Abstract
Background:
Many factors are affecting intrauterine growth. The role of Wingless-type (Wnt) inducible signaling pathway protein-1 (WISP1), a novel adipokine and placental proteoglycans in intrauterine growth, is not known. We aimed to measure umbilical cord blood levels of glucose, insulin, leptin, WISP1, and placental proteoglycans [glypican-1 (GPC1), glypican-3 (GPC3), and syndecan-1 (SDC1)] which are thought to have an important role in fetal growth and investigate their relation with birth weight.
Methods:
Full-term neonates were included in this prospective, cross-sectional study and classified as appropriate for gestational age (AGA), small for gestational age (SGA), and large for gestational age (LGA) according to their birth weight. Umbilical cord blood levels of glucose, insulin, leptin, WISP1, GPC1, GPC3, and SDC1 were measured.
Results:
Leptin levels were higher in LGA newborns compared to AGA and SGA newborns, while WISP1, GPC1, GPC3, and SDC1 levels were not different between the three groups. Leptin and GPC1 levels were higher in infants of mothers with gestational diabetes mellitus compared to infants of non-diabetic mothers, while WISP1, GPC3, and SDC1 were not different between the groups. Leptin was positively correlated with insulin, birth weight, and maternal weight. While there was a strong correlation between the WISP1, GPC1, GPC3, and SDC1 levels; there was no correlation between the birth weight, maternal weight, glucose, insulin, and WISP1, GPC1, GPC3, and SDC1 levels.
Conclusion:
Umbilical cord blood levels of GPC1, GPC3, SDC1, and WISP1 were not different between SGA, AGA, and LGA infants. The significance of serum levels of these adipokines and proteoglycans remains to be elucidated.
Keywords: Umbilical cord blood, WISP1, proteoglycan, glypican-1, glypican-3, syndecan-1
What is already known on this topic?
Umbilical cord blood level of leptin is higher in large for gestational age (LGA) newborns compared to appropriate for gestational age (AGA) and small for gestational age (SGA) newborns and leptin is positively correlated with insulin, birth weight, and maternal weight.
What this study adds on this topic?
Umbilical cord blood levels of Wingless-type (Wnt) inducible signaling pathway protein-1 (WISP1), glypican-1, glypicans-3, and syndecan-1 are not different between AGA, SGA, and LGA infants.
Leptin and glypican-1 levels are higher in infants of mothers with gestational diabetes mellitus compared to infants of non-diabetic mothers.
There is a strong correlation between the WISP1, glypican-1, glypicans-3, and syndecan1 levels but no correlation between the birth weight, maternal weight, glucose, insulin and WISP1, glypican-1, glypicans-3, and syndecan-1 levels.
Introduction
Fetal growth is a process that depends on the interaction of fetal, placental, and maternal environment and is controlled by genetic, hormonal, nutritional, and maternal factors.1,2 The developmental origins of health and disease hypothesis, also known as Barker hypothesis, proposes that suboptimal fetal environment and impaired fetal growth may have significant consequences on an offspring’s long-term health, such as obesity, metabolic syndrome, and cardiovascular disease.3 Both large for gestational age (LGA) and small for gestational age (SGA) infants have a higher risk of obesity and metabolic syndrome later in life.4,5
There is growing evidence that adipose tissue plays an important role in fetal growth.6 The placenta affects fetal growth by regulating the transfer of various nutrients to the fetus. Adipokines secreted from adipose tissue can affect inflammation and the transfer of placental nutrients to the fetus.6 Leptin is a key adipokine mainly produced in white adipose tissue. It has a critical role in energy homeostasis and neuroendocrine regulation of body fat content.7 A systematic review and meta-analysis reported a positive and moderate correlation between umbilical cord leptin levels and birth weight in different population groups.8
Wingless-type (Wnt) inducible signaling pathway protein-1 (WISP1) is a novel adipokine, whose serum levels are elevated in obesity and insulin resistance. Several studies suggest that WISP1 may have a role in the impaired glucose metabolism.9
Proteoglycans represent a special class of glycoproteins that are highly glycated. It consists of a core protein with a glycosaminoglycan chain attached by one or more covalent bonds. Glypicans are heparan sulfate proteoglycans and human genome comprises six glypican family members (glypican-1 to glypican-6).10 The placenta contains heparan sulfate and chondroitin sulfate or dermatan sulfate proteoglycans.11 Placental heparan sulfate proteoglycans enclose glypicans, syndecans, and perlecan and of the glypican family only glypican-1 (GPC1) and glypican-3 (GPC3) are expressed in the placenta.11 In a recent article, both placental expressions of GPC1 and GPC3 were reduced in SGA pregnancies when compared with appropriate for gestational age (AGA) pregnancies.11 Similarly, placental expression of syndecan-1 (SDC1) was significantly decreased in fetal growth restriction samples.12 Therefore, it is likely that fetal growth is closely related to the placental expression of glypicans.
