Abstract
Background
At the most severe end of the spectrum of congenital heart disease are patients with an univentricular physiology. They comprise a heterogeneous group of congenital heart malformations that have the common characteristic that the cardiac morphology is not equipped for sustaining a biventricular circulation.
Case summary
Here, we present a case of an adult patient after Fontan palliation, illustrative of the complex clinical course and the broad spectrum of complications that can be encountered during follow-up, highlighting the need for a multidisciplinary approach in the clinical care for these patients.
Discussion
During the surgical Fontan procedure, the inferior vena cava is connected to the pulmonary circulation, after prior connection of the superior vena cava to the pulmonary arterial circulation. The resulting cavopulmonary connection, thus lacking a subpulmonic ventricle, provides non-pulsatile passive flow of oxygen-poor blood from the systemic venous circulation into the lungs, and the functional monoventricle pumps the oxygen-rich pulmonary venous return blood into the aorta. With an operative mortality of <5% and current 30-year survival rates up to 85%, the adult population of patients with a Fontan circulation is growing. This increase in survival is, however, inevitably accompanied by long-term complications affecting multiple organ systems, resulting in decline in cardiovascular performance.
Conclusion
For optimal treatment, the evaluation in a multidisciplinary team is mandatory, using the specific expertise of the team members to timely detect and address late complications and to support quality of life.
Keywords: Case report, Congenital heart disease, Fontan circulation, Univentricular heart, Long-term complications, Fontan failure, Fontan-related liver disease
Learning points.
Follow-up of single ventricle patients with Fontan palliation can have a complex course and these patients may present with a broad spectrum of late complications affecting multiple organ systems.
Treatment and surveillance requires a well-orchestrated multidisciplinary approach in a specialized centre.
The multidisciplinary approach should focus on reduction of morbidity and mortality, but also on supporting quality of life and psychosocial impact of chronic heart disease.
In Fontan patients with (often cardiac congestion induced) liver cirrhosis, conventional imaging classification should be used with caution and correlation with other (clinical) features is pivotal prior to a diagnosis of hepatocellular carcinoma.
Primary specialities involved other than cardiology
Cardiothoracic surgery, gastroenterology, pulmonology, radiology, psychology, and dietetics.
Introduction
Congenital heart disease is the most common congenital disorder with an incidence of 1:100 in life-born children.1 At the most severe end of the spectrum are patients with an univentricular physiology, comprising a heterogeneous group of congenital heart malformations that have the common characteristic that the cardiac morphology is not equipped for sustaining a biventricular circulation. Since the Fontan palliation was first introduced in 1968, the procedure has undergone numerous modifications and the survival of patients with a single-ventricle physiology has greatly improved.2 During the Fontan procedure, the inferior vena cava is connected to the pulmonary circulation, either by construction of an intra-atrial tunnel or by placing a GORE-TEX® conduit, after prior connection of the superior vena cava to the pulmonary arterial circulation (bidirectional Glenn anastomosis) (Figure 1). The resulting cavopulmonary connection, thus lacking a subpulmonary ventricle, provides non-pulsatile passive flow of oxygen-poor blood from the systemic venous circulation into the lungs, and the functional monoventricle (that can be a morphologically left, right, or rarely an undetermined ‘true’ single ventricle) pumps the oxygen-rich pulmonary venous return blood into the aorta.
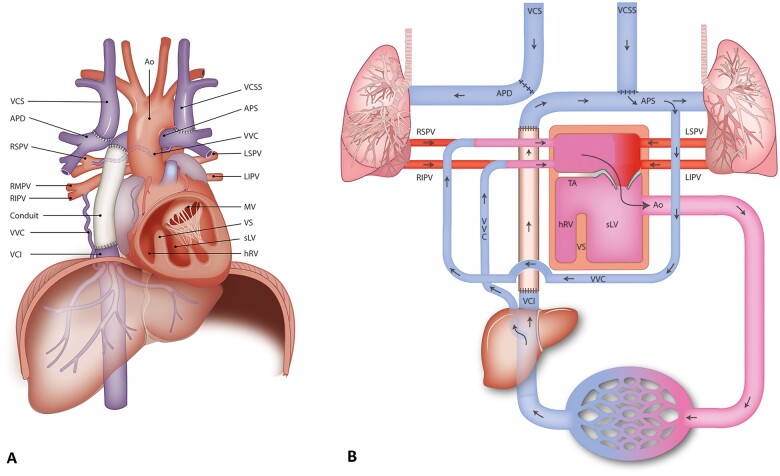
Figure 1.
(A) Graphic depiction of the anatomy of the patient. (B) Schematic depiction of the anatomy and the circulation of the patient. Ao, aorta; APD, arteria pumonalis dextra/right; APS, arteria pulmonalis sinistra/left; hRV, hypoplastic right ventricle; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RIPV, right inferior pulmonary vein; RMPV, right middle pulmonary vein; RSPV, right superior pulmonary vein; sLV, systemic (single) left ventricle; TA, tricuspid atresia; VCI, vena cava inferior; VCS, vena cava superior; VCSS, persisting vena cava superior sinistra; VS, ventricular septum; VVC, veno-venous collaterals.
