Abstract
Introduction:
It is unknown whether certain dentists account for disproportionate shares of dental opioid prescriptions and high-risk prescriptions. Identifying and characterizing such dentists could inform the targeting of initiatives to improve the appropriateness and safety of dental opioid prescribing.
Methods:
In May 2021, the authors conducted a cross-sectional analysis using the IQVIA Longitudinal Prescription Database, which reports dispensing from 92% of U.S. pharmacies, and 2 provider databases (IQVIA OneKey, National Plan and Provider Enumeration System). Analyses included opioid prescriptions from dentists dispensed in 2019 to patients aged >12 years. “High-risk” prescriptions were those considered high risk by any of 3 metrics (prescriptions to opioid-naïve patients exceeding a 3-day supply, prescriptions with daily opioid dosage ≥50 morphine milligram equivalents, opioid prescriptions with benzodiazepine overlap). Among all prescriptions and high-risk prescriptions, the authors calculated the proportion accounted for by “high-volume dentists” with prescription counts in the 95th percentile or higher. Using logistic regression, characteristics associated with being a high-volume dentist were identified.
Results:
In 2019, a total of 141,345 dentists accounted for 10,736,743 opioid prescriptions dispensed to patients aged >12 years; 4,242,634 (39.5%) were high-risk prescriptions. The 7,079 high-volume dentists, a group representing 5.0% of the 141,345 dentists, accounted for 46.9% of all prescriptions and 47.5% of high-risk prescriptions. Male sex, younger age, non-Northeast location, and specialization in oral and maxillofacial surgery were associated with higher risk of being a high-volume dentist.
Conclusions:
In 2019, high-volume dentists accounted for almost half of dental opioid prescriptions and high-risk prescriptions. Quality improvement initiatives targeting these dentists may be warranted.
INTRODUCTION
In 2016, U.S. dentists accounted for 11.2 million dispensed opioid prescriptions.1 Studies suggest many of these opioid prescriptions may be unnecessary. For example, one study found that two thirds of dental opioid prescriptions dispensed to U.S. patients aged 13–64 years are for tooth extraction, a procedure for which ibuprofen and acetaminophen are effective analgesics for most patients.2–6 Reducing unnecessary dental opioid prescribing is an important public health goal given that this prescribing increases the risk of opioid-related adverse events, such as overdose and addiction.7–11 This goal is particularly important for adolescents and young adults, for whom dentists are the most common prescribers of opioids.12
Prior studies suggest that a small group of prescribers account for disproportionate shares of opioid prescriptions and high-risk prescriptions—those that increase the risk of opioid-related adverse events.13,14 However, studies have not specifically assessed the degree to which dental opioid prescribing and high-risk prescribing is concentrated among dentists. Moreover, little is known about the demographic and practice characteristics of dentists who account for disproportionate shares of dental opioid prescriptions and high-risk prescriptions. Closing these knowledge gaps could inform the targeting of initiatives aimed at improving the appropriateness and safety of dental opioid prescribing. For example, if dental opioid prescribing and high-risk prescribing is driven by a small group of dentists, initiatives targeting these dentists may be more efficient compared with broad-based initiatives that include all dentists.
The first objective of this study is to assess the degree to which dental opioid prescribing and high-risk prescribing is concentrated among dentists. To achieve this objective, the authors analyze a 2019 national prescription dispensing database. The second objective is to identify characteristics associated with being a “high-volume dentist,” defined as dentists with prescription counts in the 95th percentile or higher among all dentists accounting for dispensed opioid prescriptions in 2019. To achieve this objective, the authors link high-volume dentists in the prescription dispensing database to 2 comprehensive clinician databases.
METHODS
Study Sample
During May 2021, the authors conducted a cross-sectional analysis of the 2019 IQVIA Longitudinal Prescription Database, 2019 IQVIA OneKey database, and National Plan and Provider Enumeration System. The first database captures all prescriptions dispensed in 2019 from 92% of U.S. retail pharmacies, 70% of mail-order pharmacies, and 70% of long-term care pharmacies. Patient demographic information include year of birth, sex, state, and method of payment for the prescription. Other data elements include de-identified patient identifiers, an IQVIA-created prescriber identifier, national provider identifier, and the practice address and specialty of prescribers. Days supplied represents the number of days the prescription covers if patients took the maximum daily number of doses allowed. For example, if a prescription is written for 1 tablet every 4–6 hours as needed for pain, the maximum daily number of daily tablets is 6, so 18 tablets would be a 3-day supply. The database does not report the case mix of dentists.
