Patients with cystic fibrosis (CF) suffer from impaired mucociliary clearance making them more susceptible to a spectrum of inhaled pathogens including bacteria, fungi and viruses [1, 2]. Therefore, patients with CF were considered at high risk for serious illness following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to “shielding or cocooning policies” [3]. Usually, patients with CF are cared for in specialised CF clinics with regular check-up visits.
Short abstract
FEV1 % predicted decreased substantially in paediatric patients with #cysticfibrosis during the first lockdown of the ongoing #SARSCoV2 pandemic in Germany. More information on consequences of repetitive shutdowns in people with cystic fibrosis is needed. https://bit.ly/3fZwuIb
To the Editor:
Patients with cystic fibrosis (CF) suffer from impaired mucociliary clearance making them more susceptible to a spectrum of inhaled pathogens including bacteria, fungi and viruses [1, 2]. Therefore, patients with CF were considered at high risk for serious illness following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to “shielding or cocooning policies” [3]. Usually, patients with CF are cared for in specialised CF clinics with regular check-up visits. It was shown that regular care by multidisciplinary CF teams with highly trained staff lead to improvements in clinical outcomes in patients with CF [4]. At our centre, patients are usually seen at least quarterly. Between March and May 2020, at the peak of the first wave of SARS-CoV-2 infection in Germany, national authorities imposed an 8-week lockdown period on the entire population. During this period, hospitals and healthcare facilities reduced elective healthcare services, including outpatient clinics, to a minimum to focus their resources on the care of patients with coronavirus disease 2019 (COVID-19). Accordingly, all scheduled visits to our CF outpatient clinic were postponed and patients were advised to stay in home isolation. Additionally, due to lockdown measures, the opportunities for patients to perform supportive therapies such as chest physiotherapy or physical exercise were limited. Hospital admission in case of severe clinical deterioration, e.g. pulmonary exacerbation, was available. Replacement of scheduled visits by, for example, video consultation or care by non-physician members of the multidisciplinary team was not yet implemented in this first lockdown period.
The aim of this study was to assess the impact of home isolation during this lockdown period on the health status of patients with CF focusing on lung function outcomes. This mono-centre observational study was performed at the CF centre of the Charité-Universitätsmedizin Berlin currently caring for 355 patients with CF (median age 23.8 years; interquartile range (IQR) 12.0–33.8 years; 133 (37.4%) patients <18 years of age). If receiving cystic fibrosis transmembrane conductance regulator (CFTR)-modulator therapy, patients had to receive this for more than 3 months before the start of the lockdown to be eligible for the study. Patients without lung transplantation or pulmonary exacerbation at the time of spirometry, and with at least one lung function test available within 3 months before and after the lockdown period were included. Spirometry was performed according to American Thoracic Society/European Respiratory Society criteria and percent predicted forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were calculated according to global lung function initiative values [5, 6]. In case more than one lung function test was available in the 3-month period pre- or post-lockdown, the mean value of each period was determined. For comparison, lung function values at the same time periods in the previous year were collected. Again, lung function values were excluded if a CFTR modulator therapy was initiated within the study period. In patients ≥18 years of age body mass index (BMI) was calculated, in those <18 years of age BMI-percentile according to age. Paired t-test was used for comparison of different time-points. The study was approved by the Ethics Committee at Charité–Universitätsmedizin Berlin (EA2/016/18) and all patients provided informed consent.
