to 5 years after diagnosis (2, 3). However, with the Food and Drug Administration (FDA) approval of two new medications that slow disease progression, pirfenidone and nintedanib, this trajectory may be changing, and better understanding the population burden and epidemiology of IPF has important implications for clinical management and outcomes.
Establishing the epidemiology of IPF has been challenging owing to disease complexity, relatively low prevalence, and heterogeneity of populations sampled. Early registry-based studies, in which IPF was likely underdiagnosed, reported incidence rates of 0.22–8.8 per 100,000 person-years and prevalence rates of 0.5–27.9 per 100,000 (4). However, more recent studies that have used large administrative databases to identify population-based cohorts have noted substantially higher incidence and prevalence rates. One study of Medicare beneficiaries over the age of 65 reported a cumulative prevalence of 494 cases per 100,000 in 2011 and an annual incidence of 94 cases per 100,000 person-years (5).
Very little is known about the population burden of IPF among U.S. Veterans. The Veterans Health Administration (VHA) is the largest integrated healthcare system in the United States and provides longitudinal comprehensive care to Veterans. It consists of 130 hospitals, more than 1,000 community-based outpatient clinics, and a network of purchased care from community providers, which together serve over 9 million patients annually (6, 7). In 2017, approximately 91% of Veterans were male and the mean age was 65 years. The mean age among the non-Veteran population was 42 years old, and less than half were male (7). Because IPF is a disease most frequently seen in older males (8), and because observational data has implicated exposures in the pathogenesis of disease (9–11), we hypothesized that the prevalence of IPF among Veterans may be higher than what has been reported in the general population.
In this study, we sought to describe the epidemiology (incidence, prevalence, and geographic distribution) of IPF among U.S. Veterans who receive care through the VHA and identify risk factors associated with IPF in the Veteran population.
Methods
The University of California San Francisco and the San Francisco Veterans Affairs (VA) Institutional Review Boards approved this study (IRB 20–30063).
Data Source and Patient Identification
We analyzed electronic health record data from Veterans who were enrolled in the VHA and had at least one inpatient or outpatient encounter at a VA facility or a non-VA facility paid for by the VA, between 2010 and 2019. We identified all patients who had an International Classification of Disease (ICD) diagnosis code for IPF (ICD-9-CM code 515, 516.3, 516.31 or ICD-10-CM code J84.111, J84.112, J84.89, J84.9, J84.10, J84.17) recorded between January 1, 2010 and December 31, 2019 (see Table E1 in the online supplement). We included the broader ICD-9 diagnosis code 515 and its ICD-10 equivalents (J84.89, J84.9, J84.10, J84.17) to improve sensitivity because these codes are often used during the initial workup of IPF (12). In our clinical experience with the VA in particular, Veterans with IPF may receive only these broader fibrosis codes.
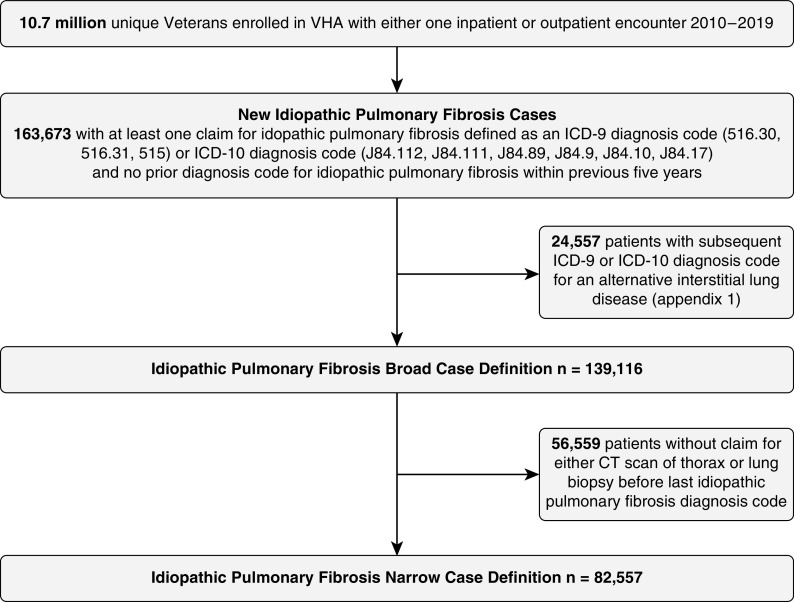
