Abstract
Objective:
To identify the expressions of SOX9 and type II collagen in spheno-occipital synchondrosis in response to quercetin, using a mouse in vitro model.
Materials and Methods:
A total of 50 one-day-old male BALB/c mice were randomly assigned to the control and experimental groups. Each group was subdivided into five different time points, which were 6, 24, 48, 72, and 168 hours, and each subgroup contained 5 mice (n = 5). In the experimental group, the spheno-occipital synchondrosis was immersed in the BGJb medium + quercetin dihydrate 1 µM. In the control group, the spheno-occipital synchondrosis was immersed in the BGJb medium. Tissue sections were subjected to immunohistochemical staining for SOX9 and type II collagen expressions.
Results:
Quantitative analysis revealed there was a statistically significant increase of 32.31% (P < .001) in the expression of SOX9 between experimental groups and control groups at 24 hours. Furthermore, there was a statistically significant increase of 22.99% (P < .001) in the expression of type II collagen between experimental groups and control groups at 72 hours.
Conclusion:
The expressions of SOX9 and type II collagen in the spheno-occipital synchondrosis can be increased by quercetin.
Keywords: Quercetin, Synchondrosis
INTRODUCTION
Phytoestrogens are plant-derived nonsteroidal compounds that bind to estrogen receptors (ERs) and have a similar activity to estrogen.1 Phytoestrogens have garnered attention with regard to their potential beneficial role in prevention and treatment of cardiovascular diseases, diabetes, obesity, osteoporosis, renal diseases, menopausal symptoms, and various cancers.2,3 The effects of phytoestrogens on osteoblast differentiation using a mouse calvaria osteoblast-like cell line can increase alkaline phosphatase activity and promote mineralization of bone in these cells.4
Quercetin, 3,3′,4′,5,7-pentahydroxyflavone; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, C15H10O7, is one of the major phytoestrogens isolated from onions (200–600 mg quercetin/kg onion), tea, nuts, berries, cauliflower, cabbage, apples, and grapes. It has been reported that only a small percentage of ingested quercetin is absorbed into the bloodstream.5
The spheno-occipital synchondrosis is initially formed in cartilage (the chondrocranium) on the ventral surface of the brain. Its position lies between the sphenoid bone and the occipital bone, which grows by endochondral bone formation.6 Over the past decade, a number of master regulator genes that control distinct pathways of endochondral bone formation have been discovered. Sox9 is a transcription factor that plays an early and essential role in chondrocyte differentiation.7 Type II collagen is the most abundant extracellular protein produced by chondrocytes8 and a major component of the extracellular cartilage.9 Transcription of type II collagen starts to be expressed following mesenchymal cell condensation that precedes cartilage formation, and thus it represents an early marker of chondrocyte differentiation.10
Recently, we demonstrated the responsive potential of the spheno-occipital synchondrosis to mechanical stimuli.11–13 These studies showed that the expression of certain growth markers, such as Sox9 and type II collagen, can be enhanced by application of mechanical force. Furthermore, they revealed that organ-cultured spheno-occipital synchondrosis can provide a good model to study the effect of various agents on endochondral growth.
We showed that quercetin increased the activity of bone-forming cells in vitro14 and increased bone formation in vivo.15 Current research on quercetin also focused on antioxidant effects, anti-inflammatory effects, anticancer effects, antihypertensive effects, antidiabetic effects, and others.16 Therefore, it is interesting to see whether it will enhance the growth and development process using the synchondrosis model. The aim of this study was to investigate the effect of quercetin on growth of the spheno-occipital synchondrosis in vitro by measuring the amount of Sox9 and type II collagen expression.
MATERIALS AND METHODS
This experiment was approved by the committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (CULATR 1743-08). A total of 50 spheno-occipital synchondroses and surrounding tissue, dissected from 1-day-old male BALB/c mice, were randomly assigned to control and experimental groups. Each group was subdivided into five different time points, which were 6, 24, 48, 72, and 168 hours, with each subgroup containing 5 synchondroses (n = 5).
Surgical Explants of the Cranial Base
According to the euthanasia guidelines of Rodent Feti and Neonates from the Laboratory Animal Unit, the University of Hong Kong, the animals were sacrificed by intracardinal injection of 0.5 mL/kg pentobarbital sodium (Dominal 20%, Alfasan, Woerden, Holland). The spheno-occipital synchondroses together with their adjacent structures were aseptically removed using the procedure described by Shum et al.17 and Rukkulchon et al.12 and incubated in a 24-well plate with or without quercetin in culture medium at 37°C with 5% CO2.
