Abstract
Personality traits and physical health both change over the lifespan. Theoretical models and empirical evidence suggest that these changes are related. The current study investigated the dynamic relations between personality traits and physical health at both the between-person and the within-person levels. Data were drawn from three longitudinal studies: the Veterans Affairs Normative Aging Study (NAS; N = 1,734), the Longitudinal Internet Studies for the Social Sciences (LISS; N = 13,559), and the Swedish Adoption/Twin Study of Aging (SATSA, N = 2,209). Using random intercept cross-lagged panel models (RI-CLPMs) and the continuous time (CT) models, after controlling the between-person variance, generally, evidence was found for bidirectional associations between changes in neuroticism and extraversion and changes in self-rated health and general disease level. Bidirectional associations between changes in neuroticism and change in cardiovascular diseases and central nervous system diseases were observed only when time was modeled as continuous. We also found within-person associations between changes in neuroticism and extraversion and changes in performance-based ratings of motor functioning impairment. According to the current findings, the dynamic within-person relations between personality traits and health outcomes were largely in the direction consistent with their between-person connections, though the within-person relationships were substantially smaller in strength when compared their between-person counterparts. Findings from the current study highlight the importance of distinguishing between-person and within-person effects when examining the longitudinal relationship between personality traits and health.
Keywords: Personality, Health, Longitudinal, Random intercept cross-lagged panel model (RI-CLPM), Continuous time model (CT)
Personality traits have long been recognized as influential predictors of multiple aspects of health, such as disease, comorbidity, and mortality risk (Atherton et al., 2014; Graham et al., 2017; Hampson & Friedman, 2008; Smith, 2006; Turiano et al., 2015). Recently, some have suggested that health may also have the potential to exert lasting impacts on personality development (Hill & Roberts, 2016). Yet little is known about how personality traits and physical health influence each other longitudinally. In addition to the presence of relatively stable variations between individuals, both personality and health are dynamic within individuals over the lifespan, and people actively shape both their own personality and health over time (Smith & Spiro, 2002; Specht et al., 2014). Such findings lead to the following questions: How are the within-individual changes in personality and changes in health related to one another? Do changes in one lead to changes in the other? Or is there a reciprocal relationship? Answers to these questions are critical in understanding the development processes of both personality and health over the lifespan, as well as providing insights into how changes in personality and changes in health are interlocked over time. In order to answer these questions, it is critical that researchers use appropriately designed studies (i.e. multiple waves of assessment of personality and health) and adopt proper approaches to modelling the dynamic longitudinal relationships between personality and health (at both the between- and within- person level). The present study investigated these questions, using data from three independent longitudinal studies, analyzed with the random intercept cross-lagged panel model (RI-CLPM; Hamaker et al., 2015) and the continuous-time version of the model (CT model; Driver et al., 2017; Voelkle et al., 2012), which disentangles within-person effects from between-person effects. To our knowledge, this is the first study that systematically investigated the longitudinal reciprocal associations between personality traits and different types of health outcomes (e.g., self-rated health, general and specific disease conditions, physiological and performance-based health assessments) at both the between-person and the within-person levels in multiple samples.
Theoretical Basis for the Longitudinal Association between Personality and Health
Development and changes in health conditions across the life course are the focus of an important class of research questions in a number of different fields (Braveman et al., 2011; Halfon & Hochstein, 2002; Halfon & Forrest, 2018). Generally, there are clear age-graded declines in health over the adulthood (Avlund et al., 2003; House et al., 1990; Yashin et al., 2007). However, considerable variation has been observed among individuals in trajectories of health over time, with some individuals declining at faster rates and at younger ages while others remain healthy until very late in life. There is a general consensus that different health trajectories are a consequence of multiple determinants involving biological, psychological, and social processes (Halfon & Hochstein, 2002).
Personality traits have received increasing attention as a potential source of individual differences in health development (Weston et al., 2020). Personality is defined as relatively enduring patterns of thoughts, feelings, and behaviors that reflect the tendency to respond in certain ways under certain circumstances (Roberts, 2009). For some people personality traits are relatively stable for the long-term but for many others this is true for shorter-term periods. In other words, there are individual differences in the enduringness of these personality patterns over long periods of time (Mroczek & Spiro, 2003; Mroczek, 2014) and personality traits change across the life span for many people (Damian et al., 2019; Graham et al., 2020; Roberts et al., 2006; Roberts & DelVecchio, 2000).
Throughout the development of the theoretical frameworks of personality, uncovering the processes that shape the dynamic variation at the within-person level has been emphasized as a key goal in personality research (Allport, 1937; Bandura, 1999; Cattell, 1957; Mischel & Shoda, 1995; Roberts, 2018). Examining the transactions or bidirectional relations between person and environment/life experiences is important for understanding the sources for the dynamics in personality traits within individuals. Several theoretical perspectives have suggested the interconnection between changes in personality traits and changes in health over the long run, as well as the possible bidirectional associations between personality traits and health over time. Viewing from the interindividual differences perspective, several models predict that personality traits are linked to health outcomes through downstream processes by impacting mechanisms that are crucial to health (Bogg & Roberts, 2013; Ferguson, 2013; Kern & Friedman, 2011; Murray & Booth, 2015; Smith, 2006). Specifically, these models indicate that individuals differing in personality traits may engage in different behaviors (e.g., health behaviors) and have different cognitive (e.g., appraisal of external circumstances and coping) and emotional reactions, resulting in between-person differences in health outcomes.
With respect to the current study, what is important is the theories that postulate a bidirectional association between health and personality constructs at the within-person level, whereby an individual’s personality traits contribute to changes in health outcomes while health may also feedback and reshape the very personality traits that shaped health in the first place (Mroczek et al., 2019). According to the corresponsive principle of the Neo-Socioanalytic model of personality development (Caspi et al., 2005; Roberts & Nickel, 2017), individuals have certain life experiences (including health experiences such as suffering from chronic diseases) because of their personality traits, and those experiences in turn can modify or change the personality traits that lead individuals to those experiences. Put differently, changing personality and changing health may operate in a feedback loop. For example, high extraversion may lead to better health, through receipt of more and better social support and social network quality, and the resulting good health may allow a person to maintain their sociability or even become more so because such a person is physically able to stay more socially connected. In turn, that higher (or maintained) extraversion reinforces better health, and so on. By the same token, high neuroticism at one occasion can lead to worse health at the next (perhaps due to chronic feelings of negative affect and stress), which leads to higher neuroticism at the next, which leads to worse health at the next, and so on. High neuroticism and low health could mutually reinforce one another over time, each shaping the other in a bidirectional manner. Usually, the corresponsive principle is framed in terms of “social selection” and “socialization” processes, whereby traits select a person into certain social experiences (e.g., relationships, careers) that in turn socialize the person, changing or reinforcing the very traits that caused the selection in the first place. In the current study, we contend that the corresponsive principle is broader than this and can operate in tandem with health experiences and not just social experiences. We hypothesize that the corresponsive principle applies to personality traits and physical health, with the two moving in concert with one another in a bidirectional fashion.
In addition, within the framework of lifespan developmental theory, bidirectionality has been suggested to be a key in understanding the transaction between personality and health over the life course (Mroczek et al., 2020). Lifespan developmental theory posits that factors such as personality traits and physical functioning are interconnected over long periods of time (Baltes, 1987). Given their dynamic nature, instead of taking the traditional notion of a simple predictor-outcome association, investigating the interrelations among personality traits and health in a bidirectional manner can help achieve a more in-depth understanding of the co-development of personality and health over time. Moreover, as the lifespan development perspective depicts, change is an intraindividual process that differs at the interindividual level (Smith & Spiro, 2002); thus, both the between-person and the within-person processes should be considered to uncover the personality-health transaction.
Empirical Evidence for the Bidirectional Association between Personality and Health
Previous research has examined the longitudinal relations between personality traits and health outcomes using diverse methods. Generally, at the between-person level, low neuroticism, high conscientiousness, extraversion, and openness were associated with better physical health outcomes (Friedman et al., 2010; Goodwin & Engstrom, 2002; Goodwin & Friedman, 2006; Murray & Booth, 2015). Compared to research that focused on the role of personality traits in predicting health outcomes, relatively few studies examined the potential influences of health or changes in health on the development of personality traits and the evidence was mixed. For example, Jokela and colleagues (2014) found that respondents showed decreases in extraversion, emotional stability, conscientiousness, and openness after the onset of chronic diseases. Similarly, Leikas and Salmela-Aro (2015) reported that those diagnosed with a chronic disease were more likely to remain higher in neuroticism and lower in extraversion when compared to their peers. It was also found that constructs closely related to health, like life satisfaction and being physically active, were prospectively predictive of adaptive personality changes (Hill & Roberts, 2016). When the prospective associations between personality traits and health were tested using a cross-lagged panel design, baseline extraversion and conscientiousness displayed positive predictive effects on later self-rated health, whereas better baseline physician-rated health predicted lower neuroticism and higher agreeableness over 12 years (Tauber, 2018). Similarly, evidence was found for the predictive effects of various health conditions on subsequent changes in the Big Five personality traits (Graham et al., 2020); however, the pattern of results (e.g., the relations between certain health conditions and personality traits) was inconsistent across different samples examined. Contrasting results have also been reported. For instance, Sutin and colleagues (2013) found that changes in most personality factors, except openness, were unrelated to the development of serious diseases. Among a comprehensive list of heath indicators (e.g., self-reported health, overall disease load, cognitive impairment), only hearing impairment was found to be related to a steeper age-related decline in extraversion in a sample aged over 80 (Berg & Johansson, 2014).
Other studies examined the bidirectional associations between trajectories of personality traits and health-related constructs. For example, when changes in personality traits were examined as predictors, declines in conscientiousness and increases in neuroticism over 10 years displayed significant associations with lower levels of perceived health (Human et al., 2013). When personality traits and health outcomes were assessed in parallel over time, increases in agreeableness, conscientiousness, and openness, and decreases in neuroticism were associated with increases in self-rated health over three years (Letzring et al., 2014). Similarly, changes in conscientiousness both at the domain and facet level (e.g., self-control, responsibility) displayed positive relations to changes in self-perceived physical health with the associations partially mediated by changes in preventative health behaviors and changes in perceived stress (Luo & Roberts, 2015; Takahashi et al., 2013). Also, evidence showed that increases in optimism were linked to improvements in self-rated health and decreases in chronic conditions over a four-year period (Chopik et al., 2015).
All told, the empirical literature has suggested possible dynamic bidirectional relations between personality traits and physical health over time, but the inconsistencies in the literature point to the need for more research. Also, although evidence for relations with health outcomes was found for all of the Big Five personality traits, overall, the associations for neuroticism and conscientiousness were replicated across studies to a greater extent when compared to personality traits in other domains, whereas the connections between agreeableness and health outcomes were less established (Murray & Booth, 2015). However, it remains unknown whether the pattern is generalizable to the dynamic relations between personality traits and health outcomes at the within-person level.
Theoretical and Methodological Considerations for the Longitudinal Association between Personality and Health
There are limitations in previous approaches used to examine the longitudinal relations between personality traits and health. In most existing studies, the relationships between personality and health were measured concurrently (time-specific associations) and were tested only at the between-person level. Classic analytic methods (e.g. traditional regression or cross-lagged panel models) are not helpful in teasing apart between-person (differences between individuals) and within-person (variability in certain constructs for individuals over time) effects, resulting in models that confound these two key sources of variance. Furthermore, although studies adopting multilevel models estimated the effects at both the between-person and within-person levels, the majority of the prospective studies only tested the unidirectional relations between personality and health (personality traits predict later health outcomes or health predicts later personality traits), thus did not control for the lagged effects of individuals’ own personality/health from earlier times (autoregressive effects). Studies employing a growth curve model to test the longitudinal associations between trajectories of personality traits and health usually focused on the between-person effects only (e.g., relations between the slopes of personality traits and the slopes of health outcomes), with the within-person effects being ignored (or treated as variability to be modeled but otherwise ignored).
Given the presence of individual differences in both personality traits and health outcomes, as well as their dynamic nature, examining their longitudinal associations at the between-person and the within-person levels shares equal importance. Specifically, while testing at the between-person level allows us to investigate who are likely to be at a risky level of certain health outcomes or show decline in health, examining at the within-person level seeks answers to how personality and health change together (e.g., whether improvements in health lead to decreases in neuroticism, and vice versa). Development in personality traits and health is an intraindividual process in itself. Although theories (e.g., the corresponsive principle of personality development, lifespan developmental theory) imply the presence bidirectional associations between personality traits and health outcomes over time, as discussed above, empirical evidence for such associations were largely drawn from studies that were not equipped with the proper design to probe the intraindividual processes. Applying findings obtained using approaches that target the between-person effects, or a mixture of between- and within-person effects, can be misleading, as effects discovered at the between-person level often cannot be generalized to the within-person level (Beck & Jackson, 2019; Fisher et al., 2018; Hamaker, 2012; Molenaar & Campbell, 2009).
After controlling for the variance at the between-person level, if no within-person effects of personality traits on health are observed, analyses and intervention on the personality-health relation may be best targeted at the interindividual level. Research on the personality-health link may inform to whom interventions may be targeted for health risk screening and prevention but provide very limited information for developing more individualized interventions that are tailored in concert with the individual’s developmental trajectory. In contrast, if the within-person effects of personality traits on health are present, information on changes in personality traits can be used to improve the precision of predicting changes in health at the individual level. The information can also be applied to inform when interventions should be implemented (e.g., when increases in neuroticism are observed), as well as developing intervention strategies that aim to improve positive development in both personality traits and health. Meanwhile, given the increasing salience of health challenges in midlife and the unavoidable health decline as people enter late stages of life, health-related experiences can be particularly relevant to individual development. Despite the robust evidence on plasticity of personality traits over the life course, more research is needed to uncover the sources of changes in personality (Bleidorn et al., 2020). Investigating the effects of health on personality traits at the within-person level can clarify the role of changes in health in driving the development of personality (whether health-related experiences act as a meaningful source for personality development). In addition, compared to the unidirectional analyses, investigating the bidirectional (or reciprocal) relationships between personality and health can provide more robust evidence about the directionality of their longitudinal associations, contributing to a deeper understanding of the co-developmental processes of personality and health. Thus, it is necessary to adopt approaches that distinguish between- from within-person effects to examine how between-person differences in personality traits and health levels are associated with each other across time, the directionality of how personality traits and health influence each other in a dynamic transactional process over the long run, as well as their time-specific associations at the within-person level after controlling for their relations at the between-person level.
Statistical Models for Dynamic Relationship
The Random Intercepts Cross-Lagged Panel Model (RI-CLPM) was developed to investigate dynamic developmental processes and the longitudinal interplay between two constructs (Hamaker et al., 2015). A conceptual overview of the RI-CLPM is shown in Figure 1. There are two main parts in a basic RI-CLPM. First, there is a latent random intercept component that captures the time-invariant component in personality (IP in Figure 1) and health (IH in Figure 1). This portion of the model incorporates stable individual differences in a given construct, namely the trait component. The second part of the model is the temporal deviations in personality (εpt in Figure 1) and health (εht in Figure 1) that reflect individuals’ time-specific deflections from their own general levels of personality traits and health. For a certain individual, his or her personality and health levels (Pt and Ht in Figure 1) at a specific time point can be expressed as Pit = μt + IPi + εpit and Hit = πt + IHi + εhit, where μt and πt are the time-specific population means for personality and health. Also, to capture changes in a certain construct, the model includes autoregressive components that estimate the within-person carry-over effects between repeated measures (b1 and b2 in Figure 1). The bivariate version of the RI-CLPM, in addition to evaluating the autoregressive effects for each variable series, also permits the estimation of the cross-lagged effects between the variable series (b3 and b4 in Figure 1), which indicate the degree to which changes in one variable can be predicted from the individual’s deviation from his or her relatively stable level on the other variable at a prior time point while controlling for the relatively stable component and prior deviation from the stable part of the variable itself. Thus, according to the specification of the model, the deviations in personality and health at a specific time point can be described as εpit = b1εpi,t-1 + b4 εhi,t-1 + uit and εhit = b2εhi,t-1 + b3εpi,t-1 + vit. In addition to the cross-lagged effects, in the present study, we also tested the time-specific associations (r in Figure 1) between deviations in personality and deviations in health at the within-person level.
Figure 1.
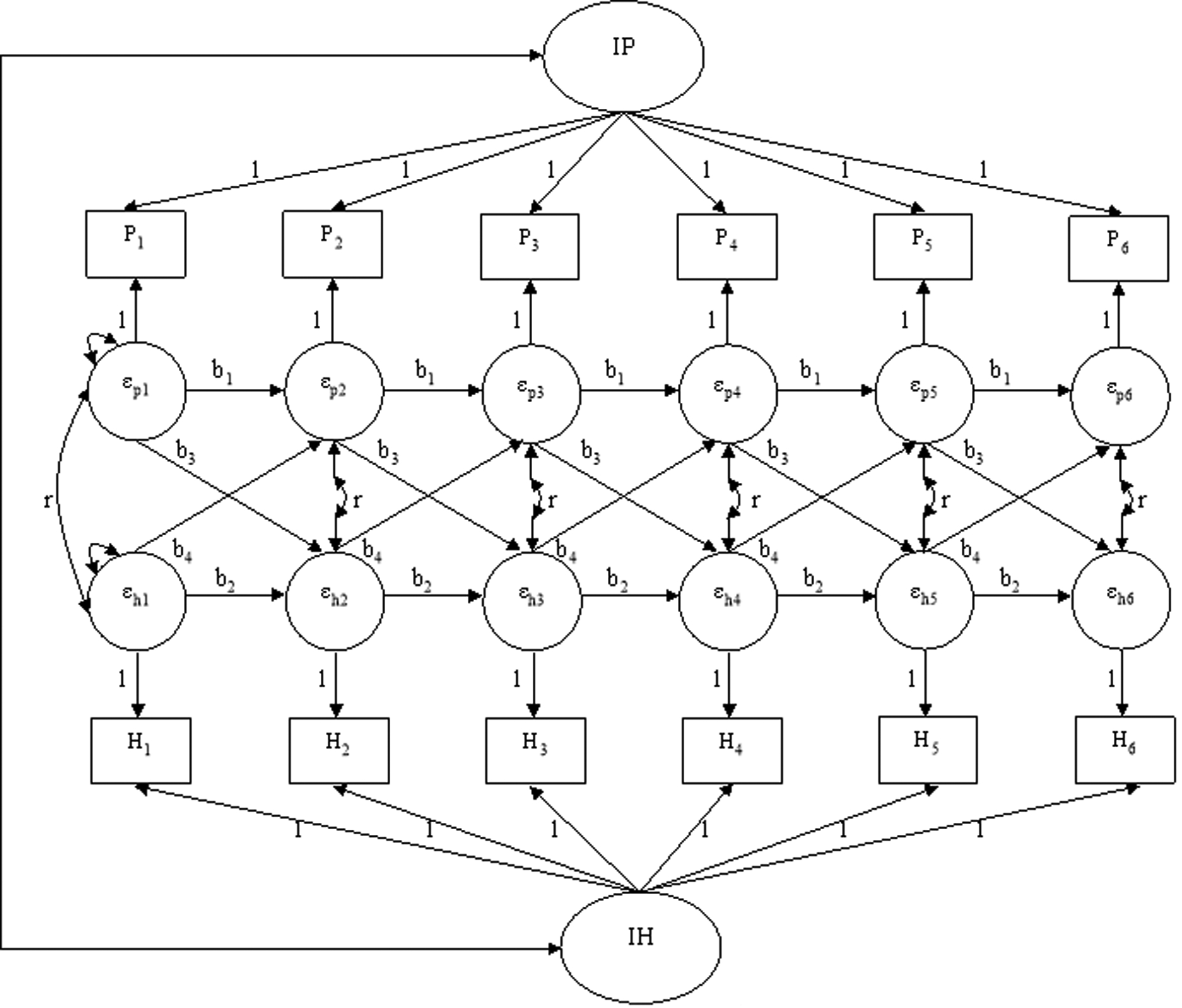
Conceptual representation of the random intercept cross-lagged panel model (RI-CLPM) of the longitudinal associations between personality traits and health outcomes. P = personality; H = health; I = intercept.
