Abstract
Background.
Although the effects of prenatal alcohol exposure (PAE) have been studied extensively, there is relatively little information available on adult mental health functioning among exposed individuals. The current study examined self-reported mental health status among midlife individuals who were prenatally exposed to alcohol and diagnosed in childhood with effects of this exposure and compares their outcomes to those of unexposed individuals.
Methods.
Participants (N=292) were recruited from two longitudinal cohorts in Atlanta and Seattle, and asked to complete an Adult Health Questionnaire that surveyed their current health and mental health status. The questionnaire could be completed either in-person or remotely and included questions about current symptoms of depression and anxiety as well as diagnosis of mental health disorders. Analysis compared a Non-Exposed Contrast group to those in two exposure groups: 1) Alcohol Exposed with Fetal Alcohol Effect (FAE) but not meeting criteria for Fetal Alcohol Syndrome (FAS) and 2) Alcohol Affected and meeting criteria for FAS .
Results.
Both alcohol-exposed groups reported higher levels of current depressive symptoms as well as higher prevalence of diagnosis of depression, anxiety, bipolar disorder and/or attention deficit/ hyperactivity disorder. No differences were noted for psychotic disorders. PAE was also associated with greater environmental stressors including higher levels of adverse childhood events and lower current socioeconomic status. Path analyses suggested that PAE was indirectly related to mood disorders with its effects being mediated by other environmental factors.
Conclusions.
PAE is associated with increased rates of mental health disorders in middle adulthood. These outcomes appear to result from multiple stressors affecting individuals made vulnerable by their early exposure. Clinical outcomes could be improved by prevention efforts directed at both prenatal alcohol use and later environmental stressors, and by early identification of PAE and its effects.
Keywords: Prenatal Alcohol Exposure, Fetal Alcohol Spectrum Disorders, Mental Health, Adverse Childhood Experiences, Adult Health
Effects of prenatal alcohol exposure (PAE) have been studied extensively. A spectrum of response to PAE has been identified and referred to as the fetal alcohol spectrum disorders (FASD). While the primary focus of research has been on the physical birth defects (Jones, Smith, Ulleland, & Streissguth, 1973), growth (Day et al., 1989) and neurocognitive development (Doyle & Mattson, 2015) that define fetal alcohol syndrome (FAS), an association between PAE and mental health disorders has been observed as well (O'Connor & Paley, 2009; Spohr, Willms, & Steinhausen, 2007; Weyrauch, Schwartz, Hart, Klug, & Burd, 2017) but remains much less thoroughly examined than other outcomes. This is particularly true for adults with PAE and FASD. There is relatively little information available about the impact of PAE on emotional status in adulthood and, in the clinically referred groups who are often the subjects of study, it can be difficult to discriminate the effects of prenatal exposure against the backdrop of social and environmental stressors that often accompany PAE.
Most information on adults with PAE has been obtained from individuals in their 20’s. Data from the Seattle Longitudinal Study on Health and Pregnancy (Streissguth, Barr, Sampson, & Bookstein, 1994), a community-based exposure study that included individuals prenatally exposed to alcohol and followed longitudinally, suggested that by the age of 21, there was an association between exposure to maternal binge drinking during pregnancy (defined as ≥ 5 drinks on at least one occasion) and having a higher level of psychiatric symptoms (Barr et al., 2006).
In a different, clinical sample, Streissguth and her colleagues (Streissguth, Barr, Kogan, & Bookstein, 1996) reported on 473 patients, ranging in in age from 3 to 51 years of age, who were seen at the University of Washington for diagnosis of effects of PAE. They found that among what they referred to as “secondary disabilities”, mental health problems were “by far the most prevalent” (p34) and were experienced by over 90% of the sample. While attention deficit/hyperactivity disorder (ADHD) was most commonly identified in childhood, by adulthood, depression, suicidal ideation, suicide attempts and panic attacks were reported more often. Famy and colleagues (Famy, Streissguth, & Unis, 1998) carried out structured clinical interviews with 25 young adults from this same cohort who were not intellectually impaired, finding that mood disorders (depression and anxiety) and psychotic disorders were the most common psychiatric diagnoses in this group. In addition to mood and anxiety disorders in individuals with PAE, substance use disorders among adults with FASD have been a concern. In fact, increased alcohol use was found in the Seattle sample among young adults (Baer, Sampson, Barr, Connor, & Streissguth, 2003).
The Atlanta Longitudinal Alcohol Study (Lynch, Kable, & Coles, 2017) compared two groups of nonexposed young adults (one non-exposed group, typically developing and the other, clinically diagnosed) to three groups of young adults with PAE at an average age of 23.28 years (SD: 1.76). Those with PAE were classified as 1) having physical features or growth retardation as well as cognitive effects (Dysmorphic), 2) cognitively affected without physical features or 3) exposed without physical or cognitive effects. Overall, all prenatally exposed young adults reported more emotional and behavioral problems than those in nonexposed contrast groups. Both groups of alcohol-exposed individuals who did not have physical features or growth retardation reported higher levels of Internalizing problems (depression and anxiety) while all alcohol groups reported more externalizing issues (attention problems/conduct disorders) than typical controls. Substance use was also higher in those who were alcohol-exposed without physical/cognitive features in comparison to both controls and other alcohol-exposed groups. Substance use was also higher in the exposure-only group in comparison to both control groups and the other PAE groups.
