ABSTRACT
Purpose:
To explore the role and mechanisms of octreotide in neurofunctional recovery in the traumatic brain injury (TBI) model.
Methods:
Rats were subjected to midline incision followed by TBI in the prefrontal cortex region. After 72 hours, the behavioural and neurological deficits tests were performed, which included memory testing on Morris water maze for 5 days. Octreotide (15 and 30 mg/kg i.p.) was administered 30 minutes before subjecting to TBI, and its administration was continued for three days.
Results:
In TBI-subjected rats, administration of octreotide restored on day 4 escape latency time (ELT) and increased the time spent in the target quadrant (TSTQ) on day 5, suggesting the improvement in learning and memory. It also increased the expression of H2S, Nrf2, and cystathionine-γ-lyase (CSE) in the prefrontal cortex, without any significant effect on cystathionine-β-synthase. Octreotide also decreased the TNF-α levels and neurological severity score. However, co-administration of CSE inhibitor (D,L-propargylglycine) abolished octreotide-mediated neurofunctional recovery, decreased the levels of H2S and Nrf2 and increased the levels of TNF-α.
Conclusions:
Octreotide improved the neurological functions in TBI-subjected rats, which may be due to up-regulation of H2S biosynthetic enzyme (CSE), levels of H2S and Nrf2 and down-regulation of neuroinflammation.
Key words: Octreotide, Trauma, Morris Water Maze Test, Prefrontal Cortex, Cystathionine-γ-Lyase, Rats
Introduction
Traumatic brain injury to the brain results from an external mechanical force that may lead to temporary or permanent impairment1. It continues to affect millions of individuals around the world every year, and its manifestations may vary depending on the severity of the traumatic impact. The incidences of traumatic brain injury are more often found in a very young age-group (0–4 y), adolescents and young adults (15–24 y) and elderly (>65 y). With the significant improvement in the medical sciences, the rate of death arising due to traumatic brain injury is significantly reduced. However, there is a growing population of patients living with significant disabilities as a result of brain injury2. Therefore, there is a need to identify new drugs or interventions that may promote healing and improve disabilities after traumatic brain injury.
Octreotide is a long-acting somatostatin analogue and a synthetic cyclic peptide consisting of eight aminoacids. It is employed to manage acromegaly, diarrhoea in patients with vasoactive intestinal peptide-secreting tumours, acute haemorrhage from oesophageal varices in liver cirrhosis, fistula by reducing gastrointestinal fistula and refractory hypoglycemia3. Apart from that, it has been shown to produce beneficial effects in brain-related problems, including idiopathic intracranial hypertension4, nociception5 and status epilepticus6, as well as to exert protection in ischemic stroke7 and severe acute pancreatitis-induced brain damage8. Accordingly, it was hypothesized that octreotide may exert beneficial effects in traumatic brain injury-subjected rats.
Neuroinflammation is a key event in traumatic brain injury-induced deleterious effects, and increase in the TNF-α levels is widely employed as a marker of neuroinflammation9 , 10. Along with the onset of neuroinflammation, there is also an imbalance in oxidative stress and endogenous antioxidants. Indeed, there is a significant decline in the levels of Nrf2, a transcriptional regulator of endogenous antioxidants, during traumatic brain injury11 , 12. There are studies suggesting that octreotide may produce beneficial effects in ischemic stroke by up-regulating the transcription factor Nrf2 and down-regulating the NF-κB expression7. Hydrogen sulfide (H2S) is a gaseous neurotransmitter that has been synthesized endogenously by cystathionine-γ-lyase and cystathionine-β-synthase13. Studies have shown the function of physiological and pathophysiological roles of H2S in the brain including traumatic brain injury14 , 15. Furthermore, other studies have pointed out the close interrelationship between H2S, neuroinflammation and Nrf216 , 17.
Accordingly, it was hypothesized to explore the role of neuroinflammation, Nrf2, H2S and its biosynthetic enzymes in octreotide-mediated beneficial effects in traumatic brain injury model.
Methods
In the present study, Wistar albino rats were employed. The experimental protocol was approved by Taiyuan Iron and Steel Group, general hospital, by the ethic no. 202078954e. Octreotide and DL-propargylglycine were procured from Sigma-Aldrich (San Luis, MO, United States). The enzyme-linked immunosorbent assay (ELISA) kits for the quantitative estimation of Nrf2, cystathionine-γ-lyase and TNF-α were obtained from Lifespan Biosciences (Seattle, WA, United States). The fluorometric assay kit of cystathionine-β-synthase was produced by BioVision (Milpitas, CA, United States). The doses of octreotide18 , 19 and DL-propargylglycine20 were selected based on literature.
