Abstract
Dysregulated activity of the transcription factors of the nuclear factor κb (NF-κB) family has been implicated in numerous cancer types, inflammatory diseases, autoimmune disease, and other disorders. As such, selective NF-κB pathway inhibition is an attractive target to researchers for preclinical and clinical drug development. A plethora of commercially and clinically available inhibitors claim to be NF-κB specific; however, such claims of specificity are rarely quantitative or benchmarked, making the biomedical literature difficult to contextualize. This imprecision is worsened because some NF-κB reporter systems have low signal-to-noise ratios. Herein, we use a robust, defined, commercially available reporter system to benchmark NF-κB agonists and antagonists for the field. We also functionally characterize a RELA fusion-positive ependymoma cell culture with validated NF-κB inhibitor compounds.
Keywords: NF-κB, RELA, ependymoma
1. Introduction
Since the discovery of nuclear factor -κB (NF-κB) in 1986 as a regulator of B-cell development (Sen and Baltimore, 1986), this group of transcription factors have been widely studied and are now understood to regulate far more than just hematopoietic activation. Dysregulation in the NF-κB transcription factor family and signaling pathway are connected to a broad spectrum of human disease states and malignancies ranging from diabetes to cancer and beyond (Hayden and Ghosh, 2004; Patel and Santani, 2009; Wang et al., 2014).
In healthy cellular contexts, the NF-κB transcriptional complex plays rolls in development across cell types to either direct proliferation or differentiation, as well as to control cell survival through gene regulation (Franzoso et al., 1997; Guttridge et al., 1999; Kanegae et al., 1998; Verma et al., 1995). NF-κB complex proteins are constitutively expressed in most differentiated cell types, and are maintained in an inactive state ready to be activated by two principal signaling cascades in response to cellular stimuli. In the classical NF-κB signaling pathway, phosphorylation of the enzyme IkB kinase 2 (IKKβ) induces a cascade of phosphorylation and ubiquitination events which results in release and activation of the p50/RelA(p65) heterodimer subunit of NF-κB. Once released, the nuclear localization signal (NLS) on p50/RelA (p65) is unmasked, and the heterodimer translocates to the nucleus where the complex binds transcriptional elements and initiates gene expression, thereby transducing stimuli into cellular response via changes in gene regulation (Brown et al., n.d.; Hayden and Ghosh, 2004; Scherer et al., 1995). Alternatively, activation of the non-canonical/alternative signaling pathway results in a p52/RelB subunit translocating to the nucleus to transcribe target genes. NF-κB is considered a rapid-acting transcription factor because the transcriptional complex exists in a poised state that allows for response to stimuli by switching from inactive to active states without requiring additional protein synthesis (Baeuerle and Baltimore, 1988).
Targeting NF-κB is an attractive therapeutic approach because aberrant pathway signaling has been observed across disease types. NF-κB transcriptionally controls hundreds of genes (Robbins et al., 2011), and has been shown to suppress both apoptotic and necrotic cell death pathways by regulating target genes that prevent cell death (Dutta et al., 2006; Luo et al., 2005). Recently, C11orf95-RELA translocations have been reported as the most common genetic alteration in supratentorial ependymoma (Parker et al., 2014), a primary neoplasm of the central nervous system that has yet to be functionally characterized with respect to cell survival (Louis et al., 2016; Taylor et al., 2009). The seven distinct fusion proteins that result from C11orf95-RELA translocations have been shown to constitutively activate RELA (p65), the active component of the NF-κB signaling complex. Thus, this newly identified genetic alteration implies a potential therapeutic target to treat supratentorial ependymoma. A wide variety of small molecule modulators of NF-κB activity have been reported (Gupta et al., 2010; Manuvakhova et al., 2011), whose ligands function to activate or inhibit NF-κB by targeting different points in the NF-κB signaling pathway. Accordingly, the associated data that characterize these small molecules are heterogeneous and difficult to compare one molecule with another.
Cell reporter assays for NF-κB signaling have sometimes been confounded by low signal to background, and/or high noise (Guttridge et al., 1999; Mitchell and Sugden, 1995), and while NF-κB inhibitors are often studied, benchmarking is rarely done. Here, we demonstrate the utility of a robust in vitro reporter of NF-κB transcription (Systems Biosciences) and identify the most specific pharmacological inhibitors of NF-κB activity. Next, we tested compounds which exhibited NF-κB agonistic or antagonistic activity and evaluated their efficacy on cells derived from a patient-derived, translocation positive ependymoma tumor cell culture in the hopes of identifying a compound lead of therapeutic value.
