Abstract
Rationale
Prior work suggests that Black patients have more severe obstructive sleep apnea (OSA) upon clinical presentation. However, the extent to which this may reflect differences in symptoms or other standard measures of OSA risk is unclear.
Objectives
We assessed for racial disparities in OSA characteristics at time of initial clinical diagnosis.
Methods
Data from 890 newly diagnosed patients with OSA at an urban academic sleep center were included in this analysis. All patients completed a standardized questionnaire on demographics and sleep-related symptoms and underwent laboratory polysomnography. Symptom severity at the time of evaluation was compared across race and sex.
Results
Black men were underrepresented in the sleep lab, making up only 15.8% of the cohort and 31.3% of Black participants (P < 0.001). Despite this, Black men had the most severe OSA with a mean apnea hypopnea index of 52.4 ± 39.4 events/hour, compared with 39.0 ± 28.9 in White men, 33.4 ± 32.3 in Black women, and 26.2 ± 23.8 in White women (P < 0.001 for test of homogeneity). Black men also had the greatest burden of OSA symptoms with the highest mean Epworth Sleepiness Scale score (12.2 ± 5.9 versus 9.4 ± 5.2 in White men, 11.2 ± 5.9, in Black women, and 9.8 ± 5.6 in White women; P < 0.001). Compared with White men, Black men were 1.61 (95% CI [1.04–2.51]) times more likely to have witnessed apneas and 1.56 (95% CI [1.00–2.46]) times more likely to have drowsy driving at the time of OSA diagnosis.
Conclusions
At the time of clinical diagnosis, Black men have greater disease severity, suggesting delay in diagnosis. Further, the greater burden of classic OSA symptoms suggests the delayed diagnosis of OSA in Black men is not due to atypical presentation. Further research is needed to identify why screening methods for OSA are not equitably implemented in the care of Black men.
Keywords: racial disparity, sex disparity, obstructive sleep apnea, referral, sleepiness
Obstructive sleep apnea (OSA) is one of the most common respiratory diseases, and the prevalence of OSA continues to rise with the worsening obesity epidemic (1). Beyond its impact on daytime sleepiness and quality of life, OSA is an independent risk factor for hypertension, congestive heart failure, and stroke (2). A wealth of evidence demonstrates that Black individuals are disproportionately impacted by these cardiovascular diseases (3) and so stand to benefit to a much greater extent from early identification and treatment of underlying OSA. In fact, preliminary studies suggest that treatment of OSA may help reduce the disparity in hypertension between Black and White individuals (4, 5). The importance of accurately diagnosing OSA in Black individuals is further highlighted by evidence that Black individuals are at higher risk for developing OSA compared with White individuals (6, 7).
The process of obtaining an OSA diagnosis in the U.S. healthcare system can be cumbersome, and evidence suggests 80–90% of people with moderate to severe OSA are undiagnosed (8). Most primary care providers do not routinely screen for OSA in their clinical practice (9, 10). Whether these barriers disproportionately impact Black individuals—even though they potentially have the most to gain from diagnosis and treatment—is unclear. However, structural factors including access to health insurance, transportation to the sleep clinic and sleep lab, ability to take time off from work for medical care or find childcare to undergo overnight sleep study, along with mistrust in the healthcare system due to a legacy of medical mistreatment that create barriers to sleep medicine care are all known to disproportionately impact Black individuals over White individuals in U.S. society.
We assumed that more severe disease at the time of clinical presentation reflect delays in clinical diagnosis. Thus, we sought to evaluate differences in OSA severity, symptoms, and co-morbidities by race at the time of initial clinical diagnosis at an urban academic sleep center.
Methods
All adult patients referred to the University Hospitals Cleveland Medical Center sleep laboratories between February 2007 and December 2010 were approached to participate in a research study evaluating genetic risk factors for sleep disorders, the results of which have been previously published (11). This analysis includes the subset of the cohort who were diagnosed with obstructive sleep apnea (apnea hypopnea index [AHI] ⩾ 5 events/hour) via polysomnography and who self-identified as either Black or White. Participants with central sleep apnea (defined as the presence of an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for central sleep apnea [327.21, 327.22, or 327.27] on the polysomnogram or a central apnea index > 5 events/hour with the central apnea index > obstructive apnea index) were excluded from the study. This work was approved by the University Hospitals Cleveland Medical Center Institutional Review Board. All participants provided written informed consent.
