Abstract
Attitudes toward deprescribing among hospitalized older patients transitioning to post-acute care in the United States are less known. This study describes older patients’ and their surrogate’s attitudes using all items of the Patient Attitudes Toward Deprescribing (PATD) questionnaire and compares perceived pill burden to the actual count of total daily pills and potentially inappropriate medications (PIMs). Overall, 93% of participants were willing to deprescribe if their physician agreed. Compared to patients, surrogates had 64% reduced odds (95%CI 0.18 to 0.74) of believing that all of the care recipient’s medications were necessary and 61% reduced odds (95% CI 0.17 to 0.88) of attributing cost as a factor in deprescribing. Perceptions of medication burden were associated with patients’ total daily pills (median 16) and PIMS (median 7), yet 61% agreed that all their medicines were necessary. Patients and surrogates typically express a willingness to deprescribe but have differing perceptions of medication appropriateness.
Keywords: polypharmacy, care transitions, assessment, medication burden
Background and Objectives
Polypharmacy, typically defined as five or more routine medications, is common among older adults, especially those with multiple comorbid conditions. Recent studies have shown that the majority of hospitalized older patients discharged to post-acute care exhibit hyper-polypharmacy, defined as taking 10 or more medications (Gamble et al., 2014; Runganga et al., 2014; Saraf et al., 2016; Gnjidic et al., 2012). Polypharmacy is associated with poor medication adherence, adverse drug events, and more frequent hospitalizations (Hilmer & Gnjidic, 2009; Morandi et al., 2013; Poudel et al., 2016). Numerous studies have demonstrated that a substantial proportion of older patients are prescribed one or more medications that are potentially inappropriate (with potential to cause harm) and/or no longer clinically necessary (Lund et al., 2015; Saraf et al., 2016). Recent systematic reviews have generally concluded that interventions to stop or reduce unnecessary medications can be effective and safe; moreover, patient-centered approaches that directly involve the patient (and/or their surrogate) in the decision process and interventions led by a trained pharmacist yield stronger evidence (Thillainadesan et al., 2018; Tjia et al., 2013).
Given the prevalence of polypharmacy in older hospitalized patients, understanding which factors influence patients’ decisions related to medications could inform how to effectively implement deprescribing interventions in clinical practice. Only a few standardized tools have been developed to measure patients’ willingness to deprescribe. Although no gold standard exists, among the earliest and most commonly applied tools is the Patient Attitudes Towards Deprescribing (PATD) questionnaire (Reeve et al., 2019; Reeve et al., 2013). The original tool includes 15 questions that assess patient attitudes toward medication necessity, comfort with use, burden, knowledge, cost, and willingness to deprescribe. Subsequently, a revised, longer version of the PATD (rPATD) was developed to include surrogate (caregiver) specific language (Reeve et al., 2016).
Most studies that assess patients’ attitudes toward deprescribing using the PATD have been conducted outside the U.S. with predominately outpatient populations. The results of these studies indicate that the majority of older patients experiencing polypharmacy and/or medication side effects express a willingness to deprescribe (Sirois et al., 2017; Turner & Tannenbaum, 2017) and report a high level of trust in their provider to make deprescribing decisions (Schiotz et al., 2018). A recent population-based survey of Medicare beneficiaries in the U.S. found that 92% of patients reported a willingness to stop taking one or more medications if their physician said it was possible; and approximately 67% reported a desire to reduce the number of medicines they were taking (Reeve et al., 2018). This is one of the few studies in the U.S. that has utilized the PATD; however, it only included two questions from the 15-question inventory and patient self-reported medications.
Several gaps in our current knowledge need to be addressed to effectively implement patient-centered deprescribing interventions for older patients. First, more research is needed to better characterize patients’ willingness to deprescribe, particularly in the U.S. Additionally, few studies have examined attitudes toward deprescribing among hospitalized and skilled nursing facility patients, both of whom experience high medication burden and frequent medication changes (Saraf et al., 2016; Stevenson et al., 2014). Moreover, given that many older hospitalized adults have a family member or other caregivers involved in their medical decisions, it’s equally important to understand surrogate attitudes and beliefs toward medications and willingness to deprescribe. Lastly, given that prior studies have indicated pharmacist-led interventions are efficacious, it is necessary to understand patients’ and surrogates’ comfort level with pharmacists initiating deprescribing (Ammerman et al., 2019; Tasai et al., 2019).
The primary aim of this study is to describe and compare patients’ or surrogate decision-makers’ attitudes toward deprescribing as measured by all 15 PATD items during their hospital stay prior to a planned transition to a post-acute care facility (PAC). This study also assessed the correlation between self-reported attitudes about medication burden and actual medication regimens. Finally, this study describes patients’ and surrogates’ comfort level with pharmacists initiating deprescribing as well preferred methods of follow-up.
Research Design and Methods
Study Setting, Participants, and Eligibility Criteria
This study was conducted as part of a larger randomized clinical intervention trial (Shed-MEDS) to reduce polypharmacy among hospitalized older patients discharged to a PAC facility. The patient-centered deprescribing protocol was conducted by nurse practitioners and pharmacists during the hospital and PAC admissions, and patients were followed for 90 days after their PAC discharge. All study measures and the intervention protocol for the larger trial have been described elsewhere (Vasilevskis et al., 2019).
Inclusion criteria for the larger, parent trial required patients to be aged 50 or older, hospitalized at an academic-affiliated hospital (Vanderbilt University Medical Center, VUMC) and referred to PAC at one of 21 regional skilled nursing facilities (SNFs) or two inpatient rehabilitation facilities (IPRs). Additionally, patients had to have: five or more medications on their pre-hospital admission medication list and a home residence in one of nine counties surrounding the medical center to facilitate a home visit during study follow-up. Full inclusion and exclusion criteria are described in the published study protocol (Vasilevskis et al., 2019). Lastly, patients must be able to speak English and have the capacity to provide self-consent or have a surrogate (i.e., family member or friend) willing to consent on their behalf. There were no additional inclusion or exclusion criteria for the purposes of this study beyond having completed baseline assessments for the parent trial. Study enrollment and data collection (including patient assessments) were completed in-person by trained research personnel typically 48-72 hours before hospital discharge. Informed written consent procedures for patients (or their respective surrogate) were approved by the medical center’s institutional review board.
Demographic, Living Situation and Health Status Measures
Research personnel used a standardized form to abstract information from patients’ electronic medical records to include demographics (age, gender, race/ethnicity), insurance status, education, literacy, living situation prior to admission, diagnoses, and medications. As part of routine medical center practice, hospital nurses administer the Brief Health Literacy Screen (BHLS) upon admission (Wallston et al., 2014). The BHLS is comprised of three standardized questions and has a score range from 3 to 15, with a lower score indicative of lower subjective health literacy. These medical record data were reviewed and verified with the patient and/or surrogate during an interview at the hospital bedside, along with education level and location prior to hospitalization (i.e., home alone or with family, assisted living facility [ALF], SNF or different acute care hospital).