Despite the many strengths of RI-CLPM, it is limited in that it treats time as a discrete variable. Parameter estimates from models are contingent on the time interval between assessments. Even if two studies are tapping into exactly the same phenomenon in the same population, they may obtain different parameter estimates if the two studies adopt different assessment intervals. To account for the unequal intervals between measurement occasions within samples, a continuous-time (CT) version of the dynamic model has been developed (Driver et al., 2017; Voelkle et al., 2012). Compared to discrete time model that assumes time progresses in discrete steps, CT model treats underlying processes as unfolding in a continuous way with respect to time (Driver et al., 2017; Ryan et al., 2018). Using stochastic differential equations, CT models provide parameter estimates that quantify how the autoregressive and cross-lagged effects change over time, thus facilitating comparisons among studies with different assessment intervals (Voelkle et al., 2012).
The Current Study
The current study investigated the longitudinal reciprocal associations between personality traits and physical health outcomes using data from three longitudinal studies. We applied the RI-CLPMs to simultaneously examine the interindividual associations between personality traits and health and the intraindividual reciprocal relations between personality traits and health across long periods of time. Specifically, we first tested (after differentiating the between-person variance from the within-person variance) how the time-invariant components of personality traits were related to the time-invariant components of health at the between-person level. Second, we examined the directionality of the dynamic associations between personality traits and health and the possibility that personality traits and health constructs influence each other in a bidirectional manner at the within-person level. Finally, we also tested whether the pattern of the time-specific relations between deviations in personality traits and deviations in health at the within-person level was different from their associations at the between-person level. To further account for the potential effects of differences in time intervals between measurement occasions, we also conducted analyses using CT models to examine the lagged effects of personality traits on rates of change of health outcomes and vice versa at the within-person level when time was treated as continuous.
In light of the mixed findings in prior work, we identified three data sources that would provide the necessary data (e.g., sufficient waves of assessment) to test both between and within-person associations between personality and health. As all three data sets included responses from thousands of participants (N = 1,734; 13,559; and 2,209: the sample sizes were determined by the availability of responses from the three longitudinal studies), the sample sizes were sufficient for the analyses conducted in the current study. Replicating findings across three distinct data sets would provide more convincing evidence to move the field forward. For Sample 1, we used data from the Veterans Affairs Normative Aging Study (NAS), a longitudinal study of aging in men, to examine the longitudinal reciprocal relations between two of the Big 5 (neuroticism and extraversion) and physical health outcomes (self-rated health and general disease level) over 23 years. For Sample 2, we tested the dynamic associations between the Big Five personality traits and physical health outcomes (self-rated health and general disease level) using data from the Longitudinal Internet Studies for the Social Sciences (LISS), a longitudinal panel study administered by CentERdata (Tilburg University, The Netherlands) with multiple assessments to follow changes in the life course and living conditions of the participants. In the current study, we used the assessments of personality traits and health over a 9-year period of time. Data of Sample 3 were drawn from the Swedish Adoption/Twin Study of Aging (SATSA), a longitudinal study designed to investigate the origins of individual differences in aging and the involvement of genetic and environmental factors underlying the aging processes. Data used in the present study covered assessment of personality traits and health over 14 years. In Sample 3, we first examined the longitudinal associations between neuroticism, extraversion, openness and self-rated health and general disease level. In addition, to further examine the dynamic associations between personality traits and physical health outcomes in a more nuanced manner, in Sample 3, we also tested the longitudinal relations between personality traits and specific health conditions (cardiovascular diseases, central nervous system diseases, and metabolic diseases), as well as health outcomes that were assessed in an objective way (allostatic load and motor functioning impairment).
Method
Participants
Data from the NAS were collected under a protocol approved by the Institutional Review Board (IRB) at Veterans Affairs (VA) Boston Healthcare System (IRB #1191; Avron Spiro III, Principal Investigator), and supported by NIA Grant R01-AG0018436 (Daniel K. Mroczek, Principal Investigator). Data from the LISS and the SATSA were publicly available and the use of which was declared by Northwestern University as exempt IRB review. Data from the NAS are subject to HIPAA privacy regulations, as well as the rules and regulations of the US Department of Veterans Affairs. De-identified data may be provided upon reasonable request. A list of publications that used the NAS data can be found at the Open Science Framework (https://osf.io/zv4bx/?view_only=13300714cd2446eba06c51b0b39990d9). Data of the LISS and the SATSA and prior publications used the data can be found at the websites of the studies (LISS: https://www.dataarchive.lissdata.nl/; SATSA: https://www.icpsr.umich.edu/web/NACDA/studies/3843).
NAS.
The NAS is a longitudinal study founded at the Boston VA Outpatient Clinic in 1963 to investigate healthy aging in men (Bossé et al., 1984). The majority were veterans (of WW II or Korea) who were free of serious mental or physical illness at the time of recruitment in the 1960s. The present study used a sample of 1,734 participants who provided usable data on personality or health measures between 1987 and 2010. The data were organized into 9 waves, and participants included in the analyses provided information in at least one wave of the assessments. The age of participants in Wave 1 ranged from 43 to 91 (M = 63.42, SD = 8.14). On average, the participants provided information in 4.52 waves (SD = 2.09, Range: 1–9).
LISS.
The LISS panel is based on a true probability sample of Dutch households drawn from the population register (Scherpenzeel & Das, 2010). The present study focused on a sample of 13,559 (54.2% female) participants who provided information on personality or health outcomes in at least one of six waves of assessment between 2008 and 2017. The age of participants who were assessed in Wave 1 ranged from 15 to 94 (M = 45.45, SD = 16.12). On average, participants completed 2.92 waves of assessment (SD = 1.92, Range: 1–6).
SATSA.
The SATSA is a longitudinal study of Swedish twins that assesses a broad spectrum of biological, psychological, and social domains to investigate the patterns and processes of health and aging (Pedersen, 2015). The data collection process of SATSA consists of two components. In the first component, participants were surveyed on personality, work environment, and an array of health measures. In the second component, in addition to being surveyed on personality and health measures, a subsample of twins also participated in waves of in-person testing, including a health examination and tests on functional capacity and cognitive abilities. In the current study, we used 6 waves of the data from the questionnaires-only assessments to examine the dynamic relations between personality traits and self-reported health outcomes (self-rated health, general disease level, and specific health conditions including cardiovascular diseases, central nervous system diseases, and metabolic diseases). Specifically, we focused on a sample 2,209 (58.9% female) individuals who provided data on personality or health outcomes in at least one of the six assessments between 1984 and 2008. The age of participants who completed the assessment in Wave 1 ranged from 26 to 93 (M = 60.13, SD = 14.03). On average, participants completed 3.64 waves of assessment (SD = 1.76, Range: 1–6).
In addition to the self-reported health outcomes, in SATSA, we also examined the between- and within-person associations between personality traits and objective health measures using 5 waves of the data from the in-person testing. Specifically, we examined a subsample of 767 participants (59.6% female) who provided data on personality traits or physiological health measures (allostatic load and motor functioning impairment) in at least one of the five assessments. The age of participants who provided data in Wave 1 ranged from 45 to 91 (M = 66.00, SD = 9.00). On average, participants had data on 3.29 waves of assessment (SD = 1.41, Range: 1–5)
Across the 3 samples, we conducted analyses to examine whether attrition resulted in unrepresentative longitudinal samples among participants who had usable data in Wave 1. Details can be found in the supplement (in the section of Attrition Analyses Across the Three Samples). Table S1 displayed the timelines of data collection of the waves used in the current study across the 3 samples. Generally, participants who provided data on more waves showed lower scores on neuroticism, higher scores on positive personality traits (e.g., extraversion, conscientiousness), and better health at baseline. The possible range of restriction resulted from attrition in both personality and health variables may lead to reduced effect sizes for focal analyses, which might make our conclusions conservative.
Measures
Personality.
Neuroticism and extraversion were assessed across the NAS, the LISS and the SATSA samples (both the main sample and the in-person testing subsample for physiological health measures). Openness was measured in both LISS and the questionnaires-only sample in SATSA. Conscientiousness and agreeableness were assessed in the LISS only.
NAS. Neuroticism and extraversion were measured using a short version (EPI-Q; Floderus, 1974) of the Eysenck Personality Inventory (Eysenck & Eysenck, 1968). Each of the two personality dimensions was assessed by 9 dichotomous items (0 = no, 1 = yes). Cronbach alphas ranged from 0.49 to 0.741, and 0.62 to 0.68 for neuroticism and extraversion across the 9 waves, respectively. LISS. Neuroticism, extraversion, openness, conscientiousness, and agreeableness were measured by the IPIP-50 that represents the Goldberg (1992) markers for the Big-Five factor structure. Each of the five personality dimensions was measured by 10 items which were rated from 1 (Very inaccurate) to 5 (Very accurate). Across the 6 waves, Cronbach alphas ranged from 0.88 to 0.89 for neuroticism, from 0.86 to 0.88 for extraversion, from 0.76 to 0.77 for openness, from 0.77 to 0.79 for conscientiousness, and from 0.80 to 0.82 for agreeableness. SATSA. Neuroticism and extraversion were measured by a short form of the Eysenck Personality Inventory (EPI; Eysenck & Eysenck, 1975; Pedersen et al., 1988). Each of the two personality domains were assessed by 9 dichotomous items. Across the 6 waves of the questionnaires-only assessment, Cronbach alphas ranged from 0.70 to 0.75 for neuroticism and 0.65 to 0.68 for extraversion. Across the 5 waves of the SATSA in-person testing sample, Cronbach alphas ranged from 0.70 to 0.76 for neuroticism and 0.63 to 0.68 for extraversion. Openness was assessed by 6 items from the NEO-Personality Inventory (NEO-PI; Bergeman et al., 1993; Costa & McCrae, 1985). Each item was rated on a five-point scale with 1 as “Exactly right” and 5 as “Not right at all”. Cronbach alphas for openness ranged from 0.64 to 0.71 across 6 waves.
Self-Rated Health.
NAS. Self-rated health was assessed by a single item asking participants to rate whether they perceive their current health state as very poor, poor, fair, good, or excellent. The item was coded such that higher scores represented better perceived health states. LISS. Self-rated health was measured by a single item that asked participants whether they described their health in general as poor, moderate, good, very good, or excellent. The item was coded on a five-point scale such that higher scores indicated better perceived health. SATSA. Self-rated health was measured by a single item asking participants rate their general state of health on a three-point scale using “good”, “mediocre”, and “bad”. The item was coded such that higher scores represented better perceived health. As previous research indicated, the single item measure of self-rated health has been used widely and demonstrated good convergence with more comprehensive self-reports (Hays et al., 2015; Lundberg & Manderbacka, 1996; Wu et al., 2013). Also, the measure of subjective health has been shown to be linked to morbidity and mortality (Idler & Benyamini, 1997; Latham & Peek, 2013).
General Disease Level.
Compared to self-rated health, the measure of general disease level was used to assess participants’ health status in a relatively objective way that captured their overall level of diagnoses of various diseases. NAS. General disease level was assessed by a modified (Bossé et al., 1987) version of the Seriousness of Illness Rating Scale (SIRS; Wyler et al., 1968). Participants were asked to list the health condition or problem that bothered them the most; the condition was then rated using SIRS, which consisted of a list of disease items weighted by the estimates of seriousness of the diseases. In the measured used in the present study, illness severity ratings ranged from 0 (no problems) to 124 (life-threatening conditions, such as cancer). LISS. General disease level was estimated as the sum of participants’ endorsement on items about diagnoses of different types of diseases, including cardiovascular diseases, respiratory diseases, musculoskeletal diseases, central nervous system related diseases, eye problems, metabolic diseases, gastro and intestinal tract diseases, and cancer or tumor. The total score ranged from 0 to 8. SATSA. General disease level was measured in Wave 1 – Wave 5 (the questionnaires-only assessment) as the sum of participants’ endorsement on items about diagnoses of a variety of diseases. In total, diseases in 13 categories were assessed, including cardiovascular diseases, respiratory diseases, musculoskeletal diseases, allergic problems, skin problems, central nervous system related problems, eye problems, metabolic diseases, gastrointestinal tract diseases, urologic diseases, cancer or tumor, ear problems, and diseases of reproductive organs. The total score ranged from 0 to 13.
Specific Disease Conditions.
Three types of specific health conditions were examined in the questionnaires-only assessment of SATSA. Cardiovascular diseases were measured by the sum of participants’ endorsement on 8 items assessing the diagnoses of a range of conditions in the cardiovascular category. Specifically, cardiovascular conditions including heart failure, angina pectoris, heart attack, phlebitis, circulation problems in limbs, thrombosis, stroke, and high blood pressure were assessed. Central nervous system diseases were measured by the sum score of 7 items that assessed diagnoses of migraines, seizures, epilepsy, Parkinson’s Disease, multiple sclerosis, speech problems, and polio. Metabolic diseases were measured by the total endorsement on 4 items assessing the diagnoses of diabetes, goiter, anemia, and gout.
Physiological Health Outcomes.
Two types of physiological health measures in SATSA in-person testings were included in the current analyses. Allostatic load was measured by using 7 biomarkers assessed in the SATSA across waves. Cardiovascular functioning was assessed by resting systolic and diastolic blood pressure. When resting blood pressure was measured twice in some waves, the average of the two readings was calculated. Functioning of metabolic system was evaluated by indicators including waist-hip ratio, total cholesterol, high density lipoprotein cholesterol (HDL), blood sugar, and triglycerides. In accordance with previous studies (McEwen, 2000; Seeman et al., 1997; Stephan et al., 2016), allostatic load index was computed by averaging z-scores for each of the indicators (standardized across waves). High values indicate higher dysregulation of the physiological systems. Motor functioning impairment was evaluated based on nurse ratings of performance of 20 motor functioning tasks and the ratings were coded on a binary scale (0 = no difficulty, 1 = having difficulty). According to previous research (Bravell et al., 2017; Finkel et al,. 2016), 3 factors were generated by using scores on the 20 motor functioning tasks with consistent loadings across waves and ages. In general, the three factors assessed impairment in fine motor movement, balance impairment, and flexibility impairment. Motor functioning impairment index was computed by the sum score of the 20 tasks.
Statistical Analysis
All analyses for measurement invariance and RI-CLPMs were conducted using Mplus 8.5 (Muthen & Muthen, 2017). The scripts for the analyses that are described can be found at https://osf.io/zv4bx/?view_only=13300714cd2446eba06c51b0b39990d9. Due to missingness in data across waves, full information maximum likelihood (FIML) was used for estimation. First, we tested measurement invariance for each personality trait over time in each sample. Details about the analyses and results (see Table S2) can be found in online supplementary materials. Overall, measurement invariance was confirmed for all traits in the three samples at the configural, metric, and scalar levels of analyses.
To examine the dynamic reciprocal relations between each personality trait and health outcome (self-rated health, general disease level, specific conditions, and physiological health indicators), across the three samples, we fitted a series of RI-CLPMs. In the SATSA, each twin pair was viewed as a cluster and robust standard errors were estimated to take the dependency within each pair of twins into consideration. The composite scores of personality traits were used as time-specific indicators for each wave of assessment. Given the diverse ages in our samples, participants’ age in Wave 1 and sex were controlled in the models. For participants who joined the study in later waves, their age in Wave 1 was computed based on the age information they provided in subsequent waves. As shown in Figure 1, in the RI-CLPM, we first constructed random intercepts for both personality and health by constraining the factor loadings of each time-specific indicators to 1. The random intercepts estimate the time-invariant parts of personality and health across waves. By allowing the two random intercepts to correlate, we estimated the between-person relationship between personality and health.
After controlling for their associations at the between-person level, the longitudinal relations between personality and health were tested at the within-person level. The latent variables (εpt & εht in Figure 1) reflect participants’ time-specific deviations from their own general levels of personality traits and health. At the within-person level, the model estimates both the autoregressive effects (b1 & b2 in Figure 1) and the cross-lagged effects (b3 & b4 in Figure 1) from one time point to another. The cross-lagged coefficients estimate the extent to which participants’ time-specific deviations from their own general levels of health can be predicted by their preceding deviations from their relatively stable levels of the personality traits, while controlling for their preceding deviations from their general health levels, and vice versa. To consider the time-specific associations between personality traits and health at the within-person level (r in Figure 1), we estimated the correlations between personality traits and health outcomes in Wave 1, as well as the contemporaneous covariances between their residuals in subsequent waves.
For each pair of personality trait and health outcome, we also fitted two additional models to further test whether constraining the cross-lagged effects between personality traits and health outcomes resulted in significant decreases in model fit. Specifically, in addition to the baseline models (both the cross-lagged effects of personality traits on health outcomes and the cross-lagged effects of health outcomes on personality traits were freely estimated), we also fitted models (Model A) in which the cross-lagged effects of personality trait on health outcome were constrained to zero (b3 = 0) and models (Model B) in which the cross-lagged effects of health outcome on personality trait were constrained to zero (b4 = 0). Model comparisons were conducted to determine the significance of the cross-effects of changes in personality traits on changes in health outcomes (baseline model vs. Model A) and the cross-effects of changes in health outcomes on changes in personality traits (baseline model vs. Model B) using chi-square difference tests. The less constrained model (baseline model) was considered to fit significantly better than the more constrained ones if the chi-square difference test was significant. To test whether the cross-lagged effects of personality traits on health outcomes and the cross-lagged effects of health outcomes on personality traits differed in magnitude, the baseline models were also compared to models in which the mutual cross-lagged effects between personality traits and health outcomes were constrained to be equal (b3 = b4 in Model C).
