Abstract
Cellular stress induced by the abnormal accumulation of unfolded or misfolded proteins at the endoplasmic reticulum (ER) is emerging as a possible driver of human diseases, including cancer, diabetes, obesity and neurodegeneration. ER proteostasis surveillance is mediated by the unfolded protein response (UPR), a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or, in the event of chronic damage, induce apoptotic cell death. Recent advances in the understanding of the regulation of UPR signalling and its implications in the pathophysiology of disease might open new therapeutic avenues.
Introduction
The secretory pathway is responsible for the synthesis of one third of all eukaryotic cell proteins, their post-translational modification and assembly into complexes, and their delivery to precise destinations within the cell or their release into the extracellular space. Proteins enter the secretory pathway by translocation from the cytosol into the endoplasmic reticulum (ER) in an unfolded state, where they undergo chaperone-assisted folding to acquire their appropriate 3D conformation. Unlike DNA replication, transcription and translation, protein folding is a highly error-prone process1. Thus, maintenance of a healthy proteome depends on complex quality control mechanisms, some of which operate at the level of the ER to promote efficient protein folding and trafficking.
ER homeostasis is constantly challenged by physiological demands and pathological insults, impacting its multiple functions in the cell as a Ca2+ reservoir, a factory for protein folding and assembly, a site for lipid and sterol biosynthesis, and as a platform for signalling and interorganelle communication. Increased protein secretion or disrupted ER protein folding can cause accumulation of unfolded or misfolded proteins in the ER lumen — a condition referred to as ‘ER stress’. To ensure protein folding fidelity and to maintain ER functions, the unfolded protein response (UPR) of eukaryotic cells evolved to a network of signal transduction pathways to reprogramme gene transcription, mRNA translation and protein modifications to relieve the load of unfolded or misfolded proteins and restore protein homeostasis (proteostasis2) (Fig. 1).
Fig. 1: The major UPR pathways initiated from the ER.
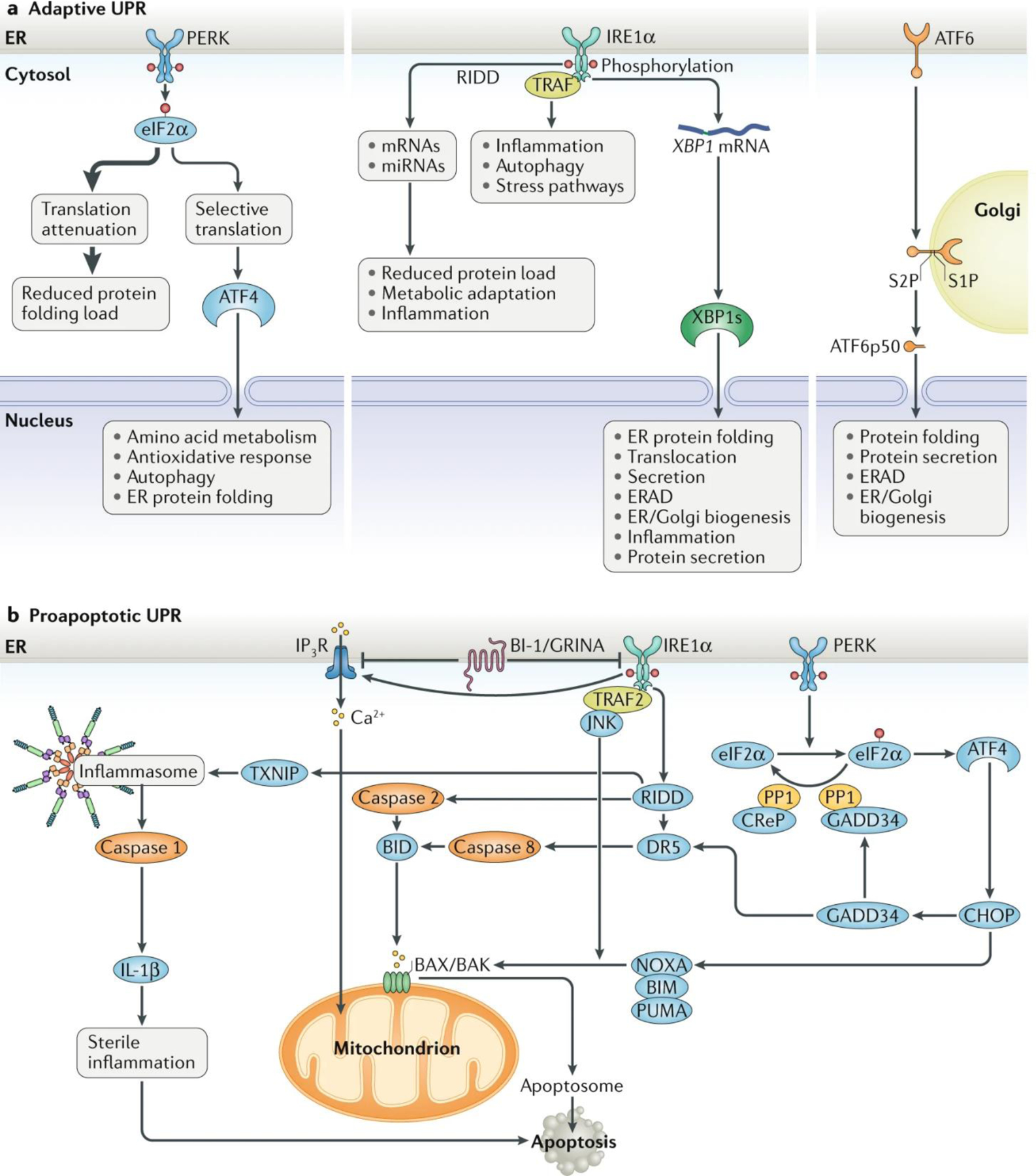
a | Adaptive unfolded protein response (UPR). Under endoplasmic reticulum (ER) stress, three major UPR branches are activated: (1) PERK phosphorylates eukaryotic translation initiation factor 2 subunit-α (eIF2α), reducing the overall frequency of mRNA translation initiation. However, selective mRNAs, such as ATF4 mRNA, are preferentially translated in the presence of phosphorylated eIF2α. ATF4 activates the transcription of UPR target genes encoding factors involved in amino acid biosynthesis, the antioxidative response, autophagy and apoptosis. (2) IRE1α RNase splices XBP1 mRNA, which encodes a potent transcription factor that activates expression of UPR target genes involved in ER proteostasis and cell pathophysiology. IRE1α RNase can also cleave ER-associated mRNAs or non-coding functional RNAs, leading to their degradation through regulated IRE1-dependent decay (RIDD), which modulates the protein folding load, cell metabolism, inflammation and inflammasome signalling pathways. The IRE1α cytosolic domain may also serve as a scaffold to recruit adaptor proteins, for example tumour necrosis factor receptor-associated factor (TRAF) family members, thereby activating inflammatory responses under non-canonical ER stress conditions. (3) ATF6 transits from the ER to the Golgi apparatus, where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P), yielding an active cytosolic ATF6 fragment (ATF6p50). This fragment migrates to the nucleus, activating transcription of the UPR target genes involved in ER protein folding homeostasis and cell physiology. Additionally, unfolded or misfolded proteins accumulated in the ER lumen may be degraded through the proteasome-based ER-associated protein degradation (ERAD) machinery that is regulated by the ATF6-mediated and/or IRE1α–X-box-binding protein 1 (XBP1)-mediated UPR branches. b | Proapoptotic UPR. Under ER stress, the PERK–eIF2α UPR branch induces translation of ATF4, which can activate expression of the proapoptotic factor CCAAT/enhancer-binding protein homologous protein (CHOP) and GADD34. GADD34 targets protein phosphatase 1 (PP1) to dephosphorylate eIF2α and thereby restore mRNA translation. Constitutive repressor of eIF2α phosphorylation (CReP) also serves as a cofactor to provide PP1 specificity for phosphorylated eIF2α under ER stress. CHOP promotes ER stress-induced apoptosis by modulating GADD34, death receptor 5 (DR5) and the members of the BCL-2 or BH3-only family, including NOXA, BIM and PUMA, to stimulate protein synthesis and exacerbating protein folding defect. Furthermore, the IRE1α UPR branch is involved in caspase 2-dependent, caspase 8-dependent or BAX/BAK-dependent apoptosis through RIDD or activation of TRAF2–JUN N-terminal kinase (JNK) signalling. The IRE1α-mediated RIDD also regulates thioredoxin-interacting protein (TXNIP) to activate inflammasome-dependent and caspase 1–IL-1β-dependent sterile inflammation, leading to apoptosis. In addition, Ca2+ release from the ER via inositol 1,4,5-trisphosphate receptor (IP3R), which interacts with the ER-located antiapoptotic proteins BAX inhibitor 1 (BI-1) and GRINA, contributes to mitochondrial reactive oxygen species release and the activation of the BAX/BAK-dependent apoptosome. miRNA, microRNA.
The UPR orchestates the enforcement of adaptive mechanisms to maintain an optimal rate of protein production and rapidly reacts to diverse stimuli, including extracellular responses to hormones, growth factors and small ligands that bind cell-surface receptors; intracellular homeostatic changes such as altered nutrient levels, energy status and redox balance; changes in cellular growth and differentiation; and disruption in the ER protein folding capacity. Activation of the UPR impacts almost every aspect of the secretory pathway, modifying the rate of protein synthesis and translocation into the ER, protein folding, maturation and quality control, protein trafficking and the elimination of misfolded proteins through the autophagy and ER-associated protein degradation (ERAD) pathways.
Here we review salient and unique features of the UPR uncovered in the past few years. We first describe how protein misfolding in the ER is sensed through the three conserved signal transducers to preserve ER functions vital for cell survival. We then give an overview of the consequences of misfolded protein accumulation by discussing recently discovered mechanisms that regulate apoptosis in cells experiencing prolonged ER stress. Recently, it has become evident that the UPR has essential cell functions beyond ER proteostasis, and therefore we discuss how the UPR controls organelle interactions, bioenergetics, cytoskeletal dynamics, the DNA damage response and cell signalling crosstalk at both the cell-autonomous and nonautonomous level. Finally, we review the emerging roles of the UPR in the pathogenesis of diseases, including metabolic syndromes, cancer, immunological disorders and neurodegenerative conditions.
ER Stress and the three UPR branches
Initially discovered in yeast (Box 1), the basic UPR pathways in mammals consist of three main signalling cascades initiated by the ER transmembrane protein sensors: IRE1α, PERK and ATF6α3. These signal-transducing proteins contain ER luminal domains that sense unfolded protein peptides (see later) and cytosolic regions that signal through the translational or transcriptional apparatus or by interacting with signalling molecules as scafold to protect cells from ER stress under physiological conditions. Here we discuss fundamental aspects of UPR signalling and the consequences that determine cell fate under ER stress. Owing to the length restriction, we are unable to acknowledge many contributions, but refer to reviews for historical or background information3,4.
Box 1. Discovery of the UPR.
Numerous independent findings converged into the discovery of the tripartite mammalian unfolded protein response (UPR).
In 1977, it was shown that glucose depletion in Rous sarcoma virus-transformed fibroblasts activated a specific set of genes, the products of which were termed ‘glucose-regulated proteins’ (GRPs)225.
In 1983, an endoplasmic reticulum (ER) protein, which was named ‘binding immunoglobulin protein’ (BiP), was found to bind immunoglobulin heavy chains in pre-B lymphocytes before immunoglobulin light chains were expressed226.
In the mid 1980s, BiP and GRP78 were found to be the same protein, a protein localized to the ER lumen and related to 70-kDa heat shock protein (HSP70)227.
In 1989, it was found that factors that inhibit cell growth and induce DNA damage activate genes encoding proteins termed ‘growth arrest and DNA damage-inducible proteins’ (GADD proteins), many of which are GRPs228.
At the same time, expression of a mutant influenza haemagglutinin that cannot fold and expression of an endogenous secreted protein prone to misfolding in the ER were shown to induce GRPs229,230. Moreover, the binding of misfolded proteins to BiP was associated with GRP gene induction230.
Together these studies showed that glucose deprivation-induced proteins (GRP gene induction) are the same as those proteins induced by accumulation of unfolded proteins in the ER (UPR gene induction) and connected the dots between protein misfolding and energy starvation. The findings provided evidence for the existence of signalling mechanisms that sense the accumulation of misfolded proteins in the ER that lead to the activation of genes among which many encode ER-resident proteins.
Yeast genetics studies identified the most conserved UPR pathway as inositol-requiring 1 (Ire1p)-mediated splicing of HAC1 (homologue of ATF and CREB) mRNA to produce a functional transcription factor for UPR gene induction231,232,233.
In 1993, the mammalian homologues of yeast Ire1p were identified as ERN1 (also known as IRE1α), which is ubiquitously expressed, and ERN2 (also known as IRE1β) with intestinal and lung epithelial cell-restricted expression234,235.
In 2001, the metazoan Hac1 homologue was identified as X-box-binding protein 1 (XBP1), for which IRE1α initiates unconventional XBP1 mRNA splicing to produce a functional transcription factor17,18,19. XBP1 was discovered as a transcription factor in B cells more than 10 years before it was identified as a target of IRE1α236.
In 1998–1999, two additional UPR transducers were identified as double-stranded RNA-activated protein kinase-like ER kinase (PERK) and activating transcription factor 6 (ATF6)9,237,238 in metazoans. The tripartite UPR restores ER proteostasis by modulating the expression of genes involved in most aspects of the secretory pathway.
UPR signalling through PERK
An immediate adaptive reaction to ER stress is initiated by PERK, a kinase that phosphorylates eukaryotic translation initiation factor 2 subunit-α (eIF2α), leading to the transient attenuation of protein synthesis3,4 (Fig. 1a). This reversible covalent modification limits the protein misfolding load by preventing the influx of newly synthesized proteins into the ER. Concomitantly, phosphorylated eIF2α initiates the translation of a growing set of specific mRNAs that harbour one or more upstream open reading frames in their 5′ untranslated regions5,6,7,8. One of these encodes ATF4, a stress-inducible transcription factor that activates the expression of genes involved in redox homeostasis, amino acid metabolism, protein synthesis, apoptosis and autophagy. ATF4 participates in a feedback loop to dephosphorylate eIF2α to restore protein synthesis through upregulation of the protein phosphatase 1 (PP1) regulatory subunit GADD34 (9,10,11) (Fig. 1b). During ER stress, GADD34 forms a complex with PP1 to dephosphorylate eIF2α12,13. Similarly to GADD34, expression of constitutive repressor of eIF2α phosphorylation (CReP), which serves as a PP1 cofactor, confers PP1 specificity for phosphorylated eIF2α. GADD34 and CReP are essential for recovery of protein synthesis as ER stress is resolved14.
