Abstract
Myocardial injury in COVID-19 is associated with in-hospital mortality. However, the development of myocardial injury over time and whether myocardial injury in patients with COVID-19 at the intensive care unit is associated with outcome is unclear. This study prospectively investigates myocardial injury with serial measurements over the full course of intensive care unit admission in mechanically ventilated patients with COVID-19. As part of the prospective Maastricht Intensive Care COVID cohort, predefined myocardial injury markers, including high-sensitivity cardiac troponin T (hs-cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and electrocardiographic characteristics were serially collected in mechanically ventilated patients with COVID-19. Linear mixed-effects regression was used to compare survivors with nonsurvivors, adjusting for gender, age, APACHE-II score, daily creatinine concentration, hypertension, diabetes mellitus, and obesity. In 90 patients, 57 (63%) were survivors and 33 (37%) nonsurvivors, and a total of 628 serial electrocardiograms, 1,565 hs-cTnT, and 1,559 NT-proBNP concentrations were assessed. Log-hs-cTnT was lower in survivors compared with nonsurvivors at day 1 (β −0.93 [−1.37; −0.49], p <0.001) and did not change over time. Log-NT-proBNP did not differ at day 1 between both groups but decreased over time in the survivor group (β −0.08 [−0.11; −0.04] p <0.001) compared with nonsurvivors. Many electrocardiographic abnormalities were present in the whole population, without significant differences between both groups. In conclusion, baseline hs-cTnT and change in NT-proBNP were strongly associated with mortality. Two-thirds of patients with COVID-19 showed electrocardiographic abnormalities. Our serial assessment suggests that myocardial injury is common in mechanically ventilated patients with COVID-19 and is associated with outcome.
SARS-CoV-2 resulting in COVID-19 is often complicated by multiorgan failure, including myocardial injury1 and thrombosis.2 , 3 Myocardial injury, based on elevated cardiac troponin (cTn),4 is reported in 4% to 37% of patients with COVID-19 and may be the consequence of viral myocarditis, type I myocardial infarction caused by atherosclerotic plaque disruption, or type II myocardial infarction caused by an imbalance between oxygen demand and supply.5, 6, 7 N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a marker of hemodynamic myocardial stress and heart failure, but it can also be elevated in patients with severe inflammatory and respiratory disease.8 , 9 Moreover, abnormal electrocardiographic (ECG) findings including arrhythmias, ST-segment deviation, and prolonged PR and QTc intervals are associated with major adverse events, infection severity, and transfer to the intensive care unit (ICU).10 , 11 Myocardial injury in patients with COVID-19 is strongly associated with in-hospital mortality.12, 13, 14 Current evidence as used in COVID-19 guidelines is based on retrospective and cross-sectional studies on myocardial injury in patients with COVID-19 without serial measurements.15 Therefore, in this study, we investigated myocardial injury development by serial cardiac biomarkers and serial electrocardiograms from intubation onward over the disease course, comparing ICU survivors and nonsurvivors.
Methods
This study is part of the Maastricht Intensive Care COVID (MaastrICCht) cohort, which is a prospective observational study that included all patients on mechanical ventilation for COVID-19 admitted to our ICU (Trial Register number NL8613). The study protocol has been described before in more detail16 and has been approved by the institutional review board of the Maastricht University Medical Centre+ (MUMC+) (METc, 2020-1565/ 300523). The manuscript was written following the “STrengthening the Reporting of OBservational studies in Epidemiology” (STROBE) guideline.17 Maastricht UMC+ is a tertiary care university teaching hospital in the Netherlands. Usually, the Maastricht UMC+ ICU had a capacity of 27 ICU beds. However, during the COVID-19 pandemic, our ICU was rapidly upgraded to a maximum of 64 beds.
All patients with respiratory insufficiency requiring mechanical ventilation and at least 1 polymerase chain reaction test positive for SARS-CoV-2 and/or a chest computed tomography scan strongly suggestive for SARS-CoV-2 infection, based on a CORADS (COVID-19 Reporting and Data System) score of 4 to 5 scored by a radiologist,18 were included. After training by qualified research staff and with daily supervision by a senior investigator, medical research interns and PhD candidates included participants and collected clinical, physiological, and laboratory variables using a predefined study protocol as previously described.16 Participants were included from March 25, 2020, to June 23, 2020.
Participants were followed up until death in the ICU or ICU discharge and categorized based on the primary outcome. Patients who were transferred to other hospitals were followed up and reclassified to the nonsurvivor group if they had died in hospital. Population characteristics and potential confounding variables have been described extensively elsewhere.1 , 2 , 16
Cardiomyocyte injury was assessed daily in serum samples using high-sensitivity cardiac troponin T (hs-cTnT) (ng/L), creatinine kinase (CK) (U/L), and creatinine kinase-MB (CK-MB) (µg/L) using the Cobas 8000 analyzer (Roche Diagnostics, Mannheim, Germany). In addition, as hemodynamic myocardial stress and heart failure biomarker, we assessed NT-proBNP (pmol/L) in serum, as measured on the Cobas 8000 analyzer. Furthermore, creatinine (µmol/L) was measured on the Cobas 8000 analyzer. Assay characteristics were all according to the package inserts. The cardiac biomarkers were scored as follows:
-
1.
Hs-cTnT: concentration at inclusion (day of intubation), lowest, highest, and delta (between lowest and highest) concentration. Normal concentration: <14 ng/L
-
2.
CK: concentration at inclusion, highest, lowest, and delta concentration. Normal concentration: 0 to 225 U/L
-
3.