2. Materials and methods
2.1. Cell Culture
HEK293 NF-κB GFP-luciferase reporter cell line (TR860A-1, System Biosciences, Palo Alto, CA, USA) were cultured in DMEM (11965–084, Sigma, St. Louis, MA, USA) supplemented with 10% FBS (26140079, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (15140– 122, Thermo Fisher Scientific). All cells were incubated at 37°C and 5% CO2. De-identified cell culture MAF1329 was created by co-authors Dr. Andrew Donson and Dr. Vladamir Amandi (University of Colorado Anschutz Medical Campus) and was established from the resection of tumor recurrence at the primary site of a temporal lobe anaplastic ependymoma from a 5 year old female patient. Cells were grown in Optimem (11058021, Thermo Fisher Scientific) supplemented with 15% FBS ad 1% P/S. Primary patient cells were used prior to passage 9.
2.2. NF-κB Antagonist or Agonist Treatment
NF-κB antagonist and agonists: TNFα (315–01A, Peprotech, Rocky Hill, NJ, USA ), Prostratin (5739, Tocris, Bristol, UK), CGS 21680 HCl (1063, Tocris), Betulinic acid (53603, Selleck Chemicals, Houston, TX, USA), PSI (4045, Tocris), Cardamonin (2509, Tocris), Bay 11–7082 (S2913, Selleck Chemicals), Bay 11–7085 (S7352, Selleck Chemicals), RO 106–9920 (1778, Tocris), TPCA-1 (S2824, Selleck Chemicals), Ikk-16 (S2882, Selleck Chemicals), PF 184 (4238, Tocris), IMD 0354 (2483, Tocris), Andrographolide (S2261, Selleck Chemicals), Costunolide (2483, Tocris), CID 2858522 (4246, Tocris), Pictilisib (S1065, Selleck Chemicals), Luteolin (S2320, Selleck Chemicals), Celastrol (1571, Tocris), Artemisinin (2668, Tocris). Cells were plated (at a seeding density of 1x104 for 96 well plates and 1x103 for 384 well plates) 12 h before treatment. Cells were treated with drugs at a range of concentrations either with fresh media (for agonists and vehicle only control) or fresh media with 5ng/ml TNFα (for antagonists and vehicle control) and incubated for 24 h. Each drug dose was performed in triplicate or quadruplicate and experiments were repeated at least three times.
2.3. Luciferase Assays
Luciferase reporter assays (E4530 or E8120, Promega, Madison, WI, USA) were performed per manufacturer instructions and a BioTek Synergy 2 plate reader (BioTek, Winooski, VT, USA) was used to measure luminescence. Luciferase activity, measured in drug lums/vehicle lums, was then plotted against log10 concentration of dose for each drug. EC50s were calculated using GraphPad Prism (San Diego, CA, USA).
2.4. Drug Cytotoxicity Assays
Cell viability following drug treatment was assayed in parallel with luciferase assays using CellTiter-Glo 2.0 (G9241, Promega) per manufacturer instructions and the BioTek Synergy 2 plate reader was used to evaluate the cytotoxic effect of the drugs. EC50s were calculated using GraphPad Prism.
2.5. Protein expression
Cultured cells were lysed using RIPA buffer (89900, Thermo Fisher) supplemented with HALT protease and phosphatase inhibitor (78441, Thermo Fisher) and total protein was determined using BCA assay (23225, Thermo Fisher). 30 μg of protein was resolved using SDS-PAGE gel (4561034, BioRad, CA) and transferred to PVDF membranes. Membranes were blocked using 5% BSA (001–000-162, Jackson ImmunoResearch Labs, PA) diluted in Tris-buffered saline with Tween-20 (BP377–500, Fisher Scientific) then incubated overnight with primary antibodies from Cell Signaling Technology (Danvers, MA) p65 (3034) or GAPDH (2118). HRP conjugated goat anti-rabbit (PI-1000, Vector laboratories) was used as a secondary antibody, and protein was visualized using Clarity ECL Substrate (1705061, Bio-Rad).
3. Results
3.1. Validation of NF-κB reporter system
Before evaluating reported NF-κB agonists and antagonists, we first validated the robustness of a HEK293 NF-κB GFP-luciferase transcriptional reporter cell line. To do this we performed luciferase reporter assays using TNFα stimulation, a well-characterized NF-κB agonist using a time course of stimulation from 4–48hrs over a dose range from 0 to 20ng/ml (data not shown). In addition to being consistent with other published findings on TNFα stimulation of NF-κB, these results established 5ng/ml TNFα for 24 h as the optimal parameter for subsequent experiments (Fig S2).