With an operative mortality of <5% and current 30-year survival rates up to 85%, the adult population of patients with a Fontan circulation is growing.1,2 This increase in survival is, however, inevitably accompanied by long-term complications affecting multiple organ systems, resulting in decline in cardiovascular performance.1,2
Here, we present a case of an adult patient after Fontan palliation, illustrative of the complex clinical course and the broad spectrum of complications that can be encountered during follow-up, highlighting the need for a multidisciplinary approach in the clinical care for these patients.
Timeline
| 1991 (age 0 years) | Male patient born with: Tricuspid atresia with hypoplastic right ventricle, pulmonary stenosis, persisting left superior vena cava, ventricular and atrial septal defect; |
| Operation (sternotomy # 1): Modified Blalock-Taussig shunt (GORE-TEX® conduit between the brachiocephalic artery and the right pulmonary artery). | |
| 1994 (age 3 years) | Operation (sternotomy # 2): Bilateral bidirectional cavopulmonary shunts, and patch augmentation of the pulmonary artery confluence. |
| 2002 (age 11 years) | Operation (sternotomy # 3): Fontan completion with atriopulmonary connection. Atriopulmonary Fontan with connection of the right atrial appendage to the left pulmonary artery, and atrial septal defect closure using a fenestrated patch. |
| 2006 (age 15 years) | Complication: Protein losing enteropathy (PLE) with hypoalbuminaemia, managed with low saturated fat and high protein diet. |
| 2007 (age 16 years) | Complication: Pulmonary embolism requiring initiation of oral anticoagulation; |
| Complication: Recurrent atrial flutter with rapid atrioventricular conduction and sinus node dysfunction, pharmacological treatment (beta-blocker); | |
| Complication: (recurrent) Episodes of cardiac decompensation. | |
| 2008 (age 17 years) | Angiographic diagnostic procedure: Heart catheterization: low pulmonary artery pressures, obstruction of the pulmonary artery confluence; the right superior vena cava drains into the right pulmonary artery, and the left superior vena cava drains into the left pulmonary artery; |
| Operation (sternotomy # 4): Conversion to extracardiac total cavopulmonary connection: 20 mm diameter GORE-TEX® tube from inferior vena cava to the left pulmonary artery; resection of atrial septum with reduction of right atrium. Concomitant radiofrequency-ablation (left and right sided), and epicardial DDD-pacemaker implantation (abdominal pulse generator); | |
| Complication: Postoperative PLE and large volumes of pleural effusion leading to re-operation (sternotomy # 5) for creation of a fenestration in the Fontan tunnel (6 mm). | |
| 2011 (age 20 years) | Complication: Tendency for PLE with hypoalbuminaemia, managed with low saturated fat and high protein diet. |
| 2014 (age 23 years) | Complication: Peripheral oedema and fatigue, with desaturation during exercise; |
| Percutaneous diagnostic procedure: Left-/right heart catheterization: veno-venous collaterals, borderline elevated pulmonary pressures. Pharmacological treatment with phosphodiesterase type 5 inhibitor. | |
| 2015–2016 (age 24–25 years) | Complication: Recurrent admissions for right-sided decompensation, phosphodiesterase type 5 inhibitor treatment discontinued; |
| Complication: Hypomagnesaemia (chronic), treated with suppletion therapy. | |
| 2015 (age 24 years) | Complications: Diagnosis of liver cirrhosis [Child-Pugh A and model for end-stage liver disease (MELD) 6]; |
| Complication: Recurrent concerns about future perspectives and depressive thoughts. | |
| 2016 (age 24 years) | Invasive diagnostic procedure: Gastroscopy: oesophageal varices grade I-II. |
| 2018 (age 27 years) | Complications: Progressive fatigue and desaturation during exercise; attributed to shunting through the veno-venous collaterals. |
| Complication: Abdominal computed tomography (CT): two foci (10 mm): suspicion for hepatocellular carcinoma (HCC), no metastases. | |
| 2019 (age 28 years) | Percutaneous intervention # 1: Coiling of veno-venous collaterals (between vena cava inferior and the pulmonary veins). |
| 2019 (age 28 years) | Percutaneous intervention # 2: Transarterial chemoembolization of two suspected early stage HCC lesions. |
| 2019–2020 (age 28–29 years) | Complication: Non-sustained ventricular tachycardias and short self-terminating paroxysms of atrial tachycardias/flutter: pharmacological treatment (beta-blocker), close follow-up. |
| 2020–2021 (age 29–30 years) | HCC diagnosis revised and discarded based on clinical course and revision of CT-scans: better compatible with focal nodular hyperplasia; close follow-up. |
Case presentation
Our patient is a 30-year-old male who was born with tricuspid atresia with a hypoplastic right ventricle, pulmonary stenosis, and a persisting left superior vena cava.
In the first years of life, he consecutively underwent (i) modified right-sided Blalock-Taussig shunt (GORE-TEX® conduit between the right brachiocephalic artery and the right pulmonary artery); (ii) bilateral bidirectional cavopulmonary shunt (of the right- and left-sided superior vena cava to the right and left pulmonary artery, respectively). Later there was an obstruction of the pulmonary artery confluence; (iii) Fontan completion with an atriopulmonary connection; and (iv) conversion to an extracardiac total cavopulmonary connection with fenestration, which later spontaneously closed (Figure 1). The patient encountered a myriad of complications over the course of his young adult life.
