Abstract
Objective
To assess the feasibility of real-time monitoring of work of breathing (WOB) indices and the impact of adjusting HFNC flow on breathing synchrony and oxygen stability in premature infants.
Study design
A prospective, observational study of infants stable on HFNC. The flow adjusted per predetermined algorithm. Respiratory inductive plethysmography (RIP) noninvasively measured WOB. A high-resolution pulse oximeter collected oxygen saturation and heart rate data. Summary statistics and mixed linear models were used.
Results
Baseline data for 32 infants, final analysis of 21 infants. Eighty-one percent with abnormal WOB. Sixty-two percent demonstrated 20% improvement in WOB. For infants with gestational age <28 weeks, an incremental increase in HFNC flow rate decreased WOB (p < 0.001) and improved oxygen saturation and stability (p < 0.01).
Conclusions
Premature infants do not receive optimal support on HFNC. The use of a real-time feedback system to adjust HFNC is feasible and improves WOB, oxygen saturation, and oxygen stability. This technology may improve the utility of HFNC in premature infants.
Introduction
Bronchopulmonary dysplasia (BPD) is a common adverse outcome for premature infants born at less than 30 weeks’ gestation or a birth weight of <1000 g [1]. The use of noninvasive respiratory support is one strategy supported by multiple large, randomized, controlled trials to prevent BPD [2–5]. High flow nasal cannula (HFNC) has gained popularity as a better tolerated, less traumatic interface but may be less effective [6–9]. This suggests that HFNC, as currently applied, does not provide optimal support to premature infants with respiratory distress.
Respiratory inductive plethysmography (RIP) measures thoracoabdominal motion and can provide an objective measurement of thoraco-abdominal asynchrony and WOB indices by recording the rib cage (RC) and abdominal (ABD) movements during breathing. RIP has been used to obtain non-invasive diagnostic measurements of WOB for both pediatric and neonatal patients [10–16]. The use of RIP as a continuous, bedside assessment of WOB has been demonstrated in the pediatric population but this technology has not been expanded into the neonatal intensive care unit. The real-time use of RIP may allow clinicians to optimize the amount of support provided by HFNC in response to the infant’s individual needs. This may result in improved outcomes.
We hypothesize that the use of RIP to make real-time adjustments to HFNC flow rates is feasible in premature infants with respiratory distress and that sequentially alternating HFNC flow rates in response to these objective measurements will be associated with improved work of breathing (WOB) indices and increased oxygen stability.
Subjects and methods
Patient population
This pilot study was conducted at the ChristianaCare Neonatal Intensive Care Unit from April 2018 to April 2019. The Institutional Review Board approved the study. Written informed consent was obtained from parents of enrolled infants prior to the initiation of the study. All infants were de-identified after demographic, clinical, RIP, and pulse oximetry data were extracted from the charts.
Inclusion criteria consisted of infants between 28 and 37 weeks’ corrected gestational age (CGA) and >4 days post-natal age who were stable on baseline HFNC (Optiflow Junior, Fisher & Paykel) settings for ≥12 h and requiring ≤40% supplemental oxygen by the clinical care team for clinical purposes independent of the study. Infants with skeletal, neuromuscular, or ABD surgical disorders that affect the accuracy of WOB measurements were excluded.
Outcomes
The primary outcome is the average phase angle, described below, using RIP technology to assess thoracoabdominal motion and WOB indices.
The secondary outcomes are oxygen saturation and oxygen variability. Oxygen saturation was measured using a high-resolution pulse oximeter (Radical: Masimo, Irvine, CA USA) that has a two-second sampling rate and two-second averaging time. We used standard deviation in oxygen saturation as a measure of oxygen variability.
Definations
RIP is a non-invasive method to determine WOB indices. RIP obtains tidal breathing measurements via thoracoabdominal motion analysis on digital signals associated with RC and ABD wall motion. RIP involves the use of two soft, elastic, cloth bands (Respibands Plus, Viasys, San Diego, CA) encircling the RC and the ABD. The bands contain a flexible sinusoidal wire that can measure motion. The relative motions of the RC and ABD bands are analyzed to determine WOB indices as previously described [10]. The presence of thoracoabdominal asynchrony is reflective of increased WOB.
The pneuRIP instrument and software package (Creative Micro Designs, Newark, DE) allows the digital transfer and instantaneous analysis of RIP data on a tablet device. The validation of this research method has been previously published and has been shown to be consistent with the traditional approach using the Respitrace system (Sensor-medics, Yorba Linda, CA) [16, 17].
