Abstract
Polycystic ovary syndrome is one of the most common endocrine and metabolic gynecological disorders, of which dysfunction of ovarian granulosa cells is a key contributing factor. The aim of the present study was to explore the role of ferrostatin-1 (Fer-1), a ferroptosis inhibitor, in a cell injury model established by homocysteine (Hcy)-induced ovarian granulosa KGN cell line and the potential underlying mechanism. Cell viability was measured using Cell Counting Kit-8 assay in the presence or absence of Hcy and Fer-1. Cell apoptosis was assessed using TUNEL staining and the expression levels of apoptosis-related proteins were measured using western blotting. To explore the effects of Fer-1 on oxidative stress in Hcy-treated ovarian granulosa cells, the levels of reactive oxygen species (ROS), malondialdehyde (MDA), lactate dehydrogenase (LDH) and glutathione (GSH) were measured using their corresponding kits. Furthermore, Fe2+ levels were assessed using Phen Green™ SK labeling and western blotting was performed to measure the protein expression levels of ferroptosis-associated proteins GPX4, SLC7A11, ASCL4 and DMT1. Subsequently, DNA methylation and ten-eleven translocation (TET) 1/2 demethylase levels were also detected to evaluate the extent of overall DNA methylation in ovarian granulosa cells after Hcy treatment. The TET1/2 inhibitor Bobcat339 hydrochloride was applied to treat ovarian granulosa cells before evaluating the possible effects of Fer-1 on TET1/2 and DNA methylation. Fer-1 was found to markedly elevate ovarian granulosa cell viability following Hcy treatment. The apoptosis rate in Fer-1-treated groups was also markedly decreased, which was accompanied by downregulated Bax and cleaved caspase-3 expression and upregulated Bcl-2 protein expression. In addition, Fer-1 treatment reduced the levels of ROS, MDA and LDH whilst enhancing the levels of GSH. Fe2+ levels were significantly decreased following Fer-1 treatment, which also elevated glutathione peroxidase 4 expression whilst reducing solute carrier family 7 member 11, achaete-scute family BHLH transcription factor 4 and divalent metal transporter 1 protein expression. Fer-1 significantly inhibited DNA methylation and enhanced TET1/2 levels, which were reversed by treatment with Bobcat339 hydrochloride. Subsequent experiments on cell viability, oxidative stress, Fe2+ content, ferroptosis- and apoptosis-related proteins levels revealed that Bobcat339 hydrochloride reversed the effects of Fer-1 on ovarian granulosa Hcy-induced cell injury. These results suggest that Fer-1 may potentially protect ovarian granulosa cells against Hcy-induced injury by increasing TET levels and reducing DNA methylation.
Keywords: polycystic ovary syndrome, ferrostatin-1, ferroptosis, oxidative stress, apoptosis
Introduction
Polycystic ovary syndrome (PCOS) is a prevalent and heterogeneous endocrine and metabolic gynecological disorder, which is also one of the most common causes of female infertility (1). PCOS affects 5–10% women of childbearing age globally and is characterized by hyperandrogenism, polycystic ovaries, irregular menstruation or amenorrhea, hirsutism and acne (2,3). In the absence of appropriate therapeutic intervention, PCOS increases the risk of acne scars, obesity, dyslipidemia, type 2 diabetes, cardiovascular disease and endometrial cancer (4,5). At present, available PCOS treatment strategies mainly focus on lifestyle changes, maintaining a healthy diet and chemotherapeutic drugs, such as metformin, oral contraceptive pills and spironolactone (6,7). However, the mechanism of PCOS pathogenesis is complex and remains to be fully elucidated (8). Therefore, it is of importance to understand the mechanisms underlying PCOS to provide novel insights into treatment strategies.
Granulosa cells are somatic cells of the sex cord and are closely associated with the development of oocytes (9). There is accumulating evidence that dysfunction of ovarian granulosa cells is an important contributing factor of PCOS (10,11). In addition, previous studies have also reported that high homocysteine (Hcy) levels, a sulfur-containing amino acid that has also been reported to be associated with insulin resistance and sex hormone level disorders (12), may be a significant triggering factor of PCOS (13,14).
Ferroptosis is a mechanism of cell death that is regulated by iron and oxidative stress that is characterized by increases in iron (Fe2+) content and lipid peroxidation (15,16) 4. Increased ferroptosis levels observed in granulosa cells is considered to be part of an important signaling pathway and mechanism in PCOS pathogenesis (17). High concentrations of Hcy has been documented to induce oxidative stress and ferroptosis in the nucleus pulposus by promoting methylase expression and glutathione (GSH) peroxidase 4 (GPX4) methylation, which prevent ferroptosis by converting lipid hydroperoxides into non-toxic lipid alcohols (18,19). Ten-eleven translocation (TET) 1 and TET2 are two dioxygenases that serve an important role in demethylating DNA sequences (20). However, their role in ovarian granulosa cell injury during PCOS remain to be elucidated. Therefore, the relationship among high Hcy levels, granulosa cell ferroptosis, TET activity and DNA methylation in PCOS pathology warrant further exploration.