The IQVIA OneKey database reports the demographic and practice characteristics of most U.S. clinicians.15 The 2019 OneKey database contains records for 171,984 dentists, including those who did and did not account for dispensed opioid prescriptions in the IQVIA Longitudinal Prescription Database. Data include year of birth and number of dentists in the practice. The National Plan and Provider Enumeration System is a comprehensive database of prescribers with a national provider identifier.16 The authors used this database to obtain information on dentist sex. The authors linked the 3 databases using the national provider identifier and IQVIA-constructed prescriber identifier. Because databases only contained de-identified patient information, the IRB of the University of Michigan Medical School exempted analyses from human subjects review.
The sample initially included all opioid analgesic prescriptions from dentists dispensed during 2019. IQVIA’s market definition was used to identify opioid analgesics (Appendix 1). Analyses excluded prescriptions to patients who resided outside of the 50 U.S. states and the District of Columbia, prescriptions from dentists who practiced outside of these locations, and prescriptions to children aged <13 years, as analyses of high-risk prescribing used a daily opioid dosage threshold that may not apply when weight-based dosing is used. Additionally, analyses excluded prescriptions with dosing information that was missing or potentially invalid (non-positive quantity or days supplied, days supplied >90). When assessing overlapping opioid and benzodiazepine prescriptions, as described in the next section, IQVIA’s market definition was used to identify dispensed prescriptions for benzodiazepines (Appendix 1).
Measures
To assess the concentration of dental opioid prescribing among dentists, the authors ranked all dentists accounting for ≥1 dispensed opioid prescription in the sample by descending number of prescriptions. Following prior work,14 dentists with prescription counts in the 95th percentile or higher were considered high-volume dentists. The proportion of dental opioid prescriptions in the sample accounted for by high-volume dentists versus other dentists was calculated.
Three metrics were used to assess the concentration of high-risk dental opioid prescribing among dentists, each of which has been employed in prior research.3,13,14,17–19 The first metric was the proportion of dental opioid prescriptions to opioid-naïve patients that exceeded a 3-day supply. This threshold derives from the Centers for Disease Control and Prevention opioid prescribing guidelines, which indicate that acute pain usually does not require more than a 3-day supply of opioids.20 Prescriptions to opioid-naïve patients exceeding this threshold are associated with increased risk of long-term opioid use.21 Following a national quality measure, opioid-naïve status was defined as the lack of dispensed opioid prescriptions in the 90 days prior to the dispensing date of the index opioid prescription.22
The second metric was the proportion of dental opioid prescriptions with daily opioid dosages ≥50 morphine milligram equivalents (MMEs), a standardized measure of opioid dosage. Prescriptions exceeding this threshold are associated with greater risk of opioid overdose.20 Daily MMEs were calculated by multiplying strength, quantity, and the Centers for Disease Control and Prevention’s MME conversion factors,23 then dividing by days supplied. Using the Centers for Disease Control and Prevention’s conversion factors, 50 daily MMEs correspond to a prescription for 10 pills containing 5 mg hydrocodone each day.23
The third metric was the proportion of dental opioid prescriptions that overlapped for ≥1 day with a benzodiazepine prescription. This metric was assessed owing to the strong link between overdose risk and concurrent exposure to opioids and benzodiazepines.24 Each opioid prescription in the sample was converted to a period of exposure that would occur if patients took medications as prescribed. Following prior literature, this period began on the dispensing date and ended on the dispensing date plus days supplied minus 1 day.14,25,26 Each dispensed benzodiazepine prescription was similarly converted to an exposure period. The numerator included any opioid prescription in the sample for which the exposure period overlapped with that of any benzodiazepine prescription. All benzodiazepine prescriptions were considered, regardless of the prescriber’s identity. For context, the authors calculated the proportion of opioid prescriptions in the sample that overlapped for ≥1 day with a benzodiazepine prescription written by the dentist who wrote the opioid prescription (e.g., a benzodiazepine prescription for perioperative anxiety).27
“High-risk prescriptions” were those classified as high risk by any of the 3 metrics.14 The proportion of high-risk prescriptions written by high-volume dentists and other prescribers was calculated. Finally, performance on each metric among the 2 groups of dentists was calculated.
