Abstract
Introduction:
Parental vaccine hesitancy can be a barrier to routine childhood immunization and contribute to greater risk for vaccine-preventable diseases. This study examines the impact of parental vaccine hesitancy on childhood vaccination rates.
Methods:
This study assessed the association of parental vaccine hesitancy on child vaccination coverage with ≥4 doses of diphtheria, tetanus toxoid, and acellular pertussis vaccine; ≥1 dose of measles, mumps, and rubella vaccine; up-to-date rotavirus vaccine; and combined 7-vaccine series coverage for a sample of children aged 19–35 months using data from the 2018 and 2019 National Immunization Survey-Child (N=7,645). Adjusted differences in multivariable analyses of vaccination coverage were estimated among vaccine hesitant and nonhesitant parents and population attributable risk fraction of hesitancy on undervaccination, defined as not being up to date for each vaccine.
Results:
Almost a quarter of parents reported being vaccine hesitant, with the highest proportion of vaccine hesitancy among parents of children who are non-Hispanic Black (37.0%) or Hispanic (30.1%), mothers with a high school education or less (31.9%), and households living below the poverty level (35.6%). Childhood vaccination coverage for all vaccines was lower for children of hesitant than nonhesitant parents, and the population attributable fraction of hesitancy on undervaccination ranged from 15% to 25%, with the highest percentage for ≥1 dose of measles, mumps, and rubella vaccine.
Conclusions:
Parental vaccine hesitancy may contribute up to 25% of undervaccination among children aged 19−35 months. Implementation of strategies to address parental vaccine hesitancy is needed to improve vaccination coverage for children and minimize their risk of vaccine-preventable diseases.
INTRODUCTION
The Advisory Committee on Immunization Practices (ACIP) recommends that children receive timely vaccinations against 14 potentially serious illnesses, including measles and pertussis, during the first 24 months of life.1 Recent studies in the U.S. show that only 44% of children were up to date with all ACIP-recommended vaccines by age 24 months in 2014; 23% were following alternate schedules and 14% were following unknown or unclassifiable patterns.2 Among children born in 2016 and 2017, only 70.5% had completed the combined 7-vaccine series (diphtheria, tetanus toxoid, and acellular pertussis vaccine [DTaP]; inactivated poliovirus vaccine; measles, mumps, and rubella vaccine [MMR]; Haemophilus influenza type b vaccine; hepatitis B vaccine; varicella vaccine; and pneumococcal conjugate vaccine) by age 24 months, which is well below the Healthy People 2020 target of 80%.3,4
Factors that may contribute to lower vaccination coverage include lack of access to vaccination services, missed opportunities for vaccination during healthcare visits, and vaccine hesitancy.5–14 Vaccine hesitancy is defined as a “delay in acceptance or the refusal of vaccination despite availability of vaccination services.”5 Vaccine hesitancy may contribute to parents modifying their children’s vaccination schedules by forgoing or delaying receipt of recommended vaccines.6–9 Lack of confidence in vaccines has led to undervaccination in several communities across the U.S. and contributed to outbreaks of measles and pertussis in recent years.10–14
A better understanding of how parents’ vaccine hesitancy beliefs are associated with decreased childhood vaccinations across different socioeconomic populations is an important step in developing tailored intervention strategies. The primary purpose of this study is to evaluate the association between parental vaccine hesitancy and childhood receipt of DTaP, rotavirus vaccine (RV), MMR, and combined 7-vaccine series. The secondary purpose is to examine differences in coverage owing to hesitancy by race/ethnicity and the population attributable fraction (PAF) of vaccine hesitancy on undervaccination of children.
METHODS
Study Sample
The National Immunization Surveys (NIS) are a group of surveys used to monitor routine vaccination coverage by age among children aged 19–35 months (NIS-Child), teens aged 13–17 years (NIS-Teen), and influenza vaccinations for children aged 6 months–17 years (NIS-Flu). This study uses data from NIS-Child, which is an annual random-digit-dial cellular telephone survey that monitors vaccines received by children aged 19–35 months in the 50 states, the District of Columbia, and some U.S. territories. The NIS is conducted in English and Spanish, if needed, by National Opinion Research Center interviewers. Otherwise, if another language is needed, respondents are connected to Language Line. The respondent is the person in the household who is most knowledgeable about the child’s vaccination history—the mother, father, or a relative (hereafter referred to as parents). Parents of eligible children were asked questions on sociodemographic characteristics of the household and to consent for NIS-Child to contact the child’s vaccination providers. Vaccination providers identified during the interview were mailed a questionnaire requesting the vaccination history from the child’s medical record, and vaccination coverage estimates were made based on provider-reported vaccination histories. Although the NIS-Child is an annual survey that is administered throughout the year, questions on vaccine hesitancy were only asked from April through June in the years 2018 and 2019. The overall Council of American Survey Research Organizations response rates for the 2018 and 2019 NIS-Child were 24.6% and 21.1%, respectively.15,16 This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and conducted in compliance with applicable federal law and CDC policy (e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501. et seq).
Measures
The association of parental vaccine hesitancy with vaccination coverage was assessed using a CDC-developed and previously validated 6-item questionnaire.17 These questions were validated as individual data-producing questions and not designed to be scaled up to a single metric and have been used in previous studies to examine vaccine hesitancy in association with childhood vaccination coverage.17–19 Interviewers asked parents 6 questions on their perceptions of childhood vaccinations (Appendix Table 1, available online).
The first question asked parents about adherence to the standard vaccination schedule: Is the child administered vaccines following a standard schedule, or some other schedule, such as the Sears Schedule? The response options were standard schedule or some other schedule. If needed, the respondents are told that the standard schedule is the vaccination schedule recommended by CDC and the American Academy of Pediatrics (AAP),1 and some other schedule refers to an alternative schedule that deviates from CDC- and AAP-recommended schedule. Although ACIP develops vaccine recommendations for children and adults and CDC sets the immunization schedules based on these recommendations, the term CDC was used in the questionnaire because it was more recognizable by most people.