Ferrostatin-1 (Fer-1) was the first aromatic amine that can effectively suppress ferroptosis, inhibit lipid peroxide accumulation and protect cells against numerous types of stress and/or toxic chemicals, such as erastin-induced cancer cell death and glutamate-induced neurotoxicity (21). Previous studies have reported the beneficial effects of Fer-1 on a number of diseases, such as dopaminergic neuroblastoma and cisplatin-induced acute kidney injury (AKI) by protecting against reactive oxygen species (ROS), apoptosis and ferroptosis (22,23). In the present study, ovarian granulosa cells treated with Hcy were used to investigate the effects of extracellular Fer-1 treatment on apoptosis, oxidative stress and ferroptosis. The mechanisms of TET activity and DNA methylation were also explored. The present study may provide a basis for the use of Fer-1 as a therapeutic agent for PCOS.
Materials and methods
Cell culture and treatment
The human ovarian granulosa KGN cell line was obtained from the American Type Culture Collection and maintained in DMEM containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2. It has been reported that Hcy levels may be a significant triggering factor of PCOS, where high concentrations of Hcy can induce oxidative stress and ferroptosis (12,18). In the present study, KGN cells were treated with various concentrations of Hcy (0.25, 0.5, 1, 2 and 4 mM, Shanghai Bang Jing Industrial Co., Ltd.; cat. no. BJ-S964101) with or without 5, 10 and 20 µM Fer-1 (APExBIO Technology LLC.; cat. no. A4371) for 24 h (24,25) at room temperature. KGN cells treated with 1 mM Hcy only were used as the model group. The HcY-induced cell models were divided into two groups, one group was added with 10 µM fer-1, the other group was added sequentially with both 10 µM Fer-1 and 40 nm final concentration of 10 µM TET1/2 inhibitor Bobcat339 hydrochloride (cat. no. T5198, TargetMol Chemicals Inc.). Both groups were incubated at 37°C for 24 h.
Cell viability assay
Cell viability was evaluated using a Cell Counting Kit-8 (CCK-8) assay. Cells were seeded into 96-well plates at a density of 6×103 cells/well. Following treatment with Hcy (0.25, 0.5, 1, 2 and 4 mM) and/or Fer-1 (5, 10 and 20 µM) for 6, 12, 24 and 48 h at 37°C, cells were incubated with CCK-8 reagent (10 µl/well; cat. no. HY-K0301, MedChemExpress LLC.) for 2 h at 37°C with 5% CO2. Absorbance of samples was examined at 450 nm using a microliter plate reader (Thermomax Molecular Devices).
Cell apoptosis assay
The TUNEL Staining kit (Beyotime Institute of Biotechnology) was used to detect cell apoptosis. Briefly, cells at a density of 2×106 were incubated with 4% paraformaldehyde for 30 min at room temperature, followed by treatment with 0.5% Triton X-100. Cells were subsequently incubated with 50 µl TUNEL reaction buffer for 1 h at 37°C. Nuclei were stained with DAPI (cat. no. C1005; Beyotime) at a concentration 5 µg/ml for 5 min at room temperature. The apoptosis-positive cells were indicated by green staining. Cells were washed 3 times with PBS for 3–5 min each and blocked with Antifade Mounting Medium (cat. no. C1005, P0126-5 ml; Beyotime). The images from randomly selected 5 fields were visualized and captured using a fluorescence microscope (magnification, ×200; Olympus Corporation).
Oxidative stress-related chemical quantification
Following cell treatment, the KGN cell culture supernatant was collected to determine the ROS, malondialdehyde (MDA), lactate dehydrogenase (LDH) and reduced GSH levels using specific commercial assay kits of ROS (cat. no. E004-1-1), MDA (cat. no. A003-1), LDH (cat. no. A020-2) and GSH (cat. no. A006-2-1) purchased from Nanjing Jiancheng Bioengineering Institute as per the procedures of manufacturer.
Fe2+ level analysis
Cellular Fe2+ levels were measured using the Phen Green™ SK reagent (cat. no. P14313, Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 50 µM according to the manufacturer's protocol. Briefly, Phen Green™ SK reagent was added to 2 ml cell-containing medium at 37°C for 1 h. The fluorescence response to Fe2+ ions was examined using a confocal laser scanning microscope (magnification, ×400; Zeiss LSM510; Carl Zeiss AG). Fluorescence randomly selected 5 fields was determined by an Image-Pro Premier software (Version 9.2; Media Cybemetics) at excitation and emission wavelengths of 488 and 520 nm, respectively.
TET1/2 secretion levels and methylated DNA quantification
ELISA was used to examine the secretion levels of TET1 and TET2 in the cell culture supernatant according to the manufacturer's protocols. These ELISA kits of TET1 (JC-E8295) and TET2 (JLC-G4351) were purchased from Gelatins Biological Reagent Co. A microplate reader was used to detect the optical density at 450 nm. DNA samples were isolated by PowerSoil DNA isolation kit (cat. no. 12888-50; Anbiosci Tech, Ltd.) and the the level of DNA methylation was detected by Methylight with a MethylFlash Methylated DNA Quantification kit (cat. no. P-1035, EpiGentek Group Inc.) according to the manufacturer's protocol. The results were normalized with the control group.