CK-MB: concentration at inclusion, highest, lowest, and delta concentration. Normal concentration: <4.9 µg/L
-
4.
NT-proBNP: concentration at inclusion, highest, lowest, and delta concentration. Normal concentration: <35 pmol/L
Within the MaastrICCht cohort, ECGs were performed at least every other day. All ECGs were recorded at 25 mm/s speed and 10-mm/mV amplitude. ECG assessment, with an appropriate scoring system, was predesigned (Supplementary Material 1) by a team of 4 physicians, including a cardiologist specialized in electrophysiology (KV), a cardiologist-intensivist (RD), and 2 cardiologists in training (MG and CG). The first 10 ECGs were assessed and discussed by all 4 physicians to score the ECG as objectively as possible. After the scoring was objective, 1 investigator assessed all ECGs using a preset protocol and solved uncertainties within the team. The ECG characteristics were scored systematically based on (1) rhythm, (2) conduction, (3) right ventricular (RV) strain, (4) repolarization abnormalities, (5) T-wave abnormalities, and (6) other. Further specification of these items can be found in the Supplementary Material.
All participants eligible for the study were included in the cohort until June 23, 2020. Data were analyzed with R version 3.6.1 (R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). First, the cohort was categorized into ICU survivors and ICU nonsurvivors. Then, the sample characteristics were described using mean and SD, median and interquartile range (IQR), or percentage, as appropriate. Finally, differences were tested using the independent-samples t test, chi-square test, Fisher's exact test, or Mann-Whitney U test, as appropriate. We used linear mixed-effects regression models with a random intercept and random slope with time to compute differences in cardiac biomarkers over time between both groups. We used an unstructured variance-covariance matrix for random effects and an autoregressive correlation structure of the first order for longitudinal measures. Using the Akaike Information Criterion, the best fitting model for change over time was selected. Biomarkers were log-transformed due to skewed residuals. We computed the crude group differences (model 1). Subsequently, we investigated potential confounding by clinical characteristics, disease severity, daily renal function, and COVID-19–related risk factors for cardiovascular disease (CVD) to challenge our hypothesis. Therefore, the model was first adjusted for gender and age (model 2), and additionally for APACHE-II score at baseline (model 3), daily creatinine concentrations (model 4), and hypertension, diabetes mellitus, and obesity (model 5). Results of the regression models were expressed as regression coefficient β, including 95% confidence interval (CI). A p value <0.05 was considered statistically significant.
Results
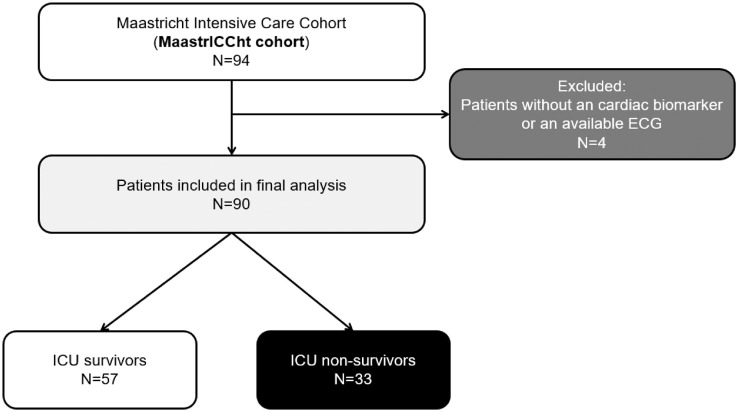
The MaastrICCht cohort included a total of 94 participants at the time of data extraction. Four patients were excluded, as there were no ECGs or biomarkers available because these patients were transported or died within 48 hours after ICU admission. Of all 90 included patients, 33 patients (37%) had died (ICU nonsurvivor group), and 57 (63%) were discharged alive from the ICU (ICU survivor group) (Figure 1 ). The nonsurvivor group was on average older, had more often a history of myocardial infarction, and had a higher APACHE II score (Table 1 ).
Figure 1.
Flowchart of the study.
Table 1.
Characteristics of the study population in ICU survivors and ICU nonsurvivors
| ICU survivors (n = 57) | ICU nonsurvivors (n = 33) | p value for difference | |
|---|---|---|---|
| Age, year | 62 (±12) | 69 (±10) | 0.006 |
| Gender, men | 42 (74%) | 28 (85%) | 0.220 |
| Time of ICU stay, days* | 22 (17) | 13 (9) | 0.001 |
| Body mass index, kg/m2 | 27.9 (±4.2) | 27.2 (±4.0) | 0.430 |
| Admission location: | |||
| Emergency room | 12 (21%) | 8 (24%) | 0.600‡ |
| Ward | 28 (49%) | 15 (45%) | 0.740 |
| Transfer from other hospital | 17 (30%) | 10 (30%) | 0.960 |
| Previous myocardial infarction | 1 (2%) | 5 (15%) | 0.020‡ |
| Cardiomyopathy/LV dysfunction | 2 (4%) | 0 (0%) | 0.530‡ |
| Coronary artery disease | 3 (5%) | 5 (15%) | 0.140‡ |
| Chronic renal disease | 1 (2%) | 1 (3%) | 1.00‡ |
| Presence of any cardiovascular risk factor§ | 25 (44%) | 21 (64%) | 0.070 |
| Apache II score, points | 15 (5.5) | 18 (5.8) | 0.040 |
| Mean arterial pressure, mm Hg (lowest in first 24 h) | 61.8 (23.9) | 63.2 (13.0) | 0.760 |
| Hydroxychloroquine administered | 38 (67%) | 23 (69%) | 0.880 |
Data are presented as mean (SD) or count (percentage), unless indicated otherwise. Differences were tested using the independent-samples t test or Pearson's chi-square test unless indicated otherwise.