The second item asked parents about overall vaccine hesitancy: Overall, how hesitant about childhood shots would you consider yourself to be? The response options were not at all hesitant, not that hesitant, somewhat hesitant, and very hesitant. This question was asked as a 4-level response because people could not commit to a dichotomous response of hesitant or not hesitant during cognitive testing. Responses for very hesitant and somewhat hesitant were combined and recoded as hesitant and responses for not that hesitant and not at all hesitant were recoded as not hesitant, because the difference between somewhat and very is not known.
The final 4 items asked parents about their perceptions toward vaccines, with yes and no as response options: Did concerns about the number of vaccines the child gets at one time impact your decision to get the child vaccinated?; Did concerns about serious, long-term side effects impact your decision to get the child vaccinated?; Do you personally know anyone who has had a serious, long-term side effect from a vaccine?; and Is the child’s doctor or health provider your most trusted source of information about childhood vaccines? Those who answered yes were considered to have the corresponding attitudes/beliefs.
Differences in coverage for children of hesitant and nonhesitant parents were estimated for DTaP ≥4 doses, MMR ≥1 dose, up-to-date RV (defined as ≥3 RV doses of any type or ≥2 Rotarix doses), and the combined 7-vaccine series by age of vaccination assessment, ranging from 19 to 35 months. Because RV was found in previous studies to have low coverage,4 it was examined to assess the impact of vaccine hesitancy on undervaccination. The combined 7-vaccine series (4:3:1:3:3:1:4) includes ≥4 doses of DTaP, ≥3 doses of inactivated poliovirus vaccine, ≥1 dose of MMR, the full series of Haemophilus influenza type b vaccine (≥3 or ≥4 doses, depending on product type), ≥3 doses of hepatitis B vaccine, ≥1 dose of varicella vaccine, and ≥4 doses of pneumococcal conjugate vaccine.
Interviewers asked parents about their child’s age, race/ethnicity, the relationship of the respondent to the child, the mother’s educational level, annual household income, and city and ZIP code of the household’s residence. Mother’s educational level was assessed because studies have identified mothers as the primary decision makers regarding childhood vaccinations, and it is used in weighting based on birth certificate data.20 Metropolitan statistical area (MSA) status (MSA principal city, MSA nonprincipal city, and non-MSA) was determined based on the city and county of the household’s residence.21 Households were classified as below the federal poverty level if their total family income was less than the federal poverty level specified for the applicable family size and number of children aged <18 years. All others were classified as at or above the poverty level.22
Statistical Analysis
Data from April to June in the years 2018 and 2019 were combined and analyzed in 2019 and 2020. Respondents from each survey were weighted to the general population and were found to have similar socioeconomic characteristics across both years.15,16 Weighted proportions of responses to each vaccine hesitancy variable were assessed overall and by sociodemographic characteristics. Differences in vaccination coverage by each parental vaccine hesitancy variable and sociodemographic differences were examined. Adjusted vaccination coverage differences and PAF were calculated from multivariable logistic regression models to determine the differences in ≥4 doses DTaP, ≥1 dose MMR, up-to-date RV, and combined 7-vaccine series coverage after adjusting for age, race/ethnicity, mother’s educational level, and poverty status. The adjusted PAF was calculated for each vaccine to assess the potential contribution of vaccine hesitancy to the observed undervaccination level. Undervaccination refers to not being up to date for DTaP, MMR, RV, or the combined 7-vaccine series and could include those who did not receive any doses of these vaccines.
PAF was calculated using the formula: p (rr − 1)/rr, where p is the proportion of hesitant individuals among the not-vaccinated group of individuals and rr denotes the relative risk comparing the proportion of those nonvaccinated among the hesitant group with the proportion of nonvaccinated among the nonhesitant group.23–25 The rr is obtained using a log-link regression model with undervaccination of each vaccine as the outcome measure and vaccine hesitancy as one of the covariates in the model. Vaccination coverage differences among hesitant and nonhesitant parents were analyzed for each vaccine by race/ethnicity.
All analyses were weighted to population totals to adjust for households having multiple cellular telephone lines, unit nonresponse, and noncoverage of noncellular telephone households.15,16 All estimates, along with 95% CIs, were calculated using SUDAAN, version 11.0.1 to account for the complex survey design. All differences were tested using 2-tailed t-tests with a significance level set at p<0.05. Only significant results (p<0.05) are described in the text.
RESULTS
For the 2018 NIS-Child, 6,336 parents were interviewed, and adequate provider data were collected for 52% (n=3,436) of interviewed parents. For the 2019 NIS-Child, 7,741 parents were interviewed and 49% (n=4,209) had adequate provider data. Overall, 23.6% of parents reported hesitancy toward child vaccinations (Table 1). Approximately one quarter reported being concerned about the number of vaccines the child receives at one time (24.3%) and serious, long-term side effects from vaccines (23.2%). In addition, 10.6% of parents reported personally knowing someone who had a serious, long-term side effect from a vaccine, 12.4% reported that their child’s doctor was not the most trusted source of information about childhood vaccines, and 5.3% were following some other vaccination schedule than those recommended by CDC and AAP. Parents who were hesitant or followed some other schedule were more likely to have concerns about the number of vaccines received at one time; have concerns about serious, long-term side effects; know someone with side effects from a vaccine; and see a doctor who is not the most trusted source of information about childhood vaccines (Appendix Table 2, available online).
Table 1.