We specified two sets of models. First, all parameters (except loadings on the random intercept factors) were allowed to be estimated freely. Second, we constrained the autoregressive, cross-lagged coefficients and the contemporaneous covariances between personality and health residuals to be equal across waves so that five parameters were estimated (b1 – b4 and r in Figure 1). According to fit indices, imposing the equality constraints did not result in substantial decreases in model fit across all the models (see Table S3 for model fit indices for the constrained and unconstrained models). Thus, we preferred the more parsimonious solutions (models with the equality constraints), which reduce model complexity, allow for consistency in findings across time, provide greater precision in estimation due to more degrees of freedom (Little et al., 2007; MacCallum et al., 2006), and allow for easier interpretation. We report point estimates and 95% confidence intervals (CIs) for all values.
To account for the potential effects of varying time intervals between measurement occasions within the samples, we also conducted analyses using the CT version of the models. CT modeling analyses were conducted using the package ctsem (Version 3.4.3; Driver et al., 2017), which interfaces with OpenMx 2.17.4 (Neale et al., 2016) in R 3.6.2. Participants’ age at baseline and sex were controlled in the models. After controlling for the personality-health associations at the between-person level, at the within-person level, the CT model estimates parameters of the drift matrix that contains both the auto-effects and the cross-effects. The auto-effects coefficients reflect the extent to which changes in personality traits/health outcomes are stable over time. The cross-effects coefficients, which are the main focus of the current study, estimate the extent to which participants’ deviations from their own general levels of personality traits at a certain point in time predict the rate of change of the developmental process of health with respect to time while controlling for their deviations from their general health levels at a preceding time point, and vice versa. Similar to the analyses using the RI-CLPMs, the baseline models that allowed the auto-effects and cross-effects parameters of the drift matrix to be estimated freely were also compared to models with either the cross-effects of personality traits on health outcomes (Model A) or the cross-effects of health outcomes on personality traits (Model B) constrained to zero.
Results
Descriptive Statistics
Tables 1–3 display the means, standard deviations, and correlations between personality traits and self-rated health and general disease level across waves in the NAS, LISS, and SATSA samples, respectively. As shown in the tables, generally, across the three samples, neuroticism exhibited negative correlations with self-rated health and positive correlations with general disease level on a cross-sectional basis. The results also suggested negative prospective associations between neuroticism and self-rated health and positive prospective associations between neuroticism and general disease level such that earlier measures of neuroticism were significantly related to subsequent health outcomes, and vice versa. Similarly, in each of the three sample, extraversion displayed positive concurrent correlations with self-rated health and negative concurrent relations with general disease level. The results also provided evidence for the prospective relations between extraversion and self-rated health and general disease level with extraversion measured earlier significantly related to subsequent general disease level, and vice versa.
Table 1.
Means, standard deviations, and correlations between neuroticism, extraversion and health outcomes in the NAS sample.
| M | SD | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 2.97 | 3.35 | 2.78 | 2.27 | 2.14 | 2.20 | 2.16 | 1.79 | 1.48 | 5.35 | 5.45 | 5.22 | 5.61 | 5.50 | 5.55 | 5.62 | 5.62 | 6.14 | ||
| SD | 2.25 | 2.32 | 2.29 | 2.16 | 2.11 | 2.06 | 2.10 | 1.97 | 1.40 | 2.30 | 2.28 | 2.21 | 2.20 | 2.09 | 2.09 | 2.22 | 2.17 | 2.13 | ||
| Self-Rate Health | ||||||||||||||||||||
| SRH1 | 4.11 | .69 | −.23* | −.17* | −.23* | −.18* | −.17* | −.12 | −.09 | −.08 | −.14 | .12* | .08* | .09* | .06 | .10 | .14* | .17* | .11 | .09 |
| SRH2 | 4.08 | .68 | −.22* | −.21* | −.25* | −.20* | −.28* | −.36* | −.23* | −.18* | −.04 | .17* | .13* | .15* | .17* | .26* | .25* | .32* | .28* | .48* |
| SRH3 | 4.06 | .67 | −.22* | −.21* | −.24* | −.21* | −.37* | −.26* | −.20* | −.32* | −.48* | .05 | .11 | .12* | .13 | .10 | .21* | .14 | .22 | .11 |
| SRH4 | 4.08 | .70 | −.21* | −.22* | −.28* | −.23* | −.23* | −.35* | −.17* | −.18* | −.09 | .14* | .16* | .14* | .13* | .21* | .20* | .24* | .25* | .27 |
| SRH5 | 4.04 | .66 | −.23* | −.21* | −.27* | −.16* | −.23* | −.23* | −.14* | −.13 | −.01 | .07 | .15* | .15* | .21* | .19* | .27* | .17* | .18* | .43* |
| SRH6 | 4.00 | .63 | −.22* | −.23* | −.27* | −.24* | −.36* | −.35* | −.23* | −.18* | −.32 | .07 | .04 | .11* | .12 | .23* | .21* | .17* | .23* | .40* |
| SRH7 | 3.94 | .64 | −.15* | −.13* | −.18* | −.12* | −.22* | −.24* | −.20* | −.08 | −.22 | .09 | .07 | .09 | .05 | .19* | .16* | .19* | .14* | .004 |
| SRH8 | 3.91 | .67 | −.17* | −.15* | −.20* | −.08 | −.15* | −.13 | −.13 | −.20* | −.18 | .01 | .08 | .002 | −.06 | .05 | .09 | .12 | .18* | −.02 |
| SRH9 | 3.95 | .63 | −.32* | −.37* | .08 | −.10 | .07 | −.24 | −.10 | .06 | −.25 | .02 | −.05 | −.05 | −.38* | −.03 | .31 | −.03 | −.19 | .11 |
| General Disease Level | ||||||||||||||||||||
| GDL1 | 44.37 | 43.27 | .20* | .13* | .14* | .17* | .19* | .17* | .12* | .09 | .22 | −.08 | −.05 | −.04 | −.06 | −.10 | −.15* | −.14* | −.07 | −.20 |
| GDL2 | 40.66 | 44.05 | .19* | .18* | .19* | .21* | .20* | .20* | .20* | .20* | −.21 | −.13* | −.11* | −.09* | −.12* | −.12* | −.17* | −.15* | −.17* | −.26 |
| GDL3 | 54.50 | 44.70 | .10 | .12 | .05 | .09 | −.02 | .18 | .12 | .22 | .07 | .05 | .02 | −.02 | −.02 | .10 | −.10 | −.05 | .10 | .12 |
| GDL4 | 65.29 | 39.27 | .14* | .17* | .22* | .09 | .11 | .18* | .17* | .03 | .44 | −.07 | −.05 | .002 | −.08 | −.04 | −.07 | −.19* | −.08 | −.25 |
| GDL5 | 42.80 | 44.14 | .15* | .14* | .19* | .13 | .20* | .25* | .13 | .02 | −.17 | −.04 | −.11* | −.06 | −.07 | −.12* | −.19* | −.02 | −.12 | −.31 |
| GDL6 | 40.88 | 43.95 | .13* | .10 | .17* | .17* | .14 | .19* | .08 | .04 | .29 | −.11* | −.04 | −.09 | −.12 | −.02 | −.09 | −.08 | −.04 | −.17 |
| GDL7 | 49.30 | 45.23 | .12* | .11* | .13* | .06 | .10* | .19* | .07 | .18* | .04 | −.05 | −.02 | −.05 | −.01 | −.02 | −.12* | −.04 | −.16* | −.16 |
| GDL8 | 40.73 | 41.56 | .10* | .09 | .14* | .04 | .09 | .21* | .16* | .13* | .10 | −.02 | −.01 | .02 | .01 | −.04 | −.03 | .01 | .01 | −.03 |
| GDL9 | 47.60 | 45.51 | .16 | .002 | .21 | .18 | .20 | .20 | .01 | −.04 | .17 | −.08 | −.21 | −.16 | −.09 | .09 | −.39* | −.08 | .10 | −.05 |
Note. N = neuroticism; E = extraversion; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
Table 3.
Means, standard deviations, and correlations between neuroticism, extraversion, openness, and self-rated health and general disease level in the SATSA sample.
| M | SD | SRH1 | SRH2 | SRH3 | SRH4 | SRH5 | SRH6 | GDL1 | GDL2 | GDL3 | GDL4 | GDL5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 2.57 | 2.56 | 2.55 | 2.54 | 2.50 | 2.46 | 2.00 | 2.61 | 2.84 | 3.09 | 3.19 | ||
| SD | .56 | .56 | .55 | .55 | .58 | .56 | 1.69 | 2.04 | 2.14 | 2.22 | 2.25 | ||
| Neuroticism | |||||||||||||
| N1 | 2.77 | 2.31 | −.28* | −.27* | −.25* | −.23* | −.15* | −.18* | .24* | .25* | .21* | .25* | .17* |
| N2 | 2.41 | 2.18 | −.23* | −.31* | −.26* | −.20* | −.15* | −.11* | .22* | .30* | .27* | .29* | .20* |
| N3 | 2.41 | 2.13 | −.25* | −.28* | −.30* | −.26* | −.17* | −.22* | .23* | .30* | .29* | .29* | .26* |
| N4 | 2.26 | 2.14 | −.24* | −.30* | −.29* | −.29* | −.23* | −.23* | .24* | .28* | .26* | .29* | .23* |
| N5 | 2.35 | 2.07 | −.22* | −.20* | −.26* | −.25* | −.30* | −.27* | .20* | .24* | .22* | .22* | .21* |
| N6 | 2.25 | 2.12 | −.16* | −.19* | −.22* | −.26* | −.24* | −.33* | .24* | .19* | .24* | .22* | .23* |
| Extraversion | |||||||||||||
| E1 | 4.78 | 2.24 | .15* | .13* | .12* | .09* | .10* | .10* | −.08* | −.04 | −.07* | −.08* | −.08* |
| E2 | 5.03 | 2.21 | .14* | .16* | .15* | .10* | .12* | .03 | −.07* | −.08* | −.07* | −.09* | −.10* |
| E3 | 5.04 | 2.21 | .15* | .15* | .13* | .13* | .11* | .14* | −.09* | −.08* | −.10* | −.10* | −.06 |
| E4 | 5.04 | 2.22 | .15* | .16* | .14* | .18* | .16* | .13* | −.11* | −.08* | −.08* | −.08* | −.08* |
| E5 | 5.16 | 2.21 | .11* | .12* | .12* | .10* | .20* | .14* | .02 | −.02 | .04 | −.01 | −.04 |
| E6 | 5.12 | 2.23 | .11* | .16* | .12* | .16* | .17* | .18* | −.03 | −.02 | .01 | −.05 | −.07 |
| Openness | |||||||||||||
| O1 | 2.98 | .69 | .08* | .05 | .05 | .08* | .13* | .14* | .001 | .01 | .05 | .05 | .05 |
| O2 | 2.96 | .72 | .09* | .10* | .12* | .11* | .11* | .12* | −.02 | −.03 | 0 | .05 | .02 |
| O3 | 2.98 | .74 | .07* | .07* | .12* | .09* | .10* | .08 | .02 | .01 | −.004 | .02 | .05 |
| O4 | 2.97 | .75 | .09* | .06* | .10* | .12* | .13* | .08* | −.003 | .001 | −.01 | .01 | .07 |
| O5 | 3.02 | .70 | −.01 | .09* | .02 | .08* | .15* | .13* | .08* | .03 | .04 | .04 | .07* |
| O6 | 3.03 | .70 | .10* | .08* | .08 | .08* | .20* | .15* | −.01 | −.03 | .04 | .04 | .06 |
Note. N = neuroticism; E = extraversion; O = openness; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
The relations between openness and health outcomes were tested in the LISS and the SATSA samples. As Tables 2 and 3 display, on both the cross-sectional and prospective basis, openness was found to be positively associated with self-rated health in the LISS and the SATSA samples. In the LISS, openness demonstrated negative concurrent and prospective connections with general disease level; however, no significant link was observed between openness and general disease level across waves in the SATSA.
Table 2.
Means, standard deviations, and correlations between the Big Five personality traits and health outcomes in the LISS sample.
| M | SD | SRH1 | SRH2 | SRH3 | SRH4 | SRH5 | SRH6 | GDL1 | GDL2 | GDL3 | GDL4 | GDL5 | GDL6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 3.18 | 3.13 | 3.09 | 3.09 | 3.10 | 3.14 | 0.43 | 0.51 | 0.58 | 0.61 | 0.58 | 0.58 | ||
| SD | .76 | .76 | .75 | .77 | .78 | .81 | 0.79 | 0.86 | 0.94 | 0.97 | 0.95 | 0.95 | ||
| Neuroticism | ||||||||||||||
| N1 | 2.59 | .68 | −.29* | −.26* | −.24* | −.26* | −.24* | −.25* | .11* | .09* | .10* | .10* | .10* | .10* |
| N2 | 2.58 | .66 | −.28* | −.28* | −.27* | −.27* | −.24* | −.24* | .09* | .09* | .10* | .09* | .09* | .09* |
| N3 | 2.55 | .67 | −.27* | −.27* | −.28* | −.28* | −.26* | −.23* | .10* | .10* | .11* | .10* | .10* | .09* |
| N4 | 2.51 | .69 | −.27* | −.27* | −.29* | −.29* | −.25* | −.24* | .09* | .09* | .10* | .09* | .09* | .08* |
| N5 | 2.55 | .70 | −.25* | −.25* | −.29* | −.29* | −.26* | −.25* | .09* | .09* | .10* | .09* | .09* | .07* |
| N6 | 2.55 | .70 | −.26* | −.24* | −.27* | −.29* | −.26* | −.28* | .11* | .11* | .11* | .10* | .09* | .07* |
| Extraversion | ||||||||||||||
| E1 | 3.30 | .63 | .11* | .12* | .10* | .09* | .11* | .10* | −.05* | −.04* | −.05* | −.05* | −.05* | −.04 |
| E2 | 3.28 | .63 | .11* | .13* | .12* | .10* | .11* | .11* | −.03 | −.05* | −.04* | −.03 | −.03 | −.03 |
| E3 | 3.25 | .63 | .11* | .12* | .12* | .12* | .10* | .10* | −.03 | −.06* | −.06* | −.05* | −.06* | −.05* |
| E4 | 3.24 | .66 | .07* | .11* | .11* | .12* | .12* | .12* | −.02 | −.04* | −.05* | −.06* | −.06* | −.05* |
| E5 | 3.25 | .66 | .09* | .11* | .11* | .13* | .14* | .14* | −.02 | −.03 | −.04* | −.04* | −.06* | −.05* |
| E6 | 3.24 | .67 | .07* | .08* | .09* | .11* | .13* | .14* | 0 | −.01 | −.02 | −.04* | −.06* | −.05* |
| Openness | ||||||||||||||
| O1 | 3.51 | .50 | .09* | .11* | .09* | .08* | .10* | .09* | −.08* | −.08* | −.10* | −.11* | −.12* | −.09* |
| O2 | 3.49 | .49 | .09* | .12* | .11* | .10* | .09* | .10* | −.08* | −.09* | −.11* | −.11* | −.11* | −.09* |
| O3 | 3.45 | .49 | .07* | .12* | .11* | .11* | .10* | .08* | −.11* | −.11* | −.11* | −.11* | −.12* | −.09* |
| O4 | 3.45 | .50 | .05* | .12* | .13* | .12* | .11* | .10* | −.09* | −.11* | −.12* | −.13* | −.12* | −.10* |
| O5 | 3.49 | .50 | .09* | .13* | .13* | .12* | .14* | .13* | −.12* | −.13* | −.15* | −.14* | −.14* | −.12* |
| O6 | 3.51 | .51 | .08* | .11* | .12* | .13* | .13* | .15* | −.09* | −.10* | −.12* | −.12* | −.12* | −.11* |
| Conscientiousness | ||||||||||||||
| C1 | 3.72 | .52 | .06* | .04* | .04* | .04* | .04* | .05* | .04* | .03* | .04* | .04* | .02 | .03 |
| C2 | 3.69 | .53 | .06* | .05* | .03 | .03 | .03 | .04* | .05* | .06* | .04* | .04* | .02 | .01 |
| C3 | 3.69 | .53 | .05* | .07* | .04* | .05* | .07* | .06* | 0 | .02 | .03* | .03 | .01 | −.01 |
| C4 | 3.71 | .53 | .04* | .08* | .07* | .06* | .06* | .07* | −.01 | .002 | .01 | .01 | .003 | −.01 |
| C5 | 3.72 | .53 | .06* | .08* | .07* | .07* | .07* | .08* | −.03 | −.03 | −.03 | 0 | −.01 | −.03 |
| C6 | 3.74 | .53 | .07* | .08* | .09* | .09* | .08* | .09* | −.02 | −.02 | −.03 | −.01 | −.02 | −.004 |
| Agreeableness | ||||||||||||||
| A1 | 3.90 | .49 | .01 | .003 | −.002 | −.02 | .01 | −.02 | .06* | .07* | .07* | .08* | .07* | .08* |
| A2 | 3.88 | .49 | −.001 | −.001 | .01 | −.02 | −.002 | −.02 | .07* | .08* | .08* | .09* | .08* | .08* |
| A3 | 3.85 | .49 | 0 | .003 | −.01 | −.02 | .01 | −.01 | .05* | .06* | .06* | .06* | .07* | .07* |
| A4 | 3.85 | .51 | −.03 | −.01 | −.01 | −.02 | −.01 | −.004 | .05* | .04* | .04* | .05* | .06* | .06* |
| A5 | 3.88 | .51 | −.02 | 0 | −.01 | −.02 | .004 | −.003 | .03 | .03 | .04* | .05* | .05* | .06* |
| A6 | 3.88 | .52 | −.01 | −.002 | .01 | .01 | .01 | .02 | .04* | .04* | .05* | .04* | .05* | .05* |
Note. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
The associations between conscientiousness and agreeableness and health outcomes were examined in the LISS only. According to the results shown in Table 2, generally, conscientiousness was significantly related to self-rated health both concurrently and prospectively such that conscientiousness assessed at earlier times were positively linked to self-rated health in later waves, and vice versa, whereas agreeableness demonstrated positive concurrent and prospective associations with general disease level.
The means, standard deviations, and correlations between personality traits and specific disease conditions in SATSA (between neuroticism, extraversion, openness and cardiovascular diseases, central nervous system diseases, and metabolic diseases) are shown in Table S4. As can be seen from the table, in addition to the positive concurrent associations, neuroticism and cardiovascular diseases, central nervous system diseases and metabolic diseases also displayed positive prospective associations with each other. However, such patterns were not observed in extraversion and openness.