3.2. Pharmacological NF-κB activation or inhibition in HEK293 cells
We next tested the efficacy of reported NF-κB antagonists and agonists to inhibit or activate NF-κB signaling, respectively. We began by identifying commercially available chemical modulators specific to NF-κB signaling. From the literature, we compiled a list of reported NF-κB antagonists and agonists (Table 1, Fig S1). To evaluate whether each drug inhibits or activates NF-κB signaling, we performed luciferase reporter assays for NF-κB transcriptional expression using the HEK293 NF-κB GFP-luciferase reporter cell line.
Table 1. EC50 values for agonists and antagonists tested on HEK293 cells.
For NF-κB reporter luciferase activity, values <1nM indicate that NF-κB expression was less than 50% expression of untreated control cells even at the lowest concentration of inhibitor, while values >100,000nM indicate no decrease in luciferase expression was observed at the highest concentration of inhibitor. For cell viability, >100,000nM indicates that 50% cell death was not observed even at the highest concentration of inhibitor.
| Inhibitor | EC50 (NF-kB reporter) nM | IC50 (cell viability) nM | Putative mechanism of Inhibition | Reference |
|---|---|---|---|---|
| Ro106–9920 | <1 | >100,000 | LPS- and TNF-a induced IkBa ubiquitination | (Swinney et al., 2002) |
| TCPA-1 | <1 | > 00,000 | IKKβ | (Birrell et al., 2005; Nan et al., 2014) |
| IMD 0354 | 292 | 1,560 | IKKβ | (Podolin, 2004; Tanaka et al., 2005) |
| PF-184 | 901 | 1,230 | IKKβ | (Sommers et al., 2009) |
| Pictalisib | 4,266 | 7,470 | PI3Kα/δ | (Folkes et al., 2008; Junttila et al., 2009) |
| BAY 11–7085 | 31,700 | >100,000 | IκB phosphorylation | (Koedel et al., 2000; Pierce et al., 1997) |
| Cardamonin | 3,290 | 27,520 | IκB phosphorylation | (Lee et al., 2006) |
| PSI | 10 | 373 | IκBα phosphorylation | (Traenckner et al., 1994) |
| Celastrol | 82 | 475 | IκBα phosphorylation | (Sethi et al., 2007) |
| Artemisinin | >100,000 | >100,000 | IκBα phosphorylation | (Wang et al., 2017) |
| Luteolin | 35,000 | >100,000 | IκBα phosphorylation | (Kim and Jobin, 2005) |
| IKK-16 | 852 | 1,510 | IKKα, IKKβ, IKK complex | (Waelchli et al., 2006) |
| CID 2858522 | 8,590 | 6,420 | Protein Kinase C induced IKKβ | (Shi et al., 2010) |
| BAY 11–7082 | 16,930 | 2,730 | IκBα phosphorylation | (Keller et al., 2000) |
| Piceatannol | >100,000 | >100,000 | IκBα phosphorylation | (Ashikawa et al., 2002) |
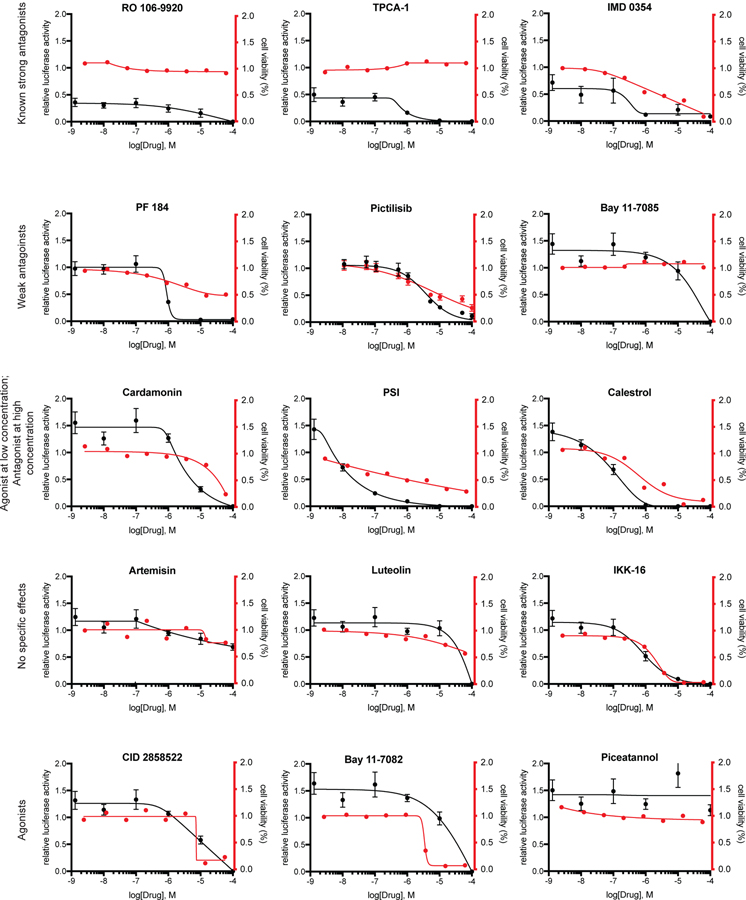
HEK293 NF-κB GFP-luciferase cells were treated with a dose range of 15 different commercially and clinically available NF-κB antagonists (Fig S1) or 4 agonists, and then the effect on NF-κB signaling was assayed. For NF-κB antagonists, cells were stimulated with TNFα in addition to drug treatment to ensure that NF-κB activity was sufficient to detect inhibition. Further, in order to distinguish between NF-κB inhibition and cytotoxic effects of the drugs, luciferase reporter assays to measure NF-κB activity were conducted in parallel with CellTiter-Glo luminescent assays to determine changes in cell viability.