Parental Vaccine Hesitancy and Association With Sociodemographic Characteristics, April–June 2018 and 2019, NIS-Child
| Sociodemographic characteristics | Hesitant about childhood vaccinations | Follow some other schedule | Concerned about the number of vaccines received at one time | Concerned about long-term serious side effects | Personally know someone with serious long-term side effect from a vaccine | Doctor is not the most trusted source of information about childhood vaccines |
|---|---|---|---|---|---|---|
| %(95% CI) | %(95% CI) | %(95% CI) | %(95% CI) | %(95% CI) | %(95% CI) | |
| Total | 23.6 (20.9, 26.3) | 5.3 (4.5, 6.1) | 24.3 (21.6, 27.0) | 23.2 (21.0, 25.4) | 10.6 (8.9, 12.3) | 12.4 (10.9, 13.9) |
| Child’s age, months | ||||||
| 19–23 (ref) | 24.9 (19.6, 31.1) | 4.9 (3.8, 6.4) | 25.9 (20.7, 32.0) | 20.3 (17.4, 23.5) | 9.5 (7.5, 12.0) | 9.8 (7.9, 12.1) |
| 24–29 | 23.1 (19.2, 27.5) | 5.6 (4.2, 7.4) | 23.1 (19.2, 27.6) | 22.8 (19.0, 27.0) | 11.7 (9.1, 14.9) | 15.2 (12.3, 18.6) |
| 30–35 | 22.8 (19.2, 26.9) | 5.4 (4.2, 6.9) | 23.9 (20.1, 28.3) | 26.0 (22.0, 30.4) | 10.6 (7.8, 14.4) | 12.2 (10.0, 14.8) |
| Child’s race/ethnicity | ||||||
| Non-Hispanic White (ref) | 16.4 (14.5, 18.5) | 5.4 (4.4, 6.7) | 19.3 (17.1, 21.7) | 20.4 (18.0, 22.9) | 11.0 (9.3, 13.0) | 13.8 (11.9, 15.8) |
| Non-Hispanic Black | 37.0 (31.2, 43.2) | 8.9 (6.1, 12.7) | 32.7 (27.1, 38.8) | 37.0 (31.3, 43.2) | 8.9 (6.2, 12.8) | 12.1 (8.7, 16.6) |
| Hispanic | 30.1 (23.0, 38.4) | 4.4 (3.1, 6.0) | 27.6 (20.5, 36.1) | 20.8 (15.8, 26.8) | 11.8 (7.7, 17.5) | 10.0 (7.0, 14.3) |
| Non-Hispanic Other | 23.3 (16.4, 32.0) | 3.4 (1.9, 6.0) | 27.1 (20.0, 35.5) | 24.4 (18.3, 31.7) | 8.7 (6.1, 12.2) | 12.7 (9.3, 17.0) |
| Relationship of respondent to child | ||||||
| Mother (ref) | 26.4 (23.4, 29.7) | 5.8 (4.9, 7.0) | 24.7 (21.7, 28.0) | 24.2 (21.4, 27.2) | 11.6 (9.6, 14.0) | 11.4 (9.7, 13.3) |
| Father | 15.4 (12.2, 19.2) | 4.6 (3.2, 6.5) | 23.1 (18.9, 27.9) | 22.2 (18.7, 26.1) | 7.1 (5.2, 9.6) | 14.4 (11.9, 17.5) |
| Other | 28.9 (18.1, 42.8) | 3.4 (1.6, 7.0) | 24.9 (14.7, 39.0) | 17.5 (11.5, 25.9) | 15.0 (9.0, 24.0) | 14.2 (8.8, 22.2) |
| Mother’s educational level | ||||||
| High school or less (ref) | 31.9 (26.8, 37.4) | 6.7 (5.2, 8.6) | 29.2 (24.0, 35.0) | 24.3 (20.7, 28.3) | 10.2 (7.7, 13.4) | 10.8 (8.6, 13.5) |
| Some college or college graduate | 26.6 (21.5, 32.5) | 4.9 (3.6, 6.5) | 26.9 (21.8, 32.7) | 31.8 (26.3, 37.9) | 14.4 (10.3, 19.8) | 16.7 (13.0, 21.3) |
| Education beyond a bachelor’s degree | 13.0 (11.0, 15.3) | 4.1 (3.2, 5.3) | 17.5 (15.4, 19.9) | 16.7 (14.6, 18.9) | 8.7 (7.3, 10.4) | 11.5 (9.9, 13.3) |
| MSA statusa | ||||||
| MSA, principal city (ref) | 25.7 (21.2, 30.7) | 4.8 (3.7, 6.1) | 26.1 (21.6, 31.2) | 21.1 (17.9, 24.7) | 8.8 (6.7, 11.6) | 10.8 (8.8, 13.2) |
| MSA, nonprincipal city | 20.3 (17.3, 23.6) | 5.5 (4.3, 7.0) | 22.2 (19.1, 25.7) | 23.9 (20.6, 27.5) | 12.1 (9.5, 15.1) | 13.7 (11.4, 16.3) |
| Non-MSA | 26.3 (21.9, 31.2) | 6.9 (4.8, 9.8) | 23.9 (19.9, 28.3) | 29.6 (25.0, 34.5) | 13.3 (10.3, 17.0) | 15.3 (12.1, 19.1) |
| Poverty statusb | ||||||
| Below poverty level (ref) | 35.6 (29.1, 42.7) | 6.5 (4.8, 8.8) | 31.1 (24.7, 38.4) | 28.4 (23.8, 33.5) | 10.8 (7.8, 14.9) | 11.9 (8.6, 16.1) |
| At or above poverty level | 18.5 (16.1, 21.2) | 4.6 (3.8, 5.6) | 22.1 (19.5, 25.0) | 21.4 (18.8, 24.2) | 10.7 (8.9, 12.8) | 12.9 (11.4, 14.6) |
Note: Boldface indicates statistical significance (p<0.05).
Significant values were obtained from t-test comparing each characteristic to the reference group.