Western blot analysis
Cells were harvested using RIPA buffer (Beyotime Institute of Biotechnology) and protein concentrations were quantified using a Bicinchoninic Acid Protein kit (Beyotime Institute of Biotechnology). Protein separation (20 µg) was performed by 10% SDS-PAGE followed by transfer onto PVDF membranes (EMD Millipore). The membranes were blocked in 5% skim milk at 25°C for 1 h, and then probed with the corresponding primary antibodies overnight at 4°C. The HRP-conjugated secondary antibody (cat. no. 7074P2; 1:2,000; Cell Signaling Technology, Inc.) was added the next day and incubated at room temperature for 1 h. Blot images were captured using Pierce™ ECL Western Blotting Substrate (cat. no. 32209; Thermo Fisher Scientific, Inc.). GAPDH was the internal control and protein band images were analyzed using the Image J software (Version 1.8.0.172; National Institutes of Health). The relative intensity for cleaved caspase-3/caspase-3 was divided by the cleaved caspase-3 value by the value of caspase-3. The relative intensity for other proteins was divided by the corresponding target value by the value of GAPDH.
Anti-Bax (cat. no. 5023T; 1:1,000), anti-Bcl-2 (cat. no. 4223T; 1:1,000), anti-cleaved caspase-3 (cat. no. 9661T; 1:1,000), anti-caspase-3 (cat. no. 14220T; 1:1,000), anti-solute carrier family 7 member 11 (SLC7A11; cat. no. 12691S; 1:1,000) and anti-GAPDH (cat. no. 5174T; 1:1,000) antibodies were provided by Cell Signaling Technology, Inc. Anti-GPX4 (cat. no. sc-166570; 1:500) and anti-achaete-scute family BHLH transcription factor 4 (ASCL4; cat. no. sc-365230; 1:500) antibodies were purchased from Santa Cruz Biotechnology, Inc. Anti-divalent metal transporter 1 (DMT1; cat. no. 20507-1-AP; 1:1,000) antibody was the product of Proteintech Group, Inc.
Statistical analysis
All statistical analyzes were performed using the GraphPad Prism 8.0 software (GraphPad Software, Inc.). Data from ≥ three independent experiments was used. Data are presented as the mean ± SD. An unpaired t-test and one way ANOVA followed by Tukey's post hoc test were performed to statistically compare two or ≥ three independent groups, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Fer-1 treatment restores viability in Hcy-induced KGN cells
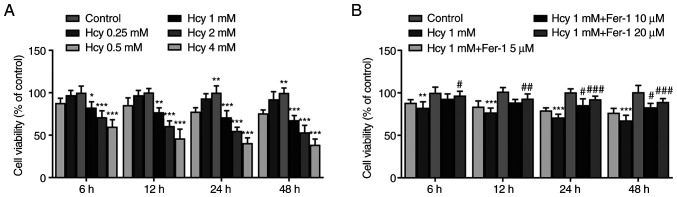
KGN cells were first treated with various concentrations of Hcy (0.25, 0.5, 1, 2 and 4 mM) for 6, 12, 24 and 48 h. Results from the CCK-8 assay demonstrated that cell viability was significantly reduced by Hcy treatment in a dose-dependent manner compared with that in the control group (Fig. 1A). At concentrations of Hcy >1 mM, cell viability decreased more potently (Fig. 1A). Therefore, 1 mM Hcy was chosen for subsequent experimentation. As presented in Fig. 1B, Fer-1 reversed the Hcy-induced decrease in cell viability in a dose-dependent manner, reaching significance at 10 and 20 µM. These results suggest that Fer-1 treatment can restore viability in Hcy-induced KGN cells.
Figure 1.
Fer-1 treatment restores viability in Hcy-induced KGN cells. (A) Cell viability was evaluated using CCK-8 assay following KGN cell treatment with different concentrations of Hcy for 6, 12, 24 and 48 h. (B) Cell viability was evaluated using CCK-8 assay following KGN cell treatment with 1 mM Hcy or 1 mM Hcy and different concentrations of Fer-1 for 6, 12, 24 and 48 h. *P<0.05, **P<0.01 and ***P<0.001 vs. Control. #P<0.05, ##P<0.01 and ###P<0.001 vs. model. Fer-1, ferrostatin-1; Hcy, homocysteine; CCK, Cell Counting Kit-8.