Traumatic brain injury model
Animals were anesthetized using 4% isoflurane, and a midline incision was made to expose the skull. Using stereotactic apparatus, the prefrontal cortex region was identified (at coordinates P = -2 and L = 1.4). Traumatic brain injury (TBI) was induced in the prefrontal cortex region using a pneumatic piston (calibrated at 40 psi of pressure and depth of 6 mm). Thereafter, the animals were allowed to recover. In sham, all procedures were performed, except inducing injury21.
Behavioural tests
To assess the functionality of the nervous system, behavioural tests were performed after 72 hours of TBI.
Neurological deficits scoring
Animals were assessed through neurological severity tests and assigned scores from 0 (normal) to 10 (the most severe form), based on total neurological deficits22 , 23.
Memory testing on Morris water maze
After three days of TBI, i.e., on the 4th day after brain injury, animals were subjected to memory testing on the Morris water maze. The animals were subjected to acquisition trials on four days (4th, 5th, 6th and 7th day after injury), and it was followed by a test for the retrieval of memory on the 8th day after injury. The escape latency time (ELT) measured on the first four days (acquisition trials) served as an index of learning. The time spent in the target quadrant (TSTQ) on the 8th day after the injury was depicted as the index of retrieval (index of memory or retention)24.
Determination of biochemical parameters
After the determination of ELT and TSTQ on the Morris water maze test, rats were sacrificed followed by the isolation of the brain. Afterwards, the brain was homogenized in a phosphate buffer solution, and the supernatant of the homogenate brain was used for the quantification of different biochemical parameters, including H2S, CSE, CBS, TNF-α and Nrf2.
H2S determination
The H2S levels in the brain homogenate were measured using monobromobimane (MBB) method coupled with reversed-phase high-performance liquid chromatography (RP-HPLC). In this method, derivatization of hydrogen sulfide with excess MBB at room temperature to form sulfide dibimane (SDB) and the fluorescence were analysed by RP-HPLC using fluorescence detectors25.
Quantification of cystathionine-β-synthase, cystathionine-γ-lyase, TNF-α And Nrf2
The expression of cystathionine-β-synthase (CBS) was determined in the brain homogenate using fluorometric activity assay kits. The expression of cystathionine-γ-lyase (CSE), TNF-α and Nrf2 was determined in the brain supernatants using commercially available ELISA kit as per manufacture instructions.
Experimental protocol
Six groups were employed, and each one was comprised of eight animals:
Sham: Under anaesthesia, a midline incision was made in the skull without induction of injury. After 72 hours, the behavioural and neurological deficits tests were performed. Afterwards, animals were subjected to memory testing on the Morris water maze for five days (4th, 5th, 6th, 7th day and 8th day). During five days of testing, ELT (4th, 5th, 6th, 7th day) and TSTQ (8th day) were measured in the Morris water maze test. On the last day, the brain was removed after killing the animals and homogenized for measuring biochemical parameters;
TBI: TBI was induced in the prefrontal cortex region of a rat by using a pneumatic piston. After 72 hours, the behavioural and neurological deficits tests were performed. Afterwards, ELT, TSTQ and biochemical parameters were measured as described in the sham control group;
Octreotide (15 mg/kg i.p.) in TBI: Octreotide (15 mg/kg i.p.)was administered 30 minutes before subjecting the rats to TBI, and its administration was continued for three days after TBI. The rest of the protocol was the same as per the sham control group;
Octreotide (30 mg/kg i.p.) in TBI: Octreotide (30 mg/kg i.p.) was administered 30 minutes before subjecting to TBI and its administration was continued for three days after TBI. The rest of the protocol was the same as per the sham control group;
D,L-propargylglycine (25 mg/kg i.p.) and octreotide (30mg/kg) in TBI: D,L-propargylglycine (25 mg/kg i.p.)was co-administered with octreotide 30 minutes before subjecting the rats to TBI, and it was continued for three days after TBI. The rest of the protocol was the same as per the sham control group;
D,L-propargylglycine (50 mg/kg i.p) and octreotide (30 mg/kg) in TBI: D,L-propargylglycine (50 mg/kg i.p.) was co-administered with octreotide (30 mg/kg) 0.5 hour before subjecting the rats to TBI, and their administration was continued for three days after TBI. The rest of the protocol was the same as per the sham control group.