Statistical Analysis
Descriptive statistics were used to describe dental opioid prescriptions in the sample. Using chi-square tests, performance on the 3 metrics of high-risk prescribing was compared between high-volume dentists and other dentists and assessed differences in characteristics between these 2 groups. Analyses focused on 6 characteristics: sex, age in years (defined as 2019 minus the year of birth and categorized as 30–39, 40–49, 50–59, 60–69, and ≥70 years), specialty (categorized as general dentistry; oral and maxillofacial surgery; and dental specialty, such as endodontics), U.S. Census region of practice location, urban/rural location of the practice (based on ZIP code and a crosswalk published by the U.S. Census Bureau28), and practice size (categorized as 1, 2, 3, 4, and ≥5 dentists in the practice). To assess which of the 6 characteristics were independently associated with being a high-volume dentist, logistic regression was used.
Among the 6 assessed characteristics, 4 had missing data for any of the dentists who accounted for ≥1 dispensed opioid prescription in the sample: urban/rural location (0.1% of dentists), sex (0.2%), practice size (14.6%), and age (33.8%). Two factors accounted for the higher rates of missing data for practice size and age, both of which were derived from OneKey. First, among all dentists who accounted for dispensed opioid prescriptions in the sample, 14.6% could not be linked to OneKey, as this database does not capture all U.S. dentists. Second, some dentists included in OneKey had missing data for year of birth. In the descriptive analysis comparing characteristics of high-volume dentists and other dentists, missing data were accounted for by creating a separate category. In the logistic regression model, missing data were accounted for by using multiple imputation with chained equations and 30 iterations. In a secondary analysis, analyses were repeated when limiting to prescriptions from oral and maxillofacial surgeons. Analyses used SAS, version 9.4; Stata, version 15.1 MP; and 2-sided hypothesis tests with α=0.05.
RESULTS
The IQVIA Longitudinal Prescription Database included 145,241,665 opioid prescriptions dispensed in 2019, of which 10,979,804 (7.6%) were written by dentists. Of these prescriptions, 243,061 (2.2%) were excluded, leaving 10,736,743 prescriptions in the sample (Appendix 2). Among the 10,736,743 prescriptions, 5,825,303 (54.3%) were for female patients, 2,099,507 (19.6%) were for patients aged 13–25 years, 3,390,601 (31.6%) were for patients aged 26–44 years, 3,485,962 (32.5%) were for patients aged 45–64 years, and 1,760,673 (16.4%) were for patients aged ≥65 years. Method of payment was cash for 966,756 (9.0%), Medicaid for 1,468,599 (13.7%), Medicare for 1,391,616 (13.0%), and commercial insurance for 6,909,772 (64.4%). Overall, 10,276,658 (95.7%) prescriptions were for opioid-naïve patients (those without dispensed opioid prescriptions in the prior 90 days). Finally, 6,330,944 (59.0%) were for hydrocodone, 2,677,411 (24.9%) were for codeine, 1,046,568 (9.7%) were for oxycodone, 662,754 (6.2%) were for tramadol, and 19,066 (0.2%) were for other opioids.
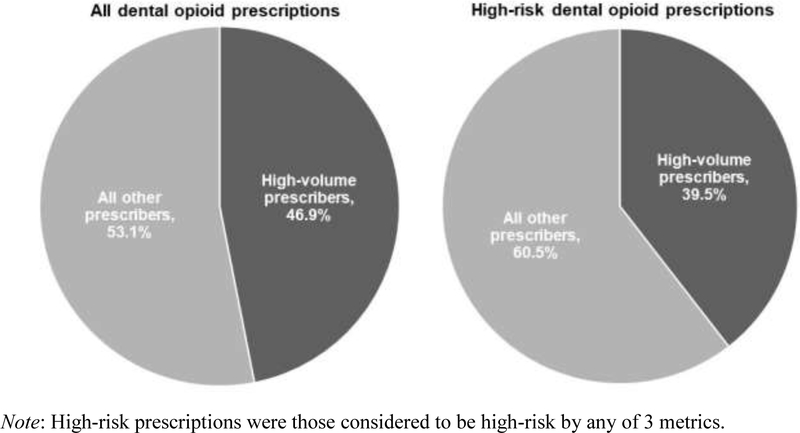
A total of 141,345 dentists accounted for the 10,736,743 prescriptions in the sample. The median number of dispensed opioid prescriptions per dentist was 18 (25th–75th percentile: 5–62). The 95th percentile was 355 prescriptions. The 7,079 high-volume dentists with prescription counts in the 95th percentile or higher—a group representing 5.0% of the 141,345 dentists—accounted for 5,037,757 (46.9%) of the 10,736,743 prescriptions. Overall, 4,242,634 (39.5%) of the 10,736,743 prescriptions were considered high risk by any of the 3 metrics. High-volume dentists accounted for 2,017,169 (47.6%) of these high-risk prescriptions (Figure 1).