Medium Culture
In the control group
The serum-free protocol was modified from Shum et al.17 using BGJb medium (Gibco, Invitrogen, New York, NY) supplemented with 2 mg/mL bovine serum albumin (BSA; A-9647, Sigma, St Louis, Mo), 100 µg/mL sodium ascorbate (A-4034, Sigma), 1 mM beta-glycerophosphate (Fluka 50020, Buchs, Switzerland), and antibiotics and antimycotics (Gibco, Invitrogen). The culture medium was changed daily and maintained at 37°C and 5% CO2 in air.
In the experimental group
The serum-free protocol similar to the control group was added with 1 µM quercetin dihydrate solution (Sigma Q-0125). The dosage was referenced from previous research of quercetin on cell culture14 and confirmed from a pilot study. The chemical was dissolved 3.3826 × 10−3 gram in 10 mL MQ H2O, autoclaved, and cooled down. The quercetin dihydrate was added 100 µL in 100 mL medium.
The tissue blocks of both groups were completely immersed in the medium at all times and incubated at 37°C under 95% air and 5% CO2 for the 6-hour, 24-hour, 48-hour, 72-hour, and 168-hour period. The medium was changed daily. Following the incubation, the tissues were fixed with 4% (w/v in PBS) paraformaldehyde at 4°C for 20 minutes, rinsed in PBS, decalcified in 10% EDTA at 4°C for 48 hours, dehydrated, and embedded in paraffin.12
Histology and Immunohistochemistry
Serial paraffin sections (5 µm) were cut in the sagittal plane, placed on poly-L-lysine–coated slides, and air dried. After that, the sections were dewaxed and rehydrated, antigenic sites were exposed by digestion with 0.1% trypsin (Sigma, T-0646) for 10 minutes at 37°C, and nonspecific binding was reduced by treating in 3% H2O2 for 10 minutes, then incubated in 3% BSA (Sigma, A-9085) blocking serum at 37°C for 30 minutes. The sections were then incubated with Sox9 antibody (sc-20095, Santa Cruz Biotechnology, Santa Cruz, Calif), dilution 1∶100, 4°C, overnight.
The reaction was detected with an avidin-biotin peroxidase complex kit (Santa Cruz Biotechnology) according to the manufacturer's instructions. Visualization was performed using 3,3-diaminobenzidine (Sigma, D-5637), followed by a rinse in running tap water. The sections were washed with TBS between each step. Negative control experiments were performed by incubating with TBS instead of the primary antibody. The primary antibody dilution of type II collagen (Santa Cruz, sc-7763) was 1∶100 and antigenic sites exposed by digestion with protease K (Sigma P-6556, 10 µg/mL), 37°C, 30 minutes. The other procedure was identical to that of Sox9.
Quantitative and Statistical Analysis
The expression of Sox9 and type II collagen in the spheno-occipital synchondrosis was localized in brown staining and measured with a true-color RGB (red-green-blue) computer-assisted image-analyzing system with a digital camera (Leica DC 300 V2.0, Wetzler, Germany) and with software (Leica Qwin Pro, version 2.6, Leica Microsystems Imaging Solutions) following the method of Rabie et al.18 Cells with at least 100 pixels of positive staining were regarded as positive. The level of positive staining was evaluated as the area in micrometers squared in the fixed measurement frame 800 × 1000 µm2 at 200× magnification (Leitz Orthoplan, Wetzler, Germany).
For each subject, five sections were measured, and in total, 400 sections were measured and quantified. The amount of staining for all sections was quantified and evaluated by one examiner. The difference between experimental and control groups was tested by t-test using SPSS statistical analysis software (SPSS for Windows, version 15.0, Chicago, Ill).
Method Error
The measurement error (Me) was calculated using Dahlberg's formula19: 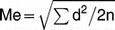 , where d is the difference between the two registrations and n is the number of double registration. Ten subjects with 30 sections were drawn randomly and were measured on the two separate occasions 1 month apart to calculate the method error. The method error was found to be 0.513 µm2. There was no significant difference (P > .05) between the two measurements.
