Abstract
Bacterial keratitis (BK) is the most common type of infectious keratitis. The spectrum of pathogenic bacteria and their susceptibility to antibiotics varied with the different regions. A meta-analysis was conducted to review the global culture rate, distribution, current trends, and drug susceptibility of isolates from BK over the past 20 years (2000–2020). Four databases were searched, and published date was limited between 2000 and 2020. Main key words were “bacterial keratitis”, “culture results” and “drug resistance”. Forty-two studies from twenty-one countries (35 cities) were included for meta-analysis. The overall positive culture rate was 47% (95%CI, 42–52%). Gram-positive cocci were the major type of bacteria (62%), followed by Gram-negative bacilli (30%), Gram-positive bacilli (5%), and Gram-negative cocci (5%). Staphylococcus spp. (41.4%), Pseudomonas spp. (17.0%), Streptococcus spp. (13.1%), Corynebacterium spp. (6.6%) and Moraxella spp. (4.1%) were the most common bacterial organism. The antibiotic resistance pattern analysis revealed that most Gram-positive cocci were susceptive to aminoglycoside (86%), followed by fluoroquinolone (81%) and cephalosporin (79%). Gram-negative bacilli were most sensitive to cephalosporin (96%) and fluoroquinolones (96%), followed by aminoglycoside (92%). In Gram-positive cocci, the susceptibility trends of fluoroquinolones were decreasing since 2010. Clinics should pay attention to the changing trends of pathogen distribution and their drug resistance pattern and should diagnose and choose sensitive antibiotics based on local data.
Keywords: bacteria, keratitis, microorganisms, antibiotic, susceptibility
1. Introduction
Infectious keratitis (IK) is a potentially sight-threatening condition, which leads to at least 1.5 to 2 million new cases of unilateral blindness every year [1,2,3]. Among them, bacterial keratitis (BK) is the most common type according to the reports from multiple regions such as UK [4,5,6,7], North & South America [8,9,10,11], Middle East [12] and Australia [13,14]. Common risk factors for BK were contact lens wear, ocular trauma, ocular surface disease, and prior ocular surgery [15]. Bacterial culture via corneal scraping samples is still the gold standard for the diagnosis of BK, which permits isolation of the causal bacteria. However, not all medical institutions are able to carry out those tests due to various limitations. Therefore, it is critical for clinicians to make empirical diagnosis to know the pathogenic microorganism and antibiotics appropriate for eradicating the infection [16].
The common organisms that cause bacterial keratitis include Staphylococcus aureus, Coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, and others [17,18,19,20,21]. The bacterial spectrum and drug susceptibility for bacterial isolates from different areas or periods are widely reported [5,8,18,22,23], but the most common pathogen of BK remains debatable. Pseudomonas spp. were demonstrated to be the most common pathogen in Malaysia [22], Iran [23] and Taiwan [16], while CoNS are reported to be the most common in UK [5,6,24] and Australia [13]. Due to the widespread use of broad-spectrum antibiotics, it is very likely that the bacterial spectrum and its resistance to antibiotics varies greatly over time and from area to area. However, a comprehensive worldwide and long-term data analysis is scarce. To analyze the trends of bacteria and drug resistance profile over time in the world, we conducted a meta-analysis to compare the positive rate of culture in medical facilities worldwide and summarize the temporal and spatial trends of microbial isolates and their susceptibility patterns since 2000.
2. Results
2.1. Literature Search and Study Characteristics
From the selected databases, 4734 potentially relevant references were identified. In total, 1156 references were excluded because of duplicates. Details of searching and de-duplications were shown in Appendix A. Search results were shown in Appendix B by reviewing the titles and abstracts, and 3459 references were excluded. After reading 119 full texts, 16 articles were excluded, and 103 papers were assessed for eligibility. A total of 42 studies were ultimately included in this meta-analysis, of which only 38 articles contained enough information for positive rate analysis, and 33 articles for drug susceptibility analysis. We used the methodological scoring system of “rate” to assess the quality of each study. The score of all studies were more than 4 points. The paper selection process was shown in Figure 1, and the characteristics of the included studies were shown in Table 1. We used the methodological scoring system of “rate” to assess the quality of each study. The score of all studies were more than 4 points. The paper selection process was shown in Figure 1, and the characteristics of the included studies was shown in Table 1. Among these data, 16 articles were reported from Asia, 11 from America, 1 from Africa, 9 from Europe and 5 from Oceania.
Figure 1.
