Abstract
Objective
Pre‐existing neutralising antibodies (NAbs) to adeno‐associated viruses (AAVs) remain an impediment for systemically administered AAV‐mediated gene therapy treatment in many patients, and various strategies are under investigation to overcome this limitation. Here, IgG‐degrading enzymes (Ides) derived from bacteria of the genus Streptococcus were tested for their ability to cleave human IgG and allow AAV‐mediated transduction in individuals with pre‐existing NAbs.
Methods
Cleavage activity of three different Ides was evaluated in vitro in serum from different species. Passively immunised mice or non‐human primates (NHP) with naturally occurring anti‐AAV NAbs were used to define the optimal IdeS dose and administration window for AAVAnc80 and AAV8 vectors in mice and AAV3B in NHPs.
Results
The selected candidate, IdeS, was found to be highly efficient at cleaving human IgG, less efficient against NHP IgG and inefficient against mouse IgG. In vivo, we observed differences in how IdeS affected liver transduction in the presence of NAbs depending on the AAV serotype. For AAVAnc80 and AAV3B, the best transduction levels were achieved when the vector was administered after IgG digestion products were cleared from circulation. However, for AAV8 we only observed a modest and transient inhibition of transduction by IdeS cleavage products.
Conclusion
Preconditioning with IdeS represents a unique treatment opportunity for patients primarily excluded from participation in gene therapy clinical trials because of elevated circulating anti‐AAV NAb levels. However, careful determination of the optimal IdeS dose and timing for the administration of each AAV serotype is essential for optimal transduction.
Keywords: AAV‐based gene therapy, IgG‐degrading enzyme, neutralising antibodies
In this study, we found that preconditioning with immunoglobulin‐degrading enzyme from Streptococcus pyogenes (IdeS) represents a unique treatment opportunity for patients primarily excluded from participation in gene therapy clinical trials due to elevated levels of anti‐adeno‐associated virus (AAV) neutralizing antibodies. However, we showed that careful determination of the optimal IdeS dose and timing for the administration of each AAV serotype is essential for optimal transduction.

Introduction
Adeno‐associated viral (AAV) vector‐based gene therapies are becoming the ‘state‐of‐the‐art’ treatment for inherited diseases including haemophilia, neuromuscular pathologies and retinopathies. 1 Several promising clinical trials are currently under way and two products, Luxturna™ and Zolgensma™, recently received market approvals in Europe and the USA. 2 An important advantage of AAV over other viral vectors is that it allows long‐term and sustained transgene expression. 3 However, one major limitation of systemic AAV administration is the presence of neutralising antibodies (NAbs) against the vector, as they interfere with AAV‐mediated gene delivery and diminish or abrogate its therapeutic effect. Even patients who qualify for primary treatment will develop NAbs that exclude the administration of a second dose of vector, if needed. 4 This particularly concerns pediatric patients, in which organ growth results in significant dilution of the vector genomes over time. Therefore, maintaining the ability to re‐administer the AAV vector is an important goal to achieve a long‐term therapeutic effect.
In recent years, strategies have been investigated to overcome the effect of anti‐AAV antibodies; however, while some of these approaches were promising in animal models, none were fully efficacious and came with limitations. 5 Hence, an effective, safe and easy‐to‐use strategy would be a big step forward.
Immunoglobulin (Ig)‐degrading enzymes (Ides) comprise a family of enzymes used by bacteria of the genus Streptococcus to escape the host immune defenses. To date, several Ides have been described, including IdeS, 6 IdeZ, 7 , 8 IgdE (family) 7 , 8 and IdeP. 9 While all Ides cleave Igs in specific sequences in the hinge region, some display a high degree of substrate specificity, reflecting the narrow host tropism of the Streptococcus species they are produced by; others have a broader specificity and degrade Ig from various species. IdeS and IdeZ cleave IgGs specifically below the hinge region by hydrolysing after Gly236 in both heavy chains, producing F(ab’)2 and two Fc/2 fragments, whereas hydrolysis by IgdE occurs at a specific site above the hinge region, thereby generating Fab and Fc fragments. 6 , 7 , 10 , 11
IdeS, also known as Mac‐1, is a 35 kDa cysteine IgG protease from Streptococcus pyogenes, 6 , 12 which cleaves all four hIgG subclasses. In vivo, full cleavage is completed within a few hours, after which IgG remains undetectable for about 24 h and then slowly rebounds to original levels within two weeks. 13 , 14 The use of IdeS in humans is considered to be safe, 13 and several clinical trials are evaluating its efficacy in transplantation immunology and autoimmune disorders such as anti‐glomerular basement membrane disease (www.clinicaltrials.com). In transplant patients with high donor‐specific antibody titres and thus highly sensitised towards the graft organ, treatment with IdeS could prevent organ rejection. 14 , 15 , 16 IdeS (imlifidase) is now indicated in the EU for desensitisation treatment of highly sensitised adult kidney transplant patients with positive crossmatch against an available deceased donor. Recently, two studies described how IdeS and IdeZ enable successful gene delivery in the presence of pre‐existing anti‐AAV antibodies. 17 , 18
Leborgne et al. 17 demonstrated in a first proof‐of‐concept study that a single dose of IdeS can reduce pre‐existing anti‐AAV NAb titres sufficiently to permit liver transduction in mice passively immunised with human intravenous IgG (IVIg) and in non‐human primates (NHP) with NAbs acquired naturally or by AAV pre‐dosing. In mice, using an AAV8 vector carrying the human factor IX (hFIX) transgene, IdeS treatment restored plasma hFIX levels to levels observed in seronegative animals. Furthermore, in seropositive NHPs using the same vector (AAV8‐hFIX) or AAV‐LK03 carrying human factor VIII as transgene (AAVLK03‐hFVIII), liver transduction could be successfully achieved after IdeS administration. Using a different Ide, IdeZ, Elmore et al. 18 were able to partially rescue transduction by AAV in passively immunised mice and in NHP harbouring naturally acquired anti‐AAV NAbs.