The phase angle is defined as the phase shift between the ABD and rib cage excursions. A phase angle of 0° represents perfect synchrony whereas a phase angle of 180° represents complete asynchrony. Based on previous studies, a value of ≥40 represents the start of asynchrony and increased WOB [18].
Study design
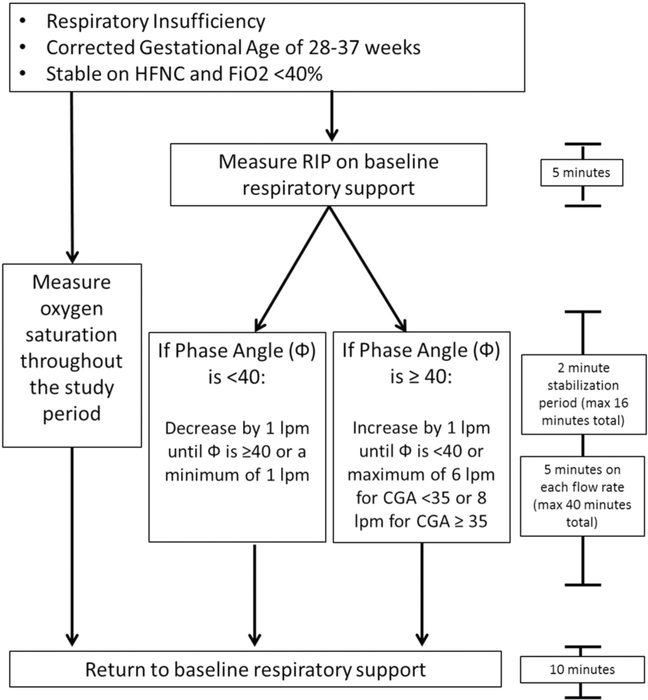
The study compared the average phase angles at commonly used HFNC flow rates in our NICU using a pre-determined algorithm (Fig. 1). As described above, two soft, elastic, cloth bands were placed around the chest and the abdomen of each infant. Pre-study (i.e., baseline) HFNC flow rates were determined by the medical team. Alterations in flow rates were sequential and determined by baseline and subsequent phase angle measurements (Fig. 1). A 2 to 5-min stabilization period occurred between each flow rate change. At the conclusion of the study, the flow rate was returned to the pre-study flow rate and each infant was monitored for 10 minutes. All study measurements were made in the supine position and each enrolled infant only participated once. The data were assessed via a tablet using pneuRIP. Data with poor signals (respiratory rate <5 or >120, labor breathing index of <1 or >5) were eliminated.
Fig. 1. Pre-determined algorithm for adjustment of HFNC based on average phase angle and gestational age.
Two elastic cloth bands were placed around the chest and the abdomen of infants that met inclusion criteria. Work of Breathing measurements were made on baseline support for 5 minutes. Flow rates were increased by 1 lpm if measured phase angle after a 2 minutes stabilization period was >40 degrees. Flow rates were decreased by 1 lpm if measured phase angle after a 2 minute stabilization period was <40 degrees. The maximum flow rate was 6 lpm for infants with a CGA of <35 weeks or 8 lpm for infants with a CGA of ≥35 weeks. The minimum flow rate for all patients was 1 lpm. The flow rate was returned to baseline support at the conculusion of the study and measurements were made for 10 minutes. Oxygen saturation was measured throughout the study using a freestanding pulse oximeter. HFNC High Flow Nasal Cannula, Fi02 Fraction of inspired oxygen, RIP Respiratory Inductuctance Plethysmography, Φ Phase Angle, LPM Liters per minute, CGA Corrected Gestational Age.
To obtain pulse oximetry data, the pulse oximeter was placed on the patient and oxygen saturation was recorded continuously using a high-resolution (2 s averaging time and 0.5 Hz sampling rate) pulse oximetry throughout the study period.
Demographic and clinical data were collected from the medical record. The hemoglobin and hematocrit were obtained from the complete blood count or blood gas obtained during routine clinical practice prior to the study. Oxygen requirement and HFNC flow rates were collected throughout the study period.
Sample size
This was a pilot study to test for the feasibility of using RIP to make objective, real-time adjustments to flow rates in premature infants. A goal sample of 30 infants with complete data was selected.