Although mental health problems are found frequently in individuals with PAE, the contribution of other factors to these outcomes also should be considered, as O’Connor has pointed out in her review (O’Connor, 2014). In 2017, Price and colleagues (Price, Cook, Norgate, & Mukherjee, 2017) reviewed the literature on the impact of adverse childhood experiences (ACEs) (Felitti et al., 1998) in individuals with PAE, finding only five articles that had addressed this issue. They concluded that while the available research was suggestive of an additive impact of ACES and PAE compared to either ACES or PAE alone, it was limited in scope and that there were design issues limiting generalizability. These researchers noted that there was no research as yet addressing the lifetime risk associated with the joint occurrence of these two risk factors. Subsequently, several studies have examined the association of ACES with PAE in children and young adults. Flannigan and colleagues (Flannigan et al., 2021) found that, in a sample referred for FASD diagnosis, a majority reported ACES in a range indicating substantial risk. Similarly, Lebel and colleagues (Lebel et al., 2019) found that two thirds of children in their sample had co-occurring PAE and postnatal adversities. In contrast, Kambeitz, et al (2019) reviewed clinical records and found that children with FASD had significantly higher ACEs than those who could not be diagnosed. In a recent registry-based cohort study of 615 individuals, ages 15 to 24 years, (Koponen et al., 2020), the intersection of prenatal substance exposure, including alcohol, ACES and diagnosis of mental and behavioral disorders was examined. The investigators found that the incidence of mental health disorders was twice as high among those with prenatal substance exposure (alcohol and polydrug use) versus nonexposed but that the difference was diminished after controlling for the effects of ACEs. Among the many risk factors measured, low birth weight, maternal risk factors and custody changes were the strongest predictors of later mental and behavioral disorders Thus, previous research has indicated that higher scores on the ACEs measure are associated with increased risk to adult mental health (Merrick, et al., 2017) and that experience of multiple stressors affect health and development (Sarkar, Patro, & Patro, 2019). Given these findings, attention to postnatal events in this population may be essential in understanding risk for later physical and mental health outcomes.
Hypotheses.
The current study examines two groups of alcohol-exposed individuals who are now in middle adulthood, those enrolled in the Atlanta Alcohol Study (Lynch et al., 2017), initiated in 1980, and those in the Seattle Fetal Alcohol Follow-Up Study clinical cohort (Streissguth et al., 1996). Our hypothesis was that, at midlife, members of these cohorts who were alcohol-exposed or who were diagnosed with FAS or Fetal Alcohol Effects (FAE, the terminology used at the time) would report a higher incidence of mental health disorders than those who were not exposed. In addition, we hypothesized that environmental factors, including early childhood adversity and social factors, would contribute to such outcomes.
Methods
Subjects.
In the current study, we report on 292 individuals who responded to a health survey between January 2017 and May 2021. Of these, 283 had responses that included all data necessary to carry out causal modeling. Participants were recruited from two existing cohorts in Atlanta and Seattle. Individuals defined as alcohol-exposed in these cohorts were known to have been diagnosed with an FASD or to have been exposed to alcohol prenatally according to maternal self-report during the prenatal period. Individuals in contrast groups were recruited either at the same time as those in the alcohol groups or at later time points. The Atlanta Longitudinal Alcohol Study included 427 low SES, predominantly African-American individuals who were born from 1980 to 1986. Of these 292 (70% alcohol-exposed) are part of the cohort recruited prenatally whose mothers provided information about alcohol use during pregnancy. The Seattle Fetal Alcohol Follow-Up Study was comprised of an original cohort of 475 individuals, predominantly White and Native American, who were 23 to 71 years old when the present study was initiated. The Seattle cohort included 178 (37.4%) diagnosed with FAS and 297 (62.7%) who were non dysmorphic but affected and classified as having FAE (using the diagnostic terminology of the time). Nonexposed contrast group members had been recruited from individuals contemporaneously participating in other studies carried out by Seattle investigators. All participants from both cohorts have been followed longitudinally.
For the current study, contact information was obtained from study records and from commercially-available databases that identified current addresses, phone numbers and other information. When contact information was confirmed, eligible participants who could be reached were informed by mail, phone or email of the opportunity to participate. For those who could be located and who volunteered for follow-up, informed consent was carried out in person or remotely using REDCap (Research Electronic Data Capture), a secure web application for building and managing online surveys and databases that is compliant with the requirements of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) (Centers for Disease Control and Prevention, 2021). Informed consent procedures were approved by the Internal Review Boards at Emory University School of Medicine and the University of Washington. With the advent of the COVID-19 pandemic, several options were made available to complete the Health Survey and Demographic Questionnaires that are the basis for this paper, including in-person or telephone interviews and remote on-line completion via REDCap. Participants were reimbursed $25 for their time in completing the surveys.
Measures.
Two surveys were used. The first is a simple demographic form that obtains basic information about the participant (e.g., date of birth, gender identity, racial identification) and their family (e.g., marital status, number of children). As part of the form, the information necessary to calculate the Hollingshead social status index (Hollingshead, 2011) was collected (i.e., the educational achievement and occupation of participants and their spouses/partners, if relevant).
The second instrument was a 190-item health survey based on the Behavioral Risk Factor Surveillance System (BRFSS) used by the Centers for Disease Control and Prevention (CDC) (CDC - BRFSS - Questionnaires ). Our goal was to survey the systemic health status of the participants. The number of questions completed depended on the participants’ responses as follow-up questions were not presented unless a health problem was acknowledged. Consistent with the BRFSS, the questions did not go “in depth” and could be as simple as “Have you ever been told by a health care professional that you have high blood pressure?” The BRFSS includes modules that assess health status in respondents and we used a number of these in our questionnaire (See Table 1).