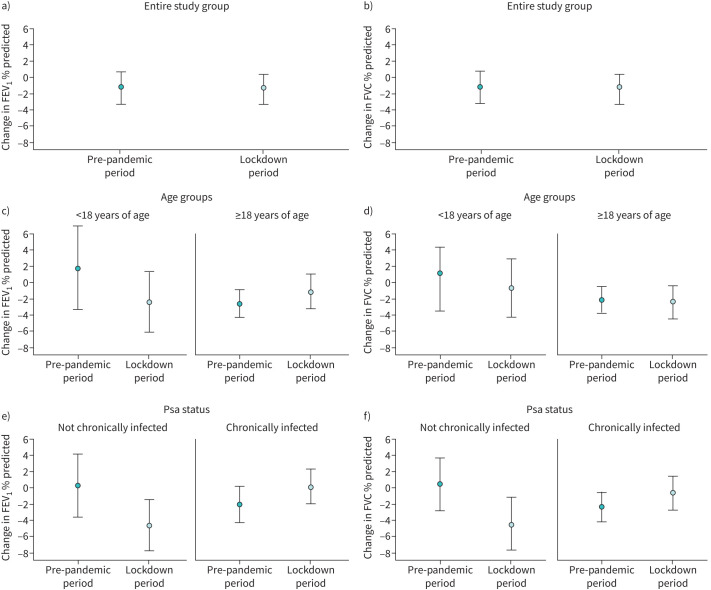
In total, 117/269 (43.5%) patients with CF were included, 73 (62.4%) were female and the median age was 23.4 years (IQR 14.2–35.4 years) with 75 patients (64.1%) being ≥18 years of age (median age 32.6 years, IQR 25.9–44.9) and 42 (35.9%) <18 years of age (median age 11.7 years, IQR 8.7–15.3). 50 (42.7%) patients received PCR-testing for SARS-CoV-2 up to the post-lockdown visit, with one out of 50 (2.0%) patients testing positive for SARS-CoV-2. 71 (60.7%) patients were chronically infected with Pseudomonas aeruginosa (Psa), four (3.4%) with Burkholderia species, and 10 (8.5%) with methicillin-resistant Staphylococcus aureus. Patients were considered chronically Psa-infected if Psa was detected in more than 50% of respiratory samples over a period of a minimum of 12 months. 55/75 (73.3%) of Psa-infected patients were ≥18 years of age compared to 16/42 (38.1%) of Psa-negative patients. During the study period, 53 patients (45.5%) received a CFTR modulator therapy, 20/42 (47.6%) paediatric patients (13 lumacaftor/ivacaftor, 2 tezacaftor/ivacaftor, 4 elexacaftor/tezacaftor/ivacaftor, 1 ivacaftor) and 33/75 (44.0%) adult patients (15 lumacaftor/ivacaftor, 14 tezacaftor/ivacaftor, 1 elexacaftor/tezacaftor/ivacaftor, 3 ivacaftor). In the whole cohort, FEV1 % predicted remained stable (mean (95% CI) pre- versus post-lockdown: 72.48% (68.40–76.57%) versus 70.99% (66.89–75.09%); p=0.080), FVC % predicted improved (84.63% (81.32–87.95%) versus 82.86% (79.48–86.23%); p=0.031). Comparing adult and paediatric patients we found that FEV1 % predicted remained stable in patients with CF ≥18 years of age (64.99% (60.18–69.79%) versus 64.33% (59.38–69.27%); p=0.526), but declined in younger patients by 3.0% (85.87% (80.16–91.58%) versus 82.88% (76.98–88.79%); p=0.05; figure 1c). FVC % predicted was stable in patients ≥18 years of age (79.93% (75.88–83.97%) versus 78.29% (74.16–82.42%); p=0.083; figure 1d) and in patients <18 years (93.04% (88.07–98.01%) versus 91.01% (85.86–96.16%); p=0.200). In CF patients without chronic Psa infection, FEV1 % predicted and FVC % predicted declined by 4.2 percentage points (80.77% (74.04–87.50%) versus 76.55% (70.05–83.06%); p=0.014) and 4.6 percentage points (89.68% (84.06–95.31%) versus 85.10% (79.33–90.88%); p=0.002), respectively, from pre- to post-lockdown visits; while in patients with chronic Psa infection, the differences were not significant (FEV1 67.12% (62.26–71.97%) versus 67.39% (62.15–72.62%); p=0.752; FVC 81.36% (77.38–85.35%) versus 81.40% (77.20–85.60%); p=0.968; figure 1e, f). In adult patients, the median BMI (IQR) before versus after lockdown was 21.2 (19.2–22.6) kg·m−2 versus 21.1 (19.1–22.8) kg·m−2 (p=0.06) and in paediatric patients it was 32.0 (17.8–49.3) percentile for age versus 32.0 (17.0–53.8) percentile for age (p=0.33).
FIGURE 1.
Comparison of change of lung function parameters in patients with cystic fibrosis over the lockdown period and over a comparable time period in the year before the pandemic in a, b) the entire study group, according to c, d) age group (<18 years of age and ≥18 years of age) and e, f) Pseudomonas aeruginosa (Psa) infection status (patients chronically and not chronically infected with Psa). Changes in forced expiratory volume in 1 s (FEV1) % predicted and forced vital capacity (FVC) % predicted are displayed as means and 95% confidence intervals.