Fer-1 treatment attenuates apoptosis in Hcy-induced KGN cells
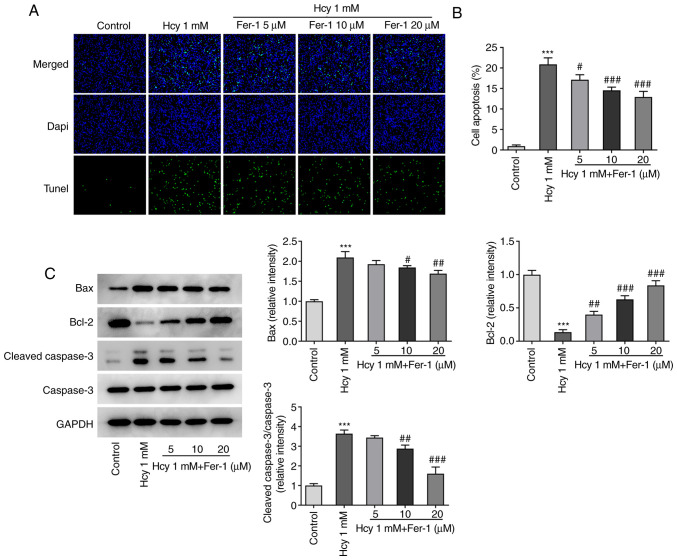
Subsequently, cell apoptosis was assessed using TUNEL staining. As demonstrated in Fig. 2A and B, the cell apoptotic rate was significantly increased in the model group compared with that in the control group. However, Fer-1 treatment significantly inhibited Hcy-triggered cell apoptosis compared with that in the model group in a dose-dependent manner. In addition, as demonstrated in Fig. 2C, Hcy treatment resulted in the significant downregulation of Bcl-2 expression and upregulation of Bax and cleaved caspase-3 expression, which was partially reversed following Fer-1 treatment in a dose-dependent manner, reaching significance at 10 and 20 µM. These results suggest that Fer-1 can potentially protect against Hcy-induced apoptosis in KGN cells.
Figure 2.
Fer-1 treatment inhibits cell apoptosis in homocysteine-treated KGN cells. (A) Cell apoptosis was evaluated using TUNEL staining, (B) which was quantified. Magnification, ×200. (C) Western blotting was performed to measure the levels of apoptosis-related protein expression. ***P<0.001 vs. Control; #P<0.05, ##P<0.01 and ###P<0.001 vs. Model. Fer-1, ferrostatin-1; Hcy, homocysteine.
Fer-1 treatment inhibits oxidative stress and ferroptosis in Hcy-induced KGN cells
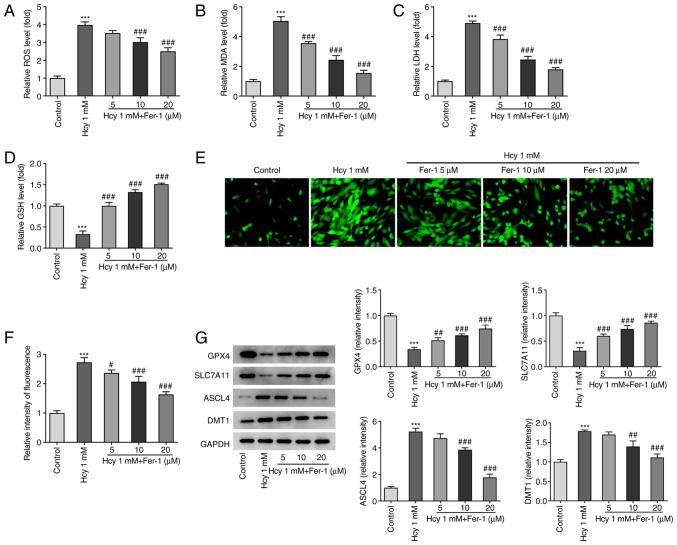
The effects of Fer-1 on oxidative stress and ferroptosis in Hcy-induced KGN cells were next investigated. As demonstrated in Fig. 3A-D, KGN cells treated with Hcy displayed significantly elevated ROS, MDA and LDH levels and reduced GSH levels compared with those in the untreated control group. However, Fer-1 treatment markedly reversed these effects of Hcy addition on oxidative stress marker levels, reaching significance at 10 and 20 µM. Furthermore, as shown in Fig. 3E and F, intracellular Fe2+ levels were significantly increased following Hcy exposure, but Fer-1 treatment significantly reduced this Hcy-induced increase in Fe2+ levels in a dose-dependent manner. In addition to significantly decreasing GPX4 and SLC7A11 expression levels, significantly increased ASCL4 and DMT1 expression levels were also observed in the Hcy model group compared with those in the control, which were significantly reversed following 10 and 20 µM Fer-1 treatment (Fig. 3G). These results suggest that Fer-1 treatment can alleviate Hcy-induced oxidative stress and suppress ferroptosis in KGN cells.
Figure 3.
Fer-1 treatment reduces oxidative stress and ferroptosis in homocysteine-induced KGN cells. The levels of (A) ROS, (B) MDA, (C) LDH and (D) GSH were measured using their corresponding commercial kits. (E) Intracellular Fe2+ levels were measured using Phen Green™ SK staining. Magnification, ×400. (F) Quantification of relative fluorescence intensity of Phen Green™ SK. (G) GPX4, SLC7A11, ASCL4 and DMT1 expression levels were measured using western blotting. ***P<0.001 vs. Control. #P<0.05, ##P<0.01 and ###P<0.001 vs. Model. Fer-1, ferrostatin-1; ROS, reactive oxygen species; MDA, malondialdehyde; LDH, lactate dehydrogenase; GSH, reduced glutathione; GPX4, glutathione peroxidase 4; SLC7A11, solute carrier family 7 member 11; ASCL4, achaete-scute family BHLH transcription factor 4; DMT1, divalent metal transporter 1.