Table 1.
Adult Health Questionnaire: Areas Queried
| Overall Physical and Mental Health (10 items) |
| Health Care Access (9 items) |
| Chronic Health Problems |
| Weight (5 items) |
| Sleep (9 items) |
| Vision (7 items)* |
| Hearing (7 items)* |
| Dental (5 items)* |
| Cardiovascular Health (Heart and Blood) (6 items) |
| Hypertension Awareness (4 items) |
| Cholesterol Awareness (5 items) |
| Other Cardiovascular Problems (3 items) |
| Cancer (3 items) |
| Stomach, Digestion, Kidney, Bladder and Bowel Problems (13 items)* |
| Endocrine Disorders |
| Diabetes (8 items) |
| Thyroid and Parathyroid Disorders (5 items) |
| Skin Problems (7 items) |
| Immune and Autoimmune Disorders (5 items) |
| Allergies (4 items) |
| Asthma (6 items) |
| Autoimmune Disorders (4 items)* |
| Arthritis (7 items) |
| Mental Health and Neurology |
| Seizure Disorders (10 items)* |
| Mental Health (14 items) |
| Symptoms of anxiety/depression (23 items) |
| Adverse Childhood Experiences (ACES) (11 items) |
Items not included in BRFSS (CDC) Questionnaires and added by authors.
In addition, because there were areas of interest for the current study that were not addressed in the BRFSS, we created our own modules to ask about neurological symptoms, vision, hearing and dental health, gastrointestinal problems, and immune system disorders. The BRFSS includes an assessment of ACEs (Felitti et al., 1998) and we included this as well. Table 1 shows the areas addressed in the survey. The Health Survey, as used in this study, is available from the authors.
Relevant to the current study, participants were asked to respond “Yes” or “No” to questions regarding whether a health care professional had ever diagnosed them with any of the following disorders: Depression, Bipolar Disorder, Anxiety Disorder, Psychosis, or ADHD. In addition, eight specific symptoms of depression/anxiety were listed, and participants were asked to identify those that they had experienced in the last two weeks. Symptoms were based on those identified in the American Psychiatric Association’s Diagnostic and Statistical Manual, 5th Edition (American Psychiatric Association, 2013). The question about suicidal ideation was not included.
Data Analysis.
Demographic data were analyzed to compare three groups of participants: 1) Contrast group of non-exposed adults; 2) Alcohol-exposed individuals not meeting criteria for FAS but exhibiting behavioral/cognitive effects, and 3) Alcohol-exposed individuals with physical effects of alcohol exposure (i.e., having “dysmorphic features”), and meeting criteria for FAS. In Seattle, dysmorphology was identified by pediatric dysmorphologists at initial diagnosis. For the current analysis, those characterized as FAS were considered to be dysmorphic. In Atlanta, an index of severity of alcohol-related dysmorphia based on a weighted dysmorphia checklist done when participants were children (Fernhoff, Smith, & Falek, 1980) was included in the model. For the analyses reported here, dysmorphology was coded as present or absent.
Chi square tests were used for analysis of categorical and ordinal data and analysis of variance was used for continuous measures. ACEs were examined individually and summed to yield a total score. Educational and vocational information for both participant and spouse, if any, was collected on the Demographic form and was used to calculate the socioeconomic status (SES) ranking for each individual (Hollingshead, 2011).
Anticipating that multiple factors would need to be taken into account in modeling mental health at midlife, we used path analysis (Sarwono, 2017) to examine the contribution of prenatal alcohol exposure on mental health outcomes while allowing for the contribution of various potentially confounding and mediating factors. Factors in such a model can be either exogenous, that is not dependent on other variables in the model although they may be correlated, or endogenous, that is, affected by other factors in the model. In the current analyses, exogenous factors include site of data collection (1) Seattle; 2) Atlanta), participants’ current age, gender Identification (1) Male; 2) Female) and PAE. Endogenous variables include, SES (Hollingshead, 2011), ACEs (Merrick et al., 2017), and presence of physical symptoms of alcohol exposure either not meeting criteria for alcohol dysmorphology or Dysmorphic/FAS, a variable included in the model to determine if individuals with physical dysmorphology are differentially impacted.
Several path analyses were done with the following binomial (1) Yes; 0) No) to predict outcome including the reported diagnosis of depression, anxiety, bipolar disorder, psychotic symptoms, and ADHD. Additionally, the continuous outcome of number of current reported symptoms of depression and anxiety (0 to 8) was also predicted. Path analysis was calculated using the AMOS function of SPSS 28.
Results
Participants.
Across sites, of the 342 individuals who could be located to date and who were approached for the study, 292 agreed to participate (85%) and 28 refused to participate. Other individuals were found but did not participate due to location, life circumstances, or incarceration and a total of 41 were identified as deceased. Of the 292 who provided data, 283 had all items available that were necessary to carry out the Path Analyses. Table 2 shows the characteristics of participants comparing those in the non-exposed contrast group with those who were alcohol exposed/FAE (without physical features) and those who could be classified as alcohol affected/FAS (having dysmorphic physical features). There were no between group difference in age at the time of the study with the average in the late 30s and there were more females than males in all groups. There were no significant differences in racial identification by group although there was a higher percentage of African-Americans in the Contrast group and more Whites in the Alcohol groups. Members of the non-exposed contrast group reported having more years of schooling and higher incomes and were more likely to be married than those in the alcohol exposed groups. Those in the Contrast group had higher Hollingshead social status ratings (Hollingshead, 2011) and reported fewer ACEs compared to those in the alcohol-exposed groups. The majority in all groups had health insurance but those in the alcohol-exposed groups were more likely to have Medicaid while Non-Exposed Contrast group members were more likely to have private insurance.