Statistical analysis
Summary statistics were calculated for baseline and demographic characteristics of the infant subjects. For categorical variables proportions were calculated and for continuous variables mean and standard deviation are reported. A linear mixed model with random intercept was used to evaluate the effect of incremental HFNC flow on phase angle. The baseline HFNC flow at which the infant entered the study varied among the infants and was adjusted for in the model as one of the model covariates. The final model included baseline HFNC flow, HFNC flow step which corresponds to one liter per minute increase in HFNC flow, gestational age, and interaction of gestational age and HFNC flow step. The number of incremental flow steps before returning to the pre-study flow rate varied among the infants. In the analysis, we included only the first three incremental steps that the final cohort of infants went through during the experiment. Similarly, two separate linear mixed models were fitted to evaluate the effect of incremental HFNC flow on oxygen saturation and oxygen saturation variability. In the analysis using the mixed linear model, an unstructured covariance pattern was used to account for possible correlation between measurement subjects.
Results
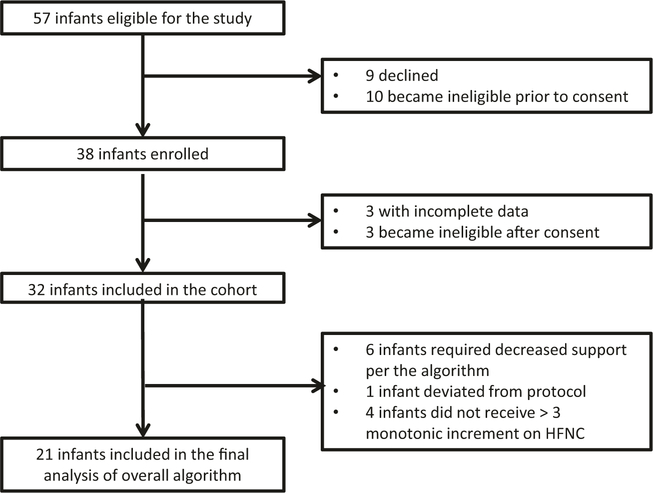
A total of 57 infants were eligible for the study. Of the eligible infants, 38 parents consented to the study and 32 infants participated in the study (Fig. 2). Baseline data for the entire cohort was analyzed. Six infants required decreased support per the algorithm, one deviated from the protocol, and four infants did not receive at least three monotonic incremental increases of HFNC flow rates. Infants who required decreased support and less than three incremental increases in HFNC rates were eliminated to simplify the data analysis and to prevent data attrition. The final analysis included 21 infants. Except for phase angle, infants included, in the final analysis, did not differ from the infants eliminated from the entire cohort. After elimination of low-quality data (low signal due to movement artifact, crying, etc.), 78% of the data was included in the final analysis.
Fig. 2. Consort diagram describing eligibility, consent, and enrollment.
Infants were screened and consent was obtained. Infants with incomplete data or who became ineligible after consent were excluded. A baseline analysis was performed on the entire 32 infant cohort and the final analysis cohort included infants that met specific statistical criteria. HFNC High Flow Nasal Cannula.
Demographic and baseline characteristics for both the cohort (n = 32) and the participants included in the final analysis (n = 21) are presented in Table 1. Eighty-one percent of infants (26/32) had abnormal WOB indices at baseline (phase angle >40°). Baseline WOB indices for both the cohort and the participants included in the final analysis are presented in Table 2. Excluding the infants that had normal WOB indices at baseline (6/21), 62% (16/26) of infants demonstrated a 20% decrease in phase angle from baseline during the step-wise incremental algorithm. Of the infants included in the final analysis, 62% (13/21) of infants demonstrated a 20% decrease in phase angle from baseline.
Table 1.
Demographic and baseline characteristics of the cohort and participants included in the final analysis. The values are mean and standard deviation unless otherwise noted.
| Entire cohort | Final analysis | ||
|---|---|---|---|
| N = 32 | N = 21 | P | |
|
| |||
| Gestational age at birth (weeks), mean (SD) | 28.2 (2.3) | 27.9 (2.4) | 0.930 |
| Birth weight (g), mean (SD) | 1066 (358) | 1134 (375) | 0.138 |
| History of PDA, n (%) | 14 (44) | 9 (43) | 0.888 |
| Diagnosis of BPD, n (%) | 19 (59) | 12 (57) | 0.722 |
| Average hemoglobin at the time of the study, mean (SD) | 12.37 (3.60) | 12.52 (3.47) | 0.730 |
| Corrected gestational age at the time of the study, mean (SD) | 33.2 (2.3) | 32.3 (2.4) | 0.142 |
| Weight at the time of the study (g), mean (SD) | 1615 (430) | 1602 (467) | 0.815 |
| Oxygen requirement at the time of the study (%), mean (SD) | 25.0 (4.5) | 24.4 (4.0) | 0.267 |
| Receiving caffeine at the time of the study, n (%) | 28 (88) | 18 (86) | 0.673 |
| Average caffeine dose (mg/kg) at the time of the study, mean (SD) | 6.77 (1.09) | 10.68 (3.43) | 0.586 |
| Female, n (%) | 17 (53) | 11 (52) | 0.907 |
| Black, n (%) | 13 (41) | 10 (48) | 0.266 |
Table 2.
