Abstract
Advances in 3D bioprinting allows not only controlled deposition of cells or cell-laden hydrogels but also flexibility in creating constructs that match the anatomical features of the patient. This is especially the case for reconstructing the pinna (ear), which is a large feature of the face and made from elastic cartilage that primarily relies on diffusion for nutrient transfer. The selection of cell lines for reconstructing this cartilage becomes a crucial step in clinical translation. Chondrocytes and mesenchymal stem cells are both studied extensively in the area of cartilage regeneration as they are capable of producing cartilage in vitro. However, such monoculture systems involve unfavorable processes and produce cartilage with suboptimal characteristics. Co-cultures of these cell types are known to alleviate these limitations to produce synergically active chondrocytes and cartilage. The current study utilized a 3D bioprinted scaffold made from combined gelatine methacryloyl and methacrylated hyaluronic acid (GelMA/HAMA) to interrogate monocultures and co-cultures of human septal chondrocytes (primary chondrocytes, PCs) and human bone marrow-derived mesenchymal stem cells (BM-hMSCs). This study is also the first to examine co-cultures of healthy human chondrocytes with human BM-hMSCs encapsulated in GelMA/HAMA bioprinted scaffolds. Findings revealed that the combination of MSCs and PCs not only yielded cell proliferation that mimicked MSCs but also produced chondrogenic expressions that mimicked PCs. These findings suggested that co-cultures of BM-hMSCs and healthy septal PCs can be employed to replace monocultures in chondrogenic studies for cartilage regeneration in this model. The opportunity for MSCs used to replace PCs alleviates the requirement of large cartilage biopsies that would otherwise be needed for sufficient cell numbers and therefore can be employed for clinical applications.
1. Introduction
In the head and neck region, elastic cartilage is key to the skeletal support of the ear, nose, and throat which are also complex 3D shapes. The elastic cartilage of the ear (auricular cartilage) has limited self-regenerative capacity. Repair and reconstruction is therefore challenged by limited donor sites in the body and donor site morbidity.1 Alloplastic implants can avoid donor site morbidity, shorten surgery time, and improve size and contour matching. The current commercially available implant Medpor (Porex Surgical Inc., Stryker, GA) has a reported fracture and exposure rate of 25 and 44%, respectively, over a 3 year period even when complemented with a temporoparietal flap and skin graft to minimize exposures.2 Autologous tissue reconstruction is therefore considered superior to alloplastic implants. However, tissue engineering on its own has had little clinical impact in the head and neck, as solutions do not address the anatomical 3D complexity of the face.
Bioprinting is a developing technology that integrates cellular delivery while ensuring that structural support is preordained through 3D printed scaffolds that are personalized to the patients’ needs. The pinna has a large and complex 3D shape which varies in its mechanical properties in its subsections.3 Attempts to address these variations have been reported previously through the use of hybrid printing.4 The ear scaffold was fabricated by extrusion printing cell-laden hydrogels “in-between” the stiff yet degradable polycaprolactone (PCL) scaffold. It has been reported previously that scaffolds made from composite hydrogels have significantly improved mechanical properties when compared to the single-component analogues. For example, the compressive modulus of 5 and 10% gelatine methacryloyl (GelMA) hydrogels increased from 4 to 36 and 32 to 72 kPa, respectively, when 2% methacrylated hyaluronic acid (HAMA) was added to each.5 Additionally, it has also been reported that softer matrices are more suitable for chondrogenic differentiation of mesenchymal stem cells (MSCs) and that primary chondrocytes (PCs) respond better to softer scaffolds compared to rigid varieties.6,7 This is particularly advantageous for reconstructing large defects (i.e., microtia) that need structural support and also have high cellular demands, as it allows methodical placement of cells, while maintaining the shape required after implantation. The challenge now is to select appropriate cell lines to ensure optimal growth, cell differentiation (chondrogenesis), and maintenance of the phenotype, which will be crucial for the later phases of clinical translation.
Early success using rudimentary applications of bioprinting has been reported using PCL as the structural scaffold and PC expansion.8 This is an exciting advancement but, at this stage, only feasible for minor defects as it relies on the availability of PCs from donor elastic cartilage for expansion. PCs dedifferentiate beyond passage 5 in vitro,9,10 whereby their phenotype changes to a fibroblast-like morphology and they lose their chondrogenic gene expression capacity.11 This leads to difficulty in expanding chondrocytes in vitro, thus limiting its clinical application.11
Bone marrow-derived MSCs are adult stem cells that hold great promise in the field of cartilage regeneration due to their chondrogenic differentiation capability and their nonimmunogenic nature.12 However, a major challenge that remains in the field of stem cell therapy is to maintain the differentiated chondrogenic phenotype outside an in vitro culturing condition. This is the main reason for the lack of translation of MSCs in widespread clinical use. Thus, there is a need for alternative chondrogenic cell sources and culture methods to maintain differentiation and provide rapid expansion.
One promising approach, as presented in this work, is the co-culture of MSCs with PCs. The presence of MSCs alongside primary PCs may provide a “synergistic effect”, thus ensuring that the chondrocyte phenotype is maintained without a continuous supply of growth factors in a cell-culturing environment.13,14 Additionally, dedifferentiation of PCs may be suppressed due to the presence of MSCs, creating a “balanced” environment. This key characteristic of MSCs, where they express low-to-intermediate levels of human leukocyte antigen (HLA) class I and are negative for cell surface expression of HLA class II molecules, makes them suitable for allogeneic therapy.15 Using the co-culturing system, scaffolds will be bioprinted to ensure the homogeneous distribution of cells and tailored to the injury site.
Typical co-culture papers examine the MSC-to-chondrocyte ratios as monocultures (i.e., 1:0 or 100%) of each cell type, 50–50 (1:1) split of each, and finally a ratio within the range of 70–80% MSCs to the corresponding proportion of chondrocytes (i.e., 30–20%). In addition, previous co-culture studies have applied animal-only,16 animal–human cell lines,11 chondrocytes from cadavers or damaged tissues (e.g., osteoarthritis), and MSCs from weaker chondrogenic potential sources (e.g., adipose) in both 2D/3D models.17−19 No studies have yet examined co-cultures of healthy human PCs with human BM-MSCs encapsulated in bioprinted scaffolds. This study aims to examine the co-cultures of healthy human PCs with human MSCs encapsulated in bioprinted scaffolds for the first time. In particular, the optimal co-culture cell ratio to produce natively similar chondrocytes will be determined, alongside cell performance, morphology, and chondrogenic potential.
2. Results
2.1. Material Characterization
5% GelMA and 2% HAMA were mixed to create the composite hydrogel GelMA/HAMA. GelMA/HAMA was characterized via rheology, examining temperature ramps between 50 and 5 °C (Figure 1A,C) or a frequency sweep (Figure 1B) against viscosity (Figure 1A) or the storage (G′) and loss (G″) moduli (Figure 1B,C). At below 18.2 °C, the storage modulus becomes much higher than the loss modulus, indicating that the bioink indeed possesses gel-like properties and is therefore suitable for extrusion printing. Swelling analysis (Figure 1D) was used to determine the stability of the material over a 35 day period. Initial weight loss observed was due to the dissolving of un-cross-linked material, which stabilized after ∼14 days. Additionally, scanning electron microscopy (SEM) was employed to image the porous properties of GelMA, HAMA, and GelMA/HAMA. The rheological data display consistent and uniform material behaviors, and the swelling data show that the material becomes stable after ∼336 h (14 days). The SEM images show the porous nature of the individual and combined materials. The pore size is related to the concentration of the hydrogel, and the higher the concentration, the denser is the cross-linked network formed by the hydrogel, resulting in smaller pores. As shown in Figure 1E–G, similar to the previously reported hydrogel microstructures,20,21 the GelMA (5%) and GelMA/HAMA (5 + 2%) hydrogels with higher concentrations reveal smaller homogeneous honeycomb-like pores throughout the entire cross-sectional area, while 2% HAMA exhibits more elongated and larger pores.
Figure 1.
Bioink characterization. Rheological and swelling data of GelMA/HAMA and the SEM images of GelMA, HAMA, and GelMA/HAMA bioinks. (A) Flow temperature ramp curve of viscosity vs temperature of GelMA/HAMA from 50 to 5 °C, reversed with a constant frequency of 1 Hz. (B) Frequency sweep curve of storage and loss moduli vs angular frequency of GelMA/HAMA with a constant sheer rate of s–1. (C) Temperature ramp of GelMA/HAMA under oscillation conditions from 50 to 5 °C and reversed curve of storage modulus (full line) and loss modulus (dashed line) vs temperature of GelMA/HAMA with a constant sheer rate of 1 s–1. (D) Swelling data of casting GelMA/HAMA (n = 6) weights over a 35 day period. Box and whisker plots show the median, 25th, and 75th percentile values for the number of reads per sample, with whiskers indicating the maximum and minimum. Graph on a logarithmic scale with an initial weight of 0.33 g. (E–G) SEM images of GelMA, HAMA, and GelMA/HAMA, taken at either ×100 (top) or ×1000 (bottom) magnification.
