Case Introduction
Our patient was a 17-year-old unaccompanied, undocumented male who arrived at the US-Mexico border and was taken into custody. Prior to that he was essentially asymptomatic, had played soccer, and was able to keep up with his peers.
He is from a small village in Guatemala, where his mother still lives. His mother and grandmother speak a local dialect and have very little in their homes. After he was taken into custody by the U.S. government, he was accompanied only by a Spanish-speaking contract worker to help him communicate.
At that time, his blood pressure in a routine intake exam was 170/89; therefore, he was transferred to a local hospital. His physical examination showed a normal-appearing, ambulatory, non-obese male with no distress. His right upper-extremity blood pressure was approximately 170/90 mmHg. However, his lower-extremity systolic blood pressure was approximately 130 mmHg. The upper- and lower-extremity saturations were normal. His cardiac examination was notable for four out of six holosystolic murmurs at the left upper sternal border, with radiation to the back of the neck and a palpable thrill. There were no abdominal bruits or masses. He had strong pulses in both upper and lower extremities, although notably with radial femoral delay, and physically, he showed no dysmorphisms, no skin findings, or neurologic findings.
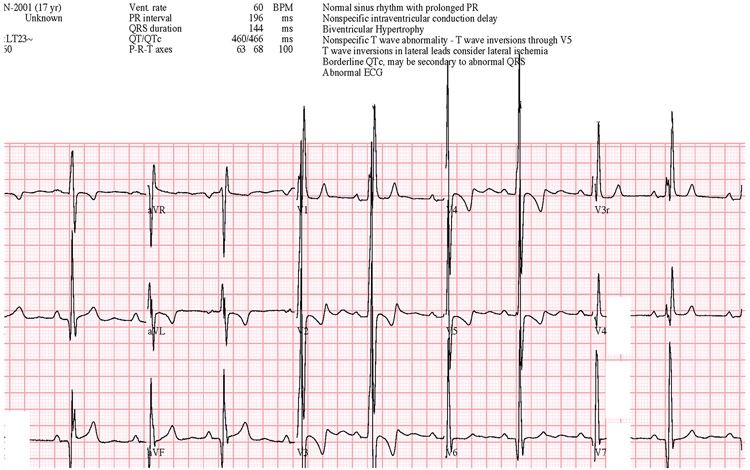
Chest radiography (Figure 1) was performed in addition to an ECG (Figure 2) initially, and the chest radiography showed cardiomegaly, likely some increased pulmonary vascular markings, but no overt abnormalities. ECG shown on the right revealed a normal sinus rhythm, ventricular hypertrophy, some nonspecific intraventricular conduction delay, most notably inverted T waves in V2 through V5.
Figure 1:
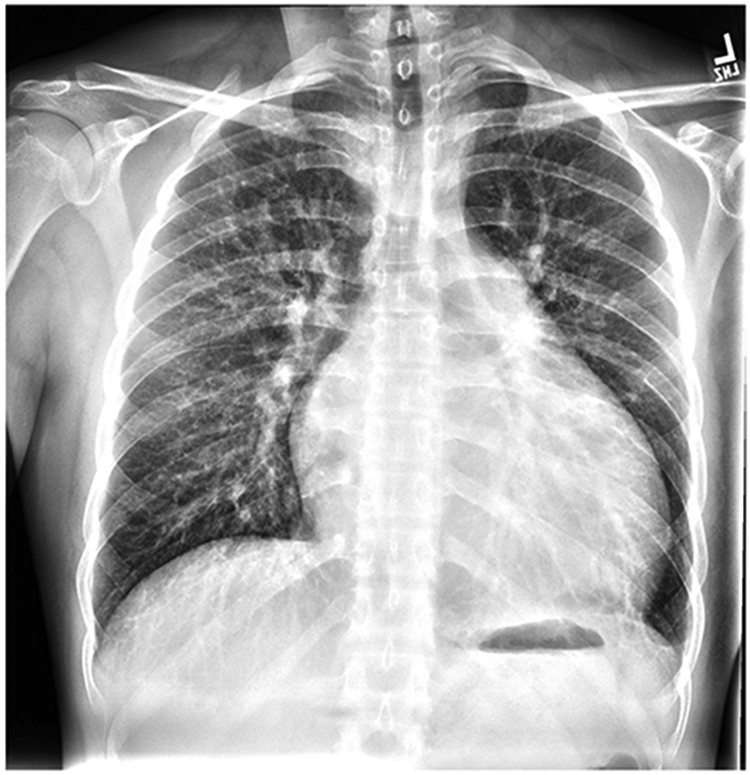
Anterior-posterior chest radiograph
Figure 2:
12-Lead electrocardiogram
Given the concerns regarding the myocardial process, an echocardiogram was performed, and is shown here. As you can see in the parasternal (Supplemental Video IA and IB) as well as apical views (Supplemental Video IC), he had a moderately dilated left ventricle with depressed function. Then, moving onto some of the other images, on the left, you can see evidence of a small restrictive