Results
Traumatic brain injury produced neurofunctional deficits and impairment in learning and memory
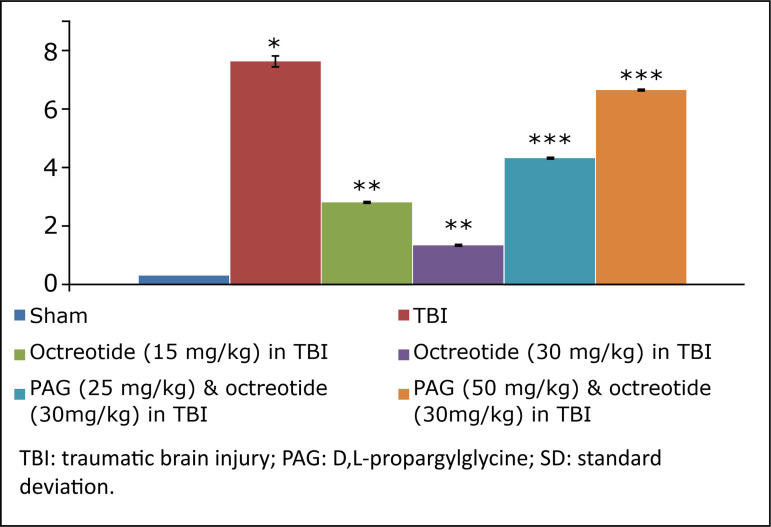
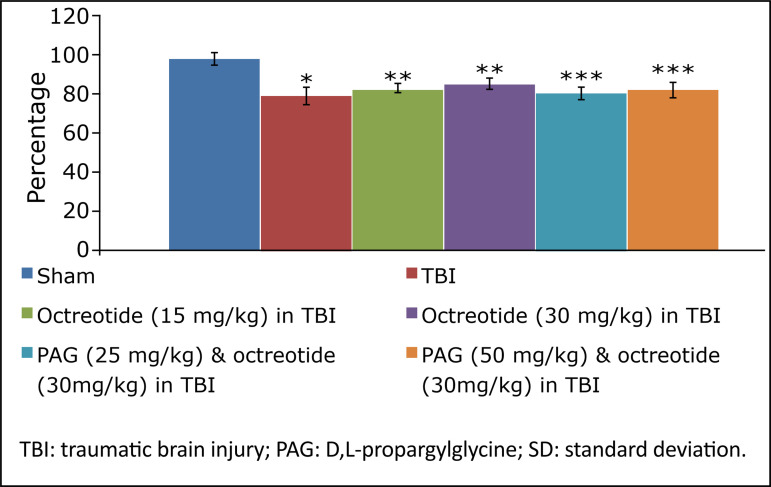
Rats were subjected to midline incision followed by TBI in the prefrontal cortex region using a stereotaxic apparatus. The TBI-subjected rats developed significant neurofunctional impairment in comparison to the sham group. There were significant behavioural alterations in terms of increase in neurological severity score (Fig. 1) assessed on the 3rd day following TBI in rats.
Figure 1. The results of neurological severity score due to TBI in different experimental groups. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
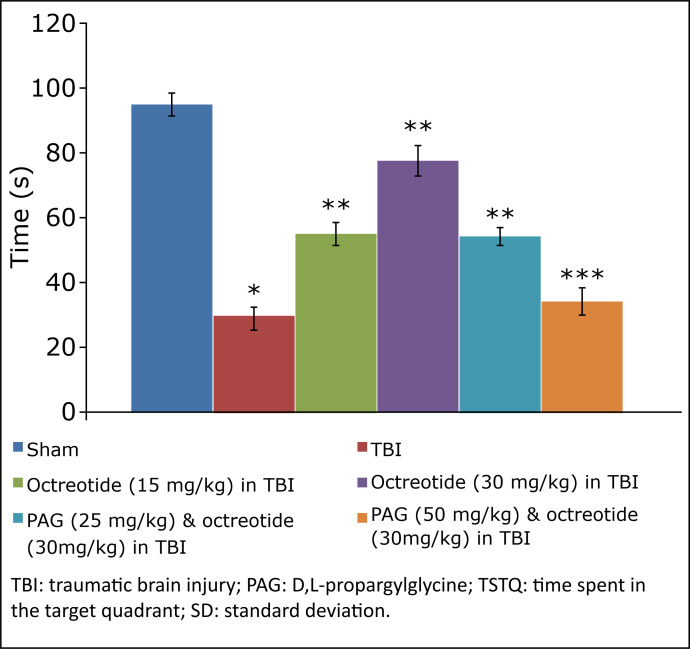
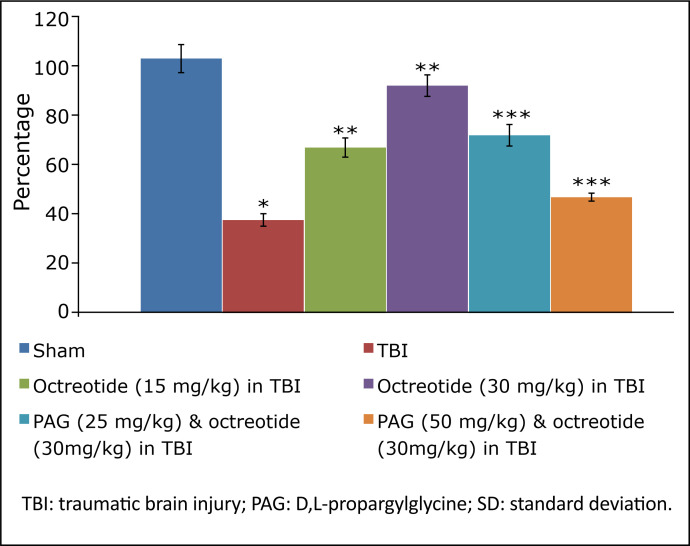
The assessment of learning and memory in terms of ELT and TSTQ in TBI-subjected animals started on the 4th day on the Morris water maze test and continued for five days (four days of acquisition and one day of retrieval). In brain injury-subjected rats, there was a deficit in the learning, as depicted by a no significant change in the ELT on the 7th day (fourth day of acquisition trial) in comparison to the 4th day (first day of trial) (Table 1). Furthermore, the parameter to assess the memory, i.e., TSTQ, was significantly lower on the 8th day in brain injury-subjected rats in comparison to the sham group, suggesting a significant impairment in memory in injury-subjected rats (Fig. 2).