2.2. Cell Viability in Printed Constructs
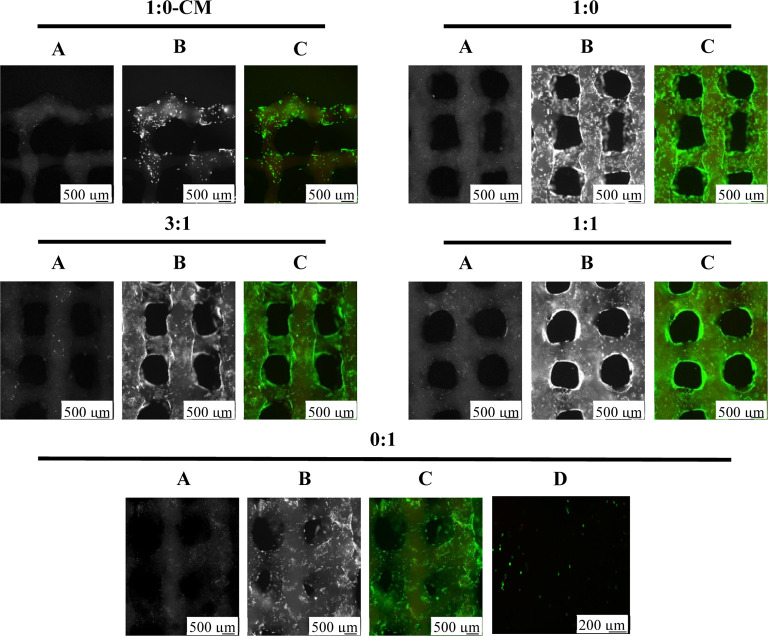
Cells were encapsulated in GelMA/HAMA hydrogels at a concentration of 2.0 × 106 cells/mL, which include monocultures of MSC and PC controls and co-cultures in differentiation media. An additional group included culturing monocultures of MSCs in manufacturer’s recommended commercial media (MSC-CM). Live/dead staining of cells indicated that cells remained viable in all scaffolds. The morphology of MSCs appeared differently when comparing between MSC-CM and MSC controls, with MSC-CM displaying smaller sizes and less consistency in shape, as shown in Figure 2.
Figure 2.
Co-culture live/dead comparison. Live/dead images of co-culture mixtures at day 35 (n = 3). Live cells fluoresce green when incubated with calcein AM and dead cells fluoresce red when incubated with propidium iodide. Images were taken with an Axiovert microscope. (A) Dead, (B) live, and (C) merged. Scale bars represent 500 μm. Confocal image (D) of day 1 (n = 3) merged live/dead stains; scale bars: 200 μm.
2.3. Cell Density and Differentiation Rates
Healthy MSCs are known to have accelerated proliferation capabilities compared to native chondrocytes. Homogeneous cell distribution in the hydrogel was confirmed by examining the day 1 DNA content, which showed that all scaffolds had initially similar cell densities (Figure 3). When the scaffolds with MSCs were cultured in differentiation media, the cultures possessed significantly greater cell densities after 21 and 35 days (Figure 3) in comparison to PC controls and MSC-CM monocultures.
Figure 3.
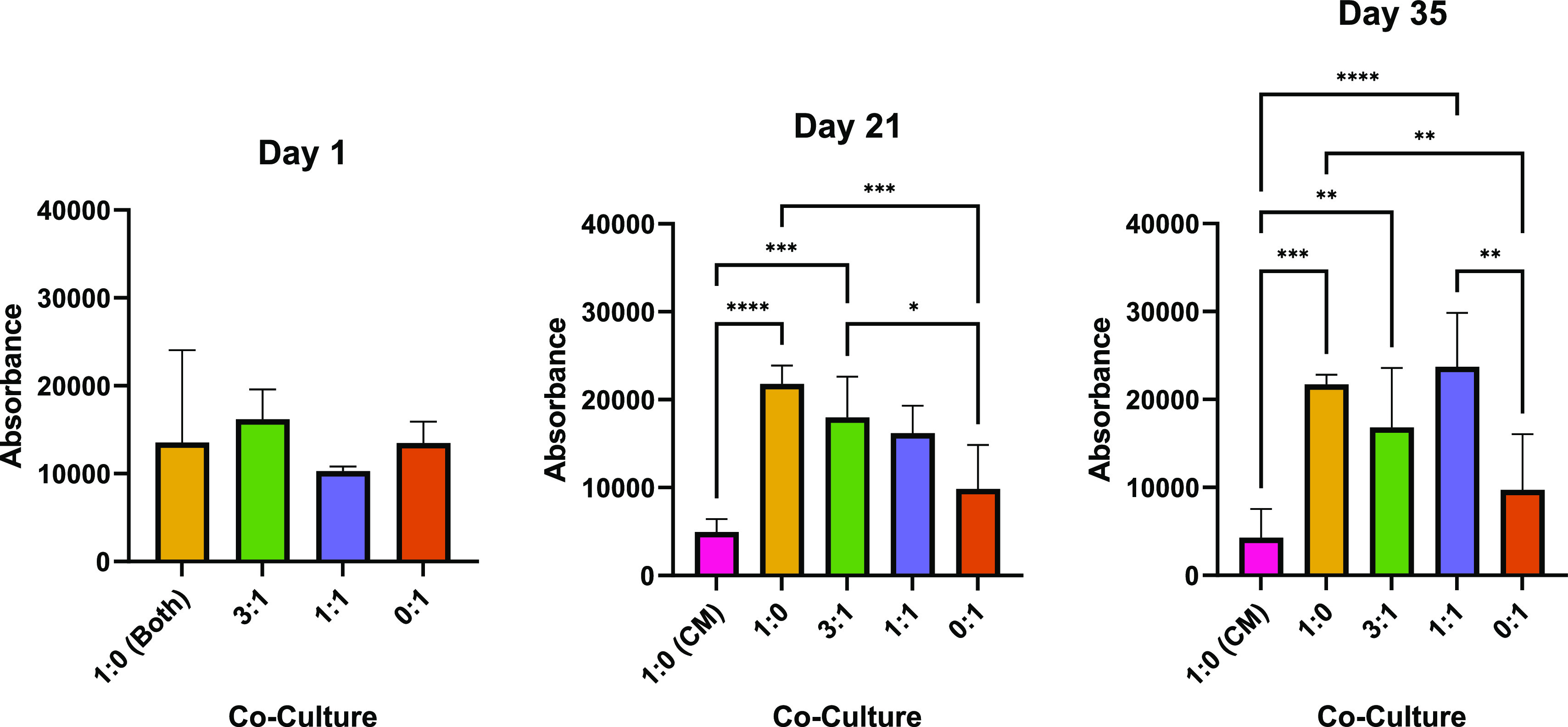
Co-culture proliferation. Bar charts demonstrates statistical significance of cell proliferation comparing the co-culture MSC/PC ratios of 1:0, 3:1, 1:1, and 0:1 (cultured in differentiation media) as well as 1:0 cultured in MSC-CM (1:0 CM) (n = 6). Error bars represent standard deviation between biological replicates, and significance was calculated with one-way ANOVA, where: * = <0.05, ** = <0.01, and *** = <0.001. 1:0 (both) refers to 1:0 scaffolds cultured in both differentiation media and MSC-CM.
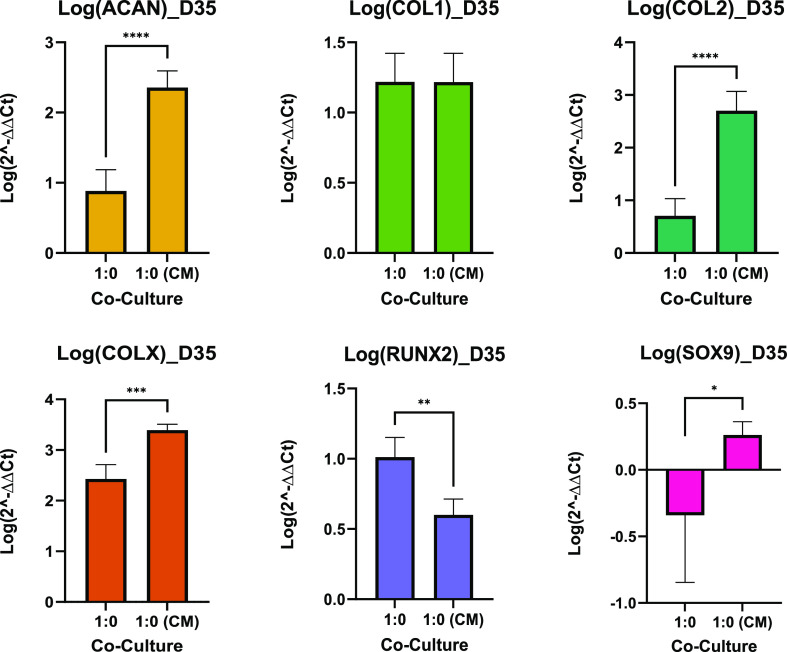
The rate of differentiation (how quickly cultures expressed chondrogenic markers from day 1) was also measured by normalizing the qPCR data of each culture group to day 1 of their respective groups, as shown in Figure 4. After 35 days, each group cultured in differentiation media differentiated at a relatively even pace. MSCs cultured in CM however differentiated quickly, indicated by the significantly higher ACAN and COL2 expression compared to the other cultures. This, along with its low proliferation rate, implies that the 1:0 (CM) group experienced an enhanced chondrogenesis.