Fer-1 treatment suppresses DNA methylation and elevates TET1/2 secretion levels in Hcy-induced KGN cells
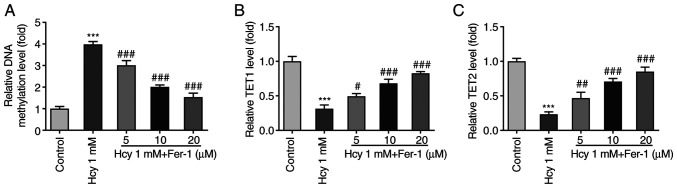
Overall DNA methylation levels in KGN cells were subsequently measured following Hcy treatment. As displayed in Fig. 4A, the DNA methylation levels were significantly enhanced in the Hcy-induced group compared with those in the control group. However, Fer-1 treatment significantly reversed this Hcy-induced DNA methylation were in a dose-dependent manner. Furthermore, TET1 and TET2 levels were significantly decreased following Hcy induction, which were subsequently restored following Fer-1 treatment in a dose-dependent manner (Fig. 4B and C). These results suggest that Fer-1 treatment can suppress DNA methylation and increase TET1/2 levels in Hcy-induced KGN cells.
Figure 4.
Fer-1 treatment suppresses DNA methylation and elevates TET1/2 levels in homocysteine-induced KGN cells. (A) DNA methylation levels were measured using MethylFlash Methylated DNA Quantification kit. The levels of (B) TET1 and (C) TET2 were assessed using ELISA. ***P<0.001 vs. control; #P<0.05, ##P<0.01 and ###P<0.001 vs. model. Fer-1, ferrostatin-1; TET, ten-eleven translocation.
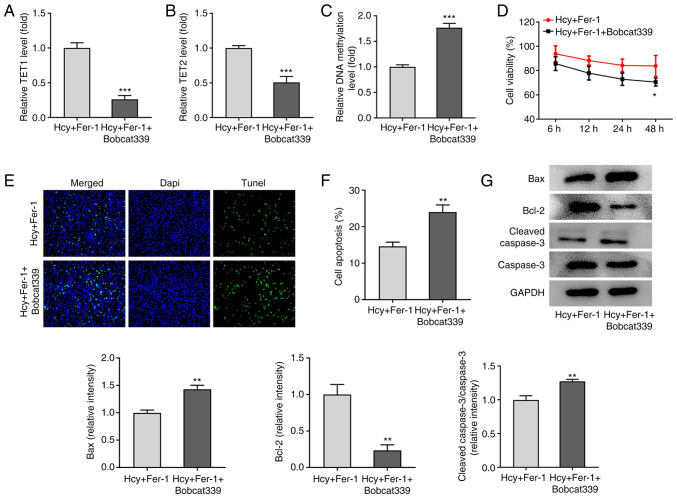
TET1/2 inhibitor Bobcat339 hydrochloride reverses the effects of Fer-1 on Hcy-induced apoptosis, oxidative stress and ferroptosis in KGN cells
To further explore the regulatory mechanism of Fer-1 on Hcy-induced apoptosis, oxidative stress and ferroptosis, the TET1/2 inhibitor Bobcat339 hydrochloride was used to treat Hcy-induced KGN cells. As shown in Fig. 5A-C, the results demonstrated that in the presence of Fer-1, Bobcat339 hydrochloride treatment significantly reduced TET1 and TET2 levels whilst significantly elevating DNA methylation levels. In addition, as presented in Fig. 5D, cell viability was significantly decreased following 48 h treatment with Bobcat339 hydrochloride in Hcy and Fer-1 treated KGN cells. TUNEL and western blotting assays demonstrated that compared with that in the Hcy and Fer-1 treated group, a significant increase in cell apoptosis in the Fer-1 + Bobcat339 hydrochloride group was observed, which is accompanied with significantly downregulated Bcl-2 and upregulated Bax and cleaved caspase-3 protein expression levels (Fig. 5E-G).
Figure 5.
Bobcat339 hydrochloride reverses the inhibitory effects of Fer-1 on homocysteine-induced apoptosis of KGN cells. The levels of (A) TET1 and (B) TET2 were measured using ELISA. (C) DNA methylation levels were determined using MethylFlash Methylated DNA Quantification kit. (D) Cell viability was assessed using a Cell Counting Kit-8 assay. (E) Cell apoptosis was evaluated using TUNEL staining, (F) which was quantified. Magnification, ×200. (G) Western blotting was performed to determine the levels apoptosis-related protein expression, which was quantified. *P<0.05, **P<0.01 and ***P<0.001 vs. Fer-1. Fer-1, ferrostatin-1; Hcy, homocysteine; TET, ten-eleven translocation.