Table 1. Effect of different interventions on escape latency time (ELT) on Morris water maze test. *p< 0.05 vs. day 1 ELT of sham; **p< 0.05 vs. day 4 ELT of sham; ***p< 0.05 vs. day 4 ELT of TBI; ****p< 0.05 vs. day 4 ELT of octreotide (30 mg/kg) in TBI.
| S. No | Groups | Day I ELT(s) | Day 4 ELT(s) |
|---|---|---|---|
| 1 | Sham | 97.6 ± 6.8 | 32.6 ± 3.4* |
| 2 | TBI | 106.2 ± 6.1 | 89.2 ± 5.1** |
| 3 | Octreotide (15 mg/kg) in TBI | 103.1 ± 5.8 | 65.2 ± 4.3*** |
| 4 | Octreotide (30 mg/kg) in TBI | 100.4 ± 4.0 | 42.3 ± 6.3*** |
| 5 | D,L-propargylglycine (25 mg/kg) and octreotide (30 mg/kg) in TBI | 102.8 ± 6.2 | 48.7 ± 5.1**** |
| 6 | D,L-propargylglycine (50 mg/kg) and octreotide (30 mg/kg) in TBI | 104.6 ± 3.7 | 72.2 ± 5.3**** |
TBI: traumatic brain injury.
Figure 2. Effect of different interventions on TSTQ measured on 8th day of Morris water maze test. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Effect of octreotide on the neurofunctional and cognitive deficit in injury-subjected rats
Octreotide (15 and 30 mg/kg i.p.) was administered 30 minutes before subjecting the rats to TBI, and its administration was continued for three days after TBI. Administration of octreotide significantly attenuated brain injury-induced behavioural deficits in terms of decrease in neurological severity score (Fig. 1) assessed on the 3rd day following TBI. Moreover, there was a significant decrease in ELT on the 7th day (4th day of acquisition trial) in comparison to ELT on the 4th day (1st day of trial) (Table 1), suggesting the improvement in learning. The value of TSTQ was also significantly increased on the 8th day in octreotide-treated rats (Fig. 2), suggesting the memory improvement.