At the age of 15, he developed a protein losing enteropathy (PLE) with hypoalbuminaemia [29 (normal range 34–48) g/L] and low total serum protein [46 (64–83) g/L]. This initially resolved with low saturated fat and high protein diet. The potency to develop PLE, however, persisted with multiple recurrences throughout the years, requiring close supervision by the dietician.
From age 15 and on, there were increasing signs of Fontan Failure, characterized by PLE, congestion, and arrhythmias, prompting further hemodynamic assessment. Echocardiography showed preserved systolic function of the single left ventricle. On cardiac catheterization, pulmonary pressures were low, however, an obstruction of the pulmonary artery confluence was demonstrated.
In this period, at age 16, an unprovoked pulmonary embolism occurred posing a chronic indication for oral anticoagulation (phenprocoumon), substituting acetylsalicylic acid 80 mg q.d. (antiplatelet agent). Also, the patient developed cardiac arrhythmias, with sinus node dysfunction and recurrent atrial flutters with rapid AV-conduction, which triggered episodes of worsening heart failure. Pharmacological treatment with beta-blocker (metoprolol, 25 mg b.i.d.) and cardiac glycoside (digoxin, 0.25 mg q.d.) was initiated.
The clinical course of the patient was extensively discussed in the multidisciplinary heart team (paediatric, adult congenital and electrophysiology cardiologists, congenital cardiothoracic surgeons). Conversion to an extracardiac tunnel, to relieve the obstruction and generate a more energy-efficient connection, with concomitant left- and right-sided radiofrequency ablation for atrial tachycardias and an epicardial DDD-pacemaker implantation (with the pulse generator implanted abdominally) to alleviate sinus node dysfunction, was preferred over heart transplantation based on current guidelines and international experience.1,3 At age 17, conversion was performed, and clinical response was good, with no arrhythmia recurrence for over 10 years.
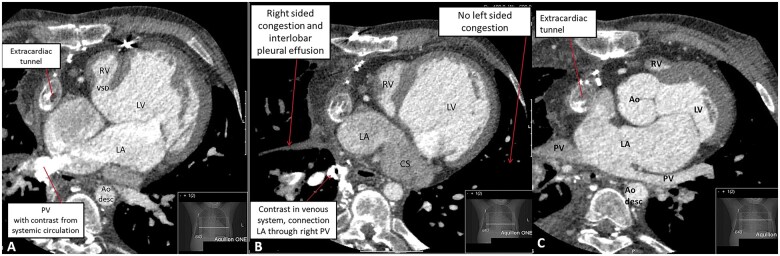
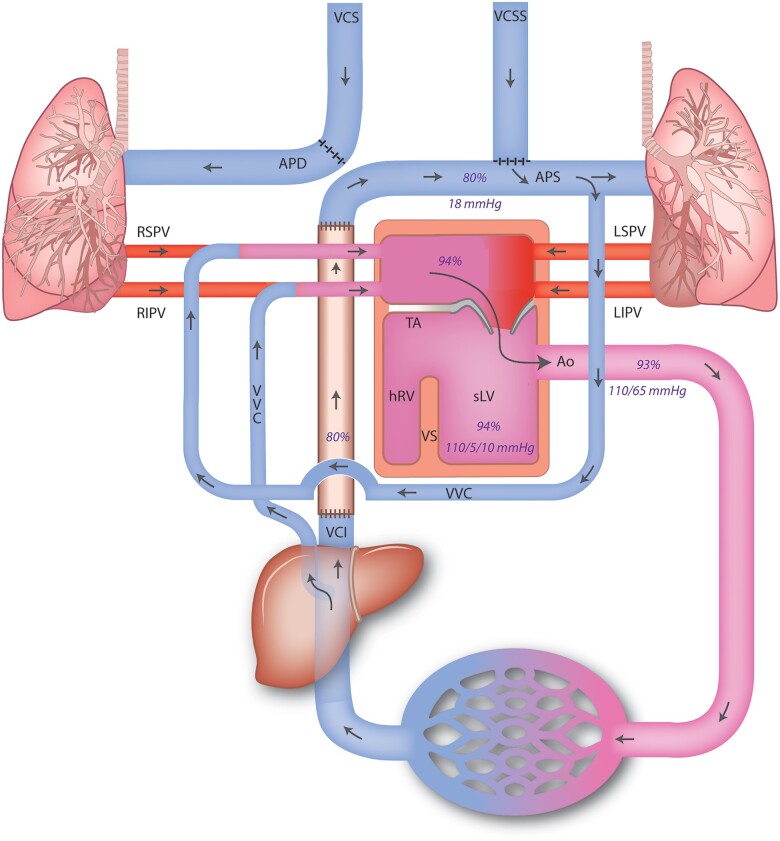
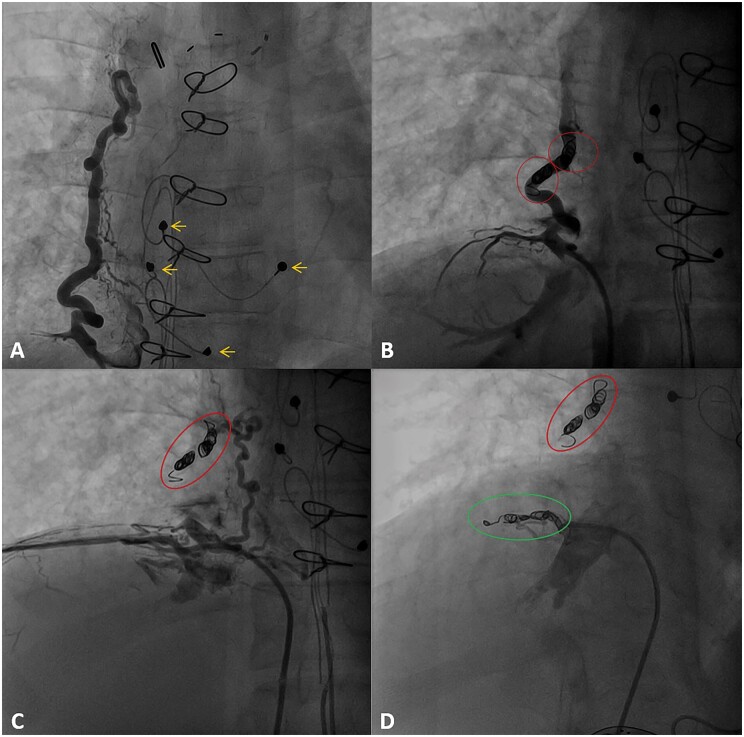
At age 23, the patient presented with increasing peripheral oedema and fatigue [New York Heart Association (NYHA) class II] that persisted despite initiation of heart failure medication (beta-blocker: metoprolol 25 mg b.i.d., angiotensin-converting enzyme inhibitors: perindopril 2 mg q.d., and diuretics: bumetanide 2 mg q.d.). Echocardiography showed moderately reduced left ventricular function, later confirmed by computed tomography (CT) scan (with simultaneous contrast administration via the femoral vein, and both brachial veins) (Figure 2), which confirmed systolic single-ventricle dysfunction (ejection fraction 37%). There were no signs of Fontan-tunnel obstruction, but congestion with right-sided pleural effusion was observed, and there was suspicion of a shunt circulation from the abdominal systemic veins to the left atrium. During cardiopulmonary exercise