In the present study, we aimed to investigate, for the first time, the umbilical cord levels of placental proteoglycans (GPC1, GPC3, SDC1), WISP1 and leptin levels in infants with SGA, AGA, and LGA.
Materials and Methods
Subjects
This prospective, cross-sectional study was carried out in term neonates who were born at Department of Gynecology and Obstetrics of Aydın Adnan Menderes University between May 2019 and December 2019. The study was conducted according to the Helsinki Declarations, with ethical approvals obtained from the Aydın Adnan Menderes University, Faculty of Medicine (no. 2019/83). Informed consent was obtained from the parents of each infant.
The gestational age was determined according to the last menstrual date and/or ultrasonography of the first trimester. Body weight of infants was measured using a portable, electronic weighing scale sensitive to the 10 g (Seca, Hamburg, Germany). Fenton growth charts were used to evaluate auxologic parameters and infants with a birth weight below the 10th percentile were defined as SGA, those between the 10th and 90th percentiles as AGA, and those with a birth weight above the 90th percentile were defined as LGA.13 Maternal weight was measured just before the birth. We defined gestational weight gain during pregnancy as the difference between weight before pregnancy and weight just before birth (measured without heavy clothing).
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the formula: (fasting glucose × fasting insulin)/405.14 All pregnant women were screened using a 50 g glucose challenge test at 24-28 weeks of gestation.15 Women with post-load glucose ≥140 mg/dL underwent a standard 100 g, 3-hour oral glucose tolerance test. The diagnosis of gestational diabetes mellitus (GDM) was made if at least 2 of 4 diagnostic criteria were met: (i) fasting plasma glucose ≥95 mg/dL, (ii) for 1 hour ≥180 mg/dL, (iii) 2 hours ≥155 mg/dL, and (iv) 3 hours ≥140 mg/dL.16
Exclusion Criteria
Infants with a major congenital anomaly or chromosomal abnormality, history or findings of any inherited metabolic disease, a history of congenital or perinatal infection, an APGAR score of <8 in the first and fifth minutes, antenatal maternal steroid prophylaxis, pregnancies complicated by preeclampsia or any chronic disease, a history of alcohol or smoking, and premature born infants (gestational age <37 weeks) were excluded from the study.
Laboratory Measurements
After birth, umbilical cord blood collected from patients was immediately centrifuged at 1500× g for 10 minutes and serum samples were separated. Serum samples were stored at −80°C before performing assays and were thawed only once prior to use. Serum glucose and insulin levels were measured by a clinical chemistry analyzer (Abbott ARCHITECT C8000 and i2000, respectively). Serum levels of leptin, SDC1, GPC3, GPC1, and WISP1/CCN4 were measured by a commercial enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (FineTest, Wuhan). Samples were measured with a micropla te reader at 450 nm wavelength (Epoch, Biotek). All assays coefficient of variation were determined with inter-assay cv: <10% and intra-assay cv: <8%, respectively.
Statistical Analysis
Statistical analyses were performed using the SPSS Statistics for Windows, Version 26.0 (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). Categorical data were presented with n and %, and numerical data with mean ± standard deviation if normally distributed, and median (25-75p) if non-normally distributed. Descriptive statistics (kurtosis and skewness), visual methods (histogram), and analytical tests (Shapiro–Wilk test) were used to determine the normal distribution of numerical variables. In the comparison of independent two groups, a student t-test was used if the data were normally distributed, and Mann–Whitney U test was used if the data were non-normally distributed. In the comparison of independent three groups, ANOVA was used if the data was normally distributed (when an overall significance was observed, Tukey’s and Games–Howell post hoc test were performed in case of homogeneity of variances, or not, respectively), and Kruskal–Wallis test was used if the data were non-normally distributed (when an overall significance was observed, Dunn’s post hoc test was performed). Pearson correlation was used if the data were normally distributed, and a Spearman correlation test was used if the data were non-normally distributed. Type I error was determined as 5% and a P value was <.05 was considered statistically significant.
