Abstract
Optical functional imaging methods such as calcium imaging have become a powerful tool for investigating neural activity in-vivo. Here, we present a design for a titanium implantable chamber with transparent silicone artificial dura which enables two-photon calcium imaging in non-human primates. This chamber accommodates imaging with high numerical aperture multiphoton objective lenses, and remains sealed, protecting the brain from the surrounding environment. In addition, we describe a tunable tissue stabilization system to apply gentle mechanical pressure to stabilize tissue during imaging. Our results suggest that two-photon calcium imaging may soon facilitate a new class of circuit and systems neuroscience experiments in non-human primates.
I. INTRODUCTION
Understanding the mechanisms by which networks of neurons give rise to perception and behavior is a primary goal of systems neuroscience, and a foundational step for developing more effective therapies to treat neurological injury and disease. Recently, advances in bioengineering have opened an exceptionally fertile experimental landscape, in which functional, structural, and behavioral information may be collected in the same organism, driven in part by the development of optical methods to record and manipulate neural activity [1]. Two-photon (2P) scanning microscopy, combined with the appearance of robust, high signal-to-noise ratio calcium reporters have enabled all-optical measurements of neural activity across worms, flies, and rodent models. [2], [3]. Despite the large array of tools and techniques available for these organisms, tools for imaging neural activity in non-human primates lag far behind [4].
Neuroscience experiments in macaques use electrodes or multielectrode arrays to sample blindly from neurons in a brain region of interest. With no way to identify the genetically-defined type of cell being recorded, it remains difficult to link the observed firing patterns to other details of circuit organization. Calcium imaging, on the other hand, may be naturally combined with viral transfection techniques to label cell types and anatomical projections in macaque brains [5]. Furthermore, whereas electrode recording samples sparsely and is biased towards a minority of highly active neurons [6], imaging can effectively record from all neurons within a field of view, providing a dense, unbiased perspective on neural population dynamics.
Translating optical imaging techniques to awake, behaving macaques presents a set of unique challenges. First, the optical window must be designed around the large diameter, high numerical aperture (NA) objective lenses typically required for multiphoton imaging. To obtain optical access to primate cortex, previously reported approaches replace a small portion of natural dura with a silicone artificial dura (AD). This approach has successfully been employed for intrinsic optical imaging [7], optogenetic stimulation [8], and acute calcium imaging [4], [9]. Building upon these techniques, we developed a novel optical imaging chamber which facilitates 2P imaging in non-human primates, with a chamber geometry optimized to accommodate large lenses. Secondly, any implanted imaging chamber must be durable enough to last for several months to years to align with typical non-human primate experimental timescales. Our design incorporates a replaceable silicone AD which is sealed from the external environment to minimize immunoreactivity.
Lastly, cardiac and respiratory rhythms cause the surface of the brain to move by several millimeters within the large craniotomy, making micron-scale imaging impossible without tissue stabilization. Cardiac and respiratory rhythms induce cortical pulsations, the magnitude of which increase with the size of the animal, making stabilization prerequisite for two photon imaging in non-human primates. In our particular case, active engagement in a motor reaching task provides another source of significant brain motion, further highlighting the need for stabilization. To address this, we developed a stabilization system which uses a circular glass cover slip to place precisely-tuned pressure on the top of the silicone window while imaging.
The work here describes a system which addresses each of these three key challenges, enabling us to capture cellular-resolution, stable 2P videos of superficial motor cortex, and facilitating optical interrogation of neural activity using calcium reporters in future work.
II. CHAMBER DESIGN
The design of our implantable chamber is optimized to provide optical access to an approximately 1 cm diameter circle of cortical tissue while providing a watertight and hygienic barrier to protect the brain. In addition, the design enables us to remove and replace the silicone AD if necessary for cleaning or removing tissue growth, and is durable enough to last for several months to years to align with non-human primate experimental timescales.
The primary consideration driving the design of the titanium imaging chamber is the large size and short working distances (4–8 mm) of current objective lenses (Fig. 1A). Previously reported functional imaging experiments in primates have relied on either: A) acute experimental preparations with a large craniotomy [7], [4], or B) microprobe objective lenses designed to penetrate through a small craniotomy [9]. Microprobe objectives feature short working distances (~200μm), limited field of view, and low numerical aperture (0.2 NA), and are sub-optimal for imaging below layer 1 in primate cortex.