testing (CPET), pre-existing ventilation-perfusion mismatch (i.e. widened alveolar-arterial oxygen gradient) worsened at exercise and resulted in inefficient ventilation, dead space ventilation and increasing hypoxaemia (peripheral oxygen saturation dropped to 85% during maximal exercise, confirmed by arterial blood gas samples). Altogether, the findings during CPET were suggestive of (anatomical) shunting. Diagnostic catheterization followed, showing no significant coronary artery disease, borderline elevated pulmonary pressures (mean pulmonary artery pressure 13 mmHg) and no signs of tunnel obstruction/stenosis (Figure 3). Veno-venous collaterals (VVC) between the vena cava inferior and the right inferior pulmonary vein (Figure 4A and B) and between the left inferior branch of the pulmonary artery and the right superior pulmonary vein were identified (Figure 1). After consultation in a multidisciplinary and multicentre expert team (pulmonary hypertension specialists, congenital cardiologists, and congenital cardiothoracic surgeons), the VVC were initially managed conservatively. The main reasons for conservative treatment at that point were (i) the consideration that the VVC may provide alleviation of the systemic venous return, and (ii) the high chance of recurrence. Medical treatment was optimized using diuretics (bumetanide 2.5 mg q.d.) and phosphodiesterase type 5 inhibitor (sildenafil 5 mg b.i.d. in titration scheme to 20 mg t.i.d.), considering that even a small reduction in pulmonary vascular resistance can improve cardiac filling pressures.4 Therapy was switched to tadalafil (5 mg q.d. in titration scheme to 40 mg q.d.) due to side effects, and later, treatment was discontinued altogether after recurrent episodes of right-sided congestion. A potential explanation for this was that sildenafil/tadalafil increased intrapulmonary shunting.
Figure 2.
(A) Computed tomography angiography showing the anatomical connections of the mildly dilated left (functionally) single ventricle connected to the aorta, the hypoplastic right ventricle and the extracardiac tunnel. In addition, the subaortic ventricular septal defect is shown. (B) Computed tomography angiography during contrast showing extensive collateral opacification paraesophageal (right), opacification of right inferior pulmonary vein and a segmental right superior pulmonary vein from the right upper lobe. These findings suggested a shunt circulation from the abdominal systemic veins to the left atrium. (C) Computed tomography angiography showing dilated left (mono) atrium and dilated coronary sinus, as well as relatively dilated right pulmonary veins. Congestion with interstitial thickening of the interlobar septa and bronchial cuffing with pleural fluid on the right side, no signs of congestion in the left lung and slim pulmonary veins. Ao, aorta; Ao desc, aorta descendens; CS, coronary sinus; LA, left atrium; LV, left ventricle; PV, pulmonary vein; RV, right ventricle (hypoplastic); VSD, ventricular septal defect.