The agents artemisin, luteolin, pictilisib and IKK-16 exhibited no specific effect on HEK-293 cells in regard to cell viability or NF-κB expression (Fig 1). Interestingly, treatment with CID 2858522, Bay 11–7082, Bay 11–7085 or piceatannol resulted in an agonistic effect on NF-κB expression at low or mid-range micromolar concentrations, with no cytotoxic effect at low or mid-range micromolar concentrations. Similarly, cardamonin, which has been reported to suppress IKK expression and IκBα phosphorylation (Qin et al., 2012), the proteasome inhibitor PSI, and the classical NF-κB pathway inhibitor celastrol, were all found to act as agonists and increased pathway activity when used at low micromolar concentrations without effecting cell viability (Fig 1, Table 1).
Fig 1. Examination of NFκB antagonists in HEK293 cells.
Inhibition of NF-κB signaling was examined in HEK293 cells that stably express a NF-κB luciferase reporter and were graphed versus a measurement of cell viability by a non-luciferase luminescence assay (CTG). Cells were stimulated with TNFα and treated with either antagonist or vehicle (DMSO) for 24 h. Graphs are representative of a single experiment where each condition was performed in triplicate. Each experiment was repeated 3 times. Mean ± S.E.M.
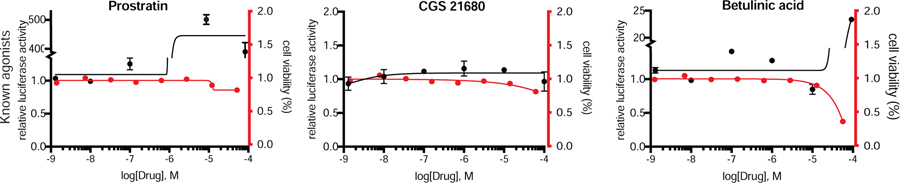
We additionally benchmarked several agonists of NF-κB (Fig 2). Prostratin, the protein kinase C agonist (Jiang and Dandekar, 2015) works upstream of NF-κB and showed activity at 1000nM. Conversely, the reported NF-κB activator betulinic acid (Kasperczyk et al., 2005) was only effective at the highest tested dose (100,000nM). The adenosine A2A receptor inhibitor CGS 21680 (Bortoluzzi et al., 2016) had no effect (Fig 2).
Fig 2. Examination of NF-κB agonists in HEK293 cells.
NF-κB signaling was examined in HEK293 cells that stably express a NF-κB luciferase reporter and were graphed versus a measurement of cell viability by a non-luciferase luminescence assay (CTG). Cells were treated with either antagonist or vehicle (DMSO) for 24 h. Graphs are representative of a single experiment where each condition was performed in triplicate. Each experiment was repeated 3 times. Mean ± S.E.M.
Of all agents tested, the selective IKKβ inhibitors Ro 106–9920 (< 1nM), IMD-0354 (292nM), TPCA-1 (<1nM) and PF 184 (901nM) were observed to work antagonistically and did not result in a decrease of cell viability at low concentrations, indicating a specificity for NF-κB without creating cytotoxic off-target effects (Fig 2). Taken together, we found that Ro 106–9920, TPCA-1 and IMD 0354 were most potent in reducing NF-κB signaling activity in HEK-293 reporter cells without decreasing cell viability.