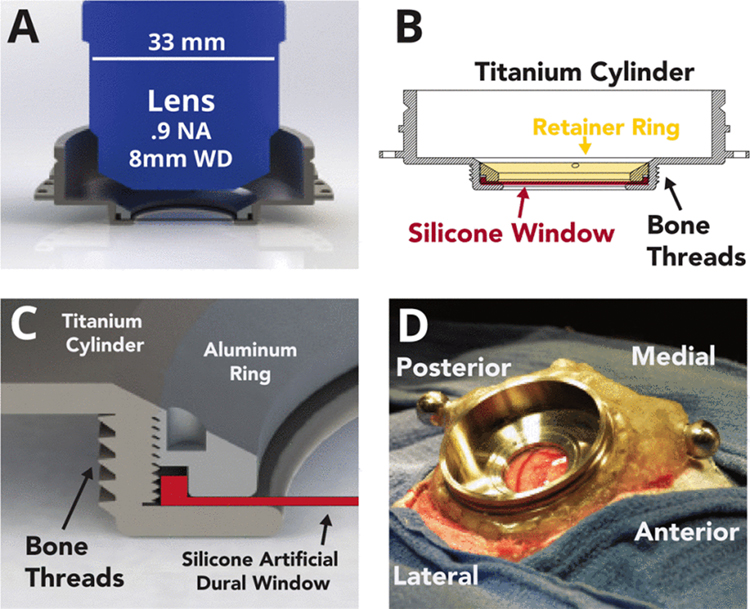
Fig. 1. Design of the imaging chamber.
(A) Side profile rendering of cylindrical chamber shown accommodating a long working distance (WD) multiphoton objective. (B) Side profile schematic illustrating the lower lip that supports the edge of the silicone AD from below, and the threaded retainer ring that secures it in place. (C) Detail rendering of the chamber and threaded retainer ring design. (D) Chamber and AD implanted onto monkey S centered over motor and premotor cortex.
Multiphoton-optimized objective lenses typically feature high numerical apertures (> 0.8, which require the lens geometry to feature a blunt tip and a large lens body diameter (often > 3 cm, Fig. 1A). Consequently, the size of the required craniotomy and the subsequent area of cortex accessible for imaging is constrained not only by the size of the window, but also by the geometry of the objective lens, implant, and thickness of skull.
Our implant design maintains a low profile above the skull, extending laterally to provide room for a large objective lens to translate. This geometry provides imaging access to an approximately 1 cm diameter region in the center of the window, though this can vary from 6–12 mm depending on the lens geometry, lens working distance, and the depth of the brain relative to the skull surface.
Existing AD implant designs for non-human primates use a removable silicone insert that is held in place by a flange inserted beneath the surrounding skull and dura to prevent dural regrowth and to permit window replacement once opaque tissue growth impedes optical access [7], [4]. This design, however, creates a fluid-air interface between the brain and the internal space of the chamber that both presents an infection risk and may accelerate the rate of tissue regrowth. To avoid these issues, our chamber design seals the interface around the silicone AD. The silicone insert is circular and is held in the chamber from below by a thin metal lip, and from above by a threaded retainer ring (Fig. 1B,C). This ring is tightened to seal the window, and can be loosened to remove and replace the window for cleaning. The ring and lip also serve to grip the edge of the silicone window tightly and prevent it from coming loose when pressure is applied to stabilize tissue for imaging.
The chamber, AD, and ring are first assembled and then implanted under sterile technique in a procedure developed for imaging studies. All experimental and surgical procedures were approved by the Stanford University Institutional Animal Care and Use Committee (IACUC). A neurosurgical drill is used to create a circular craniotomy (28.5 mm) over the motor cortical region. Appropriate techniques are utilized to temporarily reduce intracortical blood pressure. A circular durotomy is performed to match the size of the silicone window (19 mm) and the dural edges are cut back and folded under to suppress regrowth.
The outer diameter of the chamber features bone threads (Fig. 1B) which allow it to screw securely into the craniotomy. Titanium mandibular trauma straps (Synthes) are secured to the skull surrounding the implant, and dental acrylic is used to secure the chamber to these anchor straps. Additional fixtures required for head-fixation are also anchored within the dental acrylic.