Figure 4.
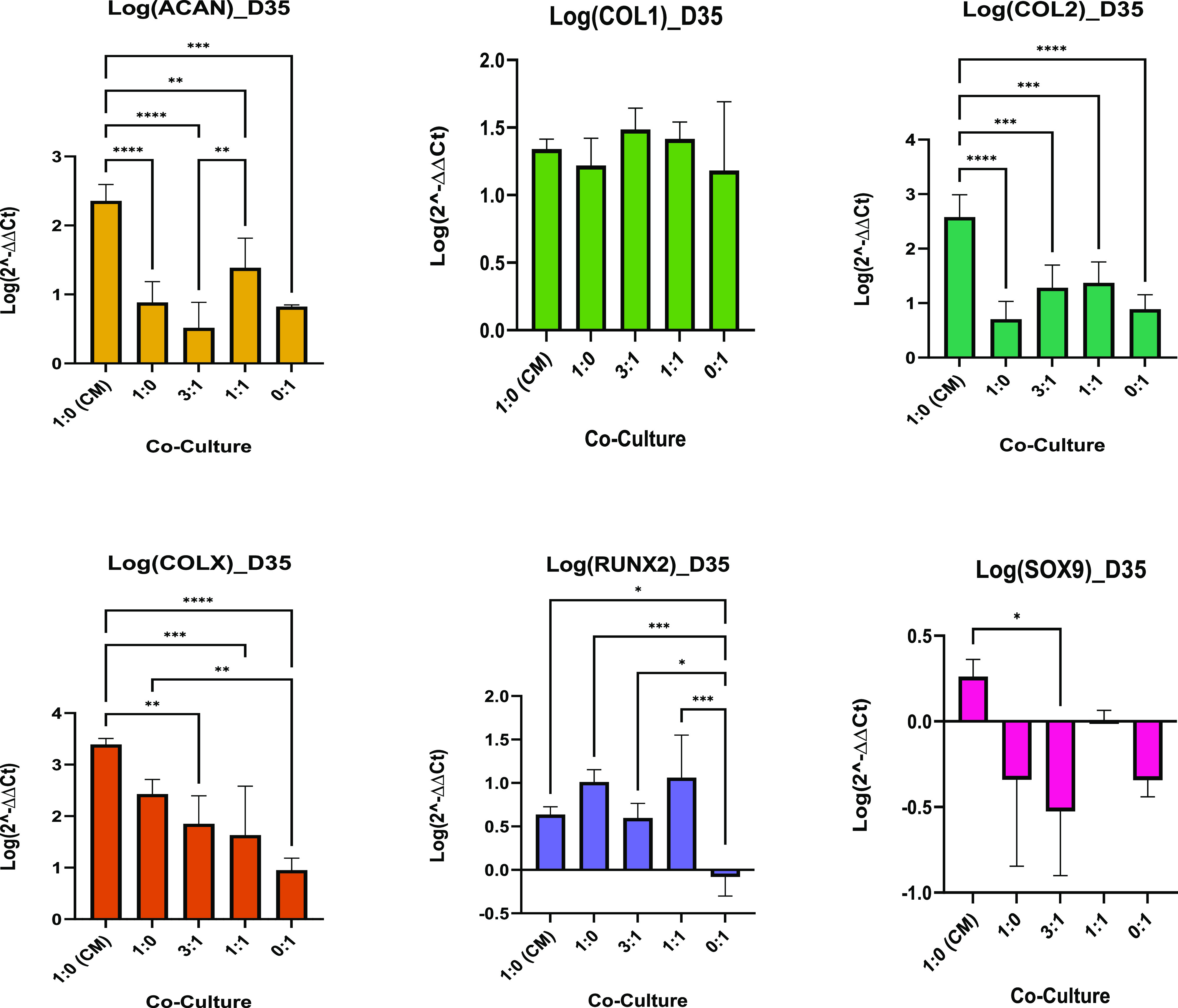
Co-culture gene expression rates. Data are shown as a mean of the logarithm of 2–ΔΔCT values for the relative gene expression of genes of interest (n = 6). Bar chart demonstrates the statistical significance of gene expression comparing the co-culture MSC/PC ratios of 1:0, 3:1, 1:1, and 0:1 (cultured in differentiation media). These were also compared to MSC-CM monocultures. Data normalized to day 1 of the corresponding cell ratio. Error bars represent standard deviation between biological replicates, and significance was calculated with one-way ANOVA, where * = <0.05, ** = <0.01, and *** = <0.001.
MSC-CM culture had a significantly greater upregulation of ACAN and COL2 compared to other cultures and was also the only culture that did not downregulate the expression of SOX9. Both the MSC monocultures had a significantly greater COLX expression compared to PC controls (0:1). There was no significant difference in COL1 expression across cultures.
2.4. Cell Morphology
Chondrocytes have a rounded morphology and are situated within lacunae. In native cartilage, chondrocytes are surrounded by the extracellular matrix (ECM) that they produce, including proteoglycans and collagen. Paraffin-embedded scaffolds were sectioned at 5 μm and stained for toluidine blue which is specific for the highly sulfated proteoglycans of cartilage matrices. The background is stained blue, and mast cells are stained purple/violet as depicted in Figure 5. As shown, MSC controls (1:0) produced the least proteoglycans.
Figure 5.
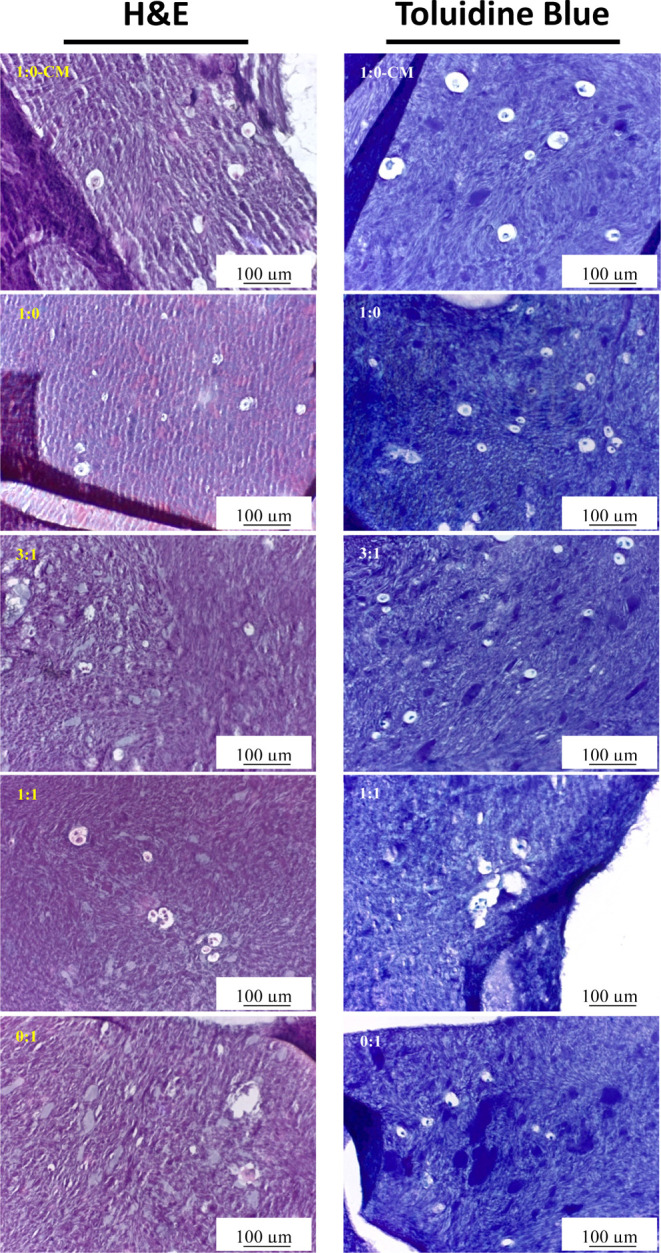
Histology. H&E (left) and toluidine blue (right) images of cell cultures (n = 3) under the microscope, ×20 magnification; scale bars represent 100 μm. H&E staining was used to determine ECM deposition, and toluidine blue staining was used to evaluate the synthesized GAGs in the bioink (observed with purple/violet tinge) after 35 days of culture.
