Abstract
Aim
Plantar enthesophyte is a common degenerative disorder. Surgical and medical treatment options are associated with either poor outcome or high percentage of relapse. Observations have indicated a beneficial effect of radiation therapy. We therefore wanted to evaluate pain reduction using orthovolt or cobalt-based radiation treatment for painful plantar enthesophyte and determine long-term response as well as prognostic parameters in this condition.
Methods
We identified a total of 102 consecutive patients treated for a total of 117 symptomatic heel spurs. 59 patients were treated with cobalt radiation, 31 patients with orthovolt therapy and 12 patients with both radiation systems. Primary outcome measure was pain reduction being scored using the modified Rowe Score prior therapy, at the end of each treatment series as well as after 6 weeks. Secondary outcome measure was long-term outcome, evaluated in patients with a follow-up period of longer than 3 years.
Results
Before radiation therapy, 61 patients (60.4%) had a score of 0, significant strong pain. At the time of completion of radiation treatment, 3 patients (2.7%) were pain-free (score of 30), whereas 8 patients (7.9%) had still severe pain (score 0). 6 weeks after radiation therapy, 33 patients (32.7%) were pain-free and 8 patients (7.9%) had severe pain (score 0), while at the time data of collection, 74 patients (73%) were free of pain and 1 patient (1%) had strong pain (score 0). Duration of pain before the start of radiation treatment was a significant prognostic factor (p = 0.012) for response to treatment.
Conclusion
Radiotherapy of painful plantar enthesophyte is a highly effective therapy with little side effects providing long-term therapeutic response. The only significant prognostic parameter for response to treatment is the duration of pre-radiation therapy pain. Early integration of radiation therapy in the treatment seems to result in superior pain reduction.
Keywords: Plantar enthesophyte, Heel spur, Radiation therapy, Benign disease, Pain, Photon therapy, Electrons
Introduction
Plantar enthesophyte is a painful degenerative disorder evolving over a long time [1–4] and is localized at the tuber calcanei [5] (Fig. 1).
Fig. 1.
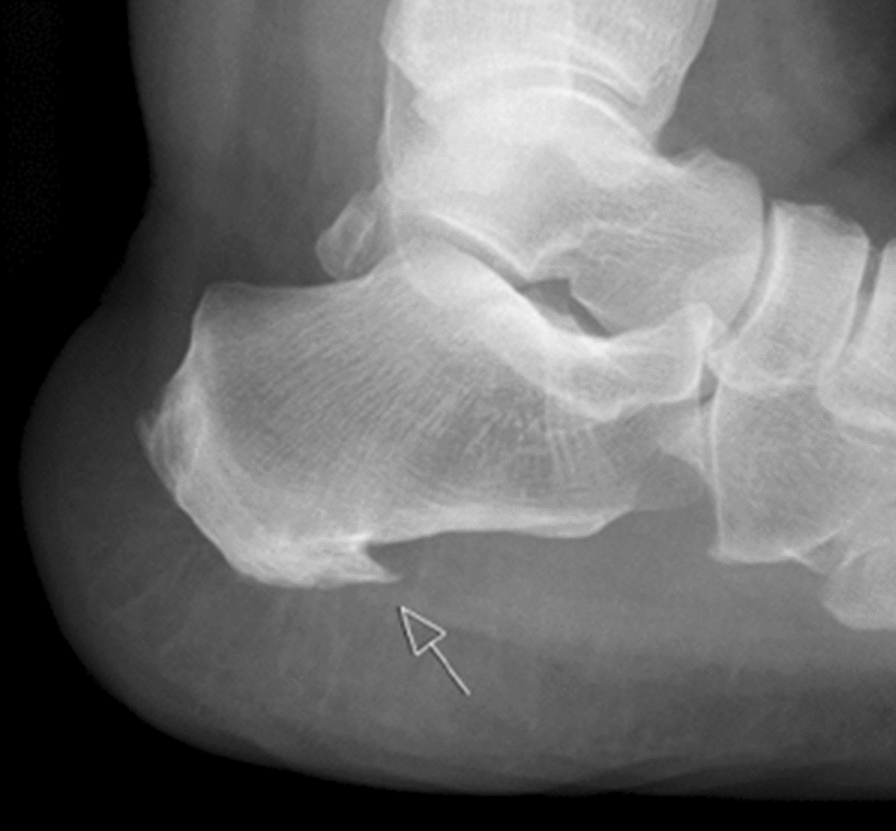
X-ray of plantar enthesophyte. The X-ray of the calcaneus shows a heel spur with an inflammatory reaction surrounding the insertion of the inferior aponeurosis (arrow)
Plantar enthesophyte, often described as heel spur, was first described by Plettner [6]. He investigated radiological findings of exostosis at the plantar sole and parts of the calcaneus. Nowadays, plantar enthesophyte are one of the most common causes of pain in the heel region.
The plantar enthesophyte develops as a reactive formation of bone due to mechanical stress with degenerative changes and microtraumas at the insertion of the tendons of the abductor hallucis and the brevis flexor digitorum muscles as well as the plantar aponeurosis and can therefore be identified as a result of a plantar fasciitis. Nevertheless, this term is misnomer, since the plantar fascia is an aponeurotic rather than a fascial layer. Plantar enthesophytes are located at the calcaneus where the plantar aponeurosis inserts at the medial tuber calcanei or at the insertion of the Achilles tendon in a dorsal position. Combinations of the two locations have also been described [7]. Risk factors are female sex (female:male = 3:1) [8], age above 40 years, obesity, weight bearing occupation [9] as well as anatomical deformity of the foot such as talipes valgus, talipes planus and flatfoot [7].
The majority of patients with plantar enthesophyte remain asymptomatic. If clinically apparent, typical symptoms are pressure pain at the medial part of the arch of the foot radiating to the calf and the sole of the foot. The pain is often described as burning or stinging, emerging while walking barefoot or after resting. Therefore, the diagnosis of plantar fasciitis is mostly a clinical one. Any further diagnostic approach should be tailored according to the clinical picture and includes foremost plain X-ray. Additional diagnostical regimen may include technetium bone scintigraphy (revealing a fasciitis) as well as MRI, though both techniques are rarely used.
If symptoms are present, physical activity may improve them. Conservative or invasive therapeutic options have been implemented in the therapy of plantar enthesophytes. Primary treatment usually consists of conservative management including orthotics, local or systemic analgesics, corticosteroids [10], antiphlogistics [11, 12], physiotherapy [13, 14] and stretching exercises [14, 15]. Massages, local thermotherapy, ultrasound and electrical stimulation therapy are often prescribed for several times [16, 17]. Nafe and other authors were able to demonstrate a temporary pain reduction effect of extracorporeal shockwave therapy [3, 4, 18–22]. However, at least in Germany, the latter therapeutical approach is rarely compensated by health insurances. Despite high rates of recurrences, surgical management was used for chronic pain condition after conservative therapy, but is usually not a standard method in the treatment of a painful plantar enthesophyte [1, 10, 23, 24]. Despite an increasing number of physicians successfully using radiation treatment in degenerative inflammatory disorders, the role of radiotherapy in this context is currently under discussion [25–27].