Demographics, social history, medical history, sleep habits, and symptoms were obtained from a standardized questionnaire completed by all participants, which included the Epworth Sleepiness Score (ESS) (12). Body mass index (BMI) was calculated using measured height and weight.
Participants were asked about the presence and frequency of snoring, witnessed apneas, drowsy driving, and unrefreshing sleep using the Berlin Questionnaire (13). Participants were asked if they had been told if they snore and given choices of yes, no, or unsure. A positive response was compared with a negative response or being unsure. Patients reported frequency of snoring and feeling tired or fatigued after sleep in categories of never or nearly never, 1–2 times a month, 1–2 times a week, 3–4 times a week, or nearly every day. Responses were categorized as regular snoring or unrefreshing sleep if symptoms occurred at least 3–4 times a week. The questions regarding witnessed apnea or drowsy driving had binary (yes/no) choices. Comorbid conditions including hypertension, diabetes, depression, coronary heart disease, and congestive heart failure were documented using self-report of a physician diagnosis.
Overnight polysomnography was performed using the Rembrandt polysomnographic system. The recording montage included (F4-M1, C4-M1, O2-M1, F3-M2, C3-M2, and O1-M2) bilateral electrooculography, submental and bilateral anterior tibial electromyography, thoracic and abdominal respiratory inductance plethysmography, and finger pulse oximetry. Nasal airflow and nasal pressure were measured using an oro-nasal thermistor and a nasal cannula, respectively. Polysomnography was performed according to American Academy of Sleep Medicine (AASM) standards with sleep scored manually in 30-second epochs, and respiratory events were scored using AASM criteria. Apnea was defined as cessation of airflow for 10 seconds, and hypopnea was defined as a ⩾50% reduction in airflow accompanied by a ⩾3% drop in oxyhemoglobin saturation and/or arousal as per the alternative criteria in the 2007 AASM scoring manual (14).
Statistical Analysis
The cohort was stratified into four groups by race and sex. No imputation was performed for missing data. Comparisons across groups were performed using analysis of variance for continuous variables across all four groups or t test within each sex, while chi-squared tests were used for categorical variables. Univariate logistic regression was used to estimate the relative risk of witnessed apnea and drowsy driving in Black men compared with White men. All analyses were conducted in SAS version 9.4.
Results
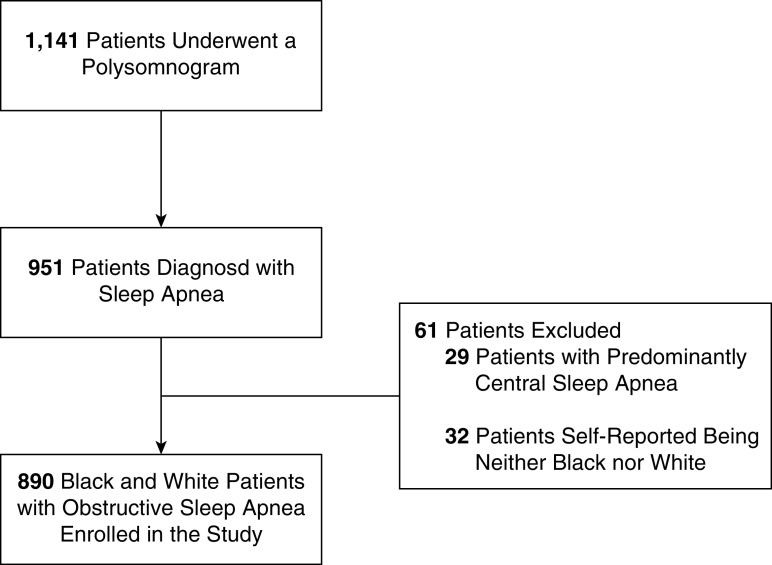
A total of 1,141 individuals presenting for evaluation of sleep disorders were enrolled into the study (Figure 1). Of these, 951 (83%) participants had an elevated AHI based on polysomnography. We excluded 29 participants with central sleep apnea. This analysis was restricted to 890 participants after excluding 32 participants who self-reported a racial identity other than Black or White or who did not report their race.