3.3. Cell viability of MAF1329 fusion positive ependymoma treated with NF-κB inhibitors
C11orf95-RELA fusion proteins in ependymoma have been shown to spontaneously translocate to the nucleus and drive an abnormal NF-κB transcriptional program and cell growth (Parker et al., 2014). To examine whether antagonism of the NF-κB pathway in ependymoma would result in a reduction in cell proliferation, we next investigated the effects of three previously benchmarked compounds on MAF1329, a patient-derived ependymoma cell culture.
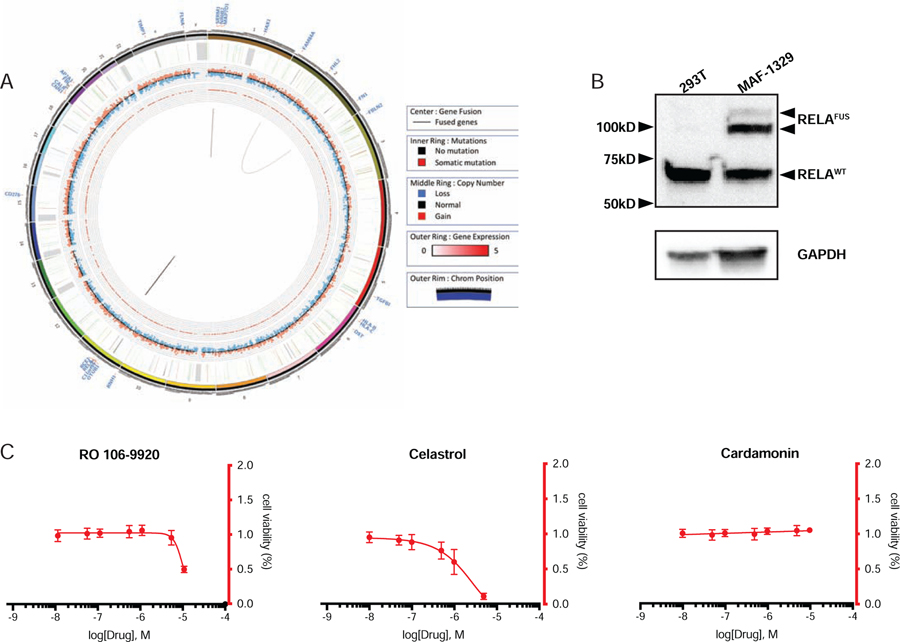
To characterize the MAF1329 cell culture RNA sequencing was performed and confirmed the presence of C11orf95-RELA chromosomal translocation (Table 2). Whole genome sequencing was additionally performed and revealed structural variations in chromosomes 1, 2 and 11 (Fig 3A). To validate the presence of a RELA fusion protein, western blot analysis for RELA was conducted and detected the presence of both wild type and two distinct fusion positive RELA proteins (Fig 3B).
Table 2. Fusion predictions from RNAseq results.
Summary of RNA sequencing results identifying C11orf95-RELA fusion in the ependymoma primary patient cell culture MAF1329. Complete results have been deposited in Gene Expression Ombibus.
| #Fusion Name | Junction Read Count | Spanning Frag Count | Left Breakpoint | Right Breakpoint | FFPM |
|---|---|---|---|---|---|
| C11orf95--RELA | 14 | 8 | chr11:63765807:- | chr11:65662205:- | 0.5068 |
| C11orf95--RELA | 13 | 8 | chr11:63764868:- | chr11:65662205:- | 0.4838 |
Fig 3. Characterization of MAF1329 ependymoma cells.
(A) Circos plot depiction of tumor genome MAF1329 (B) Western blot analysis confirmed presence of wild type RELA as well as C11orf95-RELA fusion protein in ependymoma primary cell culture MAF1329. (C) Cell viability of MAF1329 cells treated with NF-κB antagonists specifically targeting IκBα phosphorylation.
To examine the efficacy of inhibiting NF-κB activity on cell viability of MAF1329, we chose to test three compounds reported to inhibit the activation of IκBα, the functional repressor of RELA (Fig 3C). Ro 106–9920 was chosen due to the strong antagonistic effect on NF-κB signaling without reducing cell viability in HEK-293 cells, while celastrol and cardamonin were chosen due to the mixed agonist and antagonist effects exerted on NF-κB activity at different concentrations in the reporter cell line assay (Fig 1). We found that using cardamonin to block phosphorylation of IκBα resulted in no change in cell viability, while only moderate decreases in cell viability were observed at the highest concentrations of RO 106–9920 (IC50 10,000nM) and celastrol (IC50 1,700nM).