International Classification of Diseases (ICD) 9 and 10 diagnostic criteria from the last 12 months were used to calculate the Charlson comorbidity score for each participant (Charlson et al., 1987). Cognitive impairment was assessed by research personnel with the Brief Interview for Mental Status (BIMS), which has a total score range from 0 to 15, with scores below 13 indicative of impairment (Saliba et al., 2012). Research personnel also administered the Vulnerable Elders Survey (VES-13), which is a screening tool to assess functional status based on self-reported ability to perform activities of daily living. VES-13 scores range from 1 to 10, with a lower score indicative of better health (Saliba et al., 2001).
Medication Measures
Multiple data sources were used to compile a comprehensive list of all pre-hospital medications at study enrollment. Specifically, each participant’s enrollment medication list included all pre-hospital medications documented in the hospital medical record and any additional medications identified via patient/surrogate interview and/or pharmacy records (refill history). All prescribed (scheduled and as needed) and over-the-counter medications (including vitamins and herbal supplements) administered by any route other than topical were included in the patient’s baseline medication list.
Based on the comprehensive list of all pre-hospital medications, a Drug Burden Index (DBI) score was calculated per participant for anticholinergic and sedative medications (Hilmer et al., 2007). The DBI is the sum of each individual anticholinergic/sedative medication’s daily dose divided by the sum of the minimum effective dose (as estimated by the FDA minimum recommended dose) and the patient’s daily dose. Additionally, each medication was also identified as a potentially inappropriate medication (PIM) per the Beers’ Criteria (By the American Geriatrics Society Beers Criteria Update Expert, 2015). Each participant’s comprehensive pre-hospital medication list was also used to calculate the total number of capsules and/or tablets taken daily. This ‘total pill count’ measure was based only on scheduled medications; thus, medications taken as-needed were excluded from this count. Additionally, non-oral medications were excluded from the daily pill count because the PATD questions to which this count was compared (items 12 and 13) specifically refer to “capsules/ tablets per day” both verbally and visually. Thus, the ‘total pill count’ was calculated to reflect the total number of capsules/ tablets participants must consume on a daily basis as opposed to the number of different medications they might be taking (e.g., a medication taken twice per day yields a pill count of two).
Research personnel also asked the patient or surrogate standardized questions about the need for assistance with medication management (i.e., “Does anyone help you with your medicines?”). Assistance with medications could include transportation to the pharmacy, picking up medicines, reminders to take medicines, and/or organizing medicines (e.g., pill box). Participants who resided in an assisted-living facility (ALF) wherein a staff member provided medication management (delivery and/or reminders) were considered as receiving help. Additionally, research personnel asked about difficulty paying for medications (i.e., “How difficult is it for you/your family to pay for your medicines on a monthly basis?”). Response options related to difficulty paying range from “very difficult” to “not at all difficult”.
Patient and Surrogate Attitudes Toward Deprescribing
The Patients’ Attitudes Toward Deprescribing (PATD) is a structured tool that can be self-administered or administered via interview to either the patient or their surrogate. The PATD was administered via in-person interview by research personnel during the patient’s hospital stay for the purposes of this study. The development and validation of the tool has been described previously (Reeve et al., 2013). Following the initiation of this study, a revised longer version of the PATD was developed for both patients and surrogates (rPATD), with a total of 22 and 19 items respectively (Reeve et al., 2016). However, the original (15-item) version had already been approved by both the IRB and Data Safety and Monitoring Board for the parent trial prior to the rPATD publication (Vasilevskis et al., 2019).
The original PATD has a total of 15 items. The first ten items are statements with a 5-point Likert scale response option from “strongly agree” to “strongly disagree” shown in Table 2. The question format was modified by the study team for surrogates to refer to their care recipient (e.g., “I am comfortable with the number of medicines my care recipient is taking.”). Two questions are related to the patient/surrogate perception of their total number of medications: “How many different tablets/capsules per day would you consider to be a lot?” and “What is the maximum number of tablets/capsules that you would be comfortable taking per day?”. The first of these questions has response options provided in ranges (i.e., 5-10, 10-15, 15-20, 20-25, and >25), whereas, the second question displays six different pictures, each depicting a different number of tablets/capsules as response options (i.e., 4, 8, 12, 16, 20, and 24).
Table 2.
Patient and Surrogate Responses to First 10 PATD Questions (N=337)
| Interview Question | Overall Responses (N=337) | Patient Responses (N=285) | Surrogate Responses (N=52) | Significance |
|---|---|---|---|---|
| N (Percent) | N (Percent) | N (Percent) | p-values | |
| 1. I feel that I am taking a large number of medicines | 238 (70.8) | 207 (72.9) | 31 (59.6) | 0.067 |
| 2. I am comfortable with the number of medicines that I am taking | 194 (57.6) | 167 (58.6) | 27 (51.9) | 0.446 |
| 3. I believe that all my medicines are necessary | 207 (61.4) | 182 (63.9) | 25 (48.1) | 0.043 |
| 4. If my doctor said it was possible I would be willing to stop one or more of my regular medicines | 313 (92.9) | 265 (93.0) | 48 (92.3) | 0.774 |
| 5. I would like to reduce the number of medicines that I am taking | 261 (77.4) | 228 (80.0) | 33 (63.5) | 0.012 |
| 6. I feel that I may be taking one or more medicines that I no longer need | 111 (32.9) | 95 (33.3) | 16 (30.8) | 0.751 |
| 7. I would accept taking more medicines for my health conditions | 263 (78.0) | 222 (77.9) | 41 (78.8) | 1.000 |
| 8. I have a good understanding of the reasons I was prescribed each of my medicines | 277 (82.2) | 237 (83.2) | 40 (76.9) | 0.323 |
| 9. Having to pay less would play a role in my willingness to stop one or more of my medicines | 120 (35.7) | 110 (38.7) | 10 (19.2) | 0.007 |
| 10. I believe one or more of my medicines is giving me side effects | 128 (38.0) | 110 (38.6) | 18 (34.6) | 0.643 |
N (Percent) with response options “strongly agree” or “agree”.
The remaining three questions are related to the patient’s history of stopping medicines, comfort level with a pharmacist facilitated process of stopping one or more medicines, and finally, their preferred method of follow-up if a medication was stopped.