, where d is the difference between the two registrations and n is the number of double registration. Ten subjects with 30 sections were drawn randomly and were measured on the two separate occasions 1 month apart to calculate the method error. The method error was found to be 0.513 µm2. There was no significant difference (P > .05) between the two measurements.
RESULTS
Immunostaining for Sox9 Expression
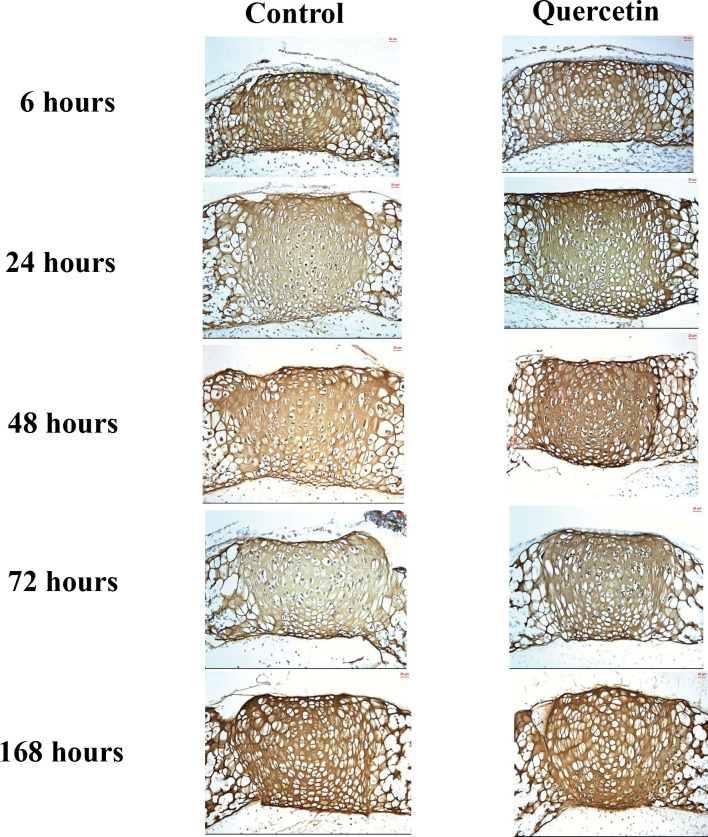
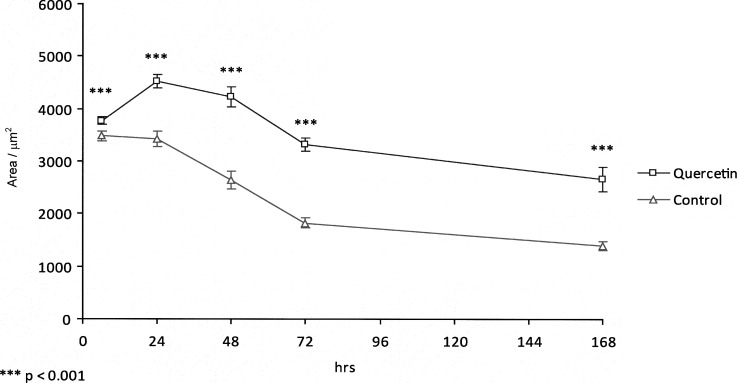
In both the experimental and control groups, the expression of Sox9 was detected predominantly in proliferating chondrocytes and prehypertrophic chondrocytes (Figure 3). The level of Sox9 expression increased dramatically from 6 hours to 24 hours in the experimental group, reaching a peak at 24 hours and then declining gradually until 168 hours. Meanwhile, the expression of Sox9 in the control group decreased gradually from 6 hours to 168 hours. By comparison, there was maximum expression at 24 hours in the experimental group, but expression decreased with time in the control group. There was a statistically significant increase (P < .001) of 32.31% in the expression of Sox9 between control and experimental groups at 24 hours, which was sustained until 168 hours (Figure 2).