Hematoxylin and eosin (H&E) and toluidine blue stain nuclei purple and blue, respectively, and reveal the cell size and morphology. H&E stains the matrix reddish-pink and toluidine blue stains the matrix purple when in the presence of proteoglycans. Discerning each cell type in scaffolds that had been cultured in differentiation media could not be achieved. Interestingly, MSCs in CM appeared much smaller than the other cultures as observed with fluorescent imaging (Figure 2); however, this phenotype was not observed in Figure 5.
2.5. Gene Expression
The chondrogenic potential of monocultures and co-cultures can be revealed from the relative upregulation and downregulation of specific chondrogenic positive and negative markers, respectively. Such positive markers for chondrogenesis include ACAN for proteoglycan production and COL2 for type II collagen production. Negative markers include COLX for hypertrophy and RUNX2, which is an osteoblast positive marker. From COL1 and SOX9 expressions, deductions can be made about the chondrocytes, such as a considerably greater expression in COL1 resulting in a more fibrocartilage-like chondrocyte and that a downregulated expression of SOX9 can limit COL2 expression. Monocultures and co-cultures were interrogated after 1, 21, and 35 days. qPCR was normalized to day 1 data of MSC controls to statistically compare the gene expressions of each culture. As shown in Figure 6, qPCR results reveal that after 21 days, none of the cultures produced significantly different expressions for the positive chondrogenic marker ACAN; however, the other positive chondrogenic marker COL2 was produced significantly greater in PC controls (0:1) compared to the MSC control (1:0) and 1:1 culture. MSC controls produced significantly greater COL1 expression compared to the other cultures, and PC controls produced the least. Additionally, both co-cultures had the greatest gene expression levels for hypertrophy (COLX), whereas the gene was not upregulated in either of the monocultures. None of the cultures expressed the osteoblast marker RUNX2, and SOX9 was downregulated in both co-cultures.
Figure 6.
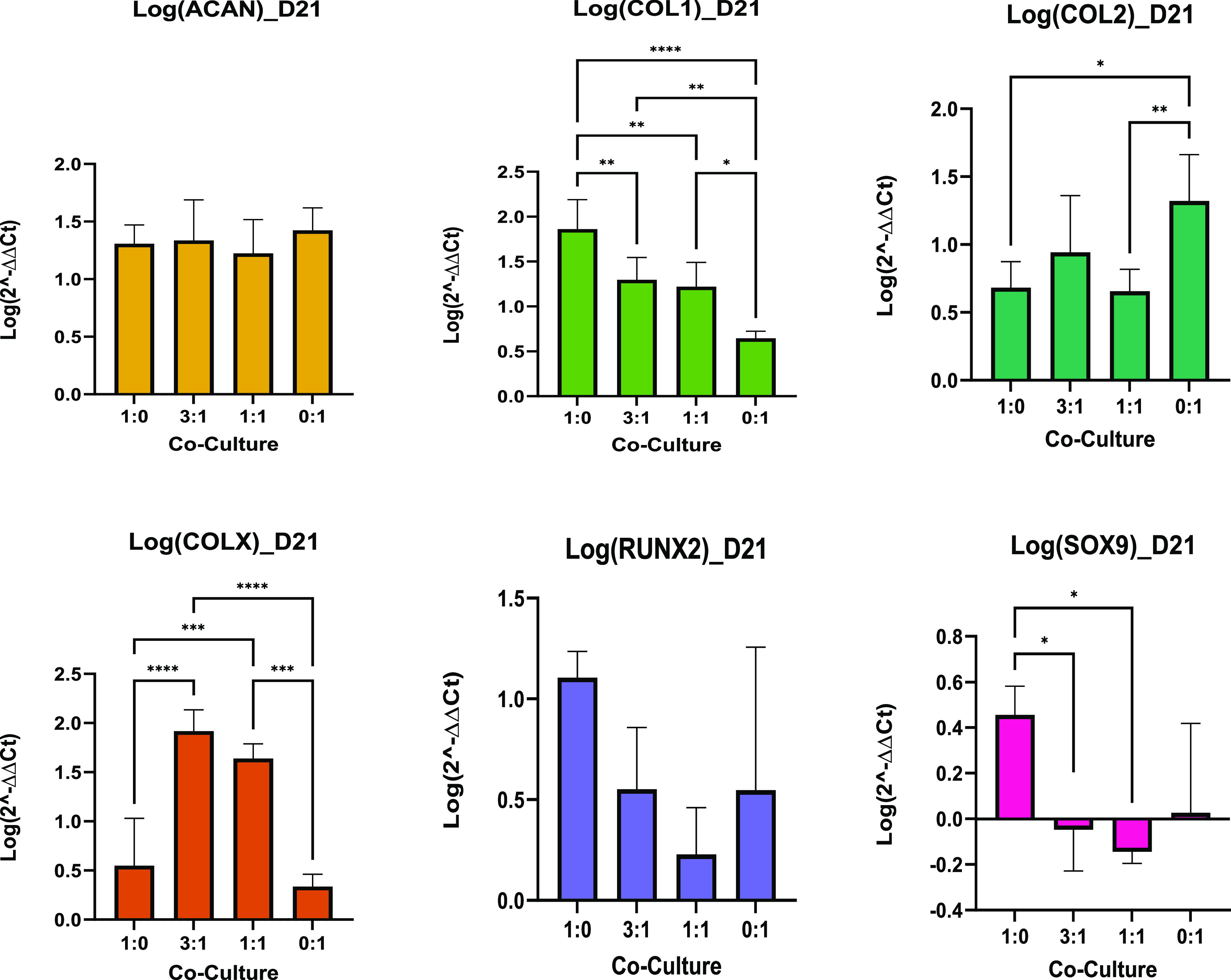
Co-culture day 21, qPCR. Data are shown as a mean of the logarithm of 2–ΔΔCT values for the relative expression of genes of interest (n = 6). Bar charts demonstrate statistical significance of gene expression comparing the co-culture MSC/PC ratios of 1:0, 3:1, 1:1, and 0:1 cultured in differentiation media at day 21. Error bars represent the standard deviation between biological replicates, and significance was calculated with one-way ANOVA, where * = <0.05, ** = <0.01 and *** = <0.001.
Figure 7 shows that at day 35, however, there was a significant difference between the MSC and PC controls for ACAN and COL2, and no significant difference between the cultures for COL1 expression was shown, and all cultures downregulated SOX9 expression. All cultures expressed an upregulation for COLX but none were significantly different.
Figure 7.

Co-culture day 35, qPCR. Data are shown as the mean of the logarithm of 2–ΔΔCT values for the relative expression of genes of interest (n = 6). Bar charts demonstrate statistical significance of gene expression comparing co-culture MSC/PC ratios of 1:0, 3:1, 1:1, and 0:1 cultured in differentiation media at day 35. Error bars represent standard deviation between biological replicates, and significance was calculated with one-way ANOVA, where * = <0.05, ** = <0.01, and *** = <0.001.
Comparison of the MSC controls and MSCs in CM revealed that the MSC-CM cultures significantly improved the expression of ACAN and COL2 and also significantly increased COLX expression, as shown in Figures 8 and 9. This indicated that there is a need to select media specifically for the cell types being utilized. However, more characterization needs to be done to understand what media should be used specifically for co-cultures.
Figure 8.
MSC culturing comparison––day 21, qPCR. Data are shown as the mean of the logarithm of 2–ΔΔCT values for relative expression of genes of interest (n = 6). Bar charts demonstrate statistical significance of gene expression comparing MSCs in differentiation media to MSCs in MSC-CM at day 21. Error bars represent standard deviation between biological replicates, and significance was calculated with Student’s t test, where * = <0.05, ** = <0.01, and *** = <0.001.
Figure 9.
MSC culturing comparison—day 35, qPCR. Data are shown as the mean of the logarithm of 2–ΔΔCT values for relative expression of genes of interest (n = 6). Bar charts demonstrate statistical significance of gene expression comparing MSCs in differentiation media to MSCs in MSC-CM at day 35. Error bars represent standard deviation between the biological replicates, and significance was calculated with Student’s t test, where * = <0.05, ** = <0.01, and *** = <0.001.