Figure 1.
Percentage of dental opioid prescriptions and high-risk prescriptions accounted for by high-volume dentists.
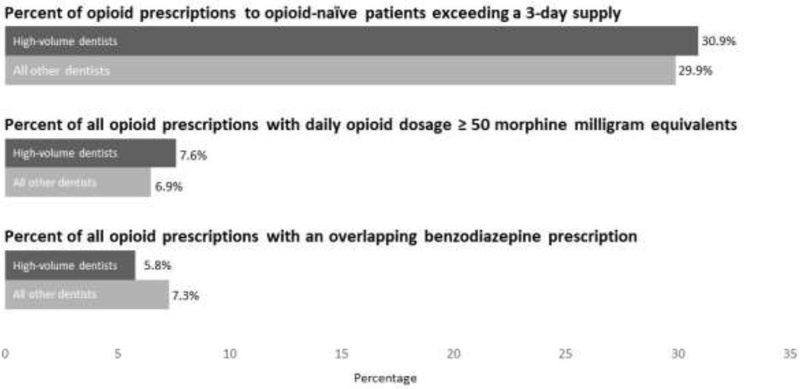
Figure 2 displays performance on the 3 metrics of high-risk prescribing among high-volume dentists and other dentists. Of 4,852,278 and 5,424,380 prescriptions to opioid-naïve patients written by high-volume dentists and other dentists, 1,496,870 (30.9%) and 1,619,502 (29.9%) exceeded a 3-day supply. Of 5,037,757 and 5,698,986 prescriptions written by high-volume dentists and other dentists, 381,422 (7.6%) and 368,840 (6.5%) had daily MMEs ≥50, while 291,056 (5.8%) and 417,314 (7.3%) overlapped with a benzodiazepine prescription for ≥1 day. Though all differences were significant, the median absolute difference in performance among the 3 metrics was just 1.1 percentage points. Among the 10,736,643 opioid prescriptions in the sample, 708,380 (6.6%) overlapped with any benzodiazepine prescription for ≥1 day, while 225,969 (2.1%) overlapped with a benzodiazepine prescription written by the dentist who accounted for the opioid prescription. Appendix 3 contains additional analyses of high-risk prescriptions.
Figure 2.
Performance on 3 metrics of high-risk opioid prescribing among high-volume dentists versus other dentists.
Table 1 shows demographic and practice characteristics of high-volume dentists and other dentists. Compared with the latter, high-volume dentists were more likely to be male (90.5% vs 68.7%), to be oral and maxillofacial surgeons (46.9% vs 1.9%), and to practice in the South (47.1% vs 34.8%; p<0.05 for all characteristics). Although the proportion of high-volume dentists in younger age groups was higher compared with other dentists, missing data for age precluded inferences based on unadjusted comparisons.
Table 1.
Unadjusted Comparison of Demographic and Practice Characteristics Between High-Volume Dentists and Other Dentists
| Characteristic | High-volume dentists | Other dentists | All dentists accounting for ≥1 dispensed opioid prescription in sample |
|---|---|---|---|
| N=7,079 n (%) | N=134,266 n (%) | N=141,345 n (%) | |
| Sex | |||
| Male | 6,406 (90.5) | 92,256 (68.7) | 98,662 (69.8) |
| Female | 670 (9.5) | 41,673 (31.0) | 42,343 (30.0) |
| Unknown | 3 (0.0) | 337 (0.3) | 340 (0.2) |
| Age, years | |||
| 30–39 | 1,267 (17.9) | 19,393 (14.4) | 20,660 (14.6) |
| 40–49 | 1,402 (19.8) | 22,062 (16.4) | 23,464 (16.6) |
| 50–59 | 1,370 (19.4) | 21,697 (16.2) | 23,067 (16.3) |
| 60–69 | 1,031 (14.6) | 20,315 (15.1) | 21,346 (15.1) |
| 70–75 | 227 (3.2) | 4,875 (3.6) | 5,102 (3.6) |
| Unknown | 1,782 (25.2) | 45,924 (34.2) | 47,706 (33.8) |
| Specialty | |||
| General dentistry | 3,697 (52.2) | 130,492 (97.2) | 134,189 (94.9) |
| Dentistry/anesthesiology | 0 (0.0) | 10 (0.0) | 10 (0.0) |
| Dentistry/endodontics | 24 (0.3) | 564 (0.4) | 588 (0.4) |
| Dentistry/orthodontics | 1 (0.0) | 48 (0.0) | 49 (0.0) |
| Dentistry/pedodontics | 0 (0.0) | 155 (0.1) | 155 (0.1) |
| Dentistry/periodontics | 36 (0.5) | 445 (0.3) | 481 (0.3) |
