Summary
Some bacteria have coevolved to establish symbiotic or pathogenic relationships with plants, animals or humans. With human association, the bacteria can cause a variety of diseases. Thus, understanding bacterial phenotypes at the single‐cell level is essential to develop beneficial applications. Traditional microbiological techniques have provided great knowledge about these organisms; however, they have also shown limitations, such as difficulties in culturing some bacteria, the heterogeneity of bacterial populations or difficulties in recreating some physical or biological conditions. Microfluidics is an emerging technique that complements current biological assays. Since microfluidics works with micrometric volumes, it allows fine‐tuning control of the test conditions. Moreover, it allows the recruitment of three‐dimensional (3D) conditions, in which several processes can be integrated and gradients can be generated, thus imitating physiological 3D environments. Here, we review some key microfluidic‐based studies describing the effects of different microenvironmental conditions on bacterial response, biofilm formation and antimicrobial resistance. For this aim, we present different studies classified into six groups according to the design of the microfluidic device: (i) linear channels, (ii) mixing channels, (iii) multiple floors, (iv) porous devices, (v) topographic devices and (vi) droplet microfluidics. Hence, we highlight the potential and possibilities of using microfluidic‐based technology to study bacterial phenotypes in comparison with traditional methodologies.
Microfluidics is an emerging technique that can complements current biological assays. Here, we review some key microfluidic‐based studies describing the effects of different microenvironmental conditions on bacterial phenotypes. We also highlight the potential and possibilities of using microfluidic‐based technology to study bacterial phenotypes in comparison with traditional methodologies.

Introduction
Bacteria are microscopic organisms that inhabit all known habitats on Earth, either interacting with natural environments or leading to beneficial or harmful effects in specific hosts. For instance, some environmental bacteria grow attached to plants, and a relationship is created between both entities. Usually, this is established as a symbiotic interaction, in which nutrients and protection are provided (Backer et al., 2018; Vives‐Peris et al., 2020), but some studies have described non‐symbiotic relationships between plants and bacteria (Lamouche et al., 2018; Deng et al., 2020). This kind of positive relationship has also been established between bacteria and animals or humans; for example, the formation of the intestinal microbiota that facilitates digestion, outcompetes pathogenic microorganisms and provides essential nutrients (Debré and Gall, 2014). In this context, research on the beneficial relationship between the microbiota and the human host has resulted in biotechnological applications, such as probiotic bacteria, which contribute to maintaining human health by battling harmful microorganisms, healing gut and skin tissue or strengthening the epithelial barrier (Lukic et al., 2017). On the other hand, some pathogenic bacteria have evolved to cause disease, either by producing virulence factors, developing mechanisms to survive in unfavourable environments or by manipulating the host immune system, among others (Kudva et al., 2016; Johnson, 2018). Even though our in‐depth knowledge of pathogens has led to vaccine and antibiotic development to treat many infectious diseases (Henriques‐Normark and Normark, 2014), additional research is needed to cope with the emerging threat of antimicrobial resistance, emerging and neglected infectious diseases and research into fastidious pathogens.
Understanding bacterial interactions with cells under physiological conditions is essential to study certain bacterial phenotypes. Traditional microbiological in vitro techniques include microscopy, cell infection models and recent molecular, cellular and immunological assays (Houpikian and Raoult, 2002; Balouiri et al., 2016). These methods have provided and continue to provide inestimable information, contributing to gaining insight into the molecular and cellular microbiology of the host‐bacteria interplay. However, traditional methodologies have some limitations. On the one hand, although culture procedures have improved with time, bacteria still exist that cannot be cultured under in vitro conditions (Ito et al., 2019; Bodor et al., 2020). On the other hand, in vitro and ex vivo models are not able to fully recreate the physiological environment, such as gastric acidity, whose pH increases while digestion occurs (Vinderola et al., 2017). Consequently, in vitro and in vivo results do not always correlate. In addition, some phenotypes, such as biofilm formation, are traditionally studied under simplistic environments that do not mimic complex physiological scenarios (Lebeaux et al., 2013). Nevertheless, the recent advent of microfluidics can help to solve some of these limitations, to not only advance fundamental microbiological research but also help in the development of therapeutic interventions.
Microfluidics is known as the technique that handles microscale fluid flows (Sackmann et al., 2014), allowing the integration, miniaturization and automation of several processes (Halldorsson et al., 2015). Working with manufactured microdevices involves the use of small volumes (on the microliter scale), assuring better control of environmental conditions and saving reagents, biological material, residues and space (Shin et al., 2012). However, the main advantage of these microfluidic devices is the possibility of recreating biological environments more realistically than traditional macroscopic cultures in flasks, well plates or dishes (Halldorsson et al., 2015). This approximation can be implemented by three‐dimensional cultures of microorganisms embedded in hydrogels that simulate the extracellular matrix or by introducing a flow to mimic interstitial fluid flow that transports nutrients and other microorganisms (Huang et al., 2010). In addition, the physical (rigidity, pressure forces, fluid flows, etc.) and chemical (pH, attraction and repulsion factors, etc.) conditions can be modified to study adaptations to the changing environment (Velve‐Casquillas et al., 2010).
The materials used in the pioneering microfluidic devices were silicon and glass. Nevertheless, the opacity of silicon makes it incompatible with microscopy studies. Moreover, both glass and silicon are characterized by their fragility, difficult bonding protocols and high cost. Altogether, their use has been limited, paving the way for the use of new materials (Sackmann et al., 2014). Since the 1990s, the most utilized material has been polydimethylsiloxane (PDMS), a mineral‐organic polymer from the siloxane family (Friend and Yeo, 2010). PDMS presents numerous beneficial properties: optical transparency, gas permeability, flexibility, chemical inertness, biocompatibility, ease of bonding and unbonding to other materials, the possibility of making its surface more hydrophilic and low cost (Weibel et al., 2007). Thus, PDMS has been considered a great candidate for microfluidics studies. Other materials used for this application are thermoplastics, paper and wax (Sackmann et al., 2014).
Microfluidic devices have great versatility in terms of design depending on the field of study in which they are applied. The diverse patterns found in the literature have been divided into six groups throughout this review: (i) devices with linear channels, subdividing for one, two and three parallel channels; (ii) devices with mixing channels; (iii) devices with multiple floors; (iv) porous devices; (v) topographic devices and (vi) droplet microfluidics (Table 1).
Table 1.
Summary of different articles that used microfluidics‐based approaches to study different phenomena. Research topic icons were constructed by Freepik (chemotaxis, aerotaxis, magnetotaxis, pH taxis, growth tests and biofilm formation), Good Ware (rheotaxis) and fps web agency (thermotaxis) from www.flaticon.com.
| Linear channels |

|
Cheng et al. (2007), Ahmed and Stocker (2008), Lanning et al. (2008), Roggo et al. (2018) | Kim et al. (2016), Menolascina et al. (2017), Stricker et al. (2019) | Kaya and Koser (2012), Caspi (2014) | Myklatun et al. (2017), Rismani Yazdi et al. (2018b), Miller et al. (2019) | Jeong et al. (2014), Murugesan et al. (2017), Paulick et al. (2017) | Zhuang et al. (2015), Parvinzadeh Gashti et al. (2016) | Balaban et al. (2004), Wang et al. (2010), Aldridge et al. (2012), Lambert and Kussel (2014), Matsumoto et al. (2016), Kim et al. (2019), Li et al. (2019), Simmons et al. (2020) | Jeong et al. (2014) |
| Mixing channels |
|
Mao et al. (2003), Englert et al. (2009) | Lam et al. (2009), Golchin et al. (2012) | ||||||
| Multiple floors |

|
Adler et al. (2012) | Salman et al. (2006), Demir and Salman (2012) | Hyun et al. (2008) | |||||
| Porous devices |

|
Long and Ford (2009), Wright et al. (2014) | Durham et al. (2012), Dehkharghani et al. (2019) | Aufrecht et al. (2019) | |||||
| Topographic devices |

|
Marcos et al. (2009), Rusconi et al. (2010), Yazdi and Ardekani (2012), Ishikawa et al. (2014), Marty et al. (2014), Zhang et al. (2019) | Rismani Yazdi et al. (2018a) | Takeuchi et al. (2005), Cho et al. (2007) | Yazdi and Ardekani (2012), Hansen et al. (2016), Rath et al. (2017), Zhang et al. (2019) | ||||
| Droplet microfluidics |
|
Cao et al. (2015), Kaushik et al. (2017), Mahler et al. (2019) | Chang et al. (2015), Jin et al. (2018), White et al. (2019) | ||||||
| Chemotaxis | Aerotaxis | Rheotaxis | Magnetotaxis | Thermotaxis | pH taxis | Growth tests | Biofilm formation | ||

|
|

|

|

|

|
|

|
Our main objective is to compile diverse studies on bacterial behaviour (Table 1), including taxis processes, which are defined as directed bacterial movement towards or away from an external stimulus or gradient (Krell et al., 2011). Depending on the external stimulus, different taxis can be stimulated. If there is a chemical compound, it is chemotaxis; an oxygen gradient, aerotaxis; a flow current, rheotaxis; a light stimulus, phototaxis; a magnetic field, magnetotaxis; a temperature stimulus, themotaxis and a pH stimulus, pH taxis. We also included studies in relation with growth assays under different environments, in the presence of diverse compounds and on the formation of biofilms, known as microbial communities established in the self‐produced extracellular substances (Mirzaei et al., 2020).
Microfluidic devices with linear channels
Devices with linear channels are the most basic design and consist of straight channels with openings at each end. Multiple channels can be added in parallel to create more versatile devices. In this review, we focused on devices with one, two and three channels.
Single‐channel devices
As the name suggests, these devices consist of a unique central channel into which bacteria are introduced. There are two main approximations with this design. On the one hand, a tactic signal gradient can be generated inside the channel. On the other hand, researchers can use several devices so that each device presents a different condition inside the channel. In the first case, bacterial behaviour is analysed along the channel, whereas in the second approximation, bacterial behaviour is compared across different devices.
Chemotaxis was among the pioneering studies carried out on microfluidic devices. Generation of a gradient inside the channel by the addition of a signal molecule on one end and bacteria on the other end allows for observations of chemotactic movements along the channel. The chemotactic response of Escherichia coli to aspartate and serine was discovered by the J. Adler laboratory in the 1960s (Adler, 1966, 1973). This line of investigation was continued by several researchers (Segall et al., 1982; Liberman et al., 2004), resulting in a deep characterization and description of the stages and molecules involved in the process. Microfluidic assays using gradients inside channels have been able to reproduce previously published results by traditional methods. For example, Ahmed and Stocker introduced E. coli bacteria on one aperture, whereas at the opposite opening, they introduced α‐methylaspartate and l‐serine as chemoattractants (Fig. 1A). The E. coli responded to this chemical gradient, increasing its speed by 35% (Ahmed and Stocker, 2008).
Fig. 1.