To refine the initial cohort, we used an algorithm similar to those used in other IPF population-based studies (5, 12, 13). Patients were considered to have IPF if they did not have any other diagnosis code for an alternative interstitial lung disease (Table E1) after the first IPF diagnosis code. This subgroup was labeled “broad case definition.” Among these Veterans, those who had procedure code for a lung biopsy or a computed tomographic (CT) scan of the thorax (Table E2) before the last IPF diagnosis were labeled “narrow case definition” (Figure 1). Of note, Veterans have several options for healthcare coverage, including dual enrollment, through both VA and non-VA (Medicare, Medicaid, or employer sponsored) health insurance plans. Estimates of dual use have ranged from 20% to 56% depending on patient demographics and services provided (14–16). Thus, for dual users, if CT scans or lung biopsies were conducted outside the VHA, these patients may not be captured by the narrow case definition.
Figure 1.
Veterans Health Administration idiopathic pulmonary fibrosis sample selection. VHA = Veterans Health Administration; ICD = International Classification of Disease; CT = computed tomography.
Covariates including age, sex, race, ethnicity, rural versus urban residence, location of medical care (VA medical facility, state, and region), and smoking history were obtained from electronic heath records. The VA defines rurality using the U.S. Department of Agriculture’s Rural-Urban Community Areas (RUCA) system (17). RUCA codes classify U.S. census tracts using measures of population density, urbanization, and daily commuting. All Veterans were categorized into rural versus urban residence based on home address at time of IPF diagnosis. A positive smoking history was defined as an ICD-9 or ICD-10 diagnosis code for tobacco use disorder any time prior to IPF diagnosis. We also included a positive tobacco use assessment from the health factor database as indicative of prior smoking history.
Statistical Analysis
We calculated the annual incidence and prevalence of IPF among Veterans between 2010 and 2019 using both the broad and narrow case definitions. Annual incidence was calculated by dividing the number of new cases of IPF by the number of patients without a prior diagnosis who had at least one encounter with the healthcare system during that calendar year. For annual prevalence, we first identified the number of patients who had at least one encounter with the healthcare system during the calendar year. From that denominator, we identified the number of patients who had an ICD diagnosis for IPF within the prior 5 years (numerator). IPF prevalence among each state’s Veteran population was calculated in 2014 (midpoint) and 2019 (most recent). We chose 2014 for midpoint prevalence to capture data prior to the ICD-9 to ICD-10 transition that occurred in October 2015.
To examine factors associated with IPF among the Veteran population, we performed multivariate logistic regression including age, race, ethnicity, smoking history, and rural residence as explanatory variables. Because of nonlinearity of the continuous variable age, we presented stratified odds ratios by decade. Because of interaction between sex and multiple variables (age, ethnicity, and tobacco use), and owing to the smaller proportion of females in this cohort, we stratified the multivariable models by sex and estimated marginal differences in IPF prevalence between males and females using regression standardization. All analyses were conducted using StataCorp Analysis Software version 16.1.
Results
Approximately 10.7 million Veterans who received care through the VHA between 2010 and 2019 were eligible for inclusion in this study (Figure 1). Among these Veterans, we identified 139,116 incident diagnoses of IPF using the broad case definition (1.26%) and 82,557 incident cases of IPF using the narrow case definition (0.77%). Demographics were similar between the broad and narrow case definition cohorts (Table 1). Compared to Veterans without IPF, Veterans with IPF were older and more likely to be non-Hispanic, White, and male. Approximately 80% of Veterans with IPF were current or former smokers.
Table 1.
