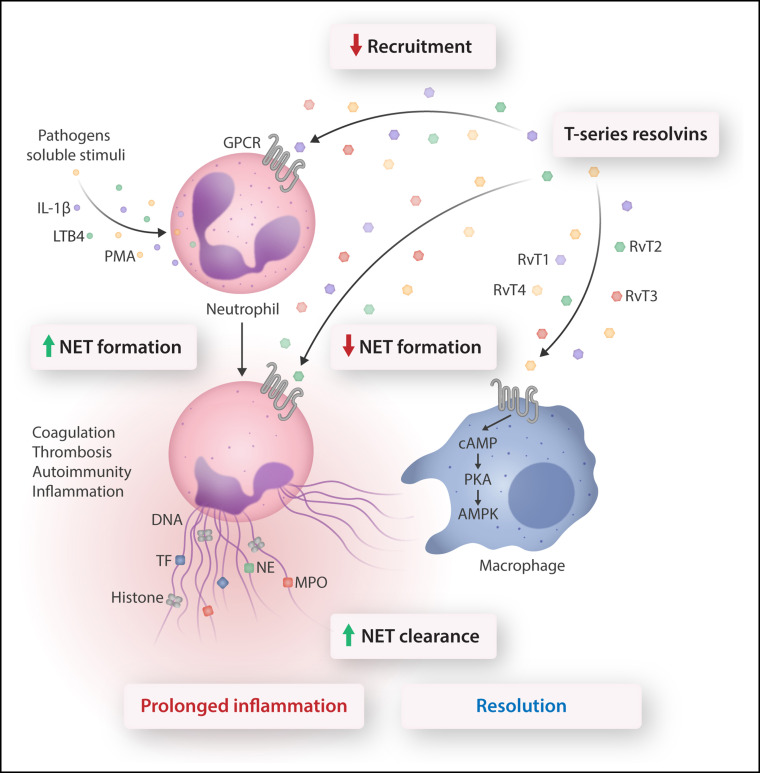
In this issue of Blood, Chiang et al 1 report that the T-series of resolvins (RvTs) reduce the formation of neutrophil extracellular traps (NETs) and enhance NET clearance by macrophages, thereby identifying a novel mechanism for the resolution of infections and coagulopathies.
Neutrophils play a key role in host defense against invading pathogens and are rapidly deployed to sites of infection or injury. However, many of the defense mechanisms they use to destroy microorganisms are potentially deleterious to the host. Among these mechanisms is release of NETs (also known as NETosis), which consist of a nucleic acid scaffold decorated with histones and granular proteins to entrap and kill bacteria, viruses, and fungi.2 Because the effects of NET components are not restricted to microbes, excessive or uncontrolled NET formation can inflict damage to the surrounding tissue, thus maintaining a pro-inflammatory and pro-thrombotic environment that underlies various pathologies, including autoimmune diseases,3 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–associated coagulopathies, and acute respiratory distress syndrome (ARDS).4 To ensure timely resolution of acute inflammation, neutrophils need to be disarmed and removed from the affected sites. Resolution of inflammation is an active process governed by specialized pro-resolving lipid mediators (lipoxins, resolvins, protectins, and maresins), proteins (eg, annexin A1), and gaseous mediators (eg, hydrogen sulfite and carbon monoxide), which predominantly act on phagocytes and other immune cells.5, 6, 7
By analyzing lipid profiles in resolution exudates, the authors previously identified a new series of resolvins that originate from a central intermediate of n-3 docosapentaenoic acid and possess a unique structure, a hydroxyl group on the carbon-13 position, termed the 13-series resolvins: RvT1, RvT2, RvT3, and RvT4.8 In their current study, Chiang et al synthesized and validated RvTs and compared their potencies to modulate NETosis by using a newly developed microfluidic device that allows NET formation to be monitored in whole human blood. In this assay, NETs are visualized by staining with Sytox Green and are then automatically identified in fluorescence images on the basis of their “long string” features. Because the 4 RvTs are synthesized in near equivalent amounts in human peripheral blood,8 Chiang et al used a mixture of RvTs to demonstrate marked reductions in NET formation evoked by phorbol 12-myristate 13-acetate (PMA), a well-known inducer of suicidal NETosis (see figure ).2 The inhibitory actions were comparable to that of resolvin D2. When tested separately, RvT1, RvT2, and RvT4, but not RvT3, also reduced PMA-stimulated NETosis in whole blood as well as interleukin-1β or leukotriene B4 (LTB4)–evoked NET release from isolated human neutrophils. In these assays, RvT1 was the most potent, and RvT3 was significantly less potent than the other RvTs. RvT1 reduced the amount of both extracellular DNA and DNA-bound myeloperoxidase (MPO), critical components of NET assembly. It is unclear how RvT1 signaled to attenuate breakdown of the nuclear envelope, which is thought to precede liberation of DNA,2 as well as release of myeloperoxidase from primary granules, which requires activation of the Src kinase Hck.9 RvT1 reduction of MPO release is consistent with earlier studies that reported reduced MPO and neutrophil elastase release by 15-epi-lipoxin A4 and 17-epi-resolvin D1.9
Conventional neutrophil host defense includes the recruitment cascade, chemotaxis, phagocytosis, and microbial killing. Stimulation of neutrophil receptors by microorganisms or soluble mediators evokes release of NETs. NETs trap bacteria, viruses, and fungi, thereby aiding in containing local infections. However, NET components could also contribute to maintaining an inflammatory environment, trigger autoimmunity, and activate coagulation resulting in thrombosis. These would lead to persistent inflammation and damage to the host. Chiang et al show that RvTs (derived from a common intermediate of n-3 docosapentaenoic acid) exert multipronged actions to counter aberrant neutrophil responses. Thus, RvTs, acting through unidentified G protein–coupled receptors (GPCRs), inhibit neutrophil recruitment, reduce NET formation, and enhance NET clearance by M0 macrophages. In macrophages, NET uptake is closely associated with activation of the cAMP-PKA-AMPK pathway that mediates phagocytosis. Reducing NET formation and facilitating NET clearance without hindering neutrophil phagocytosis led to timely resolution and return to homeostasis, suggesting the therapeutic potential for RvTs in pathologies associated with aberrant NET formation. AMPK, AMP-activated protein kinase; cAMP, cyclic adenosine monophosphate; IL-1β, interleukin-1β; NE, neutrophil elastase; PKA, protein kinase A; TF, tissue factor. Professional illustration by Somersault18:24.