Figure 3.
Schematic depiction of the circulation of the patient with pressures and saturations as measured during cardiac catheterization. Ao, aorta; APD, arteria pumonalis dextra/right; APS, ateria pulmonalis sinistra/left; hRV, hypoplastic right ventricle; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; sLV, systemic (single) left ventricle; TA, tricuspid atresia; VCI, vena cava inferior; VCS, vena cava superior; VCSS, persisting vena cava superior sinistra; VS, ventricular septum; VVC, veno-venous collaterals.
Figure 4.
(A) Angiographic projection (AP 2.2°) showing collateral flow from the inferior vena cava to the right pulmonary vein. Epicardial pacemaker leads indicated by yellow arrows. (B) Angiographic projection (AP 8.4°) showing collateral flow immediately after coiling with four coils within the red circles (VortX pushable coil Boston Scientific 6 mm × 6.5 mm). Note the reduced distal contrast opacification in the collateral vessel. (C) Angiographic projection (AP 8.4°) showing a second small collateral from the inferior vena (subdiaphragmatic) cava to the right pulmonary vein. Previously placed coils indicated by the red circle. (D) Angiographic projection (RIO 27°) showing veno-venous collaterals after additional coiling of smaller collateral with three coils indicated by the green circle (VortX pushable coil Boston Scientific 6 mm × 6.5 mm) Previously placed coils indicated by the red circle.
At age 24, the gastroenterologist was consulted due to new liver enzyme disturbances [alkaline phosphatase 130 (<115) U/L, gamma-glutamyl transferase 92 (<55) U/L, aspartate transaminase 41 (<35) U/L, alanine transaminase 34 (<45) U/L, albumin 30 (34–48) g/L]. Liver cirrhosis was diagnosed and staged as Child-Pugh A and model for end-stage liver disease (MELD) 6. During gastroscopy, four small oesophageal varices were observed. These findings were monitored biyearly through laboratory follow-up and abdominal ultrasound and a yearly gastroscopy.
At age 27, there was progression of fatigue with resting peripheral saturation of 89-91%. After consultation in the multidisciplinary team (pulmonary hypertension, congenital cardiologists, and congenital cardiothoracic surgeons), a heart catheterization was scheduled to re-evaluate the shunting through the VVC and assess the feasibility of coiling. Measurements again showed borderline pressures in the Fontan circulation and no gradient over the Fontan conduit. After balloon-occlusion of the collaterals, the arterial saturation increased to 96% and subsequently two collaterals were successfully coiled (Figure 4). After coiling, the resting saturation increased to 93–97% and complaints of fatigue and exertional dyspnoea diminished. Exercise test afterwards showed desaturation to 90% at maximal effort, as compared to 85% pre-procedurally.
At age 28 and 29, short self-terminating paroxysms of atrial tachycardias/flutter recurred, and a non-syncopal non-sustained ventricular tachycardia was documented at pacemaker read-outs. The arrhythmias were asymptomatic, and after consultation with our electrophysiology team, pharmacological approach was initiated with beta-blocker (metoprolol 50 mg q.d.), without arrhythmia recurrence to date. Magnetic resonance imaging (MRI) for assessment of late gadolinium enhancement was considered, however due to the presence of epicardial pacemaker leads deemed not feasible.
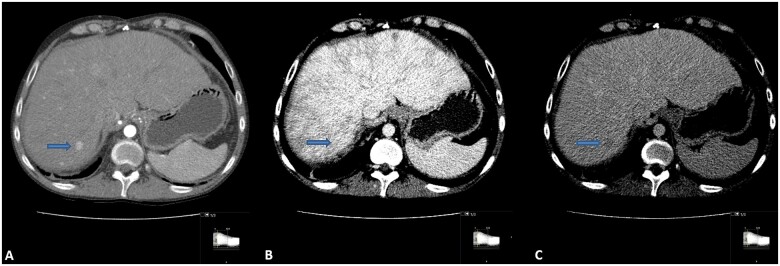
Concurrent at age 28, new hyperechogenic lesions were observed in the liver on the abdominal ultrasound. A CT scan was made to assess these lesions, as MRI was not feasible due to incompatible pacemaker leads. Two focal lesions of ∼10 mm in diameter in liver segments VII and VIII were seen and deemed highly suspicious for hepatocellular carcinoma (HCC) with evident hypervascularity and washout (Figure 5), classifying Liver Imaging Reporting And Data System (LI-RADS) 4–5. Furthermore, the liver had a nutmeg appearance (characteristic of congestive heart failure i.a.), and multiple small focal lesions with hypervascularity without washout were seen, most likely dysplastic or regeneration nodules. No metastases were documented on additional CT scan of the thorax. Oncological serum markers were low [alpha fetoprotein 3 (<7) ug/L, carbohydrate 19.9 5.1 (<27.0) kU/L]. Biopsy of the lesions was considered yet renounced due to the small lesion size and ensuing high likelihood of false negative results/low predictive value, as well as additional high risk of periprocedural complications, whereas the HCC diagnosis was considered highly likely based on radiological appearance. The patient was discussed extensively in a multidisciplinary team (congenital cardiologists, congenital cardiothoracic surgeons, gastroenterologists, oncologists, oncological-, and transplantation surgeons), and declined for liver transplantation due to the high risk of perioperative mortality. Radiofrequency ablation was deemed not feasible due to the multiple small lesions. Transarterial chemoembolization was chosen as the mode of (palliative) treatment. Simultaneously, the option of heart–liver transplantation was explored in cooperation with other specialized transplantation centres internationally, as combined heart–liver transplantations are not performed in the Netherlands.