The correlations between neuroticism, extraversion, and physiological health indicators in SATSA are presented in Table S5. As the table displays, in addition to the concurrent relations, some evidence was found for the prospective associations between neuroticism assessed in earlier waves and allostatic load and motor functioning impairment in subsequent waves, and vice versa. Prospective associations between extraversion measured in earlier waves and subsequent motor functioning impairment were also observed.
Taken together, across the samples, the correlations between personality traits and health outcomes suggested the presence of bidirectional associations over time as personality traits assessed at earlier time were prospectively related to subsequent health outcomes and vice versa. The results provide justifications for the following analyses.
Longitudinal Associations between Personality and Health
Using the RI-CLPMs, we next examined the dynamics between personality traits and health outcomes over time. We used the comparative fit index (CFI) and the root mean square error of approximation (RMSEA) to evaluate model fit. It has been recommended a CFI equal to or greater than .95 and an RMSEA equal to or smaller than .05 as indicators of good fit (Hu & Bentler, 1999). As shown in Tables 4–8, fit indices suggested reasonable to good fit across all the models in the samples. Specifically, CFI ranged from .966 to .979 in NAS, from .982 to .991 in LISS, and from .952 to .991 in SATSA. RMSEA ranged from .022 to .023 in NAS, from .018 to .031 in LISS, and from .020 to .045 in SATSA.
Table 4.
Standardized path coefficients in the random intercept cross-lagged panel models for the within-person relations between personality traits and self-rated health and general disease level in the NAS sample.
| Model | PredictorT | OutcomesT+1 | β1 | 95% CI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| N & SRH | .973 | .023 | ||||
| N | N | .24* | [.18, .30] | |||
| SRH | −.06* | [−.11, −.02] | ||||
| SRH | SRH | .16* | [.09, .22] | |||
| N | −.04 | [−.09, .01] | ||||
| E & SRH | ||||||
| E | E | .23* | [.18, .28] | .979 | .022 | |
| SRH | .04 | [−.01, .09] | ||||
| SRH | SRH | .15* | [.09, .22] | |||
| E | .01 | [−.03, .06] | ||||
| N & GDL | .966 | .023 | ||||
| N | N | .24* | [.18, .30] | |||
| GDL | .06* | [.01, .11] | ||||
| GDL | GDL | .10* | [.03, .17] | |||
| N | .02 | [−.03, .08] | ||||
| E & GDL | .972 | .023 | ||||
| E | E | .23* | [.18, .28] | |||
| GDL | .01 | [−.04, .06] | ||||
| GDL | GDL | .10* | [.03, .17] | |||
| E | .001 | [−.05, .05] |
Note. N = neuroticism; E = extraversion; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
Table 8.
Standardized path coefficients in the random intercept cross-lagged panel models for the within-person relations between personality traits and physiological health outcomes in the SATSA subsample.
| Model | PredictorT | OutcomeT+1 | β1 | 95% CI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| N & AL | .978 | .032 | ||||
| N | N | .29* | [.15, .44] | |||
| AL | −.07 | [−.14, .01] | ||||
| AL | AL | .37* | [.23, .51] | |||
| N | −.03 | [−.12, .06] | ||||
| E & AL | .991 | .022 | ||||
| E | E | .15* | [.06, .25] | |||
| AL | .03 | [−.04, .10] | ||||
| AL | AL | .36* | [.22, .50] | |||
| E | .04 | [−.05, .12] | ||||
| N & MFI | .952 | .044 | ||||
| N | N | .31* | [.16, .46] | |||
| MFI | .12* | [.04, .20] | ||||
| MFI | MFI | .38* | [.26, .50] | |||
| N | .06 | [−.04, .15] | ||||
| E & MFI | .962 | .045 | ||||
| E | E | .17* | [.07, .27] | |||
| MFI | −.14* | [−.21, −.07] | ||||
| MFI | MFI | .38* | [.26, .50] | |||
| E | −.09* | [−.17, −.01] |
Note. N = neuroticism; E = extraversion; O = openness; AL = allostatic load; MFI = motor functioning impairment.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
The Association between Personality and Health at the Between-Person Level
We tested the longitudinal associations between personality traits and health outcomes using the RI-CLPMs. Consistent with our expectation, at the between-person level, the time-invariant component of neuroticism was negatively related to the time-invariant component of self-rated health (r = −.36, 95% CI [−.42, −.30] in NAS, r = −.44 [−.46, −.41] in LISS, and r = −.45 [−.51, −.38] in SATSA), while the time-invariant component of extraversion exhibited a positive association with the time-invariant component of self-rated health (r =.18 [.11, .25] in NAS, r = .16 [.13, .18] in LISS, and r = .20 [.13, .27] in SATSA). The results indicated that individuals with higher levels of neuroticism or lower levels of extraversion were more likely to have lowered health ratings compared to those with lower levels of neuroticism or higher levels of extraversion across the three samples. According to results from LISS and SATSA, the time-invariant components of openness were positively associated with the time-invariant component of self-rated health in LISS, but not in SATSA (r = .09 [.07, .12] in LISS and r = .07 [0, .14] in SATSA). As findings from LISS indicated, the time-invariant components of conscientiousness and agreeableness were positively associated with the time-invariant component of self-rated health (r = .15 [.13, .18] for conscientiousness and r = .07 [.04, .10]).
Consistent with our expectation, the time-invariant component of neuroticism was positively related to the time-invariant component of general disease level (r = .31 [.24, .38] in NAS, r = .22 [.17, .26] in LISS, and r = .34 [.28, .41] in SATSA), suggesting that individuals with higher levels of neuroticism tended to experience more diseases compared to those with lower levels of neuroticism across the three samples. The time-invariant component of extraversion demonstrated a negative association with the time-invariant component of general disease level in NAS and SATSA, but not in LISS (r = −.13 [−.20, −.05] in NAS, r = .01 [−.04, .05] in LISS, and r = −.08 [−.15, −.02] in SATSA). Similar to self-rated health, inconsistencies were also observed in the associations between the time-invariant component of openness and the time-invariant component of general disease level such that a negative relation was found in LISS (r = −.05 [−.09, −.01]) while a positive association emerged in SATSA (r = .10 [.03, .17]). Findings from LISS revealed that the time-invariant component of conscientiousness was negatively related to the time-invariant component of general disease level (r = −.06 [−.11, −.02]). Contrary to our expectation, the time-invariant component of agreeableness (r = .06 [.01, .10]) was also found to be positively linked to the time-invariant component of general disease level.
When specific disease conditions were examined in SATSA, the time-invariant component of neuroticism exhibited a positive association with the time-invariant component of cardiovascular diseases (r = .22 [.13, .30]) such that individuals scored higher on neuroticism were more likely to have the diagnoses of cardiovascular diseases than those with lower neuroticism. However, the time-invariant components of extraversion and openness were not related to the time-invariant component of cardiovascular disease (r = −.01 [−.07, .05] for extraversion and r = .05 [−.02, .12] for openness). Similarly, the time-invariant component of neuroticism, but not extraversion or openness, was positively linked to the time-invariant components of both central nervous system diseases (r = .17 [.09, .25] for neuroticism, r = −.06 [−.13, .01] for extraversion, and r = −.01 [−.08, .06] for openness) and metabolic diseases (r = .15 [.08, .21] for neuroticism, r = −.01 [−.07, .06] for extraversion, and r = .03 [−.04, .10] for openness).
In the in-person testing subsample of SATSA in which physiological health indicators were tested, neither neuroticism nor extraversion was associated with allostatic load (r = −.07 [−.22, .08] for neuroticism and r = .02 [−.11, .14] for extraversion) or motor functioning impairment (r = .18 [−.03, .40] for neuroticism, r = .03 [−.12, .18] for extraversion) at the between-person level.
The Dynamic Associations between Personality and Health at the Within-Person Level
After partitioning the between-person effects from the within-person effects, we examined the longitudinal relations between personality traits and health outcomes at the within-person level. Tables 4–8 present the standardized path coefficients and 95% CIs for the within-person effects tested for all the personality traits and health outcomes across the samples (estimates of the standardized path coefficients from the unconstrained models can be seen in Tables S6–S8). In the RI-CLPM, the autoregressive coefficients (e.g., personalityT -> personaityT+1 in Tables 4–8) indicate the extent to which deviation from the level of a construct at one occasion predicts deviation from the relatively stable level at the next occasion. As shown in the tables, across all the samples, after controlling for the time-invariant components, the autoregressive coefficients demonstrated positive within-person carry-over effects in all of the personality traits, self-reported health outcomes (self-rated health, general disease level, and specific disease conditions), and physiological health outcomes (allostatic load and motor functioning impairment).
Personality and Self-Rated Health.
In regard to the dynamics between the personality traits and health outcomes, the cross-lagged coefficients in Tables 4–8 (e.g., personalityT -> healthT+1) suggested that within-person changes in neuroticism significantly predicted within-person changes in self-rated health across three samples. Specifically, as the results revealed, in NAS (Table 4), occasions when individuals scored higher than their general level of neuroticism preceded occasions when they scored lower in self-rated health (β = −.06 [−.11, −.02]) than their general levels, suggesting that when individuals were one within-person standard deviation higher in neuroticism than their general levels on one occasion, they tended to score 0.06 within-person standard deviation lower than their average health perception. Furthermore, we found evidence for bidirectional associations in the dynamics between neuroticism and self-rated health over time in LISS and SATSA. As Tables 5 and 6 indicate, at the within-person level, occasions on which individuals demonstrate higher-than-general scores on neuroticism preceded occasions in which individuals scored lower-than-general in self-rated health (β = −.04 [−.06, −.02] in LISS and β = −.06 [−.09, −.02] in SATSA). Simultaneously, times at which individuals scored higher-than-general in self-rated health were followed occasions on which individuals displayed lower-than-general in neuroticism (β = −.06 [−.08, −.05] in LISS and β = −.04 [−.08, −.01] in SATSA). In NAS, extraversion was not associated with self-rated health at the within-person level (Table 4). However, bidirectional relations were observed between extraversion and self-rated health in both LISS and SATSA such that times at which individuals showed higher-than-general scores in extraversion were preceded and followed by times at which individuals scored higher-than-general in self-rated health (β = .04 [.02, .06] in LISS and β = .05 [.01, .08] in SATSA from extraversion to self-rated health, and β = .04 [.02, .06] in LISS and β = .05 [.01, .09] in SATSA from self-rated health to extraversion). The relations between changes in openness and changes in self-rated health were examined from the LISS and the SATSA samples at the within-person level. As can be seen from Tables 5 and 6, in both LISS and SATSA, deviations in self-rated health displayed within-person effects on changes in openness (β = .04 [.02, .05] in LISS and β = .08 [.03, .12] in SATSA) such that when individuals showed elevations in openness relative to their average levels, they were likely to have their later health evaluation better than their general levels. However, the within-person effects of openness on changes in self-rated health were only found in LISS but not in SATSA (β = .04 [.02, .06] in LISS and β = .03 [−.01, .07] in SATSA). As expected, evidence was found for within-person bidirectional associations between conscientiousness and self-rated health (Table 5) such that occasions on which individuals scored higher on conscientiousness than their own general levels were preceded and followed by occasions on which individuals rated better perceptions of health (β = .02 [.001, .039] from conscientiousness to self-rated health and β = .04 [.03, .06] from self-rated health to conscientiousness). Agreeableness was not connected to self-rated health at the within-person level.
Table 5.
Standardized path coefficients in the random intercept cross-lagged panel models for the within-person relations between personality traits and self-rated health and general disease level in the LISS sample.
| Model | PredictorT | OutcomesT+1 | β1 | 95% CI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| N & SRH | .991 | .019 | ||||
| N | N | .27* | [.25, .30] | |||
| SRH | −.04* | [−.06, −.02] | ||||
| SRH | SRH | .19* | [.17, .21] | |||
| N | −.06* | [−.08, −.05] | ||||
| E & SRH | .991 | .020 | ||||
| E | E | .25* | [.22, .27] | |||
| SRH | .04* | [.02, .06] | ||||
| SRH | SRH | .19* | [.16, .21] | |||
| E | .04* | [.02, .06] | ||||
| O & SRH | .991 | .019 | ||||
| O | O | .19* | [.17, .21] | |||
| SRH | .04* | [.02, .06] | ||||
| SRH | SRH | .19* | [.16, .21] | |||
| O | .04* | [.02, .05] | ||||
| C & SRH | .988 | .022 | ||||
| C | C | .22* | [.19, .24] | |||
| SRH | .02* | [.001, .039] | ||||
| SRH | SRH | .19* | [.16, .21] | |||
| C | .04* | [.03, .06] | ||||
| A & SRH | .991 | .018 | ||||
| A | A | .21* | [.19, .23] | |||
| SRH | .01 | [−.01, .03] | ||||
| SRH | SRH | .19* | [.16, .21] | |||
| A | 0 | [−.02, .02] | ||||
| N & GDL | .984 | .029 | ||||
| N | N | .28* | [.25, .30] | |||
| GDL | .02* | [.005, .033] | ||||
| GDL | GDL | .73* | [.71, .75] | |||
| N | .06* | [.04, .09] | ||||
| E & GDL | .985 | .030 | ||||
| E | E | .25* | [.22, .27] | |||
| GDL | −.01 | [−.027, .002] | ||||
| GDL | GDL | .73* | [.71, .75] | |||
| E | −.05* | [−.07, −.02] | ||||
| O & GDL | .984 | .029 | ||||
| O | O | .19* | [.17, .21] | |||
| GDL | −.01 | [−.025, .002] | ||||
| GDL | GDL | .73* | [.71, .75] | |||
| O | −.03* | [−.056, −.003] | ||||
| C & GDL | .982 | .031 | ||||
| C | C | .22* | [.19, .24] | |||
| GDL | −.03* | [−.04, −.01] | ||||
| GDL | GDL | .73* | [.71, .75] | |||
| C | −.06* | [−.09, −.04] | ||||
| A & GDL | .984 | .029 | ||||
| A | A | .21* | [.19, .23] | |||
| GDL | −.01 | [−.02, .01] | ||||
| GDL | GDL | .73* | [.71, .75] | |||
| A | −.03 | [−.052, .001] |
Note. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
Table 6.
Standardized path coefficients in the random intercept cross-lagged panel models for the within-person relations between personality traits and self-rated health and general disease level in the SATSA sample.
| Model | PredictorT | OutcomeT+1 | β1 | 95% CI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| N & SRH | .977 | .028 | ||||
| N | N | .26* | [.20, .33] | |||
| SRH | −.06* | [−.09, −.02] | ||||
| SRH | SRH | .27* | [.21, .34] | |||
| N | −.04* | [−.08, −.01] | ||||
| E & SRH | .980 | .029 | ||||
| E | E | .22* | [.17, .28] | |||
| SRH | .05* | [.01, .08] | ||||
| SRH | SRH | .28* | [.22, .35] | |||
| E | .05* | [.01, .09] | ||||
| O & SRH | .978 | .028 | ||||
| O | O | .15* | [.09, .21] | |||
| SRH | .03 | [−.01, .07] | ||||
| SRH | SRH | .24* | [.18, .29] | |||
| O | .08* | [.03, .12] | ||||
| N & GDL | .970 | .043 | ||||
| N | N | .21* | [.14, .28] | |||
| GDL | .07* | [.02, .12] | ||||
| GDL | GDL | .44* | [.38, .49] | |||
| N | .08* | [.02, .13] | ||||
| E & GDL | .975 | .041 | ||||
| E | E | .14* | [.08, .19] | |||
| GDL | .02 | [−.02, .07] | ||||
| GDL | GDL | .45* | [.40, .51] | |||
| E | .02 | [−.04, .08] | ||||
| O & GDL | .970 | .042 | ||||
| O | O | .13* | [.05, .21] | |||
| GDL | −.01 | [−.06, .04] | ||||
| GDL | GDL | .45* | [.40, .51] | |||
| O | −.05 | [−.11, .02] |
Note. N = neuroticism; E = extraversion; O = openness; SRH = self-rated health; GDL = general disease level.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
Personality and General Disease Level.
In regard to general disease level, similar to self-rated health, occasions when individuals scored higher than their general level of neuroticism preceded occasions when they suffered from more diseases (β = .06 [.01, .11]) than their general levels in NAS (Table 4). Moreover, bidirectional associations in the dynamics between neuroticism and general disease level over time emerged in LISS and SATSA. As Tables 5 and 6 suggest, at the within-person level, occasions on which individuals scored lower on neuroticism (β = .02 [.005, .033] in LISS and β = .07 [.02, .12] in SATSA from neuroticism to general disease level, β = .06 [.04, .09] in LISS and β = .08 [.02, .13] in SATSA from general disease level to neuroticism) than their own general levels were preceded and followed by occasions on which individuals endorsed fewer diseases. Although occasions at which individuals experienced elevations in general disease level were followed by occasions at which individuals exhibited decreases in extraversion and openness (β = −.05 [−.07, −.02] for extraversion and β = −.03 [−.056, −.003] for openness) in LISS, generally, across all samples, no evidence was found for the within-person association between extraversion, openness, and general disease level. The longitudinal associations between changes in conscientiousness and agreeableness and changes in general disease level were examined in LISS. Within-person bidirectional associations between conscientiousness and general disease level were observed (Table 5) such that occasions on which individuals scored higher on conscientiousness than their own general levels were preceded and followed by occasions on which individuals endorsed fewer diseases (β = −.03 [−.04, −.01] from conscientiousness to general disease level and β = −.06 [−.09, −.04] from general disease level to conscientiousness). No evidence was found for within-person links between agreeableness and general disease level.
Personality and Specific Health Conditions.
In addition to general disease level, we further investigated the dynamic connections between personality traits and some specific disease conditions at the within-person level in SATSA. As shown in Table 7, despite of their associations with general disease level at the between-person level, changes in neuroticism, extraversion, and openness were not related to changes in specific disease conditions at the within-person level.
Table 7.
