Abstract
Objective:
To determine the in vitro oxidative stress induced by conventional and self-ligating brackets made of different materials.
Materials and Methods:
The concentration of oxidative stress marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in DNA of murine fibroblast cells L929 after in vitro exposure to three types of conventional and four types of self-ligating brackets was assessed. To determine viability and changes in the number of cells before and after exposure, trypan blue dye was used. Analysis of variance (ANOVA) was used for statistical analysis.
Results:
No significant difference in cell viability was noted between metal, ceramic, and polymeric conventional brackets, and self-ligating brackets made of combinations of those materials, but viability was significantly higher compared with positive controls (P < .05). The conventional sapphire ceramic bracket (Inspire Ice) showed high viability, the largest increase in the number of cells, and the lowest oxidative stress. A higher concentration of markers of oxidative stress was observed in full metal conventional and self-ligating brackets (MiniSprint and Speed) and in conventional polyurethane brackets (Quantum) compared with negative controls (P < .05).
Conclusion:
All types of orthodontic brackets, regardless of the constituent materials, are a source of oxidative stress in vitro, but the highest stress was induced in the full metal and polyurethane brackets. Conventional ceramic brackets show the highest degree of biocompatibility compared with polymeric and metal brackets and self-ligating brackets made from combinations of these materials.
Keywords: Oxidative stress, Genotoxicity, Mutagenicity, Orthodontic brackets
INTRODUCTION
Physiologic and biochemical processes of the oral cavity provide an ideal milieu for biodegradation of orthodontic appliances,1–5 thereby facilitating the release of oxidation products associated with adverse health effects, such as cytotoxicity and genotoxicity.1,6,7
Orthodontic brackets usually are made of alloys based on iron, chromium, molybdenum, and nickel, and esthetic variants of polymers and ceramics are available.8 Self-ligating brackets contain passive or active clips made of stainless steel or nickel-titanium or cobalt-chromium alloys, and may be coated with rhodium in an effort to improve their appearance.9 To improve esthetics and to retain mechanical properties, self-ligating brackets are made of various combinations of these metallic and esthetic materials.10 The biological effects of such brackets composed of combinations of different materials have not yet been studied sufficiently.
Oxidative stress is a disturbance in the balance of oxidation-reduction processes in the body, where the shift in balance is directed toward oxidation.11 This disturbance results in excessive production of free radicals, which are highly reactive and which further initiate auto-oxidation of lipids and damage proteins, nucleic acids, carbohydrates, and other organic molecules. Sources of free radicals may be found in various physiologic processes and physical and chemical agents, such as oxygen metabolism, phagocytosis, chemotaxis, apoptosis, coagulation, hypoxia, hyperoxia, cigarette smoke, medication, nutrition, pesticides, metals, and radioactive and ultraviolet (UV) irradiation.12 Probably the most important biological target of oxidative stress is DNA, which suffers pyrimidine dimerization, modification or loss of bases, single-stranded and double-stranded breaks, breaks of hydrogen bonds, and oxidation of nitrogen bases.11 It is believed that long-term oxidative damage to DNA plays an important role in various pathophysiologic processes including cancer, atherosclerosis, neurodegenerative disorders, infectious and autoimmune diseases, diabetes, aging, and hemolysis.11,13
The aim of this study was to determine the in vitro oxidative stress induced by conventional and self-ligating brackets made of different materials.
MATERIALS AND METHODS
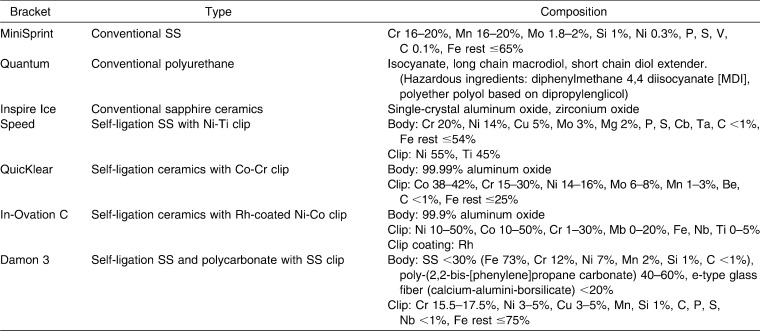
The sample consisted of three types of conventional and four types of self-ligating brackets: conventional: (1) stainless steel (MiniSprint, Forestadent, Phorzheim, Germany), (2) monocrystalline sapphire ceramic (Inspire Ice, Ormco, Orange, Calif), and (3) polyurethane (Quantum, Masel, Carlsbad, Calif); self-ligating: (1) stainless steel body with nickel-titanium clip (Speed, Strite Industries, Cambridge, Canada), (2) aluminum oxide ceramic with cobalt-chromium clip (QuicKlear, Forestadent, Phorzheim, Germany), (3) aluminum oxide ceramic with nickel-cobalt clip coated with rhodium (In-Ovation C, Dentsply GAC, Bohemia, NY), and (4) polycarbonate-stainless steel brackets (Damon 3, Ormco) (Table 1).