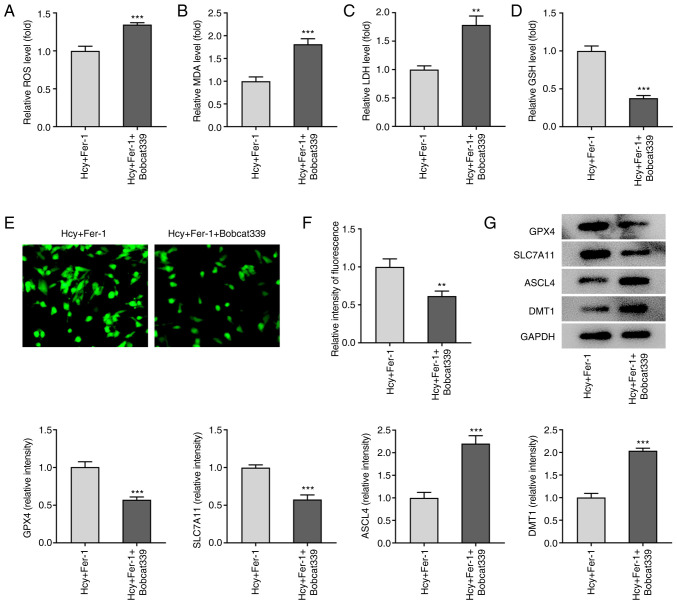
Furthermore, as demonstrated in Fig. 6A-D, Bobcat339 hydrochloride significantly enhanced ROS, MDA and LDH levels but reduced GSH levels compared with those in the Fer-1 group in Hcy-stimulated KGN cells. Fe2+ levels were also significantly decreased following Bobcat339 hydrochloride treatment, whilst GPX4 and SLC7A11 protein expression levels were significantly decreased (Fig. 6E-G). By contrast, ASCL4 and DMT1 protein expression levels were significantly increased by Bobcat339 hydrochloride treatment compared with those in the Fer-1 group in Hcy-stimulated KGN cells (Fig. 6E-G). These results suggest that Bobcat339 hydrochloride can reverse the effects of Fer-1 on Hcy-induced apoptosis, oxidative stress and ferroptosis in KGN cells.
Figure 6.
Bobcat339 hydrochloride reverses the inhibitory effects of Fer-1 on ferroptosis in homocysteine-induced KGN cells. The levels of (A) ROS, (B) MDA, (C) LDH and (D) GSH were determined using their corresponding commercial kits. (E) Intracellular Fe2+ levels were determined using Phen Green™ SK staining. Magnification, ×400. (F) Quantification of the relative Phen Green™ SK fluorescence intensity. (G) Protein expression levels of GPX4, SLC7A11, ASCL4 and DMT1 were measured using western blotting, which was quantified. **P<0.01 and ***P<0.001 vs. Fer-1. Fer-1, ferrostatin-1; ROS, reactive oxygen species; MDA, malondialdehyde; LDH, lactate dehydrogenase; GSH, reduced glutathione; GPX4, glutathione peroxidase 4; SLC7A11, solute carrier family 7 member 11; ASCL4, achaete-scute family BHLH transcription factor 4; DMT1, divalent metal transporter 1.
Discussion
PCOS is a heterogeneous disease that affects the female endocrine and reproductive system (26). The present study demonstrated that treatment with the ferroptosis inhibitor, Fer-1, ameliorated the Hcy-induced decrease in KGN cell viability and induction of cell apoptosis, in addition to inhibiting oxidative stress and ferroptosis in Hcy-induced cells. Furthermore, the results demonstrated that Fer-1 treatment suppressed DNA methylation whilst elevating TET1/2 levels in Hcy-induced KGN cells. Treatment with the TET1/2 inhibitor, Bobcat339 hydrochloride, reversed the protective effects of Fer-1 on Hcy-induced KGN cell damage. These findings suggest that the protective properties of Fer-1 against KGN cell Hcy-induced injury were mediated by TET activity and DNA demethylation.
Granulosa cells are specifically located around oocytes and serve an important role in oocyte maturation and ovulation (27). Dysfunction of ovarian granulosa cells is considered to be an important contributing factor of PCOS pathogenesis (28,29). The ovarian granulosa cell line KGN has been widely applied to explore the pathogenesis and regulatory mechanism of PCOS in previous studies (30–32). Iron metabolism serves a key role in endocrine disorders such as hypogonadotropic hypogonadism, hypothyroidism, and hypoparathyroidism (17). Compared with those in healthy individuals, patients with PCOS were previously found to have elevated serum iron concentrations (33). Ferroptosis is a unique type of programmed necrosis that is characterized by lipid peroxidation-induced cell death, which depends on the availability of iron and ROS (34). Ferroptosis has been associated with a number of pathophysiological states, including cancer, neurodegeneration and cardiovascular and endocrine diseases (35–38). Lipid peroxidation is a key process that occurs during ferroptosis, whereas ROS accumulation is considered to be an indicator of ferroptosis (39). GPX4, SLC7A11, ASCL4 and DMT1 are to be important components in ferroptosis regulation (40,41). GPX4 inhibits ferroptosis by using GSH as a reductase to catalyze the reduction of lipid peroxides (16). SLC7A11, a component of System Xc, can inhibit ferroptosis by importing the extracellular oxidized form of cysteine into the cell, reducing it to the cysteine that synthesizes the major antioxidant GSH (40). Doll et al (42) previously suggested that ACSL4 downregulation can induce ferroptosis and can be used to predict sensitivity to ferroptosis in GPX4-knockdown Pfa1 cells. As an important component of ferroptosis, the divalent metal transporter DMT1 can regulate intracellular iron levels and has been found to be essential for maintaining iron homeostasis (43). In a recent study, increased ferroptosis levels have been reported in granulosa cells during PCOS (17). In addition, high concentrations of Hcy can induce oxidative stress and ferroptosis in the nucleus pulposus by promoting the expression of methylases and enhancing methylation of the GPX4 gene (18). Fer-1 is a lipophilic radical scavenger that is a potent inhibitor of ferroptosis (34). Fer-1 can inhibit peroxidation induced by traces of lipid hydroperoxides in iron and liposomes (35). Several studies previously reported the beneficial effects of Fer-1 in dopaminergic neuroblastoma and cisplatin-induced AKI as a result of inhibiting ROS accumulation, apoptosis and ferroptosis (22,23). The present study, to the best of our knowledge, was the first to explore the effects of Fer-1 on PCOS by using a Hcy-induced KGN cell model. The results demonstrated that Fer-1 treatment inhibited apoptosis, oxidative stress and ferroptosis in Hcy-induced KGN cells.