III. SILICONE ARTIFICIAL DURAL WINDOW
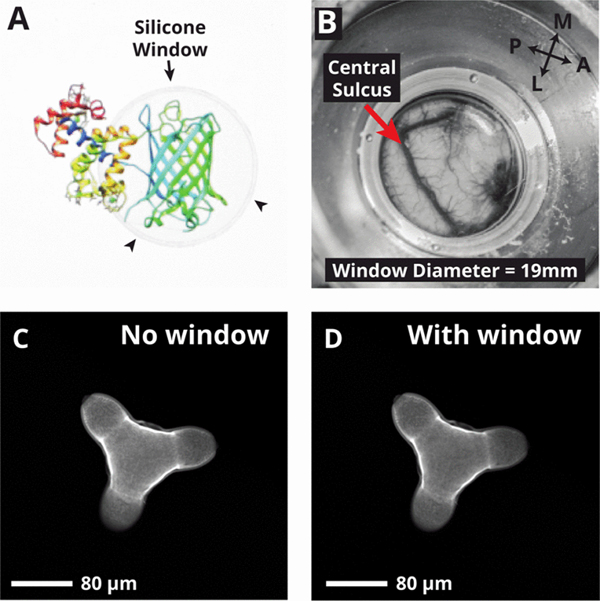
The purpose of the AD window is to maintain a seal between the brain and the environment while providing an optically transparent window (Fig 2A). We selected a silicone compound for this purpose due to its combination of optical transparency, durability, and biocompatibility (Shin-Etsu silicone type KE1300-T). The AD is 300μm thick, representing a trade-off between durability and optical losses through the silicone. To characterize optical losses and two-photon imaging performance using the window, we imaged fluorescent calibration slides and quantified the average image intensity with and without the window present. Using this approach, we measured a signal intensity drop of 8.6% with the window present. For biological samples, this results in only modest drop in intensity in resulting images (Fig. 2C, 2D).
Fig. 2.
Silicone artificial dural window. (A) Picture of the silicone window overlaid on an image of the GCaMP6m protein on a computer screen, demonstrating optical transparency. (B) View of cortical surface (central sulcus and motor cortex) and vasculature seen through the AD. (C, D) Two-photon images of a pollen grain imaged with no window (C) and with silicone AD window (D).
When implanted, the window provides unobstructed view of a 19 mm diameter circle of the surface of the brain (Fig. 2B). Due to the geometric constraints imposed by large imaging lenses, the area of cortex accessible for imaging is restricted to a 5–12 mm circle at the center of implant.
IV. STABILIZATION
A major concern for translating optical physiology from rodent to non-human primate models is the need to ensure that the tissue remains stable while imaging, particularly for the case of awake, behaving monkeys. Tissue motion relative to the microscope can arise from multiple sources. First, for large craniotomies, such as those required in optical imaging, fluctuations in intracranial pressure due to cardiac and respiratory rhythms can lead to cyclic movements of the brain surface by up to several millimeters. This motion is orders of magnitude larger than the depth of the focal plane for a 2P system (2–5 μm), necessitating mechanical tissue stabilization during imaging. Motion within the imaging plane is also a concern, but if small enough, is correctable offline using image registration algorithms.
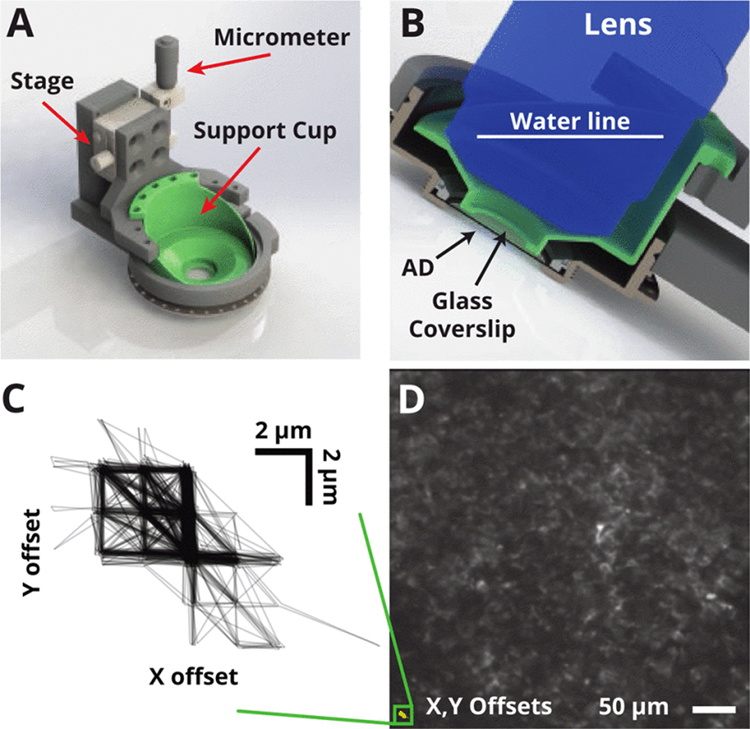
We designed a cortical stabilizer system to ensure tissue stability by providing downward pressure on the top of the silicone window via a circular glass coverslip (Fig 3A). This device mounts to the outer rim of the chamber and uses a micron-precision linear stage to push the coverslip against the top surface of the silicone AD. The silicone AD is naturally elastic, allowing the center of the window to depress by 1–3 mm as the tissue stabilizer places light pressure at the center.