Baseline work of breathing indices data of the cohort and the participants included in the final analysis, mean (SD), *single tail.
| Cohort | Analysis | ||
|---|---|---|---|
| N = 32 | N = 21 | P | |
| Mean (SD) | Mean (SD) | ||
|
| |||
| Phase Angle (°) | 71 (30.5) | 85 (22) | 0.000 |
| Respiratory Rate (bpm) | 26 (12) | 27 (14) | 0.643 |
| Sp02 (%) | 93 (4) | 93 (4) | 0.677 |
| Heart Rate (bpm) | 161 (10) | 161 (11) | 0.870 |
There was no correlation between phase angle and oxygen saturation (R = −0.025, p = 0.83) or phase angle and oxygen stability (R = 0.02, p = 0.87). Length of exposure to increased flow rates was not associated with phase angle, oxygen saturation, or oxygen saturation variability. The effect of incremental HFNC flow on phase angle was significantly impacted by gestational age. Controlling for the baseline HFNC flow rate with which the infants entered the study, infants born at a gestational age of <28 weeks, a unit increase inflow (i.e., step) was associated with a decrease in the phase angle by 9.3° (p < 0.001). In contrast, an increase in flow was associated with an increase in the phase angle by 11.9° in infants born at ≥28 weeks. Incremental HFNC flow also significantly improved the oxygen saturation and reduced oxygen saturation variability. A one liter per minute increase in HFNC flow rate was associated with increased oxygen saturation (saturation increased by 1%, p < 0.01) and increased oxygen stability (decreased oxygen saturation variability by 23%, p < 0.01) (Fig. 3). Gestational age was not associated with oxygen saturation or oxygen stability. Gestational age did not modify the effect of incremental HFNC flow rates on either oxygen saturation or oxygen stability.
Fig. 3. Variability in oxygen saturation measured in terms of standard deviation at different HFNC flow (L/min) levels (p < 0.00, n = 21 infants).
A one liter per minute increase in HFNC flow rate was assocaited with decreased oxygen saturation variability or decreased standard deviation, representing increased oxygen stability. A one liter per minutes increase in HFNC flow rate resulted in a 23% decrease in oxgyen saturation variability. HFNC High Flow Nasal Cannula, SD Standard Deviation.
In a post hoc analysis after adjusting for baseline HFNC, infants born at a gestational age of ≥28 weeks compared to infants born at a gestational age of <28 weeks demonstrated increased WOB indices (phase angle 58 ± 34 vs. 87 ± 34°, p < 0.001) and heart rate at baseline (158 ± 9 vs. 165 ± 10 bpm, 1-sided test, p < 0.05). There was no significant difference in baseline oxygen saturation, oxygen stability, respiratory support, or oxygen requirement for infants born at <28 weeks compared to infants born at ≥28 weeks. There was no significant difference in the CGA at the time of the study (33.1 ± 2.1 vs. 33.3 ± 2.5 weeks).
Discussion
This prospective pilot study of premature infants demonstrates that the use of RIP to make objective, real-time adjustments to flow rates in premature infants is feasible. We demonstrated that most infants are not at optimal HFNC flow rates and respond variably and unpredictably to incremental flow changes. Our study suggests that infants born at <28 weeks gestation are more likely to demonstrate improved WOB indices and synchrony with increasing HFNC flow rates and that increased HFNC flow significantly improves oxygen saturation and stability.
Premature infants with respiratory insufficiency have abnormal breathing patterns (asynchrony) due to a highly compliant rib cage, stiff, non-compliant lungs, and poor compensation by inspiratory rib cage muscles [19]. Many infants in this study demonstrated asynchronous breathing patterns on baseline respiratory support settings that the clinical team perceived as adequate. This finding highlights the inability of clinicians to accurately assess effective breathing patterns and WOB indices and to provide appropriate noninvasive respiratory support to premature infants.