3. Discussion
Cartilage is an aneural and avascular tissue, and the chondrocytes that reside within rely on diffusion for nutrient transfer and thus possess poor proliferative capabilities. Consequently, the presence of any defects will result in the damaged area being altered to an unnatural fibrous cartilage; as a result, regenerative treatments of defective cartilage remain a challenge for surgeons.22 Biofabrication has emerged as a promising alternative to the current treatments, where constructs can be printed into intricate shapes and sizes, with properties tailored to fit the complex nature of the cartilage. Scaffolds have also been exploited for chondrogenesis studies; however, the use of co-cultures is yet to be fully investigated.23 The porous nature of the scaffolds allows the signaling molecules and cytokines secreted from PCs to interact with the cytokines and morphogenetic factors from MSCs, thereby supporting the induction of chondrogenesis and chondrocyte proliferation.23−28 Such scaffold systems have been used for the promotion of articular chondrocyte redifferentiation,16 MSC differentiation, and prevention of hypertrophy using a variation of bovine, rabbit, and human MSCs and chondrocytes.29,30
Scaffolds for biofabrication studies are often created using hydrogels. Hydrogels are a class of hydrophilic polymer materials that can absorb large volumes of water and swell without disintegrating.31 The characteristics of hydrogels used for biofabrication include biocompatibility and biodegradability as well as being nonimmunogenic, noninflammatory, and nontoxic32 and can be referred to as “bioinks”. The bioink GelMA/HAMA has been shown to be highly suitable for cell attachment and differentiation for cartilage regeneration.33,34 GelMA/HAMA is also classified as a non-Newtonian fluid,35 a property that makes such materials ideal for bioprinting. This behavior of GelMA/HAMA can be characterized via rheological data, swelling analysis, and SEM images.
In this study, 5% GelMA was combined with 2% HAMA to produce a porous, printable bioink. From the observations and interpretations of Figure 1B,C, at and below 18.2 °C, the storage modulus is much higher than the loss modulus. This demonstrates that the GelMA/HAMA bioink possesses gel-like properties and is thus a suitable bioink for extrusion printing.36 The position of this temperature in relation to the bioink’s gelation region indicates that the viscosity of the material is strongly dependent on the temperature within this range. The low-viscosity, liquid-like behavior at 37 °C observed in Figure 1A allows for cells to be mixed into the bioink. Following cross-linking, SEM images, as shown in Figure 1E–G, show that the GelMA-only gel has smaller pore sizes than the HAMA-only gel and that GelMA/HAMA gels possess structural cues and pore sizes between those of the singular components, consistent with a previous study.37 Thus, a hybridized matrix is produced as GelMA and HAMA chains coalesce to produce an interior network that is capable of absorbing water, thereby leading to larger voids as a consequence of the sizes of the ice crystals expanding during freezing. The data shown in Figure 1D demonstrate the initial degradation of the submerged material as it absorbs the surrounding phosphate-buffered saline (PBS) but becomes stable around day 14 (336 h). This suggests a weak but effective cross-linking network, which could potentially allow for the gas and nutrient exchanges that are essential for cell growth and survival.38
For cells to survive within a 3D culture, the platform must be porous enough to allow gas and nutrient exchange. In the current study, PCs were acquired from healthy septal cartilage. As depicted in Figure 2, there was no observed difference in live/dead cells in co-cultures or MSC controls after day 35. This result indicated that the printing process does not significantly reduce the viability of the encapsulated cells compared to previously reported processes such as inkjet and laser printers.39 This is corroborated by Bian et al.,17 where live/dead staining of human cells revealed that all MSCs and co-cultures (4:1) remained viable after 35 days in hyaluronic acid (HA) hydrogels. In the same study, it was shown that PC monocultures did not survive to day 35; however, this is likely a result from the PCs having been digested from injured articular cartilage. In the current study, monocultures and co-cultures were, therefore, all capable of surviving the printing process and remained viable after 35 days in culture.
It is known that chondrocytes have poor regenerative capabilities, largely due to the slow cell division rate.32 In contrast, MSCs have excellent proliferation rates but need to undergo extensive chondrogenic induction to differentiate.27,28,40 In this study, MSCs and PCs were expanded in different culture media but were all cultured in differentiation media after printing. The results shown in Figure 3 depict that, after 21 and 35 days, proportional cell proliferation of MSC controls (1:0) and co-cultures was not significantly different. There were also no significant differences between PC controls and MSC-CM. However, when comparing MSC controls and co-cultures to PC controls and MSC-CM, a significant difference was observed. MSCs improving the cell proliferation of PCs in co-cultures has been reported previously,11,16,41 where co-cultures displayed great promise as the trophic effect of MSCs increased the chondrogenic potential of PCs, thereby alleviating the limitations with PC harvest and expansion. This is consistent with the result from Figure 3, where co-culture cell densities were not significantly different to that of MSC controls. The ingredients of MSC-CM are not available, and it is possible that the formulation includes factors that inhibit the proliferative ability of MSCs to direct chondrogenic differentiation. Such inhibitors are not used in differentiation media; so, proliferation and differentiation occur simultaneously, and the MSCs promote and support the proliferation capabilities of PCs in co-cultures. The results of this study, therefore, show that co-cultures can match the proliferative properties of MSC controls, which were significantly greater than that of PC controls, suggesting that co-cultures could replace PC monocultures to increase the PC cell numbers during culture. This has wide clinical implications, as surgeons will not be required to harvest such large cartilage biopsies that are otherwise essential for the chondrocyte numbers required, thereby reducing the size and severity of the tissue damage at the harvesting site.
Chondrocytes are the sole cell type within cartilage and play a key role in the development, maintenance, and repair of the ECM. The ECM plays an important role in chondrogenesis; therefore, whether the cells can produce key ECM components such as type II collagen (COL2) and aggrecan (ACAN) is an important indication of successful chondrogenesis.42 The efficacy as well as the efficiency for PCs, differentiated MSCs, and co-cultures to develop COL2 and ACAN can be determined by measuring the genetic material (mRNA/cDNA) via qPCR. In this study, the efficiency of chondrogenesis was evaluated by normalizing the qPCR data of each cell group at day 35 with their respective day 1 qPCR outputs. The results showed that cells that were cultured in differentiation media differentiated at a relatively even pace. In comparison, MSC-CM monocultures differentiated significantly faster (Figure 4). Taken together with its low proliferation rate, it is therefore hypothesized that MSC-CM monocultures experienced an enhanced chondrogenesis. The correlation of an enhanced chondrogenesis with limited proliferation is supported by Acharya et al.,28 where although this trend was observed with co-cultures, a decrease in the proliferation rate but a more efficient MSC differentiation was still displayed. Following 21 days in culture, all cells in differentiation media expressed equal expression levels of ACAN, and only the two co-cultures expressed COLX (Figure 6). By day 35, however, cell hypertrophy was equally upregulated across all groups consistent with the previous findings.43 In addition, PC controls expressed significantly greater ACAN and COL2 compared to MSC controls but was not significantly different when compared to the co-cultures (Figure 7). This result is in line with those published by Lopa et al.,19 where the study found that the co-cultures of A-MSCs and PCs did not enhance COL2 expression levels compared to the monocultures. Moreover, comparing MSC controls to MSCs in CM revealed that the MSC-CM group expressed significantly greater ACAN and COL2, both at day 21 and day 35. Furthermore, after 35 days of culture, both these cultures upregulated COLX expression, with MSC-CM being more apparent. These results indicated that, as there was no significant difference in the expression of the chondrogenic positive markers ACAN and COL2 between the co-cultures and PC controls, co-cultures could potentially be employed to increase the chondrogenic potential of MSCs to mimic the expressions of PC monocultures. Consequently, co-cultures are promising for subsequent in vivo studies and clinical applications as these data suggest that these cultures are capable of producing ECM-essential components that can regenerate cartilage.
4. Conclusions and Future Directions
Regenerating defective cartilage for clinical applications remains a challenge. As aforementioned, both MSCs and chondrocytes possess limitations in monoculture that can be overcome in co-cultures. In this study, MSC and PC monocultures as well as co-cultures with ratios 3:1 and 1:1, respectively, were utilized. An additional group, MSC-CM, was used to compare how chondrogenesis differs when MSCs are cultured in either commercial media or differentiation media. Interestingly, MSC-CM monocultures did not proliferate but possessed chondrogenic genetic markers similar to PC controls, indicating that they were more genetically and behaviorally similar to PC controls. In this study, MSC monocultures cultured in differentiation media had significantly greater proliferation performance compared to PC controls. The addition of MSCs to PCs to create co-cultures also increased the cell density as compared to controls. In addition, the upregulation of the chondrogenic positive markers were significantly greater in the PC controls compared to MSC controls, with no significant difference between the PC controls and co-cultures. These results showed that the combination of MSCs and PCs can yield cell proliferation similar to that of MSC controls. Simultaneously, the combination of MSCs and PCs produces chondrogenic expressions that match that of PC controls. The greatest limitation to co-culture studies is the challenge to quantitatively determine the cell numbers of differentiated and undifferentiated cells. Future studies, therefore, will utilize flow cytometry as a possible solution to quantitatively determine these cell numbers.
