Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on zearalenone hydrolase (ZenA) produced by Escherichia coli DSM 32731 when used as a feed additive for all terrestrial animals. The production strain E. coli DSM 32731 is genetically modified and harbours a kanamycin resistance gene. No viable cells of the production strain were detected in the final product, but uncertainty remains on the presence of recombinant DNA in the final product. The ZenA contained in the additive is safe for all terrestrial animal species up to the maximum use levels of (in U/kg complete feed): 100 U/kg in chickens for fattening; 150 U/kg in laying hens, turkeys for fattening and rabbits; 200 U/kg in pigs; 250 U/kg in dairy cows; 400 U/kg in veal calf (milk replacer), cattle for fattening, sheep, goats, horses and cats; and 450 U/kg in dogs. Based on the ADME and toxicological data, the FEEDAP Panel considers that the use of the ZenA contained in the additive in animal nutrition is safe for the consumers. The endotoxin content in the additive poses a risk by inhalation for users handling the additive. The additive is not a skin/eye irritant nor a skin sensitiser. Due to its proteinaceous nature, the additive should be considered as a potential respiratory sensitiser. The ZenA contained in the additive and the resulting breakdown products of its enzymatic activity do not represent a safety concern for the environment. The production strain harbours an antimicrobial resistance gene and uncertainties remain on the possible presence of its recombinant DNA in the final product; therefore, the FEEDAP Panel cannot conclude on safety of the additive for the target species, the consumer, the user and the environment.
Keywords: technological additive, substances for reduction of the contamination of feed by mycotoxins, zearalenone hydrolase, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Biomin GmbH 2 for authorisation of the product zearalenone hydrolase produced by Escherichia coli DSM 32731, when used as a feed additive for all terrestrial animals (category: technological additive; functional group: substances for reduction of the contamination of feed by mycotoxins).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 13 February 2020.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of zearalenone hydrolase produced by Escherichia coli DSM 32731, when used under the proposed conditions of use (see Section 3.1.10).
1.2. Additional information
The additive zearalenone hydrolase (ZenA) has not been previously assessed or authorised as a feed additive in the European Union.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 3 in support of the authorisation request for the use of ZenA as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the ZenA in animal feed. The Executive Summary of the EURL report can be found in Annex A. 4
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of ZenA is in line with the principles laid down in Regulation (EC) No 429/2008 5 and the relevant guidance documents: Technical guidance: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018b) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The enzyme ZenA is produced by E. coli DSM 32731 and is intended to be used as a technological additive (functional group: substances for reduction of the contamination of feed by mycotoxins) in feed for all terrestrial animals.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The production organism, a genetically modified strain of Escherichia coli, is deposited at the Leibniz Institute DSMZ‐German Collection of Microorganisms and Cell Cultures under the accession number DSM 32731. 6
The identity of the production strain was confirmed ■■■■■ a well‐known E. coli B strain commonly used for protein expression. 7
The antimicrobial susceptibility of the production strain to the battery of antimicrobials recommended by EFSA for Enterobacteriaceae (EFSA FEEDAP Panel, 2018a) was tested by broth microdilution. All minimum inhibitory concentration (MIC) values were equal or lower than the corresponding cut‐off values, except for kanamycin which exceeded the cut‐off value by six dilution steps. 8
A kanamycin resistance gene ■■■■■. No other acquired antibiotic resistance gene was identified in the genome of the production strain by comparing the genome sequences of E. coli DSM 32731 ■■■■■. 7
Information related to the genetically modified microorganism
3.1.2. Characterisation of the recipient or parental microorganism
The parental strain of E. coli DSM 32731 ■■■■■. 9 The parental strain ■■■■■ has been described to be free from pathogenic factors and enterotoxins associated with virulence 10 and is not expected to raise safety concerns.
3.1.3. Characterisation of the donor organisms
■■■■■. 11
■■■■■. 12
3.1.4. Description of the genetic modification
■■■■■.
■■■■■. The structure of the genetic modification was confirmed by alignment of the WGS of the production and the parental strains. 7
■■■■■. 7
3.1.5. Characterisation of the active substance
The active substance of the additive is the enzyme ZenA belonging to the enzyme class 3.1.1. Another name of the additive is zearalenone lactonase.
The enzyme detoxifies zearalenone (ZEN) by opening the toxins’ lactone ring by hydrolysis (Figure 1).
Figure 1.
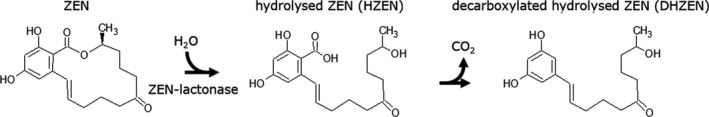
Reaction of zearalenone detoxification. The enzyme zearalenone hydrolase degrades zearalenone (ZEN) to hydrolysed zearalenone (HZEN) which may decarboxylate spontaneously resulting in decarboxylated hydrolysed zearalenone (DHZEN)
3.1.6. Manufacturing process
Zearalenone hydrolase is produced by E. coli DSM 32731 in a controlled fermentation process. ■■■■■. 13
3.1.7. Characterisation of the additive
The additive is composed of ZenA and maltodextrin as a carrier. The ZenA is specified with a minimum activity of 7 U 14 /g.
Data on batch to batch variation for the content of the active substance of five batches of the additive confirmed compliance with the specifications (average of 8.7 U/g, range: 7.6–9.2 U/g). 15
To investigate the chemical purity of the additive, analyses of heavy metals (lead (Pb), cadmium (Cd), mercury (Hg)), arsenic (As) 16 and mycotoxins (deoxynivalenol, ZEN, aflatoxins B1, B2, G1 and G2, ochratoxin A, fumonisins B1 and B2, HT‐2 toxin and T‐2 toxin) 17 were performed on three production batches. The results were in all cases below the specifications established by the applicant (< 2 mg/kg for As, < 5 mg/kg for Pb, < 0.5 mg/kg for Cd and < 0.1 mg/kg for Hg) and the respective limit of quantification (LOQ) 18 or limit of detection (LOD) 19 of the analytical method applied. The same three batches were tested for the potential presence of microbial contaminants. 20 Results showed compliance with the specifications established by the applicant: < 10 CFU/g for coliforms; no detection in 25 g for E. coli and Salmonella spp.; and < 100 CFU/g for yeasts and filamentous fungi.
The presence of viable cells of the production strain was tested in three batches of the product in triplicate. 21 ■■■■■. No colonies were observed in any of the samples of the final product.
The presence of recombinant DNA of the production strain was tested in three batches of the product in triplicate. 22 ■■■■■. No recombinant DNA of the production strain was detected in three batches of the final product. However, the FEEDAP Panel considers that the amount of sample tested is not representative and is not in the position to draw a conclusion. Consequently, uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product.