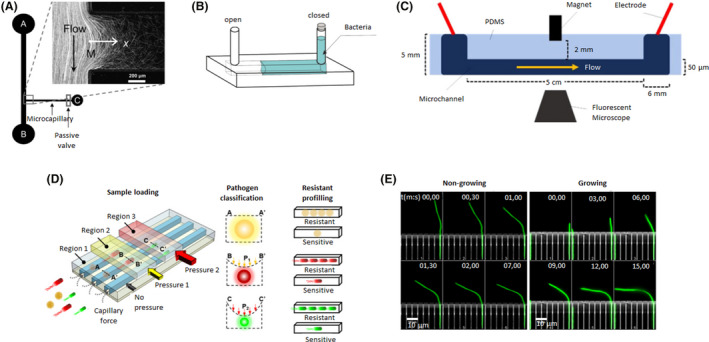
Microfluidic device designs of individual and linear channels. A. The bacterial flow travelled from reservoir A to B, whereas, chemoattractants were added to reservoir C. A fraction of the bacterial population swam into the capillary where chemotaxis was studied. Adapted from (Ahmed and Stocker, 2008). B. An oxygen gradient was generated by capturing an air bubble on one of the ends of the channel. The opposite end was filled with a bacterial solution. Adapted from (Stricker et al., 2019). C. A magnet was incorporated in the middle of the microfluidic device to attract magnetotactic bacteria that flowed through the channel. Adapted from (Miller et al., 2019). D. A microfluidic device with different regions where pressure could be applied. Depending on the pressure exerted, the channels contracted to different sizes and were able to trap bacteria as a function of their size and shape. Three different bacteria (yellow, red and green) were trapped in regions 1, 2 or 3 (with increasing pressure from 1 to 3). Then, antibiotics were added to examine drug resistance and determine if they were resistant or sensitive. Adapted from (Li et al., 2019). E. A microfluidic device with a side chamber of the same size as the E. coli body trapped single bacteria while their flagellum remained freely mobile in the central channel. Hydrodynamic forces were applied to non‐growing and growing bacteria (green) to determine their bending force and filament deformation at different times (mm, ss: 00,00–15,00). Adapted from (Caspi, 2014). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
Aerotactic assays were carried out by Stricker et al., who designed a microdevice in which an air bubble was captured in one of the ends, whereas, the rest of the channel was filled with a Shewanella oneidensis suspension (Fig. 1B), a facultative anaerobe. This design had two configurations: both openings were closed, or only the end with the bacteria was closed. The oxygen diffused from the bubble to the medium, creating a gradient. Hence, depending on the configuration, the oxygen supply was limited and variable over time or limitless and constant respectively. S. oneidensis migrated towards the bubble until it established itself at a point where the oxygen conditions were optimal, maintaining its motility. In the case of limited oxygen, as the bacteria consumed the oxygen, they entered a non‐motile vibration state. The authors concluded that the aerobic–anaerobic transition determined the motile and non‐motile states of the bacteria (Stricker et al., 2019).
The integration of multiple units on a unique microfluidic platform allows for the study of multiple conditions at the same time. For example, rheotactic and magtetotactic effects on E. coli have been analysed using multiple single channels through which a flow of bacteria passes. Under resting conditions, E. coli migrated on circular trajectories; with moderate laminar flow, they migrated in the opposite direction of the flow, and under high flow conditions, the bacteria were dragged in the same direction of flow (Kaya and Koser, 2012). It has also been demonstrated that flow interferes with magnetic stimuli. Miller et al. added a magnet on the centre in the middle of the channel and bound E. coli to magnetic microbeads (Fig. 1C). In this way, the authors could isolate bacteria by passing them through the channel. They determined that switching the flow has the highest recruitment ratio (Miller et al., 2019). Following a similar strategy, Chen and De La Fuente mimicked the natural environment of Xylella by applying a continuous flow in two parallel channels. By increasing the calcium concentration to 2 mM, they confirmed that the twitching speed was significantly increased. In addition, RNA‐seq determined that calcium regulated the transcription of genes related to the type IV pili mechanism, pathogenicity and host adaptation (Chen and De La Fuente, 2020).
These devices have also been used to test drug resistance. Pseudomonas aeruginosa was cultured inside four independent channels, each containing different dilutions of an antimicrobial compound. The results correlated with standard antimicrobial susceptibility tests and produced results within 3 h. Additionally, by using microfluidics, the authors detected alternative phenotypes linked to the use of some drugs, such as bacterial elongation in the presence of ciprofloxacin or piperacillin or spheroplast and bulge formation in the presence of meropenem (Matsumoto et al., 2016).
Microfluidics offers high flexibility in terms of design, making it suitable for numerous research approaches. Developing smaller channels and adjusting them to the measurements of the studied bacteria (Fig. 1D) allows the capture of bacteria according to their size and shape for identification by electron microscopy and testing of their resistance to antibiotics (Balaban et al., 2004; Li et al., 2019). Moreover, a single bacterium could be trapped to examine its generational growth. Working with E. coli under a continuous flow of nutrients that simulates the exponential phase of in vitro growth, it was discovered that the division rate of the initial cell remained constant over time, contradicting previous results that indicated the rate decreases (Wang et al., 2010).
Another approach to trap bacteria is by designing small side chambers perpendicular to the central channel where the bacteria are analysed. Multiple E. coli were introduced into these lateral chambers to study their adaptative memory mechanisms when carbon nutrients were removed from the central channel. The authors found that E. coli presented phenotypic memory by which the bacteria maintained the production of lactose utilization enzymes for ten generations, reducing lag phases in response to switching fluctuations in glucose‐ or lactose‐rich environments. E. coli also showed response memory, by which gene expression was maintained after the removal of inducers, promoting bacterial adaptation to short‐term changes in the surroundings (Lambert and Kussel, 2014). Single bacteria can also be caught in these side chambers. For instance, Aldridge et al. trapped a single Mycobacterium smegmatis in each chamber and discovered that these bacteria did not show symmetrical division, instead preferentially elongating their ‘old pole’. This results in populations with different sizes and elongation rates, which are affected differently by antibiotics (Aldridge et al., 2012). On the other hand, Caspi drew a chamber size similar to E. coli body size, managing to catch bacteria oriented in such a way that their flagellum remained in the central channel (Fig. 1E). By applying hydrodynamic forces through the central channel, bending forces and filament deformation were calculated. At low flow rates (200 µl h−1), twice as much force was needed to deform non‐growing bacteria to the same extent as growing bacteria. He deduced that this result is due to the difference between the elastic response of non‐growing cells, which could recover their initial conformation when the flow was stopped, in comparison to the plastic‐elastic of the growing cells, which were irreversibly deformed (Caspi, 2014).
The interaction of bacteria with bacteriophages was recently studied by Simmons et al. in 2020 using linear devices due to the highly controlled conditions offered by microfluidic techniques. E. coli was seeded on the microfluidic channel for 48 h with a constant flow to allow biofilm formation. Then, T7 bacteriophages were introduced to infect the bacteria, and biofilm development was analysed for 48 h. Combining their results with computational tools, the authors were able to develop a simulation of the population dynamics of phages that took into account environmental factors such as the ratio of bacterial growth and phage replication, nutrient diffusion or phage susceptibility (Simmons et al., 2020).
Two‐channel devices
This kind of chip presents a configuration that is based on two different channels that may or may not be connected. In the case of being connected, two inputs converge into only one outlet. Lanning and colleagues (2008) used a channel‐connected device to introduce bacteria and bacteria with chemoattractants into each inlet to study the response of E. coli to α‐methylaspartate and aspartate, obtaining results similar to those obtained by traditional methods. Following a similar approach, Gashti et al. entered Streptococcus salivarius through a single inlet, managing to develop a biofilm only at the upper part of the central channel. This central channel contained fluorescein, a pH indicator that showed a decrease in environmental pH in the biofilm area (Fig. 2A and B). Then, they removed glucose from the medium and observed that the pH returned to physiological conditions in approximately 30 min. However, when glucose was introduced again, the medium acidified within a few minutes to a pH close to 5 (Parvinzadeh Gashti et al., 2016).
Fig. 2.
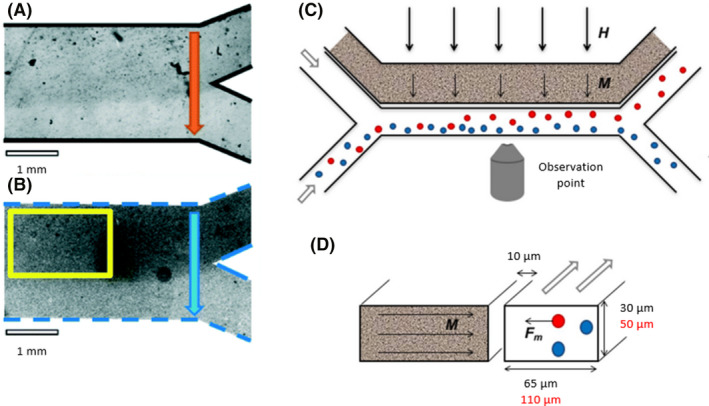
(A) A Y‐shaped microfluidic device with biofilm growth into the upper part of the common channel visualized by bright‐field transmission microscopy (A) and radiometric pH imaging (B). Adapted from (Parvinzadeh Gashti et al., 2016). (C) A microfluidic device with two linear channels, in which one contained a ferrofluid (grey channel) and the other channel contained bacteria (white channel). An external magnetic field (H) magnetized the ferrofluid (M), creating a gradient field. Magnetotactic bacteria (red) sensed the magnetic field and suffered an attractive force (F m), whereas, the nonmagnetic bacteria (blue) were not affected. Longitudinal view (C) and transversal view (D). Adapted from (Myklatun et al., 2017). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
If the channels are independent, a different fluid passes through each channel, but each channel has some effect on the other. For instance, if a magnetotactic bacterium, such as Magnetospirillum gryphiswaldense, was exposed to an orthogonal magnetic field created by a ferrofluid, the bacteria would be attracted to the channel in which the ferrofluid circulated (Fig. 2C and D; Myklatun et al., 2017).
Three‐channel devices
The inclusion of a third channel provides more possibilities because it allows the generation of a gradient in the central channel by adding a signal molecule to a lateral channel, whereas, the opposite lateral channel maintains the culture conditions and acts as a control. In this way, bacteria are seeded in the central channel, and subsequently, the bacterial movement in response to the gradient can be analysed. E. coli, as a model bacterium, has been used in multiple examples to create different gradients and study a wide range of bacterial phenotypes. Chemotactic responses were determined by adding α‐methylaspartate, l‐serine and aspartate to one channel, generating a chemical gradient (Fig. 3A). It was demonstrated that the strongest migratory response occurred in the presence of l‐serine, and the concentrations of α‐methylaspartate and aspartate had to be increased to observe significant migration (Cheng et al., 2007; Roggo et al., 2018). The relationship between nutrients and thermotaxis was studied by the incorporation of water channels with different temperatures, creating a profile gradient between 32 and 37°C. E. coli are attracted to higher temperatures, so they migrated in that direction. In the first stage, nutrient consumption was higher under those conditions due to an increase in its metabolic activity. Thus, in the second stage, a nutrient gradient was generated, with a higher concentration in cold zones. In this latter stage, the bacteria migrated towards low‐temperature areas (Murugesan et al., 2017; Paulick et al., 2017).
Fig. 3.