Data Analyses
All data were managed and analyzed using the Research Electronic Data Capture (REDCap) platform (Harris et al., 2009), Microsoft Excel, IBM® SPSS (Version 25), and R (http://www.r-project.org). All patient characteristics and PATD responses were compared between the patient and surrogate report groups using independent samples, two-sided Wilcoxon rank sum test for continuous variables and Fisher exact test for categorical variables. A p-value of 0.05 was considered evidence of statistical significance. For groupwise comparisons of the first ten PATD questions, responses were collapsed into two categories as follows: “strongly agree” and “agree” were combined into one category; while, “unsure”, “strongly disagree” and “disagree” were combined into a second category. Groupwise comparisons for the remaining PATD questions are also reported.
Responses to PATD questions 1, 12, and 13 were compared with objective daily pill count using side-by-side boxplots, stratified by patient/surrogate respondent. Linear regression was used to quantify the statistical significance of the association between the objective daily pill count (dependent variable) and PATD responses, and whether this association was modified by type of respondent (patient versus surrogate) to indicate an interaction.
For any items where a significant difference was observed in unadjusted comparisons between patient and surrogate responses, logistic regression was used to examine the adjusted association between respondent (patient versus surrogate) and grouped PATD responses, accounting for potential confounders including patient age, insurance status, assisted living facility (ALF) residence, dementia diagnosis and total number of pre-hospital medications. The results of these analyses are presented using odds ratios with 95% confidence intervals (CI).
Results
Participants and Setting
During 39 months of study enrollment, research personnel screened 8,350 hospitalized patients for eligibility, and 1,212 met all study inclusion criterion. Of those 1,212 patients who met criteria, research personnel approached 1,150 (95%) patients and received written consent for 349 (30.4%) patients. Of consented participants, 96% (N=337) completed the PATD during their hospital stay, with 285 completed by the patient and 52 completed by a surrogate. The remaining 12 cases had incomplete PATD data— either the patient was unwilling or unable to respond to the questions with no surrogate available (N=8) or the interview was incomplete (N=4)— and thus, were excluded from analyses.
The characteristics of study participants are shown in Table 1 across all participants (N=337) and by respondent groups. Overall, the median age was 76.4 (IQR: 69.3, 85.6), and the majority were female (63%) and White (85%). Almost 85% had Medicare, and the majority (82%) lived at home (alone or with family) prior to hospitalization. Approximately 44% had a high school education or less, and the median BHLS score was 12 (IQR: 9, 15), suggesting moderate to high subjective health literacy. The median BIMS score also was high (14, IQR: 13, 15), with 14.5% having a dementia diagnosis. The median VES-13 total score was 7 (IQR: 3, 7), suggesting moderate impairment in their self-reported functional health status (not shown in Table 1; patient respondent group only). Based on their diagnoses, participants had a median comorbidity score of 6 (IQR: 5, 9), which is indicative of multiple chronic health conditions.
Table 1.
Characteristics of Participants (N=337)
| Variable | Overall | Patient Responses | Surrogate Responses | P-value |
|---|---|---|---|---|
| N | 337 | 285 | 52 | |
| Demographics and Living Situation | ||||
| Age (median [IQR]) | 76.4 [69.3, 84.6] | 75.3 [67.3, 82.7] | 83.2 [77.0, 89.0] | <0.001 |
| Percent Female | 211 (62.6) | 178 (62.5) | 33 (63.5) | 1.000 |
| Percent White | 285 (84.6) | 235 (82.5) | 50 (96.2) | 0.011 |
| Percent Medicare | 287 (85.2) | 237 (83.2) | 50 (96.2) | 0.011 |
| Living Situation Prior to Hospitalization (%) | <0.001 | |||
| Home with family/ friend | 179 (53.1) | 148 (51.9) | 31 (59.6) | |
| Home alone | 98 (29.1) | 93 (32.6) | 5 (9.6) | |
| SNF or Other Hospital | 19 (5.6) | 16 (5.6) | 3 (5.8) | |
| Assisted Living Facility | 41 (12.2) | 28 (9.8) | 13 (25.0) | |
| Percent High School or Less | 148 (43.9) | 123 (43.2) | 25 (48.1) | 0.545 |
| Brief Health Literacy Screen Score (median [IQR]) (3-15) | 12.0 [9.0, 15.0] | 12.5 [9.0, 15.0] | 7.0 [5.0, 12.5] | <0.001 |
| Health Status | ||||
| Cognitive Status, BIMS (median [IQR]) (0-15) | 14.0 [13.0, 15.0] | 14.0 [13.0, 15.0] | 9.00 [6.0, 12.0] | <0.001 |
| Percent Dementia diagnosis | 49 (14.5) | 24 (8.4) | 25 (48.1) | <0.001 |
| Charlson Score (median [IQR]) | 6.0 [5.0, 9.0] | 6.0 [5.0, 9.0] | 6.5 [5.0, 8.3] | 0.902 |
| Medications | ||||
| Count of Pre-hospital Medicines (median [IQR]) | 16.0 [12.0, 20.0] | 17.0 [13.0, 21.0] | 12.0 [9.8, 15.3] | <0.001 |
| Proportion with hyper-polypharmacy (10 or more) | 297 (88.1) | 258 (90.5) | 39 (75.0) | 0.004 |
| Count of PIMS (Beers) (median [IQR]) | 7.0 [5.0, 9.0] | 7.0 [5.0, 9.0] | 4.5 [4.0, 7.0] | <0.001 |
| Prehospital DBI (median [IQR]) | 3.1 [1.8, 4.4] | 3.2 [1.9, 4.7] | 2.3 [1.5, 3.1] | <0.001 |
| Help with medications (%) | 219 (65.0) | 170 (59.6) | 49 (94.2) | <0.001 |
| Difficulty paying for medications (%) | 76 (22.9) | 71 (25.2) | 5 (10.0) | 0.017 |
| Count of scheduled pills/day (median [IQR]) | 17 [12, 22] | 17 [13, 23] | 13 [9, 17] | <0.001 |
Help with medication management includes those who receive staff assistance in an ALF care setting prior to hospitalization.
Difficulty Paying: Proportion reflects responses of “very difficult” or “somewhat difficult”
The median total number of pre-hospital medications was 16 (IQR: 12, 20) per participant, and 88.1% met criteria for hyper-polypharmacy. Of the total number of medications, a median of 7 (IQR: 5, 9) per participant were categorized as PIMs with all participants (100%) taking at least one PIM. Participants’ median DBI was 3.05 (IQR: 1.84, 4.44), indicating a high anticholinergic and sedative drug burden. Most participants (65%) received help with medication management, and approximately 23% reported difficulty paying for their medications (i.e., responses of “somewhat difficult” or “very difficult”). The median scheduled daily pill count (i.e., number of tablets/capsules taken daily) was 17 (IQR: 12, 22).