Characteristics of U.S. Veterans with incident idiopathic pulmonary fibrosis (2010–2019)
| IPF Cohort |
||||
|---|---|---|---|---|
| Broad Case Definition | Narrow Case Definition | Control Population* | ||
| Total cases (n) | 139,116 | 82,557 | 10,650,206 | |
| Demographics | ||||
| Mean age at diagnosis in yr, (SD) | 70.7 (11.8) | 70.2 (11.0) | 61.5 (18.9) | |
| Sex, n (%) | ||||
| Male | 129,598 (93%) | 76,779 (93%) | 9,767,864 (91%) | |
| Female | 9,513 (7%) | 5,776 (7%) | 918,867 (9%) | |
| Race, n (%) | ||||
| White | 108,071 (78%) | 64,435 (78%) | 7,068,839 (66%) | |
| Black or African American | 14,338 (10%) | 8,989 (11%) | 1,479,129 (14%) | |
| Asian/Native Hawaiian or Pacific Islander/American Indian | 2,472 (2%) | 1,468 (2%) | 264,775 (3%) | |
| Unknown | 14,235 (10%) | 7,665 (9%) | 1,874,966 (18%) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 5,651 (4%) | 3,544 (4%) | 556,823 (5%) | |
| Not Hispanic or Latino | 120,343 (87%) | 72,036 (87%) | 8,347,958 (78%) | |
| Unknown | 13,122 (9%) | 6,977 (8%) | 1,782,928 (17%) | |
| Rural, n (%) | 55,598 (43%) | 32,252 (42%) | 3,463,456 (34%) | |
| Region, n (%) | ||||
| West | 27,900 (20%) | 15,074 (18%) | 2,369,694 (22%) | |
| Midwest | 36,531 (26%) | 19,131 (23%) | 2,439,238 (23%) | |
| South | 54,512 (39%) | 30,576 (37%) | 4,032,728 (38%) | |
| Northeast | 20,096 (14%) | 17,776 (22%) | 1,840,928 (17%) | |
| Surgical or Transbronchial Lung Biopsy | 3,029 (2%) | 2,895 (4%) | 14,502 (0.1%) | |
| Tobacco Use | 109,895 (79%) | 69,144 (84%) | 5,543,334 (52%) | |
Definition of abbreviations: IPF = idiopathic pulmonary fibrosis; SD = standard deviation.
Control population defined as Veterans with no IPF diagnosis codes. All P values less than 0.001 comparing broad case definition to control population. P values were calculated with t test for continuous variables and chi-square test for categorical variables.
VHA Incidence and Prevalence of Idiopathic Pulmonary Fibrosis
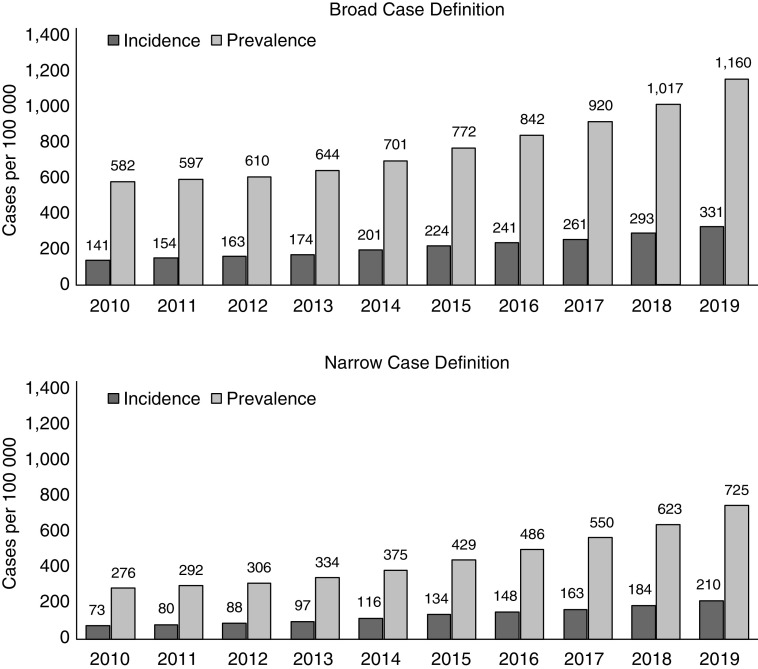
The annual incidence and prevalence of IPF increased between 2010 and 2019 (Figure 2). Among the broad case definition subgroup, incidence increased from 141 cases per 100,000 person-years in 2010 to 331 cases per 100,000 person-years in 2019. Annual prevalence also rose from 582 cases per 100,000 in 2010 to 1,160 cases per 100,000 in 2019. The incidence and prevalence of IPF was lower in the narrow case definition cohort; however, the increase in incidence and prevalence rates over time mirrored that seen in the broad case definition. Among the narrow case definition subgroup, incidence increased from 73 cases per 100,000 person-years in 2010 to 210 cases per 100,000 person-years in 2019. Annual prevalence rose from 276 cases per 100,000 in 2010 to 725 cases per 100,000 in 2019.