Standardized path coefficients in the random intercept cross-lagged panel models for the within-person relations between personality traits and specific conditions in the SATSA sample.
| Model | PredictorT | OutcomeT+1 | β1 | 95% CI | CFI | RMSEA |
|---|---|---|---|---|---|---|
| N & CVD | .968 | .038 | ||||
| N | N | .20* | [.13, .27] | |||
| CVD | .03 | [−.01, .07] | ||||
| CVD | CVD | .56* | [.49, .64] | |||
| N | .06 | [0, .12] | ||||
| E & CVD | .976 | .034 | ||||
| E | E | .14* | [.08, .19] | |||
| CVD | .03 | [−.01, .06] | ||||
| CVD | CVD | .56* | [.49, .64] | |||
| E | −.04 | [−.09, .02] | ||||
| O & CVD | .969 | .037 | ||||
| O | O | .13* | [.05, .21] | |||
| CVD | −.01 | [−.05, .04] | ||||
| CVD | CVD | .56* | [.49, .64] | |||
| O | −.06 | [−.13, .01] | ||||
| N & CNS | .965 | .034 | ||||
| N | N | .20* | [.13, .27] | |||
| CNS | .04 | [−.01, .09] | ||||
| CNS | CNS | .40* | [.25, .55] | |||
| N | .04 | [−.02, .11] | ||||
| E & CNS | .974 | .031 | ||||
| E | E | .14* | [.08, .20] | |||
| CNS | −.03 | [−.08, .02] | ||||
| CNS | CNS | .41* | [.25, .56] | |||
| E | −.02 | [−.08, .05] | ||||
| O & CNS | .970 | .032 | ||||
| O | O | .13* | [.05, .21] | |||
| CNS | .02 | [−.03, .07] | ||||
| CNS | CNS | .40* | [.25, .56] | |||
| O | −.04 | [−.02, .011] | ||||
| N & MTD | .985 | .024 | ||||
| N | N | .19* | [.13, .26] | |||
| MTD | .02 | [−.03, .07] | ||||
| MTD | MTD | .21* | [.08, .33] | |||
| N | .01 | [−.04, .06] | ||||
| E & MTD | .991 | .020 | ||||
| E | E | .14* | [.08, .19] | |||
| MTD | −.04 | [−.08, 0] | ||||
| MTD | MTD | .21* | [.08, .33] | |||
| E | −.01 | [−.06, .03] | ||||
| O & MTD | .986 | .024 | ||||
| O | O | .13* | [.05, .21] | |||
| MTD | −.01 | [−.06, .05] | ||||
| MTD | MTD | .21* | [.09, .33] | |||
| O | −.01 | [−.07, .04] |
Note. N = neuroticism; E = extraversion; O = openness; CVD = cardiovascular diseases; CNS = central nervous system diseases; MTD = metabolic diseases.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
Personality and Physiological Health Outcomes.
We also tested the within-person associations between neuroticism, extraversion, and physiological health outcomes that were evaluated in objective ways. As presented in Table 8, neither neuroticism nor extraversion was linked to allostatic load at the within-person level. In terms of motor functioning impairment, according to the results, occasions on which individuals displayed higher-than-general levels in neuroticism preceded occasions on which individuals experienced increases in motor functioning impairment (β = .12 [.04, .20]). On the contrary, deviations in motor functioning impairment did not exhibit significant effects on changes in neuroticism. Bidirectional associations were observed between changes in extraversion and changes in motor functioning impairment at the within-person level such that occasions on which individuals showed decreases in extraversion were preceded and followed by occasions on which individuals experienced increases in motor functioning impairment (β = −.14 [−.21, −.07] from extraversion to motor functioning impairment and β = −.09 [−.17, −.01] from motor functioning impairment to extraversion).
Model Comparisons.
Table 9 presents the results for model comparisons testing the significance of the dynamic within-person effects between personality traits and health outcomes by constraining the cross-lagged effects of personality traits on health outcomes and vice versa to zero. According to the model comparison indices, across all samples, constraining the cross-lagged effects to zero resulted in significant decreases in model fit when significant effects between changes in personality traits and changes in health outcomes were found as suggested by confidence intervals. Thus, despite their small effect sizes, the within-person effects should not be ignored for modeling the dynamic associations between personality traits and health outcomes over time. However, as shown in Table S9, no consistent patterns were found across samples regarding to testing equivalence in the strength of the reciprocal associations between changes in personality traits and changes in health outcomes at the within-person level.
Table 9.
Chi-square difference tests for testing the cross-lagged effects between personality traits and health outcomes at the within-person level in the random intercept cross-lagged panel models across all samples.
| b3=0 | b4=0 | |||||
|---|---|---|---|---|---|---|
| Δχ2 | Δdf | p-value | Δχ2 | Δdf | p-value | |
| NAS | ||||||
| N & SRH | 6.771 | 1 | .009 | 1.986 | 1 | .159 |
| E & SRH | 2.552 | 1 | .110 | .357 | 1 | .550 |
| N & GDL | 4.721 | 1 | .030 | .605 | 1 | .437 |
| E & GDL | .092 | 1 | .762 | .002 | 1 | .964 |
| LISS | ||||||
| N & SRH | 18.252 | 1 | < .001 | 50.799 | 1 | < .001 |
| E & SRH | 16.338 | 1 | < .001 | 16.872 | 1 | < .001 |
| O & SRH | 17.090 | 1 | < .001 | 16.198 | 1 | < .001 |
| C & SRH | 4.115 | 1 | .043 | 22.321 | 1 | < .001 |
| A & SRH | .630 | 1 | .427 | .002 | 1 | .964 |
| N & GDL | 6.882 | 1 | .009 | 21.374 | 1 | < .001 |
| E & GDL | 2.991 | 1 | .084 | 11.180 | 1 | < .001 |
| O & GDL | 2.898 | 1 | .089 | 4.616 | 1 | .032 |
| C & GDL | 13.894 | 1 | < .001 | 20.326 | 1 | < .001 |
| A & GDL | .689 | 1 | .407 | 3.682 | 1 | .055 |
| SATSA1 | ||||||
| N & SRH | 10.058 | 1 | .002 | 5.813 | 1 | .016 |
| E & SRH | 5.417 | 1 | .020 | 5.895 | 1 | .015 |
| O & SRH | 2.515 | 1 | .113 | 11.637 | 1 | .001 |
| N & GDL | 8.789 | 1 | .003 | 8.445 | 1 | .004 |
| E & GDL | 1.212 | 1 | .271 | .447 | 1 | .503 |
| O & GDL | .176 | 1 | .675 | 1.991 | 1 | .158 |
| N & CVD | 2.335 | 1 | .127 | 3.498 | 1 | .061 |
| E & CVD | 1.900 | 1 | .168 | 1.797 | 1 | .180 |
| O & CVD | .113 | 1 | .737 | 2.845 | 1 | .092 |
| N & CNS | 2.656 | 1 | .103 | 2.094 | 1 | .148 |
| E & CNS | 1.026 | 1 | .311 | .298 | 1 | .585 |
| O & CNS | .890 | 1 | .345 | 1.583 | 1 | .208 |
| N & MTD | .819 | 1 | .366 | .117 | 1 | .733 |
| E & MTD | 3.276 | 1 | .070 | .333 | 1 | .564 |
| O & MTD | .059 | 1 | .808 | .229 | 1 | .633 |
| N & AL | 2.933 | 1 | .087 | .340 | 1 | .560 |
| E & AL | .708 | 1 | .400 | .618 | 1 | .432 |
| N & MFI | 7.896 | 1 | .005 | 1.444 | 1 | .229 |
| E & MFI | 44.378 | 1 | < .001 | 6.0756 | 1 | .014 |
Note. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level; CVD = cardiovascular diseases; CNS = central nervous system diseases; MTD = metabolic diseases; AL = allostatic load; MFI = motor functioning impairment. b3 refers to estimates of the cross-lagged effects of personality traits on health outcomes; b4 refers to estimates of the cross-lagged effects of health outcomes on personality traits.
As MLR was used in SATSA to account for the cluster effects of twin pairs, the Satorra-Bentler scaled chi-square difference tests were used.
Time-Specific Associations between Personality and Health at the Within-Person Level
Table 10 presents the time-specific associations between personality traits and health outcomes at the within-person level across all samples. As the table shows, overall, contemporaneous associations between deviations in personality traits and deviations in health outcomes at the within-person level demonstrate patterns similar to that found at the between-person level in terms of direction and significance. However, the magnitude of the time-specific associations between personality traits and health outcomes at the within-person level was substantially smaller than those at the between-person level. Despite of the overall consistency, some differential findings still emerged in the time-specific associations at the within-person from those observed at the between-person level. For example, although agreeableness was related to self-rated health and general disease level at the between-person level, time-specific associations between deviations in agreeableness and deviations in the two health outcomes were not significant at the within-person level. Similarly, neuroticism was significantly linked to central nervous system diseases and metabolic diseases at the between-person but not at the within-person level. Finally, deviations in neuroticism and extraversion were significantly connected to deviations in motor functioning at the within-person level despite their nonsignificant relations at the between-person level, indicating that at the times individuals reported values higher than their general levels of neuroticism or lower than their own general levels of extraversion, they tended to experience motor functioning impairment that was more severe than their typical levels.
Table 10.
Standardized correlation coefficients in the random intercept cross-lagged panel models for the contemporaneous associations between personality traits and health outcomes at the within-person level.
| NAS | LISS | SATSA | ||||
|---|---|---|---|---|---|---|
| r1 | 95% CI | r1 | 95% CI | r1 | 95% CI | |
| Self-rated health | ||||||
| N & SRH | −.06* | [−.11, −.02] | −.09* | [−.11, −.07] | −.14* | [−.17, −.10] |
| E & SRH | .05* | [.01, .09] | .05* | [.03, .07] | .08* | [.05, .12] |
| O & SRH | .05* | [.03, .07] | .07* | [.03, .11] | ||
| C & SRH | .04* | [.03, .06] | ||||
| A & SRH | .04 | [−.01, .02] | ||||
| General disease level | ||||||
| N & GDL | .02 | [−.02, .07] | .05* | [.03, .07] | .13* | [.08, .17] |
| E & GDL | .003 | [−.04, .05] | −.03* | [−.05, −.01] | 0 | [−.05, .04] |
| O & GDL | −.03* | [−.05, −.02] | −.01 | [−.06, .04] | ||
| C & GDL | −.04* | [−.06, −.02] | ||||
| A & GDL | −.004 | [−.02, .01] | ||||
| Specific disease conditions | ||||||
| N & CVD | .10* | [.06, .14] | ||||
| E & CVD | −.02 | [−.06, .02] | ||||
| O & CVD | −.03 | [−.07, .02] | ||||
| N & CNS | .03 | [−.02, .08] | ||||
| E & CNS | −.04 | [−.08, .01] | ||||
| O & CNS | .04 | [−.02, .09] | ||||
| N & MTD | .01 | [−.04, .05] | ||||
| E & MTD | −.03 | [−.08, .01] | ||||
| O & MTD | −.01 | [−.06, .04] | ||||
| Physiological health indicators | ||||||
| N & AL | −.06 | [−.14, .02] | ||||
| E & AL | .01 | [−.06, .09] | ||||
| N & MFI | .13* | [.06, .20] | ||||
| E & MFI | −.11* | [−.19, −.04] | ||||
Note. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level; CVD = cardiovascular diseases; CNS = central nervous system diseases; MTD = metabolic diseases; AL = allostatic load; MFI = motor functioning impairment.
p ≤ .05.
Due to slight differences in the standardized coefficients across waves, the average of the coefficients is presented.
Continuous Time Modeling
Between-Person Associations.
Results for the longitudinal relations between personality traits and health outcomes in the LISS and the SATSA samples are presented in tables in the Appendix2. As shown in Table A1, overall, patterns similar to those found in the RI-CLPMs emerged for the associations between the time-invariant components of personality traits and the time-invariant components of health outcomes at the between-person level, with only few exceptions. Despite the nonsignificant relations between the time-invariant component of openness and the time-invariant components of self-rated health in SATSA in RI-CLPMs, in CT models, consistent with the observation in LISS, openness was positively related to self-rated health at the between-person level in SATSA. Rather than displaying a positive association as in the RI-CLPMs, the time-invariant component of agreeableness was not related to the time-invariant component of general disease level in LISS in CT model. Finally, openness showed a positive association with cardiovascular disease at the between-person level when CT model was applied; however, nonsignificant relation was found in RI-CLPM.
Personality and Self-Rated Health.
Tables A2–A5 display the parameter estimates, 95% CIs, model fit and model comparison indices for the cross-effects tested for all the personality traits and health outcomes (including models with the cross-effects constrained to zero) in the LISS and the SATSA samples using CT models. As Tables A2 and A3 depict, in general, the patterns for the dynamic associations between changes in personality traits and changes in self-rated health at the within-person level were congruent with those found in RI-CLPMs. However, rather than observing bidirectional relations between changes in extraversion and changes in self-rated health in both samples, in CT models, increases in self-rated health predicted subsequent rate of elevations in extraversion in both samples, with bidirectional relations found in LISS only. Also, when CT model was used, increases in self-rated health predicted subsequent rate of increases in conscientiousness. In contrast, changes in conscientiousness showed no effects on subsequent changes in self-rated health.
Personality and General Disease Level.
For general disease level, as presented in Tables A2 and A3, bidirectional associations between changes in neuroticism and changes in general disease level at the within-person level were found in SATSA but not in LISS in CT models. Using CT models, in both LISS and SATSA, increases in general disease level demonstrated effects on the rate of decreases in openness on later occasions (which was found in LISS but not SATSA when RI-CLPMs were applied). However, different from using RI-CLPM in which bidirectional relations between changes in conscientiousness and changes in general disease level emerged, no such associations were found when CT model was applied.
Personality and Specific Disease Conditions.
Discrepancies between findings from RI-CLPMS and those from CT models were observed in the dynamic associations between personality traits and specific conditions at the within-person level in SATSA. While no significant links were found between changes in personality traits and changes in specific conditions at the within-person level in SATSA using RI-CLPMs, dynamic relations were found in CT models. Specifically, as Table A4 displays, changes in neuroticism and changes in cardiovascular diseases and central nervous system diseases were connected in a bidirectional manner such that elevations in neuroticism were positively related to subsequent rate of changes in cardiovascular diseases and central nervous system diseases and vice versa. Also, increases in cardiovascular diseases were related to rate of decreases in openness at later times, whereas increases in extraversion predicted subsequent rate of decreases in central nervous system diseases. As in findings from RI-CLPMs, personality traits and metabolic diseases were not interconnected at the within-person level.
Personality and Physiological Health Outcomes.
Table A5 shows the results for the cross-effects between personality traits and physiological health outcomes at the within-person level in CT models. In accordance with the patterns found in RI-CLPMs, increases in neuroticism and decreases in extraversion were linked to rate of increases in motor functioning impairment at later times. While bidirectional associations were observed between changes in extraversion and changes in motor functioning impairment at the within-person level using RI-CLPM, bidirectional relations were found between changes in neuroticism and changes in motor functioning impairment in CT model.
Summary
Table 11 provides a summary of both the between-person and within-person associations between personality traits and health outcomes in both RI-CLPMs and CT models across the three samples. The table indicates the significance and direction of the effects on all the parameters.
Table 11.
Summary of results of the associations between personality traits and health outcomes at the between-person and within-person levels across the three samples.
| Between-Person P <-> H | Within-Person P -> H | Within-Person H -> P | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAS | LISS | SATSA | NAS | LISS | SATSA | NAS | LISS | SATSA | ||||||||||
| RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | RI-CLPM | CT | |
| Self-rated health | ||||||||||||||||||
| N | − | − | − | − | − | − | − | − | − | − | / | − | − | − | − | |||
| E | + | + | + | + | + | + | / | / | + | + | + | / | / | / | + | + | + | + |
| O | + | + | / | + | + | + | / | / | + | + | + | + | ||||||
| C | + | + | + | / | + | + | ||||||||||||
| A | + | + | / | / | / | / | ||||||||||||
| General disease level | ||||||||||||||||||
| N | + | + | + | + | + | + | + | − | + | + | / | + | / | + | + | |||
| E | − | / | / | − | − | / | / | / | / | / | / | − | − | / | / | |||
| O | − | − | + | + | / | − | / | / | − | − | / | − | ||||||
| C | − | − | − | / | − | / | ||||||||||||
| A | + | / | / | / | / | / | ||||||||||||
| Cardiovascular diseases | ||||||||||||||||||
| N | + | + | / | + | / | + | ||||||||||||
| E | / | / | / | / | / | / | ||||||||||||
| O | / | + | / | / | / | − | ||||||||||||
| Central nervous system diseases | ||||||||||||||||||
| N | + | + | / | + | / | + | ||||||||||||
| E | / | / | / | − | / | / | ||||||||||||
| O | / | / | / | / | / | / | ||||||||||||
| Metabolic diseases | ||||||||||||||||||
| N | + | + | / | / | / | / | ||||||||||||
| E | / | / | / | / | / | / | ||||||||||||
| O | / | / | / | / | / | / | ||||||||||||
| Allostatic load | ||||||||||||||||||
| N | / | / | / | / | / | / | ||||||||||||
| E | / | / | / | / | / | / | ||||||||||||
| Motor functioning impairment | ||||||||||||||||||
| N | / | / | + | + | / | + | ||||||||||||
| E | / | / | − | − | − | / | ||||||||||||
Note. “+” indicates a positive effect; “−” indicates a negative effect; “/” indicates a nonsignificant effect, blank indicates information was not available in the sample. RI-CLPM = random intercept cross-lagged panel model; CT = continuous time model; N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness.
Discussion
The current study investigated the dynamic associations between personality traits and health outcomes over time in three independent samples. Using RI-CLPMs and CT models, we examined nuances in the personality-health links by separating the stable effects at the between-person level from dynamic processes at the within-person level using longitudinal data from three studies. Across these three studies, overall, at the between-person level, the associations between personality traits and self-rated health, general disease level, and specific disease conditions were consistent with previous findings (Murray & Booth, 2015; Smith, 2006) such that individuals who were high on negative traits (e.g., neuroticism) were more likely to display negative health outcomes, whereas those scored high on positive traits (e.g., conscientiousness) tended to demonstrate better health outcomes. In addition to the between-person associations, our results further demonstrated that changes in personality traits and changes in different types of health outcomes were interconnected with each other at the within-person level after controlling for the between-person effects. Generally, the dynamic within-person relations between personality traits and health outcomes were in the direction consistent with their between-person connections (with only few exceptions), though the within-person relationships (both the cross-lagged and the time-specific links) were substantially smaller in strength when compared their between-person counterparts. Moreover, evidence was also found for the bidirectional dynamic associations between personality traits and health outcomes in within-person changes.
Relations between the Time-Invariant Components of Personality and Health
As expected, across the three studies, at the between-person level, positive personality traits (e.g., extraversion, conscientiousness) showed positive relations to self-rated health and negative relations to general disease level (except extraversion and general disease level in LISS), whereas negative trait (e.g., neuroticism) displayed negative associations with self-rated health and positive associations with general disease level. The findings suggest that there may be overlapping influences of constant or cumulative factors (e.g., genetic factors, cumulative environmental influences) contributing to individual differences in the relatively stable levels of both personality traits and health outcomes. Results at the between-person level help with identifying individuals for whom we can expect better health status according to their levels of certain personality traits, as well as for whom health related monitoring and intervention may be needed. The patterns of associations of neuroticism with self-rated health and general disease level and relations between extraversion and self-rated health were well replicated across three studies. Although some inconsistencies were present, generally, extraversion was also connected to individual differences in general disease level in the NAS and the SATSA samples. Consistent with previous findings (Friedman & Kern, 2014; Murray & Booth, 2015; Smith, 2006), at the between-person level, high conscientiousness was associated with high self-rated health and low general disease level. The connections between high conscientiousness and positive health outcomes may be partially explained by the links between conscientiousness and health behaviors and adherence at the interindividual level (Bogg & Roberts, 2004; Hill & Roberts, 2011). Openness exhibited relations with general disease level in opposite directions in the LISS and the SATSA samples. Openness has been found to be related to both positive and negative experiences (Lüdtke et al., 2011). It is possible that the association between openness and general disease level is contingent upon other related life experiences (e.g., positive and negative life events). Also, the inconsistencies in extraversion and openness among samples may be explained by differences in personality measures. Extraversion was measured by the IPIP-50 in the LISS while by the EPI in the NAS and the SATSA. Similarly, openness was assessed by different measures in the LISS and the SATSA (IPIP-50 and NEO-PI, respectively). Given that different facets of the traits are emphasized by different inventories, it is possible that different facets of extraversion and openness may show differential links to general disease level, which stimulates a call for more facet-level research in the future.