Table 2:
Characteristics of Participants by Group (N=292)
| Non Exposed Contrast (n=98) |
Alcohol Exposed/FAE (n=116 ) |
Alcohol Affected/FAS (n=78) |
Statistic | p value | |
|---|---|---|---|---|---|
| Age (yrs) M (SD) (n=285) | 37.54 (3.98) | 37.87 (6.54) | 38.81 (5.83) | F(2,282)=1.06 | p=.347, ns |
| Gender (% male) | 40.9% | 35.4% | 49.4% | X2(2) =3.68 | p=.158, ns |
| Race (%) 1 | X2(10) =17.45 | p=.065 | |||
| Native American | 5.1% | 9.5% | 9.0% | ||
| Black/African-American | 48.0% | 31.0% | 37.2% | ||
| Pacific Islander | 0 | 0 | 1.3% | ||
| White | 37.8% | 43.1% | 44.9% | ||
| Mixed Race | 4.1% | 13.8% | 6.4% | ||
| Ethnicity1 (%) | X2(6) =6.57 | p=.36, ns | |||
| Non-Hispanic | 89.8% | 87.1% | 94.9% | ||
| Years Education (%) | X2(16) =65.66 | p=.000 | |||
| 9th grade or less | 3.1% | 5.4% | 3.9% | ||
| Partial High School | 8.2% | 20.7% | 20.5% | ||
| High School | 16.3% | 24.1% | 35.9% | ||
| Graduate | 18.4% | 36.6% | 30.8% | ||
| Post HS /technical | 29.6% | 6.9% | 6.4% | ||
| College Graduate | 5.1% | 0.9% | 0 | ||
| Graduate School | 14.3% | 5.2% | 1.3% | ||
| Graduate Degree (Did not respond) | (5.1%) | (3.4%) | (1.3%) | ||
| Marital Status (%) | X2(12) =23.37 | p=.025 | |||
| Never Married | 33.7% | 41.4% | 61.5% | ||
| Divorced/Separated | 11.2% | 15.5% | 11.6% | ||
| Widowed | 0 | 0.9% | 1.3% | ||
| Living with Partner | 13.3% | 13.8% | 5.1% | ||
| Married (Did not respond) | 36.7% (5.1%) | 25.0% (3.4%) | 19.2% (1.3%) | ||
| Number of biological children | 2.41 (1.27) | 2.60 (1.43) | 2.17 (1.34) | F(2,180)=1.43 | p=.242. ns |
| Monthly Family | X2(16) =48.88 | p=.000 | |||
| Income (%) | |||||
| <$500 | 1.0% | 6.9% | 6.4% | ||
| $501-1000 | 5.1% | 12.9% | 28.2% | ||
| $1001-2000 | 9.2% | 16.4% | 14.1% | ||
| $2001-3000 | 13.3% | 12.1% | 10.3% | ||
| $3001-4000 | 6.1% | 12.9% | 12.8% | ||
| >$4000 | 56.9% | 16.4% | 15.4% | ||
| Refused/Don’t know | 23.5% | 22.3% | 12.9% | ||
| Hollingshead SES rating 2 (N=283) | 40.61 (15.41) | 29.86 (12.68) | 26.50 (11.03) | F(2,275)=27.24 | p<.000 |
| Total Adverse Childhood Experiences (ACES) M (SD) (N=285) | 2.66 (3.49) | 6.59 (5.61) | 5.43 (4.44) | F(2,282)=18.93 | p<.000 |
| Do you have Health Insurance? “Yes” % | 79.6% | 75% | 83.3% | X2(6) =6.33 | p=.387 |
| What type of Health insurance do you have? (%) | X2(10) =39.59 | p<.000 | |||
| Private Insurance | 59.2% | 36.2% | 23.1% | ||
| HMO Prepaid | 1.0% | 0.9% | 0 | ||
| Government (Medicaid, HIS) | 18.4% | 35.3% | 59% | ||
| Other/Don’t know | 1.0% | 2.6% | 1.3% |
Some did not respond to this question so percentages do not total 100%.
Ratings range from 8 to 66 with higher ratings indicating greater social status based on Occupation and Education. Ratings are averaged for families with rankings of both spouses (Hollingshead, 2011).
Mental Health Status.
Presence of a diagnosed mental health disorder was assumed if participants responded “yes” to questions in this form, “Has a doctor, nurse, or other health care professional said that you have…..?” In addition, participants responded to eight questions about symptoms of depression and anxiety experienced over the last two weeks, and these were summed with possible scores ranging from 0 to 8. Results are shown in Table 3 and indicate that prenatal alcohol exposure was associated with significantly higher reported diagnosis of depression, bipolar disorder, anxiety disorder and ADHD but not psychotic disorders. In addition, the number of current symptoms of anxiety or depression was significantly higher for the alcohol-exposed groups.