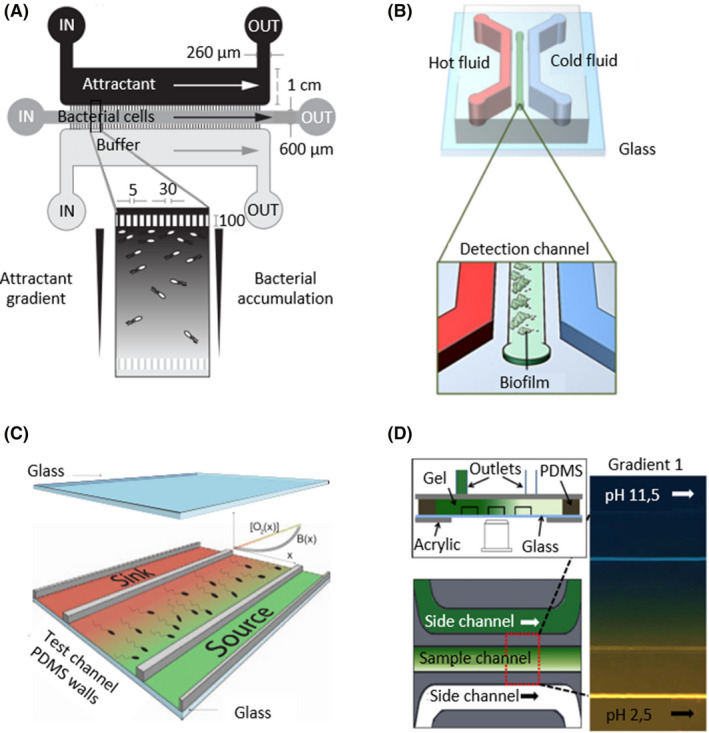
Microfluidics devices with three channels can generate gradients by adding different conditions to each lateral channel.
A. The upper channel was filled with a chemical attractant, and the bottom channel was filled with buffer, generating a chemical gradient in the central channel that was filled with a bacterial solution. Bacterial accumulation was studied in the central channel to determine chemotaxis. Adapted from (Roggo et al., 2018). B. Lateral channels were filled with hot (red) and cold (blue) fluids to generate a thermal gradient in the central channel. Bacteria were seeded on the central channel, and biofilms were closely formed at higher temperatures. Adapted from (Jeong et al., 2014). C. A linear oxygen concentration flowed from the source (green) to the sink (red), creating a gradient in the test channel where the bacterial aerotactic response was analysed. Adapted from (Menolascina et al., 2017). D. Side channels were filled with solutions with different constant pH values, 11.5 (blue) and 2.5 (yellow), which diffused through the PDMS walls, generating a pH gradient in the sample channel. Adapted from (Zhuang et al., 2015). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
In addition to E. coli, other bacteria have been studied within these devices. Developing a temperature gradient, Jeong et al. established three biofilm formation patterns for Antarctic marine bacteria (Fig. 3B). Pseudoalteromonas arctica aggregated at high temperatures, approximately 37–41°C, Pseudoalteromonas sp. associated at cold temperatures, 0–18°C, and biofilm formation of Shewanella frigidimarina was not affected by temperature (Jeong et al., 2014). By applying reduced‐oxygen media though one lateral channel, the aerotactic responses of Bacillus subtilis, E. coli and S. oneidensis, all of which are facultative anaerobes, were determined (Fig. 3C). While Bacillus and Escherichia migrated towards the presence of oxygen, S. oneidensis migrated to low‐oxygen areas (Kim et al., 2016; Menolascina et al., 2017). pH and antibiotic gradients have also been developed within these devices. Serratia marcescens was exposed to a pH gradient to establish its optimal pH at 7–7.3 (Fig. 3D; Zhuang et al., 2015), and P. aeruginosa was grown in the presence of different drug gradients to quantify its minimum inhibitory concentration (Kim et al., 2019).
Furthermore, microfluidics enables the integration of several stimuli in a single device, allowing the study of different stimuli competing in one single chip. Rismanu Yazdi et al. revealed that the capacity of Magnetospirillum magneticum to overcome a current depends on the flow, magnetic field and relative orientation. The bacterium could overcome a flow 2–3 times higher when swimming perpendicular to it. However, if a magnetic field was applied, its magnetotactic response allowed it to overcome the counter direction flow (Rismani Yazdi et al., 2018b).
Microfluidic devices with mixing channels
Microfluidic devices with mixing channels are more complex than those explained above since they require a network of channels, which is challenging in terms of both device design and fabrication. They consist of a series of interconnected microchannels that allow diffusive mixing. This channel network is used to generate a linear concentration gradient (Fig. 4A) or specific microenvironments whose conditions require precise control (Fig. 4B).
Fig. 4.
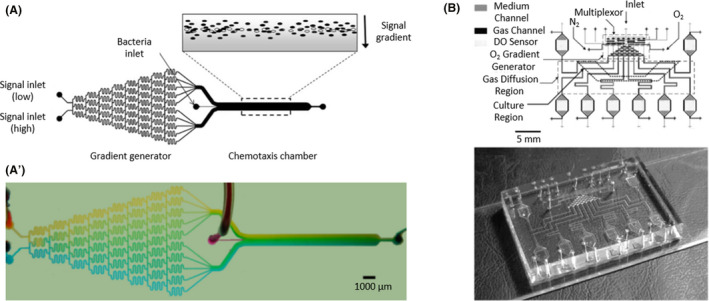
(A) A microfluidic device with a network of interconnected channels to mix different inlets and generate a highly controlled conditions as an output in the chemotaxis chamber. Live (black ovals) and dead (white ovals) bacteria were introduced in the chemotaxis channel to track migration in response to a signal gradient (grey). (A′) Representation of the gradient formation using blue and yellow food dyes, transforming into a range of greens. Adapted from (Englert et al., 2009). (B) A microfluidic device with two layers of PDMS (medium and gas). The medium layer contained a series of channels with a culture region and a density optical sensor (DO sensor). The gas layer consisted of an inlet for each oxygen (O2) and nitrogen (N2) and a multiplexor that created an oxygen gradient to grow aerobic and anaerobic bacteria. Adapted from (Lam et al., 2009). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
The E. coli response to chemical gradients of L‐aspartate and nickel(II) ions (Ni + 2) was tested. Bacteria were attracted to low concentrations of L‐aspartate and deterred from high concentrations of l‐aspartate and Ni + 2. By genetic manipulation, researchers obtained bacteria with knockouts of membrane receptors and flagella proteins, corroborating Adler's discovery (Adler, 1966) that the Tar receptor sensed l‐aspartate and Ni + 2 (Mao et al., 2003; Englert et al., 2009). On the other hand, with an oxygen mixture, researchers created different highly controlled aerobic and anaerobic environments to grow several bacteria, such as E. coli (facultative anaerobe), Actinomyces viscosus (aerobe), Streptococcus mutans (anaerobe) and Fusobacterium nucleatum (anaerobe). They achieved optimal growth conditions for each bacteria using a differential oxygenator, providing high concentrations of oxygen for aerobic bacteria, low concentrations for facultative anaerobic bacteria and a nitrogen (N2) flow for obligate anaerobes (Lam et al., 2009; Golchin et al., 2012).
Devices with multiple floors
Different layers of PDMS, with the same or distinct designs, can be successively bonded to create a microfluidic device with more than one floor. This allows the study of the relationship between two conditions at the same time or to recreate 3D conditions between different tissues. For instance, the development of a circuit of water currents with a temperature gradient located over a layer of PDMS that confined a bacterial suspension allowed the study of the influence of temperature and nutrients on the migration of E. coli (Fig. 5A). E. coli moved towards hot temperatures until it consumed the nutrients in that area; subsequently, E. coli travelled to cold areas where there were still nutrients. Moreover, at high temperatures, the intracellular pH decreased, leading to a drop in their migratory speed (Salman et al., 2006; Demir and Salman, 2012).
Fig. 5.
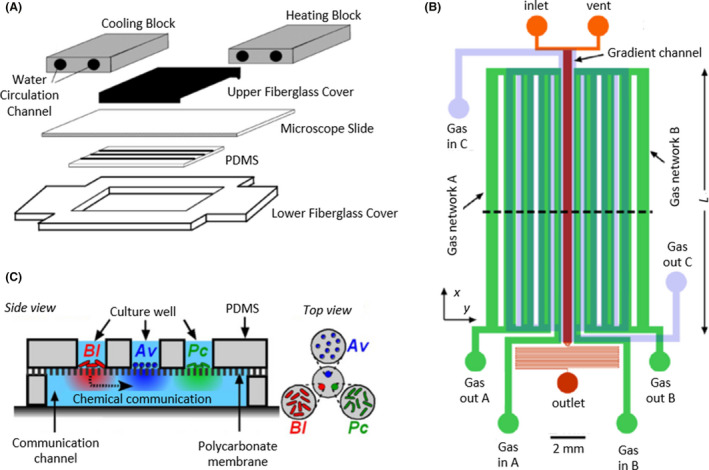
Microfluidic devices with multiple floors.
A. The bottom floor made by PDMS consisted of a camera to grow bacteria and confined by two fiberglass covers, whereas, the upper floor was a water circuit with two blocks, a cooling and a heating block (grey), to create a temperature gradient. Adapted from (Salman et al., 2006). B. Microfluidic device with four floors to control bacterial culture and the oxygen environment: two lower layers, 37 µm and 150 µm deep, were filled with bacterial culture (red and orange), a 150 µm deep layer was a channel circuit for gases A and B (green) and a 340 µm deep layer was a circuit for gas C (blue). Adapted from (Adler et al., 2012). C. Side and top view of a microfluidic device with two layers, one for bacterial culture and the other to allow chemical communication, separated by a polycarbonate permeable membrane. The upper floor consisted of three chambers with three independent bacterial cultures, Av (Azotobacter vinelandii in blue), Bl (Bacillus licheniformis in red) and Pc (Paenibacillus curdlanolyticus in green), whose chemicals diffused to the main chamber of the bottom floor. Adapted from (Hyun et al., 2008), copyright (2008) National Academy of Sciences, U. S. A. All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
Instead of a water circuit, a channel network to generate an oxygen gradient can be bonded to a layer for bacterial culture (Fig. 5B). Adler and colleagues (2012) used this approximation to examine E. coli under different aerobic and microaerobic environments, although they discovered no response from the bacterium.
Furthermore, this configuration allows the study of intercellular communication. Jung Kim et al. designed a device in which the upper floor consisted of three independent chambers and the bottom floor consisted of a large camera confined by PDMS walls. A permeable membrane that permitted the chemical diffusion of molecules separated the two layers (Fig. 5C). Different bacteria were seeded on the three chambers of the upper floor, and because of the permeable membrane, chemical communication existed between them. Significant differences were observable when bacterial colonies were interrelated or isolated. For example, Azotobacter vinelandii, Bacillus licheniformis and Paenibacillus curdlanolyticus colonies were unstable, and their population size decreased or was maintained at the initial levels over time when grown individually, but when they were connected, these colonies were stable with increasing population sizes. This fact suggested the existence of syntrophic interactions between these bacterial communities (Hyun et al., 2008).
Porous microfluidic devices
The versatility of microfluidics designs permits the use of non‐flat surfaces, increasing the scope of studies and allowing the possibility to recreate more realistic environments. For example, porous devices are characterized by a surface full of tiny holes on which bacteria adapt to grow or migrate. These designs are typically utilized to evaluate chemo‐ and rheotaxis.
Regarding chemotactic responses, E. coli was cultured with an α‐methylaspartate gradient, showing higher attraction in porous devices than in plain surfaces. This fact suggested that the flow in porous media increased transverse migration for chemotactic bacteria (Long and Ford, 2009). Listeria monocytogenes was also observed in porous devices under acetate gradients (Fig. 6A). Listeria’s flagella showed changes at acetate concentrations of 10–100 mM, modifying their motility and causing the bacterium to spin (Wright et al., 2014).
Fig. 6.