perimembranous ventricular septal defect (Supplemental Video ID). The top right is a suprasternal view (Supplemental Video IE). The ascending and transverse arch with head and neck vessel branching can be seen. However, there was no connection to the descending aorta, and there is flow going in and out of the left pulmonary artery. In addition, in the subcostal view, one can see a reversal of flow during diastole in the descending aorta (Supplemental Video IF).
Next, a chest CT was performed to better understand what was going on, and as you can see here, there is a type A, interrupted aortic arch with the descending aorta reconstituted by a large patent ductus arteriosus (Figure 3A), and significant collateral vessels, as shown in the pictures on the right (Figure 3B), coming off the internal mammary arteries.
Figure 3.
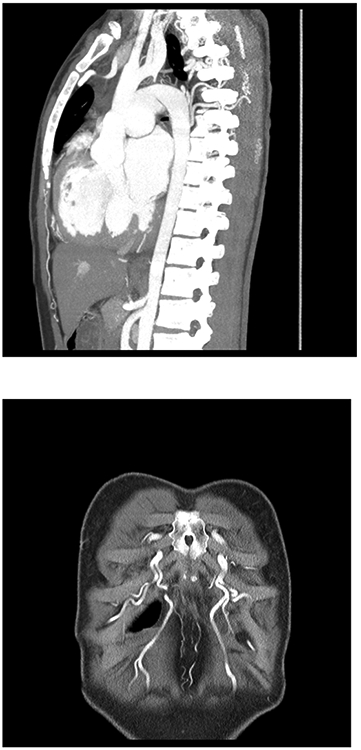
A: Preoperative chest CT with angiography, sagittal view
B: Preoperative chest CT with angiography, coronal view
Discussion: Initial diagnostic considerations
Dr. Leopold: What is our diagnosis? Again, to summarize, the patient's asymptomatic with severe systemic hypertension, a blood pressure gradient from the upper to lower extremities with radial femoral delay, but strong pulses. Anatomically, there is a type A interrupted arch with a descending artery reconstituted by a PDA and torturous collateral vessels and a small ventricular septal defect. Other findings include moderate hypertrophy, left ventricular hypertrophy (LVH), and depressed ejection fraction. I will turn it back over to my colleague for further discussion.
Dr. Zachariah: At this point, we have an anatomic diagnosis, and we have a management dilemma. How do we manage a 17-year-old who has been this way, presumably, since before birth? First, is surgical repair indicated? Full surgical repair is feasible from the perspective of surgeons. However, the question is, as physicians, should we take that chance or not? The second, and perhaps related, question is, given the interruption of the aortic arch and continuation with a patent ductus arteriosus, apparently massive collateral flow leading to no saturation gradient, and reversal of flow from the descending aorta back into the pulmonary artery, how do we manage his blood pressure from a medical perspective?