ICU = intensive care unit.
Median and first and third quartiles.
Fisher's exact test.
Diabetes mellitus, hypertension, dyslipidemia, smoking, obesity.
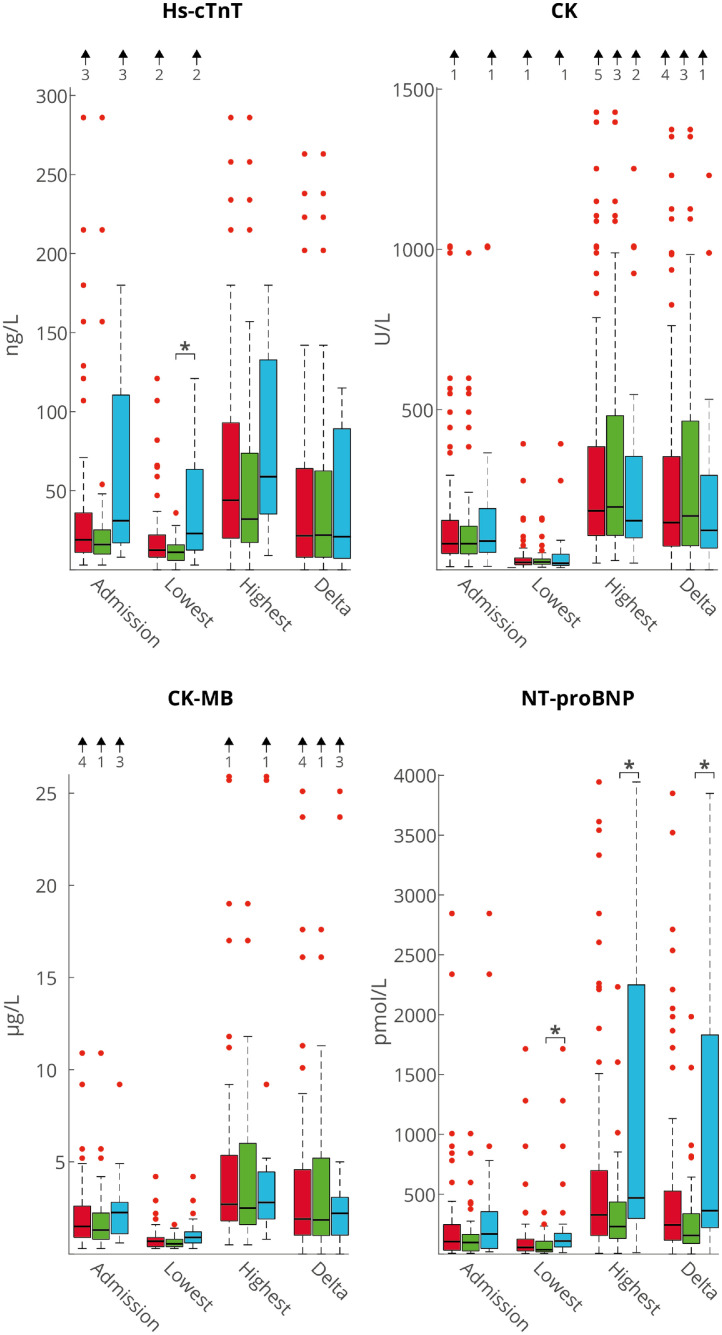
In total, we studied 628 serial ECGs and 1,565 hs-cTnT, 1615 CK, 1,535 CK-MB, and 1,559 NT-proBNP concentrations. Figure 2 shows the median with interquartile range (IQR) cardiac biomarker concentrations at admission and lowest, highest, and delta (change between lowest and highest) during ICU stay for ICU survivors and nonsurvivors. Hs-cTnT at admission was 23 (15 to 144) ng/L for nonsurvivors and 14 (9 to 22) ng/L for survivors (p = 0.177). The lowest hs-cTnT was significantly higher in nonsurvivors versus survivors (23 [12 to 65] ng/L vs 11 [6-16] ng/L, p = 0.001). For CK and CK-MB, the concentrations did not differ between the 2 groups. Log-CK and log-CK-MB concentrations are depicted in Supplementary Table 1 panel A and B. For NT-proBNP, the highest, lowest, and delta concentrations were all significantly higher in nonsurvivors compared with survivors.
Figure 2.