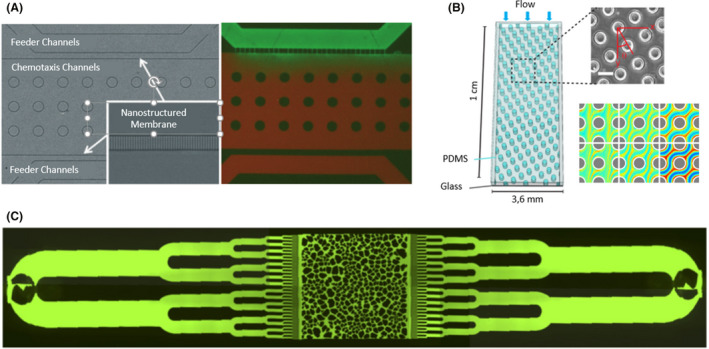
Microfluidic devices with porous surfaces. A. A porous chemotaxis channel printed 800 nm in diameter and connected with two feeder channels by a nanostructured membrane. To demonstrate that a chemical gradient could be generated within the chemotaxis channel, the fluorescent dyes fluorescein (green) and Texas Red (red) were used to create a colour gradient in the device. Adapted from (Wright et al., 2014). B. A microfluidic device that consisted of a square camera with circular pillars 65 µm in diameter. Blue arrows indicate the direction of bacterial fluid flow, and coloured lines indicate the trajectories of the swimming bacteria. Adapted from (Dehkharghani et al., 2019). C. A microfluidic device with multiple channels that ended on a central camera with a heterogeneous distribution of pores to mimic realistic surroundings, such as soil. Taken from (Aufrecht et al., 2019). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
If the hole distribution is homogenous (same diameter and same separation distance), the microfluidic device simulates ideal flows in porous surfaces (Fig. 6B). Dehkharghani and colleagues (2019) investigated the dispersion dynamics of B. subtilis, noting that hydrodynamic gradients hindered transverse bacterial dispersion, promoting dispersion in the same plane and direction as the current.
However, realistic environments are not homogenous, and pore sizes and distances vary. A heterogeneous design recreated soil surroundings, in which the trend of E. coli growing faster when close to channels with higher flow was observed (Durham et al., 2012). This approach could also imitate the infiltration of rainwater into soil (Fig. 6C). Aufrecht et al. worked with a wild‐type Pantoea sp. isolated from the rhizosphere and its extracellular polymeric substance (EPS) knockout mutant. No differences were observable between the two strains regarding the growth area. Both tended to choose a preferential route until the accumulated bacteria blocked this route, so the fluid flow redirected to other routes, supplying nutrients to slow‐growing bacteria. The only difference noticed between the wild‐type and EPS mutant was that the mutant bacteria associated in long chains in the presence of a flow stimulus, whereas, they remained independent in static zones (Aufrecht et al., 2019).
Topographic microfluidic devices
Again applying the design flexibility offered by microfluidics, topographic devices are characterized as showing an irregular design. This could be due to the non‐linear configuration of their channels or the printing of a great variety of patterns on their surfaces. For the first case, we found two different studies in which a single‐channel device was developed with streamer shapes (Fig. 7A). Marcos et al. used this design to examine rheotaxis on Leptospira biflexa, a bacterium from the order Spirochaetales characterized by a spiral shape similar to a helix. Parabolic flow on the non‐motile bacterium made it drift perpendicularly to the transverse plane, lining up the helices with the current lines. The alignment direction depended on the chirality of the helices and the velocity of the flow (Marcos et al., 2009). Rusconi et al. also worked with this configuration but evaluated P. aeruginosa biofilm formation. Bacterial aggregation tended to be the more linear possibility, occupying the centre of the channels and touching only the inner corners, without distinguishing between round or sharp corners (Rusconi et al., 2010).
Fig. 7.
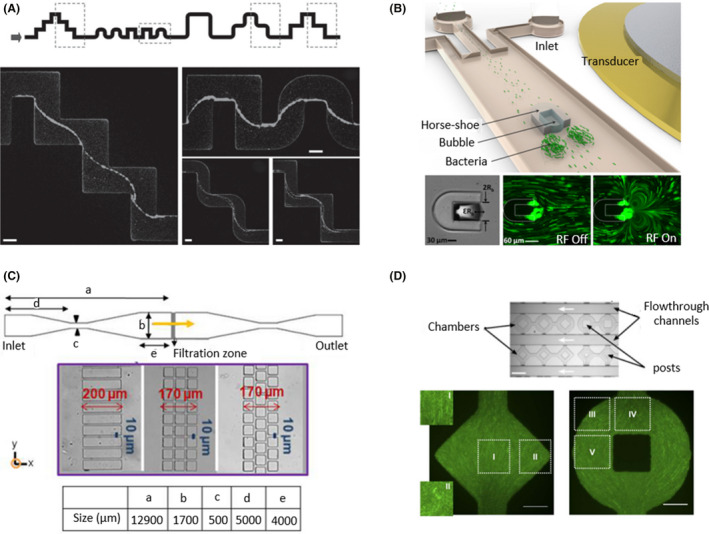
Topographic microfluidic devices with irregular shapes.
A. A single‐channel microfluidic device with a streamer shape to examine the formation of biofilms in contact with sharp and rounded corners. Images were taken in the middle horizontal plane after 12 h of a constant flow of 0.75 µm/min (from left to right). Scale bars correspond to 100 µm. Taken from Rusconi et al. (2010). B. A horseshoe shape was printed on the middle of the channel to create a vertical flow in which bacteria were trapped, developing biofilms. The trapped bubble oscillated with an amplitude of ɛRb and a diameter of 2Rb. The vortices were generated by the application of a 1.9 µm radio frequency (RF) signal. Green microbeads were used to track trajectories when the transducer was off and of (RF Off/RF On). Adapted from (Yazdi and Ardekani, 2012). C. A linear device with a filtration zone in the centre of the channel with different geometries and distributions of rectangular and square posts. Bacteria were forced to pass through the filtration zone in the direction of the yellow arrow as a recreation of filtration processes. Adapted from (Marty et al., 2014). D. A microfluidic device with geometrical chambers that may include central posts was designed to determine bacterial organization depending on chamber shape. The flow ran through the flowthrough channels in the direction of the white arrows. Bacteria were oriented 90° regions I, II, IV and V and 45° in region III with respect to the x‐axis. Scale bars correspond to 50 µm. Adapted from (Cho et al., 2007). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
However, Ishikawa et al. designed a streamer‐shaped device with different heights. From a linear three‐channel device, they modified the height of the central channel, switching two different channels successively. This led to a change in the speed of the flow, affecting the drift movement of E. coli from one lateral channel to the other (Ishikawa et al., 2014). Another way that was found to vary the conditions of fluid flow was to print a horseshoe shape in the centre of the channel and trap an air bubble within it (Fig. 7B). By means of acoustic flow of the bubble, a vortical flow was generated, capturing bacteria that formed biofilms after a few minutes (Yazdi and Ardekani, 2012). Zhang et al. also caught bacteria to examine E. coli biofilm formation. In this case, they added small holes where the bacteria attached and formed biofilms depending on the shear stress applied. Under static conditions or low flow, E. coli grew in a planktonic way, whereas under high flow, they grew in communities, forming biofilms (Zhang et al., 2019).
These devices have also been used to observe how bacteria avoid obstacles to move forward. Yazi et al. printed different flat and curved patterns on the surface of a device. They observed that against curved obstacles, Magnetospirillum magneticum maintained its axial direction, whereas when it faced flat obstacles, it switched its direction to backwards until it overcame the obstacle. As M. magneticum is a magnetotactic bacterium, researchers evaluated the effects of magnetic fields on the redirection of its migration. Using a microdevice with hexagonal posts, M. magneticum showed random migration under resting conditions, but when a magnetic field was applied, the bacteria realigned parallel to the field after interacting with the posts (Rismani Yazdi et al., 2018a). Following this approach, Marty et al. incorporated rectangular and square posts with different distributions to simulate infiltration processes (Fig. 7C). Dead bacteria or inert particles tended to be stuck in the bottleneck area at the top of the posts without going through them. In comparison with the rectangular straight channel distribution, the square connected design presented dead zones with a low flow rate, enhancing the accumulation of bacteria. In addition, the nonaligned square configuration showed tortuous flow areas, in which the flow direction changed, stimulating the formation of colonies (Marty et al., 2014).
The development of non‐linear‐shaped microfluidic devices could be used to control bacterial shape. Regarding single‐cell studies, Takeuchi and colleagues (2005) demonstrated that E. coli can grow after adapting itself to the channel shape, such as circle, sinusoidal or zig‐zag, and maintain its shape after being released from the channel. Focusing on colonies, Cho et al. observed that under high densities, E. coli colonies reorganized to propel nutrient transport, enhancing the growth population. Their devices consisted of geometrical chambers that may incorporate a central post (Fig. 7D). The E. coli distribution was dependent on the chamber shape (Cho et al., 2007). Hansen et al. designed rounded chambers with different diameters, and discovered that P. aeruginosa showed better growth in small wells (5–20 µm diameter) than in larger wells (100 µm diameter and beyond). In contrast, E. coli formed colonies in wells with a diameter greater than 80 µm. In addition, intercommunity relations were studied with E. coli and showed competitive interactions in 40 µm diameter wells but not in smaller pores (Hansen et al., 2016).
Finally, an irregular surface can be an additional material incorporated into the chamber of a microfluidic device, such as a titanium disk, previously treated with an abrasive diamond to generate a uniform pattern where bacteria could attach. Titanium was selected due to its use in medical oral implants. Streptococcus gordonii, Streptococcus oralis, Streptococcus salivarius, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were grown on titanium disks until the formed biofilms, and their morphology were analysed. S. gordonii and S salivarius grew in homogeneous patterns, S. oralis demonstrated tower‐like structures, P. gingivalis formed macrocolonies, and A. actinomycetemcomitans showed an open and loose structure (Rath et al., 2017).
Droplet microfluidic devices
Droplet‐based microfluidics are characterized for their isolation and confinement of a single bacteria or small populations into individual droplets. These devices include an immiscible two‐phase system: an organic liquid, usually oil, that fills the channel and an aqueous liquid that breaks intro droplets when it is introduced into the system (Kaminski et al., 2016). Droplets can be generated by different mechanisms, such as T‐junctions, which consist of two inlets that mix the organic and aqueous liquids on the joint (Bai et al., 2016), or flow‐focusing geometries that lie in adjustable geometric patterns that allow controlled flow conditions (Lashkaripour et al., 2019). Poisson statistics govern the ratio of organism encapsulation, determining the probability of isolating one or more cells per drop as a function of the starting concentration and occupied fraction of the droplets (Collins et al., 2015).
Droplet‐based microfluidics is the most recent and innovative approach among microfluidic devices and shows significant differences in comparison with the previously described designs. On the one hand, droplet‐based methodology is still in an optimization phase, with several studies determining the optimal parameters for growing bacteria. On the other hand, although the possibility of generating gradients inside the drop has been demonstrated (Chong et al., 2016), no studies have focused on tactic behaviours. Instead, the predominant assays are oriented to grow tests and biofilm formation. Finally, droplet‐based devices introduce the opportunity for the application of some biomolecular techniques.