Figure 1.
Study flow diagram.
The mean age of the study participants was 51.4 ± 14.5 years and mean AHI was 36.4 ± 32.1 events/hour. As expected in a clinical population of patients with OSA, BMI was elevated with a mean of 37.5 ± 9.9 kg/m2. Over 50% of the cohort had hypertension, and diabetes mellitus was present in just under one quarter. The cohort was fairly equally split between Black (50.6%) and White participants (49.4%). Among White participants, 56% with OSA were male, while among Black participants, only 31% with OSA were male (P < 0.001). Thus, the overall demographics of patients with OSA was 15.8% Black men (n = 141), 27.9% White men (n = 248), 34.7% Black women (n = 309), and 21.6% White women (n = 192).
The demographic characteristics of participants stratified by race and sex are displayed in Table 1. Black participants were on average 5 years younger than White participants, with mean age ranging from 48.1 ± 13.9 years in Black men to 54.9 ± 13.3 years in White women (P < 0.001 for global comparison). BMI also differed across race and sex; mean BMI ranged from 33.9 ± 8.3 kg/m2 in White men to 41.4 ± 9.8 kg/m2 in Black women (P < 0.001 for global comparison). Hypertension and diabetes were more common in Black participants compared with White participants, while coronary artery disease was more common in men compared with women. In fact, Black women had the highest prevalence of diabetes (28.3%) while White women had the lowest (17.8%, P = 0.008). Depression prevalence varied substantially, ranging from 24.6% in Black men to 50.3% in White women (P < 0.001 for global comparison).
Table 1.
Demographics of participants
| Characteristic | Men |
Women |
||
|---|---|---|---|---|
| Black (n = 141, 15.8%) | White (n = 248, 27.9%) | Black (n = 309, 34.7%) | White (n = 192, 21.6%) | |
| Age (years) (n = 890) |
48.1 ± 13.9 | 53.2 ± 14.5 | 49.2 ± 14.8 | 54.9 ± 13.3 |
| Body mass index (kg/m2) (n = 888) |
38.0 ± 9.6 | 33.9 ± 8.3 | 41.4 ± 9.8 | 35.7 ± 10.2 |
| College degree (n = 872) |
23 (17.2%) | 129 (52.7%) | 38 (12.6%) | 90 (47.1%) |
| Employed (n = 850) |
85 (65.9%) | 172 (70.8%) | 134 (45.4%) | 115 (62.8%) |
| Regular bed partner (n = 846) |
80 (60.6%) | 163 (68.8%) | 116 (39.6%) | 108 (58.7%) |
| Co-morbid condition | ||||
| Depression (n = 873) |
33 (24.6%) | 74 (30.1%) | 107 (35.4%) | 96 (50.3%) |
| Hypertension (n = 878) |
82 (59.0%) | 115 (46.4%) | 195 (64.4%) | 88 (46.8%) |
| Diabetes (n = 883) |
29 (21.0%) | 46 (18.6%) | 87 (28.3%) | 34 (17.8%) |
| Coronary artery disease (n = 864) |
13 (9.6%) | 30 (12.5%) | 20 (6.7%) | 11 (5.9%) |
| Heart failure (n = 873) |
18 (13.1%) | 20 (8.1%) | 34 (11.3%) | 13 (6.8%) |
Values provided as mean ± standard deviation or number (percentage).