Effect of octreotide on biochemical parameters in brain injury-subjected rats
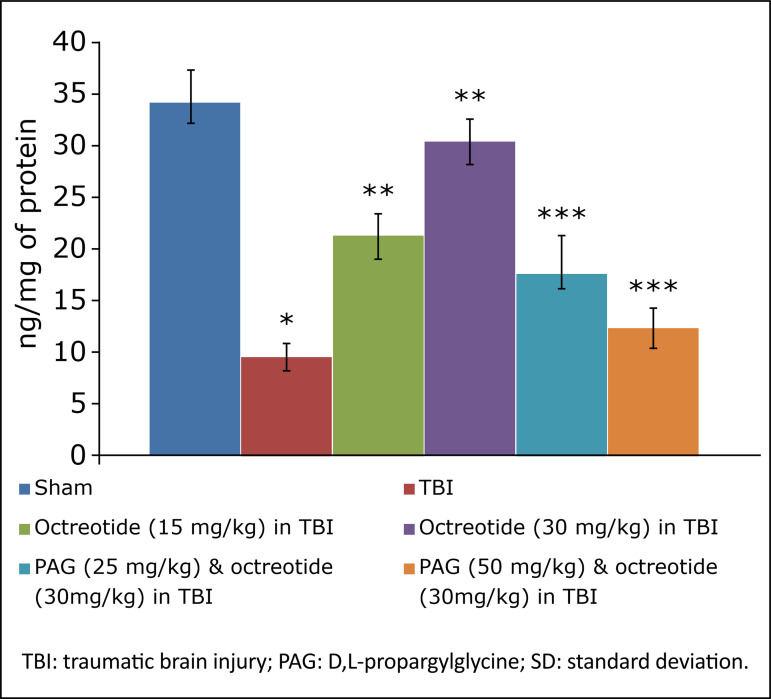
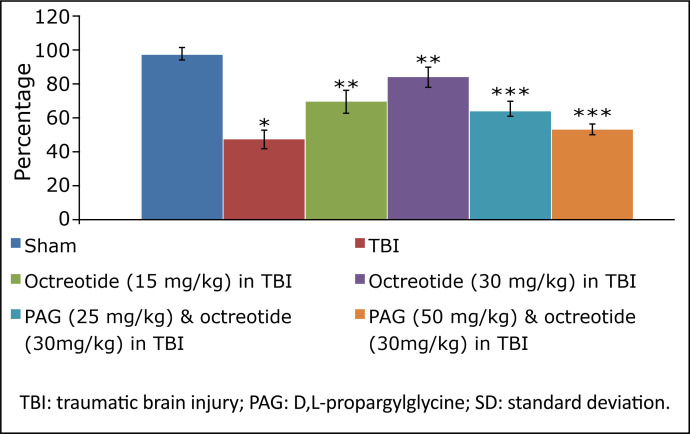
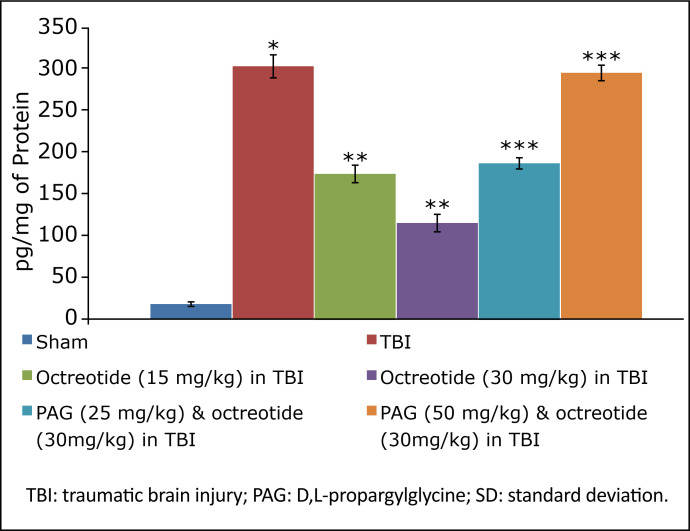
TBI led to a significant decrease in the levels of H2S (Fig. 3), CSE (Fig. 4), and with no significant change in CBS expression (Fig. 5). There were significant decrease in the Nrf2 (Fig. 6) and increase in the TNF-α levels (Fig. 7) in TBI-subjected rats. Administration of octreotide in brain injury-subjected rats exhibited significant rise in levels of H2S (Fig. 3) along with increase in the activity of CSE, an enzyme responsible for the synthesis of H2S (Fig. 4). However, administration of octreotide did not exhibit significant effect on the activity of another H2S biosynthesizing enzyme, CBS (Fig. 5). Moreover, octreotide restored the expression of Nrf2 (Fig. 6) and decreased neuroinflammation as assessed by a decrease in the TNF-α levels in TBI-subjected animals.
Figure 3. Effects of different interventions on the hydrogen sulfide levels in the brain due to TBI in different experimental groups. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Figure 4. Effects of different interventions on the cystathionine-γ-lyase in the brain due to TBI in different experimental groups. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Figure 5. Effects of different interventions on the cystathionine-β-synthase in the brain due to TBI in different experimental groups. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Figure 6. Effect of different interventions on the Nrf2 levels measured in prefrontal cortex region of brain. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Figure 7. Effect of different interventions on the TNF-α levels measured in prefrontal cortex region of brain. Values are given in mean ± SD. *P<0.05 vs. sham; **P<0.05 vs. TBI; ***P<0.05 vs. octreotide (30 mg/kg) in TBI.