Comparisons between the patient and surrogate report groups revealed multiple significant differences for the characteristics shown in Table 1. Specifically, patients for whom a surrogate completed the PATD on their behalf were older (P<0.001), with a higher proportion White (P=0.011) and with Medicare insurance (P=0.011). They also had a lower health literacy (P<0.001) and a greater proportion resided in an assisted living facility (P<0.001). Consistent with these findings, patients in the surrogate-report group also had a lower BIMS total score (P<0.001) and higher proportion with a dementia diagnosis (P<0.001) indicating more cognitive impairment in this group. A significantly greater proportion of this group also received help with medication management (P <0.001), but fewer reported having difficulty paying for medications (P = 0.017). Lastly, patients in the surrogate-report group had significantly fewer pre-hospital medications (median 12 versus 17; P<0.001) and PIMs (median 4.5 versus 7; P<0.001) along with a significantly lower (median 2.33 versus 3.23; P<0.001) DBI. Similarly, the surrogate-report group had a significantly lower number of scheduled daily pills (median 13 versus 17; P<0.001). All other characteristics shown in Table 1 were comparable between the two groups.
Patient and Surrogate Reported PATD Responses
Table 2 shows both the patient and surrogate responses of “strongly agree” or “agree” to each of the first ten PATD questions. Overall, the majority of patients and surrogates (71%) reported that they (or their care recipients) were taking a large number of medicines, which is consistent with the finding that 88.1% of this study sample met criteria for hyper-polypharmacy. In addition, respondents who reported taking a large number of medicines were, in fact, taking significantly more pre-hospital scheduled daily pills (Figure 1a, P<0.001), and this association did not differ for patients versus surrogates (P=0.979). Despite the large number of medicines per patient, more than half of respondents (58%) still reported being comfortable with the number of medicines they were taking. Additionally, 61% of respondents believed that all their medicines were necessary, with a lower proportion among surrogates (48% versus 64%, unadjusted P=0.043). After adjustment for potential confounders including age, ALF residence, total number of pre-hospital medications, Medicare insurance status, and dementia, surrogates remained significantly less likely to agree that all medicines were necessary (adjusted OR 0.36: 95% CI 0.18 to 0.74, P=0.005, Table 4).
Figures 1.
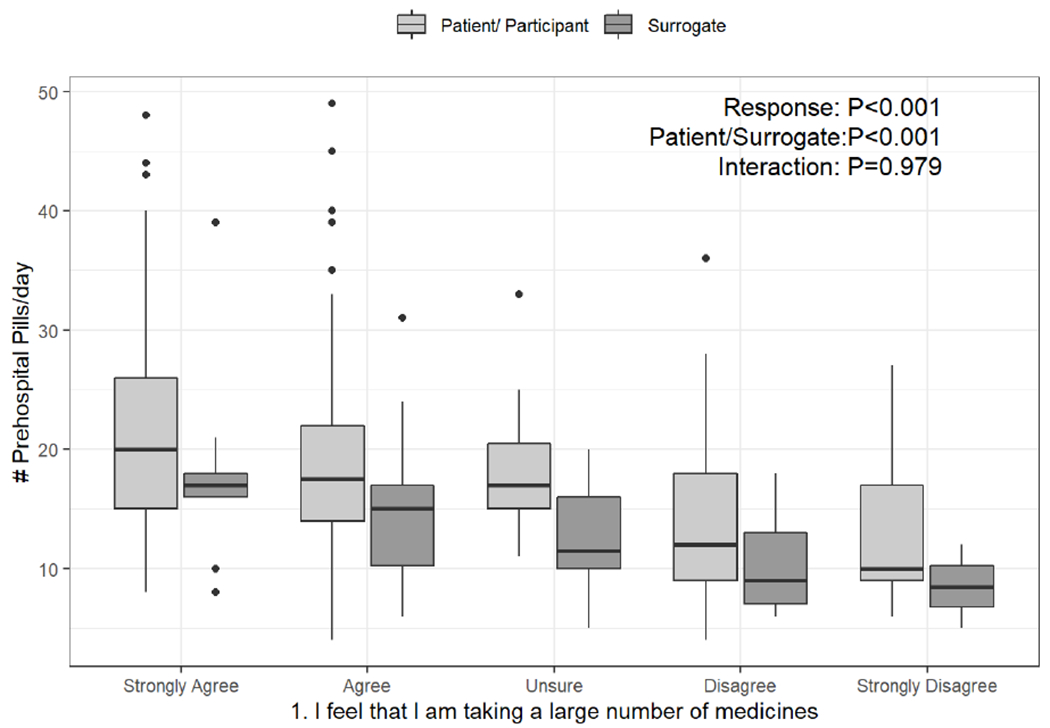
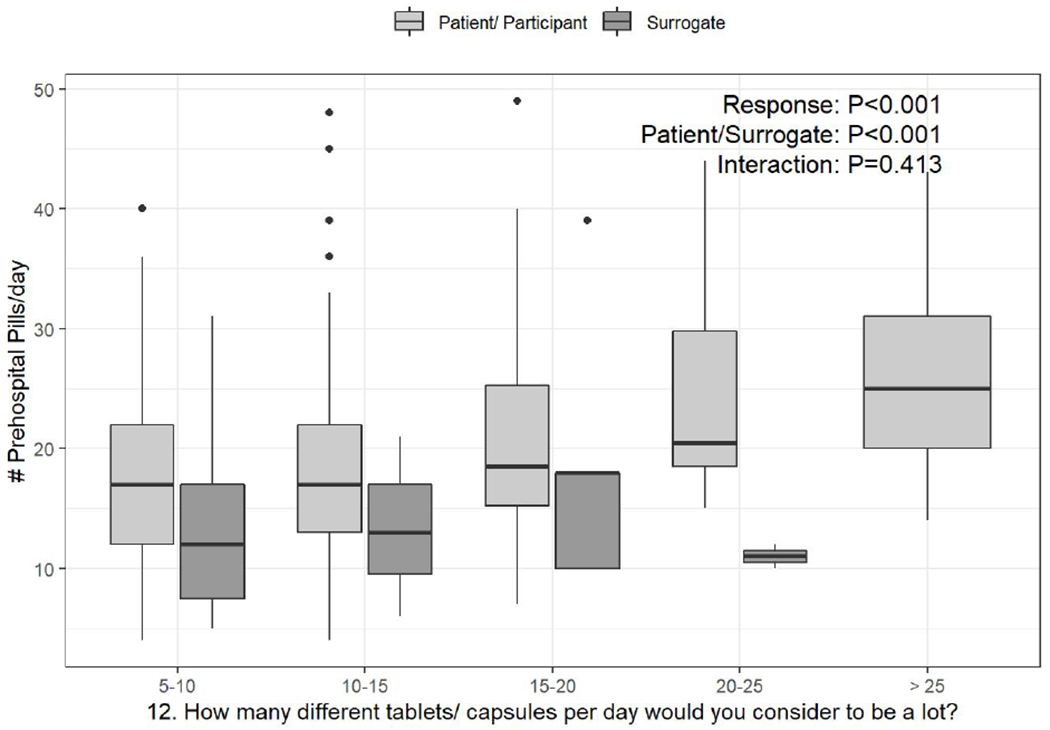
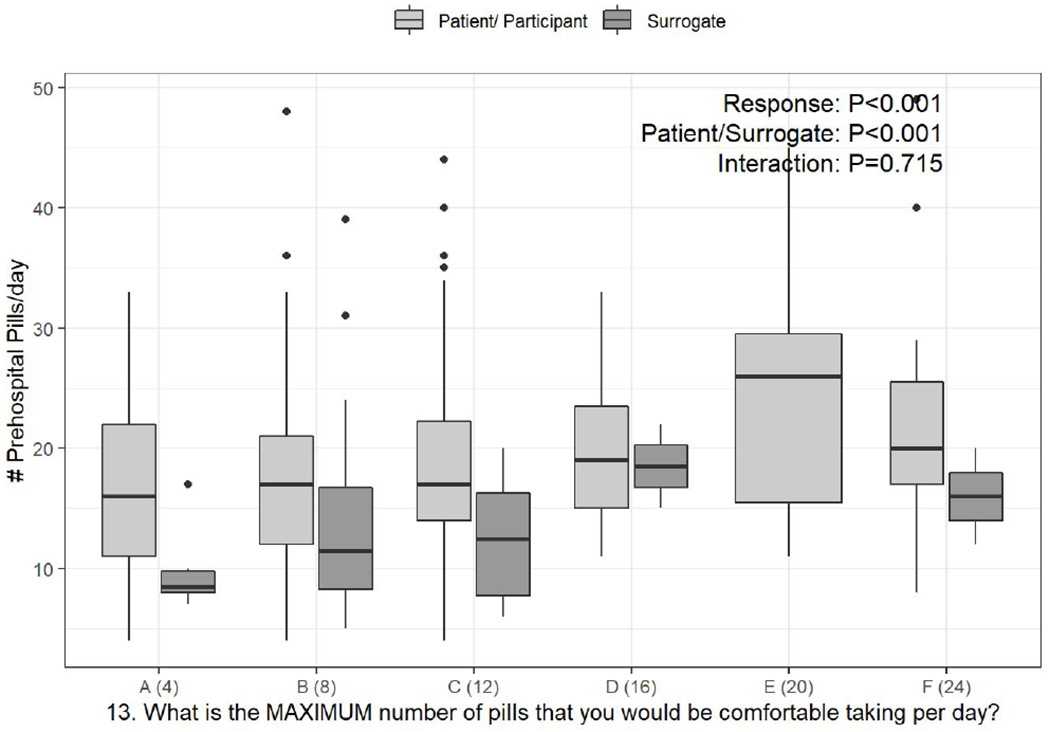
a-c. Box-and-whisker plots of the counts of pre-hospital pills per day, stratified by response to PATD questions 1, 12, and 13 and whether the survey was completed by the patient or surrogate. Linear regression was used to test the associations of daily pill count versus PATD response, patient/surrogate status, and their interaction (i.e., is the association between pill count and PATD response different for patients versus surrogates?).
Categories presented on the X-axis are the response categories participants were provided from the original version of the PATD.