Here, we further explore the potential of three Ides, IdeS, IdeZ and IgdE, for the elimination of pre‐existing anti‐AAV NAbs. IdeS was selected based on its superior cleavage of hIgG. While data obtained from a passive immunization mouse model supported previous findings, our results from both in vitro and in vivo studies cast doubt on NHP being a suitable model for IdeS studies. Importantly, this study highlights the importance of refining both the IdeS dose and the time window for AAV administration to achieve optimal AAV‐mediated transduction for each particular AAV serotype.
Results
In vitro analysis of Ide‐mediated cleavage of human IgG
The efficiency of commercially available Ides, IdeS, IdeZ and IgdE, to cleave hIgG was compared. To this end, purified hIgG and human serum (hS) were digested in vitro, and cleavage was assessed by western blot (WB) and Coomassie blue staining (Figure 1). Digestion by both IdeS and IdeZ resulted in the disappearance of intact hIgG; however, while the majority of hIgG was cleaved to F(ab')2, a small proportion still existed as single‐cleaved IgG (scIgG), especially in the IdeZ‐digested sample. IgdE was efficient on purified hIgG, but when using serum, a high proportion of IgG remained uncut. Based on these in vitro results, IdeS was selected for further studies, and both IdeZ and IgdE were discarded from further analysis.
Figure 1.

In vitro selection of best Ides for human IgG. Purified human IgG (hIgG) or human serum was digested with the Ides indicated as per the manufacturer's instructions. Samples were run under non‐reducing conditions in SDS‐PAGE (4–15%), and IgG cleavage was visualised by Coomassie blue stain or WB. Purified hIgG and untreated serum were loaded as controls. Sc, single‐cleaved. One out of the three repeat experiments is shown.
To optimise cleavage conditions, purified hIgG or hS was digested with different IdeS:hIgG ratios (µg:µg; Figure 2a, Supplementary figure 1). Complete cleavage, i.e. absence of IgG signal and appearance of the F(ab’)2 band, was achieved at ratios between 1:12.5 and 1:50, and it was only partial at 1:500. IdeS activity was comparable for all sera tested (N = 3 donors).
Figure 2.

Effect of timing on AAVAnc80 transduction efficiency in passively immunised and IdeS‐treated mice. (a) Purified human IgG, human serum or purified mouse IgG were digested with different ratios of IdeS:IgG (µg:µg) for 30 min at 37°C. Cleavage products were visualised by WB under non‐reducing conditions using a h/NHP‐specific anti‐IgG‐CH1 antibody (human) or anti‐mouse F(ab’)2. One out of the three human sera tested (Human serum #1) is shown. (b) Schematic overview of experiment: Mice were passively immunised with human serum (hS) and treated with IdeS (0.25 or 1 mg kg−1) 24 h later (D0). One (D1), three (D3) or seven (D7) days later, AAV‐Luc (1012 VG kg−1) was administered. Control animals received PBS, AAV only or hS followed by AAV. Animals were bled at the indicated time points after IdeS treatment and sacrificed on day 21 after AAV administration. (c) Cleavage of human IgG was followed by visualising hF(ab’)2 fragments in WB using a hF(ab’)2‐specific antibody. The experiment was performed once, N = 1 (PBS) or 2 (other groups) mice per group. (d) AAVAnc80‐specific NAbs in serum were determined at the time points indicated. Numbers reported are mean NAb titres on the day of vector administration. Luciferase activity in vivo was measured on days 7, 14 and 20 post‐AAV administration (e) and ex vivo upon sacrifice in liver extracts (f). Dotted lines indicate background levels of PBS‐injected animals (e and f only). Anti‐AAVAnc80 NAb titre of hS 1:1807. Two‐way ANOVA with multiple comparison and Bonferroni correction was used for luciferase activity in vivo and the Mann–Whitney U‐test was performed for all other panels. P‐values are only provided when significant: *< 0.05; **< 0.01; ***< 0.001; ****< 0.0001; C+, purified hIgG; hscIgG, human single‐cleaved IgG. (d–f) The experiment was performed once with 1 mg kg−1 and twice with 0.25 mg kg−1, N = 4 mice per group in each of the experiments.
Additionally, the efficacy of IdeS on purified mouse IgG (mIgG) was analysed in vitro using the same conditions as for hIgG (Figure 2a). Mouse IgG was not cleaved at any of the ratios tested, and hence the efficacy of IdeS to cleave mIgG in serum was not tested. Based on these results, mouse was not considered a suitable model to test the potential of IdeS to enable AAV transduction in subjects with pre‐existing NAbs.
IdeS dose and timing of AAV administration are important factors for IdeS efficacy in vivo
Subsequently, the effect of IdeS treatment on the transduction of two liver tropic AAVs (AAVAnc80 and AAV8) was tested in vivo. Since IdeS did not cleave mIgG, we passively immunised mice with hS positive for anti‐AAV neutralising activity and then administered IdeS or PBS 1 day later (Figure 2b). Animals were injected with 0.25 mg kg−1 of IdeS, a dose shown to be fully active in humans, 13 , 14 , 15 or a fourfold higher dose of 1 mg kg−1. Cleavage of hIgG was followed over time by WB detection of hIgG‐derived F(ab’)2 fragments using a hF(ab’)2‐specific antibody (Figure 2c). The level of cleavage was dose‐dependent: 12 h after treatment with the low dose (0.25 mg kg−1), faint bands of hF(ab’)2 and intact hIgG were detected, while the high dose (1 mg kg−1) resulted in complete cleavage. By day 1, the hF(ab’)2 band had disappeared in mice treated with the high dose, whereas for the low dose, traces of uncut hIgG were still visible at this time point.
Once the optimal time for vector administration had been determined, the effect of NAb cleavage on AAVAnc80 transduction was addressed. Animals injected with hS (D‐1) received a high or a low dose of IdeS 24 h later (D0) as described, and a single dose of AAVAnc80‐Luc [1012 viral genomes (VG) kg−1] one (D1), three (D3) or seven (D7) days after IdeS administration. The negative control group received hS and vector but no IdeS, and the positive control group was administered with AAVAnc80‐Luc only (maximum levels of transduction). NAbs were measured in all animals pre‐IdeS administration (D0) as well as prior to AAV vector injection (D1, D3 or D7; Figure 2d). One day after hS injection, all recipients had a mean NAb titre of 1:92. Low dose administration of IdeS reduced NAb levels progressively from pre‐treatment levels of 1:92 to 1:32 on D1, 1:27 on D3 and < 1:15 on D7, whereas the high dose of IdeS efficiently reduced NAb titres below 1:15 as early as D1.