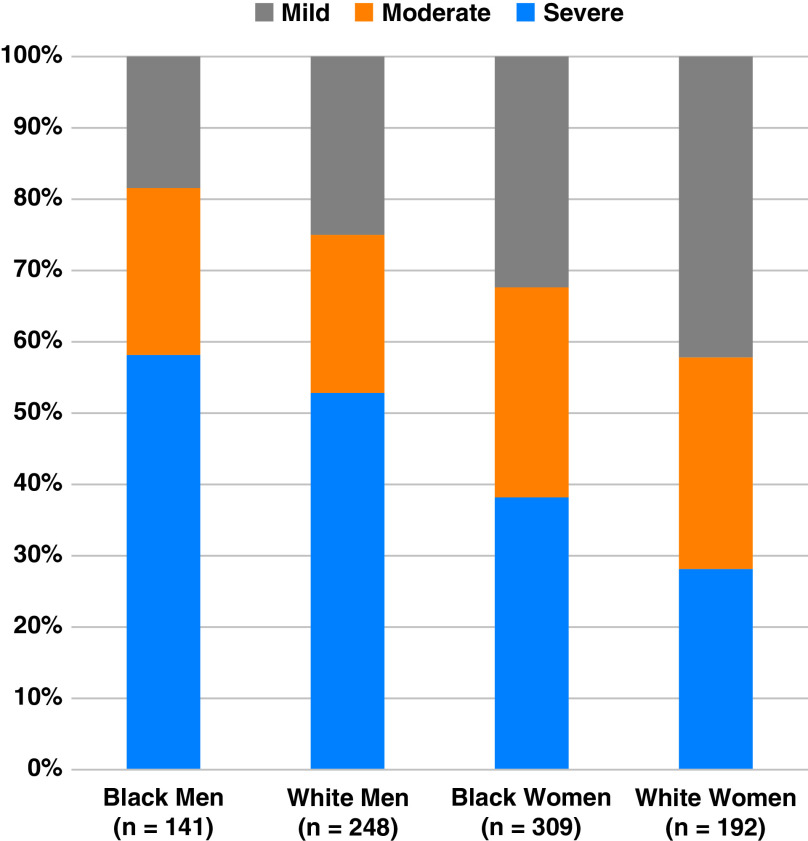
Despite their younger age, Black men had the greatest AHI, arousal index, and percent time with oxygen saturation < 90%, as well as the lowest minimum oxygen saturation at time of diagnosis (Table 2). Among Black men, mean AHI was 52.4 ± 39.4 events/hour compared with 39.0 ± 28.9 events/hour in White men, 33.4 ± 32.3 events/hour in Black women, and 26.2 ± 23.8 events/hour in White women (P < 0.001 for global comparison). When categorized, similar proportions of Black men and White men had severe OSA (58.2% versus 52.8%, P = 0.31, Figure 2). However, severe OSA was more prevalent in Black women compared with White women (38.2% versus 28.1%, P = 0.02).
Table 2.
Obstructive sleep apnea severity and symptoms
| Men |
Women |
||||||
|---|---|---|---|---|---|---|---|
| Black (n = 141, 15.8%) | White (n = 248, 27.9%) | P Value | Black (n = 309, 34.7%) | White(n = 192, 21.6%) | P Value | Global P Value | |
| AHI, events/h (n = 890) |
52.4 ± 39.4 | 39.0 ± 28.9 | < 0.001 | 33.4 ± 32.3 | 26.2 ± 23.8 | 0.004 | <0.001 |
| Arousal index, events/h (n = 847) |
39.7 ± 30.0 | 31.4 ± 20.7 | 0.005 | 24.6 ± 20.1 | 23.4 ± 15.4 | 0.47 | <0.001 |
| Minimum SpO2, % (n = 859) |
78 ± 11 | 82 ± 7 | <0.001 | 81 ± 8 | 83 ± 7 | 0.01 | <0.001 |
| Total sleep time with SpO2 < 90%, % (n = 832) |
14.8 ± 24.4 | 9.2 ± 16.7 | 0.02 | 6.3 ± 14.6 | 7.5 ± 16.7 | 0.44 | <0.001 |
| Epworth Sleepiness Scale score (n = 831) |
12.2 ± 5.9 | 9.4 ± 5.2 | <0.001 | 11.2 ± 5.9 | 9.8 ± 5.6 | 0.009 | <0.001 |
| Snores (n = 880) |
125 (90.6%) | 225 (92.2%) | 0.58 | 279 (91.2%) | 158 (82.3%) | 0.003 | 0.004 |
| Regular snoring (n = 737) |
108 (87.1%) | 174 (84.5%) | 0.51 | 214 (82.3%) | 120 (81.6%) | 0.86 | 0.58 |
| Witnessed apnea (n = 864) |
93 (68.4%) | 138 (57.3%) | 0.03 | 143 (47.5%) | 66 (35.5%) | 0.009 | <0.001 |
| Unrefreshing sleep (n = 869) |
97 (72.4%) | 165 (67.9%) | 0.37 | 218 (71.9%) | 145 (76.7%) | 0.24 | 0.25 |
| Drowsy driving (n = 876) |
49 (35.5%) | 63 (26.0%) | 0.05 | 63 (20.7%) | 40 (20.9%) | 0.94 | 0.005 |
Definition of abbreviations: AHI = apnea hypopnea index; SD = standard deviation; SpO2 = oxygen saturation as measured by pulse oximetry.