UPR signalling through IRE1α
IRE1α, a type 1 ER transmembrane protein kinase/endoribonuclease, oligomerizes and autophosphorylates to elicit its RNase activity under ER stress15,16. IRE1α excises a small 26-nucleotide intron from the mRNA encoding the transcription factor X-box-binding protein 1 (XBP1) in metazoans and thereby shifts the translational open reading frame17,18,19. This processing event results in the expression of an active XBP1 transcription factor (termed ‘XBP1s’ in metazoans for the spliced form) that upregulates genes involved in ER protein translocation, folding and secretion, as well as degradation of misfolded proteins3 (Fig. 1a).
In a process known as regulated IRE1-dependent decay (RIDD), IRE1α can also cleave a small set of mRNAs or precursor microRNAs (miRNAs), leading to their degradation20,21,22,23. RIDD may serve as an avenue to lower mRNA abundance and hence protein folding load in the ER. While the quantitative impact of RIDD on ER protein folding homeostasis remains to be determined, it was found to regulate multiple cellular processes by cleaving selected mRNAs in a cell type-dependent and stimulus-dependent manner20,21,24. For both XBP1 and RIDD-regulated mRNAs and precursor miRNAs, a common consensus CUGCAG sequence motif within a stem–loop structure is a key feature of the IRE1α cleavage site. IRE1α was recently found to form complexes with the components of the translational and translocational machineries, including signal recognition particle RNA, ribosomal RNAs and transfer RNAs25,26. However, whether the IRE1α–small RNA complexes are of biological significance remains to be further investigated. Additionally, IRE1α also associates with adapter proteins to undergo crosstalk with other stress response pathways, including macroautophagy and the MAPK pathway27.
UPR signalling through ATF6
On ER stress, full-length ATF6 (ATF6p90) transits from the ER to the Golgi apparatus, where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P) to release a fragment containing a basic leucine zipper (bZIP) transcription factor, termed ‘ATF6p50’, that translocates to the nucleus to induce gene expression28,29 (Fig. 1a). ATF6p50 and XBP1s act in parallel, but also overlapping pathways to regulate transcription of genes encoding ER chaperones and enzymes that promote ER protein translocation, folding, maturation and secretion, as well as degradation of misfolded proteins25,30,31. In addition, XBP1s and ATF6p50 promote ER and Golgi apparatus biogenesis to increase the secretory capacity of the cell under ER stress32,33,34. Overall, the UPR represents a combination of signalling pathways that maintain ER proteostasis and sustain cell function under ER stress by adjusting the ER folding capacity in a dynamic manner.
Cell death control under ER stress
When the capacity of the UPR to sustain proteostasis is overwhelmed, cells enter apoptotic programmes4. Numerous mechanisms have been proposed to sensitize cells to ER stress-induced apoptosis, where a network of upstream events rather than a single pathway controls cell demise under irreversible ER damage (Fig. 1b).
ER stress triggers activation of the canonical apoptosis pathway, involving the conformational activation of the proapoptotic members of the BCL-2 family at the mitochondria, BAX and BAK, with concomitant assembly of the apoptosome and the activation of executer caspase 3. BAX–BAK-double-knockout cells and Bax–Bak-double-knockout mice are resistant to cell death on ER stress35,36. However, the signals communicating ER stress to mitochondria to induce cell death are highly debated. BH3-only proteins of the BCL-2 family, including BIM, PUMA, NOXA and BID, are important factors that mediate ER stress-induced apoptosis in various cellular systems, where the activation mechanisms involve transcriptional upregulation and post-translational modifications of the proapoptotic BH3-only proteins37,38. Triple-knockout cells for the BH3-only proteins BIM, PUMA and BID are fully resistant to ER stress-induced apoptosis, and the proapoptotic factor CHOP, which is regulated by ATF4 under ER stress, is able to induce the expression of BIM to trigger cell death39,40. Further, ATF4 and CHOP activate genes encoding translational components to increase protein synthesis in stressed cells, leading to enhanced reactive oxygen species production and proteotoxicity and thus cell death11,41. Although a molecular switch that transits cell adaptation to cell death programmes under irreversible ER stress has been proposed, the nature of this mechanism remains speculative. Indeed, cells stimulated with pharmacological ER stress exhibit simultaneous prosurvival and proapoptosis signals42. However, experiments performed with low doses of ER stress-inducing reagents demonstrated that the signalling events trigged by long-acting, low-dose ER stressors differ from those in cells subjected to short-term, highly cytotoxic ER stress43. ER stress that falls below the threshold may not be able to elicit an effective PERK-dependent cell death programme42. Indeed, a recent study showed a new pathway that regulates ER stress-mediated cell death initiated by a hyaluronidase, an extracellular matrix component, that was independent of canonical UPR activation44.
miRNAs also contribute to ER stress-induced apoptosis, where sustained RIDD degrades miRNAs that negatively control caspase 2 levels and thioredoxin-interacting protein (TXNIP), leading to sterile inflammation or NACHT, LRR and pyrin domain-containing 3 (NLRP3) inflammasome activation and subsequent cell death22,45,46,47 (Fig. 1b). Hyperphosphorylated IRE1α may deplete essential ER components through RIDD, such as the chaperone BiP, sensitizing cells to apoptosis24. The activation kinetics of the PERK and IRE1α signalling pathways may serve as a switch to trigger apoptosis. Under prolonged ER stress, attenuation of XBP1 mRNA splicing inactivates a major UPR prosurvival avenue, whereas PERK−CHOP signalling persists48. The activity of death receptor 5 (DR5) and that of the downstream initiator caspase 8 were shown to regulate apoptosis under ER stress49,50. In this model, RIDD activity can degrade the mRNA encoding DR5, which is induced by CHOP51. Under sustained ER stress, RIDD is attenuated in certain cellular systems to allow increased expression of DR5 and activation of apoptotic programmes51,52. Finally, Ca2+ release from the ER sets the threshold of stress signalling to transit into a proapoptotic response (Fig. 1b). Mitochondrial Ca2+ uptake sensitizes cells for cytochrome c release to trigger apoptosome formation through the opening of the permeability transition pore. A group of conserved cell death regulators of the TMBIM or BAX inhibitor 1 (BI-1) family53, known as BI-1/TMBIM-6 and GRINA/TMBIM3, can attenuate ER stress-induced apoptosis by reducing Ca2+ release from the ER54,55. Although a variety of mechanisms by which UPR regulates apoptosis have been proposed, the contribution of individual UPR pathways is modest, suggesting the existence of cell type-specific networks in determining cell fate under ER stress.
Stress sensing mechanisms
Two main models have been proposed to describe the ER stress sensing mechanisms by IRE1α and PERK: a direct recognition model and an indirect model where the ER stress sensing process is directly coupled to the folding machinery56,57 (Fig. 2). Importantly, the luminal domains that sense ER protein misfolding are structurally conserved. Indeed, replacement of the luminal domain of IRE1α (termed ‘Ire1p’ in yeast) with the luminal domain of mammalian PERK, for which there is no yeast homologue, confers ER stress activation of the UPR58.
Fig. 2: Regulation of IRE1α and PERK signalling.
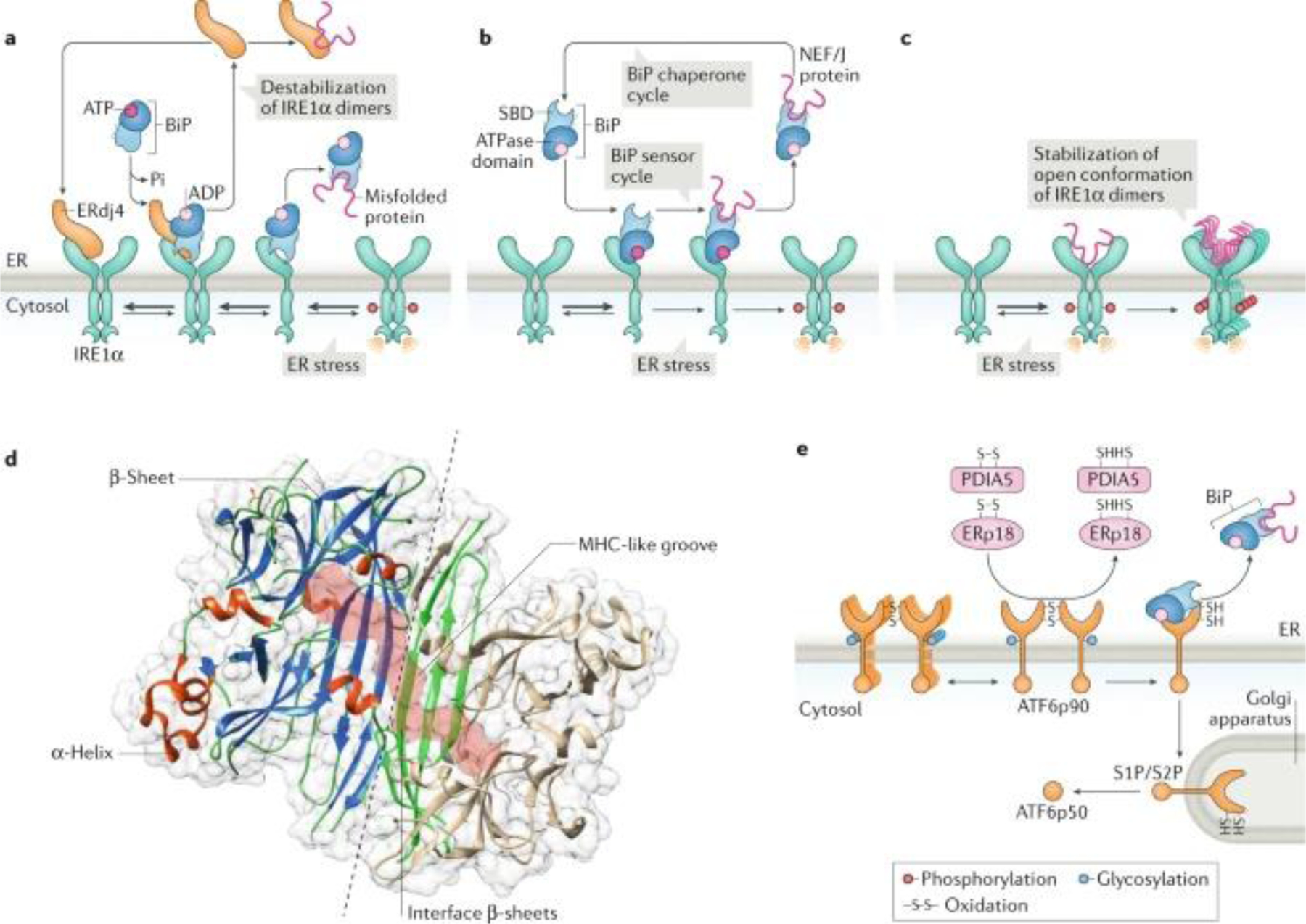
a | Indirect endoplasmic reticulum (ER) stress sensing model. In resting cells, the ER stress sensor IRE1α is maintained in an inactive state through its association with the ER chaperone BiP (also known as GRP78). On accumulation of unfolded proteins, BiP preferentially binds to unfolded protein peptides, thereby releasing the ER stress sensor to allow its spontaneous dimerization and activation. In this model, BiP has the capacity to destabilize IRE1α dimers and maintain the unfolded protein response transducer in an inactive state. In addition, the BiP co-chaperone ERdj4 is required for BiP binding to IRE1α and repression of IRE1α activation. b | Alternatively, BiP might bind misfolded proteins through the substrate-binding domain (SBD), which transduces a signal to the ATPase domain to release the repressive interaction over IRE1α and PERK. c | A direct recognition model proposes that unfolded proteins bind directly to the luminal domains of IRE1α, facilitating the assembly of highly ordered IRE1α clusters. This may orient the cytosolic region of the dimer to create a ribonuclease site and generate an mRNA docking region. d | The 3D structure of the ER luminal domain of yeast Ire1p is shown, depicting the dimeric interphase (dashed line) and the major histocompatibility complex (MHC) class-I like groove (pink surface), where misfolded peptides might bind. Protein Data Bank accession number 2BE1. e | ATF6 is regulated by its glycosylation and redox state, in addition to the binding of various disulfide isomerases, including PDIA5 and ERp18. ATF6p90, full-length AFT6; J protein, J-domain protien; NEF, nucleotide exchange factor; S1P, site-1 protease; S2P, site-2 protease.
In the indirect model, IRE1α monomers are prone to form dimers, resulting in transphosphorylation of the kinase domain, followed by a conformational change in the RNase domain that activates canonical UPR signalling4. Twenty years ago, correlative data suggested that ER stress sensors are maintained in an inactive state under resting conditions through their physical interaction with the ER chaperone BiP56 (Fig. 2a). On ER stress, BiP associates with unfolded or misfolded proteins in the ER, thereby releasing PERK and IRE1α to allow their homodimerization or the translocation of ATF6 to the Golgi apparatus through COPII vesicles59,60. However, in yeast, BiP binding to Ire1p may be dispensable to activate the UPR61, as mutations that abrogate the BiP−Ire1p interaction do not significantly affect the ER stress sensing process62 (reviewed in57). Alternatively, BiP binding was suggested to participate in the attenuation of UPR signalling under prolonged ER stress to deactivate Ire1p and sequester its inactive form, contributing to the dissociation of Ire1p clusters61,63. By contrast, mutagenesis studies with mammalian cells suggested that disruption of the BiP–IRE1α interaction results in basal UPR activation even in the absence of ER stress59. Recent studies have reinforced the idea that BiP plays a major role in ER stress sensing. ERdj4 (also known as DNAJB9) was identified as an ER luminal co-chaperone required for the formation of the BiP–IRE1α complex64. In this model, ERdj4 associates with IRE1α and recruits BiP by stimulating ATP hydrolysis (Fig. 2a). Then unfolded proteins compete for BiP to favour a ‘default’ dimeric and active state of IRE1α64. Thus, binding of ERdj4 and BiP has the capacity to destabilize IRE1α dimers to maintain them in an inactive monomeric state65.