Figure 5.
Computed tomography showing one of the focal lesions (blue arrows) suspicious of hepatocellular carcinoma with characteristic hypervascularity in arterial phase (A). The lesion is iso-attenuating in the portal venous phase (B). There is washout in the delayed phase (C).
Transarterial chemoembolization was performed through super selective angiography of the right hepatic artery (posterior division) (Figure 6). Despite the selective approach, the small lesions could not be visualized. Therefore, 50 mg of doxorubicin were injected towards segment VII/VIII. Strict follow-up with watchful waiting policy was conducted. At the follow-up CT scan 6 weeks after the procedure only one residual lesion in segment VII could be identified. During subsequent follow-up scans up until 1 year after initial diagnosis and treatment, the lesion in segment VIII had not recurred, and the lesion in segment VII remained unchanged. Oncological markers remained low, and his clinical condition remained stable. As these findings were inconsistent with the typical clinical course of HCC, findings were extensively re-evaluated, eventually resulting in revision of the HCC diagnosis based on clinical course and revision of CT scans. In retrospect, the diagnosis was better compatible with focal nodular hyperplasia.
Figure 6.
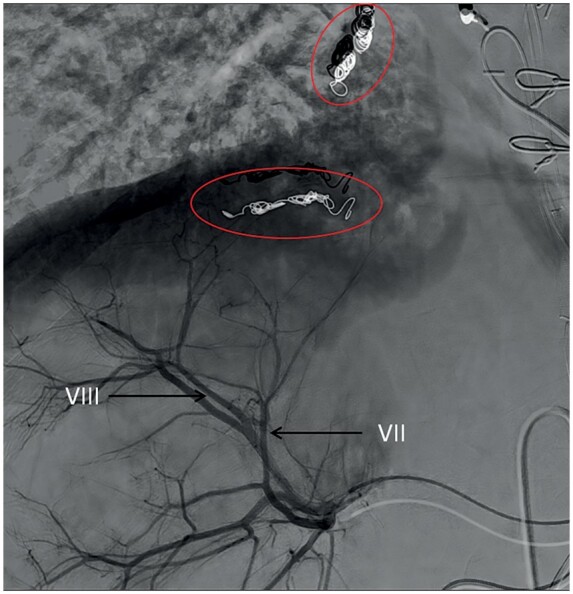
Angiographic visualization showing transarterial chemoembolization through the posterior division of the right hepatic artery (black arrows) towards segment VIII (projected over segment VII). Note the previously placed coils in the veno-venous collaterals (red circle).
Over the years, the intensive course of the complications and corresponding investigations and procedures, as well as the strict dietary restrictions, resulted in recurrent concerns about future perspectives, quality of life,and depressive thoughts of the patient, wherefore consultations with psychiatrists, psychologists and social workers were initiated.
Currently, the patient functions in NYHA class I–II and has significantly reduced exercise capacity with a validity of 49% (90 Watt) and VO2max of 16.9 mL/min/kg (36% of predicted). Oxygen saturation at rest is 92%. Echocardiography shows a mildly decreased systolic function of the dilated left ventricle (ejection fraction 51%). His medical treatment currently consists of a beta-blocker (metoprolol 50 mg q.d.), angiotensin-converting enzyme inhibitor (perindopril 2 mg q.d.), potassium-sparing diuretic (spironolactone 50 mg b.i.d.), loop diuretic (bumetanide 1.5–2 mg t.i.d.), anticoagulation therapy (phenprocoumon), and magnesium citrate (400 mg t.i.d.) for suppletion of chronic hypomagnesaemia. The patient is seen biyearly in the outpatient clinic with physical examination, including evaluation of the peripheral saturation, and yearly echocardiography, CPET, and laboratory work-up including haematology, renal function, liver panel, albumin, and heart failure markers. Additionally, consultation with the gastroenterologist, psychologist, and dietician is continued.
Discussion
Tricuspid atresia with hypoplastic right ventricle is an uncommon cyanotic congenital heart defect and accounts for about 1% of all cases of congenital heart defects.5 Without early intervention, mortality rates are high. Palliation in the form of the non-contractile Fontan circulation is dependent on passive flow towards the lungs. Therefore, any condition that elevates the resistance of the Fontan tunnel or the downstream pulmonary circulation, contributes to failing of the Fontan circulation.2 This will increase the systemic venous pressures significantly, leading to a plethora of complications effecting multiple organ systems, with detrimental effects on the exercise capacity, quality of life and increased mortality.2,6 Given the high and seemingly inevitable occurrence of complications, structured follow-up is required. Yet, evidence in guidelines for treatment and surveillance is limited and is based mostly on small and retrospective studies, case reports and expert consensus. Our patient is illustrative of the broad spectrum of complications observed in the adult patient with Fontan circulation, emphasizing the necessity of a multidisciplinary approach.