Quantification of in vivo luciferase expression revealed that AAV‐mediated liver transduction did not occur in groups that received hS but no IdeS prior to vector administration (Figure 2e), while a clear luciferase signal was detected in the positive control group. In the groups receiving hS followed by the high dose of IdeS, no significant differences in luciferase expression were observed between the positive control group and the group that received AAVAnc80‐Luc on D7; however, expression was lower in animals that received the vector on D1 or D3. In groups treated with the low dose of IdeS, the highest levels of luciferase activity were observed when AAV was administered at D7 post‐IdeS. In comparison to the high dose group, luciferase activity was significantly lower in the low dose group administered with AAV on D7, suggesting a dose‐dependent effect. In all groups, levels remained stable during the observation period (Figure 2e). On the day of sacrifice, ex vivo luciferase activity was analysed in homogenised liver, corroborating the results obtained in vivo (Figure 2f). Analysis of AAV genomes and luciferase mRNA levels in liver correlated with luciferase activity (Supplementary figure 2).
Next, we tested the effect of IdeS on liver transduction by AAV8 using the same experimental setting as for AAVAnc80, i.e. same hS and administration scheme, but using only the high dose of IdeS (1 mg kg−1). Under these conditions, pre‐IdeS NAb titres against AAV8 in mice were about 1:200, and treatment with IdeS resulted in a reduction of NAbs to <1:15 on D1, D3 and D7, except for one mouse at D3. Surprisingly, quantification of in vivo luciferase activity revealed that no inhibition of transduction had occurred (Supplementary figure 3). Hence, the experiment was repeated using a hS with a higher anti‐AAV8 NAb titre. Pre‐IdeS titres averaged 1:569 (Figure 3a), and treatment with IdeS (1 mg kg−1) resulted in a reduction of AAV8‐specific NAb titres to 1:40 on D1 and <1:15 on D3 and D7 (Figure 3a). These titres were sufficient to significantly inhibit in vivo luciferase expression in the group injected with hS but not IdeS. Furthermore, when the vector was administered on D1 and D3 post‐IdeS, a significant reduction of luciferase expression was observed in IdeS‐treated mice compared to the positive group (AAV only) 7 days after AAV8 administration (Mann–Whitney U‐test P < 0.05) but not when vector was administered on D7 post‐IdeS (Figure 3b). However, at the end of the study, luciferase activity in all IdeS‐administered groups was comparable to that of the positive control group. This indicated that the digestion products present in circulation on D1 and D3 (Supplementary figure 4) only partially and transiently inhibited AAV8‐mediated liver transduction (Figure 3b). Ex vivo luciferase activity in liver homogenates, analysis of AAV genomes and luciferase mRNA levels corroborated these findings (Figure 3c and Supplementary figure 5).
Figure 3.

Restored AAV8 liver transduction upon IdeS treatment of passively immunised mice. Mice were injected with human serum (hS) with an anti‐AAV8 NAb titre of 1:8975 and were treated with 1 mg kg−1 of IdeS 24 h later (D0). On day one (D1), three (D3) or seven (D7) post‐IdeS administration, mice were injected with AAV8‐Luc (1012 VG kg−1). Positive control animals received AAV only and negative controls received hS followed by AAV. A group of mice received PBS only and was used to determine background levels. Mouse serum was obtained pre‐IdeS and pre‐AAV administration and mice were sacrificed on D21 after AAV administration. (a) AAV8‐specific NAbs in serum were determined at the indicated time points. Numbers are mean NAb titres before AAV administration. Luciferase activity in vivo was measured on D7, 14 and 20 post AAV administration (b) and upon sacrifice ex vivo in liver extracts (c). Dotted lines indicate background levels of PBS‐injected animals. The Mann–Whitney U‐test was used for anti‐AAV8 NAbs, and two‐way ANOVA with multiple comparison and Bonferroni correction was performed for luciferase activity in vivo. P‐values are only provided when significant: *< 0.05; **< 0.01; ***< 0.001; ****< 0.0001; C+, purified hIgG; hscIgG, human single‐cleaved IgG. N = 4 mice per group, the experiment was performed once.
To further understand the potential inhibitory effect of IgG cleavage products still present in the bloodstream at the time of AAV transduction, their neutralising capacity was evaluated in vitro. Human sera containing NAbs against AAV serotypes Anc80, 8, 2, 3B, 5 and 9 were digested with IdeS and their neutralising activity was assessed (Figure 4). Albeit reduced, cleavage products on average retained more than 50% of their neutralising capacity (Figure 4a–d and f). Interestingly, two out of three sera positive for NAbs against AAV5 did not show any reduction in neutralising activity (Figure 4e). A possible direct effect of IdeS on transduction was excluded by comparing the efficacy of AAV transduction in the absence or presence of the enzyme only (data not shown).
Figure 4.

In vitro analysis of neutralising activity of IdeS‐treated human sera. Human sera with known NAb titres for different serotypes were digested with IdeS in vitro (N = 4 per serotype). Undigested (hS) and digested sera (hS + IdeS) were then used in an anti‐AAVAnc80 (a), AAV8 (b), AAV2 (c), AAV3B (d), AAV5 (e) and AAV9 (f) NAb assay to determine the remaining neutralising activity of digestion products. One out of the four sera tested for AAV5 turned out to be seronegative and is not shown in the figure. The lines are matching pre‐ and post‐digestion NAb titres from the same serum. The % of remaining neutralising activity after IdeS treatment is given for each individual serum, and the average for each serotype is shown.
Evaluation of IdeS efficacy for reduction of neutralising activity in NHP
We next evaluated the capacity of IdeS to abrogate the anti‐AAV NAb inhibition effect on AAV transduction in a pre‐clinically relevant model with natural immunity to AAV, namely NHP.
Cleavage of purified IgG from NHP was analysed in vitro (Figure 5a). Although cleavage was observed, digestion of all IgGs was only achieved at IdeS:hIgG ratios (µg:µg) higher (1:6.25–1:3.125) than those used for the digestion of hIgG (Figure 5b). When testing IgG digestion in NHP serum (N = 4), IdeS was found to be 5‐ to 10‐fold less active than that in human (Figure 5c).