Values provided as mean ± SD or number (percentage). P values presented both comparing values within each sex and for a global test of homogeneity.
Figure 2.
Severity of obstructive sleep apnea by race and sex.
Black men also had the greatest burden of OSA symptoms (Table 1). Mean Epworth Sleepiness Scale score was highest in Black men at 12.2 ± 5.9 followed by Black women at 11.2 ± 5.9, White women at 9.8 ± 5.6, and White men (9.4 ± 5.2, P < 0.001 for global comparison). Both witnessed apneas and drowsy driving were more common in Black men than in the other three groups. Compared with White men, Black men had roughly 60% greater odds of reporting witnessed apneas (OR 1.61, 95% CI [1.04–2.51]) and drowsy driving (OR 1.56, 95% CI [1.00–2.46]) at the time of OSA diagnosis.
Discussion
In this large cohort study of newly diagnosed patients of an urban academic medical system with OSA, we found significant differences across race and sex in both symptoms and severity of OSA. In terms of disease severity, Black men had not only the highest AHI but also the greatest level of sleep fragmentation and the greatest degree of hypoxemia. However, despite a high burden of disease, Black men were the least represented among the four subgroups. In addition, Black women were 1/3 more likely to have severe OSA at the time of diagnosis compared with White women. These findings suggest that there is a substantial delay in diagnosing and evaluating OSA among both Black men and women compared with their White counterparts.
The delay in clinical diagnosis cannot be ascribed to atypical clinical presentation in that classic OSA symptoms were highly prevalent in both Black men and women. While snoring was near universal across groups, Black individuals, and particularly Black men, had greater levels of sleepiness, drowsy driving, and witnessed apneas. Racial disparities in excessive daytime sleepiness and drowsy driving have been well described (15, 16), and may relate in part to other contributors such as greater rates of shiftwork and shorter sleep durations (17). In terms of comorbidities, Black participants had higher rates of those comorbidities most closely associated with OSA, including greater severity of obesity and higher prevalence of hypertension. Given the greater symptom burden and greater comorbidities, the clinical suspicion for OSA would be expected to be much greater for Black participants in our cohort, raising the question why they were not evaluated and initiated on treatment earlier.
This interpretation that greater disease severity at diagnosis means greater delay in diagnosis assumes that OSA severity both based on physiologic indices and by symptoms progresses over time. While long-term natural history studies of untreated OSA are lacking, epidemiologic data do indicate that OSA severity increases as individuals age, in part due to increasing obesity over time (18, 19). In addition, short term studies do support the notion that untreated OSA worsens (20, 21).
Our findings are consistent with that of prior research. In a cohort of patients with OSA who presented to an academic sleep center in Detroit, Black patients were also found to have a higher mean AHI compared with White patients at the time of diagnosis. Black men younger than 39 years of age and between 50 and 59 years of age were found to have higher AHI than White men of the same age ranges (22). In addition to confirming the effects of race and sex on OSA, our study expands upon this work by further exploring the effect of race and sex on OSA symptoms at the time of diagnosis. Similarly, a prior study demonstrated greater OSA severity and symptoms at the time of clinical diagnosis in a safety-net sleep clinic as compared with a clinic serving a middle-class population with health insurance (23).