Accumulating evidence supports the notion that aberrant gene methylation is a key contributing factor in the pathogenesis of PCOS (44,45). Methylation of numerous genes regulating vital ovarian functions has been previously found to be altered according to the DNA methylome profiling data of granulosa cells (46). Therefore, DNA methylation was analyzed in the present study. The results of the present study demonstrated elevated DNA methylation levels in Hcy-induced KGN cells. However, Fer-1 treatment dose-dependently reduced this methylation induced by Hcy. Furthermore, TET1 and TET2 are two dioxygenases that serve significant roles in decreasing DNA methylation (47). The present study demonstrated that Fer-1 notably increased both TET1 and TET2 levels, which suggested that Fer-1 may exert an inhibitory effect on DNA methylation in Hcy-induced ovarian granulosa cells. To verify the mechanism of Fer-1 in DNA methylation further, Bobcat339 hydrochloride, an inhibitor of TET1 and TET2, was used to treat KGN cells under Hcy-treated conditions (48). The results demonstrated that Bobcat339 hydrochloride reversed the protective effects of Fer-1 against Hcy-induced apoptosis, oxidative stress and ferroptosis in KGN cells.
In conclusion, results of the present study demonstrated that the ferroptosis inhibitor Fer-1 may alleviate Hcy-induced ovarian granulosa cell injury. This protective effect may be due to the enhancement of TET levels and DNA methylation. The present study provided an experimental basis for the application of Fer-1 as a potential therapeutic agent for the clinical treatment of PCOS. However, the present study is a preliminary investigation on the possible regulatory effects of Fer-1 on DNA methylation in Hcy-induced ovarian granulosa cells. In-depth study of the DNA methylation profile in granulosa cells after Hcy and Fer-1 treatment must be performed in future studies. Additionally, subsequent studies will need to focus on the collection of clinical data and the usage of granulosa cells from the follicular fluid of the ovary in patients with PCOS.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by Ningxia Natural Science Foundation (grant no. 2021AAC03353), Ningxia Science and technology benefit people project (grant no. 2020CMG03006) and First-Class Discipline Construction Founded Project of NingXia Medical University and the School of Clinical Medicine (grant no. NXYLXK2017A05).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QS and LC designed the study and performed the experiments. LC and RL drafted and revised the manuscript. QS analyzed the data. RL performed the literature search and analyzed the data. QS and LC confirm the authenticity of the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;105:e2695–e2709. doi: 10.1210/clinem/dgaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 4.Ignatov A, Ortmann O. Endocrine risk factors of endometrial cancer: Polycystic ovary syndrome, oral contraceptives, infertility, tamoxifen. Cancers (Basel) 2020;12:1766. doi: 10.3390/cancers12071766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 6.Witchel SF, Oberfield SE, Peña AS. Polycystic ovary syndrome: Pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3:1545–1573. doi: 10.1210/js.2019-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine Society Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What's new? Adv Clin Exp Med. 2017;26:359–367. doi: 10.17219/acem/59380. [DOI] [PubMed] [Google Scholar]
- 9.Eppig JJ. Reproduction: Oocytes call, granulosa cells connect. Curr Biol. 2018;28:R354–R356. doi: 10.1016/j.cub.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100:23–38. doi: 10.1016/j.fertnstert.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondapaneni V, Gutlapalli SD, Poudel S, Zeb M, Toulassi IA, Cancarevic I. Significance of homocysteine levels in the management of polycystic ovarian syndrome: A literature review. Cureus. 2020;12:e11110. doi: 10.7759/cureus.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–746. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 14.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/S0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv Q, Niu H, Yue L, Liu J, Yang L, Liu C, Jiang H, Dong S, Shao Z, Xing L, Wang H. Abnormal ferroptosis in myelodysplastic syndrome. Front Oncol. 2020;10:1656. doi: 10.3389/fonc.2020.01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Wang F, Li D, Yan Y, Wang H. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered. 2021;12:4983–4994. doi: 10.1080/21655979.2021.1956403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z, Wei X, Huang B, Shan Z, Liu J, Fan S, et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med. 2020;160:552–565. doi: 10.1016/j.freeradbiomed.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collignon E, Canale A, Al Wardi C, Bizet M, Calonne E, Dedeurwaerder S, Garaud S, Naveaux C, Barham W, Wilson A, et al. Immunity drives TET1 regulation in cancer through NF-κB. Sci Adv. 2018;4:eaap7309. doi: 10.1126/sciadv.aap7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabiraj P, Valenzuela CA, Marin JE, Ramirez DA, Mendez L, Hwang MS, Varela-Ramirez A, Fenelon K, Narayan M, Skouta R. The neuroprotective role of ferrostatin-1 under rotenone-induced oxidative stress in dopaminergic neuroblastoma cells. Protein J. 2015;34:349–358. doi: 10.1007/s10930-015-9629-7. [DOI] [PubMed] [Google Scholar]