MSA status was determined based on household-reported county of residence and was grouped into 3 categories: MSA principal city, MSA nonprincipal city, and non-MSA. MSA and principal city were as defined by the U.S. Census Bureau (https://www.census.gov/programs-surveys/metro-micro.html). Non-MSA areas include urban populations not located within an MSA as well as completely rural areas.
Households were classified as below the federal poverty level if their total family income was less than the federal poverty level specified for the applicable family size and number of children aged <18 years. All others were classified as at or above the poverty level. (https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html).
MSA, metropolitan statistical area; NIS, National Immunization Survey.
Hesitancy toward childhood vaccines was associated with child’s race/ethnicity, respondent relationship to the child, mother’s educational level, and the household’s poverty status (Table 1). Compared with non-Hispanic White populations (16.4%), a higher proportion of non-Hispanic Black (37.0%) and Hispanic (30.1%) populations were hesitant toward child vaccinations. Mothers (26.4%) were more likely to be hesitant than fathers (15.4%), and mothers who had less than high school education (31.9%) were more likely to be hesitant than mothers who had more than college education (13.0%). Parents living in households below the poverty level (35.6%) were more likely to be hesitant than those in households at or above poverty level (18.5%).
Child vaccination coverage was significantly lower among hesitant parents than nonhesitant parents for all vaccine series (Table 2 and Appendix Figure 1, available online). In adjusted multivariable models, the percentage point difference in vaccination coverage between hesitant and nonhesitant parents ranged from 7.9 for ≥1 dose of MMR to 16.3 for up-to-date RV (Table 2 and Appendix Figure 1, available online). PAF was highest for ≥1 dose of MMR (24.6%) and lowest for the combined 7-vaccine series (14.8%) (Figure 1). Those who followed an alternative vaccine schedule had a 30–percentage point difference in combined 7-series coverage compared with those who followed the ACIP- and AAP-recommended schedule (Table 2). Parents with other vaccine hesitancy concerns had differences in vaccine coverage ranging from 2.7 to 11.5 percentage points (Table 2).
Table 2.
Prevalence of Vaccination Coverage by Parental Hesitancy Response, April–June 2018 and 2019, NIS-Child
| Parental hesitancy variables | DTaP ≥4 doses | MMR ≥1 dose | Up-to-date RV (by 8 months)a | Combined 7-vaccine seriesb | ||||
|---|---|---|---|---|---|---|---|---|
| %(95% CI)c | Vaccination coverage difference(95% CI) | %(95% CI)c | Vaccination coverage difference(95% CI) | %(95% CI)c | Vaccination coverage difference(95% CI) | %(95% CI)c | Vaccination coverage difference(95% CI) | |
| Level of hesitancy | ||||||||
| Hesitant | 72.4 (66.9, 77.9) | −14.0 (−19.6, −8.5) | 85.8 (81.8, 89.8) | −7.9 (−12.4, −3.5) | 62.6 (56.1, 69.0) | −16.3 (−22.9, −9.7) | 61.0 (55.0, 67.0) | −15.9 (−22.1, −9.8) |
| Not hesitant | 86.5 (84.3, 88.6) | ref | 93.8 (92.1, 95.4) | ref | 78.8 (76.6, 81.1) | ref | 77.0 (74.6, 79.4) | ref |
| Follow some other schedule | ||||||||
| Yes | 58.5 (50.8, 66.2) | −25.7 (−33.4, −18.1) | 75.8 (69.2, 82.5) | −17.2 (−23.9, −10.6) | 50.0 (42.2, 57.9) | −26.5 (−34.5, −18.5) | 44.6 (36.9, 52.3) | −30.3 (−38.1, −22.4) |
| No | 84.3 (81.5, 87.0) | ref | 93.1 (91.5, 94.7) | ref | 76.5 (73.7, 79.4) | ref | 74.9 (72.0, 77.7) | ref |
| Concerns about the number of vaccines received at one time | ||||||||
| Yes | 75.3 (70.9, 79.7) | −9.4 (−14.0, −4.9) | 90.1 (87.3, 92.9) | −2.7 (−6.3, 0.9) | 66.2 (60.1, 72.4) | −11.3 (−17.7, −5.0) | 65.0 (59.4, 70.6) | −10.7 (−16.6, −4.9) |
| No | 84.7 (81.8, 87.6) | ref | 92.8 (90.8, 94.7) | ref | 77.6 (75.1, 80.0) | ref | 75.7 (73.0, 78.5) | ref |
| Concerns about serious long-term side effect from a vaccine | ||||||||
| Yes | 74.1 (67.5, 80.8) | −9.4 (−16.3, −2.5) | 85.9 (82.3, 89.6) | −7.7 (−11.5, −3.9) | 67.6 (62.5, 72.7) | −9.3 (−14.8, −3.8) | 65.7 (60.9, 70.6) | −9.4 (−14.5, −4.2) |
| No | 83.5 (80.8, 86.3) | ref | 93.7 (92.1, 95.2) | ref | 76.9 (73.9, 79.8) | ref | 75.1 (72.1, 78.1) | ref |
| Personal knowledge of someone who has had a serious long-term side effect from a vaccine | ||||||||
| Yes | 74.1 (67.5, 80.8) | −9.4 (−16.3, −2.5) | 81.6 (75.0, 88.1) | −11.5 (−18.1, −4.8) | 67.5 (59.5, 75.5) | −7.9 (−16.2, 0.5) | 65.3 (58.2, 72.4) | −8.4 (−15.8, −0.9) |
| No | 83.5 (80.8, 86.3) | ref | 93.0 (91.6, 94.5) | ref | 75.3 (72.5, 78.2) | ref | 73.6 (70.8, 76.5) | ref |
| Doctor is not the most trusted source of information about childhood vaccines | ||||||||
| Yes | 74.7 (68.7, 80.7) | −9.0 (−14.9, −3.0) | 82.7 (77.0, 88.5) | −10.4 (−16.2, −4.6) | 69.3 (63.8, 74.9) | −6.1 (−12.0, −0.2) | 65.1 (58.9, 71.2) | −9.0 (−15.3, −2.6) |
| No | 83.7 (80.9, 86.4) | ref | 93.1 (91.7, 94.5) | ref | 75.4 (72.5, 78.3) | ref | 74.1 (71.2, 76.9) | ref |
Note: Boldface indicates statistical significance (p<0.05).