| Dentistry/prosthodontics | 0 (0.0) | 54 (0.0) | 54 (0.0) |
| Oral and maxillofacial surgery | 3,321 (46.9) | 2,498 (1.9) | 5,819 (4.1) |
| Census region of practice location | |||
| Northeast | 929 (13.1) | 22,173 (16.5) | 23,102 (16.3) |
| Midwest | 1,425 (20.1) | 27,980 (20.8) | 29,405 (20.8) |
| South | 3,333 (47.1) | 46,769 (34.8) | 50,102 (35.4) |
| West | 1,392 (19.7) | 37,344 (27.8) | 38,736 (27.4) |
| Urban/rural practice location | |||
| Urban | 6,993 (98.8) | 131,136 (97.7) | 138,129 (97.7) |
| Rural | 80 (1.1) | 3,027 (2.3) | 3,107 (2.2) |
| Unknown | 6 (0.1) | 103 (0.1) | 109 (0.1) |
| Practice size | |||
| 1 | 2,376 (33.6) | 49,142 (36.6) | 51,518 (36.4) |
| 2 | 1,588 (22.4) | 29,896 (22.3) | 31,484 (22.3) |
| 3 | 896 (12.7) | 14,118 (10.5) | 15,014 (10.6) |
| 4 | 519 (7.3) | 7,204 (5.4) | 7,723 (5.5) |
| 5 | 310 (4.4) | 3,740 (2.8) | 4,050 (2.9) |
| 6 | 172 (2.4) | 2,256 (1.7) | 2,428 (1.7) |
| 7 | 101 (1.4) | 1,329 (1.0) | 1,430 (1.0) |
| 8 | 58 (0.8) | 907 (0.7) | 965 (0.7) |
| 9 | 36 (0.5) | 593 (0.4) | 629 (0.4) |
| 10–99 | 147 (2.1) | 3,271 (2.4) | 3,418 (2.4) |
| ≥100 | 141 (2.0) | 1,948 (1.5) | 2,089 (1.5) |
| Unknown | 735 (10.4) | 19,862 (14.8) | 20,597 (14.6) |
p<0.05 for all 6 characteristics according to chi-squared tests. Sex data derived primarily from the National Plan and Provider Enumeration System. In a small number of cases, these data were missing. In these cases, sex data derived from the IQVIA OneKey database. Data on age and practice sized derived from OneKey. Data on specialty derived from the IQVIA Longitudinal Prescription Database. Data on Census region of practice location and urban/rural practice location derived primarily from the IQVIA Longitudinal Prescription Database, but derived from OneKey when these data were missing.
Table 2 shows results from the logistic regression model assessing characteristics associated with being a high-volume dentist. Male dentists were more likely than female dentists to be high-volume dentists (AOR=3.48, 95% CI=3.19, 3.81). Dentists aged 30–39 years were more likely to be high-volume dentists than older dentists (e.g., 40–49 years versus 30–39 years: AOR=0.87, 95% CI=0.80, 0.95). Dentists practicing in regions other than the Northeast were more likely to be high-volume dentists than those practicing in the Northeast (e.g., South versus Northeast: AOR=2.75, 95% CI=2.52, 3.02). Dental specialists (AOR=1.64, 95% CI=1.27, 2.14) and oral and maxillofacial surgeons (AOR=46.05, 95% CI=43.09, 49.20) were more likely to be high-volume dentists compared with general dentists. Compared with dentists in solo practice (i.e., practice size of 1), dentists in practices with 3 dentists (AOR=1.16, 95% CI=1.06, 1.27) or 4 dentists (AOR=1.26, 95% CI=1.12, 1.42) were more likely to be high-volume dentists. AORs were positive but non-significant for practice size of 2 and ≥5 dentists versus solo practice. There was no association between urban/rural practice location and being a high-volume dentist.
Table 2.