Results
A total of 84 term neonates were included in the study. Seventeen infants were SGA (20%), 49 were AGA (58%), and 18 were LGA (22%). Median gestational age was 38 weeks and mean birth weights of the SGA, AGA, and LGA infants were 2388, 3321, and 4055 g, respectively. Demographic and clinical features of the subjects are given in Table 1. Mean serum glucose levels were similar between SGA, AGA, and LGA infants. Median serum insulin level was higher in LGA infants than those of SGA infants (P = .010). Similarly, median leptin levels of LGA infants were found higher compared with the AGA and SGA infants (P = .002, Figure 1). Besides, serum levels of WISP1, GPC1, GPC3, and SDC1 levels were not different between SGA, AGA, and LGA infants (Table 1). Also, similar results were observed when infants of mothers with GDM were not included in the analysis (data were not given).
Table 1.
Demographic, Clinic and Laboratory Characteristics of the Subjects
| SGA (n = 17) | AGA (n = 49) | LGA (n = 18) | P * | |
|---|---|---|---|---|
| Gestational age (week) | 38 (37-39) | 38 (38-39) | 38 (37-38) | .328 |
| Gender | ||||
| Female (%) | 10 (58.8) | 22 (44.9) | 7 (38.9) | .471 |
| Male (%) | 7 (41.2) | 27 (55.1) | 11 (61.1) | |
| Maternal age (year) | 31.6 ± 5.6 | 32.8 ± 4.4 | 31.9 ± 4.5 | .574 |
| Maternal weight at birth (kg) | 65.0 (60.8-76.0) | 79.5 (72.3-84.8) | 102.5 (92.0-112.0) | <.001 a |
| Maternal weight gain during pregnancy (kg) | 9.0 (7.0-12.8) | 13.0 (10.0-15.8) | 15.0 (10.3-18.8) | .026 b |
| Glucose (mg/dL) | 51.5 ± 11.1 | 59.7 ± 16.8 | 62.2 ± 15.7 | .101 |
| Insulin (µIU/mL) | 3.3 (2.7-3.8) | 4.7 (3.6-7.3) | 7.5 (1.8-16.6) | .010 b |
| HOMA-IR | 0.4(0.3-0.6) | 0.8 (0.5-1.3) | 1.4 (0.5-2.2) | .010 b |
| Leptin (pg/mL) | 281.5 (107.9-662.8) | 569.5 (202.2-911.3) | 1203.8 (699.5-1372.5) | .002 c |
| WISP1 (pg/mL) | 171.3 (129.6-230.8) | 152.2 (129.6-185.3) | 156.8 (132.3-189.0) | .169 |
| Glypican-1 (pg/mL) | 267.9 (267.2-299.2) | 267.0 (266.5-280.0) | 267.1 (266.8-296.4) | .099 |
| Glypican-3 (ng/mL) | 4.3 (2.0-6.5) | 2.7 (2.0-4.9) | 4.4 (1.9-8.8) | .283 |
| Syndecan-1 (ng/mL) | 7.2 ± 2.1 | 6.4 ± 2.6 | 6.7 ± 2.0 | .492 |
Bold values are statistical significant values and correct.
SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; WISP1, WNT1-inducible-signaling pathway protein 1.
*Normally distributed data were given as mean ± SDS (one-way ANOVA test), non-normally distributed data were given as median [25p-75p] (Kruskal–Wallis test). *Categorical variables were expressed as n (%) (chi-squared test).
aSGA versus AGA, AGA versus LGA, SGA versus LGA; bSGA versus LGA; cAGA versus LGA, SGA versus LGA.
Figure 1.