In this retrospective study, we tried to clarify the following questions:
Which parameters influence the clinical outcome (pain reduction) in the radiation treatment (RT) of plantar enthesophytes? We identified radiation parameters (single fraction and overall fraction dose, overall treatment time, field size (radiologically vs. clinically guided fields), type of radiation) as well as clinical parameters (sex, age, treatment prior to radiation, duration of pain prior to radiation).
Patients and methods
During 2 years, 102 patients were treated for overall 117 plantar enthesophytes. 28 male (27.2%) und 74 female (71.8%) patients with a mean age of 51.5 years (range: 20–80 years, median 52 years) were included in this study. 87 patients had a uni-lateral plantar enthesophytes (55 right, 32 left) and 15 patients had bilateral plantar enthesophytes. The correct diagnoses were confirmed using X-ray by two radiologists. 59 patients were treated with cobalt radiation (60Co) and 31 with X-rays with an orthovoltage irradiation (300 kV). 12 patients were treated with both energies.
Radiation treatment was indicated in patients aged 20 to 50 years when conservative methods failed to reduce clinically evident pain and patients experienced a loss of function in everyday life.
Radiation treatment with both techniques consisted of 6 fractions of 0.5–1 Gray (Gy) given 2–3 times a week up to total dose of 3 or 6 Gy. In detail, patients being treated with cobalt radiation were irradiated 3 times a week with a dose/fraction of 0.5 or 1 Gy up to a total dose of 3 or 6 Gy, depending on their pain intensity. Higher doses of radiation were prescribed for patients with persistent and severe pain. They always received a total of 6 fractions. Orthovoltage irradiation was applied 2 to 3 times per week with a dose/fraction of 1 Gy and a total dose of 3 or 6 Gy, resulting in a total of 3 or 6 fractions which were applied. In addition, the therapeutic effect of local steroid injection was evaluated. In 12 patients both 60-cobalt and orthovoltage therapy were administered. One reason for it was the availability of the machine. 12 patients started with orthovoltage irradiation, but as the machine was unavailable cobalt irradiation (60Co) was prescribed.
If only temporary or partial pain relief was obtained, a second course was offered to the patients. This was given after a median time gap of 5.4 (range 3–12) months after initial treatment.
Pain and function were evaluated using a modified score of Rowe et al. [28]. Table 1 shows the criteria used in this measurement. Patients were contacted directly or interviewed by telephone and completed the questionnaire retrospectively for the following four points in time: (1) before start of radiation; (2) on the last day of radiation; (3) 6 weeks after radiation; (4) during follow-up (at least 3 years after radiation treatment).
Table 1.
Modified pain and function score according to Rowe et al. [32]
| Criteria | Response level | Score | |
|---|---|---|---|
| Pain | At rest | None | 30 |
| Mild | 20 | ||
| Moderate | 10 | ||
| Severe | 0 | ||
| Pain in motion | None | 0 | |
| Mild | 30 | ||
| Moderate | 20 | ||
| Severe | 10 | ||
| Pain when applying pressure to the heel | None | 0 | |
| Mild | 30 | ||
| Moderate | 20 | ||
| Severe | 10 | ||
| Medical aids | None | 15 | |
| Orthopedic insoles, sole padding | 10 | ||
| One walking aid (cane or forearm support) | 5 | ||
| Two walking aids | 0 | ||
| Everyday activities | Normal, no constraints | 15 | |
| Small constraints | 10 | ||
| Moderate constraints | 5 | ||
| Complete constraints | 0 | ||
| Gait | No limping, normal gait with no constraints | 20 | |
| Mild pain and limping after > 1 km | 10 | ||
| Moderate pain and limping after < 1 km | 5 | ||
| Severe pain, no normal gait possible | 0 |
Score 120–140: excellent; Score 90–120: good; Score 60–90: moderate; Score 30–60 mild; Score 0–30 severe
We statistically evaluated radiation-associated factors (single and overall dose, treatment time, field size, type of radiation, pain medication) and clinical parameters (sex, age, prior treatment before radiation, duration of pain before radiation treatment) possibly influencing the clinical outcome using Microsoft-Excel 2010 and SPSS-20.
Data were expressed at mean ± standard error of the mean (SEM). Statistical significance was assessed by Mann–Whitney U and t-test. p-values less than or equal to 0.05 were considered statistically significant (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). With the Kruskal–Wallis test, we examined the influence of radiotherapy on pain relief in different groups. Normal distribution was assessed using the D’Agostino–Pearson and Kolmogorov–Smirnov test.
Results
In this retrospective analyses, we identified 102 consecutive patients treated in our institutions within 2 years. Table 2 shows our patients characteristics. Diagnosis was performed using established clinical parameters. Ideally, confirmation of diagnosis with MR technique revealing marrow and soft tissue edema along the proximal plantar fascia may underline this. Since clinical evidence and treatment decision is routinely done without this diagnostic feature because it is not necessary for clinical treatment, it is not performed widely. 102 patients were treated for a number of 117 symptomatic plantar enthesophytes. 59 patients were treated with 60-cobalt therapy, 31 patients with orthovolt therapy and 12 patients with both radiation systems.
Table 2.
Patients characteristics
| Criteria | No. of patients | (%) |
|---|---|---|
| Patients | 102 | 100 |
| Female | 74 | 71.8 |
| Male | 28 | 27.2 |
| Spurs | 117 | 100 |
| Right-sided | 55 | 54.1 |
| Left-sided | 32 | 31.6 |
| Bilateral | 15 | 14.3 |
| Pre-treatment | 134 | 100 |
| Orthopedic insoles | 48 | 35.8 |
| Corticoid infiltrations | 25 | 18.6 |
| Antiphlogistics (oral) | 34 | 25.3 |
| Physiotherapy | 3 | 2.2 |
| Shockwave therapy | 6 | 4.5 |
| Massage | 5 | 3.7 |
| Ultrasound therapy | 4 | 2.9 |
| Antiphlogistics (ointment) | 5 | 3.7 |
| Corticosteroids (oral) | 2 | 1.5 |
| Surgical therapy | 2 | 1.5 |
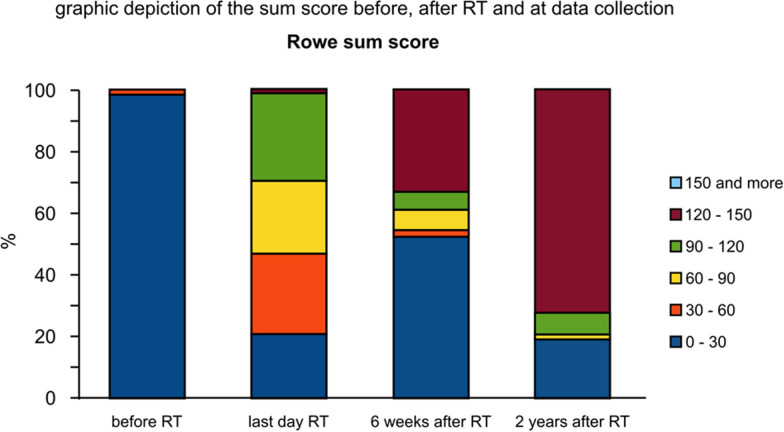
Prior to RT treatment, 100 patients (99%) presented with a Rowe S-Score between 0 and 30 (severe pain) and only 1 patient (1%) had a S-Score between 30 and 60 (mild pain) (Fig. 2).The pretreatment group of 134 patients was set to 100% because it includes patients with double or more different pre-treatments.