4. Discussion
Cellular assays are an important tool in drug development which provide information on the phenotypic effects of compounds such as proliferation, cytotoxicity or senescence. These tests are so widely used and accepted that commercial drug distributors often use the readout as evidence of effective drug treatment. However, these in vitro tests do not directly evaluate the inhibition or activation of a specific target in response to a compound. Our studies aimed to evaluate compounds commonly studied as purported NF-κB inhibitors.
Using a luciferase reporter system in HEK293 human embryonic kidney cells, we found that only 3 of the 15 compounds tested decreased NF-κB activation without affecting cell proliferation. Although the putative mechanism of action for these three compounds are different, these compounds achieved the same result of reducing NF-κB pathway activity. Two of the compounds found to effectively reduce NF-κB activity are TCPA-1 and IMD 0354, which work by inhibiting IKKβ (Podolin, 2004; Tanaka et al., 2005). Inactivated IKKβ leaves the NF-κB complex unphosphorylated and non-ubiquitinated, sequestered in the cytoplasm. The third compound is the small molecule Ro 106–9920, which acts by blocking the ubiquitination and subsequent degradation by the S26 proteasome of IκBα (Swinney et al., 2002). Blocking ubiquitination at this step similarly results in preventing the NF-κB complex from translocating to the nucleus.
Given the current understanding of supratentorial ependymoma, we reasoned that testing NF-κB inhibitors might provide information on an appropriate therapeutic target for this disease. In our experiments, we found that direct blockade of IκBα phosphorylation using the compounds cardamonin or celastrol was not effective in reducing the cell viability of fusion positive ependymoma. Given the current lack of understanding surrounding the RELA-C11orf95 fusion protein, we leave open the possibility the IκBα inhibitory complex is constitutive and/or not susceptible to feedback inhibition, and thus non-responsive to upstream NF-κB pathway inhibition in fusion positive cells.
4.1. Conclusions
We hope that the standardized compound characterization approach we have presented will be beneficial to the field when running in vitro experiments. Our finding suggests a necessity of this diligence because purported NF-κB inhibitors may in fact be non-specific, pleiotropic compounds. Our experiments comparing NF-κB activation to cell viability demonstrate that the cell death observed is not always a result of NF-κB inhibition. To our knowledge, our report is the first to have tested NF-κB inhibitors in a standardized way.
Separately, the functional evaluation of a supratentorial ependymoma cell line that we present is to be used as a waypoint for understanding the role of NF-κB in ependymoma and other pediatric cancers’ disease progression. RELA fusions in ependymoma may indeed be pathogenic, and scientists are just beginning to uncover the downstream target genes that drive this cancer.
Supplementary Material
Fig S1. Structures of compounds used in study.
Hek-293 cells transfected with NF-κB-GFP-LUC reporter were treated with varying concentrations of TNF-α. At 24 h luminescence was measured.
Acknowledgements
This work was supported in part by NCI P01 grant P01CA165995, R01 grant 5R01CA143082, Golf Fights Cancer, Storm The Heavens Fund, and Project Haystack.
Footnotes
Competing Interests Statement: The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Compliance with ethical standards
No human subjects or animals were used in this study.
Additional Information
Data Deposition and Access
Reads from RNA sequencing experiments were deposited in Gene Expression Omnibus accession number GSE136559
References
- Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB, 2002. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J. Immunol 169, 6490–7. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D, 1988. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-κB transcription factor. Cell 53, 211–217. 10.1016/0092-8674(88)90382-0 [DOI] [PubMed] [Google Scholar]
- Birrell MA, Hardaker E, Wong S, Mccluskie K, Catley M, De Alba J, Newton R, Haj-Yahia S, Tao K, Watts CJ, Shaw RJ, Savage TJ, Belvisi MG, Belvisi G, 2005. I-B Kinase-2 Inhibitor Blocks Inflammation in Human Airway Smooth Muscle and a Rat Model of Asthma. Am J Respir Crit Care Med 172, 962–971. 10.1164/rccm.200412-1647OC [DOI] [PubMed] [Google Scholar]