Table 4:
Logistic Regression Results for PATD Agree or Strongly Agree Responses that Differed between Patients and Surrogates
| PATD Question Item | OR | 95%CI | P - Value |
| Question 3: “I believe that all of my medicines are necessary” | 0.36 | 0.18, 0.74 | 0.005 |
| Question 5: “I would like to reduce the number of medicines that I am taking” | 0.56 | 0.26, 1.18 | 0.126 |
| Question 9: “Having to pay less would play a role in my willingness to stop one or more of my medicines” | 0.39 | 0.17, 0.88 | 0.024 |
All models are adjusted for Age, Assisted Living Facility Residence, Pre-hospital medication count, Insurance Status (Non-Medicare vs. Medicare), and Dementia Diagnosis. Results demonstrate the change in odds of Agreeing or Strongly agreeing to the specific PATD question of a surrogate respondent when compared to a patient respondent.
The overwhelming majority of both groups (93%) reported a willingness to stop one or more of their regular medicines, if their doctor agreed, with no difference between patients and surrogates. Most respondents (78% overall) stated that they would like to reduce their number of medicines, with a lower proportion among surrogates (64% vs 80%, unadjusted P=0.012). However, after adjusting for potential confounders, there was no significant difference between patient and surrogate respondents (adjusted OR 0.56: 95% CI 0.26 to 1.18, P=0.126; Table 4). About one-third in both groups (33%) felt that they might be taking one or more medicines they no longer needed. In fact, despite the general positive attitude toward deprescribing and the high prevalence of hyper-polypharmacy, most (78%) stated that they would accept taking even more medicines for their/care recipient’s health conditions. The majority (82%) reported that they had a good understanding of the reasons they were prescribed each of their medicines. Furthermore, 38% believed that one or more of their medicines was giving them side effects, with both groups being comparable. Lastly, only 36% reported that having to pay less would play a role in stopping medicines. This proportion was smaller among the surrogate group (19% versus 39%, unadjusted P = 0.007). In adjusted analysis the odds were 61% lower in surrogates (adjusted OR 0.39: 95% CI 0.17 to 0.88, P=0.024, Table 4) and mirrored the pattern of responses to the “difficulty paying” question (shown in Table 1), which was also low for both groups.
Table 3 reports descriptive statistics for patient and surrogate responses to PATD questions 11 through 15. Among patient and surrogate respondents, 42% and 47% respectively reported they had tried to stop a regular medicine, and of those 63% reported that they remained off the medicine, 22% restarted the medicine, and 6% started a different medicine. Nearly half of respondents (49.4%) stated that they consider 5-10 pills to be “a lot,” and 35.6% considered eight pills to be the maximum number they would be comfortable taking per day. For the PATD question related to their comfort level with a pharmacist stopping one or more of their regular medicines, 53% reported being “comfortable.” Lastly, the most preferred (49.1%) method of follow-up after deprescribing was “face-to-face appointments.” These responses were comparable between both groups.
Table 3.