Three batches of the additive were analysed for endotoxin activity (by Limulus amoebocyte lysate assay) which ranged from 91,433 to 94,368 IU/g. 23
The additive under assessment is an off‐white granulate. Bulk density measured in three batches of the additive ranged from 576 to 626 kg/m3. 24 The dusting potential of the additive measured in three batches of the additive by ‘Stauber–Heubach’ method ranged from 340 to 415 mg/m3. 25
3.1.8. Stability and homogeneity
Shelf‐life of ZenA was investigated in five batches of the additive stored at 22 and 37°C in its original packaging over a period of 24 months, following the enzymatic activity. 26 The initial activity of the enzyme ranged from 74.2 to 78.4 U/g (thus about 10× the specification). 27 The enzyme activity was not affected when stored at 22°C but an average loss of 9% was observed when stored at 37°C.
The stability of one batch of the additive in a premixture (for poultry, pigs and ruminants when supplemented at 500 U/kg) and a mineral feed (for piglets when supplemented at 20,000 U/kg) stored at 22 ± 2°C over a period of 12 months was investigated. 28 The activity of ZenA was not affected when stored under these storage conditions.
The stability of the additive incorporated at 20 U/kg in complete feed for chickens for fattening, pigs and dairy cows (one batch each, no indication if in mash or pelleted form), and stored at 22 ± 2°C under dry conditions (packaging was not described) was investigated over a period of 3 months. 29 The basal diet of chickens for fattening consisted of maize and soybean meal; that of pigs for fattening on wheat, soybean meal and barley; and the one for dairy cows consisted of whole meal from rape, wheat and maize concentrate. The activity of ZenA was not affected when stored under these storage conditions except the feed for chickens for fattening that showed a loss of 4%. The stability of the additive during feed conditioning was not studied.
The capacity of the additive to homogeneously distribute in feed for piglets (not specified if it was in mash or pelleted form) was investigated (intended inclusion level of 60 U/kg), based on 20 sub‐samples. 30 The coefficient of variation of the enzymatic activity was 8%.
3.1.9. Interference with the analysis of mycotoxins in feed
The possible interference of the additive with the analytical determination of ZEN in feed was investigated. A naturally contaminated maize with ZEN was homogenised and divided in two portions; one was left untreated and the other received the additive at the dose of 100 U/kg feed. ZEN was analysed in both samples using a quantitative LC–MS/MS‐based method. 31 The acetonitrile/water 80/20 (v/v) solution used in the initial extraction of ZEN was found to inhibit the action of ZenA as no differences in ZEN concentration in comparison with the untreated control were observed. Given the specificity of the hydrolase, the analytical determination of other structural classes of mycotoxins would not be affected.
3.1.10. Conditions of use
The additive is proposed to be used in premixtures and compound feed at a minimum inclusion level of 10 U/kg complete feed for all terrestrial animal species. No maximum level is proposed.
3.2. Safety
3.2.1. Safety of the production organism
The production strain has been properly identified at species level as E. coli and differed from the recipient strain by overproducing ZenA and resistance to kanamycin ■■■■■ The parental strain, ■■■■■ is non‐pathogenic and does not produce enterotoxins associated with virulence. No viable cells of the production strain were detected in the final product. Nevertheless, the FEEDAP Panel cannot exclude the presence of recombinant DNA from the production strain in the product.
Since the genetic modification introduces an antimicrobial resistance gene in the production strain and that uncertainties remain on the possible presence of recombinant DNA from the production strain in the final product, the FEEDAP Panel cannot conclude on the safety for the target species, consumers, users and the environment with regard to the production strain.
3.2.2. Studies on metabolites resulting from degradation of zearalenone using zearalenone hydrolase
Zearalenone hydrolase is able to modify the toxic structure of ZEN by opening its lactone ring, forming hydrolysed zearalenone (HZEN), which can spontaneously be converted to decarboxylated hydrolysed zearalenone (DHZEN).
The applicant submitted data to detect metabolites other than HZEN and DHZEN, following the degradation of ZEN in the presence of the additive. 32 ZenA alone, ZEN + ZenA and ZEN alone were incubated for 4 h at 37°C in a Teorell Stenhagen buffer/BSA solution, followed by an inactivation step at 99°C for 10 min. Analysis of the supernatant of incubation samples was carried out by HPLC‐DAD with detection at 200 and at 270 nm wavelengths. Only HZEN (about 95%) and DHZEN (about 5%) were detected in both cases. The LOD method was not given, but no other peaks with a signal to noise ratio greater than 3 were detected. Based on these results, the FEEDAP Panel concludes that, apart from HZEN and DHZEN, no other metabolites are formed in significant amounts by the action of ZenA on ZEN.
In addition, an in vitro study using the Caco‐2 cell line, 33 was carried out to evaluate the potential absorption of the two metabolites, HZEN and DHZEN, resulting from the degradation of ZEN by ZenA at the intestinal level. It was observed that both compounds, HZEN and DHZEN presented a lower coefficient of permeability, respectively 73 and 5 times lower than ZEN in the direction of absorption (apical – basal) of the cell line model. Additionally, the efflux rate was higher for HZEN, as compared to ZEN. The in vitro low absorption and high efflux of HZEN suggests a low absorption in vivo. Although the potential absorption of DHZEN is higher than that of HZEN, it is formed in a lower amount, about 5%, thus it is expected that a very low amount would be absorbed in vivo.
3.2.3. Toxicological studies
The applicant has submitted toxicological data on the additive, on ZEN and on the metabolites produced following the degradation of ZEN by the additive. The studies are described in separate sections below.
3.2.3.1. Toxicological studies conducted with zearalenone hydrolase
For the toxicological studies, two different concentrate production batches of the additive under assessment were used: a production batch with activity 550 U/g (about 60× the activity of the ZenA in the additive) for the bacterial reverse mutation assay; and a production batch with activity of 890 U/g (about 100× the activity of the ZenA in the additive) for the in vitro micronucleus test and the 90‐day study.
Genotoxicity studies
Bacterial reverse mutation test
In order to investigate the potential of ZenA to induce gene mutations in bacteria, two studies with the Ames test were performed according to OECD Test Guideline (TG) 471 (OECD, 1997) and following Good Laboratory Practice (GLP) in Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102. 34 ZenA was tested at six concentration levels ranging from 31.6 to 5,000 µg/plate in two independent experiments, applying the plate incorporation and pre‐incubation methods in the presence or absence of metabolic activation. Appropriate positive and negative controls were evaluated concurrently. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix. No precipitate and toxicity were observed in any experimental condition. No increase in the mean number of revertant colonies was observed at any tested concentration in any tested strains with or without S9‐mix. The Panel concludes that the test item did not induce gene mutations in bacteria under the experimental conditions employed in this study.