Table 1.
Composition of Brackets According to Manufacturer's Material Safety Data Sheet
Five maxillary brackets of each type were weighed, autoclaved, and immersed into artificial saliva Glandosane (Stade Arzneumittel, Vienna, Austria) with pH 6.75 ± 0.15 (adjusted and controlled with 10 N NaOH buffer solution), placed into sterile airtight polyethylene tubes, stored at 37°C, and kept in stationary conditions for 30 days. According to International Organization for Standardization (ISO) standards, 1 mL of saliva per 0.2 g of brackets was used.14 Artificial saliva, which served as a negative control, was stored in the same way. After the immersion period, the brackets were removed and the artificial saliva was stored at 4°C until further analysis was conducted.
The study was performed on murine fibroblast cells L929. All cells were grown in culture in Dulbecco modified Eagle medium (Sigma Chemicals, St Louis, Mo) supplemented with 10% fetal bovine serum (Gibson BRL, Grand Island, Nebr), 2 mM l-glutamine, 11 mM sodium bicarbonate, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were cultivated in 100 mm cell culture dishes (Sigma Aldrich Co, St Louis, Mo) in a humidified atmosphere containing 5% CO2 at 37°C for 24 hours to obtain cell monolayer. A total of 200 µL of each sample of saliva was added to cell culture dishes (2 × 106 cells each). The medium of cell culture without any test material served as the absolute control. Artificial saliva was used as the negative control, while the positive control was 0.1 mM H2O2, both in 200 µL. Samples were incubated for 48 hours. All tests were conducted in triplicate and were repeated twice.
To determine the number of cells and their viability, a 20 mL cell suspension was mixed with 80 µL 10% solution of trypan blue dye (Sigma Aldrich), and cells were counted using a Neubauer microchamber (Brand GMBH, Wertheim, Germany) with the light microscope Olympus BM-2 (Olympus, Center Valley, Pa).
For isolation of genomic DNA, the GenElute Mammalian Genomic DNA Kit (Sigma Aldrich) was used. As the number of cells increased from 2 to more than 5 × 106 in 2 days, 5 × 106 cells were taken from each of the samples. RNA was removed from genomic DNA by adding 20 µL solution of RNase A. According to the manufacturer's instructions, cells were resuspended and lysed. With the addition of 95% ethanol (Kemika, Zagreb, Croatia), binding of DNA to the silicate membrane columns was enabled, the remaining contamination was removed by washing, and DNA was eluated with tris-ethylenediaminetetraacetic acid (EDTA) buffer.
The quantity and quality of extracted DNA were determined at wavelengths of 260 nm and 280 nm on a NanoVue spectrophotometer (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). For further analysis, extracted DNA was used at a concentration of 0.1 mg/mL. The sample was converted to single-stranded DNA by incubating the DNA sample at 95°C for 5 minutes, and then rapidly cooling it on ice. DNA was digested into nucleosides by incubating denatured DNA with 10 U nuclease P1 (Sigma Aldrich) for 2 hours at 37°C in 20 mM sodium acetate pH 5.2, and was treated with 5 U alkaline phosphatase (Sigma Aldrich) for 1 hour at 37°C in 100 mM tris buffer pH 7.5. The reaction mixture was centrifuged for 5 minutes at 6000 g, and the supernatant was used in measuring the concentration of markers of oxidative stress (8-OHdG).