RIP measures thoracoabdominal motion and may be used to objectively measure WOB indices in real-time at the bedside. RIP has been used to assess pulmonary function in the neonatal patient population and to measure changes in WOB indices in response to various interventions [20–24] Recent studies have used this technology as a real-time, feedback tool in the pediatric patient [25] and the feasibility of machine learning to automatically identify normal and asynchronous breathing patterns has been demonstrated [13, 15]. This is the first study to use RIP as a bedside tool to provide individualized flow support to neonates based on objective WOB measurements.
The use of HFNC has gained popularity as a method of noninvasive respiratory support because it causes less nasal and face trauma and is perceived as better tolerated [26]. Studies have shown that HFNC flow improves respiratory gas exchange and lung mechanics, results in less drying and damage to the nasal mucosa, decreases bronchospasm/pulmonary resistance, and improves cell physiology [27–31]. In addition, during exhalation, HFNC flow creates a reservoir of gas in the anatomical dead space of the nasopharynx and upper airway, improving respiratory efficiency, breathing synchrony, and WOB indices [27, 32, 33]. In neonates, some studies have demonstrated that HFNC is equivocal to other noninvasive modalities as a primary mode of respiratory support or after extubation [7, 8]. In contrast, some studies have suggested increased treatment failure with the use of HFNC, especially in the extremely premature [6, 9]. Our study suggests that maximized HFNC support adjusted to individual needs improves oxygenation and oxygen stability. We also demonstrated that an increased HFNC support improves WOB indices for infants born at <28 weeks’ gestation. These findings suggest that observed limitations of HFNC may reflect insufficient support rather than a failure of HFNC as a noninvasive respiratory support modality.
Strengths of our study include its prospective design, use of pneuRIP technology to objectively measure breathing patterns and WOB indices, and measurement of oxygen saturation using a high-resolution pulse oximeter rather than vital sign documentation. Limitations of our study include small sample size, heterogeneity of the patient population, and the statistical model assuming no first or higher-order carry-over effect of previous flow rates. However, in the analysis, an unstructured covariance pattern was used to account for possible correlation. In addition, the flow rate algorithm used in this study failed to consistently improve WOB indices. This may be due to scarce information on WOB indices and clinical optimization in the preterm population and suggests that a superior model may exist.
In conclusion, premature infants, in our sample, are not receiving optimal support when on HFNC. The use of an automated, point-of-care, non-invasive respiratory support system that responds to individual and variable WOB indices is feasible and results in improved oxygen stability among premature infants with respiratory insufficiency. However, further research is needed to optimize the feedback algorithm to allow instantaneous adjustments to breath to breath variations in WOB indices.
Acknowledgements
THS receives funding from NIH COBRE grant P30GM114736. KS is supported by an Institutional Development Aware (IDeA) from the National Institute of General Medical Sciences of the NIH grant U54-GM104941 (PI Binder-Macleod).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196 (2):147.e1–8. [DOI] [PubMed] [Google Scholar]
- 2.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700–8. [DOI] [PubMed] [Google Scholar]
- 3.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069–76. [DOI] [PubMed] [Google Scholar]
- 5.Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain WA, Marks JD. Approaches to noninvasive respiratory support in preterm infants: from CPAP to NAVA. Neoreviews. 2019;20(4):e213–e21. [DOI] [PubMed] [Google Scholar]
- 7.Manley BJ, Owen LS, Davis PG. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med. 2014;370 (4):385–6. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev 2016;2:Cd006405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts CT, Owen LS, Manley BJ, Frøisland DH, Donath SM, Dalziel KM, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. 2016;375(12):1142–51. [DOI] [PubMed] [Google Scholar]
- 10.Allen JL, Greenspan JS, Deoras KS, Keklikian E, Wolfson MR, Shaffer TH. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;11(1):37–43. [DOI] [PubMed] [Google Scholar]