5. Materials and Methods
5.1. Material Preparation
GelMA and HAMA were supplied from Translational Initiative for Cellular Engineering and Printing (TRICEP). GelMA and HAMA were processed as described previously by O’Connell et al.(34)
100 g of gelatine (from porcine skin; gel strength ∼300 g bloom from PB Leiner, USA) was dissolved in 1 L of 0.1 M PBS (10% w/v, pH = 7.8) at 50 °C under mechanical stirring. Then, 50 g of methacrylic anhydride was added to the gelatine solution over a period of 3 h with continuously stirring while protecting the content from light. The reaction was continued for additional 3 h. Following this, 1 L of Milli-Q was added to the reaction mixture, and the resulting solution was stirred overnight at room temperature. The final product was purified to remove salts and other impurities. The aqueous solution was then adjusted to pH 7 ± 0.2 using NaOH (5 M). Finally, the purified GelMA solutions were lyophilized to produce white porous materials with 80% yield. Substitution and degree of functionalization were confirmed by 1H NMR (D2O, 1% w/v), and the degree of functionalization was observed to be 70% ± 4. The molecular weight of GelMA, analyzed by gel permeation chromatography, was 141 kDa.
Hyaluronic acid (20 g) was dissolved in Milli-Q water (2.6 L) at 60 °C under mechanical stirring for 3 h. Methacrylic anhydride (150 g) was added dropwise over a period of 3 h after the reaction mixture was cooled to room temperature. The reaction mixture was stirred at room temperature overnight, while the pH was maintained at 8 by the addition of NaOH (5 M). HAMA was then purified, and pH was adjusted to 7 ± 0.2 before freeze drying. The degree of functionalization was confirmed by 1H NMR (D2O, 0.5% w/v), and the resulting degree of functionalization was 20 ± 4%.
5.2. Cell Sources
Human cartilage tissue is commonly removed and discarded in routine ENT surgery such as septoplasty. During this procedure, human nasal septal cartilage (PC: male, age 43) was harvested with consent provided and ethics approved (HREC 2018-023). Human bone marrow-derived MSCs (BM-hMSCs) were purchased from Lonza at passage 2 (P2, male, age 25) and plated as per manufacturer’s instructions. P2 cells were subcultured to passage 4 (P4) and stored in liquid nitrogen in freezing solution [90% fetal bovine serum (FBS, Bovogen) and 10% dimethyl sulfoxide (DMSO, Sigma)] at densities of 5.0 × 105 cells/mL.
5.3. Cell Isolation and Expansion
Chondrocyte expansion medium (expansion media) was prepared by supplementing Dulbecco’s modified Eagle’s medium (DMEM)-high glucose (Invitrogen) with 10% FBS, 1% GlutaMAX, 1% nonessential amino acids (NEAA), 1% Pen strep (P/S, 10,000 U/mL, Gibco), 0.4 mM l-proline, and 0.1 mM l-ascorbic acid (AsAP). BM-hMSC expansion medium (MSC-EM) was prepared by supplementing DMEM-low glucose (Invitrogen) with 10% FBS and 1% P/S. Chondrocyte differentiation medium was prepared by supplementing DMEM-high glucose with 1% NEAA, 1% P/S, 0.01 M 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid, 0.04 mM l-proline, 1% insulin transferrin selenium, 0.1 μM dexamethasone, 0.13% bovine serum albumin, and 0.2 mM AsAP, as well as 10 ng/mL transforming growth factor-β3. Finally, commercial MSC differentiation medium (StemCell Technologies) was prepared as per the manufacturer’s instructions without modifications.
To isolate PCs, cartilage harvested from the patient was preweighed and supplemented with PBS + 10% (w/v) P/S (10,000 U/mL, Gibco). The samples were then diced to approximately 1 mm3 pieces and rinsed further with PBS + P/S before transferring into a digestion medium (DMEM-high glucose + 10% FBS + 0.15% (w/v) collagenase type II) overnight in a humidified incubator at 37 °C, with 5% CO2. The solution was then filtered through a 100 μm cell strainer and centrifuged at 700 g for 5 min. This step was repeated three times before suspending the cells in the expansion medium. Cells were expanded to P2 and cells not used for experimentation were stored in liquid nitrogen in freezing solution for later use. P2 PCs were expanded to 80% confluency and passaged to P3 via cell detachment using 0.25 mM trypsin (Gibco) and replated.
BM-hMSCs, hereafter referred to as MSCs, were thawed (5.0 × 105) and resuspended in MSC-EM, expanded to 80% confluency, and passaged to P5 via cell detachment using 0.25 mM trypsin (Gibco) and replated. At this time, MSCs were further cultured in MSC-EM supplemented with 10 ng/mL basic fibroblast growth factor (bFGF, Partech) to precondition MSCs for chondrogenic differentiation. All cells were cultured at 37 °C with 5% CO2 in a humidified incubator. The medium was changed twice weekly.
5.4. Bioink Preparation and Characterization
150 mg of the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, Sigma) was dissolved in 10 mL of PBS for stock solutions of 1.5% (w/v). GelMA and HAMA were mixed to create 5% GelMA (w/v) and 2% HAMA (w/v) composite hydrogels. Briefly, 1.2 g of GelMA was mixed and dissolved in 24 mL of PBS (Sigma) supplemented with 1% P/S at 40 °C on a heated magnetic stirrer for 1 h. Subsequently, 0.48 g of HAMA was added, and the mixture was left at 40 °C on a heated magnetic stirrer overnight.
The rheological behavior of the bioink was analyzed using an AR-G2 rheometer (TA Instruments, DE) equipped with a Peltier plate thermal controller. A 2°/40 mm cone-and-plate geometry was used in all measurements. The solutions were allowed to reach the equilibrium temperature 2 min prior to performing the experiments. Storage modulus (G′) and loss modulus (G″) were measured as a function of temperature and frequency by varying, respectively, the temperature at a constant frequency) the temperature at a constant shear rate, and the frequency at a constant temperature. Temperature ramp experiments were conducted at a rate of 3 °C min–1 from 50 to 5 °C as well as a subsequent 5 to 50 °C (also at a rate of 3 °C min–1) at a fixed strain and frequency of 1% and 1 Hz, respectively, or a shear rate of 1 s–1. Frequency sweep experiments were conducted at a fixed strain of 1% from 0.01 to 10 Hz. A temperature of 30 °C was selected for conducting experiments to ensure the ink maintained a gel-like structure.
5.5. Swelling of Hydrogels
GelMA/HAMA was transferred to circular molds of 1 cm diameter and 0.5 mm height. The molds were cross-linked with 405 nm visible blue light (set at a height of 5 cm, 792 mJ) for 60 s. The casts were removed from the molds and weighed and then transferred to 1 mL of PBS and placed in a dry incubator. PBS was changed three times a week. The cast wet weight of swollen hydrogels was recorded after gently removing the surface liquid after 0, 3, 6, 24, 48, 168, 336, 504, 672, and 840 h.
5.6. Scanning Electron Microscopy
The morphological analysis of 5% GelMA, 2% HAMA, or GelMA/HAMA was performed using a low-vacuum scanning electron microscope JEOL JSM-6490LV operating in high-vacuum mode. After the preparation of the sample as mentioned above, the cross sections of samples were imaged at the frozen state by breaking the samples in liquid nitrogen (samples were immersed in liquid nitrogen for 30 s and then cut by a sharp blade; total immersion time ∼ 45 s). An accelerating voltage of 15 kV was applied at a working distance of 10 mm. The resulting images were acquired in SE2 mode at either ×100 or ×1000 magnification.
5.7. Bioprinting of Scaffolds
Scaffolds were printed using P5 MSCs and P3 PCs at a density of 2.0 × 106 cells/mL. MSC/PC ratios of 1:0 (hereafter referred to as MSC control), 3:1, 1:1, and 0:1 (hereafter referred to as PC controls) were used and cross-linked for 60 s at 405 nm. 1.5% (w/v) LAP was added to the GelMA/HAMA ink to achieve a final concentration of 0.03% (w/v) and was kept away from light.
The samples were printed using an EnvisionTEC 3D-Bioplotter (EnvisionTEC, GmBH). The extrusion system is equipped with two high-temperature cartridges and three low-temperature cartridges and operated by pneumatic pressures of 0–5 bar. For fabricating GelMA/HAMA scaffolds, low-viscous GelMA/HAMA was loaded into tinted polymer syringe barrels, with cells subsequently added and mixed. These were placed into the low-temperature cartridges and heated to 37 °C for 10 min to remove bubbles. The cartridges were then cooled to 20 °C and left for 30 min to calibrate. The printer stage was set to 10 °C to ensure the structural integrity of extruded layers prior to cross-linking. The cell-laden bioink was dispensed through an 18G (0.84 mm) nozzle. The scaffolds were designed using SolidWorks and sliced using the software Perfactory (RP 3.2.2945) at a slicing thickness of 50% of the nozzle diameter. The scaffolds were printed with a strand spacing of 1.5 mm and with a strand orientation of 0/90° to a final dimension of 10 × 10 × 2 mm. The printed constructs were then cross-linked with 400 nm visible blue light (set at a height of 5 cm, 792 mJ) for 60 s.