In vitro micronucleus test
An in vitro micronucleus assay was performed according to OECD TG 487 (OECD, 2016) and following GLP to evaluate the potential of ZenA to induce chromosome damage in L5178Y TK+/− mouse lymphoma cells in the absence and presence of metabolic activation. 35 The compound was tested at 125, 250 and 500 µg/mL due to precipitation observed at the end of the treatment period at 500 µg/mL and above. A short treatment (3 + 24 h of recovery) with and without S9‐mix; and a continuous treatment (24 + 0 h recovery) without S9‐mix were the experimental conditions applied. Appropriate positive and negative control chemicals were used, and the results obtained confirmed that the experimental system was sensitive and valid. No significant cytotoxicity, evaluated by determining the population doubling of cells, was observed in any experimental condition. No significant increase of micronucleated cells was induced by treatment with ZenA compared to concurrent vehicle controls. The Panel concluded that the test item did not induce chromosome damage in mammalian cells under the experimental conditions employed in this study.
Repeated dose toxicity study
A GLP‐compliant 90‐day toxicity study 36 was conducted in rats (10 females and 10 males per group) administered by gavage with ZenA at 0 (control), 100, 500 or 1,000 mg/kg bw per day (corresponding to 89, 445 and 890 U/kg bw, respectively). Food and water intake were given ad libitum. No effects were observed on mortality. Clinical observations, body weight, haematology and blood chemistry, gross and microscopic pathology did not reveal any adverse effects at any doses tested. The FEEDAP Panel noted that the study was not conducted in full agreement with the relevant OECD TG (e.g. the functional observation battery was not conducted). However, the Panel considered that there were no other observations that would justify the generation of new data. From the results of this study, a no observed adverse effect level (NOAEL) of 1,000 mg/kg bw per day (corresponding to 890 U/kg bw), the highest dose tested, was identified.
3.2.3.2. Toxicological studies conducted with zearalenone and its breakdown products
Genotoxicity studies
Bacterial reverse mutation assay with ZEN, HZEN and DHZEN
Three Ames tests were provided by the applicant to investigate the potential of ZEN, 37 HZEN 38 and DHZEN 39 to induce gene mutations in bacteria. The Ames tests followed GLP and were performed according to OECD TG 471 (OECD, 1997) using the strains Salmonella Typhimurium TA98, TA100, TA1535, TA1537 and E. coli strain WP2uvrA. The compounds were tested in two independent experiments applying the plate incorporation assay and the pre‐incubation method in the presence and absence of metabolic activation. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix. ZEN having a purity of 97%, was tested at six concentrations ranging from 10 to 2,500 µg/mL (maximum concentration was limited by solubility). Cytotoxicity was observed at 316 µg/plate and above. HZEN (purity 95%) was tested at six concentrations ranging from 31.6 to 5,000 µg/mL. Cytotoxicity was observed at 2,500 and 5,000 µg/plate. DHZEN (purity 98.7%) was tested at six concentrations ranging from 31.6 to 5,000 µg/mL. Cytotoxicity was observed at 316 μg/plate and above without metabolic activation and at 5,000 μg/plate with metabolic activation. Overall, no increase in the mean number of revertant colonies was observed with any test item, in any tested strain, at any tested concentration with or without S9‐mix. The Panel concluded that ZEN, HZEN and DHZEN did not induce gene mutations in bacteria under the experimental conditions employed in the studies.
In vitro micronucleus test
Three in vitro micronucleus tests were provided by the applicant to evaluate the potential of ZEN, 40 HZEN 41 and DHZEN 42 to induce chromosome damage. The studies were performed according to OECD TG 487 (OECD, 2016) and following GLP in Chinese Hamster V79 cells in the absence and presence of metabolic activation. Detailed results for the three compounds are reported below.
Based on the results of a preliminary cytotoxicity assay, three concentrations of ZEN (purity 97%) were selected for the analysis of micronuclei applying a short treatment (4 + 20 h of recovery) with and without metabolic activation (S9‐mix) (25, 27.5 and 30 μg/mL and 17.5, 22.5 and 27.5 μg/mL, respectively) and a continuous treatment without S9‐mix (24 + 0 h recovery) (10, 15, 22.5 and 25 μg/mL). Cytotoxicity up to 60% reduction of cell proliferation compared to the concurrent negative control was observed at the highest concentrations tested. The positive controls induced statistically significant increases in the frequency of micronuclei, demonstrating the sensitivity of the test system and the efficacy of the S9‐mix. ZEN induced a dose‐related statistically significant increase of micronuclei after short and continuous treatment in the absence of S9‐mix. In the presence of metabolic activation, a statistically significant increase of micronuclei was observed at all the concentrations tested. The increase was not dose‐related; however, the Panel noted that a narrow range of concentrations was applied. Based on these results, the Panel concluded that ZEN induced chromosome damage in mammalian cells under the experimental conditions employed in this study.
Based on the results of a preliminary cytotoxicity assay, three concentrations of HZEN (purity 95%) were selected for the analysis of micronuclei applying a short treatment (4 + 20 h of recovery) with and without metabolic activation (S9‐mix) (1,000, 1,500, 1,750 and 2,000 μg/mL and 500, 1,000 and 2,000 μg/mL, respectively) and a continuous treatment without S9‐mix (24 + 0 h recovery) (100, 250 and 500 μg/mL). Cytotoxicity up to 60% reduction of cell proliferation compared to the concurrent negative control was only observed after continuous treatment at the highest concentration tested. The positive controls induced statistically significant increases in the frequency of micronuclei, demonstrating the sensitivity of the test system and the efficacy of the S9‐mix. HZEN induced a dose‐related statistically significant decrease of micronuclei after short treatment in the presence of S9‐mix. The values of micronuclei were below the historical control range. In the absence of metabolic activation, the frequencies of micronuclei were comparable to the values observed in the negative controls. Based on these results, the Panel concluded that HZEN did not induce chromosome damage in mammalian cells under the experimental conditions employed in this study.
Based on the results of a preliminary cytotoxicity assay, three concentrations of DHZEN (purity 98.7%) were selected for the analysis of micronuclei applying a short treatment (4 + 20 h of recovery) with and without metabolic activation (S9‐mix) (50, 100 and 125 μg/mL and 50, 125, 175 and 205 μg/mL, respectively). Cytotoxicity up to 59% reduction of cell proliferation compared to the concurrent negative control was observed at the highest concentrations tested. The positive controls induced statistically significant increases in the frequency of micronuclei, demonstrating the sensitivity of the test system and the efficacy of the S9‐mix. DHZEN induced a dose‐related statistically significant increase of micronuclei after short treatment in the absence of S9‐mix. In the presence of metabolic activation, a statistically significant increase of micronuclei was observed at the highest concentration tested. The increase of micronuclei was dose related, as demonstrated statistically by a dose‐trend test (p = 0.025). Based on these results, the Panel concluded that DHZEN induced chromosome damage in mammalian cells under the experimental conditions employed in this study.
Oestrogenic activity
ZEN exerts oestrogenic activity by binding to oestrogen receptors. The applicant submitted studies and papers retrieved in the literature to support the lack of concern about oestrogenicity for the metabolites formed following ZenA activity.