Prof. Touyz: Let’s start with the sociological factors, environmental factors, and psychological factors compounding this very interesting and complex case. I would like to ask you a question, if I may. So we have here, for all intents and purposes, a young, healthy 17-year-old who plays soccer, etc., and did not complain of anything abnormal. He underwent blood pressure measurement incidentally, and the results were found to be elevated. What prompted you to measure the blood pressure in the lower limbs? This was obviously critical from a diagnostic point of view.
Dr. Zachariah: Thank you for this question. In fact, the upper- versus lower-extremity blood pressure gradient was measured even before he came to our institution. Therefore, we do not know the precise reason for this measurement. However, on physical examination, a radial–femoral delay was extremely evident. It was the type of exam for which you brought medical students to the bedside, because it is a characteristic case. It is my impression that this was part of the reason that upper- versus lower-extremity blood pressures were obtained. However, I am not sure about the timing, as to whether the four extremity blood pressures were obtained before or after further cardiac evaluation was performed in light of the murmur and other findings. On examination, his pulses were indeed bounding, and there was radial femoral delay. This may have been the triggering point for obtaining the four extremity blood pressures.
Prof. Touyz: Thank you for your explanation. We have a question in chat; we saw that you presented a chest X-ray to us. Was there any evidence of rib notching to suggest the chronicity and severity of this clinical problem?
Dr. Zachariah: Thank you for this excellent question. Notching was difficult to discern on this particular youngster's X-ray, even though we looked for it very carefully. In retrospect, some X-rays, especially those of the right-side chest, perhaps show some notching at rib four or five. Scott, maybe we can go backwards and show that picture again. Perhaps in the middle sections on the right, some rib notching can be discerned a little bit off, a little bit more laterally, but it is very hard to tell in this particular X-ray. I think because of the congestion in the pulmonary arteries or prominence of the pulmonary arteries, rib notching becomes a little bit difficult to ascertain. However, this is a great question, and this is a classic case in which such a finding should appear. In fact, torturous vessels were demonstrated in other imaging modalities.
Prof. Touyz: Thank you. Another question in chat: in a 17-year-old hypertensive patient, would you not perform a CT of the whole aorta anyway?
Dr. Zachariah: No. As a general rule, an otherwise healthy-appearing 17-year-old who presents with hypertension in the outpatient clinic would not routinely undergo a CT scan.1 Simply because in this day and age, hypertension is a valid concern in youth. Of course, in American epidemiological studies from the National Health and Nutrition Examination Survey, the prevalence of elevated blood pressures has been reported to be in the order of one-in-six to one-in-seven youth under 18 years of age.2,3 Therefore, it is an extremely prominent problem. An additional workup is recommended when the expected features are not present. For example, this young man was not otherwise obese and did not otherwise have a sedentary lifestyle. His dietary habits were unknown when we took him, and they might have indicated additional workup.1 However, a CT scan would still not be one of our early workup points. We would probably start with an echocardiogram to evaluate cardiac structure and function, because coarctation should be visible in echocardiography. If body habitus or other impediments existed, cross-sectional imaging may be performed. However, a CT scan is not a routine part of the hypertension workup for the average case of elevated blood pressure in youth.
Prof. Dominiczak: This patient, as we saw in your images, had massive LVH. This is a strong indicator that this is a chronic severe problem.
Dr. Zachariah: This is indeed evidence of chronicity. Elevated blood pressure in youth shows co-existent LVH in 30% to 55% of the cases.1,2 However, epidemiological data is confounded by the fact that many, almost half, of the youth with elevated blood pressure are also obese, and obesity is also a chronic driver of LVH.4 However, this question about his LVH does give us pause about how longstanding this issue is, especially in the absence of obesity, as we noted. We believe that this will become a problem very shortly. In fact, one of the key questions that we would like to ask about this patient is that in the absence of a continuity in the aorta, any antihypertensives administered to this patient are going to be confounded by the fact that there is no connection directly from the left ventricle to the descending aorta, where most of these medicines work. What will be the effect of this on the coronary perfusion pressure? He had LVH and presumably had a higher left ventricular end-diastolic pressure (EDP). Coronary perfusion pressure is defined as the difference between mean arterial pressure and left ventricular end-diastolic pressure. His EDP is elevated. We must believe that. Thus, what will be the cost-benefit of pharmacologically lowering this youngster's blood pressure?