Regarding optimization studies, static and dynamic cultures have been tested by circulating a constant flow of perfluorinated oil through the main chamber. Oxygen transference was determined by oxygen‐sensitive near‐infrared luminescence (NIR) measurements, showing a greater rate in dynamic cultures. In addition, the biomass of four bacteria was monitored in both cultures. B. subtilis and Pseudomonas fluorescens, both obligate aerobes, only grew on dynamic cultures, whereas E. coli, a facultative anaerobe, and Streptomyces aureofaciens, an aerobe, also grew more in dynamic cultures (Mahler et al., 2015). Continuing the optimization process, Pratt et al. examined the stability and size of drops over time by designing a microarray full of parallel channels with connected wells where droplets are stored and then soaking the devices in water‐saturated oil. This created conditions in which P. aeruginosa could grow for 21.5 h within the drop (Pratt et al., 2019). The E. coli standard growth curve in the flask was reproduced using droplet‐based microfluidics by trapping drops in a serpentine channel previously sorted by drop size. On both sides of the channel, an electrode was located that simulated an oscillatory effect, supplying a dynamic environment (Ho et al., 2020). Isolation of individual organisms in drops allows the promotion of slow‐growing bacteria. By separating these bacteria from fast‐growing bacteria that deplete resources, they are prevented from being displaced. Watterson et al. isolated bacteria from faecal microbiota transplants, and by optical density, they selected those that showed slower growth. Genome identification showed the presence of bacteria previously uncultured by traditional methods (Watterson et al., 2020).
As previously mentioned, droplet‐based microfluidics studies have not focused on the tactic responses of bacteria. However, Dong et al. designed a combined device that consisted of two parts. The first part was similar to a three‐channel device where an aspartate chemical gradient was generated to attract and sort fluorescent E. coli. Second, a droplet generator encapsulating E. coli by the T‐junction technique and droplets were analysed as a function of the presence or absence of fluorescence (Fig. 8A). They determined that the cheZ gene is essential for the chemotactic ability of E. coli (Dong et al., 2016).
Fig. 8.

Droplet‐based microfluidic devices. A. A microfluidic device divided into two parts. Part I (blue) consisted of three inlets that created an aspartate chemical gradient, allowing the sorting of E. coli depending on their chemotactic response. The bacteria that were attracted by aspartate passed through a channel to part II (pink), where droplets were generated by mixing the fluorinated oil FC‐40 with medium and bacteria. The droplets were imaged, analysed and cultured. Adapted from (Dong et al., 2016). B. Single bacteria were encapsulated in droplets containing antibiotics and a fluorescent viability indicator. Subsequently, there was an in‐line incubation that permitted bacterial replication, followed by fluorescence detection in which droplets were either empty (blue circles) or included bacteria (pink circles). Adapted from (Kaushik et al., 2017). C. A design starting with a droplet generation system by a T‐junction followed by a serpentine with hydrophilic patterns of different sizes where bacteria attached. Biofilm formation was based on two phases. First, there was a seeding process in which P. aeruginosa PAO1 was encapsulated and sessile droplets adhered to hydrophilic patterns. The second phase consisted of generating droplets of media to allow bacterial growth until biofilms developed. Adapted from (Jin et al., 2018). All figure panels were reproduced/adapted with permission from the corresponding publisher and/or journal. Credits for these figures are provided in the References section of the manuscript.
Toxicology and antibiotic resistance have been tested in these devices, analysing the growth rates inside drops. Streptomyces acidiscabies and Psychrobacillus psychrodurans, bacteria that are resistant to heavy metal ions, were exposed to Cu2+, and their dissolved oxygen consumption and cellular density were monitored. Although oxygen consumption decreased in the presence of 0.5 mM Cu2+, it was not completely inhibited, confirming that these bacteria were resistant to metallic ions (Cao et al., 2015). Kaushik et al. designed a device to determine antibiotic effects on bacteria. It consisted of two inlets to generate drops with a single bacterium, followed by a serpentine where bacteria reproduced a couple of times and a camera for fluorescence detection at the end (Fig. 8B). Resazurin was used as a growth reporter because it is reduced by the intracellular electron receptors NADH and FADH and converted into fluorescent resorufin. Through fluorescence analysis, the droplets were characterized as positive or negative depending on the presence or absence of bacteria respectively. E. coli was tested with 4 µg ml−1 gentamycin, and the positive droplet ratio significantly decreased (Kaushik et al., 2017). Bacteria can also be used as reporters to detect antimicrobial compounds secreted by other organisms. Environmental microbes were isolated, and fluorescent E. coli and B. subtilis, gram‐positive and gram‐negative bacteria, respectively, were introduced into the droplets. As a function of fluorescence intensity, it was possible to determine the presence of antimicrobial molecules. Afterwards, the organisms are sequenced and identified (Mahler et al., 2019). Unlike traditional methods that require several experimental steps, these platforms integrate droplet formation, bacterial incubation and growth detection in an uninterrupted system.
Biofilm formation has been studied inside droplets using B. subtilis as a model bacterium. It was described that these bacteria first swam individually, started to aggregate in 12 h around the drop edges, and finally sporulated in 48 or 72 h (Chang et al., 2015). Bacteria can also be attached to hydrophilic patterns to grow biofilms adherent to surfaces. P. aeruginosa droplets were generated and passed through a channel where they became affixed to circle patterns. Subsequently, a media flow was introduced to allow bacterial growth until the biofilms developed (Fig. 8C). In comparison with the microfluidic devices previously described, this approach eliminated corner effects and avoided channel plugs (Jin et al., 2018). Biofilms have also been formed around oil droplets to study the interactions between bacteria and oil molecules that usually appear in flow environments. Alcanivorax aggregated in a wrinkly formation surrounding the drop, Marinobacter biofilms were oriented ±45° from the leading stagnation point with 50 µm thickness, and Pseudomonas formed a tail that dispersed in 14 h (White et al., 2019).
Biomolecular techniques have been applied within this system, mainly in order to detect the presence of bacteria in samples. For example, using fluorescent antibodies against Salmonella enterica serovar typhimurium in freshcut produce water allowed the detection of contamination and avoided foodborne contamination (Harmon et al., 2020). Fluorescence resonance energy transfer (FRET) based on RNA has also been used to analyse samples. When a droplet contained bacteria‐secreted RNases, they cleaved RNA and generated fluorescence (Ota et al., 2019). These devices also allowed sequencing of the complete genome of isolated bacteria and were able to identify soil bacteria uncultured by traditional methods (Hosokawa et al., 2017).
Advantages and disadvantages of microfluidics compared to traditional methods
Microfluidics is a versatile technique that is gaining momentum in the field of microbiological research. The reproducibility of results previously obtained by traditional methods provides confidence and robustness to this tool. A clear example of this fact is the migration assays in which the chemotactic response of E. coli to different compounds is studied. Traditional methods are based on inoculating bacteria onto agar plates to analyse the kinetics of colony formation over time. The chemoattractant may be contained in the agar plate itself or may have been used to pre‐treat the bacterial sample following the method proposed by Adler (1973). This method consists of introducing a capillary with chemoattractant into a bacterial solution that is subsequently deposited on an agar plate, and after its incubation, the number of colonies is quantified. By means of this methodology, Mesibov and Adler (1972) demonstrated that E. coli was attracted to several amino acids, including l‐aspartate, showing a peak at a concentration of 10 mM. Wolfe and Berg used the strategy of incorporating l‐aspartate into agar plates and determined that E. coli shows maximum attraction at 10 µM (Wolfe and Berg, 1989). On the other hand, through the individual monitoring of bacteria grown in microcapillaries, both Roggo's and Cheng's teams corroborated the attraction of E. coli to l‐aspartate at such concentrations, also highlighting migratory responses at intermediate concentrations such as 0.1 and 1 mM (Cheng et al., 2007; Roggo et al., 2018). Thus, the similarity and reproducibility of the results obtained by both methods make microfluidics a reliable tool.
However, although both methods are capable of determining optimal concentrations of chemoattractant, agar methods have limitations, such as the impossibility of calculating migration rates. This calculation is unfeasible since individual cells cannot be followed; instead, colony growth is evaluated. Partridge et al. decided to transfer colonies on agar plates to liquid cultures. They inoculated part of the cultures in small crystal chambers in order to monitor the bacteria individually, determining a migration speed of approximately 20–25 µm s−1 (Partridge et al., 2019). Nevertheless, this approach is incompatible with the generation of chemical gradients. Therefore, it was necessary to rely on microfluidic techniques, such as a single‐channel device designed by Ahmed and Stocker, with which they created gradients of α‐methylaspartate in a range of concentrations between 0.1 and 1 mM and obtained migration speeds between 0.6 and 13.8 µm s−1 (Ahmed and Stocker, 2008).
While traditional methods are limited to working on flat 2D surfaces, such as culture flasks, Petri dishes or well plates, microfluidics offers a new variety of materials with promising properties, among which PDMS stands out. Due to its biocompatibility and gas permeability, it is ideal for cellular and microbiological cultures, but in addition, its optical transparency allows visualization and analysis by microscopy. However, its major advantage is the possibility of combining it with other materials, resulting in great versatility in device design. Thus, microfluidics offers the option of working in heterogeneous or even three‐dimensional conditions, on which different stimuli, such as fluid flow or tactic gradients, can be applied. This advantage in culture conditions has been exposed by comparing two strategies to grow bacteria. On the one hand, following a traditional method, Bible and colleagues (2016) isolated Pantoea sp. from the rhizosphere of Populus deltoides and grew it on an agar plate. On the other hand, Aufrecht et al., after isolating Pantoea sp., cultured it on a surface with heterogeneous pores that simulate a more realistic soil distribution (Aufrecht et al., 2019). In addition, Aufretch applied a flow stream over the system to recreate rainwater filtration. While Pantoea sp. grew homogeneously on the agar plate and formed colonies (Bible et al., 2016), in the microfluidic device, it tended to grow by selecting preferential routes depending on the availability of nutrients provided by the stream flows (Aufrecht et al., 2019). Altogether, it is tempting to conclude that microfluidics offers the possibility of replicating more realistic environments than those offered by traditional methods.
The difference in volume and bacterial numbers used in microfluidics as opposed to conventional methods is also an aspect to consider. Microfluidics uses volumes in the microliter range, while traditional methods usually use volumes in the millilitre range. This small size turns microfluidic devices into portable tools and offers a number of advantages, such as economic savings in reagents, materials and space or a more accurate control of the biological and physical conditions of the study. From a biological perspective, macroscopic cultures contain a high number of bacteria, in the thousands or millions, giving rise to inevitable heterogeneity in the study population. In microfluidic assays, the number of bacteria is reduced to hundreds or even individual bacteria, achieving results that are more precise on the single‐cell scale. In the physical environment, specific conditions can be achieved, making the growth of several bacteria possible, including oligotrophic bacteria, which are still a challenge for traditional two‐dimensional cultures (Overmann et al., 2017). In addition, microfluidics allows almost instantaneous changes in environmental conditions within the device, allowing the study of bacterial adaptation. Lambert and Kussel designed a microfluidic device composed of a central channel and lateral culture chambers of 25 µm3. By adding a flow current through the main channel, they were able to generate transitions in the media conditions in less than 250 milliseconds. As a result, they studied the adaptive response of E. coli to fluctuating changes in glucose‐ or lactose‐enriched broth media on a time scale of 1 to 10 generations (Lambert and Kussel, 2014). On the other hand, Phillips et al. studied the same adaptive response in 1 ml of E. coli cultures, making daily inoculations to perform the media changes. They conducted these experiments over approximately 3000 generations (Phillips et al., 2016). In this way, the greater control over the studied condition results is reflected in the reduced duration of the experiments.