Importantly, RvTs given together with Staphyloccus aureus, a well-established stimulus for NETosis in vivo,2 significantly reduced neutrophil accumulation as well as bacterial titers in dorsal air pouches along with markedly lower amounts of long-string NETs in the exudates, which indicates efficient control of infection. The main implication of these findings is that by targeting NETosis without blocking phagocytosis, RvTs can ensure rapid resolution and prevent bystander injury to the host. However, additional studies will be necessary to assess the therapeutic window and potential of RvTs given after infection.
How NETs are cleared is incompletely understood. Human macrophages can engulf NETs through endocytosis without inducing cytokine secretion.2 Conversely, impaired NET clearance by macrophages is associated with sustained inflammation, for example, as reported in ARDS.10 In line with this understanding, Chiang et al report that human monocyte-derived M0 macrophages have higher capacity to ingest NETs than either the pro-inflammatory M1 or the anti-inflammatory M2 phenotypes. The authors show that RvTs in low nanomolar concentrations enhance the uptake of NETs by M0 macrophages with RvT2 being the most potent, followed in potency by RvT1, RvT2, and RvT4. RvT2 activates the cAMP-PKA-AMPK pathway, which mediates phagocytosis in macrophages by resolvin D2 and maresin 1.7 Consistent with the findings in human macrophages, RvT2 enhanced NET uptake by peritoneal macrophages in mice, which indicates that this RvT is fully operational in the in vivo setting and underscores the translational potential of RvTs.
Although the authors' data infers the involvement of specific receptors for RvTs, these receptors still need to be identified. Pro-resolving lipids signal through distinct G protein–coupled receptors, sometimes in a cell type–specific manner. For instance, resolvin E1 signals through BLT1 in neutrophils and ChemR23 in macrophages to enhance phagocytosis.5, 7 Because pharmacologic blockade of the resolvin D2 receptor GPR18 or the LTB4 receptor BLT1 did not affect the actions of RvTs, and because RvT1 did not antagonize LTB4 signals via BLT1, it is unlikely that RvTs interacted with these receptors. The differences in the potency of RvTs in attenuating NET release and clearance might indicate involvement of different receptors on neutrophils and macrophages. Future studies might focus on these receptors as well as on the potential interplay of RvTs with other pro-resolving circuits.
Chiang et al unveil a novel mechanism in the context of regulation by specialized pro-resolving mediators of host defense during infection. So far, microbes have been fought with pathogen-specific therapies, antibiotics, or antivirals, depending on the nature of invading pathogens, but the adverse consequences of damage from immune activation have not been addressed This study expands the discoveries made by the Serhan laboratory over the years by identifying the members and functions of the superfamily of specialized pro-resolving lipid mediators.5, 7 Herein, Chiang et al provide compelling evidence on the multipronged actions of 13-series resolvins, and RvT2 in particular, which partly overlap with those of other pro-resolving lipids but also include suppression of NET formation and facilitation of macrophage clearance of NETs in the context of bacterial and viral infections. These findings have considerable therapeutic potential and broad application for mitigating the deleterious consequences of aberrant NET formation.
Funding support for this article was provided by the Canadian Institutes of Health Research (MOP-102619).
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Chiang N, Sakuma M, Rodriguez AR, Spur BW, Irimia D, Serhan CN. Resolvin T-series reduce neutrophil extracellular traps. Blood. 2022;139(8):1222–1233. doi: 10.1182/blood.2021013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. 2016;12(7):402–413. doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middleton EA, He X-Y, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perretti M, Cooper D, Dalli J, Norling LV. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol. 2017;13(2):87–99. doi: 10.1038/nrrheum.2016.193. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Gupta SK, Perretti M, et al. The atlas of inflammation resolution (AIR) Mol Aspects Med. 2020;74:100894. doi: 10.1016/j.mam.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21(9):1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekheri M, El Kebir D, Edner N, Filep JG. 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A. 2020;117(14):7971–7980. doi: 10.1073/pnas.1920193117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grégoire M, Uhel F, Lesouhaitier M, et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J. 2018;52(2):1702590. doi: 10.1183/13993003.02590-2017. [DOI] [PubMed] [Google Scholar]