In an alternative model, BiP has a dual function in the regulation of the UPR: it directly senses ER stress by binding to unfolded or misfolded protein peptides via its substrate-binding domain and then transduces this information to IRE1α and PERK through the ATPase domain, triggering the dissociation of the sensor–BiP complex66,67 (Fig. 2b). These observations implicated BiP as a sensor of ER stress and suggested an allosteric mechanism for UPR induction. A follow-up study suggested that the physical interaction of BiP with the luminal domains of IRE1 and PERK may switch BiP function from being part of a chaperone cycle that operates as an ER stress sensor68. This switch might prevent the binding of BiP to its co-chaperones and guanine nucleotide exchange factors, in addition to preventing ATPase stimulation.
The 3D structure of the ER luminal domain of yeast Ire1p contains a binding pocket that extends across a dimerization interface reminiscent of the peptide-binding groove of the major histocompatibility complex (MHC)15. This observation led to the hypothesis that unfolded or misfolded proteins may directly bind to the luminal domain of Ire1p to promote the formation of stable dimers and oligomers (Fig. 2c,d). Mutagenesis analysis of the Ire1p groove suggested that yeast Ire1p binds peptides that have amino acid patterns reminiscent of those predicted to occupy internal positions in folded proteins69. Additional studies suggested a two step model where BiP dissociation from Ire1p is needed for Ire1p to form clusters, which then bind misfolded proteins for UPR activation70. While similar conclusions were inferred from a recent biochemical characterization of the ER luminal domain of IRE1α, IRE1β and PERK71,72,73,74, this sensing mechanism model remains to be validated in living cells. Importantly, IRE1α forming a peptide-binding groove, as observed in the crystal structure of yeast Ire1p, was not supported by the X-ray structure of the luminal domain of human IRE1α. The X-ray structure suggests that the MHC class I-type groove is not exposed to solvent, which is incompatible with the binding of a protein peptide16. In addition, other studies failed to detect the binding of misfolded proteins to the ER luminal domain of IRE1α in vitro59. Whether direct peptide binding to IRE1α can activate its function remains an open question, but it is possible that under ER stress the UPR is activated by a combination of both BiP-dependent and BiP-independent recognition mechanisms. Finally, a recent study suggested that DR5 signals through its intracellular accumulation by directly recognizing unfolded proteins in the ER lumen, triggering apoptosis75. Thus, DR5 may operate as an ER stress sensor to engage a terminal UPR.
Recent studies revealed that additional ER-resident factors are involved in the activation of UPR transducers. The collagen carrier HSP47 activates IRE1α signalling under mild ER stress through a physical interaction76. HSP47 directly binds to the ER luminal domain of IRE1α, displacing the negative regulator BiP from the complex to facilitate IRE1α oligomerization76. However, HSP47 does not affect the activation of PERK, suggesting its role in selective regulation of IRE1α. As collagens are the major cargo of the secretory pathway, the involvement of HSP47 in IRE1α activation suggests a coupling of the secretion pathway with the UPR to adjust the ER protein folding capacity. In agreement with this concept, collagen 6a was identified as a major RIDD target20, and XBP1s was shown to induce the expression of the putative collagen carrier TANGO1 (ref.77). All these observations implicate the existence of a tight association between collagen biogenesis and the UPR.
Protein disulfide isomerases (PDIs) are a group of foldases that catalyse the formation and isomerization of disulfide bounds in ER client proteins. PDIA6 was shown to attenuate IRE1α and PERK signalling under prolonged ER stress through an interaction with specific oxidized cysteines in the ER lumen of IRE1α or PERK in cultured cells78. However, PDIA6 expression enhanced IRE1α signalling in response to disruption of ER Ca2+ homeostasis79. An unbiased interactome screening for ATF6 identified several PDIs as binding partners80. At the functional level, PDIA5 and ERp18 enhance ATF6 activation, possibly through a direct interaction80,81 (Fig. 2e). Altogether, available data suggest that the mechanism for ER stress sensing is highly complex and combines the protein folding machinery and UPR signal transduction molecules.
In addition, IRE1α and ATF6 can sense ER membrane lipid bilayer alterations, without the involvement of the ER luminal domain for signalling82,83,84,85. The transmembrane domains of IRE1α and ATF6 are required to respond to such membrane aberrancies83,85,86. In yeast, an amphipathic α-helix in Ire1p may sense altered ER membrane properties84. Importantly, the transcriptional responses driven by IRE1α under ER stress or lipid bilayer stress are different87, suggesting a complex integration of local perturbations to selective adaptive programmes. These findings support the notion that multiple signals may cause activation of UPR sensors in a non-canonical manner.
UPR regulation
There is increasing evidence that the proximal UPR transducers IRE1α, PERK and ATF6 can be selectively modulated by binding to specific factors or through post-translational modifications that modify their activities and/or protein stability. Thus, the threshold of ER stress that triggers the activation of each UPR sensor is determined by specific interactomes, regulating UPR signalling amplitude, kinetics and its impact on cell physiology. Recent advances have increased our understanding of how activators, repressors and post-translational modifiers function together to fine-tune UPR signalling. Although some cases rely on single reports or artificial overexpression systems, they introduce a new layer of complexity in UPR regulation that cannot be ignored.
Temporal and selective regulation of UPR transducers
Several studies suggest that the activity of all three UPR stress sensors is modulated by different specific factors. However, most of them focused on IRE1α as it is the most conserved of the UPR stress sensors (Fig. 3; Table 1; Supplementary Table 1).
Fig. 3: Regulation of IRE1α signalling through protein–protein interactions and post-translational modifications.
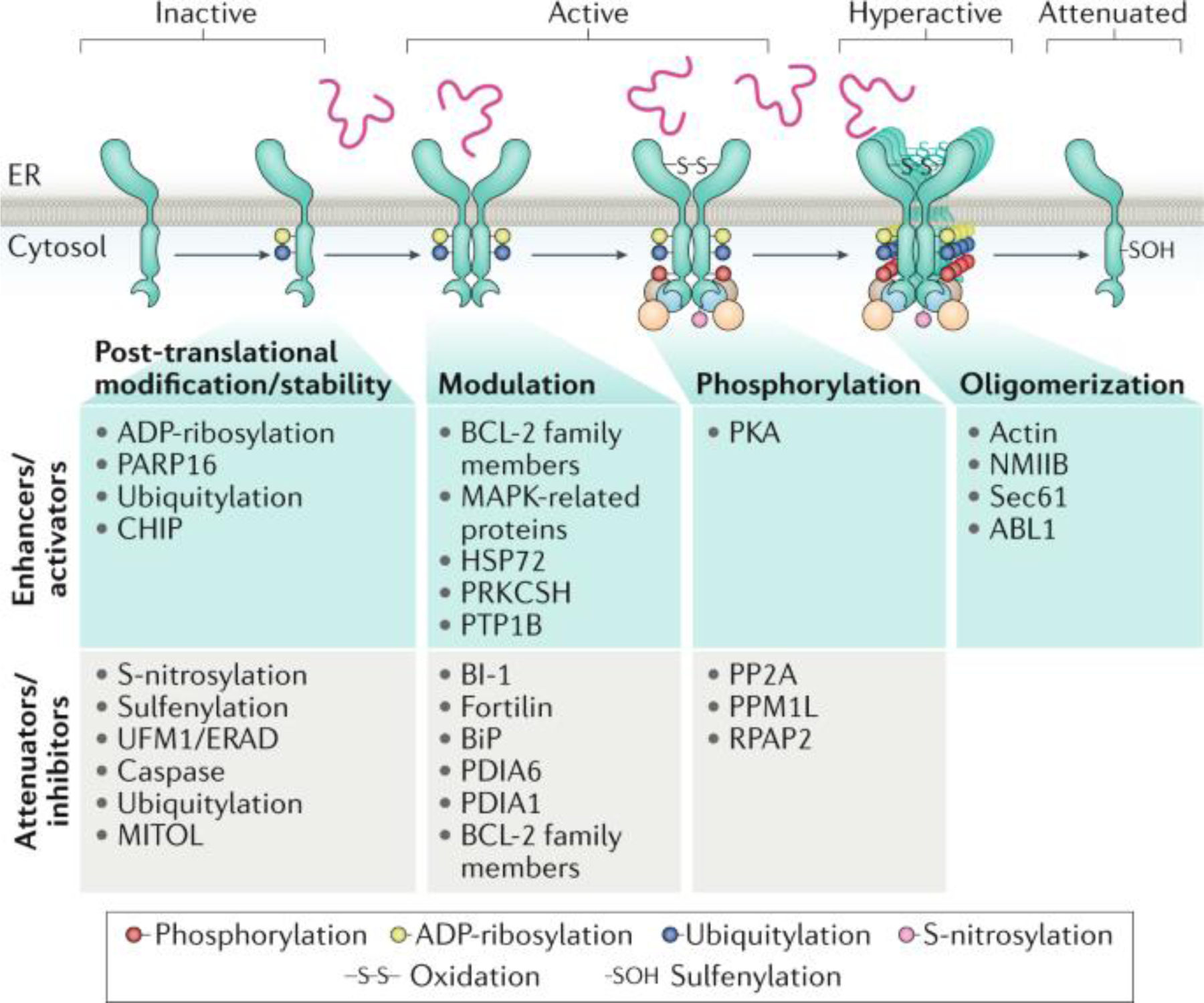
Several proteins can form a complex with IRE1α. A schematic representation is presented for negative and positive regulators of IRE1α signalling (attenuators and enhancers of unfolded protein response signalling). The effects of these regulators at different stages of the IRE1α signalling process are indicated, including IRE1α dimerization, oligomerization and phosphorylation. In addition, the occurrence of several post-translational modifications that could modify the stability or the activity of IRE1α is indicated. BI-1, BAX inhibitor 1; ER, endoplasmic reticulum; ERAD endoplasmic reticulum-associated protein degradation; NMIIB, non-muscle myosin heavy chain IIB; PKA, protein kinase A; PP2A, protein phosphatase 2A.
Table 1.
Selected IRE1α-binding partners and regulators
| Protein | Function | Endogenous complex | Ovexpressed IRE1α with (yes) or without (no) tag | Ovexpressed interactor with (yes) or without (no) tag | In vitro binding |
|---|---|---|---|---|---|
| AIP1 | MAPK signalling | Yes | – | – | – |
| BAK | Apoptosis | Yes | – | – | Yes |
| BAX | Apoptosis | Yes | – | – | Yes |
| BIM | Apoptosis | Yes | – | – | Yes |
| BI-1 | Apoptosis | Yes | – | – | Yes |
| BID | Apoptosis | – | No | Yes | Yes |
| BiP | Chaperone | Yes | – | – | Yes |
| ABL1 | Cell signalling, DNA damage, apoptosis | – | Yes | No | – |
| CHIP | Ubiquitin system | Yes | – | – | – |
| ER protein-targeting machineries | Protein synthesis | – | Yes | – | – |
| Filamin A | Actin cytoskeleton regulation | Yes | – | – | Yes |
| Fortilin | Cell survival | Yes | – | – | Yes |
| HSP47 | Collagen folding and trafficking | Yes | – | – | Yes |
| HSP90 | Chaperone | Yes | – | – | – |
| HSP70 | Chaperone | – | Yes | No | Yes |
| HRD1 | ERAD | Yes | – | – | – |
| IP3R1–IP3R3 | Calcium channel | – | Yes | No | Yes |
| JAB1 | MAPK signalling | – | Yes | Yes | – |
| JIK | MAPK signalling | – | Yes | Yes | – |
| Myosin heavy chain IIB | Actin cytoskeleton regulation | – | Yes | Yes | – |
| NMI | Cell signalling | – | No | Yes | – |
| Optineurin | Autophagy | Yes | – | – | – |
| PARP16 | Protein modification | – | Yes | Yes | – |
| PDIA1 | Disulfide bond formation | Yes | – | – | Yes |
| PDIA6 | Disulfide bond formation | Yes | – | – | Yes |
| PPM1L | Phosphatase | – | Yes | Yes | – |
| PKA | Kinase | – | – | – | – |
| PKC | Kinase | Yes | – | – | Yes |
| PSEN1 | Protein processing | – | Yes | No | – |
| PUMA | Apoptosis | Yes | – | – | Yes |
| RACK1 | Cell signalling | Yes | – | – | – |
| Sec61 | Translocon | – | Yes | Yes | Yes |
| SIG-1R | Chaperone | – | Yes | Yes | Yes |
| TRAF2 | Adapter protein, cell signalling | Yes | – | – | – |
| UFBP1 | UFM1 conjugation system | – | Yes | Yes | – |
| Ubiquitin D | Ubiquitin system | Yes | – | – | – |
| USP14 | Ubiquitin system | Yes | – | – | – |
| Yip1A | ER–Golgi apparatus trafficking | Yes | – | – | – |
Detected protein interactions with IRE1α are listed, whether between endogenous proteins or between overexpressed proteins (tagged or not tagged). If protein complexes were validated with endogenous proteins, experiments performed with overexpression systems are omited from this table. The complexes that were validated in vitro with use of purified proteins are indicated. See Supplementary Table 1 for further details and references. ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated protein degradation.
Multiple laboratories identified positive regulators of IRE1α signalling that function by controlling IRE1α dimerization, oligomerization, phosphorylation and dephosphorylation, impacting the amplitude and kinetics of the signalling response. For example, BI-1 was the first identified negative regulator of IRE1α, which was found to attenuate IRE1α signalling under prolonged ER stress by forming a protein complex with the cytosolic domain of IRE1α88,89,90,91. Fortilin inhibits IRE1α activity in a similar manner, by directly interacting with phosphorylated IRE1α, attenuating the UPR and reducing the susceptibility of cells to apoptosis92. Apoptosis regulators were also reported to directly interact with IRE1α to modulate its activity. Several members of the BCL-2 family can physically interact with IRE1α to enhance the amplitude of IRE1α downstream outputs, thereby sustaining the UPR signalling in both cultured cells and animal models35,93. Similarly, several components of the MAPK pathway can selectively modulate IRE1α activity through interacting with IRE1α (reviewed in94). Moreover, interactome analyses have identified additional regulators of IRE1α (Supplementary Table 1). For example, the non-muscle myosin heavy chain IIB protein forms a specific complex with IRE1α, promoting the formation of larger IRE1α clusters95 (Fig. 3). The tyrosine-protein kinase ABL1 is required to stabilize IRE1α oligomers, shifting the equilibrium towards a hyperactivated state that more likely catalyses RIDD, triggering cell death96.