Figure 5.
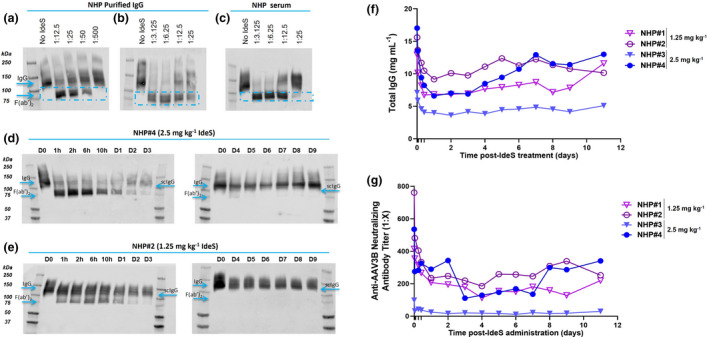
Optimisation of the therapeutic window and IdeS dose in NHP. Purified NHP IgG (a, b) or NHP serum (c) were digested at different ratios of IdeS:IgG (µg:µg) as indicated for 30 min at 37°C. Cleavage products were visualised by WB under non‐reducing conditions using an h/NHP‐specific anti‐IgG‐CH1 antibody. One exemplary NHP serum out of the four tested is shown. Four NHPs with known anti‐AAV3B NAb titres were treated with 2.5 or 1.25 mg kg−1 IdeS and bled at the indicated time points post administration. N = 2 NHP per group. (d, e) Cleavage products were visualised by WB under non‐reducing conditions using an h/NHP‐specific anti‐IgG‐CH1 antibody. One NHP treated with 2.5 mg kg−1 (d) and one treated with 1.25 mg kg−1 VTX‐PID (e) are shown. (f) Total IgG concentration in serum was quantified by nephelometry at the indicated time points, and individual data for each NHP at each time point are presented. (g) Specific anti‐AAV3B NAbs were quantified in serum. Individual data for each animal at each time point are shown. Each experiment was performed once.
Non‐human primates positive for anti‐AAV NAbs were administered with a dose of IdeS corresponding to 1.25 (NHP#1 and #2) or 2.5 mg kg−1 (NHP#3 and #4), selected based on the in vitro results and the dose used in patients (0.25 mg kg−1). 13 , 14 , 15 In both groups, IgG cleavage was detectable by WB from 1 h post administration of the protease (Figure 5d and e; corresponding SDS‐PAGE gels available in Supplementary figure 6); however, with neither dose full cleavage of IgG could be achieved. In NHP #3 and #4, administered with 2.5 mg kg−1, the lowest levels of intact IgG were found between D1 and D3 and the lowest levels of the F(ab’)2 fragment on D3 and D4, after which they were no longer detectable. The low dose resulted in even more limited IgG cleavage, accompanied by later appearance and faster clearance of F(ab’)2 and measurement of total IgG in serum confirmed these findings (Figure 5f). In addition, a dose‐dependent decrease in anti‐AAV3B NAb levels was observed 1 h post‐IdeS treatment (high dose group, 67% and 49% in NHP #3 and #4, respectively; low dose, 37% and 14% in NHP #1 and #2, respectively). For both IdeS doses, the lowest NAb levels were reached between D3 and D4 post‐IdeS administration (77% average decrease on D4; Figure 5g). Thus, 2.5 mg kg−1 was chosen as the most efficacious dose and D4 as the most appropriate time post‐IdeS treatment for AAV administration to study the sequential administration of IdeS and AAV in NHP.
The transduction efficacy of an AAV3B vector carrying a miniATP7B transgene (a therapeutic AAV vector for Wilson disease) 19 in combination with IdeS pre‐treatment was evaluated in NHP harbouring natural pre‐existing NAbs to AAV3B capsid. A total of five NHPs were used in the study. As controls, one NHP with a NAb titre of 1:1664 (#5) was injected with IdeS only and two NHP, one seronegative (#6) and one with a NAb titre of 1:107 (#7), received AAV3B‐miniATP7B only. Two NHP with anti‐AAV3B NAb levels of 1:108 (#8) and 1:696 (#9) were injected with IdeS, followed by AAV3B‐miniATP7B at a dose of 3 × 1012 VG kg−1 4 days later (Figure 6a). At the time points indicated, animals were bled to determine total IgG and anti‐AAV3B NAbs.
Figure 6.

IdeS administration cannot fully recover transduction in NHP. Two NHP with known anti‐AAV3B NAb titres were treated with 2.5 mg kg−1 IdeS followed by administration of an AAV3B vector (3 × 1012 VG kg−1) carrying a miniATP7B transgene 4 days later. One control NHP received only IdeS and a control group comprising two NHP received only AAV vector. Animals were bled at the indicated time points post IdeS administration and sacrificed on day 32 (D32). (a) Experimental set‐up. (b) Total IgG concentration in serum was quantified by nephelometry at the indicated time points and individual data for each NHP at each time point are presented. Specific anti‐AAV3B NAbs were quantified in serum (c). Individual data for each animal at each time point are shown. The arrow indicates AAV administration on D4. Transduction (d) and transgene expression (e) levels of miniATP7B were quantified by qPCR using GAPDH (d) and histone H3F3 (e) for normalisation; each data point represents the expression in one of the eight liver regions sampled. For all groups, the mean and standard deviation are shown. N = 1 NHP per group. The experiment was performed once.
The NHPs did not present with any adverse health problems during the study, and no major alterations were observed in serum biochemistry or hematological parameters (data not shown). The IgG concentrations measured in serum over time (Figure 6b) were in line with those obtained from the time course experiment (Figure 5f). Four days after IdeS treatment, IgG levels had dropped on average by 51.5% (range 46.8–56%) from basal level (D0) and then slowly recovered, returning to initial levels by the end of the study (D32). IgG cleavage was confirmed by SDS‐PAGE and, as previously observed, intact IgG was not completely cleaved at any of the time points of analysis (Supplementary figure 7). The band for the F(ab’)2 fragment disappeared after D4, and the levels of IgG started to progressively increase from D5 until complete recovery by D14.