Effect of D,L-propargylglycine on octreotide-induced improvement in neurofunctional deficits and biochemical changes in traumatic brain injury-subjected rats
Co-administration of CSE inhibitor, D,L-propargylglycine (25 and 50 mg/kg i.p.) significantly attenuated the octreotide-mediated decrease in the neurological severity score (Fig. 1). It also abolished octreotide-mediated improvement in learning (Table 1) and memory (Fig. 2) in the Morris water maze test. Furthermore, it also diminished the effects of octreotide on the H2S levels and CSE activity as depicted by a decrease in levels of H2S (Fig. 3) and CSE expression (Fig. 4). However, it did not modulate the activity of CBS in TBI-subjected rats (Fig. 5), but extinguished the octreotide-induced increase in the expression of Nrf2 (Fig. 6) and decrease in the TNF-α levels (Fig. 7) in response to TBI.
Discussion
Trauma is one of the common causes of brain injury26, and there have been different models employed by scientists to identify the pharmacological agents to mitigate the deleterious effects associated with the TBI27 , 28. In this study, traumatic injury to the brain led to significant neurofunctional impairment. There was a significant rise in the neurological severity score, assessed three days after the injury. Moreover, there was a significant impairment in the learning (increase in ELT) and memory (decrease in TSTQ) in TBI-subjected rats. Previous studies have been shown that there is impairment in behavioural functions in animals subjected to TBI29 - 31.
In this study, treatment with octreotide ameliorated TBI-induced increase in neurological severity score and decrease in cognitive functions. There was a significant decrease in the day 4 ELT and day 5 TSTQ in octreotide-treated rats. Octreotide is a somatostatin analogue, and, apart from its hormonal functions, studies have shown its widespread potential in ameliorating the pathophysiological state of different diseases32, including neuroendocrine gastrointestinal tumors33 and pancreatic tumors34, cardiovascular disease35 , 36 and Crohn’s disease37. However, to the best of our knowledge, it is the first study showing the potential of octreotide in improving neurofunctional aspects of TBI-subjected rats.
In the present investigation, treatment with octreotide also ameliorated TBI-induced biochemical alterations. In TBI-subjected rats, there was a significant decline in the expression of H2S along with the CSE activity, one of the enzymes involved in H2S synthesis. Interestingly, there was no significant change in the activity of another H2S synthesis, i.e., CBS. Accordingly, TBI may alter the activity of CSE, which might lead to a decrease in the H2S levels in the brain. Earlier studies have shown that the decrease in the H2S levels may be deleterious to the brain38 , 39. However, treatment with octreotide restored the CSE activity and increased the H2S levels, suggesting that octreotide-mediated increase in the H2S levels may contribute to improve neurofunctional aspects in TBI-subjected rats. The role of H2S in octreotide-mediated beneficial effects was further supported as the administration of D,L-propargylglycine(CSE inhibitor) abolished the effects of octreotide. Along with it, there was also a decrease in the H2S levels and CSE activity in D, L-propargylglycine administered rats.
In this study, treatment with octreotide also abolished TBI-induced increase in neuroinflammatory marker (TNF-α levels) and Nrf2 levels. It suggests that octreotide-mediated decrease in neuroinflammation and increase in endogenous antioxidant actions may contribute to improve neuronal functions in TBI-subjected rats. Moreover, D,L-propargylglycineattenuated the effects of octreotide on neuroinflammation and Nrf2. It suggests that octreotide may regulate the expression of CSE, which may increase the H2S levels to decrease neuroinflammation and increase endogenous antioxidant actions to confer neurofunctional protection against TBI in rats.
Conclusion
It is concluded that octreotide improves TBI and neurological function in the brain, possibly through up-regulation of H2S, Nrf2, and CSE in the prefrontal cortex and down-regulation TNF-α level and neuroinflammation.
Acknowledgments
Not applicable.
Footnotes
Funding: Not applicable.
Data availability statement: Data will be available upon request.
Research performed at Department of Neurosurgery, Taiyuan Iron and Steel (Group) Co., Ltd. General Hospital, Taiyuan,and Department of Neurosurgery, Hospital of Lianqin Security Force 940th, Lanzhou, China..