Table3:
Self-Reported Mental Health Outcomes in Alcohol Exposed Groups and Non-Exposed Contrast Groups.
| Non Exposed Contrast (n=98) |
Alcohol Exposed/FAE (n=116 ) |
Alcohol Affected/FAS (n=78) |
Statistic | P value | |
|---|---|---|---|---|---|
| Depression Y/N | 26.5% | 43.1% | 33.3% | X2(6) =13.04 | p<.04 |
| Current Symptoms of Depression Total M (SD) | 1.75 (2.08) | 4.06 (3.14) | 3.47 (2.54) | F(2,284)=20.42 | p<.001 |
| Bi-Polar Disorder | 4.1% | 16.4% | 16.7% | X2(6) =14.75 | p<.02 |
| Anxiety Disorder | 18.4% | 41.4% | 39.7% | X2(6) =24.87 | p<.000 |
| Psychotic Disorder | 3.1% | 6.9% | 2.7% | X2(6) =8.17 | p=.23, ns |
| Attention Deficit/Hyperactivity Disorder | 14.3% | 33.6% | 30.8% | X2(6) =15.27 | p<.02 |
Path analysis was used to model the relationship between prenatal alcohol exposure and the mental health status outcomes while taking into account other factors that might contribute to outcomes including age, gender (sex), site of data collection, SES and ACEs and dysmorphology. IBM SPSS Amos 28 was used to assess the proposed path model (for an example, see Figure 1). The goal of these analyses was to determine the direct and indirect relationships of PAE with mental health outcomes for which individuals with PAE reported significantly higher rates. Table 4 shows these relationships as well as other relationships that significantly affected mental health outcomes.
Figure 1.
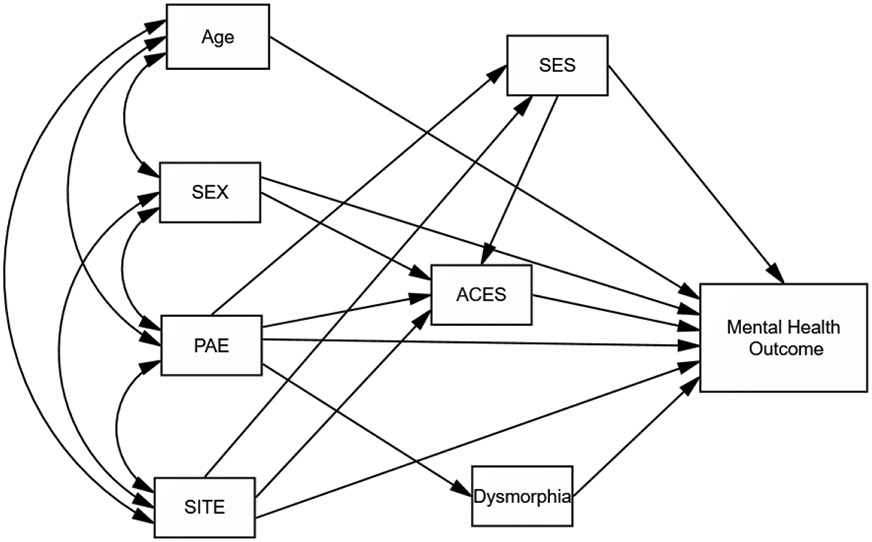
Path Analysis Model of Factors Predicting Mental Health Outcomes
Table 4:
Direct and Indirect effects of PAE and Presence of Dysmorphic Features on Mental Health Outcomes
| Variable | R-square for Outcome |
PAE Direct | Dysmorphology Direct |
Indirect PAE | Indirect Dysmorphology |
Other Endogenous |
|---|---|---|---|---|---|---|
| Depression Yes/NO | .173 | ns | ns | β = .114, p < .0081 | ns | Direct: Site (β = −.216, p < .005); ACES (β = .288, p < .003); SES (β = −.141, p < .028); Indirect: Sex (β = .057, p < .002) |
| Depression Symptoms Total Score | .229 | ns | β = −.154, p < .0132 | β = .117, p < .0093 | ns | Direct: Site (β = −.123, p < .019); ACES (β = .389, p < .003); SES ((β = −.173, p < .006): Indirect: Sex (β = .076, p < .002) |
| Anxiety | .173 | ns | ns | β = .143, p < .003 | ns | Direct: Site (β = −.218, p < .003); SES (β = −.206, p < .003): ACES (β = .222, p < .003); Indirect: Sex (β = .044, p < .002) |
| Bipolar | .130 | ns | ns | β = .148, p < .001 | ns | Direct: Age (β = −.171, p < .002); ACES (β = .225, p < .002); SES (β = −.184, p < .003); Indirect: Sex (β = .044, p < .002) |
| ADHD | .147 | β = .104, p < .093 | ns | ns | ns | Direct: Site (β = −.255, p < .002; SES (β = −.173, p < .005); Gender (β = −.193, p < .002); Indirect: Site β =.046, p < .065); |
Rejected default model χ2 (12, n = 283) = 10.743, p < .217 (RMSEA = .035, CFI=.990, GFI = .991). The above are estimates using a 1000 sample bootstrap approximation obtained by constructing two-sided bias- corrected confidence intervals.
The standardized indirect (mediated) effect of Alcohol Y/N on Depression is .114. That is, due to the indirect (mediated) effect of Alcohol Y/N on Depression, when Alcohol Y/N goes up by 1 standard deviation, Depression goes up by 0.114 standard deviations. This is in addition to any direct (unmediated) effect that Alcohol Y/N may have on Depression. The indirect (mediated) effect of Alcohol Y/N on Depression is significantly different from zero at the 0.01 level (p=.008 two-tailed). This is a bootstrap approximation obtained by constructing two-sided bias- corrected confidence intervals.