Fig. 3.
Tissue stabilization system. (A) Rendering of tissue stabilization system and chamber implant. The removable stabilization system clamps around the chamber during imaging and provides pressure via a circular glass cover slip attached to a support (green). (B) When implanted over motor cortex, the chamber sits 30 degrees off horizontal. The cover slip support (green) performs two functions: 1) applying pressure to the top of the AD, 2) holding water around the lens for index matching during imaging. (C) Frame offsets (relative to first image) acquired during a behavioral experiment in an awake macaque. Motion was confined to within ± 5 μm and discrete structure illustrates offsets are confined to a few pixels. (D) Mean intensity over all aligned images retains structure, indicating successful alignment. Alignment offsets from C) overlaid to scale (bottom left).
In practice, without the stabilizer in place, there is a layer of cerebrospinal fluid (CSF) between the surface of the brain and the AD. As the tissue stabilizer applies gentle pressure to this window, this squeezes the CSF out of this space and places the brain in contact with the window.
During reaching movements, the stabilizer reduced observed brain motion along all axes. Simple cross-correlation based image registration methods produced a very stable image recording, and the inferred translational offsets from the initial frame were restricted to a 10 × 10 μmregion in both the X and Y axes (Fig. 3C). Although it is difficult to quantify motion along the Z-axis, individual structures of only a few micrometers in size remained in focus for the duration of imaging experiments (typically 10 minutes - 1 hour), suggesting that Z motion may be restricted to several micrometers. Averaging over all timepoints in the stabilized image sequence retained the structure observed in individual images, suggesting the the motion we observe may be corrected by straightforward registration algorithms (Fig. 3D).
In summary, the stabilizer restricted brain motion to an amount comparable to that observed in typical awake rodent experiments, which is amenable to relatively straightforward image stabilization approaches.
V. RESULTS
Monkey S received the window implant in January, 2015. Imaging experiments began one week following the surgery, and continued for two months until sufficient data was collected. Beginning 4–5 weeks after implant, we observed the growth of a white, semi-translucent neomembrane, beginning at the edges of the chamber. Despite this, we were able to image the neural tissue at the center of the chamber without significant losses as this tissue had not yet covered the imaging location.
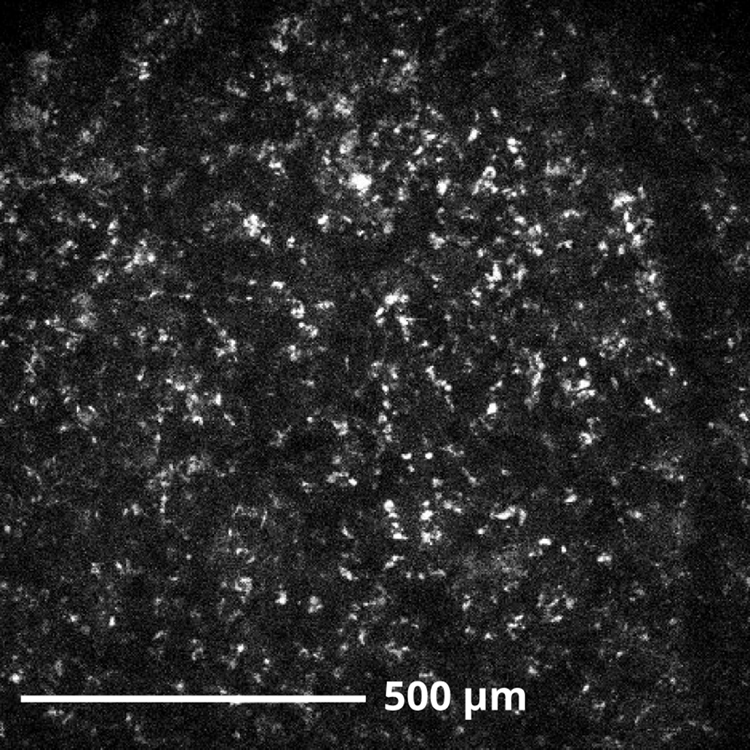
Using a custom two-photon imaging system and the cortical window, we were able to obtain images of cortical tissue and vasculature as shown in Fig. 4. The bright, punctate objects appear in both red and green channels and are suspected broad-spectrum autofluorescence from lipofuscin or another endogenous protein [10].
Fig. 4.
Example two-photon image, collected 100μm below the surface of primate motor cortical tissue. Punctate white objects are suspected autofluorescence of lipofuscin in individual neurons. Excitation frequency: 920 nm.