Oxidative DNA damage was determined using the OxiSelect Oxidative DNA Damage ELISA Kit (8-OHdG Quantitation) (Cell Biolabs, San Diego, Calif) according to the manufacturer's instructions, and optical density readings were performed by using plate readers (Start Fax 2010, Awareness Technology, Palm City, Fla) with a 620-nm filter. The amount of 8-OHdG in each sample was determined by comparing its absorbance read on the reader with that of the known standard curve. To create a standard curve, a series of dilutions of standard 8-OHdG with a concentration range of 0 to 20 ng/mL were prepared.
One-way analysis of variance (ANOVA) with a Sidak post hoc test was used for statistical analysis.
RESULTS
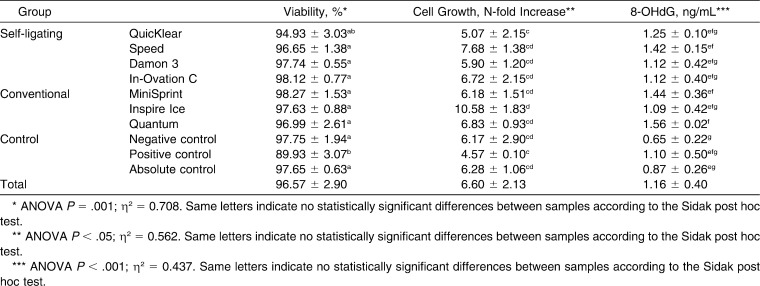
Cell viability was lowest in a positive control (89.93 ± 3.07); this is significantly different from all groups (P < .05), except the one with QuicKlear brackets (94.93 ± 3.03; Table 2; Figure 1). Inspire Ice showed the largest increase in the number of cells between initial count and that after 48-hour exposure (10.58 ± 1.83)—significantly greater than both QuicKlear (5.07 ± 2.15; P < .05) and the positive control (4.57 ± 0.10; P < .05; Table 2; Figure 2). Differences between other groups of brackets and control groups were not statistically significant.
Table 2.
Distribution of Viability, Cell Growth, and Concentration of 8-OHdG Oxidative Stress Markers Between Groups (mean ± standard deviation)
Figure 1.
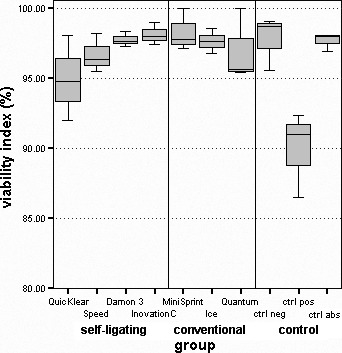
Distribution of viability index.
Figure 2.
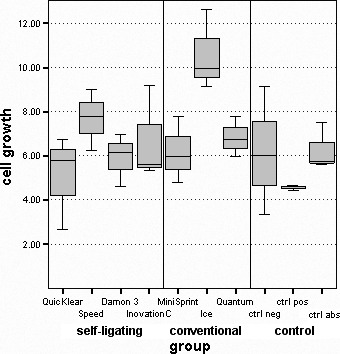
Distribution of increase in the number of cells.
A statistically significant higher oxidative stress was observed in the full metal brackets Speed (1.42 ± 0.15) and MiniSprint (1.44 ± 0.36) and in the polyurethane Quantum brackets (1.56 ± 0.02) compared with negative controls (0.65 ± 0.22; P < .05; Table 2; Figure 3). All groups of brackets had higher stress than the positive controls (1.10 ± 0.50), except Inspire Ice, which had slightly lower stress. Negative and absolute controls had the lowest levels of oxidative stress.
Figure 3.
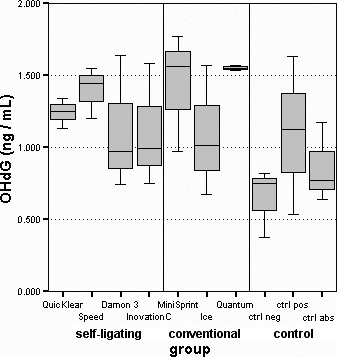
Distribution of oxidative stress marker concentrations.
The highest effect size in explaining the differences in biological effects between groups had viability (70.8%) compared with cell proliferation and oxidative stress (56.2% and 43.7%, respectively).