The standardized direct (unmediated) effect of Dysmorphic Y/N on Depression Total score is −.154. That is, due to the direct (unmediated) effect of Dysmorphic Y/N on Depression Total score, when Dysmorphic Y/N goes up by 1 standard deviation, Depression Total score goes down by 0.154 standard deviations. This is in addition to any indirect (mediated) effect that Dysmorphic Y/N may have on Depression Total score.
The standardized indirect (mediated) effect of Alcohol Y/N on Depression Total score is .117. That is, due to the indirect (mediated) effect of Alcohol Y/N on Depression Total score, when Alcohol Y/N goes up by 1 standard deviation, Depression Total score goes up by 0.117 standard deviations. This is in addition to any direct (unmediated) effect that Alcohol Y/N may have on Depression Total score.
Each path analysis involved a series of multiple regression analyses to evaluate the direct and indirect (mediated) effects of PAE on mental health status. Among the exogenous variables, site, with Atlanta being the higher value was correlated with PAE (r = −.231, p < 003) and age (r = −.270, p < .002) but the remaining variables were not significantly related to each other. Bootstrapping was done using 1000 samples to obtain an estimate of bias-corrected results at a 90% confidence interval. The Model fit was good as outcome fit statistics show: (χ2 (12, n = 283) = 10.743, p < .217; RMSEA = .035, CFI =.990;; GFI = .991).
Endogenous Relationships Common in All Models.
Site significantly predicted SES (β = −.276, p < .002) and ACEs (β = −.262 p < .002). Specifically, the Seattle site had higher SES and ACEs. As expected, PAE significantly predicted dysmorphology (β = .435, p < .002). PAE was also associated with lower SES (β = −.456, p < .001), and higher ACEs (β = .279, p < .001). Gender (sex) significantly predicted ACEs (β = .196, p < .003) with women reporting more childhood adversity.
Model Prediction of Specific Mental Health Outcomes:
Depression Diagnosis.
Total variance accounted for in depression diagnosis was R2 = .173 (p < .002). PAE did not directly predict depression but did so indirectly (β = .114, p < .008) via its impact on SES (β = .066, p < .023) and ACEs (β = .082, p < .001). Gender also had an indirect effect on depression (β = .057, p < .002) associated with its effect on ACEs (β = .056, p < .002). In addition, Site (β= −.216, p < .005), ACEs (β = .288, p < .003) and SES (β = −.141, p < .028) also directly predicted depression with the Seattle site, higher ACEs and lower SES being associated with a greater likelihood of depression diagnosis. Dysmorphology had neither a direct nor indirect relationship with depression.
Depression (Current Symptoms).
Total variance accounted for in current depression symptoms was R2 = .229 (p < .026). PAE did not directly affect depression symptoms but alcohol-related dysmorphology did (β = −.154, p < .013) with dysmorphic individuals reporting fewer symptoms. In addition, Site ( −.123, p < .019), ACEs (β = .389, p < .003) and SES (β = −.173, p < .006) also directly predicted depression symptoms with higher symptoms reported in Seattle, among those with more ACEs and lower SES. There was also an indirect effect of PAE (β = .117, p < .009) on depression symptoms via its impact on SES (β = .325, p < .004), ACEs (β = .447, p < .001), and dysmorphology (β = −.275, p < .009) . Gender also had an indirect effect on depression symptoms (β = .076, p < .002) associated with its effect on ACEs (β = .302, p < .002).
Anxiety Diagnosis:
Total variance accounted for in anxiety diagnosis was R2 = .173 (p < .039). PAE did not directly affect anxiety but did so indirectly (β = .143, p < .003) through its impact on SES (β = .095, p < .002) and ACEs (β = .063, p < .002). Gender also had an indirect effect on anxiety (β = .044, p < .002) via ACEs (β = .042, p < .002). In addition, Site ( −.218, p < .003), ACEs (β = .222, p < .003) and SES (β = −.206, p < .003) also predicted anxiety directly. That is, those in Seattle reported more anxiety as did those with more childhood adversity and lower SES. Dysmorphology had neither a direct nor indirect relationship with anxiety diagnosis.
Bipolar Disorder Diagnosis.
Total variance in the diagnosis of Bipolar Disorder was R2 = .130 (p < .045). PAE did not have a direct impact on the diagnosis but PAE did have an indirect effect (β = .148, p < .001) via its relationships with higher SES (β = .059, p < .007) and higher ACEs (β = .044, p < .005). Alcohol-related dysmorphology was not directly or indirectly related to the diagnosis. Direct effects were found on bipolar diagnosis for Age (β = −.171, p < .002), ACEs (β = .225, p < .013), and SES (β = −.184, p < .008), with a higher likelihood of diagnosis in younger individuals and those with higher ACEs and lower SES. An indirect effect was found on the diagnosis for Gender (β = .044, p < .002) via its relationship with ACEs (β = .030, p < .007).
ADHD Diagnosis.
Total variance accounted for in ADHD diagnosis was R2 = .147 (p < .046). Neither PAE nor level of alcohol-related dysmorphology had a directly or indirect effect on ADHD. Site had both a direct and indirect effect on endorsement of ADHD diagnosis (Direct β = −.255, p < .002; Indirect trend β = .046, p < .065) with trend for an indirect pathway going through SES (β = .044, p < .005). Gender had a direct effect on ADHD (β = −.193, p < .002 ) as did SES (β = −.173, p < .005). Males and those with lower SES were more likely to report an ADHD diagnosis.