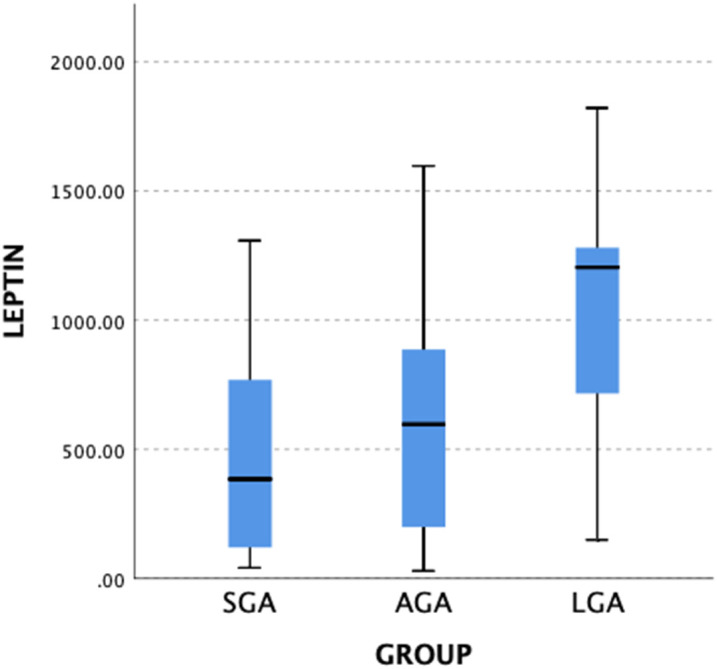
Comparison of umbilical cord leptin levels of infants. SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age. *Kruskal–Wallis test, P = .002.
Thirty infants (36%) were born to mothers with GDM. Birth weight, birth weight SDS, maternal weight at birth, serum levels of leptin, and GPC1 were higher in infants of GDM mothers compared to infants of non-diabetic mothers (Table 2). When infants of GDM mothers were compared according to their birth weight; serum WISP1, GPC1, GPC3, and SDC1 were not different between SGA, AGA, and LGA infants. Maternal weight at birth and serum leptin levels were higher in LGA infants of GDM mothers than those of AGA infants of GDM mothers (P < .001 and P = .008, respectively).
Table 2.
Comparison of Demographic, Clinic and Laboratory Characteristics of the Infants of GDM Mothers With Infants of Non-diabetic Mothers
| Infants of GDM Mothers (n = 30) | Infants of Non-diabetic Mothers (n = 54) | P * | |
|---|---|---|---|
| Gestational age (week) | 38 (37-38) | 38 (37-39) | .238 |
| Gender | |||
| Female (%) | 14 (46.7) | 25 (46.3) | .974 |
| Male (%) | 16 (53.3) | 29 (53.7) | |
| Birth weight (g) | 3526.6 ± 501.2 | 3158.5 ± 621.0 | .007 |
| Birth weight SDS | 0.7 ± 1.1 | −0.1 ± 1.2 | .003 |
| Birth weight status | |||
| SGA | 3 (10) | 14 (26) | .022 a |
| AGA | 16 (53) | 33 (61) | |
| LGA | 11 (37) | 7 (13) | |
| Maternal age (year) | 32.4 ± 4.1 | 32.4 ± 4.9 | .956 |
| Maternal weight at birth (kg) | 92.0 (83.5-103.5) | 78.0 (70.0-90.5) | .001 |
| Maternal weight gain during pregnancy (kg) | 12.0 (9.0-17.3) | 13.0 (10.0-15.0)) | .913 |
| Glucose (mg/dL) | 60.3 ± 14.4 | 57.6 ± 16.6 | .452 |
| Insulin (µIU/mL) | 6.6 (3.1-10.7) | 4.7 (3.3-7.6) | .221 |
| HOMA-IR | 0.9 (0.3-1.7) | 0.6 (0.4-1.2) | .223 |
| Leptin (pg/mL) | 848.6 (479.7-1240.5) | 597.3 (190.9-895.9) | .033 |
| WISP1 (pg/mL) | 179.1 (144.3-197.4) | 154.4 (126.8-192.8) | .139 |
| Glypican-1 (pg/mL) | 270.1 (266.9-304.5) | 267.1 (266.4-282.2) | .031 |
| Glypican-3 (ng/mL) | 2.6 (2.0-4.7) | 2.9 (1.9-5.3) | .688 |
| Syndecan-1 (ng/mL) | 6.7 ± 2.3 | 6.5 ± 2.4 | .815 |
Bold values are statistical significant values and correct.