The greater symptoms of OSA and higher severity of disease on polysomnography among Black participants compared with White participants suggests a referral bias may be present rather than a difference in clinical presentation between the two groups. The Cleveland Family Study enrolled family members of both Black and White individuals with known sleep apnea along with family members of their neighbors. Black individuals were found to have greater sleep apnea severity at a younger age than White individuals (6). These results suggest that symptoms of OSA among Black patients are present long before they arrive at sleep laboratories for evaluation.
One explanation for our findings may be that Black patients are not being referred for clinical sleep evaluation as frequently as White patients. Recent work suggests that Black patients experience more barriers in seeing their primary care physicians compared with White patients (24) that may prevent them from being fully assessed. Primary care physicians also refer their Black patients to specialists less frequently than their White patients (25). A national survey of outpatient physician visits found the percentage of visits in 2007–2010 related to sleep apnea was 25% lower among Black patients compared with White patients (26). Prior work examining primary care referrals to polysomnography among high-risk individuals did not show a difference between Black and White patients (9). However, only 19% of all of the high-risk patients underwent referral (compared with 63% for mammograms and 83% for endoscopies), suggesting a need for improvement in recognition of the importance of OSA screening across the board. The study was conducted in a safety-net hospital system where there may have been greater awareness of the effects of race, socioeconomic factors, and social determinants of health on patient outcomes.
A study examining a convenience sample of 105 independent community physicians practicing in a single large metropolitan area reported a high level of recognition of the need for OSA evaluation and a high referral rate of patients with OSA (75%) (27). Neither race, nor age, nor physician specialty was associated with referral. However, the factor most strongly associated with referral was patient inquiry about OSA, which increased referral odds more than 9-fold. Thus, since physicians appear to rely on patients to stimulate referral, not asking the physician about OSA due to such reasons as lack of awareness about it or feeling it inappropriate or uncomfortable to bring up the topic—may lead to delayed diagnosis. Studies of Black patients suggest knowledge gaps about sleep apnea are common (28–30), which makes it incumbent upon physicians to take the initiative on matters related to referral while being sensitive to biases that may be preventing them from doing so.
Knowledge gaps about the importance of OSA may also lead to uncompleted referrals. Black patients may be less likely to follow up for polysomnography after a referral is made: In a retrospective study of a hospital-based sleep clinic in New York City, only 38% of Black patients referred by their physicians arrived at the sleep clinic (31). However, among those patients who underwent polysomnography, 91% were diagnosed with OSA severe enough to require treatment.
Many people remain confused about OSA, including its diagnosis and management. A survey of Black and White couples attending a health fair in Chicago found that Black participants were more likely to believe that snoring was normal while their bed partners were less likely to report having to leave the room due to snoring (32). A focus group study conducted in Brooklyn, New York found that many Black participants considered OSA to be a form of age-associated insomnia caused by some bedtime activities (29). Regarding the sleep laboratory, many participants expressed concerns regarding being watched while they slept and sleeping in an unfamiliar environment.
Many systematic and structural barriers may also prevent patients from seeking testing for OSA (33). Nighttime access to the sleep lab when public transportation options are often limited, obtaining overnight child or eldercare to pursue overnight testing, and taking time off from work, particularly for shift workers, can all limit access. In addition, many people may feel unsafe sleeping away from home. The rise of portable monitoring has facilitated home-based diagnosis of OSA. With similar accuracy to in-laboratory polysomnography, portable monitoring may increase adherence to diagnostic sleep testing among diverse populations by removing many of the barriers people presently experience. In a study of Black patients in Chicago who underwent both home and in-lab testing, home portable testing was the preferred diagnostic modality in 87% of patients (34). Unfortunately, this diagnostic modality remains unavailable for many patients.