To compare our findings with the course of lung function in the year prior to the pandemic, we analysed lung function tests of all patients that were performed in 2019 during time periods corresponding to the “pre-lockdown” (December 2018 to February 2019) and “post-lockdown” periods (June 2019 to August 2019). In total, lung function data of 66 patients were available for this analysis. Over this time period, lung function in the entire study population remained stable. Subgroup analysis showed that lung function remained stable in paediatric patients (<18 years of age). In adult patients, FEV1 % predicted and FVC % predicted declined by −2.8 percentage points (p=0.001) and −2.3 percentage points (p=0.005), respectively. In Psa negative patients, no change in lung function was seen, whereas in Psa positive patients FEV1 % predicted declined by −2.2 percentage points (p=0.040) and FVC % predicted also declined by −2.2 percentage points (p=0.021), respectively.
To our knowledge, this is the largest study so far evaluating the effect of a lockdown period during the COVID-19 pandemic on lung function outcomes in paediatric and adult patients with CF. While lung function remained stable in the entire study population, there was a significant decline in FEV1 % predicted by 3.0 percentage points in our paediatric patients (figure 1c). Of note, patients of the Psa negative group, mainly younger than 18 years, experienced a significant decline of 4.2 percentage points of FEV1 % predicted. This decline in FEV1 % predicted exceeds the annual decline reported in registry studies (−0.5 to −2.6 percentage points) and CFTR modulator trials (−0.1 to −2.2 percentage points) [7–9] and was not observed in our study population in the year prior to the pandemic. In contrast, in patients chronically infected with Psa, lung function declined in the year prior to the lockdown, but remained stable with a trend towards improvement over the lockdown period (figure 1e, f).
It is well established that CF care and patient outcomes improve if patients are seen regularly by trained, multidisciplinary CF teams. In a centre-based analysis, more frequent monitoring and increased use of appropriate therapies were associated with an improved outcome in CF [4]. In addition to limited access to specialised CF care, supportive therapies like physiotherapy and sports opportunities were also markedly reduced during the lockdown period. A study in 327 patients with CF from Switzerland showed that lockdown measures led to substantially decreased levels of physical activity [10]. Physical exercise is routinely recommended in CF care because of its beneficial effect on general fitness, airway clearance, lung function and quality of life. Physical activity is associated with increased peak oxygen uptake, which in turn is linked to better survival in CF [11]. However, due to the implemented infection control measures, spreading of seasonal viral or bacterial infection was reduced substantially in the general population during lockdown periods. Accordingly, the number of visits to paediatricians in general practice and hospital admissions due to acute respiratory tract infections markedly declined during the lockdown period compared with previous years [12]. Preceding viral infections are an acknowledged risk factor for pulmonary exacerbations in CF [13]. With reduced exposure to respiratory pathogens, the number of pulmonary exacerbations decreased during the time of COVID-19 restrictions in patients with CF and non-CF bronchiectasis [14, 15]. Despite all this, FEV1 % predicted dropped significantly in patients without chronic Psa infection, including most of the paediatric patients in our cohort, suggesting even short lockdown periods may have adverse effects on long-term outcomes of patients with CF. Especially reduced physical activity due to home isolation with limited access to sporting activities during lockdown might play a role in the younger age group.
The majority of chronically Psa-infected patients were adults, who might have been able to dedicate more time to CF therapy during the lockdown because of home office, reduced travel time and restricted social activities. Accordingly, lung function declined in Psa-infected patients in the pre-pandemic period but was stable over the lockdown period. There was no difference in BMI (or BMI-percentile) pre- and post-lockdown.
Our study is limited by its retrospective nature and the single centre analysis.
Taken together, in the entire study group of patients with CF lung function remained stable over the first lockdown period in Germany. But our data indicate potential adverse effects of the first lockdown period, with restricted access to healthcare and sporting activities, on the subgroups of paediatric and not Psa-infected patients with CF. These findings may be aggravated by prolonged lockdown periods. Therefore, information on consequences of repetitive shutdowns and reduced availability of outpatient care over prolonged periods in patients with CF is urgently needed. This may also be relevant for other patient groups with chronic lung diseases depending on regular specialised care.