Fig. 2.
Graphic depiction of the sum score before, after RT and at final data collection
On the last day of RT treatment, 21 patients (20.8%) achieved a S-Score between 0 and 30 (mild to no improvement), 26 patients (25.7%) a S-Score between 30 and 60 (mild pain improvement), 24 patients (23.7%) a S-Score between 60 and 90 (mild to moderate improvement) and 29 patients (28.7%) had a S-Score between 90 and 120 (good pain response). Only 1 patient (1%) achieved a complete pain response with a S-Score between 120 and 150.
If patients were contacted directly or interviewed by telephone and completed the questionnaire retrospectively for 6 weeks after radiation, during follow-up (at least 3 years after radiation treatment) 53 patients (52.5%) had a S-Score of 0–30 (mild to no improvement of pain), 2 patients (2%) a S-Score between 30 and 60 (mild improvement of pain), 7 patients (6.9%) a S-Score of 60–90 (mild to moderate improvement of pain) and 6 patients (5.9%) had an S-Score of 90–120 (major improvement of pain). Overall, 33 patients (32.7%) had a complete pain remission with a S-Score of 120–150.
At the time of data collection, the results of a telephone questionnaire were the following: 19 patients (18.8%) reported a S-Score of 0–30, 2 patients (2%) a S-Score between 60 and 90, 7 patients (6.9%) a S-Score of 90–120 and 73 patients (72.3%) had a S-Score of 120–150. No patient reported a S-Score of 60–40 and 14 patients (13.9%) experienced a S-Score of 0.
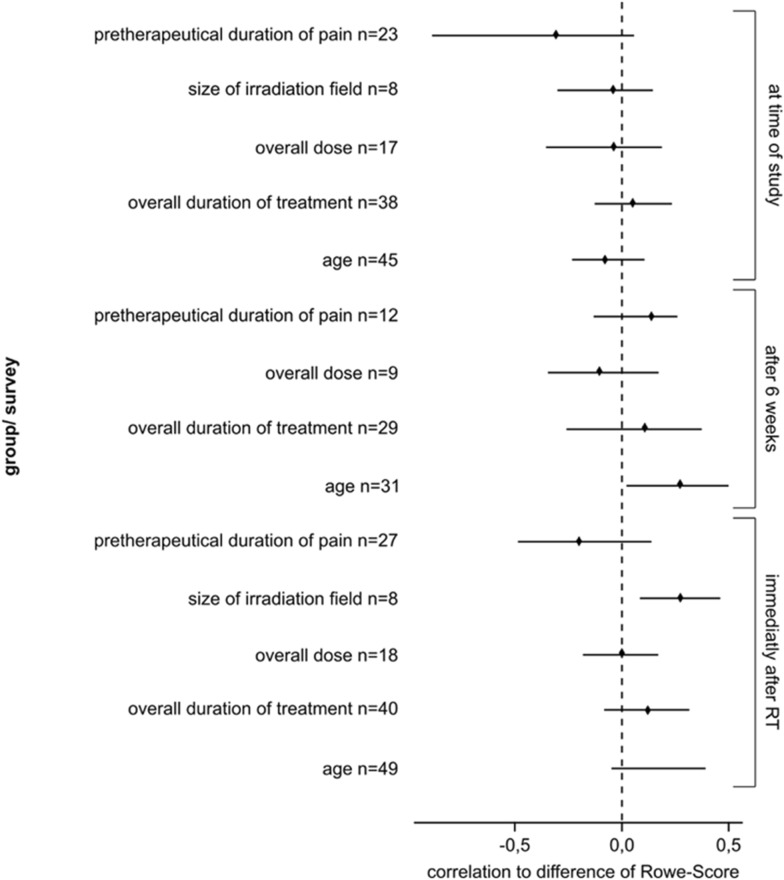
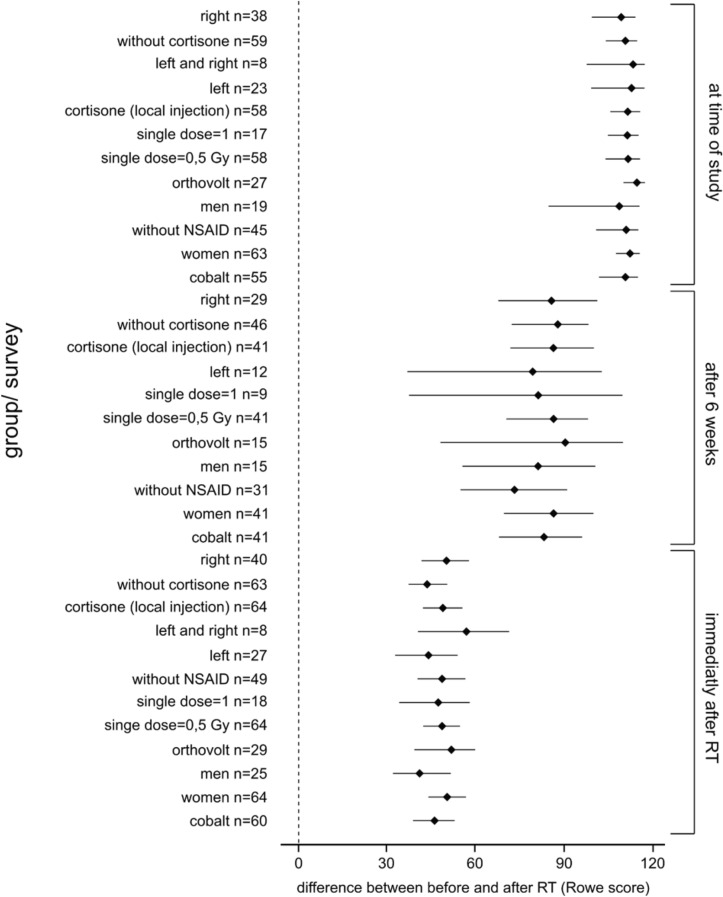
As presented in Figs. 3, 4 the best results were achieved 6 weeks after radiation therapy and if the radiation field was large.
Fig. 3.
Bootstrap confidence interval of the therapeutic results for the correlation of age, total time of treatment, total dose, radiation file and the pretherapeutic time of pain to the difference of the Rowe Scores before RT, on the last day of RT, 6 weeks after RT and at the time of the final data collection. NSAID non-steroidal anti-inflammatory drug
Fig. 4.