Patient and Surrogate Responses to PATD Questions 11 through 15 (N=337)
| Interview Question | Overall | Patient Responses | Surrogate Responses | P-value |
|---|---|---|---|---|
| N | 337 | 285 | 52 | |
| 11. Have you ever tried to stop a regular medicine (with your doctor’s knowledge)? | ||||
| No | 192 (57.5) | 166 (58.2) | 26 (53.1) | 0.533 |
| Yes | 142 (42.5) | 119 (41.8) | 23 (46.9) | |
| 12. How many different tablets/ capsules per day would you consider to be a lot? | ||||
| 5 - 10 | 164 (49.4) | 137 (48.4) | 27 (55.1) | 0.721 |
| 10 - 15 | 106 (31.9) | 91 (32.2) | 15 (30.6) | |
| 15 - 20 | 43 (13.0) | 38 (13.4) | 5 (10.2) | |
| 20 - 25 | 10 (3.0) | 8 (2.8) | 2 (4.1) | |
| 25 or more | 9 (2.7) | 9 (3.2) | 0 (0.0) | |
| 13. What is the MAXIMUM number of pills that you would be comfortable taking per day? (Response options were pictures. Reporting below the picture identification and the corresponding number of pills shown.) | ||||
| A (4) | 54 (17.0) | 48 (17.5) | 6 (14.3) | 0.129 |
| B (8) | 113 (35.6) | 91 (33.1) | 22 (52.4) | |
| C (12) | 78 (24.6) | 68 (24.7) | 10 (23.8) | |
| D (16) | 37 (11.7) | 35 (12.7) | 2 (4.8) | |
| E (20) | 18 (5.7) | 18 (6.5) | 0 (0.0) | |
| F (24) | 17 (5.4) | 15 (5.5) | 2 (4.8) | |
| 14. How comfortable would you be if a pharmacist was involved in stopping one or more of your regular medicines and provided the follow-up (informing your doctor of the progress)? | ||||
| Uncomfortable | 101 (30.1) | 88 (30.9) | 13 (25.5) | 0.562 |
| Unsure | 57 (17.0) | 46 (16.1) | 11 (21.6) | |
| Comfortable | 178 (53.0) | 151 (53.0) | 27 (52.9) | |
| 15. If one of your regular medicines was stopped, what follow-up would you like? | ||||
| Face to face appointment | 165 (49.1) | 146 (51.2) | 19 (37.3) | 0.179 |
| Phone call(s) | 120 (35.7) | 95 (33.3) | 25 (49.0) | |
| Written information | 32 (9.5) | 27 (9.5) | 5 (9.8) | |
| I wouldn’t need planned follow-up. I would be happy contacting a health professional if I had and problems | 19 (5.7) | 17 (6.0) | 2 (3.9) | |
The number of pills per day that respondents would consider to be a lot and the maximum number of pills they would be comfortable taking based on their categorical responses to these specific PATD items (questions 12 & 13, respectively) were significantly associated with the objective count of pre-hospital scheduled daily pills (Figure 1b & Figure 1c, P<0.001). This association did not differ for patients versus surrogates (Figure 1b, P=0.413 and Figure 1c, P=0.715).
Discussion and Implications
Although the PATD has been administered to various patient populations outside the United States, to our knowledge, this is the first study to report comprehensive PATD data for hospitalized older adults and their surrogates for patients transitioning from acute to post-acute care in the U.S. Eighty-eight percent of this patient population experienced hyper-polypharmacy at the time of hospital admission, and this study showed that over 90% of patients and surrogates expressed a willingness to deprescribe one or more of their medicines. Although only one-third of patients or surrogates believed they were taking at least one unnecessary medication, an objective review revealed that all patients were taking at least one potentially inappropriate medication (PIM). These findings suggest that future efforts to deprescribe will likely require enhanced education for patients, caregivers, and prescribers about medication appropriateness.
The hospital and PAC settings may provide an opportune time for deprescribing given the increased clinical oversight to monitor deprescribing. Consistent with a population-based survey of Medicare patients (Reeve et al., 2018), most older hospitalized patients in this study also report a willingness to deprescribe if their doctor said it was possible. Similar high rates of willingness to deprescribe have been found in an Italian inpatient population (Galazzi et al., 2016); however, the response of surrogates to the PATD has not been reported in prior inpatient studies (Galazzi et al., 2016; Qi et al., 2015). Deprescribing among older patients, especially in the inpatient setting, often requires the engagement of surrogate decision-makers who may also provide different types of medication assistance to the patient.
In this study, 15% of enrolled patients required a surrogate to respond on their behalf. Not surprisingly, patients requiring surrogates were older and more cognitively impaired. Although patients and surrogates expressed similar attitudes overall, there were two areas that yielded attitudinal differences. First, despite fewer total medications, surrogates were approximately 16% less likely to believe all of their care recipient’s medications were necessary. It is possible that surrogates believe that some of these medications may be contributing to the patient’s decline. They may also perceive reduced value of at least some medications in the context of cognitive impairment and life expectancy. Reducing cost does not appear to be a primary motivating factor of surrogates for potential deprescribing decisions. Compared to patient respondents, surrogates had 61% lower odds of attributing reducing cost as a role in future deprescribing decisions, even after adjusting for the lower number of medications or increased Medicare insurance status among patients with surrogates. Differences in deprescribing attitudes have potential implications in the design of future deprescribing interventions such as interventions tailored to surrogates should focus less on cost and more on the medication’s benefit to overall health and quality of life.
Perceptions of medication burden were significantly associated with their objectively measured daily pill count using the pre-hospital scheduled medications. Even at high daily pill counts (median of 17 per patient), 61% still agree that all their medicines are necessary. However, 100% of patients are prescribed at least one potentially inappropriate medication (median of 7 PIMs per patient), which suggests a substantial discordance between patients’ and surrogates’ perceptions and objective measures of medications that are potentially inappropriate. Thus, deprescribing interventions should elucidate to patients and surrogates the potential risks and side effects of medications or potential lack of indication. Prescriber engagement will be key in this process, as 93% of patients reported a willingness to deprescribe if their physician agreed.
Importantly, it may not always be the physician who is responsible for deprescribing. Pharmacist-led deprescribing trials have shown acceptance and success outside of the U.S. (Reeve et al., 2019); however, little is known about patient acceptance of pharmacists to initiate deprescribing in the U.S. health care system. Pharmacists are an increasingly important member of care teams in the U.S. and have been found to positively affect medication outcomes (Gillespie et al., 2013; Lee et al., 2013). Who facilitates deprescribing discussions may be an important factor in reducing polypharmacy among older patients. In this study, only half of patients reported being comfortable with pharmacists deprescribing medications and another 16% being unsure about their role. Patients’ stated reluctance to pharmacist-led deprescribing may be important, as trust in providers has consistently been shown to be a facilitator to deprescribing (Clyne et al., 2017; Weir et al., 2018). It is unknown if patient responses are predictive of pharmacist-specific efficacy in deprescribing. These findings highlight a continued need to engage primary healthcare providers in deprescribing decisions while continuing to educate patients about the expanding skills and competencies of clinical pharmacists as an integral member of the care team.