IRE1α also forms a complex with Sec61, a core component of the translocon machinery26. Sec61 can recruit unspliced XBP1 (XBP1u) mRNA through a ribosomal arrest sequence to bring XBP1u mRNA to the ER membrane in a signal recognition particle-dependent manner to increase the splicing efficiency of the XBP1 mRNA18,97,98. Although the protein encoded by the XBP1u mRNA is highly unstable and not detectable as it is rapidly degraded by the proteasome, it contains a membrane-interacting region that attaches to the ER membrane and brings the XBP1u mRNA close to IRE1α99. During this process, translation of the XBP1u mRNA is briefly paused, which allows the XBP1u mRNA−ribosome−nascent chain complex to be targeted to the protein-conducting channel on the ER membrane that is formed by Sec61, where XBP1u mRNA is efficiently processed by IRE1α97,100,101.
Although less explored, the activities of PERK and ATF6 are modulated by selective binding partners. For example, p58IPK, the small GTPase Rheb and transducin β-like protein 2 (TBL2) bind PERK to regulate the amplitude of PERK-mediated signalling responses at the level of eIF2α phosphorylation102,103,104. More systematic interactome screenings are needed to define the composition of UPR stress sensor-containing complexes and the dynamic nature of their assembly/disassembly under ER stress and in physiological conditions.
Post-translational regulation of the UPR
Phosphorylation of IRE1α and PERK is a well-validated crucial regulatory mechanism for the activation of the UPR. Structural and biophysical studies have shown that IRE1α undergoes homodimerization and transautophosphorylation that lead to a conformational change that promotes IRE1α RNase activity105,106. Phosphorylation of IRE1α occurs in three regions: linker, activation loop and RNase domain107. Phosphorylation of IRE1α on the activation loop enhances the accessibility of RNA substrates to the IRE1α RNase catalytic pocket and thereby facilitates IRE1α-mediated XBP1 mRNA splicing or cleavage of RIDD substrates106,107 (Fig. 1a). IRE1α can also be phosphorylated independently of ER stress at Ser724 by protein kinase A (PKA), as reported in hepatocytes in the context of glucagon biosynthesis108 and in neurons stimulated by the growth factor brain-derived neurotrophic factor (BDNF)109,110. Moreover, the levels of IRE1α phosphorylation are negatively controlled by various phosphatases, including protein phosphatase 2A111,112 (which is recruited to the ER membrane through interaction with IRE1α) or ER membrane-targeted protein phosphatase PP2Ce113 (Fig. 3). It has also been reported that in cultured cells under ER stress, oligomerization of the ER luminal domain of PERK promotes PERK transautophosphorylation of the C-terminal cytoplasmic kinase domain at multiple residues to boost its kinase activity to phosphorylate eIF2α114. However, these studies are based on cell assays for which physiological significance needs to be confirmed in vivo.
Although other post-translational modifications of ER stress sensors or transcriptional activators are less studied, emerging evidence suggests they play an important part in the regulation of UPR signalling. Metabolic challenges that increase the cellular levels of nitric oxide can reduce IRE1α RNase activity in the liver by inducing S-nitrosylation, a modification that involves the covalent attachment of nitrogen monoxide to target proteins115,116. IRE1α contains two conserved cysteine residues within the RNase domain that can be S-nitrosylated and thereby block IRE1α RNase activity under metabolic inflammation115. Furthermore, S-nitrosylation of IRE1α and PERK was implicated in cell-based models of Parkinson disease117. Whereas S-nitrosylation of IRE1α inhibits its ribonuclease activity, S-nitrosylation of PERK can activate its kinase domain and downstream phosphorylation of eIF2α117. Redox changes may induce cysteine sulfenylation in the IRE1α kinase activation loop, reducing its activity118. Other post-translational modifications also regulate the function of IRE1α in some experimental systems. IRE1α ubiquitylation is catalysed by the E3 ligase CHIP, enhancing JUN N-terminal kinase activation in cultured cells119. The stability of IRE1α can be controlled by several pathways, including the ubiquitin-fold modifier 1 (UFM1) system120, the selective autophagy receptor optineurin121 and ERAD122. ATF6 activation is dependent on its glycosylation status and reduction of luminal cysteines123. A recent report suggested that caspases can cleave IRE1α and attenuate its signalling under prolonged ER stress124. The stability of ATF6 is regulated by the XBP1-target gene WFS1 through proteasome-mediated degradation125.
UPR functions beyond ER proteostasis
In the past 5 years, many studies have reported that UPR components have multiple functions in biological processes that are beyond maintenance of ER proteostasis. The identification of novel binding partners of UPR stress sensors (Table 1; Supplementary Table 1) has provided mechanistic insights, indicating that the formation of distinct protein complexes serves as a platform for interorganellar communication and signalling crosstalk to regulate mitochondrial bioenergetics, cytoskeleton dynamics and membrane contacts. Moreover, the finding of non-canonical activation mechanisms of ER stress sensors that depend on signalling events downstream of plasma membrane receptors has revealed functions of UPR components in cell differentiation, metabolism, neuronal plasticity and angiogenesis. These studies suggest a new concept that the ER stress sensors are involved in cell physiology through mediating non-canonical UPR responses (Fig. 4).
Fig. 4: ER stress-independent functions of the UPR.
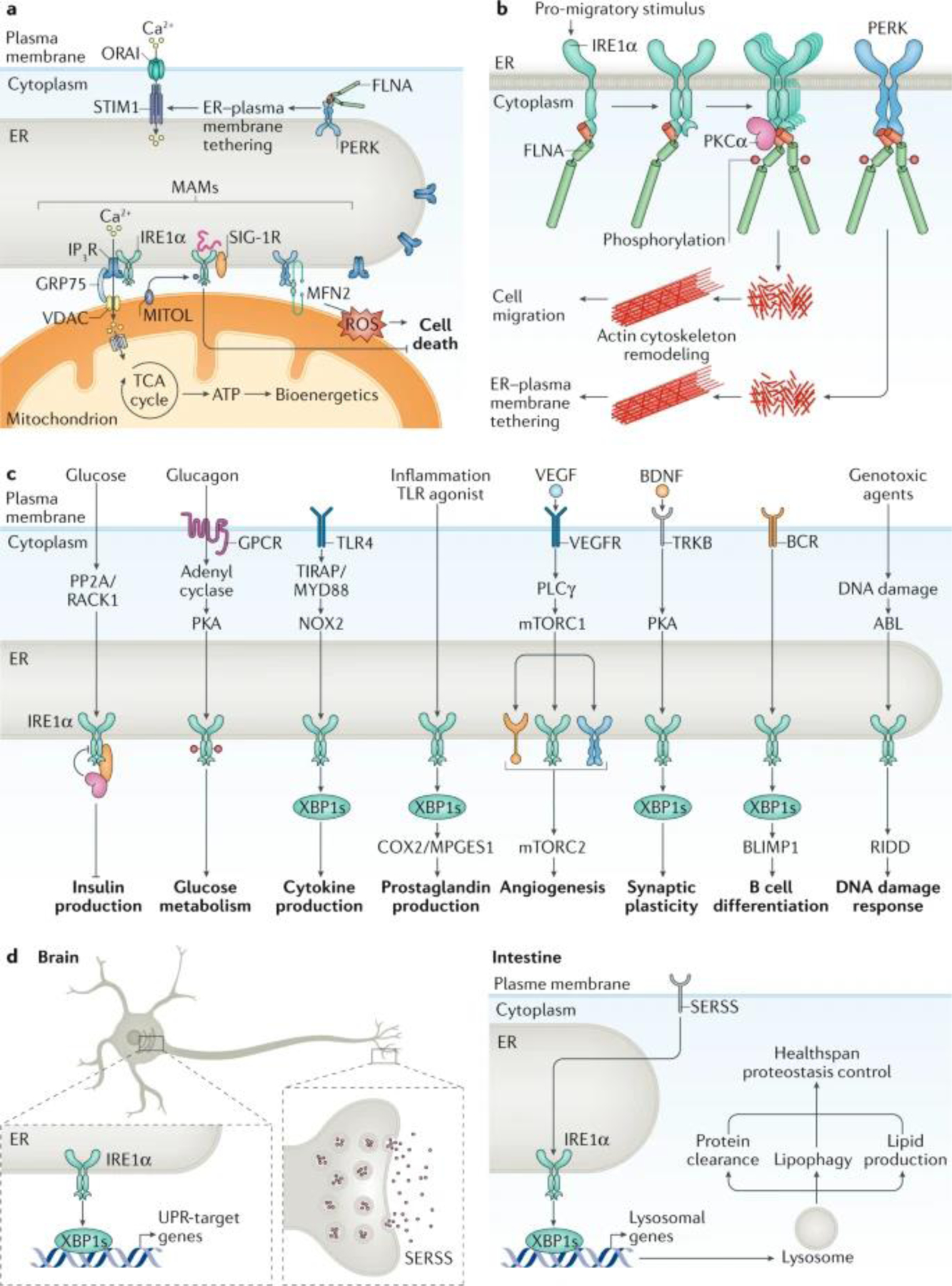
a | IRE1α and PERK localize to endoplasmic reticulum (ER)–mitochondrion contact sites that form structures known as mitochondria-associated membranes (MAMs). PERK regulates reactive oxygen species (ROS) propagation under ER stress at MAMs, in addition to affecting ER-to-mitochondrion tethering through the interaction with mitofusin 2 (MFN2). PERK also associates with filamin A (FLNA) to regulate ER–plasma membrane contact sites and calcium entry into the cell through ORAI–stromal interaction molecule (STIM) channels. The activity and stability of IRE1α is differentially regulated at MAMs through interaction with σ1 receptor (SIG-1R). IRE1α also docks the inositol 1,4,5-trisphosphate receptor (IP3R) at MAMs to control the transfer of calcium into the mitochondria and the activation of the tricarboxylic acid (TCA) cycle to produce ATP. b | Cell migration is regulated by IRE1α and PERK through the direct binding of filamin A, a regulator of actin cytoskeleton dynamics. IRE1α recruits protein kinase Cα (PKCα) as a scaffold to trigger filamin A phosphorylation, leading to its activation as a crosslinker of actin filaments. c | Plasma membrane receptor signalling pathways undergo crosstalk with unfolded protein response (UPR) signalling by leading to the activation of UPR sensors in an ER stress-independent manner. In addition, genotoxic stress might trigger a non-canonical activation of IRE1α to trigger regulated IRE1-dependent decay (RIDD) and modulate the DNA damage response. d | Cell-non-autonomous UPR activation. Expression of spliced X-box-binding protein 1 (XBP1s) in neurons signals for distal tissues to activate IRE1α−XBP1 and drive proteostatic changes that control healthspan and lifespan in simple model organisms such as Caenorhabditis elegans. XBP1s regulates different cellular processes to extend healthspan, including lipophagy, lysosomal function, proteostasis and lipid production. Neurotransmitter release mediates non-autonomous signalling downstream of XBP1s, suggesting that a secreted ER stress signal (SERSS) promotes ER stress resistance and longevity. BCR, B cell receptor; BDNF, brain-derived neurotrophic factor; GPCR, G protein-coupled receptor; mTORC1, mTOR complex 1; PKA, protein kinase A; PLCγ, phospholipase Cγ; PP2A, protein phosphatase 2A; TLR, Toll-like receptor; UFM1, ubiquitin-fold modifier 1, VDAC, voltage-dependent anion-selective channel; VEGFR, vascular endothelial growth factor receptor.
Membrane contact sites and bioenergetics
To maintain cellular homeostasis, ER and mitochondria exchange molecular signals by a physical association between the two types of organelles (Box 2). This physical association forms specific microdomains known as mitochondria-associated membranes (MAMs), which are stable structures that are found in all eukaryotic cells and cover 2−5% of the total mitochondrial surface126. These close membrane contacts facilitate the transfer of Ca2+ between the two types of organelles by generating microdomains of localized Ca2+ spikes released from the ER through inositol 1,4,5-trisphosphate receptors (Fig. 4a). Mitochondrial Ca2+ uptake modulates cellular metabolism by activating the tricarboxylic acid cycle to produce ATP. Several research groups have reported the presence of PERK or IRE1α in MAMs127, which was recently validated in vivo at the level of endogenous proteins128. PERK was found to facilitate the tethering of ER to mitochondria at MAMs, leading to increased production of reactive oxygen species in response to ER stress to promote cell death127. Another study suggested that PERK and the ER–mitochondrial tether protein mitofusin 2 form a complex that attenuates UPR signalling129. The localization of IRE1α at MAMs is stabilized by σ1 receptor (also known as SIG-1R), which may enhance UPR signalling130. The mitochondrial E3 ubiquitin ligase MITOL (also known as MARCHF5), localized at the outer mitochondrial membrane, was recently shown to attenuate IRE1α oligomerization and thereby inhibit ER stress-induced apoptosis by catalysing the ubiquitylation of IRE1α at MAMs130.
Box 2. ER-coordinated organelle physiology in metabolic disease.
The endoplasmic reticulum (ER) functions as a central organelle in the coordination of stress responses and in the maintenance of lipid homeostasis by interacting with and forming membrane contact sites with the other organelles in the cell239. ER and mitochondria interact at sites known as mitochondria-associated membranes (MAMs) to exchange metabolites and Ca2+. Recent studies suggested that MAMs could be a hub of hepatic insulin signalling and nutrient sensing240. Lipid droplets, lipid-storage and signal-transduction organelles derived from the ER, are intimately associated with the pathogenesis of metabolic disorders through processes involving multiple organelles. Mitochondria are frequently packed densely with lipid droplets in fat cells241. In response to nutrient starvation, lipid droplets, mitochondria and smooth ER in mouse embryonic fibroblasts form complexes to allow coupling of lipolysis and fatty acid oxidation242. Commonly recognized as terminal degradation stations, lysosomes move along microtubules and interface physically and functionally with the ER, lipid droplets and mitochondria in response to energy fluctuations or stress challenges241. These interorganelle contacts make possible reticulophagy, lipophagy and mitophagy: the ‘self-eating’ processes essential for protein quality control, lipid homeostasis and stress adaptation243. Functional impairment of membrane protein regulators or enzymes located in these dynamic organelle responsive systems, which can be triggered by overnutrition or other metabolic conditions, has been extensively and independently implicated in metabolic diseases, such as fatty liver disease, obesity and type 2 diabetes. From this perspective, metabolic diseases are ‘organelle diseases’.