Up to date defined as ≥3 rotavirus doses of any type or ≥2 Rotarix doses.
The combined 7-vaccine series (4:3:1:3*: 3:1:4) includes ≥4 doses of DTaP, ≥3 doses of IPV, ≥1 dose of MMR, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
Multivariable model controlling for child’s age group, race/ethnicity, mother’s educational level, and household poverty level.
DTaP, diphtheria, tetanus toxoid, and acellular pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; IPV, inactivated polio vaccine; MMR, measles; mumps, and rubella vaccine; NIS, National Immunization Survey; PCV, pneumococcal conjugate vaccine; RV, rotavirus vaccine; VAR, varicella vaccine.
Figure 1.
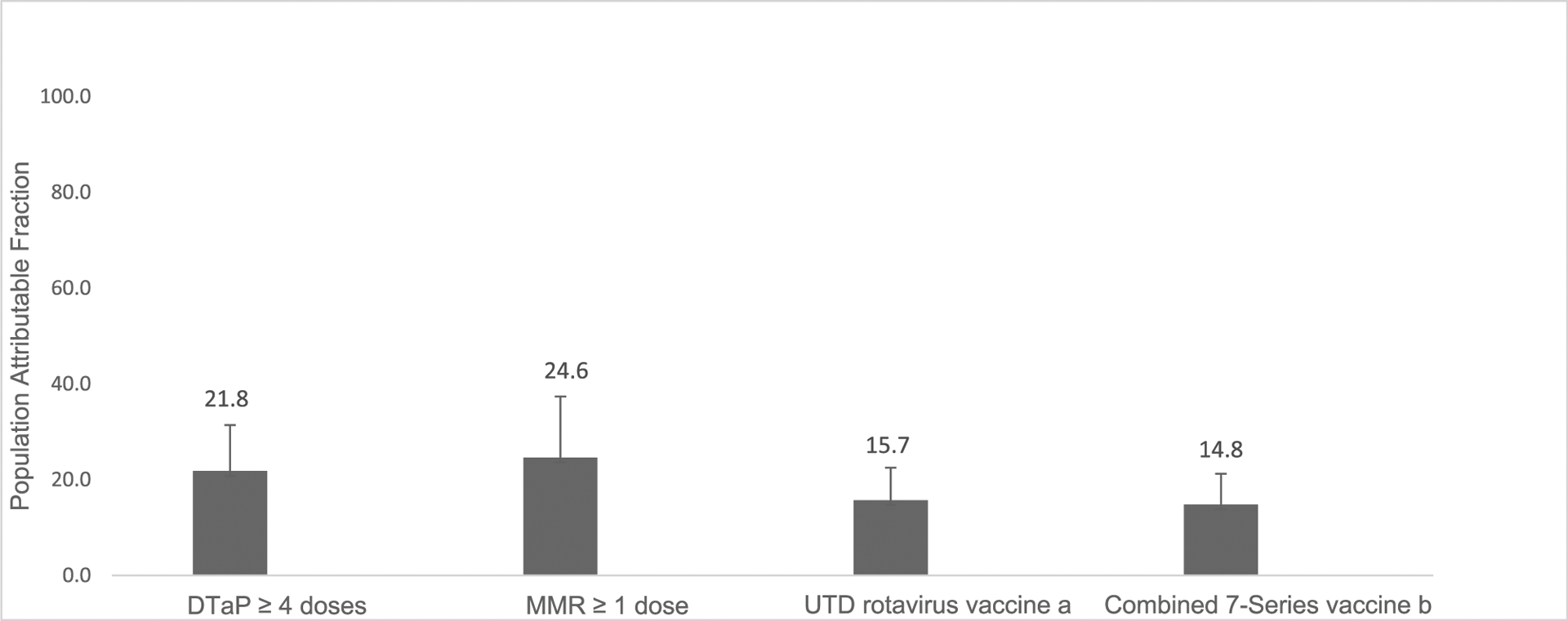
Population attributable fraction of vaccine hesitancy on undervaccination, April–June 2018 and 2019, NIS-Child.
aUTD defined as ≥3 rotavirus doses of any type or ≥2 Rotarix doses.
bThe combined 7-vaccine series (4:3:1:3*: 3:1:4) includes ≥4 doses of DTaP, ≥3 doses of IPV, ≥1 dose of MMR, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
DTaP, diphtheria, tetanus toxoid, and acellular pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; IPV, inactivated polio vaccine; MMR, measles, mumps, and rubella vaccine; NIS, National Immunization Survey; PCV, pneumococcal conjugate vaccine; UTD, up-to-date; VAR, varicella vaccine.
Overall, differences in vaccine coverage between hesitant and nonhesitant parents were highest for Hispanic (15.5%–21.0%) and non-Hispanic White populations (11.4%–19.0%), whereas coverage was not statistically different for non-Hispanic Black populations and non-Hispanic other populations (Table 3). Among non-Hispanic White and Hispanic populations, differences were highest for the combined 7-vaccine series.
Table 3.