Characteristics Independently Associated With Being a High-Volume Dentist
| Characteristic | AOR (95% CI) |
|---|---|
| Sex | |
| Female | ref |
| Male | 3.48 (3.19, 3.81) |
| Age, years | |
| 30–39 | ref |
| 40–49 | 0.87 (0.80, 0.95) |
| 50–59 | 0.76 (0.69, 0.84) |
| 60–69 | 0.58 (0.52, 0.64) |
| ≥70 | 0.43 (0.36, 0.51) |
| Specialty | |
| General dentist | ref |
| Dental specialtya | 1.64 (1.27, 2.14) |
| Oral and maxillofacial surgery | 46.05 (43.09, 49.20) |
| Census region of practice location | |
| Northeast | ref |
| Midwest | 1.75 (1.59, 1.94) |
| South | 2.75 (2.52, 3.02) |
| West | 1.26 (1.14, 1.39) |
| Urban/rural practice location | |
| Rural | ref |
| Urban | 1.20 (0.95, 1.50) |
| Practice size | |
| 1 | ref |
| 2 | 1.03 (0.96, 1.12) |
| 3 | 1.16 (1.06, 1.27) |
| 4 | 1.26 (1.12, 1.42) |
| ≥5 | 1.08 (0.98, 1.19) |
Includes anesthesiology, endodontics, orthodontics, pedodontics, periodontics, and orthodontics.
In the secondary analysis, male sex, younger age, and practice location in the South were associated with being a high-volume oral and maxillofacial surgeon (Appendix 4).
DISCUSSION
In 2019, dentists accounted for 10.7 million opioid prescriptions dispensed to U.S. patients aged >12 years. Of these prescriptions, 4 in 10 were considered high risk by any of 3 metrics. High-volume dentists—the 5% of dentists with prescription counts in the 95th percentile or higher—accounted for almost half of all dental opioid prescriptions and high-risk prescriptions. Male sex, younger age, practice location outside the Northeast, and specialization in oral and maxillofacial surgery were associated with a higher likelihood of being a high-volume dentist.
When interpreting findings, it is important to recognize that high-volume dentists may not necessarily have higher rates of opioid prescribing compared with other dentists. The number of dispensed opioid prescriptions from dentists equals their rate of prescribing multiplied by the number of opportunities to prescribe. The latter is influenced both by case mix (e.g., whether patients have surgical versus non-surgical needs) and by patient volume. For example, it is not surprising that 46.9% of high-volume prescribers were oral and maxillofacial surgeons, who frequently perform invasive procedures. Additionally, particularly busy oral and maxillofacial surgeons could be high-volume dentists even if their prescribing rates were similar to their peers. Despite these caveats, the outsized role of high-volume dentists in dental opioid prescribing suggests that closely scrutinizing their prescribing practices may be warranted, especially given that the majority of dental opioid prescriptions are written for procedures for which non-opioids are equally effective for most patients.2,3 Such scrutiny may be particularly warranted for the 53.1% of high-volume prescribers who were general dentists or dental subspecialists, as these dentists less frequently perform invasive procedures compared with oral and maxillofacial surgeons.
High-volume dentists also had an outsized role in high-risk prescribing. Importantly, however, other dentists collectively accounted for a sizable share of high-risk prescriptions, and their performance on the 3 metrics of high-risk prescribing was similar to that of high-volume dentists. These findings have important implications for the targeting of initiatives to improve the safety of dental opioid prescribing. If resources are limited, initiatives may have the most return on investment by targeting high-volume dentists, simply because they account for the largest number of high-risk prescriptions. Ideally, however, resources would be sufficient to include other dentists given that this group also frequently engages in risky prescribing. For example, quality improvement initiatives specifically for high-volume dentists, such as academic detailing and peer comparison, could be coupled with broad-based initiatives that affect all dentists, such as opioid prescribing guidelines,29 mandates to use prescription drug monitoring program databases,30 and requirements for continuing education on pain management.
Younger age and male sex were associated with a higher likelihood of being a high-volume dentist. The age finding is potentially surprising, as younger dentists who trained during the U.S. opioid epidemic may have received more training in the use of opioid alternatives for dental pain compared with older dentists. On the other hand, it is possible that younger dentists seeking to establish their practices have higher patient volume than older dentists and therefore have more opportunities to prescribe opioids. Female dentists may also have fewer opportunities to prescribe opioids because they are more likely to work part-time compared with male dentists.31 However, it is also possible that male dentists are more likely to prescribe opioids than female dentists, as sex-based differences in management style and care quality have been demonstrated in other specialties.32,33
Practicing outside of the Northeast, particularly in the South, was associated with a higher likelihood of being a high-volume dentist. This finding is consistent with literature showing geographic variation in the rate of opioid prescribing among all prescribers and among dentists specifically.34–36 Importantly, the existence of geographic variation by itself does not necessarily imply the occurrence of inappropriate prescribing. However, this variation does raise the possibility that local practice patterns, as opposed to patient need alone, play a large role in dentists’ decisions to prescribe opioids, suggesting opportunities to reduce dental opioid exposure.