DISCUSSION
Studies of biocompatibility in the orthodontic field are often focused on the corrosion of archwires and brackets, the cytotoxic effects of bracket material and bonding agents on cell culture, oral tissues, and changes in salivary parameters.1–6,15–21 Few studies have explored the genotoxicity of orthodontic appliances, usually involving stainless steel brackets.22–24 It is known that iron, copper, chromium, and vanadium undergo a redox cycle and thus directly generate free radicals, while cadmium, mercury, nickel, and lead create them indirectly.7 It seems that polymers, ceramics, and synthetic sapphire, used for esthetic components of brackets, are not inert materials. Some types of alumina ceramics cause numeric chromosome aberrations.25 Polycarbonates hydrolyze and release bisphenol A (BPA), which is associated with DNA hypomethylation, breast and prostate cancer, and disorders of the thyroid and gonads.26,27 Polyoxymethylene brackets release formaldehyde, which is toxic, allergenic, and potentially carcinogenic.16,20 Sapphire is an aluminum oxide crystal in which metals of iron, chromium, titanium, or vanadium, which determine its color, can be incorporated, and these metals can induce the production of radicals. Knowledge of the genotoxic properties of orthodontic materials, such as oxidative stress, should be an important criterion for selecting an appliance that would have safe biological effects on patients.28
The advantage of using an immortal cell line is that the cell can be grown indefinitely in culture. Performing analysis on primary cells from tissue donors (human gingival fibroblasts) does not offer such advantages and raises some ethical concerns. For initial evaluation of the biocompatibility of a material, the cellular viability test is used.14 The viability of positive control was significantly lower than that of negative and absolute controls and that for all groups of brackets, except QuicKlear. Because the body of QuicKlear brackets is composed of 99.99% aluminum oxide ceramics and a clip of 38% to 42% cobalt, 15% to 30% chromium, and 14% to 16% nickel, reduced viability of QuicKlear is probably due to release of these ions from the clip. Vitral et al.16 showed good biocompatibility of ceramic brackets in terms of cellular viability, but polycarbonate and polyoxymethylene brackets showed decreased cell viability compared with the control group and those with ceramics, although this finding was not statistically significant. Although the polyurethane bracket Quantum in our study induced lower viability, no statistically significant difference in viability was noted between brackets made of metal, ceramic, and polymeric material (and combinations); this is consistent with the findings of previous studies.16,29 It seems that corrosion extracts of stainless steel brackets do not alter viability in L929 cell culture but cause reduction of cellular metabolism.30 This suggests that mitochondrial metabolism is the target of corrosion products. Nickel ions, when in the trivalent form, are exposed to mitochondrial redox metabolism, which leads to the formation of not fully reactive oxygen radicals.30
The sapphire ceramic bracket Inspire Ice showed the greatest increase in the number of cells—significantly higher than those from QuicKlear and positive controls, which showed the smallest increase in the number of cells. This result can be explained by the composition of ceramic, which is found in monocrystalline and polycrystalline forms. The advantage of producing monocrystals (Inspire Ice) is the elimination of possible contamination or impurities.31 Some studies have reported the highest growth inhibition by nickel-chromium-cobalt alloy,29 as confirmed by our tests, in which the ceramic bracket with a cobalt-chrome-nickel clip (QuicKlear) showed the greatest inhibition of growth.
All groups of brackets induced higher oxidative stress than the positive control, except for Inspire Ice, but the differences were not statistically significant. Compared with the negative control, a higher oxidative stress level was recorded with the full metal brackets Speed and MiniSprint and with polyurethane Quantum brackets. In accordance with other studies, we were expecting to record the highest level of DNA damage with metal brackets,5,7,22,23 but the highest oxidative stress was induced by polyurethane brackets. Polyurethanes are segmented polymers formed by the reaction of polyether polyol segments with isocyanates and could be, while they are hydrolytically stable, subject to oxidative degradation. Both could be considered hazardous ingredients; the first can be nonreacted and is therefore harmful, while the other may undergo oxidation in vivo and therefore may generate reactive species such as free radicals that can further react with organic molecules in the physiologic milieu.32 Because polymers can be damaged by heat and humidity, the polyurethanes could have been affected by autoclave sterilization and made more prone to oxidation.