GDM, gestational diabetes mellitus; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; WISP1, WNT1-inducible-signaling pathway protein 1. *Normally distributed data were given as mean ± SDS (one-way ANOVA test), non-normally distributed data were given as median [25p-75p] (Kruskal–Wallis test); *Categorical variables were expressed as n (%) (chi-squared test).
aThe significance was lost after the post hoc analysis.
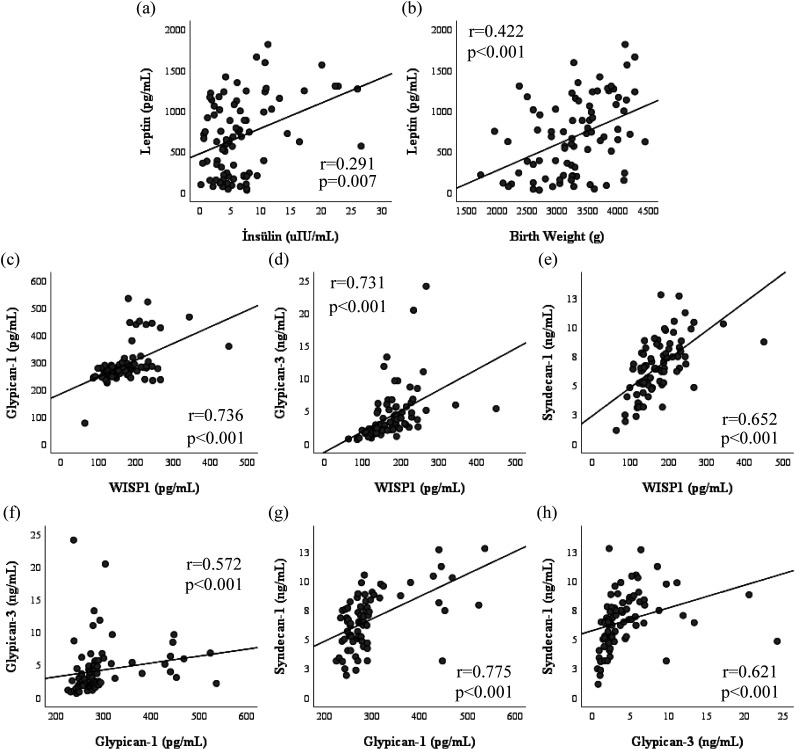
A positive correlation was found between leptin and insulin levels (r = 0.291, P = .007), birth weight (r = 0.422, P < .001), birth weight SDS (r = 0.388, P < .001), and maternal weight (r = 0.422, P < .001). Similarly, a strong positive correlation was demonstrated between WISP1 and GPC1 (r = 0.736, P < .001), GPC3 (r = 0.731, P < .001), and SDC1 (r = 0.652, P < .001). There was no correlation between the birth weight, maternal age, maternal weight, glucose, insulin levels, and serum levels of WISP1, GPC1, GPC3, and SDC1 (Figure 2).
Figure 2. a-h.
Correlation analysis was performed to analyze the correlation between leptin and insulin (a), leptin and birthweight (b), Glypican-1 and WISP1 (c), Glypican-3 and WISP1 (d), Syndecan-1 and WISP1 (e), Glypican-3 and Glypican-1 (f), Syndecan-1 and Glypican 1 (g), and Syndecan-1 and Glypican-3 (h).
Discussion
This study evaluated umbilical cord blood levels of GPC1, GPC3, SDC1, and WISP1 in infants according to the birth weight (SGA, AGA, and LGA). The evaluation of both classical adipokine (leptin) and novel adipokines and proteoglycans may provide clues for intrauterine growth. Although we know that this study cannot establish causality between the adipokine levels and long-term metabolic risk due to its cross-sectional structure, it does not change the fact that we need novel markers to predict both intrauterine growth and long-term metabolic risk in that population.
WISP1 is a member of the extracellular matrix-associated proteins which have diverse functions, such as growth, differentiation, proliferation, and apoptosis of different cell types.17,18 WISP1 has been associated with oncogenesis, bone metabolism, and fibrosis of the kidney, lung, and liver.9 Studies conducted with obese children and adults reported higher circulating WISP1 levels compared with the normal weight healthy controls.19,20 Several studies evaluated serum levels of WISP1 in patients with type 2 diabetes mellitus and reported conflicted results.19,21,22 In addition to its role in obesity, WISP1 was also linked to adipose tissue and systemic inflammation.9 Ersoy et al.23 reported that body mass index and HOMA-IR independently and positively predicted WISP1 levels in patients with polycystic ovary syndrome. Also, serum WISP1 levels were found higher in pregnant women with GDM than those of the non-GDM healthy pregnant women.24 In the present study, although WISP1 levels were higher in umbilical cord blood of infants of mothers with GDM than those of infants of mothers with non-GDM, this difference was not statistically significant. Also, serum WISP1 levels were not different between SGA, AGA, and LGA infants.