Findings suggest dentists working in practices of 3 or 4 dentists are more likely to be a high-volume dentist compared with those in solo practice. However, findings also suggest that dentists in solo practice are similarly likely to be a high-volume dentist as those working in practices of 2 or ≥5 dentists. Given these inconsistent findings, the association between practice size and being a high-volume dentist remains unclear and requires further exploration in future studies.
Limitations
Study strengths include its use of a national, all-payer prescription dispensing database and its use of 2 detailed clinician databases. However, the study had limitations. First, data on age and practice size were frequently missing. Confidence in inferences is stronger for characteristics with more complete data. Second, databases lacked information on several factors that might be associated with being a high-volume dentist, such as patient volume and case mix. However, the authors are unaware of any national database that captures these factors as well as dentists’ opioid prescribing patterns across all patients.
CONCLUSIONS
In 2019, high-volume dentists accounted for almost half of dental opioid prescriptions and high-risk dental opioid prescriptions. Focusing on these dentists may be warranted when designing initiatives to improve the appropriateness and safety of dental opioid prescribing.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Chua had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Drs. Chua, Brummett, and Nalliah report receiving honoraria from the Benter Foundation. Dr. Brummett also reports providing expert testimony and serving as a consultant for Heron Therapeutics, Vertex Pharmaceuticals, and Alosa Health. No other conflicts of interest were reported.
This manuscript was funded by a grant from the Benter Foundation (grant number 2020-02). Further support was also provided by the Substance Abuse and Mental Health Services Administration and the University of Michigan Precision Health Initiative. Dr. Chua is supported by a career development award from the National Institute on Drug Abuse (grant number 1K08DA048110-01). The funding sources played no role in the design of the study; the collection, analysis, or interpretation of the data; or the decision to approve publication of the finished manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Suda KJ, Durkin MJ, Calip GS, et al. Comparison of opioid prescribing by dentists in the United States and England. JAMA Netw Open. 2019;2(5):e194303. 10.1001/jamanetworkopen.2019.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. Benefits and harms associated with analgesic medications used in the management of acute dental pain: an overview of systematic reviews. J Am Dent Assoc. 2018;149(4):256–265.e3. 10.1016/j.adaj.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Chua KP, Hu HM, Waljee JF, Brummett CM, Nalliah RP. Opioid prescribing patterns by dental procedure among US publicly and privately insured patients, 2013 through 2018. J Am Dent Assoc. 2021;152(4):309–317. 10.1016/j.adaj.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersh EV, Moore PA, Grosser T, et al. Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain. J Dent Res. 2020;99(7):777–786. 10.1177/0022034520914254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore PA, Hersh EV. Just-in-case opioid prescribing. J Dent Educ. 2020;84(12):1327–1328. 10.1002/jdd.12500. [DOI] [PubMed] [Google Scholar]
- 6.Theken KN, Hersh EV, Lahens NF, et al. Variability in the analgesic response to ibuprofen is associated with cyclooxygenase activation in inflammatory pain. Clin Pharmacol Ther. 2019;106(3):632–641. 10.1002/cpt.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbaugh CM, Nalliah RP, Hu HM, Englesbe MJ, Waljee JF, Brummett CM. Persistent opioid use after wisdom tooth extraction. JAMA. 2018;320(5):504–506. 10.1001/jama.2018.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med. 2019;179(2):145–152. 10.1001/jamainternmed.2018.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua KP, Hu HM, Waljee JF, Nalliah RP, Brummett CM. Persistent opioid use associated with dental opioid prescriptions among publicly and privately insured US patients, 2014 to 2018. JAMA Netw Open. 2021;4(4):e216464. 10.1001/jamanetworkopen.2021.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua KP, Kenney BC, Waljee JF, Brummett CM, Nalliah RP. Dental opioid prescriptions and overdose risk in patients and their families. Am J Prev Med. 2021;61(2):165–173. 10.1016/j.amepre.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell TJ, Martins D, Tadrous M, et al. Dental opioid prescription characteristics and the risk of new, persistent use. Am J Prev Med. 2021;60(6):831–839. 10.1016/j.amepre.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiang MV, Humphreys K, Cullen MR, Basu S. Opioid prescribing patterns among medical providers in the United States, 2003–17: retrospective, observational study. BMJ. 2020;368:l6968. 10.1136/bmj.l6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua KP, Brummett CM, Conti RM, Bohnert AS. Opioid prescribing to US children and young adults in 2019. Pediatrics. 2021;148(3):e2021051539. 10.1542/peds.2021-051539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IQVIA. OneKey Reference Assets. https://www.iqvia.com/locations/united-states/solutions/life-sciences/information-solutions/essential-information/onekey-reference-assets. Published 2021. Accessed June 9, 2021.