References
- 1.Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi: 10.1016/j.mcna.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–1130. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamberts SWJ, Hofland LJ. Anniversary review: octreotide, 40 years later. Eur J Endocrinol. 2019;181(5):R173–R183. doi: 10.1530/EJE-19-0074. [DOI] [PubMed] [Google Scholar]
- 4.Panagopoulos GN, Deftereos SN, Tagaris GA, Gryllia M, Kounadi T, Karamani O, Panagiotidis D, Koutiola-Pappa E, Karageorgiou CE, Piadites G. Octreotide: a therapeutic option for idiopathic intracranial hypertension. Neurol Neurophysiol Neurosci. 2007;10:1–1. [PubMed] [Google Scholar]
- 5.Qu CL, Dang YH, Tang JS. Administration of somatostatin analog octreotide in the ventrolateral orbital cortex produces sex-related antinociceptive effects on acute and formalin-induced nociceptive behavior in rats. Neurochem Int. 2015;87:77–84. doi: 10.1016/j.neuint.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Kozhemyakin M, Rajasekaran K, Todorovic MS, Kowalski SL, Balint C, Kapur J. Somatostatin type-2 receptor activation inhibits glutamate release and prevents status epilepticus. Neurobiol Dis. 2013;54:94–104. doi: 10.1016/j.nbd.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Wang L, Zhang X, Cui L, Xing Y, Dong L, Liu Z, Li Y, Zhang X, Wang C, Bai X, Zhang J, Zhang L, Zhao X. The protection by octreotide against experimental ischemic stroke: up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80–87. doi: 10.1016/j.brainres.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 8.Jingmin O, Xiping Z, Chun W, Ping Y, Qian Y. Study of dexamethasone, baicalin and octreotide on brain injury of rats with severe acute pancreatitis. Inflamm Res. 2012;61(3):265–275. doi: 10.1007/s00011-011-0408-4. [DOI] [PubMed] [Google Scholar]
- 9.Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, Greig NH, Wang JY. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016;272:38–49. doi: 10.1016/j.jneumeth.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer PM. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med (Berl) 2019;97(12):1627–1641. doi: 10.1007/s00109-019-01851-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Jiang C, Zhang K, Lan X, Chen X, Zang W, Wang Z, Guan F, Zhu C, Yang X, Lu H, Wang J. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic Biol Med. 2019;131:345–355. doi: 10.1016/j.freeradbiomed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Sarna LK, Siow YL, O K. The CBS/CSE system: a potential therapeutic target in NAFLD? Can J Physiol Pharmacol. 2015;93(1):1–11. doi: 10.1139/cjpp-2014-0394. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Sandhir R. Hydrogen sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord Drug Targets. 2018;17(9):654–670. doi: 10.2174/1871527317666180605072018. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang S, Shan H, Zhang M. Biologic effect of hydrogen sulfide and its role in traumatic brain injury. Oxid Med Cell Longev. 2020;2020:7301615–7301615. doi: 10.1155/2020/7301615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Shi R, She X, Gu C, Chong L, Zhang L, Li R. Mineralocorticoid receptor antagonist-mediated cognitive improvement in a mouse model of Alzheimer’s type: possible involvement of BDNF-H2 S-Nrf2 signaling. Fundam Clin Pharmacol. 2020;34(6):697–707. doi: 10.1111/fcp.12576. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese V, Scuto M, Salinaro AT, Dionisio G, Modafferi S, Ontario ML, Greco V, Sciuto S, Schmitt CP, Calabrese EJ, Peters V. Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis. Antioxidants (Basel) 2020;9(12):1303–1303. doi: 10.3390/antiox9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasari A, Phan A, Gupta S, Rashid A, Yeung SC, Hess K, Chen H, Tarco E, Chen H, Wei C, Anh-Do K, Halperin D, Meric-Bernstam F, Yao J. Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors. Endocr Relat Cancer. 2015;22(3):431–441. doi: 10.1530/ERC-15-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Shorbagy MY, Nassar NN. Octreotide ameliorates inflammation and apoptosis in acute and kindled murine PTZ paradigms. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(1):61–68. doi: 10.1007/s00210-016-1303-x. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y, Zeng H, Gao L, Gu T, Wang C, Zhang H. Hydrogen sulfide attenuates atherosclerosis in a partially ligated carotid artery mouse model via regulating angiotensin converting enzyme 2 expression. Front Physiol. 