Bootstrap confidence intervals of radiation results using the modified Rowe Scores on the last day of RT, 6 weeks after RT and at data collection. Also, this investigation shows that the best results were achieved 6 weeks after RT
The long-term response was evaluated in 102 patients after a median follow-up period of 94.4 months (range 36–187 months). 16 patients received 2 irradiation series (4 patients received orthovoltage radiotherapy, 9 patients received irradiation using cobalt and 3 patients both). Furthermore, the influence of different parameters (radiotherapeutic and clinical) on the therapeutic response (pain reduction) of the irradiation of heel spurs was evaluated. There were no significant differences between the groups receiving different single doses at any time point (p-value: on the last day of RT: 0.922, 6 weeks after RT: 0.865, at data collection: 0.949). Similarly, no significant differences following the Pearson correlation could be identified between the total dose (3 or 6 Gy) and the therapeutic results according to the Rowe Scores on the last day of irradiation (r = − 0.157, p = 0.172), 6 weeks after completion of radiotherapy (r = 0.013, p = 0.905) and at data collection (r = − 0.061, p = 0.670). Moreover, no significant influence of the total treatment time on any outcome was detected using the Pearson correlation: on the last day of radiotherapy (r = 0.06, p = 0.5), 6 weeks after irradiation (r = 0.022, p = 0.87) and at data collection (r = − 0.12, p = 0.27).
Regarding the size of the radiation fields, there was a significant influence (p = 0.011, r = − 0.0282) of the field size on the clinical outcome at 6 weeks after irradiation. This indicates a better effect of radiotherapy when an extended field was used, to irradiate the complete pain expansion on the heel. Nevertheless, this effect did not influence the early outcome on the last day of irradiation (r = − 0.052, p = 0.657) and the late outcome at data collection (r = − 0.036, p = 0.806). A pretreatment of patients with oral NSAIDs had a significant effect (p = 0.047) 6 weeks after completion of radiotherapy. While patients who did not receive NSAIDs (n = 23) had an average score of 65.22 (SD = 29.52), patients undergoing NSAID therapy (n = 32) achieved an average score of 51.25 (SD = 24.05). This indicates that pretreated patients who continued NSAID therapy experienced a lesser pain improvement than patients receiving radiotherapy without NSAIDs. This effect was not significant at long-term follow-up.
Though not performed via randomization, we analyzed any effect of local steroid injection on therapeutic outcome. We identified a significant lapse of pain reduction when local corticosteroid injection on the last day of radiotherapy was administered (p = 0.036). 31 patients without prior corticoid therapy had an average score of 34.19 (SD = 16.6) compared to 24 patients who had received corticoid injections reported an average score of 23.7 (SD = 20.6). This significant difference may indicate that patients receiving local infiltrations on the last day of radiotherapy experienced less pain reduction than patients without corticoid therapy. It could also be that these patients have a more persistent plantar enthesophyte and that there has been no improvement even after many pre-therapies. This significance was also observed 6 weeks after radiotherapy as well (p = 0.001), while it was not significantly different at long-term outcome (p ≥ 0.05).
There was no significant impact of the age of the patients on the therapy outcome on the last day of radiotherapy (r = 0.17, p = 0.12) and at long-term follow-up (r = 0.078, p = 0.49). Nevertheless, there was a significant influence of the patients’ age on the therapeutic results 6 weeks after radiotherapy (r = 0.28, p = 0.041). Higher age was significantly associated with improved outcome 6 weeks after irradiation.
Using the Mann–Whitney U-test, we were able to demonstrate that neither the patients’ gender, nor the technique used for radiotherapy did influence the outcome at any time. The symptomatic time before the beginning of radiotherapy showed a significant correlation on long-term outcome (p = 0.012, r = 0.315). Patients receiving irradiation a few months after the onset of plantar enthesophyte pain benefited significantly more from radiotherapy and experienced a higher pain remission than patients with delayed irradiation.
In the present study, we have categorized pain into functional scores. The pain was subdivided into pain at rest, pain at motion and pressure pain. Overall, 33 patients (32.7%) reported a complete pain regression six weeks after RT. After a follow-up of 94.4 months (range: 36–187 months), 74 patients (73%) achieved a complete response. These results confirm the effectivity of this treatment approach. The analysis of functional scores revealed that 85.5% of the patients had none or only minor restrictions in daily activities and 89% had a normal gait pattern.
Discussion
Degenerative and inflammatory diseases of the musculoskeletal system make up 70% of all benign diseases treated by irradiation [3, 5, 6, 22, 29–40]. In Germany, more than 3500 patients annually are treated with this disease [41]. The use of low-dose irradiation when treating degenerative and inflammatory diseases has a long tradition in middle-European countries, especially in Germany [41–45].
Low-dose radiation decreases the expression and activity of glutathione peroxidase (Gpx) and nuclear factor erythroid 2-related factor 2 (Nrf2) in endothelial cells. The adhesion of peripheral blood mononuclear cells is also reduced. Large et al. provided evidence for an anti-inflammatory effect in endothelial cells stimulated by inflammation after low-dose radiation (largest effect after irradiation using 0.5 Gy) [46].
Historically, plantar enthesophytes were treated surgically in most symptomatic patients. Since surgery carries a high risk of recurrence, it is rarely performed today. Moreover, the treatment focus on symptomatic pain relief. Physiotherapy, footbed insert and medical treatment using NSAID is associated with a high therapeutic failure rate. Multiple studies in German-speaking countries reported the benefits of radiation treatment in the treatment of plantar enthesophytes in the past [6, 22, 25–27, 41, 43, 44, 47–56].
Table 3 shows the results of 19 publications between 1924 and 2004 with a total number of 3325 cases. Radiation concepts included multiple dose schemes and radiation qualities. On average, a complete pain response was achieved in 55.8% of the cases. Partial pain responses were reported in 31.2%, while 13.0% had no improvement after radiation therapy. Overall response rates varied between 65 and 100% (mean: 87.6%). We used these cases as a control group for our patients.
Table 3.