IRE1α was identified as a basal regulator of mitochondrial bioenergetics128. IRE1α regulates the transfer of Ca2+ from the ER to mitochondria, acting as a scaffold that docks the inositol 1,4,5-trisphosphate receptor at MAMs through physical interaction128. IRE1α deficiency resulted in a severe metabolic stress state and impacted the tricarboxylic acid cycle in cell culture and in the livers of mice at basal levels. An interactome screening identified filamin A as a strong PERK binding partner, and this interaction was shown to regulate the association between the ER and plasma membranes, enhancing Ca2+ uptake by cells131. These observations suggest that the subcellular distribution of PERK and IRE1α at ER subdomains is important for signalling optimization and crosstalk with other cellular processes to control Ca2+ signalling, metabolism and cell fate under ER stress.
Cytoskeleton dynamics
The actin cytoskeleton and non-muscle myosin IIB are required for the formation of IRE1α clusters95,132, but until recently it was unclear whether the UPR has reciprocal effects on the cytoskeleton. In two recent unbiased interactome screenings, filamin A was identified as the major IRE1α binder133,134 (Fig. 4b). Filamin A mediates the crosslinking of actin filaments and regulates cytoskeleton dynamics, including the formation of lamellipodia and filopodia, thus facilitating cell migration, adhesion and mechanotransduction. IRE1α regulates filamin A function through a physical interaction independent of its UPR-related enzymatic activities via a proline rich region in the IRE1α distal C-terminal region133. The formation of the IRE1α−filamin A complex dramatically impacts cell migration and cell shape in various cellular and animal models133. The interaction between PERK and filamin A, which affects Ca2+ uptake, was also shown to affect cytoskeletal dynamics and cell migration131.
Plasma membrane receptors and signalling crosstalk
Several studies have identified non-canonical ways of engaging UPR signal transducers in an ER stress-independent manner through signalling downstream of specific receptors (Fig. 4c). For example, optimal secretion of proinflammatory cytokines in macrophages is mediated by XBP1s, following IRE1α activation by Toll-like receptor signalling112,135, whereas it represses ATF4–CHOP expression136,137. Toll-like receptors can also trigger IRE1α activation and generation of functional XBP1s in myeloid leukocytes, which sustains expression of prostaglandin-endoperoxide synthase 2 (PTGS2; also know as COX2) and prostaglandin E synthase (PTGES, also known as MPGES1), two rate-limiting enzymes that control prostaglandin biosynthesis and behavioural pain responses in mice138.
IRE1α signalling can be modulated by fluctuations in glucose and glucagon levels, involving its phosphorylation via signalling events including PKA and the binding of the adapter protein RACK1 to recruit protein phosphatase 2A in pancreatic β-cells111,139. The UPR promotes angiogenesis downstream of vascular endothelial growth factor receptor through phospholipase Cγ1 (PLCγ) and the mTOR pathway, independently of ER stress140. In neurons, binding of BDNF to its receptors triggers the activation of the IRE1α−XBP1 pathway through PKA activation to induce neuronal differentiation and dendritic outgrowth, contributing to synaptic plasticity109,110. XBP1s expression is a differentiation-dependent event in plasma B cells rather than a response to increased immunoglobulin secretion141,142. These selected examples illustrate an emerging concept where UPR components may serve important cellular functions as signal transduction modules in processes that are independent of protein folding stress. However, the detailed mechanisms explaining alternative ways of activation of ER stress sensors need further investigation.
Cell-non-autonomous control of organismal proteostasis
Maintenance of organismal homeostasis depends on the integration of external and systemic signals and ability to sense cellular perturbations in order to trigger adaptive responses. Studies in model organisms implicated a new paradigm in which a neuronal UPR orchestrates the global maintenance of ER proteostasis at the organismal level in a cell-non-autonomous manner143 (Fig. 4d).
The overexpression of XBP1s in the hypothalamus protected animals against diet-induced obesity, increasing insulin sensitivity, increasing energy expenditure and reducing gluconeogenesis144. These effects were attributed, in part, to an increase in XBP1 mRNA splicing in the liver. Studies in invertebrates suggest that a loss of ER proteostasis contributes to ageing145. Overexpression of XBP1s in neurons of Caenorhabditis elegans resulted in a dramatic ~30% extension of lifespan146. Unexpectedly, these beneficial effects of neuronal XBP1s were mapped to the intestine, where the IRE1α−XBP1 branch was activated in a cell-non-autonomous manner143,146. In C. elegans, XBP1s activates transcription of lysosomal genes and may assist in clearing abnormally folded proteins147, in addition to regulating lipid metabolism and lipophagy148. A recent study in the same model organism also suggested that the expression of XBP1s in glial cells triggers cell-non-autonomous responses to engage the IRE1α–XBP1 pathway and protect against proteotoxicity and extend lifespan149. The UPR was discovered as a key mediator of the positive effects of dietary restriction in ageing148,149,150. Lastly, intestinal stem cells in flies subjected to dietary restriction triggered cell-non-autonomous activation of IRE1α and lipogenesis151. Together these studies depict a new layer of complexity, in which the neuronal UPR integrates stress signals to adjust global proteostasis and sustain organismal function. However, the actual role of the neuronal UPR in mammalian ageing remains to be established.
DNA damage response
The molecular intersection between homeostatic systems that maintain both genome integrity and proteostasis is poorly understood. A recent study reported that the selective activation of IRE1α under genotoxic stress modulates repair programmes and sustains cell survival under DNA damage. Unexpectedly, genotoxic agents exclusively engaged the RIDD activity in the absence of XBP1 mRNA splicing152. This function of IRE1α degrades mRNAs involved in the DNA damage response, impacting DNA repair, cell cycle arrest and cell death. The activation of RIDD under genotoxic stress was mediated by the recruitment of the ABL1 kinase to favour the oligomerization of IRE1α and catalyse RIDD. The protective role of IRE1α under DNA damage was validated in fly and mouse models152. Another report suggested that XBP1u has a role in regulating the levels of p53, a central regulator of the DNA damage response, by promoting its ubiquitylation and degradation153. These findings suggest a coordination between the pathways that maintain genome stability and ER proteostasis.
ER stress in physiology and disease
Altered ER proteostasis and abnormal UPR signalling have been implicated in the occurrence of a variety of human diseases, including cancer, neurodegeneration, metabolic diseases and chronic inflammation. In addition, the development of small molecules and gene therapy strategies to manipulate selective UPR components has illustrated the potential of the ER proteostasis network as a target for disease intervention (Fig. 5). In this section, we discuss selected examples depicting the emerging contribution of the UPR to a variety of human diseases.
Fig. 5: Role of the UPR in physiology and diseases.
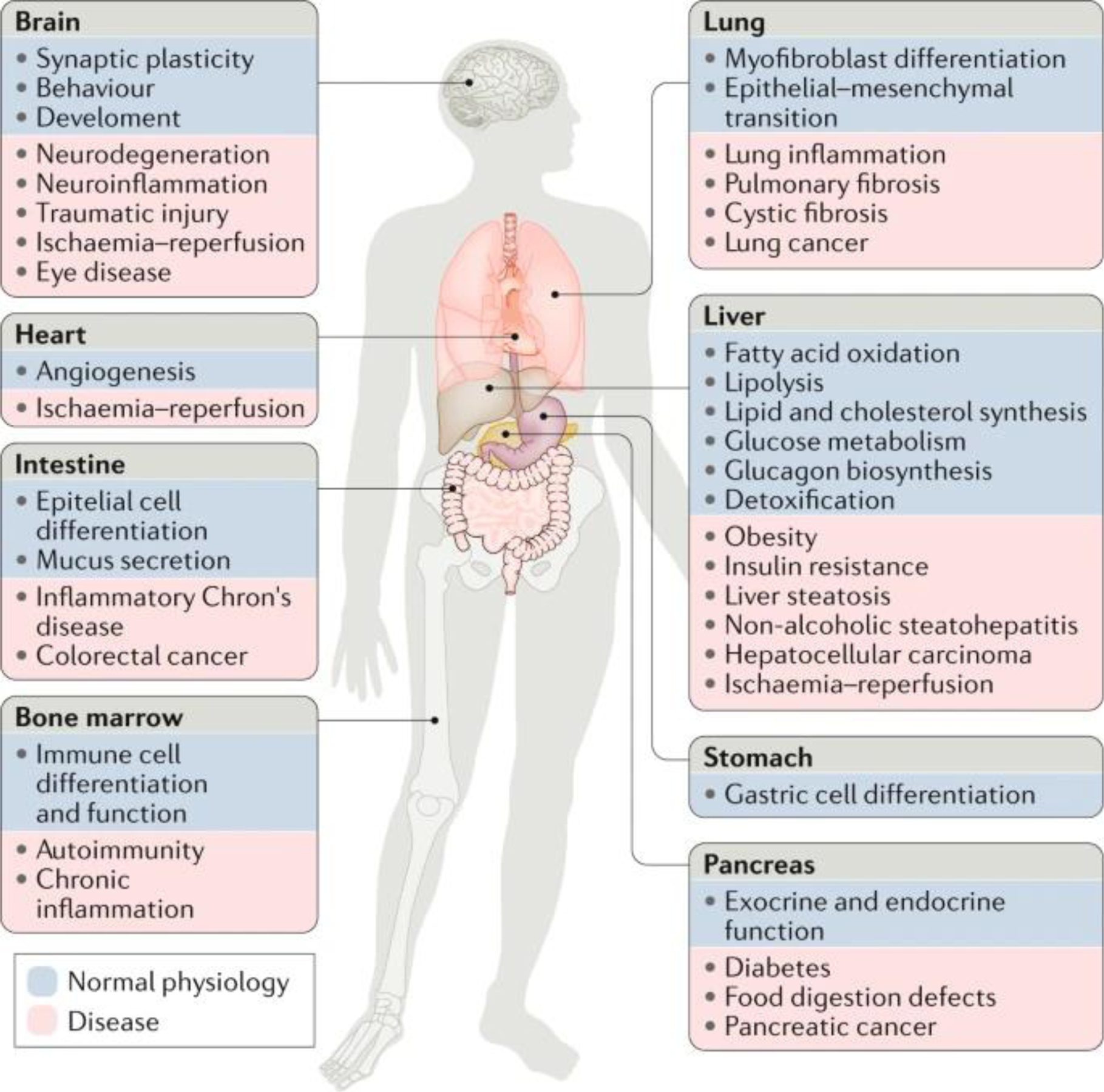
Genetic and pharmacological manipulation of major unfolded protein response (UPR) components has revealed that the UPR pathways play a part in the functions of diverse organs and cell types. Preclinical models have also shown that dysregulation of UPR signalling, mediated by specific UPR components, contributes to a variety of diseases. The figure illustrates the roles of the UPR in organ physiology (blue) or pathological conditions (pink) affecting the same tissues.
Metabolism
As the ER is a central compartment for both protein folding and lipid biosynthesis, the UPR is intrinsically associated with hepatic lipid homeostasis in the context of metabolic disease. The IRE1α-mediated UPR is required to protect the liver from stress-induced hepatic lipid accumulation in response to pharmaceutical challenges or overnutrition. IRE1α modulates expression of CCAAT/enhancer-binding protein and peroxisome proliferator-activated receptor family members to boost hepatic fatty acid oxidation, lipolysis and anti-inflammatory responses through either the XBP1s pathway or the RIDD pathway116,154. Under physiological conditions, IRE1α cleaves a subset of miRNAs that are functionally involved in inflammation and metabolism in the liver, leading to their degradation116. Furthermore, ablation of the UPR transducer ATF6 leads to hepatic steatosis owing to decreased fatty acid oxidation in mice challenged with drug-induced ER stress155,156. Similarly to ATF6, hepatic-specific cAMP-responsive element-binding protein (CREBH), another ER-located bZIP transcription factor, is activated through regulated intramembrane proteolysis under a variety of cellular stress signals29,157,158. However, cleavage and activation of CREBH did not induce UPR genes but rather induced the genes encoding functions involved in systemic inflammation and energy homeostasis157,159. In response to disruption of ER homeostasis, inflammatory challenges or circadian cues, CREBH transits from the ER to the Golgi apparatus, where it is processed to release a potent CREB transcription factor that drives expression of key genes encoding metabolic regulators or enzymes involved in hepatic lipolysis, fatty acid oxidation, lipophagy and glucose metabolism159,160,161,162,163,164. Importantly, CREBH deficiency is associated with hepatic steatosis and hyperlipidaemia in humans165,166.
Maladaptive UPR signalling is associated with diabetes and impaired survival of pancreatic β-cells. Hyperactivation of IRE1α, or loss of PERK or IRE1α function, resulted in low β-cell survival25,139,167,168. Xbp1-knockout mice develop liver dysfunction, and reconstitution of XBP1 expression in the liver bypassed embryonic lethality but resulted in a failure of exocrine pancreas function169. Loss-of-function mutations in PERK can cause a rare human diabetic condition known as Wolcott–Rallison syndrome associated with infantile-onset diabetes, similarly to what was observed in eIF2α phosphorylation-deficient or PERK-deficient mice167,170,171. Strikingly, CHOP deletion protected pancreatic β-cell function, reduced oxidative stress and prevented diet-induced diabetes in mice172. In addition, prolonged ER stress was linked to hepatic insulin resistance, where sustained IRE1α and JUN N-terminal kinase activation inhibited insulin receptor signalling173. In the context of obesity, lipids accumulated in the ER can trigger an ER stress response that is detrimental to liver function174.
Cancer
Accumulating evidence suggests that disrupted ER proteostasis is a hallmark of cancer (reviewed in175). Cancer cells rapidly metabolize glucose and proliferate, which could lead to poor vascularization of tumour mass, low oxygen supply and nutrient deprivation. In addition, overexpression of oncogenes stimulates protein synthesis and secretory demands. These oncogenic conditions are typical ER stress triggers, and UPR activation promotes the process of oncogenic transformation where all UPR signalling branches contribute to tumour growth, angiogenesis and immune evasion175,176. The high basal levels of UPR activation in cancer cells provides a survival advantage; however, it also keeps cells on a tight threshold of survival–death transition. It is possible that cancer cells require optimal UPR machinery for survival and that either inhibiting the UPR response or increasing ER stress levels may be an effective approach to repress oncogenesis. In human biopsy samples from brain cancer, breast cancer, lymphoma and multiple myeloma, high expression of XBP1s correlates with poor prognosis and low patient survival177,178,179,180.