- Bortoluzzi A, Vincenzi F, Govoni M, Padovan M, Ravani A, Borea PA, Varani K, 2016. A2Aadenosine receptor upregulation correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Res. Ther 18. 10.1186/s13075-016-1089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U, n.d. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Sci. 1995 267, 1485–1488. [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gélinas C, 2006. Current insights into the regulation of programmed cell death by NF-κB. Oncogene 25, 6800–6816. 10.1038/sj.onc.1209938 [DOI] [PubMed] [Google Scholar]
- Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJA, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ, 2008. The Identification of 2-(1 H -Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2- d ]pyrimidine (GDC-0941) as a Potent, Selective, Orally Bioavailable Inhibitor of Class I PI3 Kinase for the Treatment of Cancer. J. Med. Chem 51, 5522–5532. 10.1021/jm800295d [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U, 1997. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev 11, 3482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB, 2010. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–87. 10.1016/j.bbagrm.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS, 1999. NF-B Controls Cell Growth and Differentiation through Transcriptional Regulation of Cyclin D1. Mol. Cell. Biol 19, 5785–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S, 2004. Signaling to NF-kappaB. Genes Dev 18, 2195–224. 10.1101/gad.1228704 [DOI] [PubMed] [Google Scholar]
- Jiang G, Dandekar S, 2015. Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retroviruses 31, 4–12. 10.1089/AID.2014.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX, 2009. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell 15, 429–440. 10.1016/j.ccr.2009.03.020 [DOI] [PubMed] [Google Scholar]
- Kanegae Y, Tavares AT, Belmonte JCI, Verma IM, 1998. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature 392, 611–614. 10.1038/33429 [DOI] [PubMed] [Google Scholar]
- Kasperczyk H, La Ferla-Brühl K, Westhoff MA, Behrend L, Zwacka RM, Debatin K-M, Fulda S, 2005. Betulinic acid as new activator of NF-κB: molecular mechanisms and implications for cancer therapy. Oncogene 24, 6945–6956. 10.1038/sj.onc.1208842 [DOI] [PubMed] [Google Scholar]
- Keller SA, Schattner EJ, Cesarman E, 2000. Inhibition of NF-kB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96, 2537–2542. [PubMed] [Google Scholar]
- Kim JS, Jobin C, 2005. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 115, 375–87. 10.1111/j.1365-2567.2005.02156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Bayerlein I, Paul R, Sporer B, Pfister HW, 2000. Pharmacologic Interference with NF‐κB Activation Attenuates Central Nervous System Complications in Experimental Pneumococcal Meningitis. J. Infect. Dis 182, 1437–1445. 10.1086/315877 [DOI] [PubMed] [Google Scholar]
- Lee J-H, Jung HS, Giang PM, Jin X, Lee S, Son PT, Lee D, Hong Y-S, Lee K, Lee JJ, 2006. Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther 316, 271–8. 10.1124/jpet.105.092486 [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Webster Cavenee, K., Ohgaki H, Wiestler OD, Kleihues P, Ellison DW, 2016. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131, 803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Luo J-L, Kamata H, Karin M, 2005. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J. Clin. Invest 115, 2625–32. 10.1172/JCI26322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuvakhova MS, Johnson GG, White MC, Ananthan S, Sosa M, Maddox C, McKellip S, Rasmussen L, Wennerberg K, Hobrath JV, White EL, Maddry JA, Grimaldi M, 2011. Identification of novel small molecule activators of nuclear factor-κB with neuroprotective action via high-throughput screening. J. Neurosci. Res 89, 58–72. 10.1002/jnr.22526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T, Sugden B, 1995. Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol 69, 2968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan J, Du Y, Chen X, Bai Q, Wang Y, Zhang X, Zhu N, Zhang J, Hou J, Wang Q, Yang J, 2014. TPCA-1 Is a Direct Dual Inhibitor of STAT3 and NF-kB and Regresses Mutant EGFR-Associated Human Non-Small Cell Lung Cancers. Mol. Cancer Ther 13, 617–629. 10.1158/1535-7163.MCT-13-0464 [DOI] [PubMed] [Google Scholar]
- Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Yongjin, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, White E, Tang B, Orisme W, Gupta K, Rusch M, Chen X, Li Yuxin, Nagahawhatte P, Hedlund E, Finkelstein D, Wu G, Shurtleff S, Easton J, Boggs K, Yergeau D, Vadodaria B, Mulder HL, Becksfort J, Becksford J, Gupta P, Huether R, Ma J, Song G, Gajjar A, Merchant T, Boop F, Smith AA, Ding L, Lu C, Ochoa K, Zhao D, Fulton RS, Fulton LL, Mardis ER, Wilson RK, Downing JR, Green DR, Zhang J, Ellison DW, Gilbertson RJ, 2014. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506, 451–5. 10.1038/nature13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Santani D, 2009. Role of NF-kB in the pathogenesis of diabetes and its associated complications. Pharmacol. Reports 61, 595–603. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME, 1997. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem 272, 21096–103. 10.1074/JBC.272.34.21096 [DOI] [PubMed] [Google Scholar]