According to the current results, neuroticism, but not extraversion or openness, consistently demonstrated positive relations with all the disease conditions (cardiovascular diseases, central nervous system diseases, and metabolic diseases) tested in the current study at the between-person level. Given the widely established links between neuroticism and a broad range of health-related behaviors and biopsychosocial processes (Friedman, 2019; Lahey, 2009; Shackman et al., 2016), it is possible that in addition to being linked to mechanisms that are related to physical health in a general way, neuroticism is also related to risk/protective factors that are linked to different health conditions in a specific manner.
Within-Person Relation between Personality and Health
The primary focus of the current study was to investigate the within-person dynamic transactions between personality traits and health outcomes after accounting for their relatively stable covariances at the between-person level. Similar to the findings from previous research at the between-person level (Murray & Booth, 2015), within-person associations between personality traits and health outcomes were found for neuroticism, extraversion, openness, and conscientiousness, with neuroticism exhibited the most consistent relations across different types of health outcomes in different dynamic models and samples. However, agreeableness was not found to be linked to health outcomes at the within-person level. Depending on the specific personality traits and health outcomes tested, they were interconnected in a unidirectional or bidirectional manner over time.
The Effects of Changes in Personality on Changes in Health.
At first, results from the current study indicated that in addition to providing information on individual differences in health status, personality traits also play roles in predicting changes in health at the intraindividual level. Across samples and models (RI-CLPMs and CT models), evidence was found for the effects of deviations in personality traits at certain time points on subsequent changes in health outcomes. Furthermore, according to the current findings, the predictive effects of changes in personality traits on intraindividual changes in physical health extend beyond self-perceptions in health, the measure of which may tap into some psychological processes in addition to actual physical health and overlap with measures of personality traits. Predictive effects of changes personality traits were found across different types of health outcomes, including relatively objective measures of disease levels, assessed in both general and specific ways, as well as performance-based ratings of motor functioning impairment. As suggested by previous research, changes in personality traits may lead to changes in behaviors and other experiences, such as mental health status, that are closely related to physical health (Chow & Roberts, 2014; Takahashi et al., 2013). Through cumulative effects, changes in those health-related mechanisms result in changes in health outcomes. For example, increases in neuroticism may expose individuals to negative emotions, stress experiences, and heightened biological reactivities (Shackman et al., 2016). After being accumulated over time, the changes worsen individuals’ health conditions (both subjectively and objectively). Based on the findings, changes in personality traits may be possibly viewed as signs of onset or progression of different health conditions, the information of which can be used to guide the implementation of screenings or interventions.
The Effects of Changes in Health on Changes in Personality.
Also, the present findings provide evidence for changes in physical health as one possible source for changes in personality traits. Previous research has suggested that life experiences, such as changes in health status, lead to changes in states (e.g., emotions, perceptions), the long-term shifts of which may shape the development of personality traits (Roberts, 2018). Results from the current study indicated the predictive effects of deviations in health outcomes from one’s general level on subsequent changes in personality traits; however, the findings should be interpreted with caution as future studies are needed to investigate whether the detected effects reflect truly enduring changes in the trait components of personality. Despite the need for long-term follow-ups, the current results can be viewed as preliminary evidence for the role of changes in physical health in driving personality development given that the effects were observed in samples that were repeatedly assessed with different intervals between measurement occasions (an average interval of 1.8 years in LISS and an average interval of 4.6 years in SATSA) across different types of health assessments.
Bidirectional Relations between Changes in Personality and Changes in Health.
In the current study, results also suggested bidirectional associations between personality traits and health at the within-person level. Specifically, in both LISS and SATSA, elevations in self-rated health relative to the individuals’ overall levels at a particular time were preceded and followed by within-person decreases in neuroticism and increases in extraversion (the within-person effects of extraversion on self-rated health were not observed in SATSA when CT model was used). In SATSA, at the within-person level, the bidirectional relations were found between changes in neuroticism and changes in general disease level (in both the RI-CLPM and the CT model), and between changes in openness and changes in general disease level when the CT model was applied. Also, when time was treated as continuous, bidirectional associations were also detected between changes in neuroticism and changes in specific disease conditions, including cardiovascular diseases and central nervous system diseases, as well as changes in motor functioning impairment. The within-person bidirectional personality-health associations are in accordance with the corresponsive principle of personality development (Roberts et al., 2008; Roberts & Nickel, 2017). The reciprocal relations between personality traits and health outcomes over time provide support for the self-reinforcing aspect of the corresponsive principle such that while personality traits lead individuals to certain life experiences, the experiences may also reinforce and deepen the personality traits. Also, the findings of the within-person bidirectional associations between personality traits and health are consistent with lifespan developmental theory (Baltes, 1987; Mroczek et al., 2020) which suggests that, rather than claiming causal relationships, the developmental variables, including personality traits and health, are co-developing across time, even over the latter half of the life course. As the principle of plasticity maintains, due to the plastic and malleable nature of personality traits and health, there are dynamic processes in the associations between personality traits and health over time. The reciprocally reinforcing effects between personality traits and health may have long-term implications for healthy development and healthy aging. Individuals in the upward spirals are likely to become increasingly mature in personality, which benefits health development, and vice versa. On the contrary, a negative mutual reinforcement may result in developmental processes of personality that are deleterious to physical, psychological, and social functioning, leading to worsening health conditions.
Time-Specific Relations between Changes in Personality and Changes in Health.
In terms of the time-specific links at the within-person level, the patterns for the associations between personality traits and self-rated health were generally similar to those at the between-person level, though the effect sizes were substantially smaller. In contrast, across three studies, deviations in personality traits were less likely to connect to deviations in disease levels, either measured as the general levels or for certain specific conditions, on the concurrent basis at the within-person level, despite their significant associations at the between-person level. However, although neuroticism and extraversion and motor functioning impairment were not associated with each other at the between-person level, deviations in neuroticism and extraversion were significantly related to deviations in motor functioning impairment at a particular time. Thus, in general, the current results suggest that for certain health outcomes, the pattern of between-person associations between personality traits and health may not be generalizable to their time-specific relations at the within-person level.
Between- and Within-Person Effects Comparisons.
In the RI-CLPMs, when compared to the effect sizes at the between-person level, the magnitude of the effect sizes for the within-person relationships between personality traits and health, both the cross-lagged effects and the time-specific correlations (the estimates of the time-specific correlations at the within-person level were not biased by time effects of the measurement lags), were substantially smaller. This suggests that when examining the reciprocal relations between personality traits and health, it is important to differentiate the effects at the between-person level from those at the within-person level. Generalizing the results found at the between-person level to the within-person dynamic processes may overestimate the strength of the interconnections between personality traits and health at the within-person level. Moreover, as different patterns were found for the between-person associations and the time-specific relations at the within-person level in the links between personality traits and chronic diseases, failure to distinguish the between-person relations from the within-person links may result in inaccurate identification of the presence/absence of the within-person associations.
Inconsistencies.
As we employed different dynamic models and multiple samples to examine the within-person relations between personality traits and health outcomes, some patterns for the consistencies/inconsistencies emerged across models and samples should be noticed. Overall, significant dynamic relations between personality traits and health outcomes at the within-person level were observed across samples with different average measurement intervals and across models treating time in different ways. However, in general, higher consistencies were observed for the within-person relations between personality traits and self-rated health when compared to those between personality traits and disease-related outcomes, suggesting that the links between personality traits and self-rated health are more stable across time and sample-specific influences. For example, in LISS, bidirectional associations were found for changes in neuroticism and conscientiousness and changes in general disease level using RI-CLPMs; however, no such associations were found when CT models were used. In contrast, although no significant relation was detected between changes in personality traits and changes in specific disease conditions at the within-person level using RI-CLPMs in SATSA, bidirectional connections between changes in neuroticism and changes in cardiovascular diseases and central nervous system diseases, as well as unidirectional effects of changes in extraversion on changes in central nervous system diseases and changes in cardiovascular diseases on changes in openness were observed when CT models were used. As it has been suggested, the lagged relations modeled at the within-person level correspond to different time scales, and the lagged effects sometimes may be attenuated or exaggerated by the time-scale influences (Beck & Jackson, 2021). According to the current results, generally, we observed the predictive effects of changes of personality traits at certain occasions on subsequent changes in health outcomes in samples assessed with different measurement intervals, as well as on rates of changes in health outcomes when time was modeled as continuous, and vice versa. Future studies are needed to further explore the optimal measurement interval for studying the dynamic personality-health links. It is possible that personality traits in certain domains (e.g., conscientiousness) and certain types of health outcomes (e.g., specific disease conditions) are more sensitive to the effects of measurement interval than others such that the optimal measurement interval differs across different domains of personality traits and different types of health outcomes. Meanwhile, for some pairs of personality trait and health outcome, replicable results were obtained in different models within but not across samples. For example, using RI-CLPMs, bidirectional associations between changes in neuroticism and changes in self-rated health were found in LISS and SATSA, but only unidirectional effects of changes in neuroticism on changes in self-rated health were found in NAS. Given that the sample evaluated in the NAS was a unique sample of male veterans, making it qualitatively distinct from those assessed in the LISS and the SATSA, it is possible that certain sample-specific factors, such as life experiences that were uniquely pertinent to the sample, may play a role in moderating the within-person links between personality traits and health.
In sum, findings from the present study highlight the importance of investigating the dynamics between personality traits and health at the within-person level. Depending on the specific personality traits and health outcomes tested, they may be interconnected in a bidirectional or unidirectional manner over time. Within-person level examination provides us with unique information about the directionality for the personality-health link, as well as the processes that may play roles in shaping the development of personality traits and changes in health over time.
Practical Implications
In addition to theoretical insights, the present findings also have implications for personality interventions. Intervention efforts rest on the assumption that personality is a leading indicator of health. Results from the current study do not refute that notion. Indeed, the results provide good evidence that personality does lead health, and sometimes health leads personality. Thus, our findings complicate the traditional assumption of personality interventions. That said, interventions may focus on breaking or disrupting the bidirectional coupling, thereby stopping their deleterious effects. However, the results from the current study may spell good news for intervention efforts in that once a personality trait (or a facet thereof) has been altered in such a way as to improve health, a positive feedback loop in the form of bidirectional coupling may take over and support, or even “turbocharge”, the intervention. As with other dynamic processes, the personality-health association may be accelerated or dampened by experimental interventions.
Limitations and Future Directions
The current study has many strengths such as the use of three longitudinal samples and different dynamic models to distinguish within-person effects from between-person effects. However, there are qualifications that need to be considered when interpreting the study findings. First, we only assessed the Big Five personality traits at the domain level, and no facet-level analyses were systematically performed. According to previous research, different facets may show divergent relationships to health-related outcomes (Chopik, 2016; Sutin et al., 2018; Turiano et al., 2012). Thus, future research is needed to test the longitudinal associations between personality and health at the facet-level of traits. Second, in the present study, personality traits were measured by self-report. Previous research has suggested that aggregation of friend-rated personality traits was a better predictor of longevity when compared with self-ratings (Jackson et al., 2015). Meanwhile, objective health measures were available in SATSA only. Future research may investigate whether the current findings can be generalized to observer-reported personality traits and physiological measures of health in other samples. Third, the measure of allostatic load was mainly comprised of indicators for functioning of metabolic system, with a few other indicators for cardiovascular functioning. As suggested by previous research (McEwen, 2000; Seeman et al., 1997), optimal assessment of allostatic load should include indicators for functioning of multiple physiological systems, such as cardiovascular system, metabolic system, nervous system, and the hypothalamic–pituitary–adrenal (HPA) axis. Dominance of indicators for metabolic system in the current measure may be one possible explanation for the lack of associations between personality traits and allostatic load at both the between-person and within-person levels in SATSA, as no connection was found for personality traits and metabolic diseases in the sample. Future studies should examine the dynamic relations between personality traits and allostatic load using measures with sets of more diverse indicators. Fourth, despite the strength of using three independent samples, the effects for conscientiousness and agreeableness were only examined in LISS. Meanwhile, the analyses for specific disease conditions and physiological health outcomes were conducted in the SATSA sample only. Future studies are needed to test the replicability of the current findings. Finally, there is one caveat in interpreting the current results that we cannot make causal inferences about the longitudinal relationships between personality traits and health. In our current design, we cannot rule out the potential influences of time-varying factors that may confound the observed personality-health relations. For example, underlying biological processes or other psychological processes (e.g., perceived stress, depressive experiences) may drive the bidirectional effects in the within-person associations between neuroticism and the health outcomes. Future studies are needed to investigate the mechanisms underlying the relations between personality traits and health outcomes to better uncover their longitudinal links.
Conclusion
In summary, the current study investigated the longitudinal associations between personality traits and different types of health outcomes in three large samples. Using the RI-CLPMs and CT models, we tested the personality-health links at the between-person level and the dynamics in their longitudinal relations at the within-person level. Depending on the personality traits and health outcomes examined, evidence was found for unidirectional and bidirectional associations between changes in personality and changes in health over time. The results provide us with more in-depth understandings of how changes in personality traits are linked to changes in health, the directionality of their longitudinal associations, as well as the complexity of these relations. Future studies should examine the longitudinal relations between personality facets and health and consider biological, psychological, social, and measurement factors that may potentially moderate their longitudinal relations; only then will we have a more complete understanding of the dynamic interplay between personality and health between, as well as within, persons.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute on Aging, R01-AG018436: Personality & Well-being Trajectories in Adulthood & R01-AG064006: Boston Early Adversity and Mortality Study: Linking administrative data to long-term longitudinal studies.
Appendix
Table A1.
Parameter estimates and correlation coefficients for the relations between the time-invariant components of personality traits and the time-invariant components of health outcomes at the between-person level in the LISS and SATSA samples.
| Covariance | Standard error | Correlation coefficient | |
|---|---|---|---|
| LISS | |||
| Self-rated health | |||
| N & SRH | −.26 | .008 | −.44* |
| E & SRH | .10 | .008 | .16* |
| O & SRH | .06 | .008 | .10* |
| C & SRH | .09 | .008 | .15* |
| A & SRH | .04 | .007 | .07* |
| General disease level | |||
| N & GDL | .12 | .007 | .21* |
| E & GDL | −.01 | .008 | −.02 |
| O & GDL | −.03 | .007 | −.05* |
| C & GDL | −.05 | .007 | −.09* |
| A & GDL | .01 | .007 | .02 |
| SATSA | |||
| Self-rated health | |||
| N & SRH | −.22 | .018 | −.47* |
| E & SRH | .10 | .017 | .22* |
| O & SRH | .04 | .017 | .07* |
| General disease level | |||
| N & GDL | .18 | .017 | .37* |
| E & GDL | −.04 | .016 | −.08* |
| O & GDL | .05 | .017 | .10* |
| Cardiovascular diseases | |||
| N & CVD | .10 | .018 | .22* |
| E & CVD | −.01 | .017 | −.01 |
| O & CVD | .04 | .018 | .07* |
| Central nervous system diseases | |||
| N & CNS | .08 | .018 | .16* |
| E & CNS | −.02 | .017 | −.05 |
| O & CNS | −.01 | .018 | −.01 |
| Metabolic diseases | |||
| N & MTD | .08 | .018 | .15* |
| E & MTD | −.01 | .017 | −.01 |
| O & MTD | .02 | .019 | .04 |
| Allostatic load | |||
| N & AL | −.05 | .030 | −.10 |
| E & AL | 0 | .029 | 0 |
| Motor functioning impairment | |||
| N & MFI | .05 | .030 | .23 |
| E & MFI | −.01 | .027 | −.02 |
Note. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level; CVD = cardiovascular disease; CNS = central nervous system disease; MTD = metabolic disease; AL = allostatic load; MFI = motor functioning impairment.
p ≤ .05.
Table A2.