Anti‐AAV3B NAb levels in IdeS‐administered NHPs dropped on average 23.8% by D4 (Figure 6c). In NHP #8, the NAb titre decreased from 1:108 to 1:42 after IdeS injection, in animal #9 from 1:696 to 1:88 and in animal #5 from 1:1664 to 1:335. In NHP #6, administered with AAV only, NAb levels steadily increased after vector injection and reached a plateau from D15 (data not shown). In animal #5 that received only IdeS, the NAb levels returned to the initial values by D15 (data not shown).
Four weeks after administration of the vector, the NHPs were sacrificed and the efficacy of liver transduction and transgene expression were evaluated in eight different liver regions (two from each of the four lobes). No transduction was observed in the untreated NHP #7 with pre‐existing NAbs (1:107). However, IdeS treatment of NHP #8, an animal with pre‐existing NAb levels equivalent to those of NHP #7 at the beginning of the study, restored transduction and transgene expression (Figure 6d and e), even though not to the same level observed in NHP #6, which had no pre‐existing NAbs. This may be due to the interindividual variation in transduction. Furthermore, although IdeS treatment lowered the NAb level from 1:696 to 1:88 in NHP #9, this was still high enough to prevent an efficient transduction and thus transgene expression. This observation strongly supports the importance of a careful selection of an adequate animal model to study IdeS efficacy and the need to fine tune the IdeS treatment regimen in relation to pre‐existing NAb levels in order to allow AAV transduction.
Discussion
With the first AAV‐based gene therapy products on the market and more products likely to become available in the coming years, NAb‐mediated immunity against AAV vectors, acquired either naturally or because of a previous AAV gene therapy treatment, has remained a major obstacle for the inclusion and systemic re‐administration of many patients in need.
Recently, treatment with IdeS was shown to be a promising tool to overcome pre‐existing anti‐vector immunity in the field of gene therapy. 17 , 18 Leborgne et al. 17 showed that the administration of AAV 1 day after IdeS treatment successfully rescued liver transduction in passively immunised mice and in NHP with naturally acquired immunity to AAV. Unfortunately, the NHP studies lacked a maximum transduction control, and therefore no conclusion can be drawn as to how levels compare to seronegative animals. In all experiments, cleavage of IgG by IdeS occurred fast, but only hIgG passively transferred to mice was completely cleaved, while in NHP a proportion of IgG remained uncut and F(ab’)2 clearance was complete at about day 4 after IdeS injection.
In the present paper, we extend these findings by evaluating the IdeS dosing and the kinetics of the clearance of cleavage products in function of varying pre‐existing NAb titres using different AAV serotypes and provide new important insights in the understanding of the action of bacterial‐derived IgG proteases as well as considerations for their potential application in a gene therapy setting.
Out of three Ides tested, IdeS, IdeZ and IgdE, IdeS proved to be the most efficient enzyme for the in vitro cleavage of hIgG. Even though Elmore et al. 18 partially rescued AAV‐mediated transduction using IdeZ in both mice and NHP, in our hands, this protease had a lower efficiency than IdeS in vitro and therefore was not further evaluated in vivo. IgdE, derived from Streptococcus agalactiae, is a protease optimised for the cleavage of the hIgG1. 10 Although hIgG1 represents about 60–66% of total hIgG, the amount of intact IgG detected after IgdE digestion was higher than 40%, suggesting that only partial cleavage occurred.
While the activity of IdeS on all IgG classes has been described for purified IgG and serum IgG from NHP, 17 , 20 we found it to be approximately 5‐ to 10‐fold less efficacious than when tested on hIgG. Even though cleavage of purified mouse IgG2a/c and IgG3 in vitro was previously demonstrated, 21 we did not observe IdeS activity on purified mIgG.
A passive immunisation approach in mice was used to define the optimal conditions for AAV vector administration following IdeS treatment. To resemble a more physiological situation, we used human sera with a high anti‐AAV NAb titre instead of an IVIg preparation as did Leborgne et al. 17 Analysis of cleavage in passively immunised mice treated with IdeS at two different doses showed that 0.25 mg kg−1 was not sufficient to fully cleave passively transferred hIgG. However, a fourfold higher IdeS dose (1 mg kg−1) cleaved almost all of the hIgG within 24 h post administration. The reduced effect of IdeS in passively immunised mice with a dose fully efficacious in patients (0.25 mg kg−1) 13 , 14 , 15 might be explained by the presence of some component in mouse serum that may interfere with IdeS activity, and by administering a higher dose, we were able to partially resolve this effect. Another potential explanation is that according to the guide for dose conversion between species, the dose administered in mice is below the recommended dose. 22
We here demonstrate that the timing for vector administration following IdeS treatment is critical for the full recovery of AAVAnc80‐mediated transduction. Our results indicate that the use of IdeS to prevent the inhibitory activity of NAbs requires the evaluation of the presence of cleavage products with potential neutralising properties, in particular scIgG and F(ab’)2 fragments, since the binding of the fragment to the AAV capsid is not expected to be fully altered. Complete cleavage generally occurs within 6–24 h after the administration of IdeS 13 , 14 , 15 , 17 (Figure 2c); however, the residual neutralising activity of the resulting F(ab’)2 fragments has not been deeply explored. Our results clearly point to an important role of both scIgG and F(ab’)2 fragments in the inhibition of AAVAnc80 transduction and stress the need for their complete clearance from the circulation in order to achieve full transduction efficacy. In support of such a mechanism of action, Ide‐cleaved specific anti‐streptococcal Abs were shown to maintain protective binding activity 23 and, albeit to a variable degree, also F(ab’)2 fragments specific for other pathogens/toxins can retain their binding capacity. 24 , 25 , 26 , 27 However, for other serotypes like AAV8, only a mild and transient inhibition on liver transduction by IdeS‐cleavage products was observed at the NAb titre tested. Interestingly, in vitro data for the two serotypes revealed that the cleavage products have similar capacity to inhibit transduction.