2017;8:782–782. doi: 10.3389/fphys.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Tapia RJ, Estrada-Rojo F, Lopez-Aceves TG, Rodríguez-Mata V, Perez-Torres A, Barajas-Martinez A, Garcia-Velasco S, Ugalde-Muñiz P, Navarro L. Diurnal variation induces neurobehavioral and neuropathological differences in a rat model of traumatic brain injury. Front Neurosci. 2020;14:564992–564992. doi: 10.3389/fnins.2020.564992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez R, Santiago-Mejia J, Gomez C, San-Juan ER. A simplified procedure for the quantitative measurement of neurological deficits after forebrain ischemia in mice. J Neurosci Methods. 2005;147(1):22–28. doi: 10.1016/j.jneumeth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ya BL, Li HF, Wang HY, Wu F, Xin Q, Cheng HJ, Li WJ, Lin N, Ba ZH, Zhang RJ, Liu Q, Li YN, Bai B, Ge F. 5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia. Cell Stress Chaperones. 2017;22(1):55–65. doi: 10.1007/s12192-016-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen X, Kolluru GK, Yuan S, Kevil CG. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015;554:31–45. doi: 10.1016/bs.mie.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin North Am. 2017;97(5):1015–1030. doi: 10.1016/j.suc.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, Greig NH, Wang JY. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016;272:38–49. doi: 10.1016/j.jneumeth.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai J-X, Ma Y-B, Le N-Y, Cao J, Wang Y. Large animal models of traumatic brain injury. Int J Neurosci. 2018;128(3):243–254. doi: 10.1080/00207454.2017.1380008. [DOI] [PubMed] [Google Scholar]
- 29.Broussard JI, Acion L, De Jesús-Cortés, Yin T, Britt JK, Salas R, Costa-Mattioli M, Robertson C, Pieper AA, Arciniegas DB, Jorge R. Repeated mild traumatic brain injury produces neuroinflammation, anxiety-like behaviour and impaired spatial memory in mice. Brain Inj. 2018;32(1):113–122. doi: 10.1080/02699052.2017.1380228. [DOI] [PubMed] [Google Scholar]
- 30.Marschner L, Schreurs A, Lechat B, Mogensen J, Roebroek A, Ahmed T, Balschun D. Single mild traumatic brain injury results in transiently impaired spatial long-term memory and altered search strategies. Behav Brain Res. 2019;365:222–230. doi: 10.1016/j.bbr.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, He D, Zhao Q. Licoricidin improves neurological dysfunction after traumatic brain injury in mice via regulating FoxO3/Wnt/β-catenin pathway. J Nat Med. 2020;74(4):767–776. doi: 10.1007/s11418-020-01434-5. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Coy DH. Somatostatin and its analogs. Curr Drug Targets. 2016;17(5):529–537. doi: 10.2174/1389450116666141205163548. [DOI] [PubMed] [Google Scholar]
- 33.Oberg K. Neuroendocrine gastrointestinal tumors-a condensed overview of diagnosis and treatment. Ann Oncol. 1999;10(Suppl 2):S3–S8. doi: 10.1093/annonc/10.suppl_2.s3. [DOI] [PubMed] [Google Scholar]
- 34.Butturini G, Bettini R, Missiaglia E, Mantovani W, Dalai I, Capelli P, Ferdeghini M, Pederzoli P, Scarpa A, Falconi M. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13(4):1213–1221. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 35.Hekimsoy Z, Ozmen B, Ulusoy S. Homocysteine levels in acromegaly patients. Neuro Endocrinol Lett. 2005;26(6):811–814. [PubMed] [Google Scholar]
- 36.van Thiel, Bax JJ, Biermasz NR, Holman ER, Poldermans D, Roelfsema F, Lamb HJ, van der, Smit JW, Romijn JA, Pereira AM. Persistent diastolic dysfunction despite successful long-term octreotide treatment in acromegaly. Eur J Endocrinol. 2005;153(2):231–238. doi: 10.1530/eje.1.01955. [DOI] [PubMed] [Google Scholar]
- 37.Martelli L, Colard A, Fontaine F, Deflandre J, Bastens B, Louis E. Evaluation of the efficacy of octreotide LAR in the treatment of Crohn’s disease associated refractory diarrhea. Scand J Gastroenterol. 2017;52(5):564–569. doi: 10.1080/00365521.2017.1284893. [DOI] [PubMed] [Google Scholar]
- 38.Karimi SA, Hosseinmardi N, Janahmadi M, Sayyah M, Hajisoltani R. The protective effect of hydrogen sulfide (H2S) on traumatic brain injury (TBI) induced memory deficits in rats. Brain Res Bull. 2017;134:177–182. doi: 10.1016/j.brainresbull.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015:186908–186908. doi: 10.1155/2015/186908. [DOI] [PMC free article] [PubMed] [Google Scholar]