An in vivo screening test (performed in accordance with OECD TG 440) was carried out in piglets, 43 to compare the oestrogenic effects of HZEN and DHZEN to that of the parent compound ZEN. A total of 36 female weaned piglets (about 9 kg bw) were given six different diets containing no ZEN (control), or containing ZEN at 0.17, 1.46, 4.59 mg/kg; and HZEN or DHZEN at equimolar concentrations to that of ZEN 4.59 mg/kg for 27 days. 44 Feed and water were provided ad libitum. Weight of each piglet and vulva size were measured on days 0, 6, 9, 13, 16, 21, 23 and 26. Vulva was measured with the use of a calliper. Animals were killed at the end of the study and tissue samples (reproductive tract: vulva was removed from the reproductive tract and the total weight of the vagina, cervix, uterus and ovaries was determined) were collected. No significant differences in body weight, feed intake and feed conversion ratio were observed between the treatment groups at any time point. Significant increase (p < 0.05) in the vulva size was observed in animals receiving ZEN at the highest concentration compared to the control, during the whole experiment. ZEN, when administered at 1.46 mg/kg in the diet, induced significant increase of vulva size (p < 0.05) from day 16 to the end of the study. No significant effects compared to the control were seen in the vulva size in animals given ZEN in the diet at 0.17 mg/kg or HZEN or DHZEN. 45 A statistically significant increase in weight (absolute and relative) of the reproductive tract was observed in the group that received 4.59 mg ZEN/kg compared to control. ZEN at 0.17 or 1.46 mg/kg, HZEN and DHZEN did not induce any effects on the reproductive tract.
The oestrogenic activity of the metabolites HZEN and DHZEN formed by hydrolysis of ZEN following the zearalenone‐lactonase activity was evaluated by Fruhauf et al. (2019). 46 ZEN, HZEN and DHZEN were tested using two in vitro models, the MCF‐7 cell proliferation assay (0.01–500 nM) and an oestrogen‐sensitive yeast bioassay (1–10,000 nM). ZEN induced cell proliferation and gene transcription, no oestrogenic effects were observed when testing HZEN or DHZEN. These results would suggest that these metabolites are less oestrogenic than ZEN in vitro.
Kakeya et al. (2002) 47 demonstrated, by using the human breast cancer MCF‐7 cell assay, the absence of oestrogenic activity exerted by DHZEN even when this compound was tested at concentration 1,000 times higher than ZEN (30 nM vs. 0.03 nM, respectively).
3.2.3.3. Conclusions on the biological (ADME) and toxicological studies
The FEEDAP Panel concludes that ZenA is not genotoxic in vitro. Based on the results of a 90‐day study, a NOAEL of 890 U/kg bw and day was identified.
The in vitro studies provided allowed the following conclusions: (i) only two metabolites are formed following the action of ZenA on ZEN, HZEN was the major metabolite (about 95%) and DHZEN was detected at a much lower amount (about 5%); (ii) HZEN is not genotoxic and would be absorbed to a limited extent in the gastrointestinal (GI) tract; (iii) DHZEN, similarly to ZEN, is genotoxic, appears to be more absorbed than HZEN but less absorbed than ZEN.
Based on the results above, the FEEDAP Panel considered that the metabolites formed following the action of zearalenone hydrolase (HZEN and DHZEN) are less oestrogenic compared to the parent compound (ZEN) and would not be of toxicological concern.
Therefore, the FEEDAP Panel concluded that ZenA and ZEN metabolites, HZEN and DHZEN, resulted by the use of additive, are of no additional toxicological concern.
3.2.4. Safety for the target species
To support the safety for the target species the applicant submitted a 90‐day sub‐chronic oral toxicity study and a tolerance trial in chickens for fattening.
3.2.4.1. Derivation of a maximum safe level in feed for terrestrial animal species
The data from a 90‐day toxicity study with rats (see Section 3.2.3.1) were used for a calculation of the maximum safe concentration in feed. From that study, a NOAEL of 890 U/kg bw and day was identified; the test item in that study had a concentration of 890 U/g. 48 The maximum safe daily dose for the target species was derived following the EFSA Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b). The calculation included the application of an uncertainty factor (UF) of 100 to the NOAEL. The results showed that the lowest maximum safe additive concentration in feed would correspond to 99 U/kg feed for chickens for fattening (Table 1).
Table 1.
Maximum safe concentration of the additive in feed
| Body weight (kg) |
Feed intake (kg DM/day) |
Daily feed intake (g DM/kg bw) |
Maximum safe concentration (U/kg feed) a |
|
|---|---|---|---|---|
| Chicken for fattening | 2 | 0.158 | 79 | 99 |
| Laying hen | 2 | 0.106 | 53 | 148 |
| Turkey for fattening | 3 | 0.176 | 59 | 134 |
| Piglet | 20 | 0.880 | 44 | 178 |
| Pig for fattening | 60 | 2.20 | 37 | 214 |
| Sow lactating | 175 | 5.28 | 30 | 260 |
| Veal calf (milk replacer) | 100 | 1.89 | 19 | 414 |
| Cattle for fattening | 400 | 8.00 | 20 | 392 |
| Dairy cow | 650 | 20.00 | 31 | 254 |
| Sheep/goat | 60 | 1.20 | 20 | 392 |
| Horse | 400 | 8.00 | 20 | 391 |
| Rabbit | 2 | 0.100 | 50 | 157 |
| Dog | 15 | 0.250 | 17 | 470 |
| Cat | 3 | 0.060 | 20 | 392 |
DM: dry matter; bw: body weight.
Complete feed containing 88% dry matter, milk replacer 94.5% dry matter.
3.2.4.2. Tolerance study in chickens for fattening
A total of 600 one‐day‐old male and female Ross 308 chickens were distributed to 30 pens in groups of 20 chickens each and allocated randomly to three dietary treatments (representing 10 replicate pens per treatment of 20 chickens each). 49 Chickens were fed a starter diet (mash feed) from day 1 until day 14 and a grower diet (mash feed) from day 15 until day 35 of the experiment. The ZenA was incorporated into a basal diet based on wheat and soybean at 0, 100 (10× minimum recommended level; confirmed by analysis 98.1/78.8), and 1,500 (150× minimum recommended level; which showed analytical values of 1,210/1,140 representing about 120× the minimum recommended level) U/kg of feed. Feed and water were available ad libitum over an experimental period of 35 days. General health status and mortality were monitored throughout the study. Feed intake was recorded at days 14, 28 and 35 and body weight at days 1, 14 and 35. Average feed intake, average body weight gain and feed to gain ratio were calculated. At day 35 (end) of the experiment, blood was obtained from one chicken per pen to determine routine blood haematological 50 and biochemical 51 parameters. Data were statistically analysed with parametric (ANOVA) or non‐parametric tests (Kruskal–Wallis or Welch ANOVA). The pen was the experimental unit, and the significance level was declared at 0.05.