Cardiac biomarkers (hs-cTnT, CK, CK-MB and NT-proBNP) on day 1. Concentrations are median (IQR). Red bars depict all patients, green bars survivors, and blue bars nonsurvivors. Arrows above the figures indicate number of outliers exceeding the range of this figure.*p <0.05
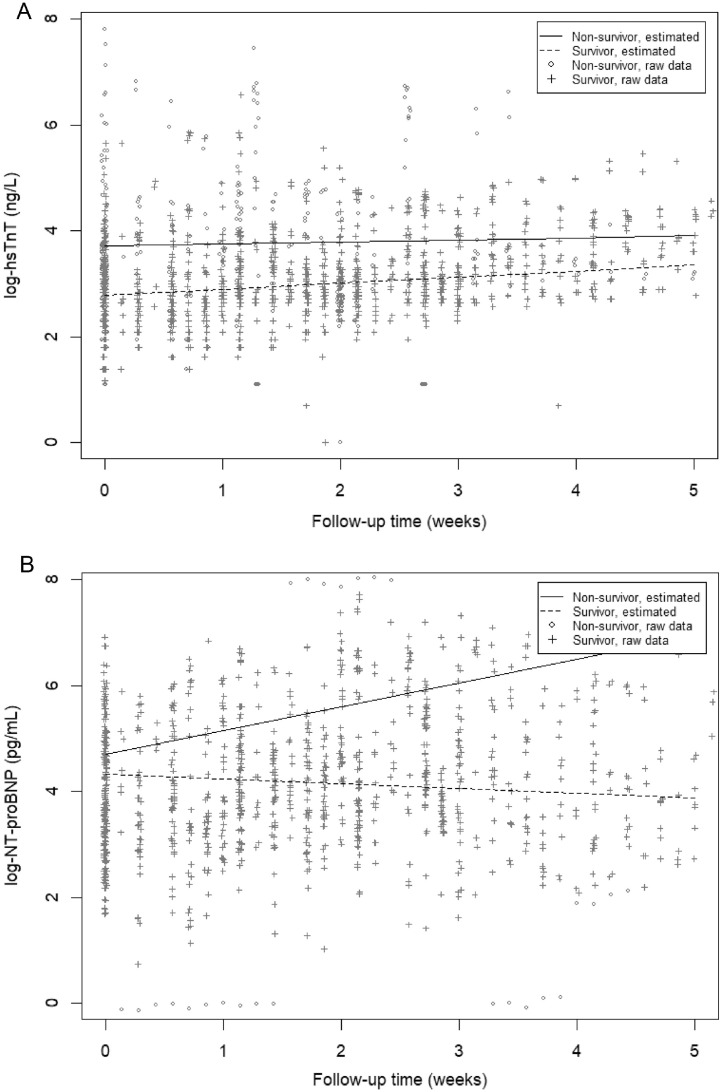
Table 2 depicts the results of the linear mixed-effect models showing the difference in biomarker concentrations at the start of mechanical ventilation and the difference in development over time between the 2 groups. Log-hs-cTnT was lower for survivors compared with nonsurvivors at day 1 (β −0.93, 95% CI −1.37 to−0.49, p <0.001), and this association remained statistically significant after adjustment for gender and age (β −0.74, 95% CI −1.17 to −0.30, p = 0.001) (model 2), APACHE II score (β −0.66, 95% CI −1.08 to −0.24, p = 0.003) (model 3), daily creatinine concentrations (β −0.47, 95% CI −0.95 to 0.00, p = 0.049) (model 4) and cardiovascular risk factors (β −0.55, 95% CI −1.04 to −0.07, p = 0.028) (model 5). The change over time in log-hs-cTnT did not differ statistically significantly between the groups (Figure 3 , Table 2). Log-NT-proBNP at day 1 did not differ statistically significantly between the 2 groups, but over time, it decreased significantly more in the survivor group (β −0.08, 95% CI −0.11 to −0.04, p <0.001). This association remained statistically significant after adjustment for gender and age (β −0.08, 95% CI −0.11 to −0.05, p <0.001), APACHE II score (β −0.08, 95% CI −0.11 to −0.05, p <0.001), daily creatinine concentrations (β −0.08, 95% CI −0.12 to −0.05, p <0.001), and COVID-19–related cardiovascular risk factors (β −0.08, 95% CI −0.12 to −0.05, p <0.001) (Figure 3, Table 2).
Table 2.
Associations between serial biomarkers over time and survival in mechanically ventilated patients with COVID-19
| Log(hs-cTnT) |
Log(CK) |
Log(CK-MB) |
Log(NT-proBNP) |
|||||
|---|---|---|---|---|---|---|---|---|
| Model | Regression Coefficient(95% CI) | p Value | Regression Coefficient (95% CI) | p Value | Regression Coefficient (95% CI) | p Value | Regression Coefficient (95% CI) | p Value |
| Model 1 | ||||||||
| ICU-nonsurvivor (reference) | ref | ref | ref | ref | ref | ref | ref | ref |
| ICU-survivor* | −0.93 (−1.37; −0.49) | <0.001 | −0.20 (−0.57; 0.17) | 0.289 | −0.41 (−0.70; −0.13) | 0.005 | −0.39 (−0.96; 0.19) | 0.182 |
| Interaction between group and time† | 0.01 (−0.01; 0.03) | 0.348 | 0.03 (0.01; 0.06) | 0.059 | 0.02 (0.00; 0.03) | 0.049 | −0.08 (−0.11; −0.04) | <0.001 |
| Model 2 | ||||||||
| ICU-nonsurvivor (reference) | ref | ref | ref | ref | ref | ref | ref | ref |
| ICU-survivor* | −0.74 (−1.17; −0.30) | 0.001 | −0.18 (−0.55; 0.20) | 0.360 | −0.29 (−0.57; 0.00) | 0.048 | −0.32 (−0.90; 0.26) | 0.279 |
| Interaction between group and time† | 0.01 (−0.01; 0.03) | 0.449 | 0.04 (0.01; 0.06) | 0.003 | 0.02 (0.00; 0.03) | 0.059 | −0.08 (−0.11; −0.05) | <0.001 |
| Model 3 | ||||||||
| ICU-nonsurvivor (reference) | ref | ref | ref | ref | ref | ref | ref | ref |
| ICU-survivor* | −0.66 (−1.08; −0.24) | 0.003 | −0.14 (−0.52; 0.24) | 0.460 | −0.25 (−0.52; 0.03) | 0.084 | −0.28 (−0.85; 0.29) | 0.331 |
| Interaction between group and time† | 0.01 (−0.02; 0.03) | 0.560 | 0.03 (0.01; 0.06) | 0.005 | 0.01 (0.00; 0.03) | 0.088 | −0.08 (−0.11; −0.05) | <0.001 |
| Model 4 | ||||||||
| ICU-nonsurvivor (reference) | ref | ref | ref | ref | ref | ref | ref | ref |
| ICU-survivor* | −0.47 (−0.95; 0.00) | 0.049 | −0.03 (−0.41; 0.35) | 0.871 | −0.13 (−0.40; 0.14) | 0.342 | −0.10 (−0.62; 0.43) | 0.717 |
| Interaction between group and time† | 0.01 (−0.02; 0.03) | 0.588 | 0.01 (0.03; 0.05) | 0.014 | 0.02 (0.00; 0.03) | 0.179 | −0.08 (−0.12; −0.05) | <0.001 |
| Model 5 | ||||||||
| ICU-nonsurvivor (reference) | ref | ref | ref | ref | ref | ref | ref | ref |
| ICU-survivor* | −0.55 (−1.04; −0.07) | 0.028 | −0.09 (−0.47; 0.29) | 0.634 | −0.15 (−0.42; 0.12) | 0.284 | −0.13 (−0.67; 0.40) | 0.631 |
| Interaction between group and time† | 0.01 (−0.02; 0.03) | 0.511 | 0.03 (0.01; 0.06) | 0.008 | 0.02 (0.00; 0.03) | 0.161 | −0.08 (−0.12; −0.05) | <0.001 |
Biomarker data were log-transformed for reasons of normality and skewed residuals.