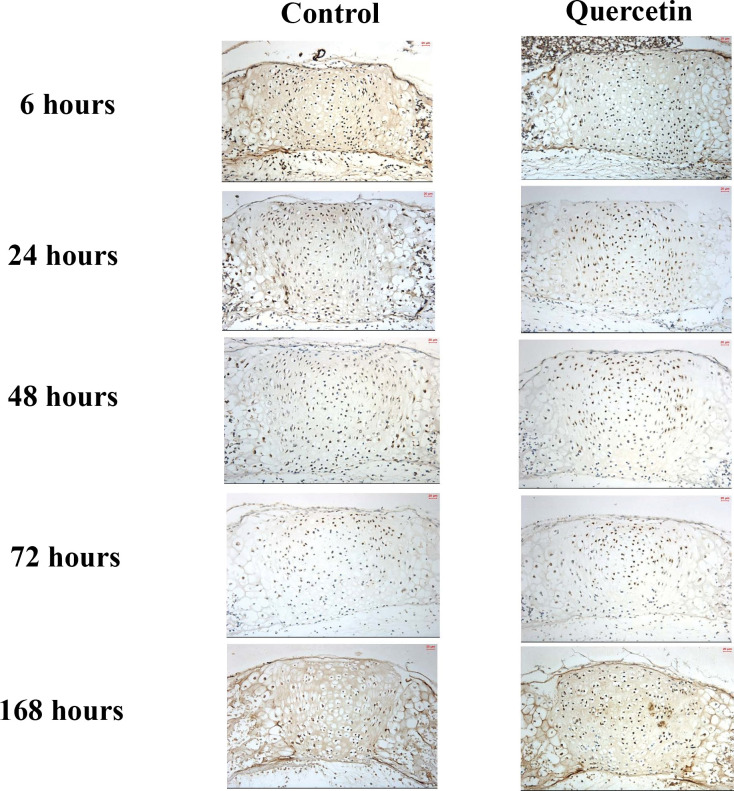
Figure 3.
Average histoimmunochemical appearance (200×) of type II collagen expression in organ culture at each experimental time point in (A) the control group and (B) the quercetin group.
Figure 2.
Graph showing levels of Sox9 expression detected by immunohistochemical staining.
Figure 1.
Average histoimmunochemical appearance (200×) of Sox9 expression in organ culture at each experimental time point in (A) the control group and (B) the quercetin group.
Immunostaining for Type II Collagen Expression
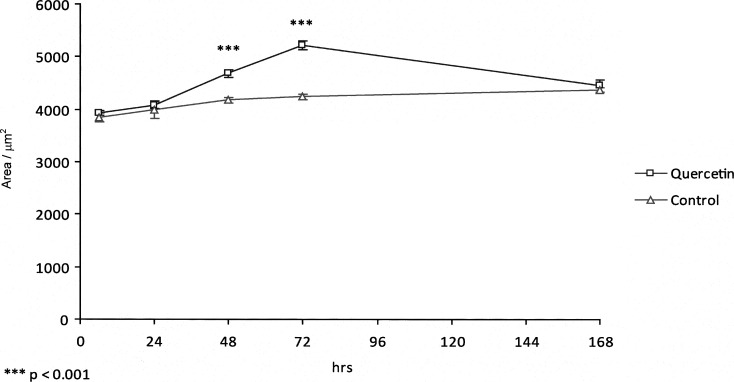
The level of expression of type II collagen in both experimental and control groups, as shown by immunohistochemical staining, was detected predominantly in the proliferative, prehypertrophic, and hypertrophic zones of the spheno-occipital synchondrosis, as shown in Figure 3. In the control group, type II collagen increased gradually from 6 hours to 168 hours, with maximum expression at 168 hours. In the experimental group, the level of type II collagen increased slightly from 6 hours to 48 hours and then dramatically to reach a peak at 72 hours. Quantitative analysis showed there was a significant increase (P < .001) of 22.99% in the expression of type II collagen at 72 hours in the experimental group compared with the control group (Figure 4).
Figure 4.
Graph showing levels of type II collagen expression detected by immunohistochemical staining.
DISCUSSION
This is the first study to demonstrate that quercetin can stimulate growth of the spheno-occipital synchondrosis through increased expression of Sox9 and type II collagen markers. In this in vitro study, we applied quercetin to the spheno-occipital synchondrosis, thus allowing the correlation of quercetin and cellular response to be studied by measuring the temporal expression of Sox9 and type II collagen. Results of the study demonstrated that quercetin stimulated an increase of Sox9 and type II collagen expression in the experimental groups compared with the control groups. Both Sox9 and type II collagen can be regarded as an indicator for growth and development. The implication is that an increase in Sox9 expression leads to greater mesenchymal cell differentiation within the chondrogenic pathway, since Sox9 expression regulates type II collagen by binding directly to 48-bp Col2a1 enhancer segments, enhancing type II collagen synthesis. This increases cartilage matrix formation and synchondrosis growth potential.20 Therefore, Sox9 is a clear indication of chondrocyte differentiation, rather than a consequence. This results in more cartilage formation, leading to increased bone formation. Rabie and Al Kalaly21 also found that the more cartilage formation, the more the possibility of formation of bone and hence more growth.