Small-molecule inhibitors of the enzymatic activity of PERK or IRE1α have demonstrated efficacy in various preclinical models of cancer181. The IRE1α−XBP1 UPR branch acts in synergy with hypoxia-inducible factor 1α in human triple-negative breast cancers to promote angiogenesis and cancer cell proliferation177. The IRE1α−XBP1 pathway also promotes prostate cancer by activating MYC signalling182 and hepatocellular carcinoma by enhancing metabolic inflammation and hepatocyte proliferation183. However, IRE1α is otherwise required to prevent oncogenesis in various cancer types. It was shown that dietary restriction limited cancer progression through IRE1α-dependent anticancer immunosurveillance184. A low-protein diet reduced tumour growth in three mouse cancer models bearing lymphoma, colorectal carcinoma and melanoma cells, respectively185, suggesting a tumour-suppressive role of the IRE1α–XBP1 pathway. A recent study revealed an intriguing scenario where XBP1s and RIDD have opposite effects on brain cancer progression through remodelling tumour stroma178. Targeting PERK signalling in human colorectal carcinoma cells reduced tumour size186, whereas suppression of translational inhibition mediated by eIF2α can selectively trigger cytotoxic effects against aggressive metastatic prostate cancer187. These selected examples illustrate the fundamental role of the UPR in the progression of cancer.
Immunology
UPR signalling intersects at many levels with the innate immune response and the adaptive immune response188. By controlling the differentiation and function of immune cells, the UPR supports the full complement of immune effectors required for self-tolerance and defence against extracellular pathogens. The IRE1α-mediated UPR is required for both early and late stages of B cell differentiation141,189. Activation of B cell receptor signals stimulates IRE1α activity to initiate splicing of XBP1 mRNA, a target of the B cell differentiation regulator BLIMP134,190. While XBP1 deficiency in B cells leads to an absence of antibody-producing plasma cells, XBP1 is marginally involved in B cell maturation or isotype switching. However, IRE1α is required for pro-B cell differentiation and survival, in addition to the differentiation of plasma cells, possibly through impacting V(D)J antigen receptor rearrangements189,191. The IRE1α−XBP1 UPR branch is also required for dendritic cell development and survival192. Loss of IRE1α-dependent RIDD, on top of XBP1 deficiency, caused intestinal dendritic cell demise, implicating the role of the RIDD pathway in dendritic cell survival under pathophysiological conditions193. Moreover, the IRE1α–XBP1 branch activates natural killer cell immunity in part by regulating the oncogenic transactivator MYC194.
The UPR is extensively involved in the signal transduction of inflammatory responses. PERK-mediated eIF2α phosphorylation attenuates overall protein synthesis and favours nuclear factor-κB activation to induce proinflammatory genes195,196. Alternatively, IRE1α and PERK signalling promote production of proinflammatory cytokines through the direct binding of XBP1 or ATF4 to the Tnfα, Il6 and Il8 gene promoters in macrophages, fibroblasts, astrocytes or epithelial cells135,197,198,199. Pathologically, the IRE1α-mediated UPR functions as a critical regulatory node governing macrophage-mediated inflammation in metabolic and autoimmune diseases. In adipose tissues, IRE1α senses metabolic and immunological states and consequently guides adipose tissue macrophage polarization200. Additionally, hyperactivation of the IRE1α–XBP1 pathway in macrophages facilitates production of proinflammatory cytokines, a major driving force of inflammatory arthritis112.
Brain physiology and neurodegenerative diseases
In neurons, the secretory pathway is essential for the production of most synaptic proteins that support neuronal connectivity and brain functions. Many studies revealed that the control of protein synthesis by eIF2α phosphorylation modulates synaptic plasticity and determines complex behaviours, including learning and memory201. Small molecules that block the translational repression mediated by phosphorylated eIF2α enhance the basal memory capacity of mice and rats202. In the brain, XBP1s was shown to regulate the transcription of a cluster of synaptic genes and neurotrophins109,110 and thus enhance learning and memory203. In the context of brain development, PERK signalling regulated neurogenesis in the cortex, and genetic ablation of PERK resulted in microcephaly204. PERK expression enhanced the generation of intermediate progenitors and projection neurons in different cortical layers, thereby impacting on overall brain architecture. As mentioned earlier, IRE1α physically interacts with filamin A, modulating cytoskeleton dynamics and cell movement133. Mutations in the gene encoding filamin A are the underlying cause of paraventricular heterotopies, a disease condition driven by altered neuronal migration during brain development205. Genetic depletion of IRE1α during brain cortex development led to a phenotype resembling filamin A deficiency, with delayed cortical migration and altered morphology of the brain133. In vitro experiments indicated that XBP1 expression drives neuronal differentiation in response to BDNF, and these effects involve in part the upregulation of BDNF on a feedforward loop203,206. Thus, the UPR is emerging as a central regulator of neuronal physiology and cognition, in addition to brain development.
Alteration to ER function is common in many neurodegenerative diseases such as Alzheimer disease, Huntington disease, Parkinson disease, prion-related disorders and amyotrophic lateral sclerosis207,208. Although all these diseases are linked to protein misfolding, most of the protein aggregates accumulate in the cytosol, not in the ER lumen. However, several studies indicated that these disease-associated protein aggregates abnormally interacted with the regulatory components of the secretory pathway, including ERAD, ATF6 signalling, ER to Golgi apparatus vesicular trafficking, chaperone function and the proteasome, among other proteostasis-related processes (reviewed in207). The occurence of chronic ER stress blocks the production of synaptic proteins by repressing protein translation through the sustained phosphorylation of eIF2α209,210 and may result in a proapoptotic reaction owing to the occurrence of unresolved stress. Genetic manipulation of the IRE1α–XBP1 pathway in mouse models has demonstrated divergent roles of the UPR in various pathological conditions affecting the nervous system, including Parkinson disease211, amyotrophic lateral sclerosis212, Huntington disease213, Alzheimer disease214, prion-related disorders210,215, spinal cord injury216 and peripheral nerve degeneration217. Similarly, genetic and pharmacological manipulation of the PERK–eIF2α pathway modifies disease progression in various models of neurodegeneration208,209. Preclinical studies suggested that use of small molecules to target different UPR signalling branches181 or that the enforcement of XBP1s or chaperone expression through gene therapy approaches delays neurodegeneration (reviewed in218). However, recent studies suggested that XBP1s may have alternative roles in the nervous system at the level of astrocytes, where it drives detrimental proinflammatory reactions and contributes to autoimmune diseases such as multiple sclerosis199.
Conclusions and perspective
The UPR is a signalling pathway that is central for the determination of cell fate — cell death or survival —under ER stress. However, the mechanisms underlying the cell survival to cell death transition under ER stress remain largely unknown (see Box 3 for outstanding questions and misconceptions). One possible explanation may involve the dynamic nature of IRE1α assembly with proteins that control signalling outputs. Ten years ago, the concept of the UPRosome was proposed94, where IRE1α-containing protein complexes are envisioned as a platform to control its activity and also mediate the crosstalk with other intracellular signalling pathways. This concept has been expanded to PERK and ATF6. Studies assessing the nature of the interactome of UPR sensors revealed novel biological functions of the pathway in diverse biological processes, such as cytoskeleton dynamics, mitochondrial bioenergetics and cell differentiation. These alternative functions could be mediated by non-canonical signalling independent of the RNase activity of IRE1α. It is anticipated that, depending on the cell type analysed and the stimuli, distinct IRE1α assemblies will form in a temporally and spatially dynamic manner. The minimal compositions of these protein complexes remains to be defined, in addition to the way they are modified in specific cell types or by physiological and pathological stimuli. It is important to note that many of the studies describing UPR-sensor binding partners rely on overexpression systems or on single studies (Table 1; Supplementary Table 1). As IRE1α and PERK are low-abundance proteins, it might be feasible to speculate that transient and dynamic interactions, rather than stable protein complexes, might explain the molecular basis and physiological significance of the IRE1α UPRosome. In addition, subcellular localized protein complexes enriched in particular ER subdomains might contain a specific subset of binding partners to control distinct cellular processes. The dynamics of IRE1α cluster assembly and disassembly were recently reported, and it was suggested that only a small fraction of IRE1α clusters form large oligomers (∼5%), that they have complex topology and that their composition changes quickly under ER stress219, supporting the existence of distinct and dynamic pools of IRE1α inside the cell.
Box 3. Outstanding questions and misconceptions.
Many researchers measure mRNA or protein induction as markers for endoplasmic reticulum (ER) stress. It is best to monitor the most proximal events in unfolded protein response (UPR) sensor activation. BiP is a poor marker for ER stress because it is an abundant and stable protein. Sensitive real-time markers for UPR activation in vivo are needed to provide insights into kinetic, physiological and pathological conditions that cause UPR activation.
Although several markers are assumed to be ER stress dependent, they are not reliable indicators of ER stress response because of signalling crosstalk. For example, phosphorylation of eukaryotic translation initiation factor 2 subunit-α (eIF2α) or ATF4 expression is mediated by PERK, but also three additional eIF2α kinases are part of the integrated stress response. In addition, CHOP (also known as GADD153) induction is frequently used as a marker for ER stress, but it can be induced under a variety of different stress conditions, such as DNA damage.
It was reported that eIF2α phosphorylation inhibits only 5′ methylguanylate (5me-G) cap-dependent mRNA translation. However, phosphorylated eIF2α inhibits both cap-dependent and cap-independent mRNA translation.
Investigators that aim to study mechanisms by which cells activate the UPR to cope with ER stress should not use pharmacological inducers of ER stress because they are not physiological, have significant adverse effects and often trigger a terminal proapoptotic response in parallel to an adaptive UPR reaction. Possible approaches to minimize this limitation include use of very low concentrations as a pulse and pharmacological agents with effects that are reversible.
There is strong interest in investigating how protein misfolding in the ER causes oxidative stress. Under these conditions, there is debate as to whether the ER lumen becomes more reducing or more oxidizing. It would be important to consider how different cell types respond and define how the demand for disulfide bond formation by protein disulfide isomerases may impact ER redox balance. More sensitive and reliable compartment-specific real-time sensors for Ca2+ and reactive oxygen species are needed.
When one is inhibiting or activating a single UPR pathway by genetic or pharmacological approaches, it is necessary to delineate possible effects on parallel signalling branches to assess the specificity of the observations to a single signalling branch.
Defining the role of IRE1α needs to consider its function as a signalling scaffold and the impact of regulated IRE1-dependent decay (RIDD) and other degradation pathways involving mRNAs and precursor microRNAs. Assessment of mRNA stability of proposed RIDD targets is required.
Are protein complexes (that is, distinct UPRosomes) formed by IRE1α, PERK and ATF6 different from cell type to cell type or by the specific ER stressor analysed? How low-abundance UPR stress sensors mediate diverse cellular functions through protein–protein interactions remains to be determined. Are these complexes localized to specific ER subdomains?
How does an adaptive UPR turn into an inducer of apoptosis? This is probably one of the most significant questions that needs to be addressed. How do ER stress sensors integrate information about the intensity and duration of the stress stimuli to determine cell fate?
Abnormal levels of ER stress are extensively linked to different human diseases, including neurodegeneration, obesity, diabetes, cancer and autoimmunity. Small molecules that specifically inhibit or activate individual UPR pathways are needed to guide studies towards therapeutic implications in humans. Different UPR pathways may be activated for unique pathophysiological processes in cell-specific and disease-specific states. For example, PERK inhibition attenuates neurodegeneration but also causes diabetes due to pancreatic β-cell failure220,221; IRE1α inhibition represses triple-negative breast cancer but may cause colorectal cancer177,222. Most significantly, ATF6 inactivating mutations in humans cause a very specific loss of photoreceptor cone cells associated with age-related colour blindness223, while Atf6-null mice exhibit no overt phenotype30. Temporal and tissue-specific gene therapies to improve ER proteostasis to deliver active UPR components (for example, XBP1s or ATF6p50) or chaperones (for example, BiP) may be an interesting strategy to avoid systemic side effects of prolonged administration of small molecules218. It is important to consider therapeutic avenues to reduce ER stress levels. For example, chemical chaperones can prevent protein aggregation, reduce protein misfolding and attenuate UPR activation. Tauroursodeoxycholic acid is the most widely used chemical chaperone to reduce ER stress. It is well tolerated and is presently being tested in ~20 clinical studies, of which the most promising are for treatment of amyotrophic lateral sclerosis and insulin resistance. Proteasome inhibitors have been intensively studied in the treatment of cancers. In particular, bortezomib (Velcade), a highly selective and reversible proteasome inhibitor, was approved for clinical use against multiple myeloma and is in clinical trials as a single agent or in combination with chemotherapeutics against other tumour malignancies224. Despite the progress, efforts to target the UPR for cancer therapy still face major challenges. A critical question is what determines the switch between prosurvival and prodeath UPR signals. Furthermore, solid tumours are highly heterogeneous. It is unclear whether the status of UPR activation reflects the tumour heterogeneity. For rational anticancer drug designs through targeting the UPR, it is necessary to answer these crucial questions.
Supplementary Material
Acknowledgements:
The authors thank H. Urra for initial figure and table design.
The authors thank J. Yong and C. Lebeaupin for critical comments on the manuscript.
Funding:
This work was directly funded by ANID/FONDAP/15150012, the Chilean Millennium Institute of Biomedical Neuroscience Institute BNI (grant P09-015-F), CONICYT–Brazil 441921/2016-7, FONDEF ID16I10223, FONDEF D11E1007 and FONDECYT 1180186 (C.H.). In addition, the authors thank the US Air Force Office of Scientific Research (grant FA9550-16-1-0384) (C.H.), the Michael J Fox Research Foundation, Target Validation Grant 12473.01 and ECOS CONICYT Cooperation grant Chile-France ECOS170032 (C.H.), the US National Institutes of Health (grants DK090313 and AR066634) (K.Z.) and the American Heart Association (grants 0635423Z and 09GRNT2280479) (K.Z.) for support. The authors acknowledge support from National Institutes of Health/National Cancer Institute grants R01DK113171, R01DK103185, R24DK110973, R01AG06219 and R01CA198103 and the Sanford Burnham Prebys Cancer Center grant P30 CA030199 (R.J.K.). R.J.K. is a member of the University of California, San Diego Diabetes Research Center (P30 DK063491) and an adjunct professor in the Department of Pharmacology, University of California, San Diego.