Prevalence of Vaccination Coverage by Race/Ethnicity Among Hesitant and Nonhesitant Parents, April–June, 2018 and 2019, NIS-Child
| Vaccines | Non-Hispanic White (n=4,453) | Non-Hispanic Black (n=545) | Hispanic (n=l,579) | Non-Hispanic Other (n=l,003) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Vaccinated among hesitant | % Vaccinated among nonhesitant | Vaccination coverage difference (95% Cl)a | % Vaccinated among hesitant | % Vaccinated among nonhesitant | Vaccination coverage difference (95% Cl)a | % Vaccinated among hesitant | % Vaccinated among nonhesitant | Vaccination coverage difference (95% Cl)a | % Vaccinated among hesitant | % Vaccinated among nonhesitant | Vaccination coverage difference (95% Cl)a | |
| DTaP ≥4 doses | 70.1 (64.2, 76.0) | 88.0 (85.3, 90.6) | −17.8 (−23.9, 11.8) | 74.4 (65.7, 83.1) | 82.5 (76.8, 88.1) | −8.1 (−18.1, 2.0) | 67.6 (51.9, 83.2) | 83.1 (77.4, 88.7) | −15.5 (−30.8, −0.1) | 84.5 (74.8, 94.3) | 89.7 (85.9, 93.4) | −5.1 (−16.2, 5.9) |
| MMR ≥1 | 83.6 (79.4, 87.7) | 95.0 (93.2, 96.7) | −11.4 (15.7, −7.1) | 87.1 (79.8, 94.4) | 90.9 (86.6, 95.1) | −3.8 (−12.1, 4.6) | 85.4 (75.7, 95.0) | 91.7 (86.7, 96.6) | −6.3 (−17.2, 4.6) | 89.5 (81.9, 97.1) | 95.0 (92.5, 97.4) | −5.5 (−13.8, 2.9) |
| Up-to-date RV (by 8 months)b | 61.8 (55.3, 68.2) | 80.6 (77.6, 83.6) | −18.8 (−25.9, −11.8) | 61.8 (50.9, 72.6) | 68.7 (61.8, 75.6) | −7.0 (−19.8, 5.8) | 60.1 (42.5, 77.8) | 79.8 (75.5, 84.1) | −19.7 (−37.4, −1.9) | 68.7 (53.1, 84.4) | 79.0 (72.9, 85.1) | −5.5 (−13.8, 2.9) |
| Combined 7-vaccine seriesc | 59.8 (53.4, 66.2) | 78.8 (75.9, 81.7) | −19.0 (−25.9, −12.1) | 62.3 (52.0, 72.5) | 70.3 (63.6, 77.0) | −8.0 (−20.1, 4.0) | 53.8 (39.4, 68.1) | 74.8 (68.8, 80.9) | −21.0 (−35.1, −7.0) | 74.4 (61.8, 87.0) | 79.8 (74.5, 85.1) | −5.3 (−19.2, 8.5) |
Note: Boldface indicates statistical significance (p<0.05).
Multivariable model controlling for child’s age group, mother’s educational level, and household poverty level.
Up to date defined as ≥3 RV doses of any type or ≥2 Rotarix doses.
The combined 7-vaccine series (4:3:1:3*: 3:1:4) includes ≥4 doses of DTaP, ≥3 doses of IPV, ≥1 dose of MMR, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
DTaP, diphtheria, tetanus toxoid, and acellular pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; IPV, inactivated polio vaccine; MMR, measles; mumps, and rubella vaccine; NIS, National Immunization Survey; PCV, pneumococcal conjugate vaccine; RV, rotavirus vaccine; VAR, varicella vaccine.
DISCUSSION
More than a quarter of surveyed parents were hesitant about vaccinating their children aged 19–35 months, and their vaccine hesitancy might have contributed to 15%–25% of undervaccination for their children. In general, vaccine hesitancy was highest among parents of non-Hispanic Black and Hispanic children, mothers with lower education, and households living below the federal poverty level. This is similar to other studies that have found disparities in race/ethnicity with respect to vaccine hesitancy and lower childhood vaccination coverage.18,19,26 Approximately 11% of parents indicated that they know someone with a serious, long-term side effect from a vaccine, which suggests that these beliefs may be based on their perceptions of risk rather than true events. Vaccine hesitancy, in addition to other barriers to vaccination, may increase the burden of vaccine-preventable diseases among populations that are already disproportionately affected by poor health outcomes.
Vaccine hesitancy is associated with lower coverage for all childhood vaccines assessed in the study, from a difference of 8 percentage points for ≥1 dose of MMR to 17 percentage points for up-to-date RV. Similarly, other studies have found lower vaccination coverage for influenza vaccine and human papillomavirus vaccine among children of hesitant parents compared with nonhesitant parents.18,19,27 Differences in vaccination coverage among hesitant and nonhesitant parents were highest among Hispanic and non-Hispanic White populations, suggesting that there are racial/ethnic disparities in the association between hesitancy and vaccination coverage, and tailored messages are needed to address hesitancy in these populations.
The PAF of hesitancy on undervaccination is highest for ≥1 dose of MMR, demonstrating that almost 25% of undervaccination in children is associated with parental vaccine hesitancy. These data suggest that parental vaccine hesitancy may have contributed to undervaccination and nonvaccination that led to multiple outbreaks of measles in several communities across the U.S.6 In the U.S., only 20 states have ≥90% vaccination coverage for 1 dose of MMR for children by age 2 years.4 In 2019, there were 1,282 cases of measles reported in the U.S., the highest reported number since 1992, and the U.S. almost lost its measles elimination status.28,29 Most of these measles cases occurred among people who were intentionally unvaccinated and in communities with low vaccination coverage rates.30 Low community vaccination rates can lead to disease outbreaks that could have been prevented.