Anticoagulation
With the low non-pulsatile flow in the Fontan conduit, atrial blood stasis and disturbed coagulation, the Fontan circulation is prone to thrombosis. There seems consensus to treat all Fontan patients with lifelong antiplatelet agents.2 Anticoagulation is recommended in patients with a history of atrial arrhythmia, atrial thrombus, and/or thromboembolic events.1,2
Arrhythmias
Supraventricular tachycardias are common, with a prevalence of 20% 10 years after Fontan palliation.7 Guidelines recommend a proactive approach towards electrophysiologic evaluation and potential ablation strategies.1 Our patient had a good response to radiofrequency ablation and pacemaker implantation, supported by pharmacological treatment. Patients with univentricular circulation also have an increased risk of ventricular arrhythmias (reported incidence of 5%), especially those with late gadolinium enhancement on MRI.7,8
Protein losing enteropathy
The incidence of PLE is 5–12%, and prognosis varies with a 5-year survival of <50% to up to 88%.2,9 Although the exact mechanism of PLE remains unclear, multiple factors have been identified, including lymphatic congestion, variability in lymphatic anatomy, elevated systemic venous pressures, altered intestinal mucosal perfusion, and a pro-inflammatory state.2 Our patient’s first episode of PLE occurred at the age of 15 years and remained rather stable over the following 15 years with strict dietary precautions and conversion to an extracardiac tunnel with fenestration.
Veno-venous collaterals
VVC occur frequently after total cavopulmonary connection, with a prevalence of 15–30%.10,11 Although still not elucidated, possible causes are angiogenesis and recanalization of embryonic remnants. It is considered that VVC both have potential beneficial (unloading of the Fontan circulation), as well as unfavourable (desaturation/cyanosis) physiological effects.10 The best approach for the treatment of VVC still remains unclear with conflicting results. Proper patient selection is important and particularly careful risk-benefit considerations should be made before embolization of VVC in patients with atriopulmonary Fontan, heterotaxia, and Fontan pressures above 18 mmHg, as these conditions have been correlated with worse outcomes and survival, which might be explained by the dependency of the cardiac output on the increased preload from shunting.11 Our patient experienced relieve of symptoms and improved peripheral oxygen saturations after successful coiling of two VVC.
Fontan-related liver diseases
Fontan-related liver disease entails hepatic disorders arising from the hemodynamic changes and systemic venous congestion as a result of the Fontan palliation, and include liver fibrosis, cirrhosis, as well as HCC.12 The incidence of Fontan-related liver diseases increases with time, with liver fibrosis present in 43% thirty years after Fontan palliation.12 Therefore, regular evaluation of liver function and imaging should be considered.1 In Fontan associated cirrhotic livers, there is an annual risk for HCC of 1.5–5%.12 For patients at increased risk of developing HCC, the LI-RADS classification is developed to assess HCC risk.13 The use of this classification in this specific patient can be argued upon due to the different (i.e., including cardiac congestion) aetiology of the cirrhosis.13–15 Although the imaging findings were characteristic, the clinical course proved the initial HCC diagnosis to be incorrect. This again stresses that in this specific patient group, LI-RADS may be misleading and should be used with caution and correlation with other (clinical) features is important prior to a diagnosis of HCC.14,15
Fontan failure
As failure of the Fontan circulation is the main cause of mortality in this patient group, and currently seems inevitable in many cases, long-term options should be considered. Firstly, in case of an atriopulmonary conduit, the option of conversion to extracardiac tunnel should be evaluated in selected patients.1 In our patient, at age 15, signs of failure were first noted, which mandated hemodynamic evaluation of the Fontan circulation.1 As our patient had atrial arrhythmias, preserved single left ventricular function, low pulmonary pressures, and an atriopulmonary connection, conversion to an extracardiac tunnel was performed with concomitant ablation and pacemaker implantation, as is supported by current guidelines.1,3
Another long-term option is cardiac transplantation. The majority of congenital heart disease patients receiving heart transplantation (70–80%) have a univentricular circulation.5,16 However, in the Netherlands, donor hearts remain scarce and only 50% of patients listed for transplantation have received a heart between 2013 and 2017, with high mortality on the waiting list of 15%.17 In cases of Fontan failure and Fontan associated liver diseases, a combined heart–liver transplant is a rare option and is described in expertise centres with carefully optimistic results.18,19 Lastly, mechanical support, mainly as bridge to transplant, remains controversial in the Fontan population, yet interest on the topic is increasing due to the persisting scarceness of donor hearts and the ageing Fontan patient.2,17 Mechanical support tackling the lack of a subpulmonary ventricle might be of the greatest value in Fontan failure, e.g. in the form of a cavopulmonary assist device. Yet, unfortunately, these devices have not been successfully developed to date.2,20 Artificial heart/total cardiac replacement strategies might be the most effective strategy for combined systolic dysfunction and Fontan failure, yet are only reported in case reports to date.2,20,21 The congenital heart disease community is aware of the ever-growing need for strategies tackling advance Fontan failure with several international initiatives trying to address this challenge.20
In conclusion, there is a broad range of cardiac and extracardiac complications in Fontan palliation to be aware of, which are common and often associated with poor clinical outcomes. For optimal treatment the evaluation in a multidisciplinary team is mandatory, using the specific expertise of the team members to timely detect and address late complications and to support the quality of life.