The differences found between AAV8 and AAVAnc80 might be associated with differences in their susceptibility to neutralisation by either intact IgGs or IgG cleavage products in vivo. As shown, while NAb titres of 1:92 against AAVAnc80 completely inhibit liver transduction (Figure 2), titres higher than 1:200 are necessary to block transduction by AAV8 (Supplementary figure 3). However, it is important to take into consideration that even though the NAb assays were performed under the same exact conditions for all the serotypes, the titres obtained from each of them cannot be directly compared in terms of inhibitory capacity in vivo. Differences in the stability of AAVAnc80 and AAV8 in circulation might also explain the observed discrepancies because the longer a vector remains in circulation, the higher the chance of transduction after the disappearance of the IgG cleavage products. In fact, when the vector was administered at D1 and D3 post‐IdeS, luciferase expression was lower than that in the positive control group (AAV only) 7 days after AAV injection, but was comparable at day 20. Additional experiments could help to clarify the mechanism underlying the different behaviour of the serotypes.
Interestingly, in vitro data for AAV5, a serotype considered to be advantageous for certain gene therapy applications as seroprevalence in the population is low, 28 , 29 revealed that digestion with IdeS did not result in a loss of neutralising activity in two out of three sera. This was independent of the initial NAb titre, and even though a limited number of samples were tested it suggests that at least for this serotype factor(s) other than IgG is/are responsible for limiting transduction.
Turning to a clinically more relevant model, the efficiency of IdeS in clearing pre‐existing anti‐AAV NAbs from the circulation was addressed in NHP. Even though we used an IdeS dose of 2.5 mg kg−1, a dose 10 times higher than the one found active in humans 13 , 14 , 15 (which is threefold higher if the dose conversion between species is applied), IgG was only partially cleaved. NAb titres were lowest on D3–D4 after administration of IdeS and, on average, dropped by 76.5% compared with basal levels. Efficient transduction was only obtained in an NHP that had pre‐existing NAbs of 1:108, but not in one with a NAb titre of 1:696. Despite presenting with a low basal NAb titre and turning seronegative after IdeS treatment, the transduction and transcription levels could not be fully rescued in the former animal; however, we cannot exclude that individual differences in the efficiency of AAV‐mediated transduction may have played a role in this case.
Taken together, our results confirm IdeS as a useful tool for achieving transient depletion of hIgG in subjects with inhibitory anti‐AAV NAb levels, thus allowing efficient AAV‐mediated transduction of the tissue targeted for gene therapy. Moreover, our data highlight the importance of adjusting both the dose of IdeS and the time window for subsequent vector administration in function of the AAV serotype selected, its dose and the corresponding inhibitory levels of NAbs.
Methods
Animals and use
Mice
C57BL/6 wild‐type males were purchased from Envigo (Barcelona, Spain). Six‐ to eight‐week‐old mice were used in all experiments. They were kept under controlled temperature and light, with water and food ad libitum. For all procedures, animals were anaesthetised by i.p. injection of a mixture of xylazine (Rompun 2%, Bayer, Barcelona, Spain) and ketamine (Ketamidor 100 mg mL−1, Equinvest; Barcelona, Spain) 1:9 v/v. All mouse studies were performed in accordance with Spanish and European legislative requirements. The experimental designs were approved by the Ethics Committee for Animal Testing of the University of Navarra.
Human serum and IgG protease (0.25 or 1 mg kg−1) were injected i.p. and AAV vector (1012 VG kg−1) was administered i.v. Blood collection was performed by submandibular bleeding, and serum samples were obtained after coagulation and centrifugation of total blood.
Non‐human primates
Six male and three female cynomolgus monkeys (Macaca fascicularis) with body weights of 2–3.5 kg and with known anti‐AAV3B NAb titres were purchased from BioPRIM (Baziège, France) and Camarney SL (Tarragona, Spain). All animals, their IDs and their respective NAb titres are listed in Supplementary table 1. They were housed communally at the animal facility at the Center of Applied Pharmaceutical Investigation for the duration of the study. They were kept under controlled temperature and light cycles, and food was provided twice daily with ad libitum access to water. For all procedures, animals were anaesthetised i.m. with ketamine (5 mg kg−1, Ketamidor 100 mg mL−1, Equinvest). Anaesthesia was maintained by inhalation of Sevofluran 2% (Abbott, Iran) in 60% O2 in a Servo Ventilator 900 D (Siemens). All in vivo experiments were approved by and performed in strict adherence to the guidelines of the Ethics Committee for Animal Testing of the University of Navarra.
IdeS (1.25 or 2.5 mg kg−1) was administered i.v. via the saphenous vein by slow infusion. AAV vector (3 × 1012 VG kg−1) was infused 4 days later. Blood collection was performed from the inguinal vein at the indicated time points.
Animals were sacrificed by i.v. administration of T61 (Intervet, Unterschleissheim, Germany). Gross necropsy was performed for visual examination of the major organs. Whole liver was weighed, and three pieces (< 20 mg each) from eight different regions were collected and snap frozen in liquid nitrogen and stored at −80°C for further analysis.
Enzymes, purified IgG and serum
Research grade IdeS (low endotoxin; #A0‐FR8‐050) was obtained from Genovis (Lund, Sweden) and Neurozon LLC (Ventura, CA, USA), and assays were run to assure both enzymes have identical cleavage efficiency. IdeZ (FabRICATOR Z; #A0‐FRZ‐020) and IgdE (FabALACTICA; #A0‐AG1‐020) were obtained from Genovis (Lund, Sweden).
Purified control IgG was purchased from the following providers: human (#I4506, Sigma‐Aldrich, St. Louis, MO, USA); mouse (BioRad, Kidlington, UK, #PMP01); NHP (#NBP1‐97047; Novus Biologicals, Abingdon, UK).
Serum samples of animal origin were obtained in‐house. Samples from patients included in the study were provided by the Biobank of the University of Navarra (Av Pio XII 55, 31008 Pamplona, Spain) and were processed following standard operating procedures approved by the Ethical and Scientific Committees under protocol number 2018.018.
IgG concentrations in human and NHP sera were determined by nephelometry (Immage800, CUN). IgG reagents (#446400; Beckman Coulter, Birmingham, UK) used for nephelometry are designed to quantify hIgG, and a calibration curve with the purified NHP control IgG (Novus Biologicals) was performed to assess the accuracy of IgG quantification in NHP samples.
AAV vectors
AAVAnc80 and AAV8 encoding the reporter protein luciferase (Luc) under the transcriptional control of cytomegalovirus (CMV) promoter were produced as described elsewhere. 19 AAV3B‐miniATP7B used in NHP studies was produced by Sirion Biotech (Planegg, Germany).