WHO recently reported that global vaccination coverage with 2 doses of the measles vaccine has decreased to less than the 95% threshold needed for herd immunity.31 In 2019, the number of measles cases globally rose by 30%, even in countries where measles had been eliminated.31 Erosion of public confidence in the use of vaccines and increasing spread of misinformation and disinformation on vaccine safety prompted WHO to declare vaccine hesitancy one of the 10 greatest threats to global health.32
Among parents of undervaccinated children, vaccine hesitancy is an important factor, but there are other factors that contribute to low vaccination coverage rates. To increase access to vaccines, immunization programs can increase awareness of the Vaccines for Children program, which provides recommended vaccines at no cost to children aged ≤18 years who are Medicaid-eligible, uninsured, American Indian/Alaska Native, or insured by health plans that do not fully cover all routine immunizations. Providers can also improve vaccination coverage by administering all recommended vaccines during the same office visit, addressing any concerns or misinformation from parents during office visits, and using other evidence-based strategies for improving vaccination coverage.33 These strategies may include notifying parents when children are due for a vaccination, establishing standing orders or policies that allow nonphysician personnel to administer vaccines, and enhancing computerized immunization information systems for tracking vaccinations.33–37 For racial and ethnic groups that may be more hesitant, tailored messages to address specific concerns and misinformation and physician recommendation of vaccines may increase vaccine confidence and uptake.
The following strategies are recommended by CDC to reduce vaccine hesitancy and strengthen vaccine confidence: (1) identify undervaccinated communities, (2) empower families in their decision to vaccinate by strengthening provider–parent vaccine conversations, and (3) address myths and misinformation.38 Public health partners should work together to identify undervaccinated communities using surveillance tools and vaccine coverage monitoring systems, characterize populations at risk for undervaccination, and use science-based strategies tailored for the population to promote vaccination, while continuing to remove barriers to vaccine access. In addition, healthcare providers should have access to appropriate resources to initiate early vaccine conversations with parents of young children and with pregnant women.39 Finally, overcoming myths and misinformation on vaccines requires educating the public and policymakers about vaccines and engaging trusted messengers to repeatedly share accurate and easily understandable information.
Limitations
The findings in this report are subject to several limitations. First, bias in estimates might remain even after weighting for household and provider nonresponse and noncoverage. Second, the vaccine hesitancy questions were only asked in the NIS-Child for 3 months in 2018 and in 2019. The small sample size among non-Hispanic Black and non-Hispanic other racial groups may explain why no significant differences in PAF were found in these groups. Third, the survey asked about hesitancy toward vaccines in general and not specifically about any particular vaccine or vaccine series. Fourth, the question on vaccine hesitancy may not fully capture the true prevalence of vaccine hesitancy in the population owing to social desirability bias or nonresponse bias. Fifth, the outcome in this study was undervaccination, but other ways of calculating nonvaccination could affect the results. Sixth, data are from a cross-sectional survey and the PAF estimates are only an approximation of the true causal relationship between hesitancy and vaccination. Finally, although the results were significant at the p<0.05 level, the number of significant results may be overestimated owing to multiple comparisons.
CONCLUSIONS
Routine vaccinations for many children may have been impacted by the coronavirus disease 2019 (COVID-19) pandemic, owing to disruptions in routine medical care and stay-at-home orders.40 Although the delivery of recommended childhood vaccines may have recovered after the initial impact of the pandemic, continued efforts should be made to ensure that children continue receiving life-saving vaccines. Increasing coverage involves addressing hesitancy and access barriers to vaccination. Countering vaccine hesitancy is critical to strengthen trust in vaccines among parents and maintain a culture that recognizes the continuing value of vaccines to prevent diseases.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Akhil Vaish from Research Triangle Institute, for his help in the review of the population attributable risk calculations in this study, and Paul Scanlon, from the National Center for Health Statistics, for his contributions to the development of the vaccine hesitancy questions.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
No financial disclosures were reported by the authors of this paper.
Footnotes
CREDIT AUTHOR STATEMENT
Kimberly H. Nguyen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing - original draft; Writing - review and editing. Anup Srivastav: Data curation; Formal analysis; Methodology; Software; Validation; Writing -review and editing. Megan C. Lindley: Methodology; Writing - review and editing. Allison Fisher: Methodology; Writing - review and editing. David Kim: Methodology; Writing - review and editing. Stacie M. Greby: Conceptualization; Methodology; Resources; Validation; Writing - review and editing. James Lee: Formal analysis; Software; Validation; Writing - review and editing. James A. Singleton: Conceptualization; Funding acquisition; Resources; Supervision; Writing - review and editing.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.08.015.
REFERENCES
- 1.Robinson CL, Bernstein H, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for children and adolescents aged 18 years or younger - United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(5):112–114. 10.15585/mmwr.mm6805a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hargreaves AL, Nowak G, Frew P, et al. Adherence to timely vaccinations in the United States. Pediatrics. 2020;145(3):e20190783. 10.1542/peds.2019-0783. [DOI] [PubMed] [Google Scholar]
- 3.Immunization and infectious diseases. Healthy People 2020, HHS, Office of Disease Prevention and Health promotion. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Updated October 21, 2021. Accessed October 27, 2021. [Google Scholar]
- 4.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination coverage by age 24 months among children born in 2016 and 2017 - National Immunization Survey-Child, United States, 2017–2019. MMWR Morb Mortal Wkly Rep. 2020;69(42):1505–1511. 10.15585/mmwr.mm6942a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler R Vaccine Hesitancy: what it means and what we need to know in order to tackle it. Geneva, Switzerland: WHO. https://www.who.int/immunization/research/forums_and_initiatives/1_RButler_VH_-Threat_Child_Health_gvirf16.pdf. Accessed September 14, 2021. [Google Scholar]