Strengths of the study include its large sample size of Black and White participants, ascertainment of race through participant self-report, and detailed collection of polysomnographic data. In addition, the study population was broadly reflective of the underlying referral population—our cohort was 50.6% White and 49.4% Black while the city of Cleveland is 40.0% White and 48.8% Black.
Limitations to this study should be considered in interpreting our results. This was a cohort study of patients referred to sleep laboratories of an urban academic medical center. The results may not be reflective of patients attending sleep laboratories of other healthcare systems. However, the results closely align with those of other investigators. Although the criteria for scoring hypopneas used in this study are outdated, a recent meta-analysis comparing the criteria used with the currently recommended scoring criteria found extremely similar diagnostic performance (35). In addition, we did not exclude patients with OSA who had comorbid obesity hypoventilation syndrome due to a lack of reliable information about this diagnosis. Comorbid conditions were noted by self-report and may have been subject to recall bias. Only Black and White participants were included in this analysis. It is unclear how other races and ethnicities are affected by OSA. Our study focused on classic symptoms of OSA and did not assess different clusters of OSA symptoms (36, 37).
Conclusions
Black individuals present with greater symptoms of OSA on clinical presentation and more severe OSA on polysomnography. Future work should determine the reasons behind delayed diagnoses and test interventions to mitigate these delays to achieve health equity. Such interventions will need to be effective among both women and men.
Footnotes
Supported by National Institutes of Health (NIH) grants HL007901, HL124767, HL127307, HL081385, MD002265, and TR002548.
Author Contributions: J.D.T., K.A.D., and S.R.P. were responsible for study concept, design, analysis, interpretation of data, and drafting of the manuscript. S.T.S., A.S., and J.C.S. assisted with data acquisition and interpretation as well as drafting of the manuscript. G.J.S. and S.R.P. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the analysis. All authors critically revised the manuscript and approved the version submitted for publication.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol . 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol . 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation . 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 4. Prasad B, Carley DW, Krishnan JA, Weaver TE, Weaver FM. Effects of positive airway pressure treatment on clinical measures of hypertension and type 2 diabetes. J Clin Sleep Med . 2012;8:481–487. doi: 10.5664/jcsm.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rasmussen-Torvik LJ, De Chavez PJD, Kershaw KN, Montag SE, Knutson KL, Kim KA, et al. The mediation of racial differences in hypertension by sleep characteristics: Chicago area sleep study. Am J Hypertens . 2016;29:1353–1357. doi: 10.1093/ajh/hpw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med . 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep . 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep . 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 9. Thornton JD, Chandriani K, Thornton JG, Farooq S, Moallem M, Krishnan V, et al. Assessing the prioritization of primary care referrals for polysomnograms. Sleep . 2010;33:1255–1260. doi: 10.1093/sleep/33.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mold JW, Quattlebaum C, Schinnerer E, Boeckman L, Orr W, Hollabaugh K. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med . 2011;24:138–145. doi: 10.3122/jabfm.2011.02.100095. [DOI] [PubMed] [Google Scholar]
- 11. Patel SR, Goodloe R, De G, Kowgier M, Weng J, Buxbaum SG, et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: the Candidate Gene Association Resource (CARe) PLoS One . 2012;7:e48836. doi: 10.1371/journal.pone.0048836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep . 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med . 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15. Hayes AL, Spilsbury JC, Patel SR. The Epworth score in African American populations. J Clin Sleep Med . 2009;5:344–348. [PMC free article] [PubMed] [Google Scholar]