Figure 2.
Annual incidence and prevalence of idiopathic pulmonary fibrosis among U.S. Veterans, 2010–2019.
Risk Factors Associated with IPF Diagnosis
Older age, White race, a history of tobacco use, and rural residence were associated with higher odds of incident IPF for both male and female Veterans (Tables 2 and 3). The risk of incident IPF increased by decade until age 80, after which a plateau effect was observed. Among female Veterans, non-Hispanic ethnicity was associated with slightly higher odds of IPF. These trends held true for both the broad and narrow case definition. The average marginal difference in prevalence of IPF between the male and female sex, accounting for interactions, was small (0.0048), and in favor of the female sex.
Table 2.
Risk factors associated with incident idiopathic pulmonary fibrosis among male Veterans
| Broad Case Definition IPF: n = 129,598 Non-IPF: n = 9,767,864 |
Narrow Case Definition IPF: n = 76,779 Non-IPF: n = 9,767,864 |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) |
P Value | Odds Ratio (95% CI) |
P Value | |
| Age | ||||
| ⩽60 yr | (Reference) | (Reference) | ||
| >60–70 yr | 2.83 (2.78–2.89) | <0.001 | 3.11 (3.04–3.19) | <0.001 |
| >70–80 yr | 4.39 (4.30–4.47) | <0.001 | 4.77 (4.65–4.89) | <0.001 |
| >80 yr | 3.21 (3.15–3.28) | <0.001 | 2.86 (2.78–2.94) | <0.001 |
| Race | ||||
| White | (Reference) | (Reference) | ||
| Black or African American | 0.90 (0.88–0.91) | <0.001 | 0.93 (0.90–0.95) | <0.001 |
| Asian/Native Hawaiian or Pacific Islander/American Indian | 0.86 (0.82–0.90) | <0.001 | 0.86 (0.81–0.91) | <0.001 |
| Ethnicity | ||||
| Hispanic or Latino | (Reference) | (Reference) | ||
| Not Hispanic or Latino | 0.99 (0.96–1.02) | 0.44 | 0.94 (0.90–0.97) | 0.001 |
| Tobacco Use | 2.94 (2.89–2.98) | <0.001 | 4.02 (3.93–4.11) | <0.001 |
| Rural Residence | 1.26 (1.25–1.28) | <0.001 | 1.21 (1.19–1.23) | <0.001 |
Definition of abbreviations: CI = confidence interval; IPF = idiopathic pulmonary fibrosis.
Table 3.