- Podolin PL, 2004. Attenuation of Murine Collagen-Induced Arthritis by a Novel, Potent, Selective Small Molecule Inhibitor of I B Kinase 2, TPCA-1 (2-[(Aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), Occurs via Reduction of Proinflammatory Cytokines and Ant. J. Pharmacol. Exp. Ther 312, 373–381. 10.1124/jpet.104.074484 [DOI] [PubMed] [Google Scholar]
- Qin Y, Sun C-Y, Lu F-R, Shu X-R, Yang D, Chen L, She X-M, Gregg NM, Guo T, Hu Y, 2012. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk. Res 36, 514–520. 10.1016/j.leukres.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Robbins PD, Tilstra JS, Clauson CL, Niedernhofer LJ, 2011. NF-kB in Aging and Disease. Aging Dis 2, 449–465. 10.1126/science.1205623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW, 1995. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc. Natl. Acad. Sci. U. S. A 92, 11259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Baltimore D, 1986. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46, 705–16. 10.1016/0092-8674(86)90346-6 [DOI] [PubMed] [Google Scholar]
- Sethi G, Seok Ahn K, Pandey MK, Aggarwal BB, 2007. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-B-regulated gene products and TAK1-mediated NF-B activation. Blood 109, 2727–2735. 10.1182/blood-2006-10 [DOI] [PubMed] [Google Scholar]
- Shi R, Re D, Dudl E, Cuddy M, Okolotowicz KJ, Dahl R, Su Y, Hurder A, Kitada S, Peddibhotla S, Roth GP, Smith LH, Kipps TJ, Cosford N, Cashman J, Reed JC, 2010. Chemical biology strategy reveals pathway-selective inhibitor of NF-kappaB activation induced by protein kinase C. ACS Chem. Biol 5, 287–99. 10.1021/cb9003089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CD, Thompson JM, Guzova JA, Bonar SL, Rader RK, Mathialagan S, Venkatraman N, Holway VW, Kahn LE, Hu G, Garner DS, Huang H-C, Chiang P-C, Schindler JF, Hu Y, Meyer DM, Kishore NN, 2009. Novel Tight-Binding Inhibitory Factor- B Kinase (IKK-2) Inhibitors Demonstrate Target-Specific Anti-Inflammatory Activities in Cellular Assays and following Oral and Local Delivery in an in Vivo Model of Airway Inflammation. J. Pharmacol. Exp. Ther 330, 377–388. 10.1109/AIM.2017.8014218 [DOI] [PubMed] [Google Scholar]
- Swinney DC, Xu Y-Z, Scarafia LE, Lee I, Mak AY, Gan Q-F, Ramesha CS, Mulkins MA, Dunn J, So O-Y, Biegel T, Dinh M, Volkel P, Barnett J, Dalrymple SA, Lee S, Huber M, 2002. A small molecule ubiquitination inhibitor blocks NF-kappa B-dependent cytokine expression in cells and rats. J. Biol. Chem 277, 23573–81. 10.1074/jbc.M200842200 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, Itai A, Matsuda H, 2005. A novel NF-B inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood 105, 2324–2331. 10.1182/blood-2004-08-3247 [DOI] [PubMed] [Google Scholar]
- Taylor JG, Cheuk AT, Tsang PS, Chung J-Y, Song YK, Desai K, Yu Y, Chen Q-R, Shah K, Youngblood V, Fang J, Kim SY, Yeung C, Helman LJ, Mendoza A, Ngo V, Staudt LM, Wei JS, Khanna C, Catchpoole D, Qualman SJ, Hewitt SM, Merlino G, Chanock SJ, Khan J, 2009. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Invest 119, 3395–407. 10.1172/JCI39703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traenckner EB, Wilk S, Baeuerle PA, 1994. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J 13, 5433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Antwerp D Van, 1995. Rel/NF-kB/IkB: intimate tales of association and dissociation. Genes Dev 2723–2735. 10.1101/gad.9.22.2723 [DOI] [PubMed]
- Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, Hersperger R, Janser P, Revesz L, Zerwes H-G, Schlapbach A, 2006. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg. Med. Chem. Lett 16, 108–112. 10.1016/J.BMCL.2005.09.035 [DOI] [PubMed] [Google Scholar]
- Wang DJ, Ratnam NM, Byrd JC, Guttridge DC, 2014. NF-κB functions in tumor initiation by suppressing the surveillance of both innate and adaptive immune cells. Cell Rep 9, 90–103. 10.1016/j.celrep.2014.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X, 2017. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol 39, 28–36. 10.1080/08923973.2016.1267744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Structures of compounds used in study.
Hek-293 cells transfected with NF-κB-GFP-LUC reporter were treated with varying concentrations of TNF-α. At 24 h luminescence was measured.