Overall mortality (2.2%) and culling rate (2.6%) were low and not affected by treatments. There were no significant differences in the performance parameters measured/calculated: total feed intake (2,984 g for control group), final body weight (1,644 g for control group) and feed conversion rate (1.87 for control group). No differences were identified in any of the haematological parameters measured. Not dose‐related differences were observed in concentrations of urea, potassium, total protein content and phosphorus. These differences do not raise concerns.
Therefore, the supplementation of the experimental diets with the additive up to an intended level of 150× the minimum recommended level (120× as per analytical values) did not have any negative effect on the performance of chickens for fattening. However, the Panel notes that the performance of the chickens was lower than the standard for the strain (final body weight: control 1,644 g vs standard 2,144 g). 52 Therefore, this study is considered only as supporting evidence of the safety of the additive for chickens for fattening.
3.2.4.3. Risk posed by the endotoxin content of the final product
The endotoxin concentration of the final product was up to 94,368 IU/g. These values are very low when compared with ca. 1,000,000 IU/g commonly found in feedingstuffs (Cort et al., 1990). Therefore, at the proposed conditions of use of the additive in feed, the endotoxins added by the additive would be insignificant compared with the background in feed. When ingested, they do not represent any safety concern for the target species.
3.2.4.4. Conclusions on safety for the target species
The FEEDAP Panel concludes that the ZenA contained in the additive is safe for all terrestrial animal species up to the maximum use levels of 100 U/kg complete feed in chickens for fattening; 150 U/kg complete feed in laying hens, turkeys for fattening and rabbits; 200 U/kg complete feed in pigs; 250 U/kg complete feed in dairy cows; 400 U/kg complete feed in veal calf (milk replacer), cattle for fattening, sheep, goats, horses and cats; and 450 U/kg complete feed in dogs. Nevertheless, the Panel notes that uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product. The production strain contains a kanamycin resistance gene which, if present in final product, would represent a safety concern for the target species.
3.2.5. Safety for the consumer
The FEEDAP Panel concludes that ZenA is not genotoxic in vitro and does not show any toxicity based on the results of a 90‐day study. The metabolite DHZEN was found to be genotoxic, like the parent compound ZEN. However, this metabolite is produced at lower concentration (5%) compared to the other metabolite (95%). The two metabolites (HZEN and DHZEN), produced following the ZEN degradation, are less absorbed and show lower oestrogenic activity compared to the parent compound (ZEN). Consequently, no residues of concern are expected to be deposited in the food products.
Based on the above, the FEEDAP Panel considered that the use of the ZenA contained in the additive in animal nutrition is safe for the consumers.
Nevertheless, the Panel notes that uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product. The production strain contains a kanamycin resistance gene which, if present in final product, would represent a safety concern for the consumer.
3.2.6. Safety for user
3.2.6.1. Effects on the respiratory system
The applicant did not submit specific studies for the additive under assessment. However, considering the proteinaceous nature of the additive, it should be considered as a potential respiratory sensitiser.
The highest dusting potential of the additive measured was 415 mg/m3 (see Section 3.1.7). Concerning endotoxins, users can suffer from occupational respiratory disease depending on the level of endotoxins in air and dust (Rylander, 1999; Thorn, 2001). The scenario used to estimate the exposure of persons handling the additive to endotoxins in the dust, based on the EFSA guidance on user safety (EFSA FEEDAP Panel, 2012), is described in Appendix A. The threshold for the quantity of inhaled endotoxins per working day is 900 IU, derived from the provisional occupational exposure limits given by the Dutch Expert Committee on Occupational Safety (Health Council of the Netherlands, 2010) and the UK Health and Safety Executive (HSE, 2013). Based on calculations of the content of endotoxins in dust (up to 94,368 IU/g), exposure by inhalation would be 8,068 IU per 8‐h working day, indicating a risk from exposure to endotoxins for people handling the additive.
3.2.6.2. Effects on the eyes and skin
The test item used in the studies provided was a concentrate production batch of the additive with an enzymatic activity of 890 U/g (about 100× the activity of the ZenA contained in the additive).
The acute dermal toxicity of the additive was tested in a study performed following the principles of GLP and according to OECD TG 402. 53 Under the conditions of this study, the acute dermal median lethal dose (LD50) of the test item was found to be greater than 2,000 mg/kg bw in rats. According to the GHS criteria, the additive is classified as ‘Category 5 or Unclassified’ for acute dermal exposure.
The skin irritation potential of the additive was tested in an in vitro study performed following the principles of GLP and according to OECD TG 439. The additive is classified as non‐irritant to skin, in accordance with UN GHS “No Category”. 54
The eye irritation potential of the additive was tested in an in vitro study performed under GLP and according to OECD TG 437. The additive is classified as non‐irritant to eyes in accordance with UN GHS “No Category”. 55
In a skin sensitisation study following GLP and the OECD TG 429, the additive was found not to be a skin sensitiser. 56
3.2.6.3. Conclusions on safety for the user
Due to its proteinaceous nature, the additive should be considered as a potential respiratory sensitiser. The endotoxin content in the additive poses a risk by inhalation for users handling the additive. The additive was not shown to be a skin/eye irritant and a skin sensitiser.
Considering that the production strain contains a kanamycin resistance gene and that uncertainties remain on the possible presence of recombinant DNA from the production strain in the final product, the FEEDAP Panel cannot conclude on the safety of the product for the user.
3.2.7. Safety for the environment
The active substance of the additive under assessment is an enzyme that will be degraded in the digestive tract of animals. Residues of the active substance in faeces and urine can be considered negligible (if present at all).
Resulting breakdown products of the enzyme activity are of no toxicological concern. Furthermore, ZEN degrading activity resulting in similar compounds also occurs by other microorganisms. 57 Therefore, the additive does not add any new compounds to the environment.
The additive under assessment does not contain viable cells of the production strain. Nevertheless, the production strain contains a kanamycin resistance gene and uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product and this raises safety concerns for the environment. 58
3.2.7.1. Conclusions on safety for the environment
The active substance (ZenA enzyme) and the resulting breakdown products of its enzymatic activity do not represent a safety concern for the environment. Nevertheless, the production strain contains a kanamycin resistance gene and uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product that raise safety concerns for the environment.
3.3. Efficacy
The applicant provided three in vitro studies, in three different controlled conditions, and three in vivo studies (two short‐term studies in chickens for fattening and dairy cows and a long‐term study in pigs) to support the efficacy of the additive under assessment.
3.3.1. In vitro studies
3.3.1.1. Degradation of zearalenone from maize‐based culture material
The efficacy of ZenA in degrading ZEN from a maize‐based culture material was examined. 59 ZEN was extracted from the maize‐based culture material (1,027 mg ZEN/kg) with Teorell Stenhagen buffer. Extracted ZEN material (960 µL) was transferred to 12 one‐mL tubes. Nine (replicates) of them were treated with 40 µL of ZenA working solution (5 U/L); while the three (replicates) left received 40 µL of buffer solution and were used as negative controls. ZEN concentration was measured at 0, 15, 30, 45, 60, 120 and 180 min of incubation and after inactivating the enzyme. The initial concentration of ZEN was on average 7.5 μM and was reduced to half after 30 min incubation with ZenA, and to 0.03 µM after 180 min. The control samples had an initial value of 7.8 μM at start, 7.4 μM after 30 min and 8.6 μM after 180 min.