Discussion
The results of the current study confirm that individuals with prenatal alcohol exposure are more likely to report both symptoms of depression and diagnosis of mental health problems and that these problems remain observable in middle adulthood. Similarly, we find in these samples that participants are more likely to report adverse childhood experiences if they have been prenatally exposed to alcohol. Review of Table 4 suggests that while mental health problems are more common in those with prenatal alcohol exposure or with a diagnosis of FAS, the relationship is typically an indirect one. Path analyses indicate that alcohol effects on mood disorders and anxiety are indirect, mediated by the postnatal environmental factors that include ACEs as well as social and economic status. For instance, alcohol exposure indirectly affects the diagnosis of depression while there are significant direct effects of participants’ age (with younger people more likely to report diagnosis), site of data collection (with individuals in Seattle more likely to report depression), ACEs (with higher adverse experiences related to depression) and SES (with lower SES rankings more likely to report depression). There is also an indirect effect of gender with women more likely to report depression. Similar patterns are observed for anxiety and bipolar disorder diagnoses. For current symptoms of depression, the inverse relationship with dysmorphology suggests that those individuals in the non-dysmorphic alcohol group were reporting more current symptoms. These results also suggest, as has been observed previously (Lynch et al., 2017; Streissguth et al., 1996), that it is not those with full FAS who are most impacted by mental health issues but rather alcohol-exposed individuals with less obvious physical effects and cognitive impairments.
We have often in the past attempted to discriminate the unique teratogenic effect of alcohol exposure on developmental outcomes, but, while important scientifically, this may have been too simple an approach in understanding the complex clinical presentation observed in FASD. When we examine the long-term effects of PAE on mental health as reported in this study, results suggest the impact of “multiple hits” on a vulnerable organism. In addition to providing a more complete picture of the long-term consequences of PAE, this perspective allows the exploration of the complicated development processes involved. In the current study, only social and demographic factors were examined. However, it is likely that these factors may be associated with increased stress for these individual and that future research should address the physiological impact of such stress on emotional function as well as any increased vulnerability to stress that may be associated with PAE.
Given that there are (at least) several etiological factors in the development of mental health disorders among those with PAE, it follows that prevention and treatment should be more broadly conceptualized. Certainly, a primary goal must be the prevention or reduction of prenatal exposure although this has proved difficult to accomplish. In addition, we need to understand that when prenatal exposure has occurred the child is more vulnerable to subsequent environmental insults. By taking preventive steps to reduce or eliminate environmental stressors early in development we may be able to mitigate lifelong consequences of PAE. Our study results also suggest that mental health issues are not the same for all of those with PAE. Further research is warranted to examine why having a diagnosis of FAS can be protective while being alcohol-exposed and non-dysmorphic yet exhibiting behavioral/cognitive problems can lead to more serious consequences.
Limitations.
There are several limitations in the current study. As this was a survey, information about mental health is based on participant self-report and not confirmed by professional diagnosis. Participants were recruited from longitudinal cohorts, both of which previously had reported mental health issues in alcohol-exposed groups. Thus, it may be most conservative to say that this study demonstrates that such effects are persistent over many years. In addition, due to the extreme difficulty in locating potential participants almost 40 years after they were first recruited, the current group who were located and agreed to participate represents only about half of the total who were seen initially. It is certainly possible that those who could not be located and/or those who declined to participate may have different characteristics. Another limitation is that much of the survey was carried out during the COVID-19 pandemic which may have influenced willingness to participate in research as well as participant mental health. Despite these limitations, these results provide insight into the characteristics of those who agreed to participate and into their current health status that are of value.
The study also has several strengths. Recruitment from existing cohorts whose members’ alcohol exposure and diagnostic status was documented previously avoided questions about retrospective ascertainment of maternal alcohol use, a problem for much research in this area. Similarly, by recruiting from these cohorts, ascertainment bias was somewhat reduced in that individuals were not presenting with a current clinical concerns or other factors that may motivate adults to participate in such research. As a result, participants were likely to be more representative of the spectrum of outcomes associated with prenatal exposure. Finally, by recruiting from Atlanta and Seattle, the study allows considerable ethnic and racial diversity and avoids attributing outcomes to alcohol exposure that may be the result of unmeasured social factors.
These results suggest that mental health problems, particularly mood and anxiety disorders, are reported more commonly in those who were prenatally exposed to alcohol. The study also suggests that PAE is only one of many stressful factors occurring over the lives of individuals who report mental health disorders in midlife and that the most parsimonious way to understand these outcomes is as the result of multiple “hits” that have placed a cumulative developmental burden on vulnerable individuals, a perspective that has been proposed previously (Streissguth et al., 2004; Kambeitz, et al., 2019). These results have implications for the clinical care of individuals of all ages with PAE. Certainly, those diagnosed with FASD should be evaluated for mood and anxiety disorders as part of their medical care. In addition, because children with PAE are at even greater risk when exposed to family and social stressors, caregivers and professionals should recognize that early identification of PAE and diagnosis of FASD may reduce the risk for negative long-term mental health consequences if appropriate care is provided.
Acknowledgments
Research was supported by a Cooperative Agreement (U01AA026108) awarded by the National Institute on Alcohol Abuse and Alcoholism and by a gift from the SKK Foundation.