Initial Medical Management
At the time of the diagnosis, the patient was started on metoprolol 25 mg with some slight improvement in his blood pressure to 150s over 60s to 70s, and then he was transferred to Texas Children’s Hospital in Houston for further care. Further workup showed normal chemistry, renal ultrasound, and thyroid findings.
Given that, we decided to proceed with a full repair and deferred further anti-hypertension management until after repair. However, due to the complexities of a patient in federal custody, there was some delay in obtaining consent for the operation. During that interval, we decided to intensify medical management.
Since he showed limited improvement with beta blockade, the patient was switched to amlodipine. A follow-up echocardiogram on a low dose of amlodipine showed some reduction in his LV ejection fraction. Thus, amlodipine was increased to 10 mg. However, there was very little improvement in his blood pressure.
Surgical and postoperative management
At that time, consent was obtained, and surgery was performed. The surgery involved an aortic interposition graft to connect the transverse and descending aorta, VSD closure, and tricuspid valvuloplasty, which is quite common with this type of VSD repair. Postoperative echo performed immediately after coming out of the operating room (OR) showed moderate-to-severe LV dysfunction.
However, one week after the operation, he developed worsening signs of heart failure, including fatigue and worsening ventricular ectopy. At one week postoperatively, another echo was performed (Supplemental Videos IIA and IIB). Parasternal long and short views demonstrated severe dysfunction. At that time, he was on medications including milrinone, spironolactone, and furosemide, and he had reasonable blood pressure control. So, I will turn it back over to Dr. Zachariah for further discussion.
Pharmacotherapeutic balance between afterload reduction and coronary perfusion?
Therefore, we have now performed an anatomic surgical repair. Perhaps a consequence of this repair according to the surgical operative notes, and the repair itself was straightforward; however, as a consequence, his ventricular function had markedly and noticeably declined.
Perhaps, you do not even need to be a cardiologist to see the difference in cardiac function there. Our antihypertensive management was initially geared towards reducing myocardial oxygen demand, overall inotropy, and chronotropy with the use of beta blockade. However, when I went off service, my colleagues took over certain changes in that regimen. Perhaps this was the initial step leading to the decline in function. So, the question to the group is what should we have done better? What could we have improved in terms of management techniques? However, now that we have performed this repair and his blood pressure is now "better," how should we continue to manage him moving forward? Long-term implications?
The final question is, what have we improved long-term in this young man's life? Have we improved the risk of atherosclerotic heart disease in the future? Have we improved his stroke risk? Have we reduced the risks of hypertension, vascular function, renal function, and neurocognitive decline, which may be associated with blood pressure in young adulthood?
Have we improved any of these things or made his life worse with a noticeably worse function? With these questions, I will open it up for further discussion.
Discussion: Pharmacotherapeutic management pearls
Prof. Touyz: Yes, thank you very much. I will just have to ask you one question before we move on. You gave him the beta blocker initially, is that correct? His pulse was about 60 if I recall.
Dr. Zachariah: We did not initiate the beta blockade; it was initiated before he came to us. However, when we were on service, we elected not to change it and keep him on it. Yes.
Dr. Anna Szyndler: Why was amlodipine chosen as your hypertensive drug?
Dr. Zachariah: It is difficult for me to come up with a good answer for that. The attending physician who was on service elected to use a medication that we often use for antihypertensive indications, has a low risk of interactions with other medications, is generally well tolerated, and does not need other monitoring laboratories. Therefore, this calcium channel blocker was used. I suspect simply for comfort, since it’s something that we use.