- 23.Deng F, Sharma I, Dai Y, Yang M, Kanwar YS. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J Clin Invest. 2019;129:5033–5049. doi: 10.1172/JCI129903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B, Liu Y, Chen X, Zhao J, Han J, Dong H, Zheng Q, Nie G. Ferrostatin-1 protects auditory hair cells from cisplatin-induced ototoxicity in vitro and in vivo. Biochem Biophys Res Commun. 2020;533:1442–1448. doi: 10.1016/j.bbrc.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Jia F, Wei J, Yu Y, Yu T, Wang Y, Sun J, Luo G. Salidroside protects against homocysteine-induced injury in human umbilical vein endothelial cells via the regulation of endoplasmic reticulum stress. Cardiovasc Ther. 2017;35:33–39. doi: 10.1111/1755-5922.12234. [DOI] [PubMed] [Google Scholar]
- 26.Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100060. doi: 10.1016/j.eurox.2019.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 28.He M, Mao G, Xiang Y, Li P, Wu Y, Zhao D, Li T. MicroRNA-664a-3p inhibits the proliferation of ovarian granulosa cells in polycystic ovary syndrome and promotes apoptosis by targeting BCL2A1. Ann Transl Med. 2021;9:852. doi: 10.21037/atm-21-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Wang H, Zhou D, Shuang T, Zhao H, Chen B. Up-regulation of long noncoding RNA SRA promotes cell growth, inhibits cell apoptosis, and induces secretion of estradiol and progesterone in ovarian granular cells of mice. Med Sci Monit. 2018;24:2384–2390. doi: 10.12659/MSM.907138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Zheng Q, Sun D, Cui X, Chen S, Bulbul A, Liu S, Yan Q. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. 2019;234:7435–7447. doi: 10.1002/jcp.27501. [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Xia J, Yang Z, Chen Y, Jiang W, Yin T, Yang J. CircASPH promotes KGN cells proliferation through miR-375/MAP2K6 axis in polycystic ovary syndrome. J Cell Mol Med. 2020 Dec 28; doi: 10.1111/jcmm.16231. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8:e3145. doi: 10.1038/cddis.2017.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JW, Kang KM, Yoon TK, Shim SH, Lee WS. Study of circulating hepcidin in association with iron excess, metabolic syndrome, and BMP-6 expression in granulosa cells in women with polycystic ovary syndrome. Fertil Steril. 2014;102:548–554.e2. doi: 10.1016/j.fertnstert.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Xiong Y, Zhang Y, Wen J, Cai N, Cheng K, Liang H, Zhang W. The molecular mechanisms of regulating oxidative stress-induced ferroptosis and therapeutic strategy in tumors. Oxid Med Cell Longev. 2020;2020:8810785. doi: 10.1155/2020/8810785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do Van B, Gouel F, Jonneaux A, Timmerman K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Peng X, Zhang M, Jia Y, Yu B, Tian J. Revisiting tumors and the cardiovascular system: Mechanistic intersections and divergences in ferroptosis. Oxid Med Cell Longev. 2020;2020:9738143. doi: 10.1155/2020/9738143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12:2954. doi: 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang WS, Stockwell BR. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Q, Peng S, Sun Z, Heng X, Zhu X. Temozolomide drives ferroptosis via a DMT1-dependent pathway in glioblastoma cells. Yonsei Med J. 2021;62:843–849. doi: 10.3349/ymj.2021.62.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, Attili D, Pant A, Győrffy B, Zhan M, Carter-Su C, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016;24:447–461. doi: 10.1016/j.cmet.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao P, Yang W, Wang P, Li X, Nashun B. Characterization of DNA methylation and screening of epigenetic markers in polycystic ovary syndrome. Front Cell Dev Biol. 2021;9:664843. doi: 10.3389/fcell.2021.664843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan JX, Tan YJ, Wang FF, Hou NN, Xiang YQ, Zhang JY, Liu Y, Qu F, Meng Q, Xu J, et al. Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: A new insight into its pathogenesis. Clin Epigenetics. 2018;10:6. doi: 10.1186/s13148-018-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagvekar P, Kumar P, Mangoli V, Desai S, Mukherjee S. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin Epigenetics. 2019;11:61. doi: 10.1186/s13148-019-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole CJ, Lodh A, Choi JH, van Riggelen J. MYC deregulates TET1 and TET2 expression to control global DNA (hydroxy)methylation and gene expression to maintain a neoplastic phenotype in T-ALL. Epigenetics Chromatin. 2019;12:41. doi: 10.1186/s13072-019-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chua GNL, Wassarman KL, Sun H, Alp JA, Jarczyk EI, Kuzio NJ, Bennett MJ, Malachowsky BG, Kruse M, Kennedy AJ. Cytosine-based TET enzyme inhibitors. ACS Med Chem Lett. 2019;10:180–185. doi: 10.1021/acsmedchemlett.8b00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.