Risk factors associated with incident idiopathic pulmonary fibrosis among female Veterans
| Broad Case Definition IPF: n = 9,513 Non-IPF: n = 918,867 |
Narrow Case Definition IPF: n = 5,776 Non-IPF: n = 918,867 |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) |
P Value | Odds Ratio (95% CI) |
P Value | |
| Age | ||||
| ⩽60 yr | (Reference) | (Reference) | ||
| >60–70 yr | 4.19 (3.95–4.44) | <0.001 | 4.72 (4.38–5.09) | <0.001 |
| >70–80 yr | 5.61 (5.15–6.10) | <0.001 | 6.33 (5.69–7.03) | <0.001 |
| >80 yr | 3.70 (3.35–4.09) | <0.001 | 2.99 (2.58–3.45) | <0.001 |
| Race | ||||
| White | (Reference) | (Reference) | ||
| Black or African American | 0.86 (0.80–0.92) | <0.001 | 0.87 (0.80–0.95) | 0.002 |
| Asian/Native Hawaiian or Pacific Islander/American Indian | 0.75 (0.64–0.89) | 0.001 | 0.81 (0.66–0.99) | 0.047 |
| Ethnicity | ||||
| Hispanic or Latino | (Reference) | (Reference) | ||
| Not Hispanic or Latino | 1.20 (1.05–1.37) | 0.007 | 1.18 (1.0–1.4) | 0.051 |
| Tobacco Use | 2.79 (2.64–2.96) | <0.001 | 3.47 (3.22–3.74) | <0.001 |
| Rural Residence | 1.24 (1.17–1.31) | <0.001 | 1.20 (1.12–1.29) | <0.001 |
Definition of abbreviations: CI = confidence interval; IPF = idiopathic pulmonary fibrosis.
Geographic Distribution
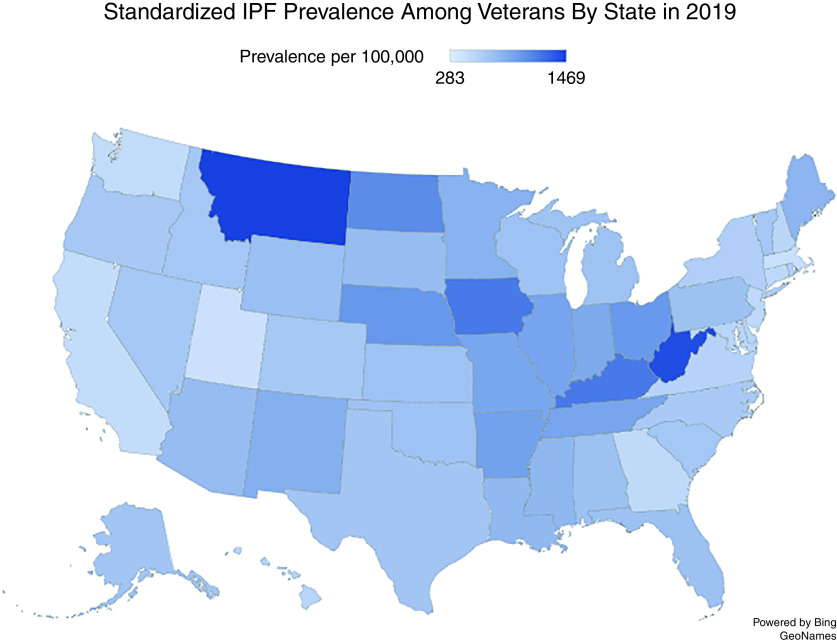
The geographic burden of IPF was heterogeneous across the United States (Figure 3), and Veterans with IPF were more likely than Veterans without IPF to live in rural areas. The 2019 standardized prevalence of IPF among the state’s Veteran population using the narrow case definition ranged from 430 cases per 100,000 in Utah (lowest) to 1,469 cases per 100,000 in Montana (highest). Compared with the midpoint prevalence in 2014, the prevalence of IPF among the Veteran population increased across all states in 2019; however, the states with the highest standardized prevalence rates (Iowa, Kentucky, Montana, and West Virginia) remained consistent between 2014 and 2019. A similar geographic distribution was seen when the broad case definition was used.
Figure 3.
Geographic distribution of prevalent idiopathic pulmonary fibrosis cases among U.S. Veterans (narrow case definition). State prevalence was calculated by identifying the number of unique Veterans with IPF in each state divided by total number of Veterans living in the state. IPF = idiopathic pulmonary fibrosis.