Dr. Joseph Flynn: What about carvedilol or an angiotensin-converting enzyme (ACE) inhibitor or a combination of these? In addition, based on studies of post-coarctation hypertension, I suspect that he will always have hypertension.
Dr. Zachariah: There is evidence to suggest that ACE inhibitors or angiotensin receptor blockade may have some modest effects on vascular remodeling,5 if this patient’s aorta has not already reached an end stage. The cardiac-remodeling effects of ACE inhibitors are well documented in hypertension,6 although they are not always fully leveraged in pediatric congenital heart disease. These effects would take time to appear. In the meantime, there may have been some unresolved concerns about renal vascular perfusion or renin-angiotensin-aldosterone effects on volume status. We would seldom use carvedilol as a beta blocker for primary antihypertensive therapy, unless significant ventricular dysfunction was present. However, none of these were documented in terms of rationale.
Prof Jan Staessen: What was the difference in blood pressure between the upper and lower limbs?
Dr. Zachariah: Yes. When he first presented, his systolic blood pressure was in the 170s, and the systolic blood pressure in the lower extremities was in the 130s. Therefore, there was a roughly 40-mmHg gradient between the upper and lower extremities at the beginning. After repair, the gradient was completely abolished. It was an excellent repair, but his overall systolic blood pressures were barely in the 112-114 range, likely due to ventricular dysfunction, and so on.
Discussion: Recontextualizing afterload reduction as heart failure management
Dr. Amar: Regarding the blood pressure level, I would decrease the antihypertensive treatment and focus on the management of heart failure. What do you think?
Dr. Zachariah: I think that is a very prescient point and exactly the way that we proceeded. Rather than focusing on blood pressure as an independent issue, we focused on it in terms of optimization of congestive heart failure care. In fact, the remainder of our two slides demonstrate what the care looked like.
Dr. Leopold: He was started on heart failure therapy as you suggested, initially with milrinone and epinephrine drops, and then, over the course of three to four weeks, converted to a regimen of oral heart failure therapies, including ACE inhibitors, diuretics, spironolactone, and metoprolol, considering arrhythmia. During that time, his exercise capacity in a six-minute walk test increased from 55% to 81% predicted. At discharge, he had normal blood pressure and a normal BNP level, with improved LV ejection fraction, as seen in these echocardiogram clips (Supplemental Video IIIA and IIIB). Cardiac MRI was performed immediately before discharge (Figure 4). The right ventricle was moderately-to-severely dilated, and the left ventricle was severely dilated. RV function was normal, but LV function showed more-than-moderate depression. There was some delayed enhancement of the basal anterior septum and inferior LV, suggestive of subendocardial ischemia in those locations. As can be seen from this sagittal image, the aorta was widely patent with no stenosis or aneurysms.
Figure 4:
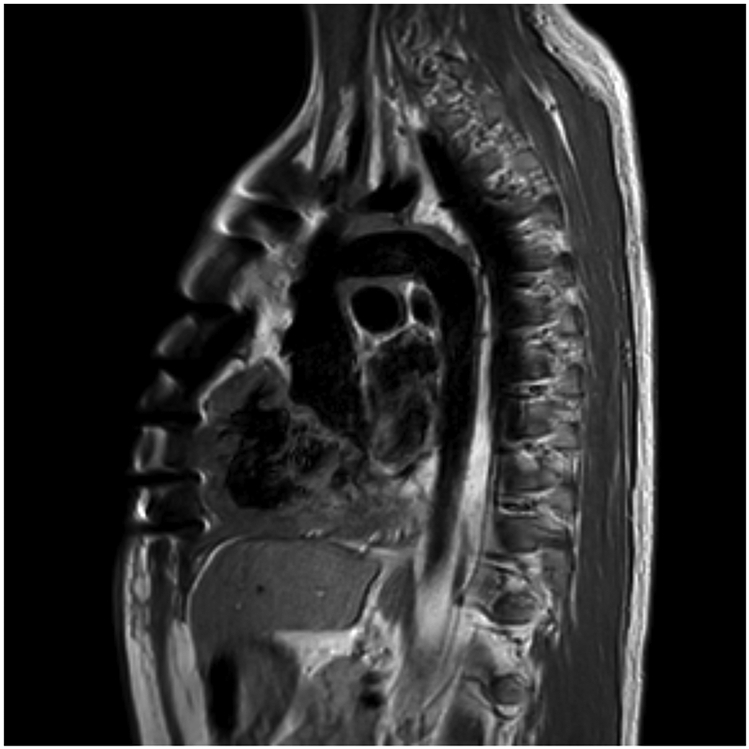
Cardiac magnetic resonance imaging, black blood sequences, sagittal view
Dr. Zachariah: We have concluded our presentation. We thank you for all of your interesting questions and inputs and the prescient comments that anticipated every one of our steps. Thank you again.