- 11.de Jongh BE, Locke R, Mackley A, Emberger J, Bostick D, Stefano J, et al. Work of breathing indices in infants with respiratory insufficiency receiving high-flow nasal cannula and nasal continuous positive airway pressure. J Perinatol 2014;34(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez A, Mulot R, Vardon G, Barois A, Gallego J. Thoracoabdominal pattern of breathing in neuromuscular disorders. Chest. 1996;110(2):454–61. [DOI] [PubMed] [Google Scholar]
- 13.Ryan L, Rahman T, Strang A, Heinle R, Shaffer TH. Diagnostic differences in respiratory breathing patterns and work of breathing indices in children with duchenne muscular dystrophy. PLoS ONE. 2020;15(1):e0226980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfson MR, Greenspan JS, Deoras KS, Allen JL, Shaffer TH. Effect of position on the mechanical interaction between the rib cage and abdomen in preterm infants. J Appl Physiol. 1992;72(3): 1032–8. [DOI] [PubMed] [Google Scholar]
- 15.Ratnagiri MV, Ryan L, Strang A, Heinle R, Rahman T, Shaffer TH. Machine learning for automatic identification of thoracoabdominal asynchrony in children. Pediatr Res. 2020. 10.1038/s41390-020-1032-1. Jul 3[Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman T, Page R, Page C, Bonnefoy JR, Cox T, Shaffer TH. pneuRIP(TM): a novel respiratory inductance plethysmography monitor. J Med Device. 2017;11(1):0110101–110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strang A, Ryan L, Rahman T, Balasubramanian S, Hossain J, Heinle R, et al. Measures of respiratory inductance plethysmography (RIP) in children with neuromuscular disease. Pediatr Pulmonol 2018;53(9):1260–8. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramaniam SL, Wang Y, Ryan L, Hossain J, Rahman T, Shaffer TH. Age-related ranges of respiratory inductance plethysmography (RIP) reference values for infants and children. Paediatr Respir Rev. 2019;29:60–7. [DOI] [PubMed] [Google Scholar]
- 19.Ambalavanan N, Van Meurs KP, Perritt R, Carlo WA, Ehrenkranz RA, Stevenson DK, et al. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J Perinatol. 2008;28(6):420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanbar J, Shalish W, Laremouille S, Rao S, Brown K, Kearney R, et al. Cardiorespiratory behavior of preterm infants receiving continuous postiive airway pressure and high flow nasal cannula post extubation: randomzied crossover study. Pediatr Res. 2020; 87(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks L, DiFiore JM, Martin R. Assessment of tidal volume preterm infants using respiratory inductance plethysmography. Pediatr Pulmonol 1997;23(6):429–33. [DOI] [PubMed] [Google Scholar]
- 22.Stick S, Ellis E, Leouef P, Sly P. Validation of respiratory inductance plethysmography for the measurement of tidal breathing parameters in newborns. Pediatr Pulmonol 1992;14(3): 187–91. [DOI] [PubMed] [Google Scholar]
- 23.Springer C, Godfrey S, Vilozni D, Bar-Yishay E, Noviski N, Avital A. Comparison of respiratory inductance thoracoabdominal compression in bronchial challenges in infants and young children. Am J Respir Crit Care Med. 1996;154(3):665–9. [DOI] [PubMed] [Google Scholar]
- 24.Martin R, DiFiore JM, Korenke CB, Randal H, Miller MJ, Brooks LJ. Vulternability of respiratory control in healthy preterm infants placed supine. J Pediatr. 1995;127(4):609–14. [DOI] [PubMed] [Google Scholar]
- 25.Robles-Rubio CA, Kearney R, Bertolizio G, Brown K. Automatic unnsupervised respiratory analysis of infant respiratory inductance plethymography signals. PLoS ONE. 2020;15(9):e0238402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotecha SJ, Adappa R, Gupta N, Watkins WJ, Kotecha S, Chakraborty M. Safety and efficacy of high-flow nasal cannula therapy in preterm infants: a meta-analysis. Pediatrics. 2015;136 (3):542–53. [DOI] [PubMed] [Google Scholar]
- 27.Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol 2006;26(8):476–80. [DOI] [PubMed] [Google Scholar]
- 29.Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH. The effects of gas humidification with high-flow nasal cannula on cultured human airway epithelial cells. Pulm Med 2012;2012: 380686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenspan JS, Wolfson MR, Shaffer TH. Airway responsiveness to low inspired gas temperature in preterm neonates. J Pediatr. 1991;118(3):443–5. [DOI] [PubMed] [Google Scholar]
- 31.Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial. J Perinatol 2006;26(8):481–5. [DOI] [PubMed] [Google Scholar]
- 32.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med 2009;103(10): 1400–5. [DOI] [PubMed] [Google Scholar]
- 33.McQueen M, Rojas J, Sun SC, Tero R, Ives K, Bednarek F, et al. Safety and long-term outcomes with high flow nasal cannula therapy in neonatology: a large retrospective cohort study. J Pulm Respir Med. 2014;4(6):216. [DOI] [PMC free article] [PubMed] [Google Scholar]