Studies conducted so far suggest that some other polymeric brackets can also release harmful substances.16,20,33,34 More BPA seems to be released from polycarbonate brackets in saliva than had been expected based on in vitro data, but the effect of estrogen is very weak.33 Nevertheless, it is suggested that long-term use of polycarbonate brackets in the mouth may not be desirable.33 However, Pithon et al. reported that polycarbonate brackets do not cause cytotoxicity in cell culture L929 after immersion brackets in culture medium up to 7 days.34 The first research measured only the amount of released BPA, not its biological effect; the second measured the biological effect, not the quantity or type of a potentially toxic substance. Also, the first simulated the length of orthodontic treatment and allowed enough time to see the release of BPA.
Our results indicate that all brackets are the source of oxidative stress, and that metal brackets, probably because of iron, chromium, and nickel ions, have higher stress, but the highest stress was induced by polymeric brackets, probably because of polyether polyol. Induction of lactate dehydrogenase and lipid peroxidation and induction of the Fenton reaction mediate oxidative stress caused by exposure to metals, particularly nickel. This last process involves the reaction of O− with an oxidative metal trace and generation of O2, which eventually reacts with hydroxyl peroxide to form the hydroxyl radical.6 Hexavalent chromium has been shown to induce the formation of 8-OHdG and formamidopyrimidine DNA glycosylase-dependent DNA strand breaks in white blood cells in vitro. Because cobalt and iron possess similar chemical properties, there is reason to assume that cobalt causes mechanisms such as Fenton for the production of reactive oxygen species.12 A recent in vitro study failed to detect the presence of DNA damage after treatment by corrosion eluates from stainless steel brackets at experimental periods of 0 to 70 days.24 However, the very manipulation of cell culture (trypsinization and media replacement) could affect viability and cell proliferation results, inducing oxidative stress.
The results of this study complement previous findings in the field of biocompatibility of appliances used in orthodontics. Still further in vivo studies concerning oxidative stress that include measurement of changes in pH of the oral cavity and concentrations of potentially hazardous ingredients during long-term exposure are needed.
CONCLUSIONS
All types of orthodontic brackets, regardless of the constituent materials, are a source of oxidative stress in vitro, but the highest stress inducers are full metal and polyurethane brackets.
Conventional ceramic brackets have the highest degree of biocompatibility compared with polymeric and metallic brackets and self-ligating brackets made from combinations of these materials.
Acknowledgments
The study was supported by the Croatian Ministry of Science (grants 062-0650444-0442 and 065-0650444-0436).
REFERENCES
- 1.Eliades T. Orthodontic materials research and applications: part 2. Current status and projected future developments in materials and biocompatibility. Am J Orthod Dentofacial Orthop. 2007;131:253–262. doi: 10.1016/j.ajodo.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Grimaudo NJ. Biocompatibility of nickel and cobalt dental alloys. Gen Dent. 2001;49:498–503. [PubMed] [Google Scholar]
- 3.Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, Jäger A, Bourauel C. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofacial Orthop. 2009;135:59–65. doi: 10.1016/j.ajodo.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Amini F, Borzabadi Farahani A, Jafari A, Rabbani M. In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res. 2008;11:51–56. doi: 10.1111/j.1601-6343.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78:345–350. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 6.Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222–237. doi: 10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 8.Russell JS. Aesthetic orthodontic brackets. J Orthod. 2005;32:146–163. doi: 10.1179/146531205225021024. [DOI] [PubMed] [Google Scholar]
- 9.Chen SS, Greenlee GM, Kim JE, Smith CL, Huang GJ. Systematic review of self-ligating brackets. Am J Orthod Dentofacial Orthop. 2010;137:726.e1–726.e18. doi: 10.1016/j.ajodo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Fleming PS, Johal A. Self-ligating brackets in orthodontics: a systematic review. Angle Orthod. 2010;80:575–584. doi: 10.2319/081009-454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. London: Oxford University Press; 2007. [Google Scholar]