Summary
An asymptomatic 17-year-old undocumented male immigrant was noted to have severe hypertension during immigration intake. Identification of a gradient between the upper and lower extremities and the radial-femoral pulse delay led to extensive work-up culminating in a diagnosis of interrupted aortic arch and VSD. Medical and surgical management resolved the patient’s hypertension but was accompanied by profound congestive heart failure. On discharge, his functional status had improved markedly.
Supplementary Material
Supplemental Video IA: Preoperative echocardiogram, parasternal long axis
Supplemental Video IC: Preoperative echocardiogram, apical 4 chamber
Supplemental Video IB: Preoperative echocardiogram, parasternal short axis
Supplemental Video ID: Preoperative echocardiogram, parasternal short-axis color comparison of ventricular septal defects
Supplemental Video IE: Preoperative echocardiogram, suprasternal notch view
Supplemental Video IIA: Postoperative echocardiogram, parasternal long axis
Supplemental Video IIB: Postoperative echocardiogram, parasternal short axis
Supplemental Video IIIB: Predischarge echocardiogram, parasternal long axis
Supplemental Video IF: Preoperative echocardiogram, subcostal view of Descending aorta
Supplemental Video IIIA: Predischarge echocardiogram, parasternal short axis
Acknowledgments
The authors are grateful to the following session audience members for contributing to the discussion: Jan A. Staessen and Laurence Amar.
Sources of Funding
JPZ was supported by the R01 HL148217.
Footnotes
Disclosures
SML and JPZ have no financial conflicts of interest.
The following case was presented on 13 April 2021 as part of the Clinical-Pathological conference chaired by Anna F. Dominiczak and Rhian Touyz at the virtual joint meeting of the European Society of Hypertension and the International Society of Hypertension. Scott Leopold and Justin P. Zachariah presented the case and led the discussion.
References
- 1.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 2.Khoury M, Khoury PR, Dolan LM, Kimball TR, Urbina EM. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142(2):e20180245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999-2012. JAMA Pediatr. 2015;169(3):272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64(15):1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA, Prevention of Events With Angiotensin-Converting Enzyme Inhibition Investigators. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49(6):1271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension. 2009;54(5):1084–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video IA: Preoperative echocardiogram, parasternal long axis
Supplemental Video IC: Preoperative echocardiogram, apical 4 chamber
Supplemental Video IB: Preoperative echocardiogram, parasternal short axis
Supplemental Video ID: Preoperative echocardiogram, parasternal short-axis color comparison of ventricular septal defects
Supplemental Video IE: Preoperative echocardiogram, suprasternal notch view
Supplemental Video IIA: Postoperative echocardiogram, parasternal long axis
Supplemental Video IIB: Postoperative echocardiogram, parasternal short axis
Supplemental Video IIIB: Predischarge echocardiogram, parasternal long axis
Supplemental Video IF: Preoperative echocardiogram, subcostal view of Descending aorta
Supplemental Video IIIA: Predischarge echocardiogram, parasternal short axis