Leptin and insulin are key factors in regulating the energy balance and growth of the fetus and newborns.25 Leptin regulates the growth and development of the fetus during pregnancy, and umbilical cord leptin levels are positively correlated with the weight and fat mass of the newborn.26 It has been shown that umbilical cord leptin levels correlate with birth weight and insulin levels in large for gestational age newborns.27,28 In the present study, we found higher levels of leptin in LGA infants when compared with AGA and SGA infants. Also, it was positively correlated with birth weight and serum insulin levels. A systematic review and meta-analysis investigating the relation between leptin and birth weight reported moderate correlation between umbilical cord leptin levels and birth weight and leptin was explained 21% of variation in birth weight.8 The results of the present study were consistent with previous studies.
Proteoglycans are structural molecules that affect the placenta’s cellular functions and the angiogenesis process.29 SDC1, GPC1, and GPC3 proteoglycans which are expressed in syncytiotrophoblast cells are essential members of the placental barrier.11 SDC1 may have an important role in modifying growth factor interaction and angiogenesis in the placenta. A few studies investigating the relation between maternal serum SDC1 levels and pregnancy outcomes reported similar plasma soluble SDC1 levels in preeclamptic and normotensive pregnant women.30,31 On the contrary, some studies reported lower serum SDC1 levels in preeclamptic pregnant women.32 To the best of our knowledge, there is no study investigating the relation between umbilical cord blood SDC1 levels and birth weight of infants in mothers with GDM and non-GDM. A study investigating the role of endothelial glycocalyx constituents in predicting GDM reported that SDC1 have no role in prediction of GDM.33 In the present study, we find no difference between umbilical cord SDC1 levels in SGA, AGA, and LGA infants as well as in infants of mothers with GDM and non-GDM. Proteoglycans in heparan sulfate structures such as GPC1 and GPC3 facilitate the binding of growth factors and coagulation factors to the cell surface.34,35 Gunatillake et al.11 have reported a significant decrease in the placental expression of GPC1 and GPC3 in SGA pregnancies compared to control pregnancies. On the other hand, Chen et al.36 have reported increased proteoglycan expression in gestational diabetes, which is associated with fetal overgrowth.36 Our study found no difference between the umbilical cord blood levels of GPC1 and GPC3 in SGA, AGA, and LGA infants, and no correlation between the levels of these proteoglycans and birthweight. To the best of our knowledge, there is no study investigating serum levels of GPC1 in patients with diabetes mellitus. In the present study, serum levels of GPC1 were higher in infants of mothers with GDM compared to infants of mothers with non-GDM.
There are some limitations in our study. The sample size was relatively small, and the design of the study was cross-sectional. Since there was no correlation between serum levels of GPC1, GPC3, WISP1 and birth weight, we thought that the small sample size did not affect the results of the study.
In conclusion, umbilical cord blood levels of GPC1, GPC3, SDC1, and WISP1 were not different between SGA, AGA, and LGA infants. The significance of serum levels of these adipokines and proteoglycans, which are thought to have an important role on fetal growth, remains to be elucidated.
Funding Statement
This work was supported by the Aydın Adnan Menderes University Research Fund (Grant no. ADU-TPF-19045).
Footnotes
Ethical Committee Approval: Ethical approval was received from the Aydın Adnan Menderes University, Faculty of Medicine (2019/83).