Summary of previous published studies
| Publication | Patients | Dose (Gy) | Technique | Response-rate (%) | CR (%) | PR (%) | NR (%) |
|---|---|---|---|---|---|---|---|
| n | ED/GD | ||||||
| Richarz (1924) [57] | 5 | – | Orthovolt | 100 | 80 | 20 | 0 |
| Pannewitz (1933) [58] | 88 | Orthovolt | |||||
| Mustakallio & Laitinien (1939) [59] | 17 | 1.0–1.5/4.0–6.0 | Orthovolt | 82 | 76 | 6 | 18 |
| Cocchi (1943) [60] | 6 | 1.8/9.0 | Orthovolt | 83 | 33 | 50 | 17 |
| Pizon (1957) [61] | 3 | – | Orthovolt | 100 | 100 | 0 | 0 |
| Wieland & Kuttig (1965) [62] | 16 | 1.0/4.0 | Cobalt-60 | 100 | 74 | 13 | 13 |
| Mitrov & Harbov (1967) [63] | 1520 | 0.5–1.5/3.0–9.0 | Orthovolt | 88 | 50 | 38 | 12 |
| Zschache (1972) [64] | 49 | 0.74–1.5/2.25–4.5 | Orthovolt | 86 | 12 | 74 | 14 |
| Mantell (1978) [65] | 26 | 2.0/10.0 | 240–300 kV | 65 | 53 | 12 | 35 |
| Basche et al. (1980) [66] | 102 | 0.3–0.5/4.0 | 120 kV | 90 | 32 | 58 | 10 |
| Sautter-Bihl et al. (1993) [67] | 15 | 0.5–1.0/2.5–6.0 | Cobalt-60 | 80 | 60 | 20 | 20 |
| Schäfer et al. (1995) [68] | 11 | 0.5–1.0/2.0–4.0 | Cobalt-60 | 72 | 13 | 59 | 27 |
| Seegenschmiedt et al. (1996) [69] | 72 | 1.0/12.0 | 250 kV | 100 | 67 | 33 | 0 |
| 98 | 0.3–0.5/3.0–5.0 | 200 kV | 95 | 72 | 23 | 5 | |
| Lederer et al. (1998) [70] | 21 | 1.0/6.0 | 4–6 MV, cobalt-60 | 91 | 43 | 48 | 9 |
| Oehler & Hentschel (2000) [71] | 258 | – | Orthovolt | 88 | 81 | 7 | 12 |
| Koeppen et al. (2000) [72] | 673 | 0.3/1.5–3.0 | 250 kV | 78 | 13 | 65 | 22 |
| Schreiber et al. (2000) [73] | 87 | 1.0/6.0 | 6MV | 86 | 67 | 19 | 14 |
| Heyd et al. (2001) [74] | 105 | 6MV | 88 | 46 | 42 | 12 | |
| Glatzel et al. (2001) [75] | 161 | 1.0/6.0–12.0 | 175 kV | 89 | 63 | 26 | 11 |
| Mücke et al. (2003) [76] | 117 | 0.5/5.0–1.0 | 6MV | 89 | 73 | 16 | 11 |
| Schneider et al. (2004) [77] | 68 | 0.25–1.0/5.0 | 10MV | 90 | 53 | 37 | 10 |
| Heyd et al. (2006) [78] | 252 | 1.0/6.0 | 6MV | 85.6 | 44.3 | 19.7 | 15.4 |
| Heyd et al. (2007) [79] | 130 | 0.5–1.0/3.0–6.0 | 6MV | 87.7 | 12.3 | ||
| Niewald et al. (2012) [80] | 66 | 1.0–0.1/6.0–0.6 | 6MV |
CR complete remission, ED single dose, GD total dose, NR no remission, PR partial remission
Despite comprehensive clinical results, the optimal radiation dose concept needs yet to be identified. In the pattern-of-care study by Micke and Seegenschmiedt [41], single doses varied from 0.3 to 1.5 Gy and total doses from 2.5 to 18.75 Gy. In most institutions, two (44%) to three (37.5%) weekly fractions of 0.5 to 1.5 Gy were utilized. However, the authors failed to demonstrate a dose relationship. Seegenschmiedt et al. [6] identified in his study the best response rates if patients received 5 Gy or 12 Gy total dose, while patients with only 3 Gy total dose had significantly worse outcome.
Heyd et al. recently published a prospective, randomized study comparing the effect of 3 Gy (6 fractions of 0.5 Gy) vs. 6 Gy (6 fractions of 1 Gy). Both groups demonstrated a significant improvement of symptoms. A different effect of the applied radiation dose was not reported.
Our study identified size of the radiation field as a significant factor for the treatment response 6 weeks after radiation (p = 0.011). At the time of long-term follow-up, this effect could no longer be demonstrated. Comparable results had not been reported previously.
Previous treatments with NSAID or local steroid injections had a detrimental effect on the response to therapy. NSAID-pretreated patients had a reduced pain response at 6 weeks after RT and corticoid injections led to a worse response at the last day of RT and 6 weeks hereafter. We were unable to obtain any comparable data from the literature.
Statistical analysis provided evidence that a short pretherapeutic pain duration [6, 22, 26, 54, 81] and an increased patients’ age [6, 26, 54] were prognostic factors for improved outcome. Glatzel and coauthors reported an improved outcome when pain duration was below 12 months or the patient’s age above 50 years [26]. Similarly, Mücke [43] and Schneider [54] also reported a significantly better pain response with a pretherapeutic pain duration of < 6 months [81].
Acute and late side effects of radiation treatment have not been studied in the present population. Some authors describe an initial pain exacerbation probably due to a probably localized acidotic tissue reaction [82]. In theory, any radiation therapy may be associated with an elevated risk of cancer [83–85]. Using the low intensity as performed here, it is unlikely for developing any malignant transformation, though unmasking this possibility may take years.
This study is limited by the retrospective study design. Furthermore, the investigated cohorts were irradiated with different concepts and techniques, which strongly reduces the validity of this study.
Furthermore, in consideration of the benign condition, the length of the follow-up is not sufficient for a conclusive evaluation concerning the role of radiotherapy. Nevertheless, this study represents real-life data from a large cohort of patients with high levels of pain from a disease that has not been sufficiently investigated by large prospective trials, providing results to help inform radiotherapy decision-making.
Conclusion
In addition to conservative treatment using physiotherapy, lifestyle changes, oral medication and orthopedic devices, radiotherapy can be regarded as a treatment option for painful plantar enthesophyte. Low radiation doses lead to a significant long-term pain reduction in more than 60% of the treated patients. In accordance with previous publications [22, 26, 43, 54, 86], we found that pretreatment pain duration was a significant prognostic factor for treatment response. In future prospective studies further associated parameters should be evaluated. In order to better assess the role of radiotherapy, prospective studies or analyses with a longer follow-up would be desirable.
Acknowledgements
This work is dedicated to Prof. em. Dr. Gerd Schmitt, former Chair of the Department of Radiation Therapy, University Hospital Düsseldorf.
Abbreviations
- Co
Cobalt
- Gy
Gray
- NSAID
Non-steroidal anti-inflammatory drugs
- RT
Radiotherapy
- SD
Standard deviation
- S-Score
Sum-Score
Authors’ contributions
FD, EB, MP, JH, JN, DJ, KM, RG, CS, BT, SC, KO, MvG, KK, AR, WB, CM wrote parts of the manuscript. FD and BT did the literature research and prepared the data for analysis. FD, MP, CM, BT, EB, DJ and JH contributed significantly to the discussion on the interpretation of the results. FD and BT contributed equally to this work as first authors. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All data and materials can be accessed via CM and FD.
Declarations
Ethics approval and consent to participate
The study was approved by the local ethical commission board.
Consent for publication
All authors gave consent for the publication.
Competing interests
All authors including the corresponding author declare that they have no competing interests. Martijn van Griensven is the chief editor of the European Journal of Medical Research. Edwin Bölke is a section editor of the European Journal of Medical Research. Christiane Matuschek is an associate editor of the European Journal of Medical Research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Freddy Djiepmo and Bálint Tamaskovics shared first authorship
Contributor Information
Freddy Djiepmo, Email: frdji100@hhu.de.
Bálint Tamaskovics, Email: Balint.Tamaskovics@med.uni-duesseldorf.de.
Edwin Bölke, Email: boelke@med.uni-duesseldorf.de.
Matthias Peiper, Email: Peiper@mail.de.
Jan Haussmann, Email: Jan.Haussmann@med.uni-duesseldorf.de.
Judith Neuwahl, Email: Judith.Neuwahl@med.uni-duesseldorf.de.
Danny Jazmati, Email: Danny.Jazmati@med.uni-duesseldorf.de.
Kitti Maas, Email: Kitti.Maas@med.uni-duesseldorf.de.
Livia Schmidt, Email: Livia.Schmidt@med.uni-duesseldorf.de.