Parameter estimates and 95% confidence intervals of the cross-effects in the continuous time models for the within-person relations between the Big Five personality traits and self-rated health and general disease level in the LISS sample.
| Pt -> Ht+1 | Ht -> Pt+1 | Model fit statistics | Model comparison statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable & Model | Estimate | 95% CI | Estimate | 95% CI | −2LL | df | AIC | ΔLL | Δdf | p-value |
| N & SRH | ||||||||||
| Baseline Model | −.10 | [−.18, −.03] | −.13 | [−.18, −.08] | 215015.3 | 97058 | 20899.32 | |||
| Model A | 0 | - | −.11 | [−.16, −.06] | 215022.4 | 97059 | 20904.45 | 7.12 | 1 | .008 |
| Model B | −.04 | [−.12, .03] | 0 | - | 215042.7 | 97059 | 20924.71 | 27.38 | 1 | < .001 |
| E & SRH | ||||||||||
| Baseline Model | .23 | [.14, .33] | .12 | [.07, .17] | 212067.0 | 97058 | 17950.97 | |||
| Model A | 0 | - | .08 | [.04, .13] | 212089.8 | 97059 | 17971.82 | 22.85 | 1 | < .001 |
| Model B | .16 | [.07, .26] | 0 | - | 212090.4 | 97059 | 17972.41 | 23.44 | 1 | < .001 |
| O & SRH | ||||||||||
| Baseline Model | .21 | [.11, .31] | .12 | [.06, .18] | 216509.4 | 97058 | 22393.41 | |||
| Model A | 0 | - | .09 | [.03, .15] | 216526.6 | 97059 | 22408.55 | 17.14 | 1 | < .001 |
| Model B | .16 | [.06, .25] | 0 | - | 216524.9 | 97059 | 22406.94 | 15.53 | 1 | < .001 |
| C & SRH | ||||||||||
| Baseline Model | .08 | [−.01, .17] | .11 | [.05, .17] | 217448.2 | 97058 | 23332.22 | |||
| Model A | 0 | - | .09 | [.04, .15] | 217451.3 | 97059 | 23333.27 | 3.05 | 1 | .081 |
| Model B | .03 | [−.05, .12] | 0 | - | 217461.6 | 97059 | 23343.60 | 13.38 | 1 | < .001 |
| A & SRH | ||||||||||
| Baseline Model | .05 | [−.03, .14] | 0 | [−.06, .06] | 219151.1 | 97058 | 25035.13 | |||
| Model A | 0 | - | −.01 | [−.07, .05] | 219152.5 | 97059 | 25034.50 | 1.37 | 1 | .242 |
| Model B | .05 | [−.03, .14] | 0 | - | 219151.1 | 97059 | 25033.13 | 0 | 1 | .998 |
| N & GDL | ||||||||||
| Baseline Model | −.05 | [−.09, −.01] | −.02 | [−.06, .02] | 197284.4 | 95045 | 7194.36 | |||
| Model A | 0 | - | 0 | [−.03, .03] | 197289.8 | 95046 | 7197.77 | 5.40 | 1 | .020 |
| Model B | −.04 | [−.069, −.001] | 0 | - | 197285.5 | 95046 | 7193.48 | 1.11 | 1 | .292 |
| E & GDL | ||||||||||
| Baseline Model | −.05 | [−.09, 0] | −.05 | [−.09, −.02] | 193233.4 | 95045 | 3143.45 | |||
| Model A | 0 | - | −.04 | [−.07, −.01] | 193237.3 | 95046 | 3145.27 | 3.82 | 1 | .051 |
| Model B | −.01 | [−.05, .03] | 0 | - | 193242.6 | 95046 | 3150.60 | 9.16 | 1 | .002 |
| O & GDL | ||||||||||
| Baseline Model | −.07 | [−.11, −.02] | −.05 | [−.090, −.002] | 197544.3 | 95045 | 7454.32 | |||
| Model A | 0 | - | −.02 | [−.06, .02] | 197552.0 | 95046 | 7459.98 | 7.66 | 1 | .006 |
| Model B | −.04 | [−.086, −.002] | 0 | - | 197548.5 | 95046 | 7456.51 | 4.19 | 1 | .041 |
| C & GDL | ||||||||||
| Baseline Model | −.02 | [−.06, .02] | .01 | [−.03, .05] | 198537.3 | 95045 | 8447.34 | |||
| Model A | 0 | - | .02 | [−.02, .06] | 198538.2 | 95046 | 8446.16 | .83 | 1 | .364 |
| Model B | −.03 | [−.06, .01] | 0 | - | 198537.5 | 95046 | 8445.53 | .19 | 1 | .660 |
| A & GDL | ||||||||||
| Baseline Model | .01 | [−.04, .05] | 0 | [−.05, .05] | 200119.9 | 95045 | 10029.86 | |||
| Model A | 0 | - | 0 | [−.05, .04] | 200119.9 | 95046 | 10027.95 | .09 | 1 | .768 |
| Model B | .01 | [−.03, .04] | 0 | - | 200119.9 | 95046 | 10027.86 | 0 | 1 | .979 |
Note. In baseline models, the cross-effects of personality traits on health outcomes and the cross-effects of health outcomes on personality traits were allowed for free estimation. In Model A, the cross-effects of personality traits on health outcomes were constrained to zero. In Model B, the cross-effects of health outcomes on personality traits were constrained to zero. Model comparisons were made for Baseline Model vs. Model A and Baseline Model vs. Model B. N = neuroticism; E = extraversion; O = openness; C = conscientiousness; A = agreeableness; SRH = self-rated health; GDL = general disease level; LL = Log Likelihood; AIC = Akaike’s Information Criterion; df = degrees of freedom.
Table A3.
Parameter estimates and 95% confidence intervals of the cross-effects in the continuous time models for the within-person relations between personality traits and self-rated health and general disease level in the SATSA sample.
| Pt -> Ht+1 | Ht -> Pt+1 | Model fit statistics | Model comparison statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable & Model | Estimate | 95% CI | Estimate | 95% CI | −2LL | df | AIC | ΔLL | Δdf | p-value |
| N & SRH | ||||||||||
| Baseline Model | −.04 | [−.086, −.001] | −.05 | [−.08, −.01] | 46991.05 | 20178 | 6635.05 | |||
| Model A | 0 | - | −.04 | [−.07, −.01] | 46995.00 | 20179 | 6637.00 | 3.96 | 1 | .047 |
| Model B | −.03 | [−.07, .01] | 0 | - | 46999.50 | 20179 | 6641.50 | 8.45 | 1 | .004 |
| E & SRH | ||||||||||
| Baseline Model | .04 | [−.01, .09] | .05 | [.02, .08] | 46533.75 | 20186 | 6161.75 | |||
| Model A | 0 | - | .04 | [.01, .07] | 46536.23 | 20187 | 6162.23 | 2.47 | 1 | .116 |
| Model B | .02 | [−.03, .07] | 0 | - | 46542.67 | 20187 | 6168.67 | 8.91 | 1 | .003 |
| O & SRH | ||||||||||
| Baseline Model | .05 | [−.01, .12] | .08 | [.04, .13] | 45721.39 | 19699 | 6323.392 | |||
| Model A | 0 | - | .07 | [.04, .11] | 45723.98 | 19700 | 6323.980 | 2.59 | 1 | .108 |
| Model B | .01 | [−.05, .07] | 0 | - | 45740.66 | 19700 | 6340.664 | 19.27 | 1 | < .001 |
| N & GDL | ||||||||||
| Baseline Model | .04 | [.003, .077] | .03 | [.001, .060] | 41817.81 | 18688 | 4441.81 | |||
| Model A | 0 | - | .02 | [−.01, .05] | 41822.36 | 18689 | 4444.36 | 4.55 | 1 | .033 |
| Model B | .03 | [−.01, .06] | 0 | - | 41822.00 | 18689 | 4444.00 | 4.19 | 1 | .041 |
| E & GDL | ||||||||||
| Baseline Model | .03 | [−.02, .08] | .01 | [−.02, .04] | 41452.42 | 18696 | 4060.42 | |||
| Model A | 0 | - | 0 | [−.03, .03] | 41453.99 | 18697 | 4059.99 | 1.57 | 1 | .210 |
| Model B | .03 | [−.02, .07] | 0 | - | 41452.61 | 18697 | 4058.61 | .19 | 1 | .662 |
| O & GDL | ||||||||||
| Baseline Model | −.02 | [−.08, .04] | −.04 | [−.085, −.003] | 40491.91 | 18211 | 4069.91 | |||
| Model A | 0 | - | −.04 | [−.074, −.002] | 40492.31 | 18212 | 4068.31 | .40 | 1 | .527 |
| Model B | .01 | [−.05, .06] | 0 | - | 40496.42 | 18212 | 4072.43 | 4.51 | 1 | .034 |
Note. In baseline models, the cross-effects of personality traits on health outcomes and the cross-effects of health outcomes on personality traits were allowed for free estimation. In Model A, the cross-effects of personality traits on health outcomes were constrained to zero. In Model B, the cross-effects of health outcomes on personality traits were constrained to zero. Model comparisons were made for Baseline Model vs. Model A and Baseline Model vs. Model B. N = neuroticism; E = extraversion; O = openness; SRH = self-rated health; GDL = general disease level; LL = Log Likelihood; AIC = Akaike’s Information Criterion; df = degrees of freedom.
Table A4.
Parameter estimates and 95% confidence intervals of the cross-effects in the continuous time models for the within-person relations between personality traits and specific conditions in the SATSA sample.
| Pt -> Ht+1 | Ht -> Pt+1 | Model fit statistics | Model comparison statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable & Model | Estimate | 95% CI | Estimate | 95% CI | −2LL | df | AIC | ΔLL | Δdf | p-value |
| N & CVD | ||||||||||
| Baseline Model | .05 | [.01, .09] | .04 | [.01, .06] | 42295.34 | 18531 | 5233.34 | |||
| Model A | 0 | - | .02 | [0, .05] | 42302.60 | 18532 | 5238.60 | 7.26 | 1 | .007 |
| Model B | .03 | [0, .06] | 0 | - | 42302.65 | 18532 | 5238.65 | 7.31 | 1 | .007 |
| E & CVD | ||||||||||
| Baseline Model | .03 | [−.01, .08] | −.01 | [−.04, .01] | 41782.19 | 18539 | 4704.19 | |||
| Model A | 0 | - | −.02 | [−.05, 0] | 41784.19 | 18540 | 4704.19 | 2.00 | 1 | .158 |
| Model B | .04 | [0, .09] | 0 | - | 41783.07 | 18540 | 4703.07 | .88 | 1 | .348 |
| O & CVD | ||||||||||
| Baseline Model | −.03 | [−.10, .03] | −.06 | [−.11, −.03] | 40819.96 | 18054 | 4711.96 | |||
| Model A | 0 | - | −.06 | [−.09, −.02] | 40821.12 | 18055 | 4711.12 | 1.16 | 1 | .281 |
| Model B | .01 | [−.04, .07] | 0 | - | 40832.75 | 18055 | 4722.75 | 12.79 | 1 | < .001 |
| N & CNS | ||||||||||
| Baseline Model | .08 | [.03, .14] | .06 | [.02, .10] | 43252.15 | 18535 | 6182.15 | |||
| Model A | 0 | - | .03 | [−.01, .06] | 43261.76 | 18536 | 6189.76 | 9.61 | 1 | .002 |
| Model B | .05 | [.001, .098] | 0 | - | 43260.48 | 18536 | 6188.48 | 8.33 | 1 | .004 |
| E & CNS | ||||||||||
| Baseline Model | −.08 | [−.15, −.02] | −.04 | [−.08, 0] | 42700.55 | 18543 | 5614.55 | |||
| Model A | 0 | - | −.02 | [−.05, .02] | 42706.59 | 18544 | 5618.59 | 6.04 | 1 | .014 |
| Model B | −.05 | [−.11, 0] | 0 | - | 42703.91 | 18544 | 5615.91 | 3.36 | 1 | .067 |
| O & CNS | ||||||||||
| Baseline Model | .05 | [−.03, .13] | .03 | [−.02, .09] | 41757.33 | 18058 | 5641.33 | |||
| Model A | 0 | - | .02 | [−.02, .06] | 41758.65 | 18059 | 5640.65 | 1.32 | 1 | .250 |
| Model B | .02 | [−.05, .10] | 0 | - | 41759.04 | 18059 | 5641.04 | 1.72 | 1 | .190 |
| N & MTD | ||||||||||
| Baseline Model | .01 | [−.04, .07] | .02 | [−.02, .07] | 43391.05 | 18546 | 6299.05 | |||
| Model A | 0 | - | .02 | [−.02, .06] | 43391.36 | 18547 | 6297.36 | .31 | 1 | .578 |
| Model B | 0 | [−.05, .05] | 0 | - | 43392.31 | 18547 | 6298.31 | 1.25 | 1 | .263 |
| E & MTD | ||||||||||
| Baseline Model | −.02 | [−.09, .04] | −.01 | [−.05, .04] | 42821.27 | 18554 | 5713.27 | |||
| Model A | 0 | - | 0 | [−.05, .04] | 42821.82 | 18555 | 5711.82 | .55 | 1 | .460 |
| Model B | −.02 | [−.08, .04] | 0 | - | 42821.42 | 18555 | 5711.42 | .14 | 1 | .706 |
| O & MTD | ||||||||||
| Baseline Model | −.04 | [−.12, .04] | −.04 | [−.10, .02] | 41864.59 | 18069 | 5726.59 | |||
| Model A | 0 | - | −.03 | [−.08, .02] | 41865.39 | 18070 | 5725.39 | .80 | 1 | .372 |
| Model B | −.02 | [−.10, .06] | 0 | - | 41866.35 | 18070 | 5726.35 | 1.76 | 1 | .185 |
Note. In baseline models, the cross-effects of personality traits on health outcomes and the cross-effects of health outcomes on personality traits were allowed for free estimation. In Model A, the cross-effects of personality traits on health outcomes were constrained to zero. In Model B, the cross-effects of health outcomes on personality traits were constrained to zero. Model comparisons were made for Baseline Model vs. Model A and Baseline Model vs. Model B. N = neuroticism; E = extraversion; O = openness; CVD = cardiovascular diseases; CNS = central nervous system diseases; MTD = metabolic diseases; LL = Log Likelihood; AIC = Akaike’s Information Criterion; df = degrees of freedom.
Table A5.
Parameter estimates and 95% confidence intervals of the cross-effects in the continuous time models for the within-person relations between personality traits and physiological health outcomes in the SATSA sample.
| Pt -> Ht+1 | Ht -> Pt+1 | Model fit statistics | Model comparison statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable & Model | Estimate | 95% CI | Estimate | 95% CI | −2LL | df | AIC | ΔLL | Δdf | p-value |
| N & AL | ||||||||||
| Baseline Model | −.06 | [−.14, .02] | .01 | [−.07, .09] | 14847.55 | 6349 | 2149.55 | |||
| Model A | 0 | - | .03 | [−.04, .11] | 14849.51 | 6350 | 2149.51 | 1.96 | 1 | .162 |
| Model B | −.06 | [−.14, .01] | 0 | - | 14847.66 | 6350 | 2147.66 | .11 | 1 | .739 |
| E & AL | ||||||||||
| Baseline Model | .10 | [−.03, .26] | .07 | [−.02, .20] | 14573.52 | 6356 | 1861.52 | |||
| Model A | 0 | - | .05 | [−.04, .15] | 14575.67 | 6357 | 1861.67 | 2.15 | 1 | .142 |
| Model B | .07 | [−.05, .20] | 0 | - | 14575.69 | 6357 | 1861.69 | 2.17 | 1 | .140 |
| N & MFI | ||||||||||
| Baseline Model | .09 | [.03, .16] | .05 | [.01, .10] | 14887.29 | 6322 | 2243.29 | |||
| Model A | 0 | − | .03 | [−.01, .07] | 14895.31 | 6323 | 2249.31 | 8.02 | 1 | .005 |
| Model B | .06 | [.01, .12] | 0 | - | 14892.29 | 6323 | 2246.29 | 5.00 | 1 | .025 |
| E & MFI | ||||||||||
| Baseline Model | −.11 | [−.21, −.01] | −.03 | [−.09, .02] | 14638.21 | 6329 | 1980.21 | |||
| Model A | 0 | - | −.01 | [−.07, .04] | 14643.01 | 6330 | 1983.01 | 4.80 | 1 | .028 |
| Model B | −.09 | [−.19, 0] | 0 | - | 14639.40 | 6330 | 1979.40 | 1.18 | 1 | .276 |
Note. In baseline models, the cross-effects of personality traits on health outcomes and the cross-effects of health outcomes on personality traits were allowed for free estimation. In Model A, the cross-effects of personality traits on health outcomes were constrained to zero. In Model B, the cross-effects of health outcomes on personality traits were constrained to zero. Model comparisons were made for Baseline Model vs. Model A and Baseline Model vs. Model B. N = neuroticism; E = extraversion; O = openness; AL = allostatic load; MFI = motor functioning impairment; LL = Log Likelihood; AIC = Akaike’s Information Criterion; df = degrees of freedom.
Footnotes
For neuroticism, Cronbach alphas ranged from 0.70 to 0.74 across Wave 1 to Wave 8. The relatively low reliability in Wave 9 was likely due to the smaller sample with older respondents in that wave, which may result in range restriction and increased sampling error.
Results for the associations between neuroticism and self-rated health/general disease level and between extraversion and general disease level in NAS are not available due to model convergence issues. In NAS, extraversion was negatively associated with general disease level at the between-person level; however, the cross-lagged effects between changes in extraversion and changes in general disease level were not significant at the within-person level.