3.3.1.2. Degradation of zearalenone in buffer simulating gastric conditions
The efficacy of ZenA with regard to the degradation of pure ZEN at pH 5.0 (simulating acidic conditions as those in the stomach) was examined. 60 ZEN concentration was measured after inactivating the enzyme at 0, 15, 30, 45, 60, 120 and 180 min at 37°C, in nine samples (replicates) containing 0.25 U ZenA per litre of gastric simulation buffer; and in three control samples (replicates) without addition of the additive. The initial concentration of ZEN in treated tubes was on average 1.11 μM and the addition of the additive reduced it to half after 60 min, and to 0.21 μM after 180 min. The control samples had an initial value of 1.12 μM at start, 1.03 μM after 30 min and 1.1 μM after 180 min, showing no modification of the concentration with time.
3.3.1.3. Degradation trial in simulated ruminal conditions
The efficacy of ZenA with regards to degradation of ZEN (originating from a ZEN culture material containing 357 mg ZEN/kg) in micro‐fermenters containing a rumen simulation matrix was examined. 61 The rumen simulation matrix consisted of a dairy total mixed ration (43% hay, 57% concentrate) and an inoculation mixture with rumen fluid (50%), water (30%) and artificial saliva (buffer, 20%). ZenA was added to four fermenters (replicates) at a concentration corresponding to 40 U/kg feed; another set of four fermenters (replicates) remained unsupplemented and acted as control. Fermentation samples were taken for measurements of ZEN, HZEN and DHZEN and alpha‐zearalenol (α‐ZEL) over a period of 30 min; DHZEN was not detected. The Mann–Whitney test was used to compare the statistical significance of the differences between the treatments.
The concentrations of the different substances at the beginning of the study were not different between the control (0.15 μM ZEN, HZEN not detected) and treated (0.13 μM ZEN, HZEN not detected) samples, but afterwards and from time 10 min the ZenA group showed significant higher values of HZEN (0.17, 0.16 and 0.13 μM at 10, 20 and 30 min, respectively) and lower values of ZEN (0.09, 0.08 and 0.09 μM at 10, 20 and 30 min, respectively) compared to control (no HZEN detected at any time, and 0.20, 0.19 and 0.21 μM ZEN at 10, 20 and 30 min, respectively). Regarding α‐ZEL, its concentration decreased over time in the group treated with the additive (0.01, 0.001, 0.003, 0.004 μM at 0, 10, 20 and 30 min, respectively) in comparison to the control where the concentration increased over time (0.01, 0.02, 0.02, 0.03 μM at 0, 10, 20 and 30 min, respectively) likely due to the reduction of its substrate (ZEN) that is metabolised by the enzyme.
3.3.1.4. Conclusions on the in vitro studies
The addition of the ZenA to maize‐based contaminated culture material in a buffer simulating gastric conditions or in a simulation of ruminal conditions, allowed to reduce the concentration of ZEN. The studies allow to conclude on the potential of the additive to degrade ZEN under different conditions.
3.3.2. In vivo studies
The applicant provided three in vivo studies: in 14‐day‐old chickens for fattening (duration 14 days), 62 in weaned piglets (duration 42 days) 63 and another in dairy cows (duration three days). 64 The aim was to test the effect of the additive at the minimum recommended inclusion level (10 U/kg feed), in feeds containing 0.4, 0.2 or 0.5 mg zearalenone/kg feed (for chickens, pigs and cows, respectively), on the content of ZEN, HZEN, DHZEN and zearalenol in the digesta/faeces of the animals. In the case of chickens, the evaluation included the crop, gizzard and excreta; while for piglets, faeces were analysed; and for dairy cows, samples of three areas of the rumen were analysed (mat, reticulum and ventral sac).
The results from the three in vivo studies showed to be in line with those obtained in vitro. Samples from the GI tract of the animals receiving the additive showed lower concentration of ZEN and α‐ZEL and increases in the HZEN. However, the studies do not allow to elucidate whether the reduction on the ZEN in digesta, excreta/faeces would be due either to the mycotoxin enzymatic degradation or to the possible absorption/uptake of the mycotoxin in the gut. The Panel considers that the measurement of ZEN in blood would be a suitable parameter to confirm that there is no absorption/uptake of the mycotoxin in the gut.
The Panel notes also other limitations in these studies, which include insufficient experimental replication in the studies to ensure enough statistical power (with no real replicates in the chickens for fattening study, and only a small number of replicates in the piglets (three pens per group) and dairy cows (four animals per group) studies). In addition, the dairy cows study had a too short overall duration (3 days) which included a wash‐out period of only one day and many samples showed compound concentrations below the LOQ/LOD (with exception of content of ZEN). Moreover, the concentrations measured were not corrected with an internal/external marker, and therefore the quantification of the concentration alone in GI samples may not provide an appropriate measurement.
3.3.3. Conclusions on the efficacy
The results from three in vitro tests showed that adding ZenA to different substrates in different conditions allows to reduce the concentration of ZEN.
Owing to the limitations identified in the in vivo studies the Panel cannot conclude on the efficacy of the additive in the target species.
4. Conclusions
The production strain E. coli DSM 32731 is genetically modified and harbours a kanamycin resistance gene. No viable cells of the production strain were detected in the final product, but uncertainty remains on the presence of recombinant DNA in the final product.
The ZenA contained in the additive is safe for all terrestrial animal species up to the maximum use levels of 100 U/kg complete feed in chickens for fattening; 150 U/kg complete feed in laying hens, turkeys for fattening and rabbits; 200 U/kg complete feed in pigs; 250 U/kg complete feed in dairy cows; 400 U/kg complete feed in veal calf (milk replacer), cattle for fattening, sheep, goats, horses and cats; and 450 U/kg complete feed in dogs.
Based on the ADME and toxicological data, the FEEDAP Panel considers that the use of the ZenA contained in the additive in animal nutrition is safe for the consumers.
The endotoxin content in the additive poses a risk by inhalation for users handling the additive. The additive was not shown to be a skin/eye irritant nor a skin sensitiser. Due to its proteinaceous nature, the additive should be considered as a potential respiratory sensitiser.
The ZenA contained in the additive and the resulting breakdown products of its enzymatic activity do not represent a safety concern for the environment.
The production strain harbours an antimicrobial resistance gene and uncertainties remain on the possible presence of recombinant DNA of the production strain in the final product. Therefore, the FEEDAP Panel cannot conclude on safety of the additive for the target species, the consumer, the user and the environment.