Experimental times can also be shortened by integrating several experimental processes into a single platform, thus also reducing the number of manipulations by researchers and therefore the risk of contamination and technical variability (Halldorsson et al., 2015). A clear example of the power of integration from microfluidics is droplet‐based devices. This can be demonstrated by comparing the approach of these devices to traditional methods in the study of antimicrobial susceptibility. In 1966, the antibiotic susceptibility test was established by means of the standardized disk diffusion method (Bauer et al., 1966), colloquially named the antibiogram, which is still used today (Kibret and Abera, 2011). This method consists of inoculating a bacterial culture onto an agar plate and placing it on a paper disk containing antibiotics that radially diffuse in the agar. After an incubation period, in the case where the bacteria are susceptible to the antibiotic, a growth inhibition zone appears whose diameter correlates with the antimicrobial power of the antibiotic tested. Kaushik et al. proposed a droplet‐based microfluidic device in which they integrated the incubation of the bacteria in contact with the antibiotic in microdrops with the detection and analysis of its susceptibility (Fig. 8B) through the fluorescence produced by resazurin when reduced by the bacterial metabolic activity (Kaushik et al., 2017). While the standardized method requires several manipulations, including inoculation, deposition of the antibiotic disk or measurement of the diameter of the inhibition zone, the microfluidic device integrates all steps in a continuous flow system where the only manipulation is the initial load of the components to be studied. In addition, the antibiogram requires at least 24 h of incubation, while microfluidic devices allow testing of antimicrobial susceptibility after 1 h. Undoubtedly, this time‐saving and minimal technical manipulation are valuable considerations for the use of microfluidic devices in a clinical context, where proper treatment of infected patients requires reliable and instant results.
Nevertheless, microfluidics still has a long way to go to overcome several of the challenges it presents. For example, despite the numerous advantages described above for PDMS, we also have also found some drawbacks. Its major limitation in the cellular field is its capacity to absorb small hydrophobic molecules or biomolecules, such as proteins, interfering with the results of the assays. This demonstration was performed by van Meer et al., comparing the absorption of four cardiac drugs when incubated in standard tissue culture grade polystyrene (TCPS) 96‐well and PDMS wells. While the adsorption in the TCPS wells was negligible, PDMS adsorbed between 20% and 80% of the compounds within 3 h (van Meer et al., 2017). PDMS is a porous material that not only absorbs molecules but also allows the passage of organic solvents that can modify the dimensions of the channels. Dangla and colleagues (2010) observed that when filling 50 µm‐high channels with the solvent hexadecane, the channels were deformed by sinking the ceiling parabolically by approximately 7 µm. As a result, protocols are being developed that modify the surface of PDMS to avoid these phenomena (Shin et al., 2018; Gökaltun et al., 2019; You et al., 2020).
Due to its recent appearance, the absence of standardized protocols in microfluidics‐based research is evident. Bacteria are mainly visualized using microscopy techniques, which require reporter‐labelled bacteria and/or specialized microscopy facilities (Subramanian et al., 2020). This restricts microfluidics assays to visual analysis and model microorganisms, which frequently do not reflect the virulent traits of pathogenic species. The assays carried out are mostly focused on the study of migration trajectories and growth rates, and it is clear that there is a lack of molecular characterization. The development of devices based on droplets has led to a great advance in this field, giving the possibility of studying bacteria at a single‐cell level. In addition, some protocols for the extraction of nucleic acids and proteins from samples confined within microfluidic devices have been published (Schilling et al., 2002; Oblath et al., 2013; Julich et al., 2016; Hosokawa et al., 2017). Nevertheless, microfluidics is far from assessing molecular changes at the intracellular level, and new techniques have to be improved to allow genomic, transcriptomic, proteomic and metabolomic research at the same level as established methods. Once these limitations are overcome, microfluidics might become an indispensable technique for research and commercial applications.
Future directions
Most microfluidic studies involving bacteria have been performed using environmental or non‐pathogenic species. This fact shows a knowledge gap in the biomedical field regarding the interaction between host cells and bacteria, both symbiotic and pathogenic. A key limitation to study pathogenic species by microfluidic devices is the requirement for specialized time‐lapse microscopy facilities within biosafety laboratories. Although this limitation could be partially overcome by using non‐pathogenic but phylogenetically related microorganisms, the application of microfluidics for antimicrobial testing or immunological research still requires the presence of pathogenic counterparts.
Some researchers have developed more complex microfluidic devices, integrating several tissues to mimic organs, which is known as organ‐on‐a‐chip. Some of the organs that have been simulated thanks to microfluidics are the gut (Kim and Ingber, 2013), lung (Huh, 2015), liver (Ho et al., 2006), endothelial vessels (Polacheck et al., 2019) and brain (Bang et al., 2019). The application of bacteria in these systems would not only result in a more complete and realistic approach to recreate complex bacterial interactions with some human organs, such as the intestinal microbiota, but would also allow for a more detailed study of the host‐bacterial interplay in vitro. A recent example of the potential of this combination is the work of Thacker et al., which includes Mycobacterium tuberculosis within a lung‐on‐a‐chip. This device consisted of two monolayers of endothelial and epithelial cells adhered to a porous membrane, recreating the alveolar surface, with a flow of macrophages and M. tuberculosis to simulate part of the immune system. They discovered the importance of the surfactant on M. tuberculosis infections, since its deficiency led to uncontrolled bacterial growth and its presence is involved in attenuating bacterial virulence (Thacker et al., 2020). This later work involving a deadly pathogen and a lung‐on‐a‐chip device opens attractive perspectives for microfluidics in biomedical applications.
Finally, some molecular biology applications can be adapted to a microfluidic platform allowing high‐throughput studies. Specifically, a microfluidic device designed by Mathai et al. allows performing multiple quantitative PCR in a single run. This system was successfully applied to decipher the microbial content in complex samples, and constitutes an interesting adaptation of laboratory‐level techniques to a microfluidic scale (Mathai et al., 2019).
Conclusions
Throughout this review, we have analysed the potential of microfluidics in the microbiological field. Its great versatility in terms of design allows the recreation of 3D microenvironments, which otherwise allows a more realistic study of certain bacterial phenotypes than traditional methods. As a result, microfluidic devices can reproduce different scenarios, including physical, chemical or biological variations that exist in natural environments. For example, the simplest devices consisting of linear channels have been used to track bacterial migration in response to tactic stimuli, analyse bacterial sensitivity to drugs or study biofilm formation. More complex chips include mixing channels or those with more than one floor that allow greater control of conditions within the channels and/or the co‐culture of various species of bacteria. In addition, the surfaces of these devices can be modified to fabricate non‐flat channels, resulting in porous devices or topographic devices that recreate natural environments, such as soil, or obstacles with specific shapes. Finally, droplet‐based microfluidic devices isolate bacteria on microdroplets, allowing single‐cell studies mainly focused on testing drug resistance and biofilm formation. Together, microfluidics offers versatile approaches to study multiple microbiological scenarios.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by the Spanish Ministry of Education, Culture and Sport (FPU grant: FPU16/04398) to S. P.‐R.; the Spanish Ministry of Science, Innovation and Universities (RTI2018‐094494‐B‐C21) to J. M. G. A. and the Spanish Ministry of Science and Innovation (PID2019‐104690RB‐I00) to J. G.‐A.
Microb Biotechnol (2022) 15(2), 395–414
Funding information
This work was supported by the Spanish Ministry of Education, Culture and Sport (FPU grant: FPU16/04398) to S. P.‐R.; the Spanish Ministry of Science, Innovation and Universities (RTI2018‐094494‐B‐C21) to J. M. G. A. and the Spanish Ministry of Science and Innovation (PID2019‐104690RB‐I00) to J. G.‐A.
References
- Adler, J. (1966) Chemotaxis in bacteria. Science 153: 708–716. [DOI] [PubMed] [Google Scholar]
- Adler, J. (1973) A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol 74: 77–91. [DOI] [PubMed] [Google Scholar]
- Adler, M. , Erickstad, M. , Gutierrez, E. , and Groisman, A. (2012) Studies of bacterial aerotaxis in a microfluidic device. Lab Chip 12: 4835–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, T. , and Stocker, R. (2008) Experimental verification of the behavioral foundation of bacterial transport parameters using microfluidics. Biophys J 95: 4481–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge, B.B. , Fernandez‐Suarez, M. , Heller, D. , Ambravaneswaran, V. , Irimia, D. , Toner, M. , and Fortune, S.M. (2012) Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufrecht, J.A. , Fowlkes, J.D. , Bible, A.N. , Morrell‐Falvey, J. , Doktycz, M.J. , and Retterer, S.T. (2019) Pore‐scale hydrodynamics influence the spatial evolution of bacterial biofilms in a microfluidic porous network. PLoS One 14: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer, R. , Rokem, J.S. , Ilangumaran, G. , Lamont, J. , Praslickova, D. , Ricci, E. , et al. (2018) Plant growth‐promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 871: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, L. , Fu, Y. , Zhao, S. , and Cheng, Y. (2016) Droplet formation in a microfluidic T‐junction involving highly viscous fluid systems. Chem Eng Sci 145: 141–148. [Google Scholar]
- Balaban, N.Q. , Merrin, J. , Chait, R. , Kowalik, L. , and Leibler, S. (2004) Bacterial persistence as a phenotypic switch. Science 305: 1622–1625. [DOI] [PubMed] [Google Scholar]
- Balouiri, M. , Sadiki, M. , and Ibnsouda, S.K. (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang, S. , Jeong, S. , Choi, N. , and Kim, H.N. (2019) Brain‐on‐a‐chip: a history of development and future perspective. Biomicrofluidics 13: 51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, A.W. , Kirby, W.M.M. , Sherris, J.C. , and Turck, M. (1966) Antibiotic susceptibility testing byt a standardized single disk method. Am J Clin Pathol 45: 493–496. [PubMed] [Google Scholar]