Results of the linear mixed-effects models show the difference in log-biomarker development over time between ICU survivors and ICU nonsurvivors, with ICU nonsurvivors as the reference category. Data are regression coefficients (β) with 95% confidence intervals (CIs). The regression coefficient β indicates the mean difference in log biomarker over time. The regression β for the between-group and time interaction indicates the changes in log biomarker over time between ICU survivors and ICU nonsurvivors (with ICU nonsurvivors as reference: ref). Model 1 is the crude model; model 2 adjusts for gender and age; model 3 additionally adjusts for APACHE-II score at baseline; model 4 also adjusts for daily creatinine concentration; model 5 also adjusts for smoking status, the presence of hypertension, diabetes, and obesity.
APACHE = Acute Physiology and Chronic Health Evaluation; CI = confidence interval; ICU = intensive care unit.
A negative regression coefficient indicates that the average log-biomarker concentration of survivors at day 1 is lower compared with the nonsurvivors.
A negative regression coefficient for the interaction term indicates that the log-biomarker concentration of survivors decreases more over time compared with the nonsurvivors. (i.e., the interaction between group and time models the change over time for both groups separately).
Figure 3.
Observed and predicted log-biomarker values over time for ICU-survivors and ICU non-survivors. Biomarkers were log-transferred due to non-normal distribution.
In general, ECG abnormalities occurred in a large number of patients with COVID-19 with the highest presence at baseline of flat T waves (n = 83, 92%), QRS fragmentations (n = 71, 79%), p-wave split (n = 39, 43%), RV strain (n = 38, 42%), and occurrence of repolarization abnormalities (n = 20, 22%). When assessing not only baseline but all ECGs, abnormalities were largely found in QRS fragmentations (n = 86, 96%), followed by p-wave split (n = 69, 77%), flat T waves (n = 67, 74%), RV strain (n = 65, 72%), and occurrence of repolarization abnormalities (n = 57, 61%). These data are summarized in in Table 3 and Supplementary Table 1. At baseline, 80 of 90 patients (89%) were in sinus rhythm and 7 patients had atrial fibrillation. Rhythm at baseline ECG did not differ between survivors and nonsurvivors (Supplementary Table 1). However, over time, 9 patients developed atrial fibrillation, 5 in survivors and 4 in nonsurvivors (p = 0.72) (Table 3). At baseline (Supplementary Table 1), left bundle branch block was present in 5 patients (6%), 2 (4%) in the survivor group and 3 (9%) in the nonsurvivor group (p = 0.35) and did not change over time (Table 3). QRS duration did not differ between survivors and nonsurvivors. The PR time at baseline did not differ statistically significantly between groups (150 ± 20 ms vs 153 ± 25 ms). When assessing baseline and all subsequent ECGs, the prevalence of first-degree atrioventricular block was comparable in both groups. At baseline, the prevalence of right bundle branch block did not differ between both groups and was seen in 2 survivors (4%) compared with 2 nonsurvivors (6%) (p = 0.62). Over time, this number did not change in both groups. Right axis deviation was also comparable between groups and present in 2 survivors (4)% and 2 nonsurvivors (6%) (p = 0.62). RV strain was found in 21 survivors (37%) and 17 nonsurvivors (52%) and was comparable between both groups (p = 0.17). Of the individual criteria, a broad S wave in I, aVL, V5, or V6 was statistically significantly more present in nonsurvivors (23 patients, 70%) compared with survivors (27 patients, 47%) (p = 0.04). When comparing all ECGs, RV strain did not differ between both groups (Table 3). At baseline, ST-segment elevation in at least 2 contiguous leads did not differ between groups and was present in 3 survivors (5%) and 1 nonsurvivor (3%) (p = 1.0). Over time, the presence of ST-segment elevation increased but remained comparable between both groups. ST-segment depression was present in 10 survivors (18%) and 7 nonsurvivors (21%) (p = 0.67).
Table 3.