Acknowledgements
We thank the staff at the Christiane Herzog Cystic Fibrosis Centre, Charité – Universitätsmedizin Berlin for their support.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: Conception and design of the study: S. Thee, M.A. Mall, M. Stahl; acquisition, analysis and interpretation of data: S. Thee, L. Busack, M.A. Mall, M. Stahl; drafting the article or revising it critically for important intellectual content: S. Thee, L. Busack, M.A. Mall, M. Stahl.
Conflict of interest: S. Thee has nothing to diclose.
Conflict of interest: L.M. Busack has nothing to diclose.
Conflict of interest: M. Mall reports grants from the German Federal Ministry of Education and Research during the conduct of the study; personal fees and other support from Boehringer Ingelheim, personal fees from Arrowhead Pharmaceuticals, personal fees and other support from Vertex Pharmaceuticals, and personal fees from Santhera, Sterna Biologicals, Enterprise Therapeutics, Antabio, Kither Biotech and Prieris Pharmaceuticals, outside the submitted work.
Conflict of interest: M. Stahl declares grants or contracts from the German Cystic Fibrosis Association Mukoviszidose e.V., Vertex Pharmaceuticals, Charité – Universitätsmedizin Berlin and the BIH, in the 36 months prior to manuscript submission.
Support statement: This study was supported in part by a grant from the German Federal Ministry of Education and Research (82DZL009B1) and the German Innovation Fund (01NVF19008). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519–2531. doi: 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- 2.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J. 2014; 44: 1042–1054. doi: 10.1183/09031936.00228013 [DOI] [PubMed] [Google Scholar]
- 3.Peckham D, McDermott MF, Savic S, et al. . COVID-19 meets cystic fibrosis: for better or worse? Genes Immun 2020; 21: 260–262. doi: 10.1038/s41435-020-0103-y [DOI] [PubMed] [Google Scholar]
- 4.Johnson C, Butler SM, Konstan MW, et al. . Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest 2003; 123: 20–27. doi: 10.1378/chest.123.1.20 [DOI] [PubMed] [Google Scholar]
- 5.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BL, Steenbruggen I, Miller MR, et al. . Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200: e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flume PA, Biner RF, Downey DG, et al. . Long-term safety and efficacy of tezacaftor-ivacaftor in individuals with cystic fibrosis aged 12 years or older who are homozygous or heterozygous for Phe508del CFTR (EXTEND): an open-label extension study. Lancet Respir Med 2021; 9: 733–746. doi: 10.1016/S2213-2600(20)30510-5 [DOI] [PubMed] [Google Scholar]
- 8.Earnest A, Salimi F, Wainwright CE, et al. . Lung function over the life course of paediatric and adult patients with cystic fibrosis from a large multi-centre registry. Sci Rep 2020; 10: 17421. doi: 10.1038/s41598-020-74502-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstan MW, McKone EF, Moss RB, et al. . Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017; 5: 107–118. doi: 10.1016/S2213-2600(16)30427-1 [DOI] [PubMed] [Google Scholar]
- 10.Radtke T, Haile SR, Dressel H, et al. . Recommended shielding against COVID-19 impacts physical activity levels in adults with cystic fibrosis. J Cyst Fibros 2020; 19: 875–879. doi: 10.1016/j.jcf.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nixon PA, Orenstein DM, Kelsey SF, et al. . The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med 1992; 327: 1785–1788. doi: 10.1056/NEJM199212173272504 [DOI] [PubMed] [Google Scholar]
- 12.Donath H, Zielen S, Wittekindt B, et al. . Effects of the SARS-CoV2-lockdown on pediatric care in the Rhine-Main area. Klin Padiatr 2021; 233: 31–36. doi: 10.1055/a-1263-1467 [DOI] [PubMed] [Google Scholar]
- 13.Wark PA, Tooze M, Cheese L, et al. . Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur Respir J 2012; 40: 510–512. doi: 10.1183/09031936.00202311 [DOI] [PubMed] [Google Scholar]
- 14.Patel S, Thompson MD, Slaven JE, et al. . Reduction of pulmonary exacerbations in young children with cystic fibrosis during the COVID-19 pandemic. Pediatr Pulmonol 2021; 56: 1271–1273. doi: 10.1002/ppul.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crichton ML, Shoemark A, Chalmers JD. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med 2021; 204: 857–859. doi: 10.1164/rccm.202105-1137LE [DOI] [PMC free article] [PubMed] [Google Scholar]