Informed Consent: Informed consent was obtained from the parents of each infant.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept - Ah.A., Ay.A.; Design - Ah.A., Ay.A.; Supervision - Ah.A., Ay.A., A.B.A., A.T., E.Z.; Resource - Ay.A.; Materials - Ay.A., A.T., Ö.Ç., E.Z., A.B.A.; Data Collection and/or Processing - Ay.A., A.T., Ö.Ç., E.Z., A.B.A.; Analysis and/or Interpretation - Ay.A., S.Ö., A.T., Ö.Ç., M.K.T., Ah.A.; Literature Search - Ah.A., Ay.A., Ö.Ç.; Writing - Ah.A., Ay.A., Ö.Ç.; Critical Reviews - Ah.A., M.K.T., Ö.Ç., S.Ö., A.B.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1. Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res. 2006;65(suppl 3):28–33.. 10.1159/000091503) [DOI] [PubMed] [Google Scholar]
- 2. Dessì A, Ottonello G, Fanos V. Physiopathology of intrauterine growth retardation: from classic data to metabolomics. J Matern Fetal Neonatal Med. 2012;25(suppl 5):13–18.. 10.3109/14767058.2012.714639) [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417.. 10.1111/j.1365-2796.2007.01809.x) [DOI] [PubMed] [Google Scholar]
- 4. Reinehr T, Kleber M, Toschke AM. Small for gestational age status is associated with metabolic syndrome in overweight children. Eur J Endocrinol. 2009;160(4):579–584.. 10.1530/EJE-08-0914) [DOI] [PubMed] [Google Scholar]
- 5. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296.. 10.1542/peds.2004-1808) [DOI] [PubMed] [Google Scholar]
- 6. Nguyen-Ngo C, Jayabalan N, Haghvirdizadeh P, Salomon C, Lappas M. Role of adipose tissue in regulating fetal growth in gestational diabetes mellitus. Placenta. 2020;102:39–48.. 10.1016/j.placenta.2020.05.006) [DOI] [PubMed] [Google Scholar]
- 7. Yildiz L, Avci B, Ingeç M. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clin Chem Lab Med. 2002;40(11):1114–1117.. 10.1515/CCLM.2002.195) [DOI] [PubMed] [Google Scholar]
- 8. Karakosta P, Chatzi L, Plana E, et al. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2011;25(2):150–163.. 10.1111/j.1365-3016.2010.01163.x) [DOI] [PubMed] [Google Scholar]
- 9. Mirr M, Owecki M. An update to the WISP-1/CCN4 role in obesity, insulin resistance and diabetes. Medicina. 2021;57(2). 10.3390/medicina57020100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224. 10.1186/gb-2008-9-5-224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunatillake T, Chui A, Fitzpatrick E, et al. Decreased placental glypican expression is associated with human fetal growth restriction. Placenta. 2019;76:6–9.. 10.1016/j.placenta.2018.12.007) [DOI] [PubMed] [Google Scholar]
- 12. Chui A, Zainuddin N, Rajaraman G, et al. Placental syndecan expression is altered in human idiopathic fetal growth restriction. Am J Pathol. 2012;180(2):693–702.. 10.1016/j.ajpath.2011.10.023) [DOI] [PubMed] [Google Scholar]
- 13. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. 10.1186/1471-2431-13-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peplies J, Jiménez-Pavón D, Savva SC, et al. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight European children from the IDEFICS cohort. Int J Obes. 2014;38(suppl 2):S39–S47.. 10.1038/ijo.2014.134) [DOI] [PubMed] [Google Scholar]
- 15. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773.. 10.1016/0002-9378(82)90349-0) [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15–S33.. 10.2337/dc21-S002) [DOI] [PubMed] [Google Scholar]
- 17. Tacke C, Aleksandrova K, Rehfeldt M, et al. Assessment of circulating Wnt1 inducible signalling pathway protein 1 (WISP-1)/CCN4 as a novel biomarker of obesity. J Cell Commun Signal. 2018;12(3):539–548.. 10.1007/s12079-017-0427-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu K, Tao Y, Li J, et al. A comparative genomic and phylogenetic analysis of the origin and evolution of the CCN gene family. BioMed Res Int. 2019;2019:8620878. 10.1155/2019/8620878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hörbelt T, Tacke C, Markova M, et al. The novel adipokine WISP1 associates with insulin resistance and impairs insulin action in human myotubes and mouse hepatocytes. Diabetologia. 2018;61(9):2054–2065.. 10.1007/s00125-018-4636-9) [DOI] [PubMed] [Google Scholar]