Roman Gelzhäuser, Email: Roman.Gelzhaeuser@uni-duesseldorf.de.
Christoph Schleich, Email: info@mvz-radiologie-viersen.de.
Stefanie Corradini, Email: Stefanie.Corradini@med.uni-muenchen.de.
Klaus Orth, Email: Klaus.Orth@gmx.de.
Martijn van Griensven, Email: M.vanGriensven@maastrichtuniversity.nl.
Amir Rezazadeh, Email: Amir.Rezazadeh@med.uni.duesseldorf.de.
Kimia Karimi, Email: Kimia.karimi@med.uni-duesseldorf.de.
Wilfried Budach, Email: Wilfried.Budach@med.uni-duesseldorf.de.
Christiane Matuschek, Email: Matuschek@med.uni-duesseldorf.de.
References
- 1.Boike AM, Snyder AJ, Roberto PD, Tabbert WG. Heel spur surgery. A transverse plantar approach. J Am Podiatr Med Assoc. 1993;83:39–42. doi: 10.7547/87507315-83-1-39. [DOI] [PubMed] [Google Scholar]
- 2.Prichasuk S. The heel pad in plantar heel pain. J Bone Joint Surg Br. 1994;76:140–142. [PubMed] [Google Scholar]
- 3.Sistermann R, Katthagen B-D. 5 Jahre Lithotripsie des plantaren Fersenspornes: Erfahrungen und Ergebnisse-eine Nachuntersuchung nach 36, 9 Monaten. Z Orthop Ihre Grenzgeb. 1998;136:402–406. doi: 10.1055/s-2008-1053675. [DOI] [PubMed] [Google Scholar]
- 4.Weil LS, Jr, Roukis TS, Weil LS, Borrelli AH. Extracorporeal shock wave therapy for the treatment of chronic plantar fasciitis: indications, protocol, intermediate results, and a comparison of results to fasciotomy. J Foot Ankle Surg. 2002;41:166–172. doi: 10.1016/s1067-2516(02)80066-7. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz M. Treatment choices for plantar fasciitis. Am Fam Physician. 1999;60:2504. [PubMed] [Google Scholar]
- 6.Seegenschmiedt MH, Keilholz L, Stecken A, Katalinic A, Sauer R. Radiotherapy of plantar heel spurs: indications, technique, clinical results at different dose concepts. Strahlenther Onkol. 1996;172:376–383. [PubMed] [Google Scholar]
- 7.Prichasuk S, Subhadrabandhu T. The relationship of pes planus and calcaneal spur to plantar heel pain. Clin Orthop Relat Res 1994. 192–6. [PubMed]
- 8.Riepert T, Drechsler T, Urban R, Schild H, Mattern R. Häufigkeit, Altersabhängigkeit und Geschlechtsverteilung des Fersensporns. Fortschr Röntgenstr. 1995 doi: 10.1055/s-2007-1015925. [DOI] [PubMed] [Google Scholar]
- 9.Gill LH. Plantar fasciitis: diagnosis and conservative management. J Am Acad Orthop Surg. 1997;5:109–117. doi: 10.5435/00124635-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Brown C. A review of subcalcaneal heel pain and plantar fasciitis. Aust Fam Physician. 1996;25(875–81):84–85. [PubMed] [Google Scholar]
- 11.Hohmann G, et al. Handbuch der Orthopädie. Stuttgart: Thieme; 1961. [Google Scholar]
- 12.Schreiber A. Entzündungen/Fersensporne. Stuttgart: Thieme; 1985. [Google Scholar]
- 13.Stepanek P, Stepanek V. Zur Problematik des Kalkaneussporns. Z Rheumaforsch. 1967;26(9):353–363. [PubMed] [Google Scholar]
- 14.Stucke K. Der Fersenschmerz. Stuttgart: Thieme; 1956. [Google Scholar]
- 15.Cornwall MW, McPoil TG. Plantar fasciitis: etiology and treatment. J Orthop Sports Phys Ther. 1999;29:756–760. doi: 10.2519/jospt.1999.29.12.756. [DOI] [PubMed] [Google Scholar]
- 16.Furey JG. Plantar fasciitis. The painful heel syndrome. J Bone Joint Surg Am. 1975;57:672–673. [PubMed] [Google Scholar]
- 17.Gudeman SD, Eisele SA, Heidt RS, Jr, Colosimo AJ, Stroupe AL. Treatment of plantar fasciitis by iontophoresis of 0.4% dexamethasone. A randomized, double-blind, placebo-controlled study. Am J Sports Med. 1997;25:312–6. doi: 10.1177/036354659702500307. [DOI] [PubMed] [Google Scholar]
- 18.Chandler TJ, Kibler WB. A biomechanical approach to the prevention, treatment and rehabilitation of plantar fasciitis. Sports Med. 1993;15:344–352. doi: 10.2165/00007256-199315050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sack GM. Über den Kalkaneussporn. Röntgenpraxis (4) 1932. pp. 158–67.
- 20.DeMaio M, Paine R, Mangine RE, Drez D., Jr Plantar fasciitis. Orthopedics. 1993;16:1153–1163. doi: 10.3928/0147-7447-19931001-13. [DOI] [PubMed] [Google Scholar]
- 21.Tisdel CL, Donley BG, Sferra JJ. Diagnosing and treating plantar fasciitis: a conservative approach to plantar heel pain. Cleve Clin J Med. 1999;66:231–235. doi: 10.3949/ccjm.66.4.231. [DOI] [PubMed] [Google Scholar]
- 22.Hammer DS, Rupp S, Kreutz A, Pape D, Kohn D, Seil R. Extracorporeal shockwave therapy (ESWT) in patients with chronic proximal plantar fasciitis. Foot Ankle Int. 2002;23:309–313. doi: 10.1177/107110070202300403. [DOI] [PubMed] [Google Scholar]
- 23.Krischek O, Rompe J-D, Herbsthofer B, Nafe B. Symptomatische niedrig-energetische Stoßwellentherapie bei Fersenschmerzen und radiologisch nachweisbarem plantaren Fersensporn. Z Orthop Ihre Grenzgeb. 1998;136:169–174. doi: 10.1055/s-2008-1051301. [DOI] [PubMed] [Google Scholar]
- 24.Ogden JA, Alvarez RG, Marlow M. Shockwave therapy for chronic proximal plantar fasciitis: a meta-analysis. Foot Ankle Int. 2002;23:301–308. doi: 10.1177/107110070202300402. [DOI] [PubMed] [Google Scholar]
- 25.Perlick LBWGG. Hochenergetische Stoßwellenbehandlung des schmerzhaften Fersenspornes. Unfallchirurg. 1998;101:914–918. doi: 10.1007/s001130050358. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber H, Böhnlein G, Ziegler K. Strahlentherapie des schmerzhaften Fersenspornes. Altenberge: Diplodocus-Verlag; 2000. [Google Scholar]
- 27.Dailey JM. Differential diagnosis and treatment of heel pain. Clin Podiatr Med Surg. 1991;8:153–166. [PubMed] [Google Scholar]
- 28.Kulthanan T. Operative treatment of plantar fasciitis. J Med Assoc Thai. 1992;75(6):337–40. [PubMed] [Google Scholar]
- 29.Oehler W, Hentschel B. Niedrigdosierte analgetische Radiotherapie von Arthrosen. Ärztebl Thüring. 2000;11:92–95. [Google Scholar]