Lead author biography

Marieke Nederend (1993) is currently a PhD candidate at the Leiden University Medical Center, the Netherlands. Her thesis is focusing an clinical outcomes in (the ageing) congenital heart disease, centralizing on Fontan circulation, and systemic right ventricular failure. During her time in the clinic, the work described in this case report was performed.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to thank Ronald Slagter for drawing the Graphical Abstract, as well as Figures 1 and 3.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Ethical statement
All procedures performed involving the human participant were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller G-P. et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur Heart J 2020;41:4153–4154. [DOI] [PubMed] [Google Scholar]
- 2. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH. et al. ; On behalf of the American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and management of the child and adult with fontan circulation: a scientific statement from the American Heart Association. Circulation 2019;140:e234–e284. [DOI] [PubMed] [Google Scholar]
- 3. van Melle JP, Wolff D, Horer J, Belli E, Meyns B, Padalino M. et al. Surgical options after Fontan failure. Heart 2016;102:1127–1133. [DOI] [PubMed] [Google Scholar]
- 4. Schuuring MJ, Vis JC, van Dijk AP, van Melle JP, Vliegen HW, Pieper PG. et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail 2013;15:690–698. [DOI] [PubMed] [Google Scholar]
- 5. Sumal AS, Kyriacou H, Mostafa A.. Tricuspid atresia: where are we now? J Card Surg 2020;35:1609–1617. [DOI] [PubMed] [Google Scholar]
- 6. Gewillig M, Goldberg DJ.. Failure of the fontan circulation. Heart Fail Clin 2014;10:105–116. [DOI] [PubMed] [Google Scholar]
- 7. Pundi KN, Pundi KN, Johnson JN, Dearani JA, Li Z, Driscoll DJ. et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis 2017;12:17–23. [DOI] [PubMed] [Google Scholar]
- 8. Rathod RH, Prakash A, Powell AJ, Geva T.. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol 2010;55:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F.. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol 2014;64:54–62. [DOI] [PubMed] [Google Scholar]
- 10. Magee AG, McCrindle BW, Mawson J, Benson LN, Williams WG, Freedom RM.. Systemic venous collateral development after the bidirectional cavopulmonary anastomosis. Prevalence and predictors. J Am Coll Cardiol 1998;32:502–508. [DOI] [PubMed] [Google Scholar]
- 11. Poterucha JT, Johnson JN, Taggart NW, Cabalka AK, Hagler DJ, Driscoll DJ. et al. Embolization of veno-venous collaterals after the Fontan operation is associated with decreased survival. Congenit Heart Dis 2015;10:E230–E236. [DOI] [PubMed] [Google Scholar]
- 12. Gordon-Walker TT, Bove K, Veldtman G.. Fontan-associated liver disease: a review. J Cardiol 2019;74:223–232. [DOI] [PubMed] [Google Scholar]
- 13. Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR. et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells ML, Hough DM, Fidler JL, Kamath PS, Poterucha JT, Venkatesh SK.. Benign nodules in post-Fontan livers can show imaging features considered diagnostic for hepatocellular carcinoma. Abdom Radiol 2017;42:2623–2631. [DOI] [PubMed] [Google Scholar]
- 15. Haeffele C, Aggarwal A, Lutchman G, Veldtman GR, Wu FM, Lui GK.. Fontan liver lesions: not always HCC. JACC Case Rep 2019;1:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM. et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol 2004;44:2065–2072. [DOI] [PubMed] [Google Scholar]
- 17. Roest S, Kaffka Genaamd Dengler SE, van Suylen V, van der Kaaij NP, Damman K, van Laake LW. et al. Waiting list mortality and the potential of donation after circulatory death heart transplantations in the Netherlands. Neth Heart J 2021;29:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Souza BA, Fuller S, Gleason LP, Hornsby N, Wald J, Krok K. et al. Single-center outcomes of combined heart and liver transplantation in the failing Fontan. Clin Transplant 2017;31:e12892. [DOI] [PubMed] [Google Scholar]
- 19. Reardon LC, DePasquale EC, Tarabay J, Cruz D, Laks H, Biniwale RM. et al. Heart and heart-liver transplantation in adults with failing Fontan physiology. Clin Transplant 2018;32:e13329. [DOI] [PubMed] [Google Scholar]
- 20. Mascio CE. Mechanical support of the failing Fontan circulation. Semin Thorac Cardiovasc Surg 2021;33:454–458. [DOI] [PubMed] [Google Scholar]
- 21. Ross HJ, Law Y, Book WM, Broberg CS, Burchill L, Cecchin F. et al. ; American Heart Association Adults With Congenital Heart Disease Committee of the Council on Clinical Cardiology and Council on Cardiovascular Disease in the Young, the Council on Cardiovascular Radiology and Intervention, and the Council on Functional Genomics and Translational Biology. Transplantation and mechanical circulatory support in congenital heart disease: a scientific statement from the American Heart Association. Circulation 2016;133:802–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.