The findings of this study should be interpreted in context and in consideration of study limitations. First, this study includes older hospitalized patients admitted to a single, academic medical center. The study sample is notably of low proportion Medicaid/uninsured and majority White. Secondly, current study results could be affected by selection bias because patients had to consent to enroll in a deprescribing intervention trial; thus, patients who refused enrollment may have different attitudes and ones biased toward less willingness to deprescribe. Additionally, surrogates were only interviewed when patients were unable to report their own attitudes; thus, we are unable to directly compare the attitudes of patients to their respective surrogates. Lastly, the original version of the PATD used in this study does not have a validated scoring system or surrogate version. Thus, future studies that use the more recently developed rPATD may address these limitations.
In conclusion, a high proportion of older hospitalized patients requiring skilled post-acute care, as well as patient surrogates, express a willingness to deprescribe at least one of their medications, yet also report all their medications were necessary and that they were comfortable with their current number of medications despite each participant having at least one potentially inappropriate medication. The results of this study have important implications for future deprescribing interventions. First, deprescribing interventions should engage surrogates/caregivers as their attitudes are similar to albeit not the same as patients and might impact long-term success. Second, deprescribing interventions should include an educational component related to medication appropriateness and empower patients/surrogates to participate in shared decision making when determining if a medication is appropriate and of benefit. Finally, patients/surrogates also should be made aware of the value pharmacists and other advanced practice providers contribute to their on-going medication management, particularly during care transitions. Future research needs to be conducted to yield a better understanding of how self-reported attitudes relate to deprescribing outcomes, which will be examined at the conclusion of the larger Shed-MEDS trial. These data will help to optimize future patient-centered deprescribing interventions geared toward both patients and their surrogates.
Acknowledgements:
We extend our thanks to the members of the Shed-MEDS team, which includes the following personnel beyond those listed as co-authors: Carole Bartoo, GNP; Jennifer Kim, GNP; Kanah Lewallen, GNP; Whitney Narramore, PharmD; Robin Parker, PharmD; Jessica Lovell, PharmD Joanna Gupta, MEd; Susan Lincoln, BS
Funding:
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institute on Aging of the National Institutes of Health award R01AG053264 which as awarded to the two co-principal investigators Drs. Vasilevskis and Simmons. The use of institutional data management system REDCap is supported by also supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences.
Footnotes
Conflict of Interest: The Authors declare that there is no conflict of interest.
IRB and Human Subjects Approval: Approval was obtained from the Institutional Review Board at Vanderbilt University, IRB approval #161571. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. This trial was prospectively registered at clinicaltrials.gov (NCT02979353).
Reference List
- Ammerman CA, Simpkins BA, Warman N, & Downs TN (2019). Potentially Inappropriate Medications in Older Adults: Deprescribing with a Clinical Pharmacist. J Am Geriatr Soc, 67(1), 115–118. 10.1111/jgs.15623 [DOI] [PubMed] [Google Scholar]
- By the American Geriatrics Society Beers Criteria Update Expert, P. (2015). American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc, 63(11), 2227–2246. 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, & MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis, 40(5), 373–383. http://www.ncbi.nlm.nih.gov/pubmed/3558716 [DOI] [PubMed] [Google Scholar]
- Clyne B, Cooper JA, Boland F, Hughes CM, Fahey T, Smith SM, & team O.-S. s. (2017). Beliefs about prescribed medication among older patients with polypharmacy: a mixed methods study in primary care. Br J Gen Pract, 67(660), e507–e518. 10.3399/bjgp17X691073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazzi A, Lusignani M, Chiarelli MT, Mannucci PM, Franchi C, Tettamanti M, Reeve E, & Nobili A. (2016). Attitudes towards polypharmacy and medication withdrawal among older inpatients in Italy. Int J Clin Pharm, 38(2), 454–461. 10.1007/s11096-016-0279-4 [DOI] [PubMed] [Google Scholar]
- Gamble J-M, Hall JJ, Marrie TJ, Sadowski CA, Majumdar SR, & Eurich DT (2014). Medication transitions and polypharmacy in older adults following acute care. Therapeutics and Clinical Risk Management, 10, 189–196. 10.2147/TCRM.S58707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie U, Alassaad A, Hammarlund-Udenaes M, Morlin C, Henrohn D, Bertilsson M, & Melhus H. (2013). Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments’ (MAI, STOPP and STARTs’) ability to predict hospitalization–analyses from a randomized controlled trial. PloS One, 8(5), e62401. 10.1371/journal.pone.0062401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, McLachlan AJ, Abernethy DR, Banks E, & Le Couteur DG (2012). High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther, 91(3), 521–528. 10.1038/clpt.2011.258 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmer SN, & Gnjidic D. (2009). The effects of polypharmacy in older adults. Clin Pharmacol Ther, 85(1), 86–88. 10.1038/clpt.2008.224 [DOI] [PubMed] [Google Scholar]
- Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, Harris TB, Hanlon JT, Rubin SM, Shorr RI, Bauer DC, & Abernethy DR (2007). A drug burden index to define the functional burden of medications in older people. Arch Intern Med, 167(8), 781–787. 10.1001/archinte.167.8.781 [DOI] [PubMed] [Google Scholar]
- Hilmer SN, Mager DE, Simonsick EM, Ling SM, Windham BG, Harris TB, Shorr RI, Bauer DC, Abernethy DR, & Health ABCS (2009). Drug burden index score and functional decline in older people. Am J Med, 122(12), 1142–1149 e1141-1142. 10.1016/j.amjmed.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Dell’Aquila G, Collamati A, Martone AM, Zuliani G, Gasperini B, Eusebi P, Lattanzio F, & Cherubini A. (2014). Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J Am Med Dir Assoc, 15(11), 825–829. 10.1016/j.jamda.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Lee JK, Slack MK, Martin J, Ehrman C, & Chisholm-Burns M. (2013). Geriatric patient care by U.S. pharmacists in healthcare teams: systematic review and meta-analyses. J Am Geriatr Soc, 61(7), 1119–1127. 10.1111/jgs.12323 [DOI] [PubMed] [Google Scholar]