- 30.Glatzel M, Bäsecke S, Krauß A, Fröhlich D. Radiotherapy of the painful plantar heel spur. Benig News. 2001;2:18–19. [Google Scholar]
- 31.Mitrov G, Harbov I. Unsere Erfahrungen mit der Strahlentherapie von nichttumorartigen Erkrankungen. Radiobiol Radiother. 1967;8:419. [PubMed] [Google Scholar]
- 32.Rowe CR, Sakellarides HT, Freeman PA, Sorbie C. Fractures of the os calcis: a long-term follow-up study of 146 patients. JAMA. 1963;184:920–923. [Google Scholar]
- 33.Seegenschmiedt MH, Katalinic A, Makoski H-B, Haase W, Gademann G, Hassenstein E. Strahlentherapie von gutartigen Erkrankungen: eine Bestandsaufnahme für Deutschland. Strahlenther Onkol. 1999;175:541–547. doi: 10.1007/s000660050038. [DOI] [PubMed] [Google Scholar]
- 34.Seegenschmiedt MH, Keilholz L, Katalinic A, Stecken A, Sauer R. Heel spur: radiation therapy for refractory pain–results with three treatment concepts. Radiology. 1996;200:271–276. doi: 10.1148/radiology.200.1.8657925. [DOI] [PubMed] [Google Scholar]
- 35.Arican M, Turhan Y, Karaduman ZO. A rare cause of heel pain: a calcaneal spur fracture. J Am Podiatr Med Assoc. 2019;109:172–173. doi: 10.7547/17-111. [DOI] [PubMed] [Google Scholar]
- 36.Behounek Ml J, Skotak M, Behounek St J. Open heel spur surgery—our experience. J Acta Chir Orthop Traumatol Cech. 2019;86:212–215. [PubMed] [Google Scholar]
- 37.Doruk AP. Role of radiotherapy in the management of heel spur. Eur J Orthop Surg Traumatol. 2017;27:569. doi: 10.1007/s00590-017-1935-7. [DOI] [PubMed] [Google Scholar]
- 38.Krol P, Franek A, Krol T, et al. Ground reaction force analysis for assessing the efficacy of focused and radial shockwaves in the treatment of symptomatic plantar heel spur. J Back Musculoskelet Rehabil. 2021;34:279–287. doi: 10.3233/BMR-191739. [DOI] [PubMed] [Google Scholar]
- 39.Luo JC, Lang BX. Case-control study on the treatment of heel spur syndrome with modified stretching manipulation combined with needle Dao loosing. Zhongguo Gu Shang. 2018;31:504–509. doi: 10.3969/j.issn.1003-0034.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Notarnicola A, Maccagnano G, Moretti L, et al. Could the presence of heel spur be a prognostic factor for outcome of extracorporeal shock wave therapy for plantar fasciitis? J Biol Regul Homeost Agents. 2019;33:1949–1954. doi: 10.23812/19-264-L. [DOI] [PubMed] [Google Scholar]
- 41.Prokein B, Holtmann H, Hautmann MG, et al. Radiotherapy of painful heel spur with two fractionation regimens: results of a randomized multicenter trial after 48 weeks’ follow-up. Strahlenther Onkol. 2017;193:483–490. doi: 10.1007/s00066-017-1116-y. [DOI] [PubMed] [Google Scholar]
- 42.Uysal B. Reply to letter to the editor about radiotherapy in the management of heel spur pain. Eur J Orthop Surg Traumatol. 2018;28:757. doi: 10.1007/s00590-017-2106-6. [DOI] [PubMed] [Google Scholar]
- 43.Zahnreich S, Rosler HP, Schwanbeck C, Karle H, Schmidberger H. Radiation-induced DNA double-strand breaks in peripheral leukocytes and therapeutic response of heel spur patients treated by orthovoltage X-rays or a linear accelerator. Strahlenther Onkol. 2020;196:1116–1127. doi: 10.1007/s00066-020-01662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micke O, Seegenschmiedt M. Radiotherapy in painful heel spurs (plantar fasciitis). Results of a national patterns of care study. Int J Radiat Oncol Biol Phys. 2004;58:828–43. doi: 10.1016/S0360-3016(03)01620-1. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer U, Micke O, Glashörster M, Rübe C, Prott F, Willich N. The radiotherapy treatment of painful calcaneal spurs. Strahlenther Onkol. 1995;171:202–6. [PubMed] [Google Scholar]
- 46.Muecke R, Micke O, Reichl B, et al. Demographic, clinical and treatment related predictors for event-free probability following low-dose radiotherapy for painful heel spurs—a retrospective multicenter study of 502 patients. Acta Oncol. 2007;46:239–246. doi: 10.1080/02841860600731935. [DOI] [PubMed] [Google Scholar]
- 47.Richarz A. Die Röntgenbehandlung der Epikondylitis und Kalkaneodynie. Fortschr Röntgenstr. 1924;32:460. [Google Scholar]
- 48.Trott K, Parker R, Seed M. The effect of X-rays on the experimental arthritis in rats; Die Wirkung von Roentgenstrahlen auf die experimentelle Arthritis der Ratte. Strahlenther Onkol. 1995;171(9):534–8. [PubMed] [Google Scholar]
- 49.Large M, Hehlgans S, Reichert S, et al. Study of the anti-inflammatory effects of low-dose radiation: the contribution of biphasic regulation of the antioxidative system in endothelial cells. Strahlenther Onkol. 2015;191:742–749. doi: 10.1007/s00066-015-0848-9. [DOI] [PubMed] [Google Scholar]
- 50.Basche S, Drescher W, Mohr K. Results of X-ray therapy of calcaneal spur. Radiobiol Radiother. 1980;21:233–236. [PubMed] [Google Scholar]
- 51.Cocchi U. Erfolge und Mißerfolge bei Röntgenbestrahlung nichtkrebsiger Leiden. Strahlentherapie. 1943;73:285. [Google Scholar]
- 52.Koeppen D, Bollmann G, Gademann G. Ein Beitrag zur Dosiswirkungsbeziehung bei der Röntgentherapie des Fersensporns. Strahlenther Onkol. 2000;176:91. [Google Scholar]
- 53.Lederer K, et al. Perkutane Radiatio des schmerzhaften Fersensporns. MTA. 1998;13:488–491. [Google Scholar]
- 54.Mantell BS. Radiotherapy for painful heel syndrome. Br Med J. 1978;2:90–91. doi: 10.1136/bmj.2.6130.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mustakallio S, Laitinen H. Über die Insertionsschmerzen IHRE Röntgen-Diagnostik und-Behandlung. Acta Radiologica. 1939;20:427–37. [Google Scholar]
- 56.Pizon P. La roentgentherapie des affections rhumatismales. Paris: Masson & Cie; 1957. pp. 142–3. [Google Scholar]
- 57.Schneider O, Stuckle CA, Bosch E, Gott C, Adamietz IA. Effectiveness and prognostic factors of radiotherapy for painful plantar heel spurs. Strahlenther Onkol. 2004;180:502–509. doi: 10.1007/s00066-004-1204-7. [DOI] [PubMed] [Google Scholar]