Discussion
This study represents the first comprehensive epidemiological analysis of IPF in the U.S. Veteran population. Among 10.7 million U.S. Veterans who received care through the VHA during the last decade, the annual incidence and prevalence of IPF substantially increased. Between 2010 and 2019, the prevalence doubled (from 582 to 1,160 cases per 100,000 using the broad case definition and from 276 to 725 cases per 100,000 using the narrow case definition), and incidence more than doubled (from 141 to 331 cases per 100,000 person-years using the broad case definition and from 73 to 210 cases per 100,000 person-years using the narrow case definition). Risk factors associated with IPF included older age, White race, rural residence, and a history of tobacco use. The effect of sex on odds of IPF was attenuated once accounting for other risk factors. We also found significant geographic heterogeneity of disease across the United States with a greater prevalence of IPF among Veterans living in rural compared with urban areas.
Prior studies that have examined the incidence and prevalence of IPF in the Medicare population (5) and among U.S. adults aged 18 to 64 (13) also demonstrated increasing prevalence over time, but little is known about the epidemiology of IPF among U.S. Veterans. One study of 760,621 Veterans who were deployed to combat operations in Iraq and Afghanistan reported that 0.3% were diagnosed with interstitial lung disease between 2002 and 2011 (18). However, the study focused exclusively on younger patients (mean age of 40 years old) with non-IPF interstitial lung disease. IPF is one of the most common interstitial lung diseases, and because prior literature has implicated age, male sex, and tobacco use in the pathogenesis, we hypothesized that incidence and prevalence may be higher when evaluating the Veteran population at large. Our estimates of incidence and prevalence are similar to what has been reported in Medicare data, suggesting that among cohorts demographically enriched for older patients, the population burden of IPF is likely higher than what has been described in registry-based studies, which have historically relied on individual patient recruitment, which has likely led to underestimation of true disease burden owing to selection bias of the referral base.
The rising incidence and prevalence of IPF among Veterans is likely due to a combination of factors, including increasing disease awareness and treatment motivation with the approval of antifibrotic therapies in 2014, an aging Veteran population, and the implementation of low-dose CT scans for lung cancer screening among smokers, which has been shown to detect incidental or subclinical pulmonary disease in up to 40% of Veterans (19). It is possible that the transitions from ICD-9 to ICD-10 codes in October 2015 also contributed to changes in coding practices, resulting in increasing incidence and prevalence rates. However, this is unlikely to be the sole driver as IPF rates were increasing before and after the transition year. With the recent expansion of lung cancer screening guidelines to include patients 50–80 years old, we hypothesize that detection of subclinical or early pulmonary fibrosis will further increase. This has important implications on healthcare systems that will need to develop the infrastructure to meet the specialty care needs of patients, both Veterans and non-Veterans, with pulmonary fibrosis.
Among Veterans, we found substantial variation in the geographic distribution of IPF across the United States. This has been noted in other large database studies, although the factors behind the geographic variability have not been elucidated (5). We also observed a higher prevalence of IPF in rural compared with urban areas. Rurality is not yet an established risk factor for IPF, but meta-analysis data has suggested an association between IPF and environmental factors such as agriculture, farming, livestock, wood dust, and metal dust (20). Exposures, whether combat, occupational, or residential, are of particular relevance to the Veteran population. Further work is needed to determine the extent to which exposures, underlying patient demographics, recognition, and/or coding differences contribute to the geographic heterogeneity of IPF and the higher prevalence of IPF in rural areas.
In our cohort, we noted that after accounting for interactions with age, race/ethnicity, and tobacco use history, the average marginal difference in prevalence between males and females was small (0.0048) and slightly favored females. Early epidemiological studies have previously demonstrated higher incidence and prevalence of IPF among men. This difference has been hypothesized to be driven by genetic and biologic predispositions or perhaps in part owing to differential exposures between men and women. However, few studies have used multivariable models to examine the association between male sex and incident IPF while controlling for other risk factors such as age and smoking. It is possible that once controlling for these risk factors, the association between male sex and IPF is attenuated. Alternatively, female Veterans may represent a unique subgroup that may not be generalizable to the non-Veteran population. The association between sex and IPF deserves further study.