ECG characteristics in serial ECGs (n = 628 ECGs) for the whole population and stratified for ICU survivors and ICU nonsurvivors
| All (n = 90) | ICU survivors (n = 57) | ICU nonsurvivors (n = 33) | p value for difference | |
|---|---|---|---|---|
|
||||
| Sinus rhythm | 83 (92%) | 53 (90%) | 30 (91%) | 0.70* |
| Supraventricular tachycardia | 12 (13%) | 7 (12%) | 5 (15%) | 0.70 |
| Atrial fibrillation | 9 (10%) | 5 (9%) | 4 (12%) | 0.72* |
| Atrial flutter | 1 (1%) | 1 (2%) | 0 (0%) | 1.0* |
| Atrial tachycardia | 3 (3%) | 2 (4%) | 1 (3%) | 1.0* |
| Escape rhythm | 4 (4%) | 2 (4%) | 2 (6%) | 0.62* |
| Paced rhythm | 1 (1%) | 1 (2%) | 0(0%) | 1.0* |
|
||||
| LBBB | 5 (6%) | 2 (4%) | 3 (9%) | 0.27 |
| QRS duration (ms) | 98 ± 16 | 98 ± 15 | 99 ± 17 | 0.49 |
| QRS>120 ms | 10 (11%) | 5 (9%) | 5 (15%) | 0.35 |
| 1st degree AV block | 14 (16%) | 7 (12%) | 7 (21%) | 0.26 |
| PR time (ms) | 150 ± 25 | 149 ± 24 | 153 ± 26 | 0.05 |
|
||||
| RBBB | 4 (4%) | 2 (4%) | 2 (6%) | 0.62* |
| Right or extreme axis | 6 (7%) | 4 (7%) | 2 (6%) | 1.0* |
| At least 2 of the following | 65 (72%) | 40 (70%) | 25 (76%) | 0.57 |
| RsR’ pattern | 33 (37%) | 19 (33%) | 14 (42%) | 0.39 |
| qR pattern | 12 (13%) | 4 (7%) | 8 (24%) | 0.027* |
| Broad S in I, aVL, V5or V6 | 67 (74%) | 39 (68%) | 28 (85%) | 0.09 |
| Slurred S in I, aVL, V5or V6 | 66 (73%) | 44 (77%) | 22 (67%) | 0.28 |
| P-pulmonale | 12 (13%) | 8 (11%) | 4 (12%) | 1.0* |
|
||||
|
57 (61%) | 36 (61%) | 21 (60%) | 0.92 |
| ST-segment elevation in at least 2 consecutive leads | 22 (24%) | 14 (25%) | 8 (24%) | 0.97 |
| ST-segment depression in at least 2 consecutive leads | 44 (49%) | 28 (49%) | 16 (49%) | 0.95 |
|
||||
| Any T-wave abnormality | 73 (81%) | 47 (83%) | 26 (79%) | 0.67 |
| T-waves inversion in at least consecutive 2 leads | 20 (22%) | 13 (23%) | 7 (21%) | 0.86 |
| Flat T-waves in at least 2 consecutive leads | 67 (74%) | 44 (77%) | 23 (70%) | 0.43 |
| Biphasic T-waves in at least 2 consecutive leads | 15 (17%) | 9 (14%) | 6 (18%) | 0.60 |
|
||||
| QTc time (ms) | 436 (55) | 430 (51) | 450 (61) | <0.001 |
| Prolonged QTc time (ms)>500 ms | 34 (38%) | 18 (32%) | 16 (49%) | 0.11 |
| Heartrate (bpm) | 86 (21) | 86 (21) | 85 (21) | 0.46 |
| Height R-wave V1 (mV) | 0.15 (0.14) | 0.15 (0.13) | 0.16 (0.17) | 0.162 |
| V2 (mV) | 0.50 (0.36) | 0.48 (0.32) | 0.55 (0.44) | 0.04 |
| Depth S-wave in I (mV) | 0.23 (0.14) | 0.22 (0.12) | 0.27 (0.14) | 0.10 |
| P-wave split | 69 (77%) | 43 (75%) | 26 (79%) | 0.71 |
| QRS fragmentations | 86 (96%) | 55 (96%) | 31 (94%) | 0.62* |
| Microvoltages | 15 (17%) | 11 (19%) | 4 (12%) | 0.56* |
| Left heart axis | 25 (28%) | 12 (21%) | 13 (39%) | 0.06 |
Data are presented as mean (standard deviation) or count (percentage), unless indicated otherwise. Differences were tested using the independent-samples t test or Pearson's chi-square test unless indicated otherwise.
ICU = intensive care unit.
Fisher's exact test.
T-wave abnormalities neither differed between both groups at baseline nor when comparing serial ECGs, with a markedly higher presence for flat T-waves in both groups (52 (91%) and 31 (94%) for survivors and nonsurvivors, respectively) (Supplementary Table 1). When comparing serial ECGs, no difference in ST-segment abnormalities and T-wave abnormalities were seen between both groups (Table 3).
At baseline, QTc was 429 ± 52 ms in the survivor group and 448 ± 58 ms in the nonsurvivor group (p = 0.118). Over time, QTc was statistically significantly of longer duration in nonsurvivors when compared with survivors (450 ± 61 ms vs 430 ± 51 ms, p <0.001) (Table 3). We did not observe a statistically significant difference in the occurrence of a prolonged QTc (>500 ms) between patients who received hydroxychloroquine and those who did not. The average heart rate was 92 beats/min on average at baseline and comparable between both groups. R-wave height in lead V2 over time was statistically significantly higher in nonsurvivors compared with survivors. At baseline, p-wave splitting, QRS-fragmentations, and microvoltages occurred in 43%, 79%, and 4%, and over time in 77%, 96%, and 17%, respectively. These characteristics did not differ between both groups (Table 3).