Glossary
- Signal recognition particle
A conserved, cytosolic ribonucleoprotein that recognizes and targets specific proteins to the endoplasmic reticulum membrane or plasma membrane
- Apoptosome
A quaternary protein complex, composed of mitochondrial cytochrome c, apoptotic protease activating factor 1 and deoxyadenosine triphosphate, formed in the process of apoptosis in response to cell death stimulus
- BCL-2 family
A protein family consisting of approximately 25 members that either promote or inhibit apoptosis by protein interactions that regulate mitochondrial outer membrane permeabilization
- Proteotoxicity
Adverse effects of aberrant or misfolded proteins that cause impairment of cell function
- Pre-B lymphocytes
A developmental stage of B lymphocytes defined by the expression of membrane μ-chains with surrogate light chains in the pre-B receptor, which is composed of two surrogate light chains and two immunoglobulin heavy chains expressed on the cell surface
- HSP70
A family of conserved ubiquitously expressed heat shock proteins that function as molecular chaperones or folding catalysts to assist protein folding or protect cells from stress
- COPII
A type of vesicle coat protein that transports newly synthesized proteins from the endoplasmic reticulum to the Golgi apparatus
- Collagens
The most abundant structural proteins in the extracellular matrix in connective tissues in mammals
- Foldases
Molecular chaperones that support the folding of nascent protein peptides in the endoplasmic reticulum
- Translocon
A complex of proteins associated with the translocation of nascent protein peptides into the luminal space of the endoplasmic reticulum from the cytosol within a cell
- Inositsol 1,4,5-trisphosphate receptors
Endoplasmic reticulum membrane glycoprotein complexes that act as the Ca2+ release channels within cells
- Lamellipodia
Thin plates of cytoplasm produced by cytoskeletal protein actin on the leading edge of a cell
- Filopodia
Thin, actin-rich cytoplasmic projections that extend beyond the edge of lamellipodia in cells
- Phospholipase Cγ1
(PLCγ). Catalyses the formation of inositol 1,4,5-trisphosphate and diacylglycerol from phosphatidylinositol 4,5-bisphosphate and plays an important role in signal transduction of receptor-mediated tyrosine kinase activators
- CCAAT/enhancer-binding protein
A member of a family of leucine zipper domain-containing transcription factors that are functionally involved in different cellular responses, such as in the control of cell growth and differentiation, metabolism and immunity
- Peroxisome proliferator-activated receptor
A member of a group of ligand-regulated transcription factors that control gene expression by binding to specific peroxisome proliferator hormone response elements within promoters
- Hepatic steatosis
A reversible condition in which excessive triglyceride fat accumulate in the liver cells, causing liver inflammation and fibrosis when the condition persists
- Innate immune response
The first line of non-specific immune response consisting of physical, chemical and cellular defences against the spread and movement of foreign pathogens
- Adaptive immune response
The acquired immune response that is primarily mediated by T and B lymphocytes to attack specific pathogens
- Isotype switching
A biological process that changes immunoglobulin production of B lymphocytes from one type to another; also known as immunoglobulin class switching
- UPRosome
A protein complex assembled at the level of IRE1α that regulates its activity and mediates the crosstalk with other signalling pathways and biological processes
Footnotes
Competing interests: The authors declare no competing interests.
Supplementary information: Supplementary information is available for this paper at https://doi.org/10.1038/s41580-020-0250-z.
References
- 1.Hebert DN & Molinari M In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol. Rev 87, 1377–1408 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Balch WE, Morimoto RI, Dillin A & Kelly JW Adapting proteostasis for disease intervention. Science 319, 916–919 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RJ Orchestrating the unfolded protein response in health and disease. J. Clin. Invest 110, 1389–1398 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ron D & Walter P Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol 8, 519–529 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Dever TE et al. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–596 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Vattem KM & Wek RC Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu PD, Harding HP & Ron D Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol 167, 27–33 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaman I et al. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113, 519–531 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Harding HP, Zhang Y & Ron D Protein translation and folding are coupled by an endoplasmic-reticulumresident kinase. Nature 397, 271–274 (1999). This study reports the discovery of the consequences of ER stress for protein translation through PERK. [DOI] [PubMed] [Google Scholar]
- 10.Harding HP et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Han J et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol 15, 481–490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novoa I, Zeng H, Harding HP & Ron D Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol 153, 1011–1022 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jousse C et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol 163, 767–775 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding HP et al. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc. Natl Acad. Sci. USA 106, 1832–1837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM & Walter P On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005). This study proposes a direct recognition model for sensing ER stress in yeast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl Acad. Sci. USA 103, 14343–14348 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida H, Matsui T, Yamamoto A, Okada T & Mori K XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001). This article is one of the first reports identifying XBP1 as a downstream target of IRE1α. [DOI] [PubMed] [Google Scholar]
- 18.Calfon M et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002). Yoshida et al. (2001) and Calfon et al. (2002) identify XBP1 as a downstream target of IRE1α. [DOI] [PubMed] [Google Scholar]
- 19.Shen X et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107, 893–903 (2001). This article is one of the first reports identifying XBP1 as a downstream target of IRE1α. [DOI] [PubMed] [Google Scholar]
- 20.Hollien J et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol 186, 323–331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollien J & Weissman JS Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006). In this study, the process known as regulated IRE1-dependent decay (RIDD) is discovered. [DOI] [PubMed] [Google Scholar]
- 22.Upton JP et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science 338, 818–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JM, Qiu Y, Yang ZQ, Li L & Zhang K Inositol-requiring enzyme 1 facilitates diabetic wound healing through modulating microRNAs. Diabetes 66, 177–192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han D et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassler JR et al. The IRE1alpha/XBP1s pathway is essential for the glucose response and protection of beta cells. PLoS Biol. 13, e1002277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta-Alvear D et al. The unfolded protein response and endoplasmic reticulum protein targeting machineries converge on the stress sensor IRE1. eLife 7, e43036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hetz C & Papa FR The unfolded protein response and cell fate control. Mol. Cell 69, 169–181 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Haze K, Yoshida H, Yanagi H, Yura T & Mori K Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Wu J et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Shoulders MD et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 3, 1279–1292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bommiasamy H et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci 122, 1626–1636 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sriburi R, Jackowski S, Mori K & Brewer JW XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol 167, 35–41 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaffer AL et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Hetz C et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312, 572–576 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Wei MC et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pihan P, Carreras-Sureda A & Hetz C BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 24, 1478–1487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R, Letai A & Sarosiek K Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol 20, 175–193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puthalakath H et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007). [DOI] [PubMed] [Google Scholar]
- 40.McCullough KD, Martindale JL, Klotz LO, Aw TY & Holbrook NJ Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol 21, 1249–1259 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marciniak SJ et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski DT et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedraza JM & van Oudenaarden A Noise propagation in gene networks. Science 307, 1965–1969 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Schinzel RT et al. The hyaluronidase, TMEM2, promotes ER homeostasis and longevity independent of the UPR(ER). Cell 179, 1306–1318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerner AG et al. IRE1alpha induces thioredoxininteracting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bronner DN et al. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43, 451–462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oslowski CM et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 16, 265–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JH et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam M, Lawrence DA, Ashkenazi A & Walter P Confirming a critical role for death receptor 5 and caspase-8 in apoptosis induction by endoplasmic reticulum stress. Cell Death Differ. 25, 1530–1531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munoz-Pinedo C & Lopez-Rivas A A role for caspase-8 and TRAIL-R2/DR5 in ER-stress-induced apoptosis. Cell Death Differ. 25, 226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu M et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang TK et al. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol. Cell 71, 629–636 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Rojas-Rivera D & Hetz C TMBIM protein family: ancestral regulators of cell death. Oncogene 34, 269–280 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Rojas-Rivera D et al. TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell Death Differ. 19, 1013–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chae HJ et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol. Cell 15, 355–366 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Bertolotti A, Zhang Y, Hendershot LM, Harding HP & Ron D Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol 2, 326–332 (2000). This study indicates that BiP is involved in the regulation of the UPR. [DOI] [PubMed] [Google Scholar]
- 57.Karagoz GE, Acosta-Alvear D & Walter P The unfolded protein response: detecting and responding to fluctuations in the protein-folding capacity of the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol 11, a033886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu CY, Wong HN, Schauerte JA & Kaufman RJ The protein kinase/endoribonuclease IRE1alpha that signals the unfolded protein response has a luminal N-terminal ligand-independent dimerization domain. J. Biol. Chem 277, 18346–18356 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Oikawa D, Kimata Y, Kohno K & Iwawaki T Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res 315, 2496–2504 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Walter P & Ron D The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Pincus D et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimata Y, Oikawa D, Shimizu Y, Ishiwata-Kimata Y & Kohno K A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol 167, 445–456 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishiwata-Kimata Y, Promlek T, Kohno K & Kimata Y BiP-bound and nonclustered mode of Ire1 evokes a weak but sustained unfolded protein response. Genes Cell 18, 288–301 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Amin-Wetzel N et al. A J-protein Co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171, 1625–1637 e1613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amin-Wetzel N, Neidhardt L, Yan Y, Mayer MP & Ron D Unstructured regions in IRE1alpha specify BiP-mediated destabilisation of the luminal domain dimer and repression of the UPR. eLife 8, e50793 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrara M, Prischi F, Nowak PR, Kopp MC & Ali MM Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife 4, e03522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopp MC, Nowak PR, Larburu N, Adams CJ & Ali MM In vitro FRET analysis of IRE1 and BiP association and dissociation upon endoplasmic reticulum stress. eLife 7, e30257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopp MC, Larburu N, Durairaj V, Adams CJ & Ali MMU UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol 26, 1053–1062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gardner BM & Walter P Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimata Y et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol 179, 75–86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karagoz GE et al. An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6, e30700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang P, Li J, Tao J & Sha B The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J. Biol. Chem 293, 4110–4121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sundaram A, Appathurai S, Plumb R & Mariappan M Dynamic changes in complexes of IRE1alpha, PERK, and ATF6alpha during endoplasmic reticulum stress. Mol. Biol. Cell 29, 1376–1388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oikawa D, Kitamura A, Kinjo M & Iwawaki T Direct association of unfolded proteins with mammalian ER stress sensor, IRE1beta. PLoS One 7, e51290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam M, Marsters SA, Ashkenazi A & Walter P Misfolded proteins bind and activate death receptor 5 to induce apoptosis during unresolved endoplasmic reticulum stress. eLife 9, e52291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sepulveda D et al. Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1alpha. Mol. Cell 69, 238–252 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Maiers JL et al. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 65, 983–998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eletto D, Eletto D, Dersh D, Gidalevitz T & Argon Y Protein disulfide isomerase A6 controls the decay of IRE1alpha signaling via disulfide-dependent association. Mol. Cell 53, 562–576 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groenendyk J et al. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci. Signal. 7, ra54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oka OB et al. ERp18 regulates activation of ATF6alpha during unfolded protein response. EMBO J 38, e100990 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Higa A et al. Endoplasmic reticulum stress-activated transcription factor ATF6alpha requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol. Cell Biol 34, 1839–1849 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Promlek T et al. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol. Biol. Cell 22, 3520–3532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volmer R, van der Ploeg K & Ron D Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl Acad. Sci. USA 110, 4628–4633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halbleib K et al. Activation of the unfolded protein response by lipid bilayer stress. Mol. Cell 67, 673–684 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Kono N, Amin-Wetzel N & Ron D Generic membrane-spanning features endow IRE1alpha with responsiveness to membrane aberrancy. Mol. Biol. Cell 28, 2318–2332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tam AB et al. The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev. Cell 46, 327–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fun XH & Thibault G Lipid bilayer stress and proteotoxic stress-induced unfolded protein response deploy divergent transcriptional and nontranscriptional programmes. Biochim. Biophys. Acta 1865, 158449 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Bailly-Maitre B et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 103, 2809–2814 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi L et al. Bax inhibitor-1 is required for resisting the early brain injury induced by subarachnoid hemorrhage through regulating IRE1-JNK pathway. Neurol. Res. 40, 189–196 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Lisbona F et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol. Cell 33, 679–691 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castillo K et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J. 30, 4465–4478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinkaew D et al. Fortilin binds IRE1alpha and prevents ER stress from signaling apoptotic cell death. Nat. Commun 8, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez DA et al. BH3-only proteins are part of a regulatory network that control the sustained signalling of the unfolded protein response sensor IRE1alpha. EMBO J. 31, 2322–2335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hetz C & Glimcher LH Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell 35, 551–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He Y et al. Nonmuscle myosin IIB links cytoskeleton to IRE1alpha signaling during ER stress. Dev. Cell 23, 1141–1152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morita S et al. Targeting ABL-IRE1alpha signaling spares ER-stressed pancreatic beta cells to reverse autoimmune diabetes. Cell Metab. 25, 883–897 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yanagitani K, Kimata Y, Kadokura H & Kohno K Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science 331, 586–589 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Shanmuganathan V et al. Structural and mutational analysis of the ribosome-arresting human XBP1u. eLife 8, e46267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanagitani K et al. Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol. Cell 34, 191–200 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Kanda S, Yanagitani K, Yokota Y, Esaki Y & Kohno K Autonomous translational pausing is required for XBP1u mRNA recruitment to the ER via the SRP pathway. Proc. Natl Acad. Sci. USA 113, E5886–E5895 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plumb R, Zhang ZR, Appathurai S & Mariappan M A functional link between the co-translational protein translocation pathway and the UPR. eLife 4, e07426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsukumo Y et al. TBL2 is a novel PERK-binding protein that modulates stress-signaling and cell survival during endoplasmic reticulum stress. PLoS One 9, e112761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huber AL et al. p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Mol. Cell 49, 1049–1059 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Tyagi R et al. Rheb inhibits protein synthesis by activating the PERK-eIF2alpha signaling cascade. Cell Rep. 10, 684–693 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee KP et al. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132, 89–100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Korennykh AV et al. The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prischi F, Nowak PR, Carrara M & Ali MM Phosphoregulation of Ire1 RNase splicing activity. Nat. Commun. 5, 3554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mao T et al. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc. Natl Acad. Sci. USA 108, 15852–15857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saito A et al. Neuronal activity-dependent local activation of dendritic unfolded protein response promotes expression of brain-derived neurotrophic factor in cell soma. J. Neurochem 144, 35–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayashi A et al. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J. Biol. Chem 282, 34525–34534 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Qiu Y et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Sci. Signal 3, ra7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiu Q et al. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J. 32, 2477–2490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu G et al. PPM1l encodes an inositol requiring-protein 1 (IRE1) specific phosphatase that regulates the functional outcome of the ER stress response. Mol. Metab 2, 405–416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui W, Li J, Ron D & Sha B The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr. D. Biol. Crystallogr 67, 423–428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L et al. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 349, 500–506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang JM et al. IRE1alpha prevents hepatic steatosis by processing and promoting the degradation of select microRNAs. Sci Signal 11, eaao4617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakato R et al. Regulation of the unfolded protein response via S-nitrosylation of sensors of endoplasmic reticulum stress. Sci. Rep 5, 14812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hourihan JM, Moronetti Mazzeo LE, Fernandez-Cardenas LP & Blackwell TK Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol. Cell 63, 553–566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu X et al. Ubiquitination of inositol-requiring enzyme 1 (IRE1) by the E3 ligase CHIP mediates the IRE1/TRAF2/JNK pathway. J. Biol. Chem 289, 30567–30577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu H et al. Ufbp1 promotes plasma cell development and ER expansion by modulating distinct branches of UPR. Nat. Commun 10, 1084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tschurtschenthaler M et al. Defective ATG16L1-mediated removal of IRE1alpha drives Crohn’s disease-like ileitis. J. Exp. Med 214, 401–422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao B et al. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 9, 480–485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nadanaka S, Okada T, Yoshida H & Mori K Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol. Cell Biol 27, 1027–1043 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shemorry A et al. Caspase-mediated cleavage of IRE1 controls apoptotic cell commitment during endoplasmic reticulum stress. eLife 8, e47084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fonseca SG et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest 120, 744–755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phillips MJ & Voeltz GK Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol 17, 69–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verfaillie T et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 19, 1880–1891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carreras-Sureda A et al. Non-canonical function of IRE1alpha determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat. Cell Biol 21, 755–767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Munoz JP et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 32, 2348–2361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takeda K et al. MITOL prevents ER stress-induced apoptosis by IRE1alpha ubiquitylation at ERmitochondria contact sites. EMBO J 38, e100999 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Vliet AR et al. The ER stress sensor PERK coordinates ER-plasma membrane contact site formation through interaction with filamin-A and F-actin remodeling. Mol. Cell 65, 885–899 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Ishiwata-Kimata Y, Yamamoto YH, Takizawa K, Kohno K & Kimata Y F-actin and a type-II myosin are required for efficient clustering of the ER stress sensor Ire1. Cell Struct. Funct 38, 135–143 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Urra H et al. IRE1alpha governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol 20, 942–953 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Tavernier Q et al. Regulation of IRE1 RNase activity by the ribonuclease inhibitor 1 (RNH1). Cell Cycle 17, 1901–1916 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinon F, Chen X, Lee AH & Glimcher LH TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol 11, 411–418 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woo CW, Kutzler L, Kimball SR & Tabas I Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol 14, 192–200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woo CW et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat. Cell Biol 11, 1473–1480 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chopra S et al. IRE1alpha-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science 365, eaau6499 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Lipson KL et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulumresident protein kinase IRE1. Cell Metab. 4, 245–254 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Karali E et al. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol. Cell 54, 559–572 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Iwakoshi NN et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol 4, 321–329 (2003). [DOI] [PubMed] [Google Scholar]
- 142.van Anken E et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18, 243–253 (2003). [DOI] [PubMed] [Google Scholar]
- 143.Morimoto RI Cell-nonautonomous regulation of proteostasis in aging and disease. Cold Spring Harb. Perspect. Biol 10.1101/cshperspect.a034074 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Williams KW et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20, 471–482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP & Hetz C Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16, 615–623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taylor RC & Dillin A XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imanikia S, Ozbey NP, Krueger C, Casanueva MO & Taylor RC Neuronal XBP-1 activates intestinal lysosomes to improve proteostasis in C. elegans. Curr. Biol 29, 2322–2338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daniele JR et al. UPR(ER) promotes lipophagy independent of chaperones to extend life span. Sci. Adv 6, eaaz1441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matai L et al. Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc. Natl Acad. Sci. USA 116, 17383–17392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Frakes AE & Dillin A The UPR(ER): sensor and coordinator of organismal homeostasis. Mol. Cell 66, 761–771 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Luis NM et al. Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep. 17, 1207–1216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dufey E et al. Genotoxic stress triggers the selective activation of IRE1α-dependent RNA decay to modulate the DNA damage response. Nat. Commun 11, 2401 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang C et al. Identification of XBP1-u as a novel regulator of the MDM2/p53 axis using an shRNA library. Sci. Adv 3, e1701383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang K et al. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 30, 1357–1375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamamoto K et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell 21, 2975–2986 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rutkowski DT et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 15, 829–840 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang K et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006). [DOI] [PubMed] [Google Scholar]
- 158.Bailey D & O’Hare P Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal 9, 2305–2321 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Zhang C et al. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology 55, 1070–1082 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zheng Z et al. CREBH couples circadian clock with hepatic lipid metabolism. Diabetes 65, 3369–3383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim H et al. Regulation of hepatic autophagy by stress-sensing transcription factor CREBH. FASEB J 33, 7896–7914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim H et al. Liver-enriched transcription factor CREBH interacts with peroxisome proliferatoractivated receptor alpha to regulate metabolic hormone FGF21. Endocrinology 155, 769–782 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim H, Zheng Z, Walker PD, Kapatos G & Zhang K CREBH maintains circadian glucose homeostasis by regulating hepatic glycogenolysis and gluconeogenesis. Mol. Cell Biol 37, e00048–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee MW et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 11, 331–339 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Lee JH et al. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat. Med 17, 812–815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cefalu AB et al. Novel CREB3L3 nonsense mutation in a family with dominant hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol 35, 2694–2699 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Harding HP et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001). [DOI] [PubMed] [Google Scholar]
- 168.Back SH et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 10, 13–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee AH, Chu GC, Iwakoshi NN & Glimcher LH XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Delepine M et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet 25, 406–409 (2000). [DOI] [PubMed] [Google Scholar]
- 171.Scheuner D et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med 11, 757–764 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Song B, Scheuner D, Ron D, Pennathur S & Kaufman RJ Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest 118, 3378–3389 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ozcan U et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). [DOI] [PubMed] [Google Scholar]
- 174.Fu S et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Urra H, Dufey E, Avril T, Chevet E & Hetz C Endoplasmic reticulum stress and the Hallmarks of cancer. Trends Cancer 2, 252–262 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Lee AS GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 67, 3496–3499 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Chen X et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature 508, 103–107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lhomond S et al. Dual IRE1 RNase functions dictate glioblastoma development. EMBO Mol. Med 10, e7929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee AH, Iwakoshi NN, Anderson KC & Glimcher LH Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang CH et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J. Clin. Invest 124, 2585–2598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hetz C, Axten JM & Patterson JB Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol 15, 764–775 (2019). [DOI] [PubMed] [Google Scholar]
- 182.Sheng X et al. IRE1alpha-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun 10, 323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu Y et al. Dual role for inositol-requiring enzyme 1alpha in promoting the development of hepatocellular carcinoma during diet-induced obesity in mice. Hepatology 68, 533–546 (2018). [DOI] [PubMed] [Google Scholar]
- 184.Rubio-Patino C et al. Low-protein diet induces IRE1alpha-dependent anticancer immunosurveillance. Cell Metab. 27, 828–842 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Niederreiter L et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J. Exp. Med 210, 2041–2056 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bi M et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nguyen HG et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl Med 10, eaar2036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bettigole SE & Glimcher LH Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol 33, 107–138 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Zhang K et al. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest 115, 268–281 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Todd DJ et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med 206, 2151–2159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brunsing R et al. B- and T-cell development both involve activity of the unfolded protein response pathway. J. Biol. Chem 283, 17954–17961 (2008). [DOI] [PubMed] [Google Scholar]
- 192.Iwakoshi NN, Pypaert M & Glimcher LH The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med 204, 2267–2275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tavernier SJ et al. Regulated IRE1-dependent mRNA decay sets the threshold for dendritic cell survival. Nat. Cell Biol 19, 698–710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dong H et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat Immunol 20, 865–878 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu S et al. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J. Biol. Chem 279, 34898–34902 (2004). [DOI] [PubMed] [Google Scholar]
- 196.Deng J et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol 24, 10161–10168 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iwasaki Y et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes 63, 152–161 (2014). [DOI] [PubMed] [Google Scholar]
- 198.Gargalovic PS et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol 26, 2490–2496 (2006). [DOI] [PubMed] [Google Scholar]
- 199.Wheeler MA et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shan B et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol 18, 519–529 (2017). [DOI] [PubMed] [Google Scholar]
- 201.Martinez G, Khatiwada S, Costa-Mattioli M & Hetz C ER proteostasis control of neuronal physiology and synaptic function. Trends Neurosci. 41, 610–624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sidrauski C et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife 2, e00498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Martinez G et al. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 14, 1382–1394 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Laguesse S et al. A dynamic unfolded protein response contributes to the control of cortical neurogenesis. Dev. Cell 35, 553–567 (2015). [DOI] [PubMed] [Google Scholar]
- 205.Fox JW et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21, 1315–1325 (1998). [DOI] [PubMed] [Google Scholar]
- 206.Hayashi A, Kasahara T, Kametani M & Kato T Attenuated BDNF-induced upregulation of GABAergic markers in neurons lacking Xbp1. Biochem. Biophys. Res. Commun 376, 758–763 (2008). [DOI] [PubMed] [Google Scholar]
- 207.Hetz C & Saxena S ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol 13, 477–491 (2017). [DOI] [PubMed] [Google Scholar]
- 208.Freeman OJ & Mallucci GR The UPR and synaptic dysfunction in neurodegeneration. Brain Res. 1648, 530–537 (2016). [DOI] [PubMed] [Google Scholar]
- 209.Halliday M et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 140, 1768–1783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moreno JA et al. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature 485, 507–511 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Valdes P et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl Acad. Sci. USA 111, 6804–6809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hetz C et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 23, 2294–2306 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vidal RL et al. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum. Mol. Genet 21, 2245–2262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Duran-Aniotz C et al. IRE1 signaling exacerbates Alzheimer’s disease pathogenesis. Acta Neuropathol. 134, 489–506 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Hetz C et al. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc. Natl Acad. Sci. USA 105, 757–762 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Valenzuela V et al. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 3, e272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Onate M et al. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep 6, 21709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Valenzuela V, Jackson KL, Sardi SP & Hetz C Gene therapy strategies to restore ER proteostasis in disease. Mol. Ther 26, 1404–1413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Belyy V, Tran NH & Walter P Quantitative microscopy reveals dynamics and fate of clustered IRE1alpha. Proc. Natl Acad. Sci. USA 117, 1533–1542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hughes D & Mallucci GR The unfolded protein response in neurodegenerative disorders – therapeutic modulation of the PERK pathway. FEBS J. 286, 342–355 (2019). [DOI] [PubMed] [Google Scholar]
- 221.Axten JM et al. Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med. Chem. Lett 4, 964–968 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Spaan CN et al. Expression of UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell proliferation and stemness by activating PERK signaling. Cell Death Dis. 10, 490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kohl S et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet 47, 757–765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Richardson PG, Mitsiades C, Hideshima T & Anderson KC Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med 57, 33–47 (2006). [DOI] [PubMed] [Google Scholar]
- 225.Shiu RP, Pouyssegur J & Pastan I Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc. Natl Acad. Sci. USA 74, 3840–3844 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Haas IG & Wabl M Immunoglobulin heavy chain binding protein. Nature 306, 387–389 (1983). [DOI] [PubMed] [Google Scholar]
- 227.Munro S & Pelham HR An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46, 291–300 (1986). [DOI] [PubMed] [Google Scholar]
- 228.Fornace AJ Jr. et al. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell Biol 9, 4196–4203 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kozutsumi Y, Segal M, Normington K, Gething MJ & Sambrook J The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988). [DOI] [PubMed] [Google Scholar]
- 230.Dorner AJ, Wasley LC & Kaufman RJ Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J. Biol. Chem 264, 20602–20607 (1989). [PubMed] [Google Scholar]
- 231.Cox JS, Shamu CE & Walter P Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993). [DOI] [PubMed] [Google Scholar]
- 232.Mori K, Ma W, Gething MJ & Sambrook J A transmembrane protein with a cdc2 +/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993). [DOI] [PubMed] [Google Scholar]
- 233.Cox JS & Walter P A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996). [DOI] [PubMed] [Google Scholar]
- 234.Tirasophon W, Welihinda AA & Kaufman RJ A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang XZ et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17, 5708–5717 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liou HC et al. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science 247, 1581–1584 (1990). [DOI] [PubMed] [Google Scholar]
- 237.Shi Y et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell Biol 18, 7499–7509 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yoshida H, Haze K, Yanagi H, Yura T & Mori K Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem 273, 33741–33749 (1998). [DOI] [PubMed] [Google Scholar]
- 239.Wu H, Carvalho P & Voeltz GK Here, there, and everywhere: the importance of ER membrane contact sites. Science 361(6401): eaan5835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Theurey P & Rieusset J Mitochondria-associated membranes response to nutrient availability and role in metabolic diseases. Trends Endocrinol. Metab. 28, 32–45 (2017). [DOI] [PubMed] [Google Scholar]
- 241.Novikoff AB, Novikoff PM, Rosen OM & Rubin CS Organelle relationships in cultured 3T3-L1 preadipocytes. J. Cell Biol 87, 180–196 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rambold AS, Cohen S & Lippincott-Schwartz J Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678–692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Settembre C, Fraldi A, Medina DL & Ballabio A Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol 14, 283–296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