The significance of type II collagen has been highlighted by studies in which mutant pro 1 (II) collagen chains have resulted in abnormal skeletal phenotypes.10 Furthermore, Barbieri et al.22 showed that a deletion of exon 48 in the mouse α1 (II) procollagen gene (Col2a1) led to a reduction in chondrogenesis and osteogenesis such as severe shortening of the limbs, resulting in reduced anteroposterior length of the animals, reduced growth of the mandible and the maxilla, incomplete closure of the hard palate, and a downward bending of the snout in transgenic mice.
Our study demonstrated a growth response pattern of the spheno-occipital synchondrosis similar to what has been reported by other studies. Cendekiawan et al.13 used tensile stress to stimulate expression of Sox9 and type II collagen, showing the same pattern of Sox9 and type II collagen expression from 6 hours to 168 hours. In the experimental groups, the expression of Sox9 and type II collagen reached a maximum level at 24 hours and 72 hours, respectively. However, the amount of expression in the experimental groups compared with the control groups in this study differs from the study of Cendekiawan et al. Our study found a significant increase of 32.31% (P < .001) in Sox9 expression between experimental and control groups. This is in contrast to the 57% (P < .001) increase reported in the study by Cendekiawan et al. In addition, we found an increased expression of 22.99% (P < .001) of type II collagen versus the 44.4% (P < .001) in their analysis.13 This suggests 1 µM of quercetin is capable of producing a similar, but attenuated, amount of Sox9 and type II collagen expression compared with tensile stress. Although the overall effect of an increase of type II collagen expression is not big and the period of exposure of quercetin is short (several days), further research is needed to optimize the dosage of quercetin and to investigate the long-term effect of quercetin on growth.
Growth of the maxilla and mandible is directly influenced by the spheno-occipital synchondrosis.23 The upper facial skeleton articulates with the anterior cranial fossa, and the mandible articulates with the midcranial fossa through the temporomandibular joint.24 The discovery that the growth of spheno-occipital synchondrosis may be modified chemically may offer a new therapeutic alternative for treatment of craniofacial deformity.
On the other hand, quercetin is a commonly used health supplement in Asia. It gained interest among researchers for its multiple health maintenance effects, for instance, prevention of cardiovascular disease, cancer, cataract, schizophrenia, and prostatitis.25–27 The discovery of the growth stimulation effect of quercetin on spheno-occipital synchondrosis opened a new area of research in growth modification as other flavonoids and phytoestrogens may also have a similar effect. The phytochemical modifier of growth is therefore a new therapeutic possibility. Further research on various gene expression responses to quercetin in spheno-occipital synchondrosis is needed to confirm the actual stimulation mechanisms and to determine the optimum dose to maximize the effect of the quercetin and also to evaluate the effect of different concentrations of quercetin solution on growth. Moreover, further studies should carry out a quantitative assessment of the amount of growth stimulation of this extract compared with control with a greater sample size.
CONCLUSIONS
The expression of Sox9 and type II collagen in the spheno-occipital synchondrosis can be increased in a response of the stimulation by quercetin.
Sox9 is an essential factor for early differentiation of chondrocytes and for type II collagen synthesis during growth of cartilage in spheno-occipital synchondrosis.
Acknowledgments
This research was supported by the Committee on Research and Conference Grants, the University of Hong Kong (CRCG 10207932. 36170.08003.323.01). We thank Dr Shuxia Li and Mr Chui Ying Yip of the Hard Tissue Laboratory for their technical assistance in the histological preparation and also Mr Shadow Yeung for his kind assistance with the statistical analysis.