The scaffolds were placed into a 24-well plate submerged in a 0.5 mL differentiation medium, where they were then returned to the humidified incubator. This was repeated three more times (for each cell ratio). Another group of MSC-only cells was also printed but submerged in 0.5 mL of MSC-CM. All media changes were performed as described in the Stemcell Technologies manufactory protocol without modification. The scaffolds were characterized via live/dead fluorescent imaging, histology analysis, biochemical analysis, and qPCR after 1, 21, and 35 days.
5.8. Live/Dead Comparison
To determine the cell viability following bioprinting, a live/dead analysis was performed on the scaffolds after days 1, 21, and 35. The medium was removed from the scaffolds and replaced with calcein AM (1 mg/mL in DMSO) and diluted to 0.005 mg/mL using fresh medium. The scaffolds were then incubated at 37 °C for 28 min. After this time, the scaffolds were removed from the incubator, and propidium iodide (1 mg/mL) was added such that it was diluted to a final concentration of 0.001 mg/mL. The scaffolds were then returned to the incubator for 6 min. Following this, the medium was removed and 1 mL of PBS was added. The scaffolds were then imaged using the Axiovert.A1 inverted microscope [Zeiss] at ×2.5 and ×5 magnifications and processed using Zen 3.0 (blue edition) and Fiji (ImageJ). In addition, day 1 scaffolds were stained as aforementioned and then mounted onto well plates with glass coverslip bottoms with Eukitt Quick-hardening mounting medium (Sigma). Images were taken with a FALCON SP8 confocal microscope, equipped with a 405 nm UV laser and a white light laser (470–670 nm) (Leica) at ×10 magnification. Images were collected and analyzed using Leica Application Suite X (LAS X) software and Fiji (ImageJ).
5.9. DNA Analysis
To quantify the DNA content, scaffolds were added to 1 mL of lysis solution [as described by Quant-iT PicoGreen dsDNA Assay Kit protocol (Thermo Fisher)] and broken down using an electric drill with pellet pestles (Sigma) in an Eppendorf tube. The supernatant was then collected and stored at −20 °C. Once all samples had been collected and stored, the samples were thawed and measured with the Quant-iT Pico Green dsDNA Reagents kit. A standard curve was prepared using a dilution factor of known cell densities. 100 μL of lysis solution was mixed with 100 μL of PicoGreen, and 100 μL of the mixture was added into an ultravision 96-well plate. After incubating for 5 min at room temperature, fluorescence was read using a plate reader (FLUOstar Omega, BMG LABTECH) set at PicoGreen with Ex 483–15 nm and Em 530–30 nm at 4.6 focal plate height, Gain 1500. The standard curve was then used to determine the scaffold cell densities.
5.10. Histology
Histological staining was performed on two samples from each group on days 1, 21, and 35. The samples were fixed in 0.5 mL of paraformaldehyde (PFA, Sigma) for 20 min at RT. PFA was subsequently removed and replaced with 1 mL of PBS and stored at 4 °C overnight. The specimens were then processed in a tissue processor (Leica, ASP300S) and embedded in paraffin wax (Leica). The cell morphology was studied, with the nucleus stained with H&E and polysaccharides stained with toluidine blue.
5.11. Gene Analysis
Gene analysis was performed using real-time qPCR. In brief, an electric drill with pellet pestles (Sigma) was employed to break the scaffolds submerged in 100 μL of PBS and 350 μL of lysis solution in an Eppendorf tube. RNA was then extracted from scaffolds using the Aurum Total RNA Mini Kit (Bio-Rad Laboratories, USA) as per the manufacturer’s protocol without modifications. The RNA yield and quality were assessed using a Nanodrop spectrophotometer ND-2000 (Bio-Rad). RNA was then normalized and converted into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad) as per the manufacturer’s protocols. For this reaction, a CFX 96 optical reaction module (Bio-Rad) was used. The collected cDNA was then analyzed on the Nanodrop system for yield and quality. All cDNA were normalized to 50 ng/μL. A C100 Touch Cylcer W/96WELL (Bio-Rad) machine was used for qPCR. qPCR was performed with normalized cDNA and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) as per the manufacturer’s protocol. Six genes were tested using specific forward and reverse primers including: aggrecan (ACAN) F: AGTATCATCAGTCCCAGAAT, R: AATGCAGAGGTGGTTTCACT, type I collagen A2 (Col1) F: TCTGGATGGATTGAAGGGACA, R: CCAACACGTCCTCTCTCACC, type-II collagen A1 (Col2) F: GGACTTTTCTCCCCTCTCT, R: GACCCGAAGGTCTTACAGGA, type X collagen (ColX) F: CCCTCTTGTTAGTGCCAACC, R: AGATTCCAGTCCTTGGGTCA, Runt-related transcription factor 2 (RUNX2) F: TCCTACTTGAGCCAGATGAC, R: GAGGCAGAAGTCAGAGGTG and SRY-Box 9 (SOX9) F: ACACACAGCTCACTCGACCTTG, R: GGGAATTCTGGTTGGTCCTCT. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) F: GAAGGTGAAGGTCGGAGTC, R: GAAGATGGTGATGGGATTTC was used as a reference gene.
Gene expression was calculated using the 2–ΔΔCt method, where the cycle threshold (Ct) was produced using the qPCR machine (Bio-Rad). It is the cycle number where fluorescence generated by the PCR product is distinguishable from the background noise. Genes of interest were then normalized to the housekeeping gene (GAPDH), followed by the normalization of the treated cells to the control group (day 1 cell group (further information can be found in the figure legend)). These values were subsequently logged to report the fold change in gene expression, and subsequent statistical analyses were performed.
5.12. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9.0.0 (GraphPad, La Jolla, CA, USA). When data sets adhered to normal distribution, two-sample t test or one-way ANOVA with Tukey’s multiple comparison test for multiple comparisons was used.
Acknowledgments
The authors would like to acknowledge the funding and bioinks from the Australian National Fabrication Facility (ANFF) Materials Node, funding from the Australian Research Council Centre of Excellence Scheme (Project CE 140100012), and support by the Australian Government Research Training Program (RTP) Scholarship. The authors acknowledge use of the facilities and the assistance of Qiang Zhu at the UOW Electron Microscopy Centre. The authors also acknowledge the Illawarra Health and Medical Research Institute (IHMRI) and Molecular Horizons for laboratory and equipment support. Special acknowledgments to Dr. Kerry Gilmore, Dr. Eva Tomaskovic-Crook, Dr. Zhilian Yue, Kalani Ruberu, Ros Canales, Jeremy Dinoro, Malachy Maher, Samuel Rathbone, Sarah Higginbottom, Danielle Warren, Emma James, and Aida Shoushtari Zadeh Naseri for laboratory and technical support. The authors declare no competing financial interest.
The authors declare no competing financial interest.