Discussion
We assessed serial cardiac biomarkers and ECG characteristics in mechanically ventilated patients of the MaastriCCht cohort and compared them in survivors to nonsurvivors. Our main findings are that (1) higher hs-cTnT at day 1 was associated with mortality (p <0.001), and this association remained significant after adjustment for gender, age, APACHE II score, daily creatinine concentrations, and COVID-19–related cardiovascular risk factors; (2) the lowest, highest, and delta NT-proBNP concentrations were higher in nonsurvivors versus survivors, with serial NT-proBNP concentrations that decreased more in survivors compared with nonsurvivors in adjusted models (p values <0.001); and (3) the presence of ECG abnormalities in all patients, irrespective of survival was high but did not essentially differ between survivors and nonsurvivors. Our findings underscore the importance of serial assessment of the cardiac biomarkers, especially NT-proBNP, as opposed to single measurements. In addition, hs-cTnT was associated with mortality on admission in the ICU.
Previous studies of myocardial injury in COVID-19 were retrospective by design,14 did not include mechanically ventilated patients,13 and solely investigated either biomarker19 or ECG parameters.20 These studies consistently showed that patients with signs of myocardial injury had an up to 10 times higher mortality rate compared with patients without myocardial injury.14 , 21 , 22 Several studies showed that 6% to 10% of patients with COVID-19 had elevated cTn concentrations and 13% to 15% had elevated NT-proBNP, and both were associated with increased mortality. Furthermore, although myocardial injury is associated with fatal outcomes, the prognosis of patients with COVID-19 with underlying CVD without myocardial injury was shown to be relatively favorable compared with those with underlying CVD with myocardial injury.12 As the risk factors of both CVD and COVID-19 disease severity are similar, adjustment for confounders such as hypertension, diabetes mellitus, and obesity is important. Renal function is modified over the course of critical illness and affects hs-cTnT, NT-proBNP concentrations, and survival, thus acting as a potential confounder.
Although the mechanism of cardiac injury in COVID-19 is not fully elucidated, the downregulation of angiotensin-converting enzyme 2 by SARS-CoV-2 may lead to increased microvascular damage, myocardial hypertrophy, atrial dilatation, and diastolic dysfunction during COVID-19 infection.23 , 24 In addition, SARS-CoV-2 elicits a host response that triggers wide-ranging immune-inflammatory thrombotic and parenchymal derangements,2 , 25 also leading to myocardial damage reflecting myocarditis, thromboembolism,2 endothelitis, cardiac arrhythmias, or myocardial ischemia (either type I or II).
Previous studies investigating ECG alterations in patients with COVID-19 primarily included hospitalized patients who were not admitted to the ICU.10 , 20 Translating these findings to patients admitted to the ICU with mechanical ventilation and hemodynamic support may be difficult because these factors may affect ECG alterations. Furthermore, previous studies did not perform serial ECGs every other day as was done in this study. We found a high incidence of ECG abnormalities in patients with COVID-19, which is in line with previous observations.10 , 20 Most ECG characteristics did not differ between survivors and nonsurvivors. This can be explained by the fact that our study population consisted of mechanically ventilated ICU patients and thus more severely affected patients with COVID-19. In contrast, other studies investigated ECGs at the emergency department or non-ICU departments, including less severe cases.26 Hydroxychloroquine use was not associated with the occurrence of a prolonged QTc (>500 ms) in our study (p = 0.362). Strikingly, we found fragmentation of the QRS in 79% of patients, whereas previously, fragmented QRS was identified in 36.8% of patients and was a predictor of cardiac mortality in patients with COVID-19.27
This study has several strengths. First, this is a comprehensive prospective cohort study. We determined biomarkers daily in all patients, thereby including many serial measurements over time. Furthermore, we performed an ECG every other day in all patients, thereby investigating ECG features and changes over time during ICU admission owing to COVID-19. Second, all ECGs (n = 632) were interpreted manually, which has several advantages, including improved accuracy over automated interpretation.28 Except for the first 10 ECGs, 1 physician manually evaluated the ECGs. However, uncertainty was solved within the team of 4 physicians. Third, we addressed potential confounding extensively by adjusting the models for gender, age, APACHE II score, daily creatinine concentrations, and COVID-19–related cardiovascular risk factors. A limitation of this study is that it is a single-center study, solely including ICU patients with COVID-19 on mechanical ventilation. This limits generalizability to other mechanically ventilated patients with COVID-19 only. Furthermore, we studied variables over time, and during admission, many interventions might influence these variables. For example, except for hydroxychloroquine, we did not report/adjust for daily medication, which can influence some ECG characteristics such as QTc time.
In conclusion, to our knowledge, this prospective study is the first to serially investigate cardiac biomarker and ECG characteristics over time in mechanically ventilated ICU patients with COVID-19. We show that higher hs-cTnT at admission and increasing NT-proBNP over time are associated with mortality, irrespective of gender, age, APACHE II score, daily creatinine concentrations, and COVID-19–related cardiovascular risk factors. Significant ECG abnormalities are prevalent in more than 2/3 of mechanically ventilated patients with COVID-19. These findings shed light on the cardiac pathophysiology over time in critically ill patients with COVID-19. This suggests that serial measurement can provide direction to physicians for clinical decision making and prognostication in future studies of patients with COVID-19.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Mohammed A. Ghossein and Rob G.H. Driessen contributed equally to this manuscript.
Netherlands Trial Register number: NL8613.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.01.030.