References
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, & Streissguth AP (2003). A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry, 60(4), 377–385. doi: 10.1001/archpsyc.60.4.377 [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O'Malley KD, Connor PD, Huggins JE, & Streissguth AP (2006). Binge drinking during pregnancy as a predictor of psychiatric disorders on the Structured Clinical Interview for DSM-IV in young adult offspring. Am J Psychiatry, 163(6), 1061–1065. doi: 10.1176/ajp.2006.163.6.1061 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2021) Public Health Professionals Gateway: Public Health Law. Health Insurance Protability and Accountability Act of 1996 (HIPAA). [Google Scholar]
- Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M (1989). Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics, 84(3), 536–541. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2771556 [PubMed] [Google Scholar]
- Doyle LR, & Mattson SN (2015). Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Review of Evidence and Guidelines for Assessment. Curr Dev Disord Rep, 2(3), 175–186. doi: 10.1007/s40474-015-0054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, & Unis AS (1998). Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry, 155(4), 552–554. doi: 10.1176/ajp.155.4.552 [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med, 14(4), 245–258. doi: 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fernhoff PM, Smith IE, & Falek A (1980). Dysmorphia Checklist. Document available through the Maternal Substance Abuse and Child Development Project, Division of Psychiatry, Emory University School of Medicine, Atlanta, GA. [Google Scholar]
- Flannigan K, Kapasi A, Pei J, Murdoch I, Andrew G, & Rasmussen C (2021). Characterizing adverse childhood experiences among children and adolescents with prenatal alcohol exposure and Fetal Alcohol Spectrum Disorder. Child Abuse Negl, 112, 104888. doi: 10.1016/j.chiabu.2020.104888 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (2011). Four Factor Index of Social Status. Yale Journal of Sociology, 8, 21–51. [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, & Streissguth P (1973). Pattern of malformation in offspring of chronic alcoholic mothers. Lancet, 1(7815), 1267–1271. doi: 10.1016/s0140-6736(73)91291-9 [DOI] [PubMed] [Google Scholar]
- Kambeitz C, Klug MG, Greenmyer J, Popova S, & Burd L (2019) Association of adverse childhood experiences and neurodevelopmental disorders in people with fetal alcohl spectrum disoders (FASD) and non-FASD controls. BMC Pediatrics, 19, doi.org/ 10.1186/s12887-019-1878-8.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen AM, Nissinen NM, Gissler M, Autti-Ramo I, Sarkola T, & Kahila H (2020). Prenatal substance exposure, adverse childhood experiences and diagnosed mental and behavioral disorders - A longitudinal register-based matched cohort study in Finland. SSM Popul Health, 11, 100625. doi: 10.1016/j.ssmph.2020.100625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel CA, McMorris CA, Kar P, Ritter C, Andre Q, Tortorelli C, & Gibbard WB (2019). Characterizing adverse prenatal and postnatal experiences in children. Birth Defects Res, 111(12), 848–858. doi: 10.1002/bdr2.1464 [DOI] [PubMed] [Google Scholar]
- Lynch ME, Kable JA, & Coles CD (2017). Effects of prenatal alcohol exposure in a prospective sample of young adults: Mental health, substance use, and difficulties with the legal system. Neurotoxicol Teratol, 64, 50–62. doi: 10.1016/j.ntt.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, & Grogan-Kaylor A (2017). Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse Negl, 69, 10–19. doi: 10.1016/j.chiabu.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, & Paley B (2009). Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev, 15(3), 225–234. doi: 10.1002/ddrr.74 [DOI] [PubMed] [Google Scholar]
- O’Connor MJ (2014). Mental Health Outcomes Associated with Prenatal Alcohol Exposure: Genetic and Environmental Factors. Current Developmental Disorders Reports, 1(3), 181–188. doi: 10.1007/s40474-014-0021-7 [DOI] [Google Scholar]
- Price A, Cook PA, Norgate S, & Mukherjee R (2017). Prenatal alcohol exposure and traumatic childhood experiences: A systematic review. Neurosci Biobehav Rev, 80, 89–98. doi: 10.1016/j.neubiorev.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Sarkar T, Patro N, & Patro IK (2019). Cumulative multiple early life hits- a potent threat leading to neurological disorders. Brain Res Bull, 147, 58–68. doi: 10.1016/j.brainresbull.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Sarwono J (2017). Path Analysis: Data Analysis Applicaiton, Using IBM SPSS and Stata. Columbia , S.C.: Sarwono Jonathan. [Google Scholar]
- Spohr HL, Willms J, & Steinhausen HC (2007). Fetal alcohol spectrum disorders in young adulthood. J Pediatr, 150(2), 175–179, 179 e171. doi: 10.1016/j.jpeds.2006.11.044 [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, & Bookstein FL (1996). Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE): Final Report. Seattle, WA: University of Washington School of Medicine. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, & Bookstein FL (1994). Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend, 36(2), 89–99. doi: 10.1016/0376-8716(94)90090-6 [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, & Young JK (2004). Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr, 25(4), 228–238. doi: 10.1097/00004703-200408000-00002 [DOI] [PubMed] [Google Scholar]
- Weyrauch D, Schwartz M, Hart B, Klug MG, & Burd L (2017). Comorbid Mental Disorders in Fetal Alcohol Spectrum Disorders: A Systematic Review. J Dev Behav Pediatr, 38(4), 283–291. doi: 10.1097/DBP.0000000000000440 [DOI] [PubMed] [Google Scholar]