- Bible, A.N. , Fletcher, S.J. , Pelletier, D.A. , Schadt, C.W. , Jawdy, S.S. , Weston, D.J. , et al. (2016) A carotenoid‐deficient mutant in Pantoea sp. YR343, a bacteria isolated from the Rhizosphere of Populus deltoides, is defective in root colonization. Front Microbiol 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor, A. , Bounedjoum, N. , Vincze, G.E. , Erdeiné Kis, Á. , Laczi, K. , Bende, G. , et al. (2020) Challenges of unculturable bacteria: environmental perspectives. Rev Environ Sci Biotechnol 19: 1–22. [Google Scholar]
- Cao, J. , Nagl, S. , Kothe, E. , and Köhler, J.M. (2015) Oxygen sensor nanoparticles for monitoring bacterial growth and characterization of dose‐response functions in microfluidic screenings. Microchim Acta 182: 385–394. [Google Scholar]
- Caspi, Y. (2014) Deformation of filamentous Escherichia coli cells in a microfluidic device: a new technique to study cell mechanics. PLoS One 9: e83775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.B. , Wilking, J.N. , Kim, S.H. , Shum, H.C. , and Weitz, D.A. (2015) Monodisperse emulsion drop microenvironments for bacterial biofilm growth. Small 11: 3954–3961. [DOI] [PubMed] [Google Scholar]
- Chen, H. , and De La Fuente, L. (2020) Calcium transcriptionally regulates movement, recombination and other functions of Xylella fastidiosa under constant flow inside microfluidic chambers. Microb Biotechnol 13: 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S.Y. , Heilman, S. , Wasserman, M. , Archer, S. , Shuler, M.L. , and Wu, M. (2007) A hydrogel‐based microfluidic device for the studies of directed cell migration. Lab Chip 7: 763–769. [DOI] [PubMed] [Google Scholar]
- Cho, H.J. , Jönsson, H. , Campbell, K. , Melke, P. , Williams, J.W. , Jedynak, B. , et al. (2007) Self‐organization in high‐density bacterial colonies: efficient crowd control. PLoS Biol 5: 2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, Z.Z. , Tan, S.H. , Gañán‐Calvo, A.M. , Tor, S.B. , Loh, N.H. , and Nguyen, N.T. (2016) Active droplet generation in microfluidics. Lab Chip 16: 35–58. [DOI] [PubMed] [Google Scholar]
- Collins, D.J. , Neild, A. , deMello, A. , Liu, A.Q. , and Ai, Y. (2015) The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation. Lab Chip 15: 3439–3459. [DOI] [PubMed] [Google Scholar]
- Dangla, R. , Gallaire, F. , and Baroud, C.N. (2010) Microchannel deformations due to solvent‐induced PDMS swelling. Lab Chip 10: 2972–2978. [DOI] [PubMed] [Google Scholar]
- Debré, P. , and Le Gall, J.‐Y. (2014) Le microbiote intestinal. Bull Acad Natl Med 198: 1667–1684. [PubMed] [Google Scholar]
- Dehkharghani, A. , Waisbord, N. , Dunkel, J. , and Guasto, J.S. (2019) Bacterial scattering in microfluidic crystal flows reveals giant active Taylor‐Aris dispersion. Proc Natl Acad Sci USA 166: 11119–11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir, M. , and Salman, H. (2012) Bacterial thermotaxis by speed modulation. Biophys J 103: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z.‐S. , Kong, Z.‐Y. , Zhang, B.‐C. , and Zhao, L.‐F. (2020) Insights into non‐symbiotic plant growth promotion bacteria associated with nodules of Sphaerophysa salsula growing in northwestern China. Arch Microbiol 202: 399–409. [DOI] [PubMed] [Google Scholar]
- Dong, L. , Chen, D.W. , Liu, S.J. , and Du, W. (2016) Automated chemotactic sorting and single‐cell cultivation of microbes using droplet microfluidics. Sci Rep 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham, W.M. , Tranzer, O. , Leombruni, A. , and Stocker, R. (2012) Division by fluid incision: Biofilm patch development in porous media. Phys Fluids 24: 91107. [Google Scholar]
- Englert, D.L. , Manson, M.D. , and Jayaraman, A. (2009) Flow‐based microfluidic device for quantifying bacterial chemotaxis in stable, competing gradients. Appl Environ Microbiol 75: 4557–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend, J. , and Yeo, L. (2010) Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökaltun, A. , Kang, Y.B. (Abraham), Yarmush, M.L. , Usta, O.B. , and Asatekin, A. (2019) Simple surface modification of Poly(dimethylsiloxane) via surface segregating smart polymers for biomicrofluidics. Sci Rep 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchin, S.A. , Stratford, J. , Curry, R.J. , and McFadden, J. (2012) A microfluidic system for long‐term time‐lapse microscopy studies of mycobacteria. Tuberculosis 92: 489–496. [DOI] [PubMed] [Google Scholar]
- Halldorsson, S. , Lucumi, E. , Gómez‐Sjöberg, R. , and Fleming, R.M.T. (2015) Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 63: 218–231. [DOI] [PubMed] [Google Scholar]
- Hansen, R.H. , Timm, A.C. , Timm, C.M. , Bible, A.N. , Morrell‐Falvey, J.L. , Pelletier, D.A. , et al. (2016) Stochastic assembly of bacteria in microwell arrays reveals the importance of confinement in community development. PLoS One 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, J.B. , Gray, H.K. , Young, C.C. , and Schwab, K.J. (2020) Microfluidic droplet application for bacterial surveillance in fresh‐cut produce wash waters. PLoS One 15: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques‐Normark, B. , and Normark, S. (2014) Bacterial vaccines and antibiotic resistance. Ups J Med Sci 119: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, C.T. , Lin, R.Z. , Chang, W.Y. , Chang, H.Y. , and Liu, C.H. (2006) Rapid heterogeneous liver‐cell on‐chip patterning via the enhanced field‐induced dielectrophoresis trap. Lab Chip 6: 724–734. [DOI] [PubMed] [Google Scholar]
- Ho, C.M.B. , Sun, Q. , Teo, A.J.T. , Wibowo, D. , Gao, Y. , Zhou, J. , et al. (2020) Development of a microfluidic droplet‐based microbioreactor for microbial cultivation. ACS Biomater Sci Eng 6: 3630–3637. [DOI] [PubMed] [Google Scholar]
- Hosokawa, M. , Nishikawa, Y. , Kogawa, M. , and Takeyama, H. (2017) Massively parallel whole genome amplification for single‐cell sequencing using droplet microfluidics. Sci Rep 7: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpikian, P. , and Raoult, D. (2002) Traditional and molecular techniques for the study of emerging bacterial diseases: one laboratory’s perspective. Emerg Infect Dis 8: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Fan, S. , Xing, W. , and Liu, C. (2010) Microfluidic cell culture system studies and computational fluid dynamics. Math Comput Model 52: 2036–2042. [Google Scholar]
- Huh, D. (2015) A human breathing lung‐on‐a‐chip. Ann Am Thorac Soc 12: S42–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, J.K. , Boedicker, J.Q. , Jang, W.C. , and Ismagilov, R.F. (2008) Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA 105: 18188–18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, T. , Shioiri, T. , Numayama‐Tsuruta, K. , Ueno, H. , Imaia, Y. , and Yamaguchi, T. (2014) Separation of motile bacteria using drift velocity in a microchannel. Lab Chip 14: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Ito, T. , Sekizuka, T. , Kishi, N. , Yamashita, A. , and Kuroda, M. (2019) Conventional culture methods with commercially available media unveil the presence of novel culturable bacteria. Gut Microbes 10: 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, H.H. , Jeong, S.G. , Park, A. , Jang, S.C. , Hong, S.G. , and Lee, C.S. (2014) Effect of temperature on biofilm formation by Antarctic marine bacteria in a microfluidic device. Anal Biochem 446: 90–95. [DOI] [PubMed] [Google Scholar]
- Jin, Z. , Nie, M. , Hu, R. , Zhao, T. , Xu, J. , Chen, D. , et al. (2018) Dynamic sessile‐droplet habitats for controllable cultivation of bacterial biofilm. Small 14: 1–8. [DOI] [PubMed] [Google Scholar]
- Johnson, D. (2018) Bacterial Pathogens and Their Virulence Factors. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Julich, S. , Hotzel, H. , Gärtner, C. , Trouchet, D. , El Metwaly, F. , Ahmed, M. , et al. (2016) Evaluation of a microfluidic chip system for preparation of bacterial DNA from swabs, air, and surface water samples. Biologicals 44: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski, T.S. , Scheler, O. , and Garstecki, P. (2016) Droplet microfluidics for microbiology: Techniques, applications and challenges. Lab Chip 16: 2168–2187. [DOI] [PubMed] [Google Scholar]
- Kaushik, A.M. , Hsieha, K. , Chena, L. , Shina, D.J. , Liaob, J.C. , and Wang, T.‐H. (2017) Accelerating bacterial growth detection and antimicrobial susceptibility assessment in integrated picoliter droplet platform. Biosens Bioelectron 97: 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, T. , and Koser, H. (2012) Direct upstream motility in Escherichia coli. Biophys J 102: 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibret, M. , and Abera, B. (2011) Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci 11: S40–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B.J. , Chu, I. , Jusuf, S. , Kuo, T. , TerAvest, M.A. , Angenent, L.T. , and Wu, M. (2016) Oxygen tension and riboflavin gradients cooperatively regulate the migration of Shewanella oneidensis MR‐1 revealed by a hydrogel‐based microfluidic device. Front Microbiol 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.J. , and Ingber, D.E. (2013) Gut‐on‐a‐Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol 5: 1130–1140. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Lee, S. , Kim, J.K. , Chung, H.J. , and Jeon, J.S. (2019) Microfluidic‐based observation of local bacterial density under antimicrobial concentration gradient for rapid antibiotic susceptibility testing. Biomicrofluidics 13: 14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell, T. , Lacal, J. , Muñoz‐Martínez, F. , Reyes‐Darias, J.A. , Cadirci, B.H. , García‐Fontana, C. , and Ramos, J.L. (2011) Diversity at its best: bacterial taxis. Environ Microbiol 13: 1115–1124. [DOI] [PubMed] [Google Scholar]
- Kudva, I.T. , Cornick, N.A. , Plummer, P.J. , Zhang, Q. , Nicholson, T.L. , Bannantine, J.P. , and Bellaire, B.H. (2016) Virulence Mechanisms of Bacterial Pathogens (5th edn.). Washington, DC: ASM books. [Google Scholar]
- Lam, R.H.W. , Kim, M.C. , and Thorsen, T. (2009) Culturing aerobic and anaerobic bacteria and mammalian cells with a microfluidic differential oxygenator. Anal Chem 81: 5918–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, G. , and Kussel, E. (2014) Memory and fitness optimization of bacteria under fluctuating environments. PLoS Genet 10: e1004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouche, F. , Gully, D. , Chaumeret, A. , Nouwen, N. , Verly, C. , Pierre, O. , et al. (2018) Transcriptomic dissection of Bradyrhizobium sp. strain ORS285 in symbiosis with Aeschynomene spp. inducing different bacteroid morphotypes with contrasted symbiotic efficiency. Environ Microbiol 21, 3244–3258. [DOI] [PubMed] [Google Scholar]
- Lanning, L.M. , Ford, R.M. , and Long, T. (2008) Bacterial chemotaxis transverse to axial flow in a microfluidic channel. Biotechnol Bioeng 100: 653–663. [DOI] [PubMed] [Google Scholar]
- Lashkaripour, A. , Rodriguez, C. , Ortiz, L. , and Densmore, D. (2019) Performance tuning of microfluidic flow‐focusing droplet generators. Lab Chip 19: 1041–1053. [DOI] [PubMed] [Google Scholar]
- Lebeaux, D. , Chauhan, A. , Rendueles, O. , and Beloin, C. (2013) From in vitro to in vivo models of bacterial biofilm‐related infections. Pathogens 2: 288–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Torab, P. , Mach, K.E. , Surrette, C. , England, M.R. , Craft, D.W. , et al. (2019) Adaptable microfluidic system for single‐cell pathogen classification and antimicrobial susceptibility testing. Proc Natl Acad Sci USA 116: 10270–10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, L. , Berg, H.C. , and Sourjik, V. (2004) Effect of chemoreceptor modification on assembly and activity of the receptor‐kinase complex in Escherichia coli. J Bacteriol 186: 6643–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, T. , and Ford, R.M. (2009) Enhanced transverse migration of bacteria by chemotaxis in a porous T‐sensor. Environ Sci Technol 43: 1546–1552. [DOI] [PubMed] [Google Scholar]