This study used the strength of the VHA’s integrated healthcare system and data repository to identify IPF cases. Accurate identification of IPF cases using billing-code-based algorithms depends heavily on the characteristics of the underlying source population (21). For example, algorithms that successfully identify IPF cases in older patients seen at tertiary care centers may not perform as well in younger community-based cohorts because of an overall lower likelihood of disease. The algorithms used in our study are adapted from prior literature that has defined their performance across varying cohorts (5, 12, 13). The use of ICD-9 code 515 and its ICD-10 equivalents (J84.89, J84.9, J84.10, and J84.17) has been a particular point of discussion. A study that cross-referenced code-based diagnosis with review of electronic health records found that among a network of Mid-Atlantic Veterans, ICD-9 codes 516.3, 516.31, and 515 were commonly used for patients with pulmonary fibrosis (22). We thus included the 515 code to optimize sensitivity in our study and believe that it more closely approximates the true population burden of disease among the Veteran population. Because the underlying VHA population is predominantly older and White, with a high prevalence of tobacco use, there is a higher pre-test probability of IPF and likely a greater diagnostic specificity of the 515 code.
Limitations
Our study has a number of limitations. First, although previously validated in other cohorts, this ICD-based algorithm has not specifically been case validated in the VHA. Reassuringly, the broad and narrow case cohorts in the VHA were demographically consistent with IPF and the conclusions internally consistent with both approaches. Additionally, the estimates of incidence and prevalence are similar to what has been observed in other large population-based studies like Medicare. Second, we refined the algorithm by requiring either a CT scan or surgical lung biopsy prior to the last IPF diagnosis code (narrow case definition). Patients who have both VA and non-VA (Medicare, Medicaid, or employer-sponsored) health insurance may have completed a CT scan or lung biopsy outside the VA Healthcare System. If these scans or biopsies were paid for by the VA, they would be captured by our algorithm; however, if they were paid for by non-VA insurance, they would not be captured by our restrictive algorithm. Prior studies have suggested that the percentage of dual users of VA and non-VA health insurance ranges from 20% to 56% depending on patient demographics and services provided (14–16). For example, most Veterans over the age of 65 qualify for Medicare and although cost-sharing is usually lower with VA insurance, patients with complex medical conditions are more likely to be dual users. We thus suspect that the narrow case definition likely underestimates the prevalence of IPF among Veterans and that the “true” prevalence lies in between the broad and narrow case definitions.
Conclusions
The past decade has seen substantial progress in our understanding and treatment of IPF. However, the efficiency and speed with which we have been able to answer research questions has been limited by access to sufficiently large, demographically and clinically diverse patient cohorts. Like many relatively rare diseases, IPF has historically been studied primarily through the use of discrete tertiary-care-based patient cohorts that take years to accumulate, number at best in the hundreds, are methodologically incompatible, and lack generalizability. Clinical data sets from electronic health records and other registry sources represent a new and exciting opportunity to change the research paradigm by providing direct access to large, real-world, population-based patient cohorts and comprehensive data sets. They are also a critical component of a transformative, practice-based model of discovery and implementation known as the learning healthcare system that has been promoted by the National Academy of Medicine, the Agency of Healthcare Research and Quality, and the Food and Drug Administration (23, 24).
In this study, we used the strength of the VA learning healthcare system to establish a population-based cohort that can serve as the foundation for future work that uses data generated from patient–healthcare system interactions to more efficiently answer questions related to epidemiology, comparative effectiveness, and predictive analytics in IPF with the ultimate goal of improving care for Veterans with interstitial lung disease.