The results from three in vitro tests showed that the additive applied to different substrates in different conditions has the potential to reduce the concentration of ZEN. Nevertheless, owing to the limitations identified in the in vivo studies, the Panel cannot conclude on the efficacy of the additive.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 11/12/2019 | Dossier received by EFSA. Zearalenone hydrolase for all terrestrial animal species by Biomin GmbH |
| 23/12/2019 | Reception mandate from the European Commission |
| 13/02/2020 | Application validated by EFSA – Start of the scientific assessment |
| 18/05/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterization, safety for the target animals, safety for the consumer, efficacy |
| 13/05/2020 | Comments received from Member States |
| 12/06/2020 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 17/05/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 16/07/2021 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterization of the additive |
| 07/09/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 26/01/2022 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- α‐ZEL
alpha‐zearalenol
- BSA
bovine serum albumin
- BW
body weight
- CFU
colonyforming unit
- CG
chemical group
- CV
coefficient of variation
- DHZEN
decarboxylated hydrolysed zearalenone
- DHZEN
decarboxylated hydrolised zearalenone
- DM
dry matter
- EURL
European Union Reference Laboratory
- FCR
feed conversion ratio
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- GLP
good laboratory practice
- HCA
α‐Hexylcinnamaldehyde
- HZEN
hydrolysed zearalenone
- HZEN
hydrolysed zearalenone
- IU
International unit
- LC‐MS
liquid chromatography‐mass spectrometry
- LD50
lethal dose 50%
- LOD
limit of detection
- LOQ
limit of quantification
- MRL
maximum residue level
- MSDS
material safety data sheet
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- RNA
ribonucleic acid
- SI
stimulation indices
- UF
uncertainty factor
- ZEN
zearalenone
- ZenA
zearalenone hydrolase
Appendix A – Safety for the user
The effects of the endotoxin inhalation and the exposure limits have been described in a previous opinion (EFSA FEEDAP Panel, 2015).
Calculation of maximum acceptable levels of exposure from feed additives
The likely exposure time according to EFSA guidance (EFSA FEEDAP Panel, 2012) for additives added in premixtures assumes a maximum of 40 periods of exposure per day, each comprising 20 s, equal to = 800 s per day. With an uncertainty factor of 2, maximum inhalation exposure would occur for 2 × 800 = 1,600 s (0.444 h per day). Again, assuming a respiration volume of 1.25 m3/h, the inhalation volume providing exposure to potentially endotoxin‐containing dust would be 0.444 × 1.25 = 0.556 m3 per day. This volume should contain no more than 900 IU endotoxin, so the dust formed from the product should contain no more than 900/0.556 = 1,619 IU/m3 .
Calculation of endotoxin content of dust
Two key measurements are required to evaluate the potential respiratory hazard associated with endotoxin content of the product (the dusting potential of the product, expressed in g/m3; the endotoxin concentration of the dust, determined by the Limulus amoebocyte lysate assay (expressed in IU/g)). If data for the dust are not available, the content of endotoxins of the product can be used instead. If the content of endotoxins of the relevant additive is IU/g and the dusting potential is b g/m3, then the content of endotoxins of the dust, c IU/m3, is obtained by the simple multiplication a × b. This resulting value is further used for calculation of potential inhalatory exposure by users to endotoxin from the additive under assessment (Table A.1) (EFSA FEEDAP Panel, 2012).
Table A.1.
Estimation of user exposure to endotoxins from the additive
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Endotoxin content IU/g product | 94,368 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.415 | Technical dossier | |
| a × b | c | Endotoxin content in the air (IU/m3) | 14,522 | |
| d | No of premixture batches made/working day | 40 | EFSA FEEDAP Panel (2012) | |
| e | Time of exposure (s)/production of one batch | 20 | EFSA FEEDAP Panel (2012) | |
| d × e | f | Total duration of daily exposure/worker (s) | 800 | |
| g | Uncertainty factor | 2 | EFSA FEEDAP Panel (2012) | |
| f × g | h | Refined total duration of daily exposure (s) | 1,600 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.44 | |
| j | Inhaled air (m3)/8‐h working day | 10 | EFSA FEEDAP Panel (2012) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.56 | |
| c × k | l | Endotoxin inhaled (IU) during exposure/8‐h working day | 8,068 | |
| m | Health‐based recommended exposure limit of endotoxin (IU/m3)/8‐h working day | 90 | Health Council of the Netherlands (2010) | |
| m × j | n | Health‐based recommended exposure limit of total endotoxin exposure (IU)/8‐h working day | 900 |
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Zearalenone hydrolase
In the current application an authorisation is sought under Article 4(1) for zearalenone hydrolase under the category/functional group 1(m) “technological additives” / “substances for reduction of the contamination of feed by mycotoxins: substances that can suppress or reduce the absorption, promote the excretion of mycotoxins or modify their mode of action”, according to the classification system of Annex I of Regulation (EC) No 1831/2003. The authorisation is sought for the use of the feed additive for all terrestrial animal species. The product is intended to be marketed by the Applicant as preparation, namely ZENzyme®. The active substance in ZENzyme® is the purified enzyme zearalenone hydrolase (EC 3.1.1.‐). According to the Applicant the preparation has a minimum guaranteed enzyme activity of 7 U/g, where:
“one unit corresponds to the enzymatic activity that hydrolyses 1 µmol zearalenone per minute in a 5 mg/L (15.71 µM) zearalenone solution in Teorell Stenhagen buffer, pH 7.5, with 0.1 mg/L BSA at 37°C”.
ZENzyme® is intended to be used in premixtures and feedingstuffs, at a minimum recommended enzyme activity of 10 U/kg feedingstuffs.
For the quantification of the zearalenone hydrolase activity in the feed additive, premixtures and feedingstuffs the Applicant proposed an activity assay, where the decay of zearalenone triggered by the enzyme at specific experimental conditions is monitored with a single laboratory validated and further verified method based on high performance liquid chromatography coupled to tandem mass spectrometry (HPLC‐MS/MS). In these experiments the enzymatic activity is determined as zearalenone degradation over an enzymatic reaction time, where the corresponding concentration of zearalenone is determined at various points in time by means of the HPLC‐MS/MS method.
Based on the experimental evidence available the EURL recommends for official control the enzymatic activity assay based on the HPLC‐MS/MS method submitted by the Applicant for the quantification of the zearalenone hydrolase activity in the feed additive, premixtures and feedingstuffs.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives, Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Bories G, Glandorf B, Svensson K, Anguita M, Brozzi R, Galobart J, Gregoretti L, Innocenti ML, Pettenati E, Pizzo F, Tarrés‐Call J, Vettori MV and López‐Gálvez G, 2022. Scientific Opinion on the safety and efficacy of a feed additive consisting of zearalenone hydrolase produced by Escherichia coli DSM 32731 for all terrestrial animal species (Biomin GmbH). EFSA Journal 2022;20(2):7157, 21 pp. 10.2903/j.efsa.2021.7157
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00008
Panel members: Giovanna Azimonti, Vasileios Bampidis Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to acknowledge the contribution to this opinion of Angelica Amaduzzi, Jürgen Gropp and Joana Revez, and the following Working Groups of the FEEDAP Panel: WG on Animal Nutrition, WG on Microbiology and WG on Toxicology.