- 12.Pilger A, Rüdiger HW. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int Arch Occup Environ Health. 2006;80:1–15. doi: 10.1007/s00420-006-0106-7. [DOI] [PubMed] [Google Scholar]
- 13.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 14.ISO . Biological Evaluation of Medical Device Part 5 Tests for In Vitro Cytotoxicity ISO document 109935. Geneva, Switzerland: International Organization for Standardization; 1999. pp. 1–7. [Google Scholar]
- 15.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M. Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–110. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 16.Vitral JC, Fraga MR, de Souza MA, Ferreira AP, Vitral RW. In-vitro study of cellular viability and nitric oxide production by J774 macrophages stimulated by interferon gamma with ceramic, polycarbonate, and polyoxymethylene brackets. Am J Orthod Dentofacial Orthop. 2010;137:665–670. doi: 10.1016/j.ajodo.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Singh DP, Sehgal V, Pradhan KL, Chandna A, Gupta R. Estimation of nickel and chromium in saliva of patients with fixed orthodontic appliances. World J Orthod. 2008;9:196–202. [PubMed] [Google Scholar]
- 18.Terhune WF, Sydiskis RJ, Davidson WM. In vitro cytotoxicity of orthodontic bonding materials. Am J Orthod. 1983;83:501–506. [PubMed] [Google Scholar]
- 19.D'Antò V, Spagnuolo G, Polito I, Paduano S, Ambrosio L, Valletta R. In vitro cytotoxicity of orthodontic primers. Prog Orthod. 2009;10:4–11. [PubMed] [Google Scholar]
- 20.Kusy RP, Whitley JQ. Degradation of plastic polyoxymethylene brackets and the subsequent release of toxic formaldehyde. Am J Orthod Dentofacial Orthop. 2005;127:420–427. doi: 10.1016/j.ajodo.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Gursoy UK, Sokucu O, Uitto VJ, Aydin A, Demirer S, Toker H, Erdem O, Sayal A. The role of nickel accumulation and epithelial cell proliferation in orthodontic treatment-induced gingival overgrowth. Eur J Orthod. 2007;29:555–558. doi: 10.1093/ejo/cjm074. [DOI] [PubMed] [Google Scholar]
- 22.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124:687–693. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Westphalen GH, Menezes LM, Prá D, Garcia GG, Schmitt VM, Henriques JA, Medina-Silva R. In vivo determination of genotoxicity induced by metals from orthodontic appliances using micronucleus and comet assays. Genet Mol Res. 2008;7:1259–1266. doi: 10.4238/vol7-4gmr508. [DOI] [PubMed] [Google Scholar]
- 24.Angelieri F, Marcondes JPC, de Almeida DC, Salvadori DMF, Ribeiro DA. Genotoxicity of corrosion eluates obtained from orthodontic bracket in vitro. Am J Orthod Dentofacial Orthop. 2011;139:4:504–509. doi: 10.1016/j.ajodo.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Tsaousi A, Jones E, Case CP. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (CoCr alloy) particles. Mutat Res. 2010;697:1–9. doi: 10.1016/j.mrgentox.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 26.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montanaro L, Cervellati M, Campoccia D, Prati C, Breschi L, Arciola CR. No genotoxicity of a new nickel-free stainless steel. Int J Artif Organs. 2005;28:58–65. doi: 10.1177/039139880502800110. [DOI] [PubMed] [Google Scholar]
- 29.Mockers O, Deroze D, Camps J. Cytotoxicity of orthodontic bands, brackets and archwires in vitro. Dent Mater. 2002;18:311–317. doi: 10.1016/s0109-5641(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 30.Costa MT, Lenza MA, Gosch CS, Costa I, Ribeiro D. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J Dent Res. 2007;86:441–445. doi: 10.1177/154405910708600510. [DOI] [PubMed] [Google Scholar]
- 31.Gautam P, Valiathan A. Ceramic brackets: in search of an ideal! Trends Biomater Artif Organs. 2007;20:122–126. [Google Scholar]
- 32.Tanzi MC, Fare S, Petrini P. In vitro stability of polyether and polycarbonate urethanes. J Biomater Appl. 2000;14:325–348. doi: 10.1177/088532820001400402. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Hase T, Imai Y. Degradation and formation of bisphenol A in polycarbonate used in dentistry. J Med Sci. 2004;51:1–6. [PubMed] [Google Scholar]
- 34.Pithon MM, dos Santos RL, Martins FO, Ruellas ACO, Nojima LI, Nojima MG, Romanos MTV. Cytotoxicity of polycarbonate orthodontic brackets. Braz J Oral Sci. 2009;8:2:84–87. [Google Scholar]