In vitro digestion of IgG by IgG proteases
About 25 µg of IgG – serum or purified controls – was incubated with IdeS or IdeZ in PBS for 30 min at 37°C as indicated. Digestion with IgdE was performed in 150 mM sodium phosphate buffer, pH 7.0 for 18 h at 37°C, according to the manufacturer’s protocol.
SDS‐PAGE and western blot
IgG cleavage was analysed by SDS‐PAGE followed by Coomassie blue staining or by western blot under denaturing or non‐denaturing conditions, as indicated. About 4–15% Mini‐PROTEAN® TGX™ Precast Protein Gels (#4561085, BioRad) were used for gel electrophoresis.
For Coomassie blue staining, a standard stain in Coomassie blue solution (0.1% Coomassie Brilliant Blue R‐250, 50% methanol and 10% glacial acetic acid) was performed.
For western blot analysis, protein was transferred using a wet transfer system (BioRad). After blocking with PBS/10% reconstituted skim milk powder, total IgG, scIgG, F(ab’)2 and Fab bands were detected with antibodies specific for each species (human, NHP: Captureselect™ biotin anti‐IgG‐CH1 conjugate, #7103202100, Thermo Fisher Scientific, Amsterdam, the Netherlands; mouse: goat anti‐mouse IgG Fab secondary Ab, #SA5‐10226, Thermo Fisher Scientific, Rockford, IL, USA), followed by either HRP conjugate (#SA10001, Thermo Fisher Scientific) or anti‐goat IgG‐peroxidase (#5420, Sigma‐Aldrich) as secondary Abs. Bands were revealed with SuperSignal West Pico Plus (#34580, Thermo Fisher Scientific) and analysed with an Odyssey imaging system Laia [(LI‐COR Biosciences (Lincon, NE, USA)].
Neutralising antibody assays
The measurement of NAbs was performed as described previously 29 with some modifications. In short, HEK293T cells were seeded in black 96‐well culture plates (#6005182; PerkinElmer, Waltham, MA, USA) coated with 0.25% poly‐L‐lysine (#A‐003‐E; Sigma‐Aldrich, Madrid, Spain) at a density of 104 cells/well in complete DMEM [supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (all GIBCO)] and incubated for 24 h at 37°C. The medium was then replaced with serum‐vector mixtures containing rAAV‐CMV‐Luc (serotype of interest, AAVAnc80, AAV8, AAV2, AAV3B AAV5 and AAV9) at MOI of 104 pre‐incubated for 2 h with heat‐inactivated, serially diluted serum samples. Cells were incubated for another 48 h at 37°C. Cells incubated with a seronegative serum and rAAV‐CMV‐Luc served as positive controls; cells incubated with medium only as negative controls. Supernatant was then removed and 50 μL (150 μg mL−1) of luciferin Quanty‐Luc (REPQLC2; InvivoGen, Morton, IL, USA) were added. Luminescence was measured after 2 min with a PhotonIMAGER™ (Biospace lab Nesles‐la‐Vallée, France) and processed with M3 Vision software. The anti‐AAV NAb titre was calculated as the lowest serum dilution that inhibited AAV transduction by at least 50% of the transduction obtained in the positive control.
Luciferase expression
In vivo
Animals were injected i.p. with 100 μL (30 mg mL−1) of luciferin (Quanty‐Luc, #REPQLC2; InvivoGen) and luciferase activity in vivo was measured non‐invasively with PhotonIMAGER™ equipment. Image analysis was performed with the M3 Vision program. A region of interest (ROI) was generated for the area to be quantified (i.e. liver region), and the same ROI was applied to all animals. The measurements were expressed as ph s−1 cm−2 sr−1.
Ex vivo
A volume of 10 μL of previously homogenised tissue samples was transferred into Röhren tubes (#55476; Sarstedt, Nümbrecht, Germany), to which 50 μL of Luciferase Assay Reagent (E4550; Promega, Madison, WI, USA) was added. Luciferase measurements were performed in the Lumat LB 9507 luminometer (Berthold) at an absorbance of 540 nm (Abs540).
Vector load
Extraction of total DNA from liver tissues was performed using the NucleoSpin Tissue kit (#740952.250; Macherey‐Nagel, Düren, Germany) according to the manufacturer´s protocol. Total RNA was extracted using the Maxwell® 16 LEV simplyRNA Tissue Kit (#AS1340; Promega) according to the manufacturer's instructions, and 1 μg RNA was reverse transcribed into complementary DNA (cDNA) using M‐MLV reverse‐transcriptase (#18057‐018; Invitrogen, Carlsbad, CA, USA).
Vector DNA genomes were quantified by means of real‐time qPCR, using GoTaq® qPCR Master Mix (#A6001; Promega) in a CFX96 Real‐Time PCR Detection System (Bio Rad) with primers specific for luciferase or ATP7B‐minigene. Mouse Gapdh or human/NHP GAPDH served as a reference gene for normalisation.
Luciferase (mouse) or miniATP7B (NHP) expression from liver cDNA was quantified by real‐time qPCR using the same specific primers for the detection of luciferase/ATP7B‐minigene. Mouse Gapdh or NHP histone H3F3A was used as housekeeping gene for normalisation.
All primers used in this study are listed in Supplementary table 2.
Statistics
Statistical analysis was performed using GraphPad Prism (San Diego, CA, USA) version 8.2. Unless stated otherwise, data are presented as mean ± standard deviation. Comparisons between two groups were made using a two‐tailed unpaired t‐test (Mann–Whitney). Two‐way ANOVA with multiple comparison and Bonferroni correction was used to assess statistical significance of in vivo luciferase activity. Statistical significance was assigned to P‐values < 0.05.
Conflict of Interest
The following authors are employees of Vivet Therapeutics: IRG, LTM, BT, AD, JPC, BB, VF and GGA.