References
- Justicz N.; Dusseldorp J. R.; Shaye D. Firmin technique for microtia reconstruction. Oper. Tech. Otolayngol. Head Neck Surg. 2017, 28, 90–96. 10.1016/j.otot.2017.03.005. [DOI] [Google Scholar]
- Reinisch J.; Lewin S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Arch. Facial Plast. Surg. 2009, 25, 181–189. 10.1055/s-0029-1239448. [DOI] [PubMed] [Google Scholar]
- Chung J. H. Y.; Kade J. C.; Jeiranikhameneh A.; Ruberu K.; Mukherjee P.; Yue Z.; Wallace G. G. 3D hybrid printing platform for auricular cartilage reconstruction. Biomed 2020, 6, 035003. 10.1088/2057-1976/ab54a7. [DOI] [PubMed] [Google Scholar]
- Chung J. H. Y.; Kade J.; Jeiranikhameneh A.; Yue Z.; Mukherjee P.; Wallace G. G. A bioprinting printing approach to regenerate cartilage for microtia treatment. Bioprinting 2018, 12, e00031 10.1016/j.bprint.2018.e00031. [DOI] [Google Scholar]
- Camci-Unal G.; Cuttica D.; Annabi N.; Demarchi D.; Khademhosseini A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. 10.1021/bm3019856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Laha M.; Mondal S.; Sengupta S.; Kaplan D. L. In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials 2009, 30, 6530–6540. 10.1016/j.biomaterials.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh E.; Hofmann S.; Stok K.; Notbohm H.; Müller R.; Rotter N. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J. Biomed. Mater. Res. 2012, 100, 38–47. 10.1002/jbm.a.33226. [DOI] [PubMed] [Google Scholar]
- Zhou G.; Jiang H.; Yin Z.; Liu Y.; Zhang Q.; Zhang C.; Pan B.; Zhou J.; Zhou X.; Sun H.; Li D.; He A.; Zhang Z.; Zhang W.; Liu W.; Cao Y. In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 2018, 28, 287–302. 10.1016/j.ebiom.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.; Plant J.; Wann A.; Bishop C.; Novak P.; Mitchison H.; Beales P.; Chapple J.; Knight M. Chondrocyte expansion is associated with loss of primary cilia and disrupted hedgehog signalling. Eur. Cell. Mater. 2017, 34, 128–141. 10.22203/ecm.v034a09. [DOI] [PubMed] [Google Scholar]
- Kang S.-W.; Yoo S. P.; Kim B.-S. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed. Mater. Eng. 2007, 17, 269–276. [PubMed] [Google Scholar]
- Pleumeekers M. M.; Nimeskern L.; Koevoet J. L. M.; Karperien M.; Stok K. S.; van Osch G. J. V. M. Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PLoS One 2018, 13, e0190744 10.1371/journal.pone.0190744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K.; Das A. K.; Chullikana A.; Majumdar A. S. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res. Ther. 2012, 3, 25. 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-y.; Wu X.-y.; Tong J.-b.; Yang X.-x.; Zhao J.-l.; Zheng Q.-f.; Zhao G.-b.; Ma Z.-j. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh B. S.; Che Omar S. N.; Ubaidah M. A.; Saim L.; Sulaiman S.; Chua K. H. Chondrogenesis of human adipose derived stem cells for future microtia repair using co-culture technique. Acta Otolaryngol. 2017, 137, 432–441. 10.1080/00016489.2016.1257151. [DOI] [PubMed] [Google Scholar]
- Le Blanc K.; Tammik C.; Rosendahl K.; Zetterberg E.; Ringdén O. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Meretoja V. V.; Dahlin R. L.; Kasper F. K.; Mikos A. G. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 2012, 33, 6362–6369. 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L.; Zhai D. Y.; Mauck R. L.; Burdick J. A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. 2011, 17, 1137–1145. 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner F.; Concaro S.; Peterbauer A.; Wolbank S.; Danzer M.; Lindahl A.; Gatenholm P.; Redl H.; van Griensven M. Human adipose-derived stem cells contribute to chondrogenesis in coculture with human articular chondrocytes. Tissue Eng. 2009, 15, 3961–3969. 10.1089/ten.tea.2009.0002. [DOI] [PubMed] [Google Scholar]
- Lopa S.; Colombini A.; Sansone V.; Preis F. W. B.; Moretti M. Influence on chondrogenesis of human osteoarthritic chondrocytes in co-culture with donor-matched mesenchymal stem cells from infrapatellar fat pad and subcutaneous adipose tissue. Int. J. Immunopathol. Pharmacol. 2013, 26, 23–31. 10.1177/03946320130260s104. [DOI] [PubMed] [Google Scholar]
- Arya A. D.; Hallur P. M.; Karkisaval A. G.; Gudipati A.; Rajendiran S.; Dhavale V.; Ramachandran B.; Jayaprakash A.; Gundiah N.; Chaubey A. Gelatin methacrylate hydrogels as biomimetic three-dimensional matrixes for modeling breast cancer invasion and chemoresponse in vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. 10.1021/acsami.6b06309. [DOI] [PubMed] [Google Scholar]
- Athirasala A.; Lins F.; Tahayeri A.; Hinds M.; Smith A. J.; Sedgley C.; Ferracane J.; Bertassoni L. E. A novel strategy to engineer pre-vascularized full-length dental pulp-like tissue constructs. Sci. Rep. 2017, 7, 3323. 10.1038/s41598-017-02532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos E. J.; Pluemeekers M.; Helder M.; Kuzmin N.; van der Laan K.; Groot M.-L.; van Osch G.; van Zuijlen P. Structural and Mechanical Comparison of Human Ear, Alar, and Septal Cartilage. Plast. Reconstr. Surg. 2018, 6, e1610 10.1097/GOX.0000000000001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazempour A.; Van Wie B. J. Chondrocytes, mesenchymal stem cells, and their combination in articular cartilage regenerative medicine. Ann. Biomed. Eng. 2016, 44, 1325–1354. 10.1007/s10439-016-1575-9. [DOI] [PubMed] [Google Scholar]
- Wing-hoi C.; Kwong-man L.; Kwok-pui F.; Pauline L. P.-y.; Kwok-sui L. TGF-β1 is the factor secreted by proliferative chondrocytes to inhibit neo-angiogenesis. J. Cell. Biochem. 2001, 81, 79–88. 10.1002/jcb.1079. [DOI] [PubMed] [Google Scholar]
- Villiger P. M.; Lotz M. Differential expression of TGF? isoforms by human articular chondrocytes in response to growth factors. J. Cell. Physiol. 1992, 151, 318–325. 10.1002/jcp.1041510213. [DOI] [PubMed] [Google Scholar]
- Böhme K.; Winterhalter K. H.; Bruckner P. Terminal differentiation of chondrocytes in culture is a spontaneous process and is arrested by transforming growth factor-β2 and basic fibroblast growth factor in synergy. Exp. Cell Res. 1995, 216, 191–198. 10.1006/excr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Cao L.; Kiani C.; Yang B. L.; Hu W.; Yang B. B. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J. Cell. Biochem. 1999, 73, 445–457. . [DOI] [PubMed] [Google Scholar]
- Acharya C.; Adesida A.; Zajac P.; Mumme M.; Riesle J.; Martin I.; Barbero A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2012, 227, 88–97. 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- Dahlin R. L.; Ni M.; Meretoja V. V.; Kasper F. K.; Mikos A. G. TGF-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 2014, 35, 123–132. 10.1016/j.biomaterials.2013.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meretoja V. V.; Dahlin R. L.; Wright S.; Kasper F. K.; Mikos A. G. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013, 34, 4266–4273. 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H.; Wang J.; Mi S. Photo processing for biomedical hydrogels design and functionality: A review. Polym. J. 2017, 10, 11. 10.3390/polym10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslahi N.; Abdorahim M.; Simchi A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016, 17, 3441–3463. 10.1021/acs.biomac.6b01235. [DOI] [PubMed] [Google Scholar]
- Wang G.; An Y.; Zhang X.; Ding P.; Bi H.; Zhao Z. Chondrocyte spheroids laden in GelMA/HAMA hybrid hydrogel for tissue-engineered cartilage with enhanced proliferation, better phenotype maintenance, and natural morphological structure. Gels 2021, 7, 247. 10.3390/gels7040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C. D.; Onofrillo C.; Duchi S.; Li X.; Zhang Y.; Tian P.; Lu L.; Trengove A.; Quigley A.; Gambhir S. Evaluation of sterilisation methods for bio-ink components: gelatin, gelatin methacryloyl, hyaluronic acid and hyaluronic acid methacryloyl. Biofabrication 2019, 11, 035003. 10.1088/1758-5090/ab0b7c. [DOI] [PubMed] [Google Scholar]
- Cooke M. E.; Rosenzweig D. H. The rheology of direct and suspended extrusion bioprinting. APL Bioeng. 2021, 5, 011502. 10.1063/5.0031475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C. D.; Di Bella C.; Thompson F.; Augustine C.; Beirne S.; Cornock R.; Richards C. J.; Chung J.; Gambhir S.; Yue Z. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016, 8, 015019. 10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- Velasco-Rodriguez B.; Diaz-Vidal T.; Rosales-Rivera L. C.; García-González C. A.; Alvarez-Lorenzo C.; Al-Modlej A.; Domínguez-Arca V.; Prieto G.; Barbosa S.; Soltero Martínez J. F. A.; Taboada P. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. 10.3390/ijms22136758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D.; Watson J. B.; Sah R. L.; Briggs K. K.. Craniofacial cartilage tissue engineering. Stem Cell Biology and Tissue Engineering in Dental Sciences; Academic Press, 2015; pp 541–552. [Google Scholar]
- Belk L.; Tellisi N.; Macdonald H.; Erdem A.; Ashammakhi N.; Pountos I. Safety Considerations in 3D Bioprinting Using Mesenchymal Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 924. 10.3389/fbioe.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. S.; Choi Y.; Kim H.-S.; Kim H. O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Leijten J. C. H.; Georgi N.; Post J. N.; van Blitterswijk C. A.; Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng. 2011, 17, 1425–1436. 10.1089/ten.tea.2010.0517. [DOI] [PubMed] [Google Scholar]
- Mardones R.; Jofré C. M.; Minguell J. J. Cell therapy and tissue engineering approaches for cartilage repair and/or regeneration. Int. J. Stem Cells 2015, 8, 48. 10.15283/ijsc.2015.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini S.; Diaz-Romero J.; Aigner T.; Heini P.; Mainil-Varlet P.; Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur. Cells Mater. 2010, 20, 245. 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]