VI. CONCLUSIONS AND FUTURE WORK
We present the design and demonstration of a sealed artificial dura chamber designed for optically imaging the brain of non-human primates. We believe this is the first demonstration of an imaging window that enables chronic multiphoton imaging in awake primates, while sealing off the brain from the external environment. The applications of this artificial dural window are not limited to multiphoton imaging. This implant design may benefit a number of experimental modalities, including single and multiphoton imaging of voltage and calcium reporters, optogenetics, or electrophysiology experiments using fragile silicon electrodes, high-density multielectrodes, or where precise localization relative to identifiable cortical landmarks is essential. Future work will be aimed at using this window to establish two-photon calcium imaging in non-human primates as a viable tool for physiology.
Translating novel tools for all-optical neural recording to non-human primates is essential to furthering our understanding of the human brain [11]. Many neural circuits, including the motor system, are finely adapted to the specific requirements of each species. Non-human primates utilize a motor system which is biomechanically similar to that of humans, and the neural structures which control movement in primates is anatomically homologous to, and thus likely to perform similar computations as that of humans. Second, non-human primates exhibit a rich repertoire of complex, highly-stereotyped movements. While rodents can be trained to perform simple reaching movements [12], it is unlikely that studying the motor system of simpler organisms like rodents will yield a mechanistic understanding of the principles underlying human motor control. Combining optical tools for neural circuit interrogation with well-controlled, precise behavior will provide powerful leverage into understanding neural circuitry in health and disease.
ACKNOWLEDGMENT
We thank M. Mazariegos, M. Wechsler, L. Yates, & S. Smith for expert surgical assistance & veterinary care, and J. Marshel, K. Ames, K. Deisseroth, W. Allen, N. Young, & L. Grossnick for useful discussions regarding optics and imaging, P. Sabes, A. Yazdan, & T. Hansen for help with the silicone mold design, and B. Davis, & E. Casteneda for administrative assistance.
This work was supported by DARPA NeuroFAST award number W911NF-14-2-0013 and an NIH Director’s Pioneer Award number 8DPIHD075623.
REFERENCES
- [1].Warden MR, Cardin J. a., and Deisseroth K, “Optical Neural Interfaces.,” Annual review of biomedical engineering, vol. 16, pp. 103–129, July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deisseroth K and Schnitzer MJ, “Engineering approaches to illuminating brain structure and dynamics.,” Neuron, vol. 80, pp. 568–77, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, and Kim DS, “Ultrasensitive fluorescent proteins for imaging neuronal activity,” Nature, vol. 499, pp. 295–300, July 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nauhaus I, Nielsen KJ, Disney AA, and Callaway EM, “Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex.,” Nature Neuroscience, vol. 15, pp. 1683–90, Dec. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gerits A and Vanduffel W, “Optogenetics in primates: a shining future?,” Trends in genetics : TIG, vol. 29, pp. 403–11, July 2013. [DOI] [PubMed] [Google Scholar]
- [6].Barth AL and Poulet J. F. a., “Experimental evidence for sparse firing in the neocortex,” Trends in Neurosciences, vol. 35, no. 6, pp. 345–355, 2012. [DOI] [PubMed] [Google Scholar]
- [7].Arieli A, Grinvald A, and Slovin H, “Dural substitute for long-term imaging of cortical activity in behaving monkeys and its clinical implications,” Journal of Neuroscience Methods, vol. 114, pp. 119–133, 2002. [DOI] [PubMed] [Google Scholar]
- [8].Ruiz O, Lustig BR, Nassi JJ, Cetin A, Reynolds JH, Albright TD, Callaway EM, Stoner GR, and Roe AW, “Optogenetics through windows on the brain in the nonhuman primate.,” Journal of Neurophysiology, vol. 110, pp. 1455–67, Sept. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heider B, Nathanson JL, Isacoff EY, Callaway EM, and Siegel RM, “Two-photon imaging of calcium in virally transfected striate cortical neurons of behaving monkey,” PLoS ONE, vol. 5, no. 11, pp. 1–13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schnell SA, Staines WA, and Wessendorf MW, “Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue.,” The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, vol. 47, no. 6, pp. 719–730, 1999. [DOI] [PubMed] [Google Scholar]
- [11].Anderson DM, “The nonhuman primate as a model for biomedical research,” Sourcebook of Models for Biomedical Research, pp. 251–258, 2008. [Google Scholar]
- [12].Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado M. a., Kane JR, Schallert T, and Jones T. a., “The Vermicelli Handling Test: A simple quantitative measure of dexterous forepaw function in rats,” Journal of Neuroscience Methods, vol. 170, pp. 229–244, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]