References
- Allport GW(1937). Personality: A psychological interpretation. New York: Henry Holt & Co. [Google Scholar]
- Atherton OE, Robins RW, Rentfrow PJ, & Lamb ME (2014). Personality correlates of risky health outcomes: Findings from a large internet study. Journal of Research in Personality, 50, 56–60. 10.1016/j.jrp.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlund K, Pedersen AN, & Schroll M (2003). Functional decline from age 80 to 85: Influence of preceding changes in tiredness in daily activities. Psychosomatic Medicine, 65(5), 771–777. doi: 10.1097/01.PSY.0000082640.61645.BF [DOI] [PubMed] [Google Scholar]
- Baltes PB (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23(5), 611–626. doi: 10.1037/0012-1649.23.5.611 [DOI] [Google Scholar]
- Bandura A (1999). A social cognitive theory of personality. In Pervin L & John O (Eds.), Handbook of personality (pp. 154–196). (2nd ed.). New York: Guilford Publications. [Google Scholar]
- Beck ED, & Jackson JJ (2020). Consistency and change in idiographic personality: A longitudinal ESM network study. Journal of Personality and Social Psychology, 118(5), 1080–1100. 10.1037/pspp0000249 [DOI] [PubMed] [Google Scholar]
- Beck ED, & Jackson JJ (2021). Within-person variability. In The handbook of personality dynamics and processes (pp. 75–100). Academic Press. 10.1016/B978-0-12-813995-0.00004-2 [DOI] [Google Scholar]
- Berg AI, & Johansson B (2014). Personality change in the oldest-old: Is it a matter of compromised health and functioning? Journal of Personality, 82(1), 25–31. doi: 10.1111/jopy.12030 [DOI] [PubMed] [Google Scholar]
- Bergeman CS, Chlpuer HM, Plomin R, Pedersen NL, McClearn GE, Nesselroade JR, … McCrae RR (1993). Genetic and environmental effects on openness to experience, agreeableness, and conscientiousness: An Adoption/Twin study. Journal of Personality, 61(2), 159–179. doi: 10.1111/j.1467-6494.1993.tb01030.x [DOI] [PubMed] [Google Scholar]
- Bleidorn W, Hopwood CJ, Back MD, Denissen JJ, Hennecke M, Jokela M, … & Zimmermann J (2020). Longitudinal experience–wide association studies—A framework for studying personality change. European Journal of Personality, 34(3), 285–300. 10.1002/per.2247 [DOI] [Google Scholar]
- Bogg T, & Roberts BW (2004). Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin, 130(6), 887. 10.1037/0033-2909.130.6.887 [DOI] [PubMed] [Google Scholar]
- Bogg T, & Roberts BW (2013). The case for conscientiousness: Evidence and implications for a personality trait marker of health and longevity. Annals of Behavioral Medicine, 45(3), 278–288. 10.1007/s12160-012-9454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé R (1984). The veterans administration normative aging study. In Mednick SA, Harway M, & Finello KM, Handbook of Longitudinal Research: Teenage and Adult Cohorts, Vol. 2. Praeger. [Google Scholar]
- Bossé R, Aldwin CM, Levenson MR, & Ekerdt DJ (1987). Mental health differences among retirees and workers: Findings from the normative aging study. Psychology and Aging, 2(4), 383–389. doi: 10.1037/0882-7974.2.4.383 [DOI] [PubMed] [Google Scholar]
- Bravell ME, Finkel D, Aslan AD, Reynolds CA, Hallgren J, & Pedersen NL (2017). Motor functioning differentially predicts mortality in men and women. Archives of gerontology and geriatrics, 72, 6–11. 10.1016/j.archger.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Braveman P, Egerter S, & Williams DR (2011). The social determinants of health: Coming of age. Annual Review of Public Health, 32(1), 381–398. doi: 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, & Shiner RL (2005). Personality development: Stability and change. Annual Review of Psychology, 56(1), 453–484. doi: 10.1146/annurev.psych.55.090902.141913 [DOI] [PubMed] [Google Scholar]
- Cattell RB (1957). Personality and motivation structure and measurement. Chicago, IL: World Book Company. [Google Scholar]
- Cheung GW, & Rensvold RB (2002). Evaluating goodness-of-fit indexes for testing measurement invariance. Structural equation modeling, 9(2), 233–255. 10.1207/S15328007SEM0902_5 [DOI] [Google Scholar]
- Chopik WJ (2016). Age differences in conscientiousness facets in the second half of life: Divergent associations with changes in physical health. Personality and Individual Differences, 96, 202–211. 10.1016/j.paid.2016.02.076 [DOI] [Google Scholar]
- Chopik WJ, Kim ES, & Smith J (2015). Changes in optimism are associated with changes in health over time among older adults. Social Psychological and Personality Science, 6(7), 814–822. doi: 10.1177/1948550615590199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow PI, & Roberts BW (2014). Examining the relationship between changes in personality and changes in depression. Journal of Research in Personality, 51, 38–46. 10.1016/j.jrp.2014.04.007 [DOI] [Google Scholar]
- Costa PT, & McCrae RR (1985). The NEO personality inventory Psychological Assessment Resources Odessa, FL. [Google Scholar]
- Damian RI, Spengler M, Sutu A, & Roberts BW (2019). Sixteen going on sixty-six: A longitudinal study of personality stability and change across 50 years. Journal of Personality and Social Psychology, 117(3), 674–695. doi: 10.1037/pspp0000210 [DOI] [PubMed] [Google Scholar]
- Driver CC, Oud JH, & Voelkle MC (2017). Continuous time structural equation modeling with R package ctsem. Journal of Statistical Software, 77(5). doi: http://hdl.handle.net/10.18637/jss.v077.i05 [Google Scholar]
- Eysenck HJ, & Eysenck S (1968). Manual for the Eysenck Personality Inventory. San Diego, CA: Educational Industrial Testing Service. [Google Scholar]
- Eysenck HJ & Eysenck SBG (1975). Manual of the Eysenck Personality Questionnaire. Hodder & Stoughton, London. [Google Scholar]
- Ferguson E (2013). Personality is of central concern to understand health: Towards a theoretical model for health psychology. Health Psychology Review, 7(sup1), S32–S70. doi: 10.1080/17437199.2010.547985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Ernsth-Bravell M, & Pedersen NL (2016). Temporal dynamics of motor functioning and cognitive aging. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 71(1), 109–116. 10.1093/gerona/glv110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AJ, Medaglia JD, & Jeronimus BF (2018). Lack of group-to-individual generalizability is a threat to human subjects research. Proceedings of the National Academy of Sciences, 115(27), E6106–E6115. 10.1073/pnas.1711978115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floderus B (1974). Psycho-social factors in relation to coronary heart disease and associated risk factors. Nordisk Hygienisk Tidskrift, Suppl 6, 7–148. [Google Scholar]
- Friedman HS (2019). Neuroticism and health as individuals age. Personality Disorders: Theory, Research, and Treatment, 10(1), 25. 10.1037/per0000274 [DOI] [PubMed] [Google Scholar]
- Friedman HS, & Kern ML (2014). Personality, well-being, and health. Annual review of psychology, 65, 719–742. 10.1146/annurev-psych-010213-115123 [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kern ML, & Reynolds CA (2010). Personality and health, subjective well-being, and longevity. Journal of Personality, 78(1), 179–216. doi: 10.1111/j.1467-6494.2009.00613.x [DOI] [PubMed] [Google Scholar]
- Goldberg LR (1992). The development of markers for the big-five factor structure. Psychological Assessment, 4(1), 26–42. 10.1037/1040-3590.4.1.26 [DOI] [Google Scholar]
- Goodwin RD, & Friedman HS (2006). Health status and the five-factor personality traits in a nationally representative sample. Journal of Health Psychology, 11(5), 643–654. doi: 10.1177/1359105306066610 [DOI] [PubMed] [Google Scholar]
- Goodwin R, & Engstrom G (2002). Personality and the perception of health in the general population. Psychological Medicine, 32(2), 325–32. 10.1017/S0033291701005104 [DOI] [PubMed] [Google Scholar]
- Graham EK, Rutsohn JP, Turiano NA, Bendayan R, Batterham PJ, Gerstorf D, … Mroczek DK (2017). Personality predicts mortality risk: An integrative data analysis of 15 international longitudinal studies. Journal of Research in Personality, 70, 174–186. 10.1016/j.jrp.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, Weston SJ, Gerstorf D, Yoneda TB, Booth T, Beam CR, … Mroczek DK (2020). Trajectories of big five personality traits: A coordinated analysis of 16 longitudinal samples. European Journal of Personality, 34(3), 301–321. doi: 10.1002/per.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenole N, & Brown A (2014). The consequences of ignoring measurement invariance for path coefficients in structural equation models. Frontiers in psychology, 5, 980. 10.3389/fpsyg.2014.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N, & Forrest CB (2018). The emerging theoretical framework of life course health development. Handbook of life course health development (pp. 19–43) Springer, Cham. [PubMed] [Google Scholar]
- Halfon N, & Hochstein M (2002). Life course health development: An integrated framework for developing health, policy, and research. The Milbank Quarterly, 80(3), 433–479. doi: 10.1111/1468-0009.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker EL (2012). Why researchers should think “within-person”: A paradigmatic rationale. In Mehl MR & Conner TS (Eds.), Handbook of research methods for studying daily life (p. 43–61). The Guilford Press. [Google Scholar]
- Hamaker EL, Kuiper RM, & Grasman RPPP (2015). A critique of the cross-lagged panel model. Psychological Methods, 20(1), 102–116. doi: 10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- Hampson SE, & Friedman HS (2008). Personality and health: A lifespan perspective. In John OP, Robins RW, & Pervin LA (Eds.), Handbook of personality: Theory and research (p. 770–794). The Guilford Press. [Google Scholar]
- Hays RD, Spritzer KL, Thompson WW, & Cella D (2015). US general population estimate for “excellent” to “poor” self-rated health item. Journal of General Internal Medicine, 30(10), 1511–1516. 10.1007/s11606-015-3290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, & Roberts BW (2011). The role of adherence in the relationship between conscientiousness and perceived health. Health Psychology, 30(6), 797. 10.1037/a0023860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, & Roberts BW (2016). Personality and health: Reviewing recent research and setting a directive for the future. In Schaie KW & Willis SL (Eds.), Handbook of the psychology of aging (Eighth Edition) (pp. 205–218). San Diego: Academic Press. 10.1016/B978-0-12-411469-2.00011-X. [DOI] [Google Scholar]
- Horn JL, & McArdle JJ (1992). A practical and theoretical guide to measurement invariance in aging research. Experimental aging research, 18(3), 117–144. 10.1080/03610739208253916 [DOI] [PubMed] [Google Scholar]
- House JS, Kessler RC, & Herzog AR (1990). Age, socioeconomic status, and health. The Milbank Quarterly, 68(3), 383–411. doi: 10.2307/3350111 [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Human LJ, Biesanz JC, Miller GE, Chen E, Lachman ME, & Seeman TE (2013). Is change bad? Personality change is associated with poorer psychological health and greater metabolic syndrome in midlife. Journal of personality, 81(3), 249–260. 10.1111/jopy.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL, & Benyamini Y (1997). Self-rated health and mortality: a review of twenty-seven community studies. Journal of Health and Social Behavior, 21–37. 10.2307/2955359 [DOI] [PubMed] [Google Scholar]
- Jackson JJ, Connolly JJ, Garrison SM, Leveille MM, & Connolly SL (2015). Your friends know how long you will live: A 75-year study of peer-rated personality traits. Psychological Science, 26(3), 335–340. doi: 10.1177/0956797614561800 [DOI] [PubMed] [Google Scholar]
- Jokela M, Hakulinen C, Singh-Manoux A, & Kivimäki M (2014). Personality change associated with chronic diseases: Pooled analysis of four prospective cohort studies. Psychological Medicine, 44(12), 2629–2640. doi: 10.1017/S0033291714000257 [DOI] [PubMed] [Google Scholar]
- Kern ML, & Friedman HS (2011). Personality and pathways of influence on physical health. Social and Personality Psychology Compass, 5(1), 76–87. doi: 10.1111/j.1751-9004.2010.00331.x [DOI] [Google Scholar]
- Kline RB (2005). Principles and practice of structural equation modeling (second ed.). The Guilford Press, New York. [Google Scholar]
- Lahey BB (2009). Public health significance of neuroticism. American Psychologist, 64(4), 241. 10.1037/a0015309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham K, & Peek CW (2013). Self-rated health and morbidity onset among late midlife US adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68(1), 107–116. 10.1093/geronb/gbs104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikas S, & Salmela-Aro K (2015). Personality trait changes among young finns: The role of life events and transitions. Journal of Personality, 83(1), 117–126. doi: 10.1111/jopy.12088 [DOI] [PubMed] [Google Scholar]
- Letzring TD, Edmonds GW, & Hampson SE (2014). Personality change at mid-life is associated with changes in self-rated health: Evidence from the Hawaii personality and health cohort. Personality and Individual Differences, 58, 60–64. 10.1016/j.paid.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD, Preacher KJ, Selig JP, & Card NA (2007). New developments in latent variable panel analyses of longitudinal data. International Journal of Behavioral Development, 31(4), 357–365. doi: 10.1177/0165025407077757 [DOI] [Google Scholar]
- Lüdtke O, Roberts BW, Trautwein U, & Nagy G (2011). A random walk down university avenue: Life paths, life events, and personality trait change at the transition to university life. Journal of Personality and Social Psychology, 101(3), 620–637. doi: 10.1037/a0023743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg O, & Manderbacka K (1996). Assessing reliability of a measure of self-rated health. Scandinavian Journal of Social Medicine, 24(3), 218–224. doi: 10.1177/140349489602400314 [DOI] [PubMed] [Google Scholar]
- Luo J, & Roberts BW (2015). Concurrent and longitudinal relations among conscientiousness, stress, and self-perceived physical health. Journal of Research in Personality, 59, 93–103. 10.1016/j.jrp.2015.10.004 [DOI] [Google Scholar]
- MacCallum RC, Browne MW, & Cai L (2006). Testing differences between nested covariance structure models: Power analysis and null hypotheses. Psychological Methods, 11(1), 19–35. doi: 10.1037/1082-989X.11.1.19 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2000). Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology, 22(2),108–124. 10.1016/S0893-133X(99)00129-3 [DOI] [PubMed] [Google Scholar]
- Mischel W, & Shoda Y (1995). A cognitive-affective system theory of personality: reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychological review, 102(2), 246. 10.1037/0033-295X.102.2.246 [DOI] [PubMed] [Google Scholar]
- Molenaar PC, & Campbell CG (2009). The new person-specific paradigm in psychology. Current Directions In Psychological Science, 18(2), 112–117. 10.1111/j.1467-8721.2009.01619.x [DOI] [Google Scholar]
- Mroczek DK, & Spiro III A (2003). Modeling intraindividual change in personality traits: Findings from the normative aging study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 58(3), P153–P165. 10.1093/geronb/58.3.P153 [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Weston SJ, Willroth EC (2020). A lifespan perspective on the interconnections between personality, health, and optimal aging. In Hill P, Allemand M (eds), Personality and healthy aging in adulthood. International Perspectives on Aging, vol 26. Springer, Cham. 10.1007/978-3-030-32053-9_12. [DOI] [Google Scholar]
- Mroczek DK, Graham EK, Turiano NA, & Oro-Lambo MO (2019). Personality development in adulthood and later life. In Handbook of personality: Theory and research (4th ed.). Guilford. [Google Scholar]
- Mroczek DK (2014). Personality plasticity, healthy aging, and interventions. Developmental Psychology, 50(5), 1470–1474. doi: 10.1037/a0036028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AL, & Booth T (2015). Personality and physical health. Current Opinion in Psychology, 5, 50–55. 10.1016/j.copsyc.2015.03.011 [DOI] [Google Scholar]
- Muthén LK, & Muthén BO (2017). 1998–2017. mplus user’s guide. Muthén & Muthén: Los Angeles, CA. [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, … & Boker SM (2016). OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika, 81(2), 535–549. 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL (2015). Swedish Adoption/Twin Study on Aging (SATSA), 1984, 1987, 1990, 1993, 2004, 2007, and 2010. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 05–13. 10.3886/ICPSR03843.v2 [DOI] [Google Scholar]
- Pedersen NL, Plomin R, McClearn GE, & Friberg L (1988). Neuroticism, extraversion, and related traits in adult twins reared apart and reared together. Journal of Personality and Social Psychology, 55(6), 950. 10.1037/0022-3514.55.6.950 [DOI] [PubMed] [Google Scholar]
- Roberts BW (2018). A revised sociogenomic model of personality traits. Journal of personality, 86(1), 23–35. 10.1111/jopy.12323 [DOI] [PubMed] [Google Scholar]
- Roberts BW, Wood D, & Caspi A (2008). The development of personality traits in adulthood. In John OP, Robins RW, & Pervin LA (Eds.), Handbook of personality: Theory and research (3rd ed., pp. 375–398). New York: The Guilford Press. [Google Scholar]
- Roberts BW (2009). Back to the future: Personality and assessment and personality development. Journal of Research in Personality, 43(2), 137–145. 10.1016/j.jrp.2008.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, & DelVecchio WF (2000). The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin, 126(1), 3–25. doi: 10.1037/0033-2909.126.1.3 [DOI] [PubMed] [Google Scholar]
- Roberts BW, & Nickel LB (2017). A critical evaluation of the neo-socioanalytic model of personality. In Specht J (Ed.), Personality development across the lifespan (pp. 157–177) Academic Press. 10.1016/B978-0-12-804674-6.00011-9 [DOI] [Google Scholar]
- Roberts BW, Walton KE, & Viechtbauer W (2006). Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin, 132(1), 1–25. doi: 10.1037/0033-2909.132.1.1 [DOI] [PubMed] [Google Scholar]
- Ryan O, Kuiper RM, & Hamaker EL (2018). A continuous-time approach to intensive longitudinal data: what, why, and how?. In Continuous time modeling in the behavioral and related sciences (pp. 27–54). Springer, Cham. 10.1007/978-3-319-77219-6_2 [DOI] [Google Scholar]
- Scherpenzeel A, & Das M (2011). True longitudinal and probabilitybased internet panels: Evidence from the Netherlands. In Das M, Ester P, & Kaczmirek L (Eds.), Social and behavioral research and the Internet: Advances in applied methods and research strategies (pp. 77–104). New York: Taylor & Francis. [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, & McEwen BS (1997). Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Archives of Internal Medicine, 157(19), 2259–2268. doi: 10.1001/archinte.1997.00440400111013 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–1314. doi: 10.1037/bul0000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Song H, & Lewis MD (2019). The impact of partial factorial invariance on cross-group comparisons. Assessment, 26(7), 1217–1233. 10.1177/1073191117711020 [DOI] [PubMed] [Google Scholar]
- Smith TW (2006). Personality as risk and resilience in physical health. Current Directions Psychological Science, 15(5), 227–231. doi: 10.1111/j.1467-8721.2006.00441.x [DOI] [Google Scholar]
- Smith TW, & Spiro I, Avron. (2002). Personality, health, and aging: Prolegomenon for the next generation. Journal of Research in Personality, 36(4), 363–394. 10.1016/S0092-6566(02)00014-4 [DOI] [Google Scholar]
- Specht J, Bleidorn W, Denissen JJA, Hennecke M, Hutteman R, Kandler C, … Zimmermann J (2014). What drives adult personality development? A comparison of theoretical perspectives and empirical evidence. European Journal of Personality, 28(3), 216–230. doi: 10.1002/per.1966 [DOI] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2016). Allostatic load and personality: A 4-year longitudinal study. Psychosomatic medicine, 78(3), 302. doi: 10.1097/PSY.0000000000000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Zonderman AB, Ferrucci L, & Terracciano A (2013). Personality traits and chronic disease: Implications for adult personality development. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68(6), 912–920. 10.1093/geronb/gbt036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, & Terracciano A (2018). Facets of conscientiousness and objective markers of health status. Psychol & Health, 33(9), 1100–1115. doi: 10.1080/08870446.2018.1464165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Edmonds GW, Jackson JJ, & Roberts BW (2013). Longitudinal correlated changes in conscientiousness, preventative health-related behaviors, and self-perceived physical health. Journal of Personality, 81(4), 417–427. doi: 10.1111/jopy.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber B (2018). Predictors of Personality Development in Mid and Late Adulthood. The Role of Life satisfaction, Cognition and Health–an Investigation of Differentiating Effects of Aging. Findings from the” Interdisciplinary Longitudinal Study on Adult Development and Aging (ILSE)” (Doctoral dissertation). 10.11588/heidok.00022832 [DOI] [Google Scholar]
- Turiano NA, Chapman BP, Gruenewald TL, & Mroczek DK (2015). Personality and the leading behavioral contributors of mortality. Health Psychology, 34(1), 51–60. doi: 10.1037/hea0000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Spiro A, & Mroczek DK (2012). Openness to experience and mortality in men: Analysis of trait and facets. Journal of Aging and Health, 24(4), 654–672. doi: 10.1177/0898264311431303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkle MC, Oud JH, Davidov E, & Schmidt P (2012). An SEM approach to continuous time modeling of panel data: Relating authoritarianism and anomia. Psychological Methods, 17(2), 176. 10.1037/a0027543 [DOI] [PubMed] [Google Scholar]
- Weston SJ, Graham EK, Turiano NA, Aschwanden D, Booth T, Harrison F, … Mueller S (2020). Is healthy neuroticism associated with chronic conditions? A coordinated integrative data analysis. Collabra: Psychology, 6(1). 10.1525/collabra.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wang R, Zhao Y, Ma X, Wu M, Yan X, & He J (2013). The relationship between self-rated health and objective health status: A population-based study. BMC Public Health, 13(1), 320. 10.1186/1471-2458-13-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler AR, Masuda M, & Holmes TH (1968). Seriousness of illness rating scale. Journal of Psychosomatic Research, 11(4), 363–374. doi: 10.1016/0022-3999(68)90033-0 [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, & Ukraintseva SV (2007). Health decline, aging and mortality: How are they related? Biogerontology, 8(3), 291–302. 10.1007/s10522-006-9073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