- 16. Genaurdi Mv, Althouse AD, Sharbaugh MS, Ogilvie RP, Patel SR. Exploring the mechanisms of the racial disparity in drowsy driving. Sleep Health . 2018;4:331–338. doi: 10.1016/j.sleh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol . 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 18. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA . 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 19. Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med . 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 20. Svanborg E, Larsson H. Development of nocturnal respiratory disturbance in untreated patients with obstructive sleep apnea syndrome. Chest . 1993;104:340–343. doi: 10.1378/chest.104.2.340. [DOI] [PubMed] [Google Scholar]
- 21. Kales A, Cadieux RJ, Bixler EO, Soldatos CR, Vela-Bueno A, Misoul CA, et al. Severe obstructive sleep apnea--I: Onset, clinical course, and characteristics. J Chronic Dis . 1985;38:419–425. doi: 10.1016/0021-9681(85)90137-7. [DOI] [PubMed] [Google Scholar]
- 22. Pranathiageswaran S, Badr MS, Severson R, Rowley JA. The influence of race on the severity of sleep disordered breathing. J Clin Sleep Med . 2013;9:303–309. doi: 10.5664/jcsm.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg H, Fleischman J, Gouda HE, De La Cruz AE, Lopez R, Mrejen K, et al. Disparities in obstructive sleep apnea and its management between a minority-serving institution and a voluntary hospital. Sleep Breath . 2004;8:185–192. doi: 10.1007/s11325-004-0185-1. [DOI] [PubMed] [Google Scholar]
- 24. Wisniewski JM, Walker B. Association of simulated patient race/ethnicity with scheduling of primary care appointments. JAMA Netw Open . 2020;3:e1920010. doi: 10.1001/jamanetworkopen.2019.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landon BE, Onnela JP, Meneades L, O’Malley AJ, Keating NL. Assessment of racial disparities in primary care physician specialty referrals. JAMA Netw Open . 2021;4:e2029238. doi: 10.1001/jamanetworkopen.2020.29238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care survey 1999-2010. Sleep . 2014;37:1283–1293. doi: 10.5665/sleep.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams NJ, Nunes JV, Zizi F, Okuyemi K, Airhihenbuwa CO, Ogedegbe G, et al. Factors associated with referrals for obstructive sleep apnea evaluation among community physicians. J Clin Sleep Med . 2015;11:23–26. doi: 10.5664/jcsm.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sawyer AM, Canamucio A, Moriarty H, Weaver TE, Richards KC, Kuna ST. Do cognitive perceptions influence CPAP use? Patient Educ Couns . 2011;85:85–91. doi: 10.1016/j.pec.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw R, McKenzie S, Taylor T, Olafiranye O, Boutin-Foster C, Ogedegbe G, et al. Beliefs and attitudes toward obstructive sleep apnea evaluation and treatment among blacks. J Natl Med Assoc . 2012;104:510–519. doi: 10.1016/s0027-9684(15)30217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baron KG, Gilyard SG, Williams JL, Lindich D, Koralnik L, Lynch EB. Sleep-related attitudes, beliefs, and practices among an urban-dwelling African American community: a qualitative study. Sleep Health . 2019;5:418–425. doi: 10.1016/j.sleh.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jean-Louis G, von Gizycki H, Zizi F, Dharawat A, Lazar JM, Brown CD. Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med . 2008;4:421–425. [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman M, Bliznikas D, Klein M, Duggal P, Somenek M, Joseph NJ. Comparison of the incidences of obstructive sleep apnea-hypopnea syndrome in African-Americans versus Caucasian-Americans. Otolaryngol Head Neck Surg . 2006;134:545–550. doi: 10.1016/j.otohns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 33. Billings ME, Cohen RT, Baldwin CM, Johnson DA, Palen BN, Parthasarathy S, et al. Disparities in sleep health and potential intervention models: a focused review. Chest . 2021;159:1232–1240. doi: 10.1016/j.chest.2020.09.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garg N, Rolle AJ, Lee TA, Prasad B. Home-based diagnosis of obstructive sleep apnea in an urban population. J Clin Sleep Med . 2014;10:879–885. doi: 10.5664/jcsm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mansukhani MP, Kolla BP, Wang Z, Morgenthaler TI. Effect of varying definitions of hypopnea on the diagnosis and clinical outcomes of sleep-disordered breathing: a systematic review and meta-analysis. J Clin Sleep Med . 2019;15:687–696. doi: 10.5664/jcsm.7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J . 2014;44:1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pien GW, Ye L, Keenan BT, Maislin G, Björnsdóttir E, Arnardottir ES, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic Sleep Apnea Cohort. Sleep . 2018;41:1–13. doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]