- 58.Wieland C, Kuttig H. High-voltage therapy in arthroses and inflammations. Strahlentherapie. 1965;127:44–48. [PubMed] [Google Scholar]
- 59.Zschache H. Ergebnisse der Röntgenschwachbestrahlung. Radiobiol Radiother. 1972;13:181–186. [PubMed] [Google Scholar]
- 60.Lee AY, Akileswaran L, Tibbetts MD, Garg SJ, Van Gelder RN. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology. 2015;122:524–530. doi: 10.1016/j.ophtha.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Focosi D, Maggi F, Albani M, et al. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J Clin Virol. 2010;47:189–192. doi: 10.1016/j.jcv.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 62.Mustakallio SLH. Über die insertionsschmerz—ihre Diagnostik und Behandlung. Acta Radiol. 1939;20:427–437. [Google Scholar]
- 63.Cocchi U. Erfolg und Mißerfolg bei Röntgenbestrahlung nichtkrebsiger Leiden. Strahlentherapie. 1943;73:255–84. [Google Scholar]
- 64.Solodky ML, Galvez C, Russias B, et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieland CKH. Hochvolttherapie bei Arthrosen und Entzündungen. Strahlentherapie. 1965;127:44–48. [PubMed] [Google Scholar]
- 66.Mitrov GHI. Unsere Erfahrungen mit der Strahlenbehandlung von nichttumorartigen Erkrankungen. Radiobiol Radiother. 1967;8:419–422. [PubMed] [Google Scholar]
- 67.Chang TJ, Yang DM, Wang ML, et al. Genomic analysis and comparative multiple sequences of SARS-CoV2. J Chin Med Assoc. 2020;83:537–543. doi: 10.1097/JCMA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 68.Bs M. Radiotherapy for painful heel syndrome. Br Med J. 1978;2:90–91. doi: 10.1136/bmj.2.6130.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basche S, Drescher W, Mohr K. Ergebnisse der Röntgenstrahlentherapie bei Fersensporn. Radiobiol Radiother. 1980;21:233–236. [PubMed] [Google Scholar]
- 70.Sautter-Biehl MLLH, Scheurig H, Heinze GH. Analgetische Bestrahlung degenerativ entzündlicher Skeletterkrankungen. Dtsch med Wschr. 1993;118:493–498. doi: 10.1055/s-2008-1059354. [DOI] [PubMed] [Google Scholar]
- 71.Schäfer UMO, Glashörster M, et al. Strahlentherapeutische Behandlung des schmerzhaften Fersensporns. Strahlenther Onkol. 1995;171:202–206. [PubMed] [Google Scholar]
- 72.Seegenschmiedt MHKL, Katalinic A, Stecken A, Sauer R. Heel Spur: radiation therapy for refractory pain-results with three treatment concepts. Radiology. 1996;200:271–276. doi: 10.1148/radiology.200.1.8657925. [DOI] [PubMed] [Google Scholar]
- 73.Lederer KNU, Walter K, et al. Perkutane Radiatio des schmerzhaften Fersensporns. MTA. 1998;13:488–491. [Google Scholar]
- 74.Oehler WHB. Niedrigdosierte analgetische Radiotherapie von Arthrosen. Ärzteblatt Thüringen. 2000;11:92–95. [Google Scholar]
- 75.Koeppen DBG, Gademann G. Ein Beitrag zur Dosiswirkungsbeziehung bei der Röntgentherapie des Fersensporns. Strahlenther Onkol. 2000;176(Sondernr 1):91. [Google Scholar]
- 76.Schreiber H, Böhnlein G, Ziegler K. Strahlentherapie des schmerzhaften Fersenspornes. In: Seegenschmiedt MH, Makoski HB, editors. 10. Kolloquium Radioonkologie/Strahlentherapie Radiotherapie von gutartigen Erkrankungen. Altenberge: Diplodocus-Verlag; 2000. pp. S186–187. [Google Scholar]
- 77.Heyd R, Strassmann G, Filipowicz I, Borowsky K, Martin T, Zamboglou N. Radiotherapy in the management of inflammatory calcaneal spurs: results of a prospective study. In: Seegenschmiedt MH, Makoski HB, editors. 15. Kolloquim Radioonkologie/Strahlentherapie, Radiotherapie von gutartigen Erkrankungen. Altenberge: Diplodocus-Verlag; 2001. [Google Scholar]
- 78.Glatzel M, Bäsecke S, Krauß A, Fröhlich D. Radiotherapy of painful plantar heel spur. Benig News. 2001;2:18–19. [Google Scholar]
- 79.Mücke R, Schönekaes K, Micke O, Berning D, Heyder R. Radiotherapy of painful heel spurs—a retrospective study of 117 patients treated with 6-MeV-photons. Strahlenther Onkol. 2003;179:774–778. doi: 10.1007/s00066-003-1126-9. [DOI] [PubMed] [Google Scholar]
- 80.Schneider O, Stückle CA, Bosch E, Gott C, Adamietz IA. Effectiveness and prognostic factors of radiotherapy for painful plantar heel spurs. Strahlenther Onkol. 2004;180:502–509. doi: 10.1007/s00066-004-1204-7. [DOI] [PubMed] [Google Scholar]
- 81.Heyd R, Tselis N, Ackermann H, Röddiger SJ, Zamboglou N. Funktionelle Ergebnisse nach Megavoltbestrahlung beim Fersensporn. Strahlenther Onkol. 2006;182:733–739. doi: 10.1007/s00066-006-1569-x. [DOI] [PubMed] [Google Scholar]
- 82.Heyd R, Tselis N, Ackermann H, Rödiger S, Zamboglou N. Radiation therapy for painful heel spurs. Stahlenther Onkol. 2007;183:3–9. doi: 10.1007/s00066-007-1589-1. [DOI] [PubMed] [Google Scholar]
- 83.Mucke R, Schonekaes K, Micke O, Seegenschmiedt MH, Berning D, Heyder R. Low-dose radiotherapy for painful heel spur. Retrospective study of 117 patients. Strahlenther Onkol. 2003;179:774–778. doi: 10.1007/s00066-003-1126-9. [DOI] [PubMed] [Google Scholar]
- 84.Scherer E. Biologische Grundlagen und neuere Ergebnisse der Entzündungsbestrahlung und der funktionellen Röntgentherapie. Strahlentherapie. 1955;97:349–361. [PubMed] [Google Scholar]
- 85.Smklsakas R. Radiotherapie bei plantarem Fersensporn. Strahlenther Onkol. 1996;172:376–383. [PubMed] [Google Scholar]
- 86.Niewald M, Seegenschmiedt MH, Micke O, Graeber S, Muecke R, Schaefer V, Scheid C, Fleckenstein J, Licht N, Ruebe C. Randomized, multicenter trial on the effect of radiation therapy on plantar fasciitis (painful Heel Spur) comparing a standard dose with a very low dose: mature result after 12 months follow-up. Int J Radiat Oncol Biol. 2012 doi: 10.1016/j.ijrobp.2012.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials can be accessed via CM and FD.