- Lukic, J. , Chen, V. , Strahinic, I. , Begovic, J. , Lev‐Tov, H. , Davis, S.C. , et al. (2017) Probiotics or pro‐healers the role of beneficial bacteria in tissue repair. Wound Repair Regen 25: 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler, L. , Niehs, S. , Martin, K. , Weber, T. , Scherlach, K. , Agler‐Rosenbaum, M. , et al. (2019) Highly parallelized microfluidic droplet cultivation and prioritization on antibiotic producers from complex natural microbial communities. bioRxiv. Doi: 10.1101/2019.12.18.877530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler, L. , Tovar, M. , Weber, T. , Brandes, S. , Rudolph, M.M. , Ehgartner, J. , et al. (2015) Enhanced and homogeneous oxygen availability during incubation of microfluidic droplets. RSC Adv 5: 101871–101878. [Google Scholar]
- Mao, H. , Cremer, P.S. , and Manson, M.D. (2003) A sensitive, versatile microfluidic assay for bacterial chemotaxis. Proc Natl Acad Sci USA 100: 5449–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos , Fu, H.C. , Powers, T.R. , and Stocker, R. (2009) Separation of microscale chiral objects by shear flow. Phys Rev Lett 102: 158103. [DOI] [PubMed] [Google Scholar]
- Marty, A. , Causserand, C. , Roques, C. , and Bacchin, P. (2014) Impact of tortuous flow on bacteria streamer development in microfluidic system during filtration. Biomicrofluidics 8: 14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai, P.P. , Dunn, H.M. , Venkiteshwaran, K. , Zitomer, D.H. , Maki, J.S. , Ishii, S. , and Sadowsky, M.J. (2019) A microfluidic platform for the simultaneous quantification of methanogen populations in anaerobic digestion processes. Environ Microbiol 21: 1798–1808. [DOI] [PubMed] [Google Scholar]
- Matsumoto, Y. , Sakakihara, S. , Grushnikov, A. , Kikuchi, K. , Noji, H. , Yamaguchi, A. , et al. (2016) A microfluidic channel method for rapid drug‐susceptibility testing of Pseudomonas aeruginosa . PLoS One 11: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer, B.J. , de Vries, H. , Firth, K.S.A. , van Weerd, J. , Tertoolen, L.G.J. , Karperien, H.B.J. , et al. (2017) Small molecule absorption by PDMS in the context of drug response bioassays. Biochem Biophys Res Commun 482: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menolascina, F. , Rusconi, R. , Fernandez, V.I. , Smriga, S. , Aminzare, Z. , Sontag, E.D. , and Stocker, R. (2017) Logarithmic sensing in bacillus subtilis aerotaxis. npj Syst Biol Appl 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov, R. , and Adler, J. (1972) Chemotaxis toward amino acids in Escherichia coli. J Bacteriol 112: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. , Weiss, A.A. , Heineman, W.R. , and Banerjee, R.K. (2019) Electroosmotic flow driven microfluidic device for bacteria isolation using magnetic microbeads. Sci Rep 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei, R. , Mohammadzadeh, R. , Sholeh, M. , Karampoor, S. , Abdi, M. , Dogan, E. , et al. (2020) The importance of intracellular bacterial biofilm in infectious diseases. Microb Pathog 147: 104393. [DOI] [PubMed] [Google Scholar]
- Murugesan, N. , Dhar, P. , Panda, T. , and Das, S.K. (2017) Interplay of chemical and thermal gradient on bacterial migration in a diffusive microfluidic device. Biomicrofluidics 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myklatun, A. , Cappetta, M. , Winklhofer, M. , Ntziachristos, V. , and Westmeyer, G.G. (2017) Microfluidic sorting of intrinsically magnetic cells under visual control. Sci Rep 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblath, E.A. , Henley, W.H. , Alarie, J.P. , and Ramsey, J.M. (2013) A microfluidic chip integrating DNA extraction and real‐time PCR for the detection of bacteria in saliva. Lab Chip 13: 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota, Y. , Saito, K. , Takagi, T. , Matsukura, S. , Morita, M. , Tsuneda, S. , and Noda, N. (2019) Fluorescent nucleic acid probe in droplets for bacterial sorting (FNAP‐sort) as a high‐throughput screening method for environmental bacteria with various growth rates. PLoS One 14: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmann, J. , Abt, B. , and Sikorski, J. (2017) Present and future of culturing bacteria. Annu Rev Microbiol 71: 711–730. [DOI] [PubMed] [Google Scholar]
- Partridge, J.D. , Nhu, N.T.Q. , Dufour, Y.S. , and Harshey, R.M. (2019) Escherichia coli remodels the chemotaxis pathway for swarming. MBio 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinzadeh Gashti, M. , Asselin, J. , Barbeau, J. , Boudreau, D. , and Greener, J. (2016) A microfluidic platform with pH imaging for chemical and hydrodynamic stimulation of intact oral biofilms. Lab Chip 16: 1412–1419. [DOI] [PubMed] [Google Scholar]
- Paulick, A. , Jakovljevic, V. , Zhang, S. , Erickstad, M. , Groisman, A. , Meir, Y. , et al. (2017) Mechanism of bidirectional thermotaxis in Escherichia coli . eLife 6: 26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, K.N. , Castillo, G. , Wünsche, A. , and Cooper, T.F. (2016) Adaptation of Escherichia coli to glucose promotes evolvability in lactose. Evolution 70: 465–470. [DOI] [PubMed] [Google Scholar]
- Polacheck, W.J. , Kutys, M.L. , Tefft, J.B. , and Chen, C.S. (2019) Microfabricated Blood Vessels for Modeling the Vascular Transport Barrier. Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt, S.L. , Zath, G.K. , Akiyama, T. , Williamson, K.S. , Franklin, M.J. , and Chang, C.B. (2019) DropSOAC: Stabilizing microfluidic drops for time‐lapse quantification of single‐cell bacterial physiology. Front Microbiol 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath, H. , Stumpp, N. , and Stiesch, M. (2017) Development of a flow chamber system for the reproducible in vitro analysis of biofilm formation on implant materials. PLoS One 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rismani Yazdi, S. , Nosrati, R. , Stevens, C.A. , Vogel, D. , and Escobedo, C. (2018a) Migration of magnetotactic bacteria in porous media. Biomicrofluidics 12: 11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rismani Yazdi, S. , Nosrati, R. , Stevens, C.A. , Vogel, D. , Davies, P.L. , and Escobedo, C. (2018b) Magnetotaxis enables magnetotactic bacteria to navigate in flow. Small 14: 1–10. [DOI] [PubMed] [Google Scholar]
- Roggo, C. , Picioreanu, C. , Richard, X. , Mazza, C. , van Lintel, H. , and van der Meer, J.R. (2018) Quantitative chemical biosensing by bacterial chemotaxis in microfluidic chips. Environ Microbiol 20: 241–258. [DOI] [PubMed] [Google Scholar]
- Rusconi, R. , Lecuyer, S. , Guglielmini, L. , and Stone, H.A. (2010) Laminar flow around corners triggers the formation of biofilm streamers. J R Soc Interface 7: 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann, E.K. , Fulton, A.L. , and Beebe, D.J. (2014) The present and future role of microfluidics in biomedical research. Nature 507: 181–189. [DOI] [PubMed] [Google Scholar]
- Salman, H. , Zilman, A. , Loverdo, C. , Jeffroy, M. , and Libchaber, A. (2006) Solitary modes of bacterial culture in a temperature gradient. Phys Rev Lett 97: 1–5. [DOI] [PubMed] [Google Scholar]
- Schilling, E.A. , Kamholz, A.E. , and Yager, P. (2002) Cell lysis and protein extraction in a microfluidic device with detection by a fluorogenic enzyme assay. Anal Chem 74: 1798–1804. [DOI] [PubMed] [Google Scholar]
- Segall, J.E. , Manson, M.D. , and Berg, H.C. (1982) Signal processing times in bacterial chemotaxis. Nature 296: 855–857. [DOI] [PubMed] [Google Scholar]
- Shin, S. , Kim, N. , and Hong, J.W. (2018) Comparison of surface modification techniques on polydimethylsiloxane to prevent protein adsorption. Biochip J 12: 123–127. [Google Scholar]
- Shin, Y. , Han, S. , Jeon, J.S. , Yamamoto, K. , Zervantonakis, I.K. , Sudo, R. , et al. (2012) Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc 7: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, E.L. , Bond, M.C. , Koskella, B. , Drescher, K. , Bucci, V. , and Nadella, C.D. (2020) Biofilm structure promotes coexistence of phage‐resistant and phage‐susceptible bacteria. mSystems 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, L. , Guido, I. , Breithaupt, T. , Mazza, M.G. , and Vollmer, J. (2019) Hybrid sideways/longitudinal swimming in the monoflagellate Shewanella oneidensis: from aerotactic band to biofilm. Physics (College Park Md). [DOI] [PMC free article] [PubMed]
- Subramanian, S. , Huiszoon, R.C. , Chu, S. , Bentley, W.E. , and Ghodssi, R. (2020) Microsystems for biofilm characterization and sensing – a review. Biofilm 2: 100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, S. , DiLuzio, W.R. , Weibel, D.B. , and Whitesides, G.M. (2005) Controlling the shape of filamentous cells of Escherichia Coli. Nano Lett 5: 1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker, V.V. , Dhar, N. , Sharma, K. , Barrile, R. , Karalis, K. , and McKinney, J.D. (2020) A lung‐on‐chip model of early M. tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLife 9: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velve‐Casquillas, G. , Le Berre, M. , Piel, M. , and Tran, P.T. (2010) Microfluidic tools for cell biological research. Nano Today 5: 28–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinderola, G. , Gueimonde, M. , Gomez‐Gallego, C. , Delfederico, L. , and Salminen, S. (2017) Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria. Trends Food Sci Technol 68: 83–90. [Google Scholar]
- Vives‐Peris, V. , de Ollas, C. , Gómez‐Cadenas, A. , and Pérez‐Clemente, R.M. (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39: 3–17. [DOI] [PubMed] [Google Scholar]
- Wang, P. , Robert, L. , Pelletier, J. , Dang, W.L. , Taddei, F. , Wright, A. , and Jun, S. (2010) Robust growth of Escherichia coli . Curr Biol 20: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson, W.J. , Tanyeri, M. , Watson, A.R. , Cham, C.M. , Shan, Y. , Chang, E.B. , et al. (2020) Droplet‐based high‐throughput cultivation for accurate screening of antibiotic resistant gut microbes. eLife 9: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel, D.B. , DiLuzio, W.R. , and Whitesides, G.M. (2007) Microfabrication meets microbiology. Nat Rev Microbiol 5: 209–218. [DOI] [PubMed] [Google Scholar]
- White, A.R. , Jalali, M. , and Sheng, J. (2019) A new ecology‐on‐a‐chip microfluidic platform to study interactions of microbes with a rising oil droplet. Sci Rep 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, A.J. , and Berg, H.C. (1989) Migration of bacteria in semisolid agar. Proc Natl Acad Sci USA 86: 6973–6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E. , Neethirajan, S. , Warriner, K. , Retterer, S. , and Srijanto, B. (2014) Single cell swimming dynamics of Listeria monocytogenes using a nanoporous microfluidic platform. Lab Chip 14: 938–946. [DOI] [PubMed] [Google Scholar]
- Yazdi, S. , and Ardekani, A.M. (2012) Bacterial aggregation and biofilm formation in a vortical flow. Biomicrofluidics 6: 44114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, J.B. , Lee, B. , Choi, Y. , Lee, C.S. , Peter, M. , Im, S.G. , and Lee, S.S. (2020) Nanoadhesive layer to prevent protein absorption in a poly(dimethylsiloxane) microfluidic device. Biotechniques 69: 47–52. [DOI] [PubMed] [Google Scholar]
- Zhang, X.Y. , Sun, K. , Abulimiti, A. , Xu, P.P. , and Li, Z.Y. (2019) Microfluidic system for observation of bacterial culture and effects on biofilm formation at microscale. Micromachines 10: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, J. , Carlsen, R.W. , and Sitti, M. (2015) PH‐taxis of biohybrid microsystems. Sci Rep 5: 11403. [DOI] [PMC free article] [PubMed] [Google Scholar]