REFERENCES
- 1.Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;62:877–887. doi: 10.1079/PNS2003309. [DOI] [PubMed] [Google Scholar]
- 2.Hathena S. J, Velasquez M. T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 3.Duncan A. M, Phipps W. R, Kurzer M. S. Phyto-oestrogens. Best Pract Res Clin Endocrinol Metab. 2003;17:253–271. doi: 10.1016/s1521-690x(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 4.Kanno S, Hirano S, Kayama F. Effects of phytoestrogens and environmental estrogens on osteoblastic differentiation in MC3T3-E1 cells. Toxicology. 2004;196:137–145. doi: 10.1016/j.tox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Price K. R, Rhodes M. J. C. Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J Sci Food Agric. 1997;74:331–339. [Google Scholar]
- 6.Proffit W. R, Fields H. W, Sarver D. M. Contemporary Orthodontics 4th ed. Philadelphia, Pa: Mosby; 2007. Concept of growth and development; p. 44. [Google Scholar]
- 7.Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre V, Zhou G, Mukhopadhyay K, et al. An 18-base-pair sequence in the mouse proalpha1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996;16:4512–4523. doi: 10.1128/mcb.16.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upholt W. B. The type II collagen gene. In: Olsen B. R, Nimni M. E, editors. CollagenMolecular Biology Vol IV. Boca Raton, Fla: CRC Press; 1989. p. 31. [Google Scholar]
- 10.Garofalo S, Vuorio E, Metsaranta M, et al. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Nat Acad Sci U S A. 1991;88:9648–9652. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei W. Y, Wong R. W. K, Rabie A. B. M. Factors regulating endochondral ossification in the spheno-occipital synchondrosis. Angle Orthod. 2008;78:215–220. doi: 10.2319/020707-59.1. [DOI] [PubMed] [Google Scholar]
- 12.Rukkulchon B. K, Wong R. W. K. Effect of tensile force on expression of PTHrP and thickness of hypertrophic zone in organ-cultured mouse spheno-occipital synchondroses. Arch Oral Biol. 2008;53:690–699. doi: 10.1016/j.archoralbio.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Cendekiawan T, Wong R. W. K, Rabie A. B. M. Temporal expression of SOX9 and type II collagen in spheno-occipital synchondrosis of mice after mechanical tension stimuli. Angle Orthod. 2008;78:83–88. doi: 10.2319/012507-36.1. [DOI] [PubMed] [Google Scholar]
- 14.Wong R. W. K, Rabie A. B. M. Effect of quercetin on preosteoblasts and bone defects. Open Orthop J. 2008;2:27–32. doi: 10.2174/1874325000802010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong R. W. K, Rabie A. B. M. Effect of quercetin on bone formation. J Orthop Res. 2008;26:1061–1066. doi: 10.1002/jor.20638. [DOI] [PubMed] [Google Scholar]
- 16.Kelly G. S. Quercetin. Alt Med Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 17.Shum L, Wang X, Kame A. A, et al. BMP4 promotes chondrocyte proliferation and hypertrophy in the endochondral cranial base. Int J Dev Biol. 2003;47:423–431. [PubMed] [Google Scholar]
- 18.Rabie A. B. M, She T. T, Hägg U. Functional appliance therapy accelerates and enhances condylar growth. Am J Orthod Dentofacial Orthop. 2003;123(1):40–48. doi: 10.1067/mod.2003.45. [DOI] [PubMed] [Google Scholar]
- 19.Dahlberg G. Statistical Methods for Medical and Biological Students. London, UK: George Allen and Unwin; 1940. [Google Scholar]
- 20.Cheah K. S, Lau E. T, Au P. K, et al. Expression of the mouse alpha 1 (II) collagen gene is not restricted to cartilage during development. Development. 1991;111:945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- 21.Rabie A. B, Al-Kalaly A. Does the degree of advancement during functional appliance therapy matter? Eur J Orthod. 2008;30:274–282. doi: 10.1093/ejo/cjm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbieri O, Astigiano S, Morini M, et al. Depletion of cartilage collagen fibrils in mice carrying a dominant-negative Col2a1 transgenic affects chondrocyte differentiation. Am J Physiol Cell Physiol. 2003;285:1504–1512. doi: 10.1152/ajpcell.00579.2002. [DOI] [PubMed] [Google Scholar]
- 23.Rosen H. M, Whitaker L. A. Cranial base dynamics in craniofacial dysostosis. J Maxillofac Surg. 1984;12:56–61. doi: 10.1016/s0301-0503(84)80212-x. [DOI] [PubMed] [Google Scholar]
- 24.Lavelle D. Oxford Handbook of Applied Dental Sciences. Oxford, UK: Oxford University Press; 2003. Craniofacial development; p. 3. [Google Scholar]
- 25.Hertog M. G. L, Hollman P. C. H. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996;50:63–71. [PubMed] [Google Scholar]
- 26.Formica J. V, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]