- 16.Centers for Medicare and Medicaid Services. National Plan & Provider Enumeration System. https://nppes.cms.hhs.gov. Published 2021. Accessed June 9, 2021.
- 17.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N, Vujicic M, Blatz A. Multiple opioid prescriptions among privately insured dental patients in the United States: evidence from claims data. J Am Dent Assoc. 2018;149(7):619–627.e1. 10.1016/j.adaj.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012–2017. N Engl J Med. 2019;380(11):1043–1052. 10.1056/nejmsa1807069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pharmacy Quality Alliance. Initial Opioid Prescribing for Long Duration (IOP-LD). https://www.pqaalliance.org/assets/Measures/PQA_Measures_Overview.pdf. Published 2020. Accessed March 25, 2021.
- 23.Data resources: analyzing prescription data and morphine milligram equivalents (MMEs). Centers for Disease Control and Prevention. https://www.cdc.gov/drugoverdose/resources/data.html. Published 2020. Accessed January 2, 2021.
- 24.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua KP, Brummett CM, Conti RM, Bohnert A. Association of opioid prescribing patterns with prescription opioid overdose in adolescents and young adults. JAMA Pediatr. 2020;174(2):141–148. 10.1001/jamapediatrics.2019.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua KP, Brummett CM, Ng S, Bohnert ASB. Association between receipt of overlapping opioid and benzodiazepine prescriptions from multiple prescribers and overdose risk. JAMA Netw Open. 2021;4(8):e2120353. 10.1001/jamanetworkopen.2021.20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teoh L, Thompson W, Hubbard CC, Gellad W, Finn K, Suda KJ. Comparison of dental benzodiazepine prescriptions from the U.S., England, and Australia from 2013 to 2018. Am J Prev Med. 2021;61(1):73–79. 10.1016/j.amepre.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Relationship files. U.S. Census Bureau. https://www.census.gov/geographies/reference-files/2010/geo/relationship-files.html#par_list. Published 2021. Accessed May 15, 2021.
- 29.Guan Q, Campbell T, Martins D, et al. Assessing the impact of an opioid prescribing guideline for dentists in Ontario, Canada. J Am Dent Assoc. 2020;151(1):43–50. 10.1016/j.adaj.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. Impact of a mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS One. 2015;10(8):e0135957. 10.1371/journal.pone.0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surdu S, Mertz E, Langelier M, Moore J. Dental workforce trends: a national study of gender diversity and practice patterns. Med Care Res Rev. 2021;78(1_suppl):30S–39S. 10.1177/1077558720952667. [DOI] [PubMed] [Google Scholar]
- 32.Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for Medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206–213. 10.1001/jamainternmed.2016.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilligan MA, Neuner J, Sparapani R, Laud PW, Nattinger AB. Surgeon characteristics and variations in treatment for early-stage breast cancer. Arch Surg. 2007;142(1):17–22. 10.1001/archsurg.142.1.17. [DOI] [PubMed] [Google Scholar]
- 34.Suda KJ, Zhou J, Rowan SA, et al. Overprescribing of opioids to adults by dentists in the U.S., 2011–2015. Am J Prev Med. 2020;58(4):473–486. 10.1016/j.amepre.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard CC, Evans CT, Calip GS, et al. Characteristics associated with opioid and antibiotic prescribing by dentists. Am J Prev Med. 2021;60(5):648–657. 10.1016/j.amepre.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schieber LZ, Guy GP Jr., Seth P, et al. Trends and patterns of geographic variation in opioid prescribing practices by state, United States, 2006–2017. JAMA Netw Open. 2019;2(3):e190665. 10.1001/jamanetworkopen.2019.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.