- 20. Wang AR, Yan XQ, Zhang C, et al. Characterization of Wnt1-inducible signaling pathway protein-1 in obese children and adolescents. Curr Med Sci. 2018;38(5):868–874.. 10.1007/s11596-018-1955-5) [DOI] [PubMed] [Google Scholar]
- 21. Barchetta I, Cimini FA, Capoccia D, et al. WISP1 is a marker of systemic and adipose tissue inflammation in dysmetabolic subjects with or without type 2 diabetes. J Endocr Soc. 2017;1(6):660–670.. 10.1210/js.2017-00108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klimontov VV, Bulumbaeva DM, Fazullina ON, et al. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal. 2020;14(1):101–109.. 10.1007/s12079-019-00536-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahin Ersoy G, Altun Ensari T, Vatansever D, Emirdar V, Cevik O. Novel adipokines WISP1 and betatrophin in PCOS: relationship to AMH levels, atherogenic and metabolic profile. Gynecol Endocrinol. 2017;33(2):119–123.. 10.1080/09513590.2016.1223286) [DOI] [PubMed] [Google Scholar]
- 24. Sahin Ersoy G, Altun Ensari T, Subas S, et al. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30(8):942–946.. 10.1080/14767058.2016.1192118) [DOI] [PubMed] [Google Scholar]
- 25. Guzmán-Bárcenas J, Hernández JA, Arias-Martínez J, et al. Estimation of umbilical cord blood leptin and insulin based on anthropometric data by means of artificial neural network approach: identifying key maternal and neonatal factors. BMC Preg Childbirth. 2016;16(1):179. 10.1186/s12884-016-0967-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shekhawat PS, Garland JS, Shivpuri C, et al. Neonatal cord blood leptin: its relationship to birth weight, body mass index, maternal diabetes, and steroids. Pediatr Res. 1998;43(3):338–343.. 10.1203/00006450-199803000-00005) [DOI] [PubMed] [Google Scholar]
- 27. Wolf HJ, Ebenbichler CF, Huter O, et al. Fetal leptin and insulin levels only correlate inlarge-for-gestational age infants. Eur J Endocrinol. 2000;142(6):623–629.. 10.1530/eje.0.1420623) [DOI] [PubMed] [Google Scholar]
- 28. Özdemir ZC, Akşit MA. The association of ghrelin, leptin, and insulin levels in umbilical cord blood with fetal anthropometric measurements and glucose levels at birth. J Matern Fetal Neonatal Med. 2020;33(9):1486–1491.. 10.1080/14767058.2018.1520828) [DOI] [PubMed] [Google Scholar]
- 29. Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem. 2000;275(51):40344–40356.. 10.1074/jbc.M006398200) [DOI] [PubMed] [Google Scholar]
- 30. Greeley ET, Rochelson B, Krantz DA, et al. Evaluation of Syndecan-1 as a novel biomarker for adverse pregnancy outcomes. Reprod Sci. 2020;27(1):355–363.. 10.1007/s43032-019-00032-5) [DOI] [PubMed] [Google Scholar]
- 31. Hassani Lahsinoui H, Amraoui F, Spijkers LJA, et al. Soluble syndecan-1 and glycosaminoglycans in preeclamptic and normotensive pregnancies. Sci Rep. 2021;11(1):4387. 10.1038/s41598-021-82972-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kornacki J, Wirstlein P, Wender-Ozegowska E. Levels of syndecan-1 and hyaluronan in early- and late-onset preeclampsia. Preg Hypertens. 2019;18:108–111.. 10.1016/j.preghy.2019.08.165) [DOI] [PubMed] [Google Scholar]
- 33. Long DS, Hou W, Taylor RS, McCowan LM. Serum levels of endothelial glycocalyx constituents in women at 20 weeks’ gestation who later develop gestational diabetes mellitus compared to matched controls: a pilot study. BMJ Open. 2016;6(12):e011244. 10.1136/bmjopen-2016-011244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001;11(3):19R–23R.. 10.1093/glycob/11.3.19r) [DOI] [PubMed] [Google Scholar]
- 35. Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573(3):241–246.. 10.1016/s0304-4165(02)00390-2) [DOI] [PubMed] [Google Scholar]
- 36. Chen CP, Chang SC, Vivian Yang WC. High glucose alters proteoglycan expression and the glycosaminoglycan composition in placentas of women with gestational diabetes mellitus and in cultured trophoblasts. Placenta. 2007;28(2-3):97–106.. 10.1016/j.placenta.2006.02.009) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a