Adopted: 26 January 2022
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Biomin GmbH, Erber Campus 1, 3131, Getzersdorf, Austria.
FEED dossier reference: FAD‐2019‐0088.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad‐2019‐0088_zearalenone_hydrolase.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex II.22.
Technical dossier/Section II/Annex II.27.
Technical dossier/Section II/Annex II.32.
Technical dossier/Section II/Annex II.24.
Technical dossier/Section II/Annex II.30.
Technical dossier/Section II/Annex II.21.
Technical dossier/Section II/Annex II.28.
Technical dossier/Section II/Annex II_36‐Annex II_45.
One unit corresponds to the enzymatic activity that hydrolyses 1 μmol zearalenone per minute in a 5 mg/L (15.71 μM) zearalenone solution in Teorell Stenhagen buffer, pH 7.5, with 0.1 mg/L bovine serum albumin (BSA) at 37°C.
Technical dossier/Section II/Annex_03. Determination of zearalenone using LC‐MS/MS analytical method.
Technical dossier/Section II/Annex II_04, Annex II_05, Annex II_06.
Technical dossier/Section II/Annex II_07.
LOQ in mg/kg: 0.5 for As and Pb, 0.1 for Cd and 0.01 for Hg.
LOD in μg/kg: 20 for deoxynivalenol and for fumonisins B1 and B2; 4 for zearalenone; 0.2 for aflatoxins (B1, B2, G1 and G2) and for ochratoxin A; and 2 for T‐2 Toxin and HT‐2 Toxin.
Technical dossier/Section II/Annex II_08, Annex II_09 and Annex II_10.
Technical dossier/Supplementary information September 2021/Answers to supplementary information request of 20210715 and Annex xviii.
Technical dossier/Supplementary information September 2021/ Answers to supplementary information request of 20210716.
Technical dossier/Section II/Annex II_14, Annex II_15 and Annex II_16. One International Unit (IU) is equivalent to one Endotoxin Unit (EU).
Technical dossier/Section II/Annex II_20.
Technical dossier/Section II/Annex II_17, Annex II_18 and Annex II_19.
Technical dossier/Section II/Annex II_46.
The respective report indicates that the test batches were produced aiming to an enzymatic activity of 80 U/g.
Technical dossier/Section II/Annex II_47.
Technical dossier/Section II/Annex II_48.
Technical dossier/Section II/Annex II_49.
Technical dossier/Section II/Annex II_50.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex IV.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex V.
Technical dossier/Section III/Annex III 20.
Technical dossier/Section III/Annex III 21.
Technical dossier/Section III/Annex III_22.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex VI.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex VII.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex VIII.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex IX.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex X.
Technical dossier/Supplementary Information (May 2021)/Section III/Annex XI.
Technical dossier/Section III/Annex III 12‐13.
Technical dossier/Section III/Annex III 14 and 15.
Technical dossier/Section III/Annex III 15 and 16.
Technical dossier/Annex III/Annex III 12.
Technical dossier/Section III/Annex III_23.
Technical dossier/Section III/Annex III_17.
Technical dossier/Section III/Annex_III_01 Report Tolerance BR61 and Annexes III_02‐10 and Supplementary information May 2021
White blood cell count (WBC), differential white blood cell count.
Glucose, urea, creatinine, total protein, albumin, globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), glutamate dehydrogenase, gamma‐glutamyltransferase (GGT), bile acids, triglycerides, lactate dehydrogenase (LDH), creatine kinase, sodium, potassium, chloride, calcium, phosphorus.
Ross 308 broilers performance objectives 2014, ‘as hatch.’ available online: https://www.winmixsoft.com/files/info/Ross‐308‐Broiler‐PO‐2014‐EN.pdf
Technical dossier/Section III/Annex III.19.
Technical dossier/Section III/Annex III 29.
Technical dossier/Section III/Annex III 30.
Technical dossier/Section III/Annex III 31.
Technical dossier/Section III/Annex III 23.
Technical dossier/Section II/Annex II 11 and 12.
Technical dossier/Section IV/Annex IV 1.
Technical dossier/Section IV/Annex IV 2.
Technical dossier/Section IV/Annex IV 4, 5, 6.
Technical dossier/Section IV/Annex_IV_07 Report Efficacy BR0217 and Annexes_IV_08‐18.
Technical dossier/Section IV/Annexes IV.28 to IV.38.
Technical dossier/Section IV/Annexes IV.18 to IV.27.
References
- Cort N, Fredriksson G, Kindahl H, Edqvist LE and Rylander R, 1990. A Clinical and Endocrine Study on the Effect of Orally Administered Bacterial Endotoxin in Adult Pigs and Goats. Journal of Veterinary Medicine Series A, 37, 130–137. 10.1111/j.1439-0442.1990.tb00884.x [DOI] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2015. Scientific Opinion on the safety and efficacy of L‐lysine monohydrochloride, technically pure, produced with Escherichia coli CGMCC 3705 and L‐lysine sulphate produced with Corynebacterium glutamicum CGMCC 3704 for all animal species, based on a dossier submitted by HELM AG. EFSA Journal 2015;13(7):4156, 25 pp. 10.2903/j.efsa.2015.4156 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhauf S, Novak B, Nagl V, Hackl M, Hartinger D, Rainer V, Labudová S, Adam G, Aleschko M, Moll WD, Thamhesl M and Grenier B, 2019. Biotransformation of the mycotoxin zearalenone to its metabolites hydrolyzed zearalenone (HZEN) and decarboxylated hydrolyzed zearalenone (DHZEN) diminishes its estrogenicity in vitro and in vivo . Toxins, 11, 481. 10.3390/toxins11080481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Council of the Netherlands , 2010. Endotoxins. Health‐based recommended occupational exposure limit. Publication no 2010/04OSH, 100 pp.
- HSE (Health and Safety Executive) , 2013. Occupational hygiene implications of processing waste at materials recycling facilities (exposure to bioaerosol and dust). Research report RR977. Available online: http://www.hse.gov.uk/research/rrpdf/rr977.pdf
- Kakeya H, Takahashi‐Ando N, Kimura M, Onose R, Yamaguchi I and Osada H, 2002. Biotransformation of the mycotoxin, zearalenone, to a non‐estrogenic compound by a fungal strain of Clonostachys sp. Bioscience, Biotechnology, and Biochemistry, 66, 2723–2726. [DOI] [PubMed] [Google Scholar]
- OECD , 1997. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris.
- OECD , 2016. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris.
- Rylander R, 1999. Health effects among workers in sewage treatment plants. Occupational and Environmental Medicine, 56, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J, 2001. The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflammatory Response, 50, 254–261. [DOI] [PubMed] [Google Scholar]