- 6.Kennedy A, Lavail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011;30(6):1151–1159. 10.1377/hlthaff.2011.0396. [DOI] [PubMed] [Google Scholar]
- 7.Bedford H, Attwell K, Danchin M, Marshall H, Corben P, Leask J. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine. 2018;36(44):6556–6558. 10.1016/j.vaccine.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau JA, Bednarczyk RA, Masawi MR, et al. Vaccinating my way –use of alternative vaccination schedules in New York State. J Pediatr. 2015;166(1):151–156. 10.1016/j.jpeds.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Wallace AS, Mantel C, Mayers G, Mansoor O, Gindler JS, Hyde TB. Experiences with provider and parental attitudes and practices regarding the administration of multiple injections during infant vaccination visits: lessons for vaccine introduction. Vaccine. 2014;32(41):5301–5310. 10.1016/j.vaccine.2014.07.076. [DOI] [PubMed] [Google Scholar]
- 10.Mbaeyi S, Cohn A, Messonnier N. A call to action: strengthening vaccine confidence in the United States. Pediatrics. 2020;145(6): e20200390. 10.1542/peds.2020-0390. [DOI] [PubMed] [Google Scholar]
- 11.Atwell JE, Salmon DA. Pertussis resurgence and vaccine uptake: implications for reducing vaccine hesitancy. Pediatrics. 2014;134 (3):602–604. 10.1542/peds.2014-1883. [DOI] [PubMed] [Google Scholar]
- 12.Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vaccin Immunother. 2013;9(8):1755–1762. 10.4161/hv.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33(suppl 4): D66–D71. 10.1016/j.vaccine.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui M, Salmon DA, Omer SB. Epidemiology of vaccine hesitancy in the United States. Hum Vaccin Immunother. 2013;9(12):2643–2648. 10.4161/hv.27243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Immunization Survey-Child: a user’s guide for the 2018 public-use data file. Atlanta, GA: Centers for Disease Control and Prevention; January 2020. https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NISPUF18-DUG.pdf. Published January 2020. Accessed January 18, 2020. [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Immunization Survey-Child: a user’s guide for the 2019 public-use data file. Atlanta, GA: Centers for Disease Control and Prevention; December 2020. https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-PUF19-DUG.pdf. Published December 2020 Accessed March 28, 2020. [Google Scholar]
- 17.Scanlon P, Jamoom E. The cognitive evaluation of survey items related to vaccine hesitance and confidence for inclusion on a series of short question sets. Atlanta, GA: Centers for Disease Control and Prevention. https://wwwn.cdc.gov/QBank/report/Scanlon_NCHS_2019_VAX.pdf. Published June 2019. Accessed May 5, 2020. [Google Scholar]
- 18.Santibanez TA, Nguyen KH, Greby SM, et al. Parental vaccine hesitancy and childhood influenza vaccination. Pediatrics. 2020;146(6): e2020007609. 10.1542/peds.2020-007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen KH, Santibanez TA, Stokley S, et al. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine. 2021;39(17):2416–2423. 10.1016/j.vaccine.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matoff-Stepp S, Applebaum B, Pooler J, Kavanagh E. Women as health care decision-makers: implications for health care coverage in the United States. J Health Care Poor Underserved. 2014;25(4):1507–1513. 10.1353/hpu.2014.0154. [DOI] [PubMed] [Google Scholar]
- 21.Metropolitan and micropolitan. U.S. Census Bureau. https://www.census.gov/programs-surveys/metro-micro.html. Updated October 8, 2021. Accessed October 27, 2021.
- 22.Poverty thresholds. U.S. Census Bureau. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html. Updated October 8, 2021. Accessed October 27, 2021.
- 23.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York, NY: John Wiley & Sons, 1982. [Google Scholar]
- 24.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions [published correction appears in Am J Public Health. 2008;98(12):2119]. Am J Public Health. 1998;88(1):15–19. 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaish A, Khajvou O. Confidence intervals for population attributable fractions. Alexandria, VA: American Statistical Association. JSM -Survey Research Methods Section; 2017. http://www.asasrms.org/Proceedings/y2017/files/593887.pdf. Published 2017. Accessed November 6, 2020. [Google Scholar]
- 26.Williams JTB, Rice JD, Lou Y, et al. Parental vaccine hesitancy and vaccination disparities in a safety-net system. Pediatrics. 2021;147(2): e2020010710. 10.1542/peds.2020-010710. [DOI] [PubMed] [Google Scholar]
- 27.Kempe A, Saville AW, Albertin C, et al. Parental hesitancy about routine childhood and influenza vaccinations: a national survey. Pediatrics. 2020;146(1):e20193852. 10.1542/peds.2019-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Measles cases and outbreaks. Centers for Disease Control and Prevention. https://www.cdc.gov/measles/cases-outbreaks.html. Updated October 21, 2021. Accessed October 27, 2021.
- 29.Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks-United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(40):893–896. 10.15585/mmwr.mm6840e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zucker JR, Rosen JB, Iwamoto M, et al. Consequences of undervaccination - measles outbreak, New York City, 2018–2019. N Engl J Med. 2020;382(11):1009–1017. 10.1056/NEJMoa1912514. [DOI] [PubMed] [Google Scholar]
- 31.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17. 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Ten threats to global health in 2019. Geneva, Switzerland: WHO. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed August 3, 2020. [Google Scholar]
- 33.Vaccination. The Community Guide. https://www.thecommunityguide.org/topic/vaccination. Accessed September 5, 2020.
- 34.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149–207. 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- 35.Szilagyi PG, Albertin C, Humiston SG, et al. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204–213. 10.1016/j.acap.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011;40(5):548–555. 10.1016/j.amepre.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Rao S, Fischman V, Kaplan DW, Wilson KM, Hyman D. Evaluating interventions to increase influenza vaccination rates among pediatric inpatients. Pediatr Qual Saf. 2018;3(5):e102. 10.1097/pq9.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Building confidence in COVID-19 vaccines: vaccinate with confidence: strategy to reinforce confidence in COVID-19 vaccines. Atlanta, GA: Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/covid-19/vaccinate-with-confidence.html. Updated October 26, 2021. Accessed October 27, 2021. [Google Scholar]
- 39.Making a strong flu vaccine recommendation. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/professionals/vaccination/flu-vaccine-recommendation.htm. Updated September 14, 2021. Accessed October 27, 2021.
- 40.Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 (19):591–593. 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