Author Contributions
Irene Ros‐Gañán: Data curation; Formal analysis; Investigation; Validation; Visualization; Writing – review & editing. Mirja Hommel: Data curation; Project administration; Supervision; Writing – original draft; Writing – review & editing. Laia Trigueros: Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – review & editing. Blanche Tamarit: Formal analysis; Methodology; Writing – review & editing. Estefanía Rodríguez‐García: Data curation; Formal analysis; Investigation. David Salas: Data curation; Formal analysis; Investigation. Guiomar Pérez: Data curation; Formal analysis; Investigation. Anne Douar: Conceptualization; Supervision; Writing – review & editing. Jean‐Philippe Combal: Conceptualization; Funding acquisition; Resources; Writing – review & editing. Bernard Benichou: Conceptualization; Writing – review & editing. Veronica Ferrer: Formal analysis; Methodology; Supervision; Validation; Writing – review & editing. Gloria Gonzalez‐Aseguinolaza: Conceptualization; Formal analysis; Funding acquisition; Methodology; Supervision; Validation; Writing – review & editing.
Supporting information
Supplementary figure 1
Supplementary figure 2
Supplementary figure 3
Supplementary figure 4
Supplementary figure 5
Supplementary figure 6
Supplementary table 1
Supplementary table 2
Acknowledgments
The authors thank África Vales for production of AAV and the Government of Navarra for supporting IRG financially (PhD grant ‘Doctorados industriales 2019–2021’). We particularly acknowledge the patients for their participation and the Biobank of the University of Navarra for its collaboration.
References
- 1. Mendell JR, Al‐Zaidy SA, Rodino‐Klapac LR et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther 2021; 29: 464–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahryari A, Jazi MS, Mohammadi S et al. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet 2019; 10: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther 2020; 28: 723–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno‐associated virus (AAV) as a vector for gene therapy. BioDrugs 2017; 31: 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mingozzi F, High KA. Overcoming the host immune response to adeno‐associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu Rev Virol 2017; 4: 511–534. [DOI] [PubMed] [Google Scholar]
- 6. von Pawel‐Rammingen U, Johansson BP, Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J 2002; 21: 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lannergård J, Guss B. IdeE, an IgG‐endopeptidase of Streptococcus equi ssp. equi . FEMS Microbiol Lett 2006; 262: 230–235. [DOI] [PubMed] [Google Scholar]
- 8. Hulting G, Flock M, Frykberg L, Lannergård J, Flock JI, Guss B. Two novel IgG endopeptidases of Streptococcus equi: Research letter. FEMS Microbiol Lett 2009; 298: 44–50. [DOI] [PubMed] [Google Scholar]
- 9. Rungelrath V, Wohlsein JC, Siebert U et al. Identification of a novel host‐specific IgG protease in Streptococcus phocae subsp. phocae . Vet Microbiol 2017; 201: 42–48. [DOI] [PubMed] [Google Scholar]
- 10. Spoerry C, Hessle P, Lewis MJ, Paton L, Woof JM, Von Pawel‐Rammingen U. Novel IgG‐degrading enzymes of the IgdE protease family link substrate specificity to host tropism of Streptococcus Species. PLoS One 2016; 11: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincents B, von Pawel‐Rammingen U, Björck L, Abrahamson M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 2004; 43: 15540–15549. [DOI] [PubMed] [Google Scholar]
- 12. Lei B, DeLeo FR, Hoe NP et al. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat Med 2001; 7: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 13. Winstedt L, Järnum S, Nordahl EA et al. Complete removal of extracellular IgG antibodies in a randomized dose‐escalation phase I study with the bacterial enzyme IdeS ‐ a novel therapeutic opportunity. PLoS One 2015; 10: e0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorant T, Bengtsson M, Eich T et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti‐HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am J Transplant 2018; 18: 2752–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jordan SC, Lorant T, Choi J et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 2017; 377: 442–453. [DOI] [PubMed] [Google Scholar]
- 16. Lonze BE, Tatapudi VS, Weldon EP et al. IdeS (Imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high‐strength donor‐specific antibody. Ann Surg 2018; 268: 488–496. [DOI] [PubMed] [Google Scholar]
- 17. Leborgne C, Barbon E, Alexander JM et al. IgG‐cleaving endopeptidase enables in vivo gene therapy in the presence of anti‐AAV neutralizing antibodies. Nat Med 2020; 26: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 18. Elmore ZC, Oh DK, Simon KE, Fanous MM, Asokan A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG‐degrading enzyme. JCI Insight 2020; 5: e139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murillo O, Moreno D, Gazquez C et al. Liver expression of a MiniATP7B gene results in long‐term restoration of copper homeostasis in a Wilson disease model in mice. Hepatology 2019; 70: 108–126. [DOI] [PubMed] [Google Scholar]
- 20. Agniswamy J, Lei B, Musser JM, Sun PD. Insight of host immune evasion mediated by two variants of group A Streptococcus Mac protein. J Biol Chem 2004; 279: 52789–52796. [DOI] [PubMed] [Google Scholar]
- 21. Nandakumar KS, Johansson BP, Björck L, Holmdahl R. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo . Arthritis Rheum 2007; 56: 3253–3260. [DOI] [PubMed] [Google Scholar]
- 22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Pawel‐Rammingen U. Streptococcal IdeS and its impact on immune response and inflammation. J Innate Immun 2012; 4: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol 1984; 51: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlesinger JJ, Chapman S. Neutralizing F(ab’)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J Gen Virol 1995; 76: 217–220. [DOI] [PubMed] [Google Scholar]
- 26. Mazuet C, Dano J, Popoff MR, Créminon C, Volland H. Characterization of botulinum neurotoxin type A neutralizing monoclonal antibodies and influence of their half‐lives on therapeutic activity. PLoS One 2010; 5: e12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cui J, Zhao Y, Wang H et al. Equine immunoglobulin and equine neutralizing F(ab′)2 protect mice from West Nile virus infection. Viruses 2016; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boutin S, Monteilhet V, Veron P et al. Prevalence of serum IgG and neutralizing factors against adeno‐associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010; 21: 704–712. [DOI] [PubMed] [Google Scholar]
- 29. Salas D, Kwikkers KL, Zabaleta N et al. Immunoadsorption enables successful rAAV5‐mediated repeated hepatic gene delivery in nonhuman primates. Blood Adv 2019; 3: 2632–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1
Supplementary figure 2
Supplementary figure 3
Supplementary figure 4
Supplementary figure 5
Supplementary figure 6
Supplementary table 1
Supplementary table 2