- Lund BC, Schroeder MC, Middendorff G, & Brooks JM (2015). Effect of hospitalization on inappropriate prescribing in elderly Medicare beneficiaries. J Am Geriatr Soc, 63(4), 699–707. 10.1111/jgs.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi A, Vasilevskis E, Pandharipande PP, Girard TD, Solberg LM, Neal EB, Koestner T, Torres RE, Thompson JL, Shintani AK, Han JH, Schnelle JF, Fick DM, Ely EW, & Kripalani S. (2013). Inappropriate Medication Prescriptions in Elderly Adults Surviving an Intensive Care Unit Hospitalization. Journal of the American Geriatrics Society, 61(7), 1128–1134. 10.1111/jgs.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Spies CD, Eckardt R, Weiss B, Pohrt A, Wernecke KD, Schmidt M, & Group P. (2020). Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: A clinical trial. Journal of Clinical Anesthesia, 61, 109632. 10.1016/j.jclinane.2019.109632 [DOI] [PubMed] [Google Scholar]
- Poudel A, Peel NM, Nissen LM, Mitchell CA, Gray LC, & Hubbard RE (2016). Adverse Outcomes in Relation to Polypharmacy in Robust and Frail Older Hospital Patients. J Am Med Dir Assoc, 17(8), 767 e769–767 e713. 10.1016/j.jamda.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Qi K, Reeve E, Hilmer SN, Pearson S-A, Matthews S, & Gnjidic D. (2015). Older peoples’ attitudes regarding polypharmacy, statin use and willingness to have statins deprescribed in Australia. International Journal of Clinical Pharmacy, 37(5), 949–957. 10.1007/s11096-015-0147-7 [DOI] [PubMed] [Google Scholar]
- Reeve E, Low LF, & Hilmer SN (2019). Attitudes of Older Adults and Caregivers in Australia toward Deprescribing. J Am Geriatr Soc, 67(6), 1204–1210. 10.1111/jgs.15804 [DOI] [PubMed] [Google Scholar]
- Reeve E, Low LF, Shakib S, & Hilmer SN (2016). Development and Validation of the Revised Patients’ Attitudes Towards Deprescribing (rPATD) Questionnaire: Versions for Older Adults and Caregivers. Drugs Aging, 33(12), 913–928. 10.1007/s40266-016-0410-1 [DOI] [PubMed] [Google Scholar]
- Reeve E, Shakib S, Hendrix I, Roberts MS, & Wiese MD (2013). Development and validation of the patients’ attitudes towards deprescribing (PATD) questionnaire. Int J Clin Pharm, 35(1), 51–56. 10.1007/s11096-012-9704-5 [DOI] [PubMed] [Google Scholar]
- Reeve E, Wolff JL, Skehan M, Bayliss EA, Hilmer SN, & Boyd CM (2018). Assessment of Attitudes Toward Deprescribing in Older Medicare Beneficiaries in the United States. JAMA internal medicine. 10.1001/jamainternmed.2018.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runganga M, Peel NM, & Hubbard RE (2014). Multiple medication use in older patients in post-acute transitional care: a prospective cohort study. Clinical Interventions in Aging, 9, 1453–1462. 10.2147/CIA.S64105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba D, Buchanan J, Edelen MO, Streim J, Ouslander J, Berlowitz D, & Chodosh J. (2012). MDS 3.0: brief interview for mental status. J Am Med Dir Assoc, 13(7), 611–617. 10.1016/j.jamda.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Saliba D, Elliott M, & Rubenstein LZ (2001). The Vulnerable Elders Survey (VES-13): A tool for identifying vulnerable elders in the community. J Am Geriatr Soc, 49, 1691–1699. http://onlinelibrary.wiley.com.proxy.library.vanderbilt.edu/doi/10.1046/j.1532-5415.2001.49281.x/pdf [DOI] [PubMed] [Google Scholar]
- Saraf AA, Petersen AW, Simmons SF, Schnelle JF, Bell SP, Kripalani S, Myers AP, Mixon AS, Long EA, Jacobsen JML, & Vasilevskis EE (2016). Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. Journal of Hospital Medicine, n/a-n/a. 10.1002/jhm.2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiotz ML, Frolich A, Jensen AK, Reuther L, Perrild H, Petersen TS, Kornholt J, & Christensen MB (2018). Polypharmacy and medication deprescribing: A survey among multimorbid older adults in Denmark. Pharmacology Research & Perspectives, 6(6), e00431. 10.1002/prp2.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois C, Ouellet N, & Reeve E. (2017). Community-dwelling older people’s attitudes towards deprescribing in Canada. Res Social Adm Pharm, 13(4), 864–870. 10.1016/j.sapharm.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Stevenson DG, Dusetzina SB, James O’Malley A, Mitchell SL, Zarowitz BJ, Chernew ME, Newhouse JP, & Huskamp HA (2014). High-Risk Medication Use by Nursing Home Residents Before and After Hospitalization. Medical Care, 52(10), 884–890. 10.1097/MLR.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasai S, Kumpat N, Dilokthornsakul P, Chaiyakunapruk N, Saini B, & Dhippayom T. (2019). Impact of Medication Reviews Delivered by Community Pharmacist to Elderly Patients on Polypharmacy: A Meta-analysis of Randomized Controlled Trials. J Patient Saf. 10.1097/PTS.0000000000000599 [DOI] [PubMed] [Google Scholar]
- Thillainadesan J, Gnjidic D, Green S, & Hilmer SN (2018). Impact of Deprescribing Interventions in Older Hospitalised Patients on Prescribing and Clinical Outcomes: A Systematic Review of Randomised Trials. Drugs & Aging, 35(4), 303–319. 10.1007/s40266-018-0536-4 [DOI] [PubMed] [Google Scholar]
- Tjia J, Velten SJ, Parsons C, Valluri S, & Briesacher BA (2013). Studies to reduce unnecessary medication use in frail older adults: a systematic review. Drugs Aging, 30(5), 285–307. 10.1007/s40266-013-0064-1 [DOI] [PubMed] [Google Scholar]
- Turner JP, & Tannenbaum C. (2017). Older Adults’ Awareness of Deprescribing: A Population-Based Survey. J Am Geriatr Soc, 65(12), 2691–2696. 10.1111/jgs.15079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevskis EE, Shah AS, Hollingsworth EK, Shotwell MS, Mixon AS, Bell SP, Kripalani S, Schnelle JF, & Simmons SF (2019). A patient-centered deprescribing intervention for hospitalized older patients with polypharmacy: rationale and design of the Shed-MEDS randomized controlled trial. BMC Health Serv Res, 19(1), 165. 10.1186/s12913-019-3995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, & Kripalani S. (2014). Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med, 29(1), 119–126. 10.1007/s11606-013-2568-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir K, Nickel B, Naganathan V, Bonner C, McCaffery K, Carter SM, McLachlan A, & Jansen J. (2018). Decision-Making Preferences and Deprescribing: Perspectives of Older Adults and Companions About Their Medicines. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 73(7), e98–e107. 10.1093/geronb/gbx138 [DOI] [PubMed] [Google Scholar]