Footnotes
Supported by the Health Services Research and Development Quality Enhancement Research Initiative (QUE 15-283), Veterans Health Administration, Washington, D.C. Additional support provided by the Nina Ireland Grant for Lung Health and National Institutes of Health (NIH) HL127131. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Author Contributions: B.K., J.S.L., E.V., K.S., H.R.C., and M.A.W. contributed to study conception, study design, and data interpretation. N.Z. and M.A.W. contributed to data acquisition. B.K., N.Z., E.V., and M.A.W. contributed to analysis. B.K. drafted the report, and all authors revised it critically. All authors approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2. Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 3. Fernández Pérez ER, Daniels CE, Schroeder DR, St. Sauver J, Hartman TE, Bartholmai BJ, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest . 2010;137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaunisto J, Salomaa ER, Hodgson U, Kaarteenaho R, Myllärniemi M. Idiopathic pulmonary fibrosis--a systematic review on methodology for the collection of epidemiological data. BMC Pulm Med . 2013;13:53. doi: 10.1186/1471-2466-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med . 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 6.Veterans Health Administration. Providing Health Care for Veterans. https://www.va.gov/health/
- 7.National Center for Veteran Analysis and Statistics. https://www.va.gov/vetdata/
- 8. Esposito DB, Lanes S, Donneyong M, Holick CN, Lasky JA, Lederer D, et al. Idiopathic pulmonary fibrosis in United States automated claims. Incidence, prevalence, and algorithm validation. Am J Respir Crit Care Med . 2015;192:1200–1207. doi: 10.1164/rccm.201504-0818OC. [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, et al. Collaborating Centers Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Am J Epidemiol . 2000;152:307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 11. Kim JS, Podolanczuk AJ, Borker P, Kawut SM, Raghu G, Kaufman JD, et al. Obstructive sleep apnea and subclinical interstitial lung disease in the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Am Thorac Soc . 2017;14:1786–1795. doi: 10.1513/AnnalsATS.201701-091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ley B, Urbania T, Husson G, Vittinghoff E, Brush DR, Eisner MD, et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis. Case validation and improvement. Ann Am Thorac Soc . 2017;14:880–887. doi: 10.1513/AnnalsATS.201610-764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J . 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 14. Hynes DM, Koelling K, Stroupe K, Arnold N, Mallin K, Sohn MW, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care . 2007;45:214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 15. Thorpe CT, Gellad WF, Mor MK, Cashy JP, Pleis JR, Van Houtven CH, et al. Effect of dual use of Veterans Affairs and Medicare Part D drug benefits on antihypertensive medication supply in a national cohort of Veterans with dementia. Health Serv Res . 2018;53:5375–5401. doi: 10.1111/1475-6773.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thorpe JM, Thorpe CT, Schleiden L, Cashy J, Carico R, Gellad WF, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med . 2019;179:1584–1586. doi: 10.1001/jamainternmed.2019.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Economic Research Service. Rural-urban commuting codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 18. Pugh MJ, Jaramillo CA, Leung KW, Faverio P, Fleming N, Mortensen E, et al. Increasing prevalence of chronic lung disease in Veterans of the wars in Iraq and Afghanistan. Mil Med . 2016;181:476–481. doi: 10.7205/MILMED-D-15-00035. [DOI] [PubMed] [Google Scholar]
- 19. Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, King HA, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med . 2017;177:399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 20. Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc . 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 21. Ley B, Collard HR. House of cards? Testing fundamental assumptions in idiopathic pulmonary fibrosis epidemiology. Am J Respir Crit Care Med . 2015;192:1147–1148. doi: 10.1164/rccm.201508-1636ED. [DOI] [PubMed] [Google Scholar]
- 22. Bedoya A, Pleasants RA, Boggan JC, Seaman D, Reihman A, Howard L, et al. Interstitial lung disease in a Veterans Affairs regional network; a retrospective cohort study. PLoS One . 2021;16:e0247316. doi: 10.1371/journal.pone.0247316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med . 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 24. Bindman AB. The Agency for Healthcare Research and Quality and the development of a learning health care system. JAMA Intern Med . 2017;177:909–910. doi: 10.1001/jamainternmed.2017.2589. [DOI] [PubMed] [Google Scholar]