Appendix. Supplementary materials
References
- 1.Bels JLM, van Kuijk SMJ, Ghossein-Doha C, Tijssen FH, van Gassel RJJ, Tas J, Collaborators M, Schnabel RM, Aries MJH, van de Poll MCG, Bergmans D, Meex SJR, van Mook W, van der Horst ICC, van Bussel BCT. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J Crit Care. 2021;62:38–45. doi: 10.1016/j.jcrc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulshof AM, Bruggemann RAG, Mulder MMG, van de Berg TW, Sels JEM, Olie RH, Spaetgens B, Streng AS, Verhezen P, van der Horst ICC, Ten Cate H, Spronk HMH, van Bussel BCT, Henskens YMC, Serial E. Serial EXTEM, FIBTEM, and tPA rotational thromboelastometry observations in the Maastricht intensive care COVID cohort-persistence of hypercoagulability and hypofibrinolysis despite anticoagulation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.654174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brüggemann RAG, Spaetgens B, Gietema HA, Brouns SHA, Stassen PM, Magdelijns FJ, Rennenberg RJ, Henry RMA, Mulder MMG, van Bussel BCT, Schnabel RM, van der Horst ICC, Wildberger JE, Stehouwer CDA. Ten Cate H. The prevalence of pulmonary embolism in patients with COVID-19 and respiratory decline: a three-setting comparison. Thromb Res. 2020;196:486–490. doi: 10.1016/j.thromres.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology(ACC)/American Heart Association(AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 5.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller C, Laule-Kilian K, Frana B, Rodriguez D, Scholer A, Schindler C, Perruchoud AP. Use of B-type natriuretic peptide in the management of acute dyspnea in patients with pulmonary disease. Am Heart J. 2006;151:471–477. doi: 10.1016/j.ahj.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes-Genis A, Mueller T, Richards M, Januzzi JL., Jr Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 10.Bergamaschi L, D'Angelo EC, Paolisso P, Toniolo S, Fabrizio M, Angeli F, Donati F, Magnani I, Rinaldi A, Bartoli L, Chiti C, Biffi M, Pizzi C, Viale P, Galié N. The value of ECG changes in risk stratification of COVID-19 patients. Ann Noninvasive Electrocardiol. 2021;26:e12815. doi: 10.1111/anec.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moey MYY, Sengodan PM, Shah N, McCallen JD, Eboh O, Nekkanti R, Carabello BA, Naniwadekar AR. Electrocardiographic changes and arrhythmias in hospitalized patients with COVID-19. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, De Marco A, Briani M, Bocciolone M, Bragato R, Corrada E, Gasparini GL, Marconi M, Monti L, Pagnotta PA, Panico C, Pini D, Regazzoli D, My I, Kallikourdis M, Ciccarelli M, Badalamenti S, Aghemo A, Reimers B, Condorelli G. Humanitas COVID-19 Task Force. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 14.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habets MAW, Sturkenboom HN, Tio RA, Belfroid E, Hoogervorst-Schilp J, Siebelink HJ, Jansen CW, Smits PC. How often and to what extent do admitted COVID-19 patients have signs of cardiac injury? Neth Heart J. 2021;29:5–12. doi: 10.1007/s12471-021-01571-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tas J, van Gassel RJJ, Heines SJH, Mulder MMG, Heijnen NFL, Acampo-de Jong MJ, Bels JLM, Bennis FC, Koelmann M, Groven RVM, Donkers MA, van Rosmalen F, Hermans BJM, Meex SJ, Mingels A, Bekers O, Savelkoul P, Oude Lashof AML, Wildberger J, Tijssen FH, Buhre W, Sels JEM, Ghossein-Doha C, Driessen RGH, Kubben PL, Janssen MLF, Nicolaes GAF, Strauch U, Geyik Z, Delnoij TSR, Walraven KHM, Stehouwer CD, Verbunt JAMCF, Van Mook WNKA, van Santen S, Schnabel RM, Aries MJH, van de Poll MCG, Bergmans D, van der Horst ICC, van Kuijk S, van Bussel BCT. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort (MaastrICCht) BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Kang H, Liu X, Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. 2020;92:538–539. doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angeli F, Spanevello A, De Ponti R, Visca D, Marazzato J, Palmiotto G, Feci D, Reboldi G, Fabbri LM, Verdecchia P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020;78:101–106. doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-Dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 24.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, Monneret G, Venet F, Bauer M, Brunkhorst FM, Weis S, Garcia-Salido A, Kox M, Cavaillon JM, Uhle F, Weigand MA, Flohé SB, Wiersinga WJ, Almansa R, de la Fuente A, Martin-Loeches I, Meisel C, Spinetti T, Schefold JC, Cilloniz C, Torres A, Giamarellos-Bourboulis EJ, Ferrer R, Girardis M, Cossarizza A, Netea MG, van der Poll T, Bermejo-Martín JF, Rubio I. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murat S, Babayigit E, Gorenek B. Comments on the value of ECG changes in risk stratification of COVID-19 patients. Ann Noninvasive Electrocardiol. 2021;26:e12841. doi: 10.1111/anec.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildirim A, Karaca IO, Yilmaz FK, Gunes HM, Cakal B. Fragmented QRS on surface electrocardiography as a predictor of cardiac mortality in patients with SARS-CoV-2 infection. J Electrocardiol. 2021;66:108–112. doi: 10.1016/j.jelectrocard.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willems JL, Abreu-Lima C, Arnaud P, van Bemmel JH, Brohet C, Degani R, Denis B, Gehring J, Graham I, van Herpen G, Machado H, Macfarlane PW, Michaelis J, Moulopoulos SD, Rubel P, Zywietz C. The diagnostic performance of computer programs for the interpretation of electrocardiograms. N Engl J Med. 1991;325:1767–1773. doi: 10.1056/NEJM199112193252503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.