The PRISMA flow chart of paper selection.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Authors (Years) | Country | City | Study Period | Sample Size | Positive Rate (%) | Microbiological Profiles |
|---|---|---|---|---|---|---|
| Europe | ||||||
| Schaefer (2001) | Switzerland | Lausanne | 1997–1998 | 85 | 86 |
Staphylococcus epidermidis (29.0%) Staphylococcus aureus (16.0%) Pseudomonas species (7.0%) |
| Saeed (2009) | Ireland | Dublin | 2001–2003 | 90 | 36 |
Pseudomonas species (33.3%) Coagulase negative staphylococci (12.1%) Staphylococcus aureus (9.0%) |
| Orlans (2011) | UK | Oxford | 1999–2009 | 467 | 54 | Coagulase negative staphylococci (25.8%) Pseudomonas aeruginosa (24.3%) Staphylococcus aureus (14.3%) |
| Prokosch (2012) | Germany | Münster | 2002–2009 | 346 | 43 |
Staphylococcus aureus (31.7%) Pseudomonas species (7.5%) Streptococcus pneumoniae (6.0%) |
| Otri (2013) | UK | Nottingham | 2007–2007 | 129 | 35 |
Staphylococcus aureus (18.8%) Pseudomonas aeruginosa (15.0%) Pneumococcus (9.4%) |
| Tan (2017) | UK | Manchester | 2004–2015 | 4229 | 30 | Coagulase negative staphylococci (38.5%) Pseudomonas (37.1%) Staphylococcus aureus (23.9%) |
| Ferreira (2018) | Portugal | Porto | 2007–2015 | 235 | 38 |
Staphylococcus aureus (23.1%) Corynebacterium macginleyi (20.0%) Pseudomonas aeruginosa (13.8%) |
| Tavassoli (2019) | UK | Bristol | 2006–2017 | 2116 | 38 | Coagulase negative staphylococci (49.9%) Pseudomonas species (22.0%) Streptococci (9.7%) |
| Tena (2019) | Spain | Guadalajara | 2010–2016 | 298 | 65 | Coagulase negative staphylococci (28.6%) Cutibacterium species (19.6%) Corynebacterium species (9.8%) |
| Africa | ||||||
| Capriotti (2010) | Sierra Leone | Freetown | 2005–2006 | 73 | 58 |
Pseudomonas aeruginosa (39.7%) Staphylococcus aureus (27.4%) Coagulase negative staphylococci (5.5%) |
| Asia | ||||||
| Sharma (2007) | India | Hyderabad | 2002–2002 | 170 | 62 |
Staphylococcus epidermidis (18.6%) Streptococcus pneumoniae (18.6%) Pseudomonas species (4.9%) |
| Yilmaz (2007) | Turkey | Izmir | 1990–2005 | 620 | 28 |
Staphylococcus epidermidis (26.6%) Staphylococcus aureus (24.4%) Streptococcus pneumoniae (15.5%) |
| Fong (2007) | China | Taipei | 1994–2005 | 272 | - |
Pseudomonas aeruginosa (46.7%) Cutibacterium species (8.1%) Nontuberculous Mycobacteria (6.6%) |
| Lavaju (2009) | Nepal | Dharan | 2007–2008 | 44 | 36 |
Staphylococcus aureus (70.0%) Pseudomonas species (15.0%) Acinetobactor species (5.0%) |
| Feilmeier (2010) | Nepal | Kathmandu | 2006–2009 | 468 | 15 |
Streptococcus pneumoniae (69.0%) Staphylococcus aureus (11.0%) Staphylococcus epidermidis (7.0%) |
| Dhakhwa (2012) | Nepal | Siddharthanagar | 2007 | 414 | 39 |
Staphylococcus epidermidis (29.6%) Streptococcus viridans (15.1%) Pseudomonas aeruginosa (14.0%) |
| Lin (2012) | India | Madurai | 2006–2009 | 5221 | 21 |
Staphylococcus epidermidis (31.9%) Pseudomonas aeruginosa (12.4%) Staphylococcus simulans (5.5%) |
| Politis (2016) | Israel | Jerusalem | 2002–2014 | 943 | 44 | Coagulase-negative staphylococci (43.9%) Pseudomonas aeruginosa (24.8%) Streptococcus pneumoniae (6.9%) |
| Hsiao (2016) | China | Taoyuan | 2003–2012 | 2012 | 40 |
Pseudomonas aeruginosa (24.4%) Coagulase-negative staphylococci (16.6%) Cutibacterium species (9.1%) |
| Aruljyothi (2016) | India | Madurai | 2011–2013 | 234 | 30 |
Pseudomonas aeruginosa (37.9%) Streptococcus pneumoniae (24.1%) Staphylococcus aureus (12.0%) |
| Lin (2017) | China | Guangzhou | 2009–2013 | 2973 | 12 |
Staphylococcus epidermidis (31.9%) Pseudomonas aeruginosa (12.4%) Staphylococcus simulans (5.5%) |
| Bagga (2018) | India | Hyderabad | 1991–2012 | 60 | 42 |
Staphylocci (35.0%) Corynebacteria (25.5%) Streptococci (24.0%) |
| Mun (2019) | Korea | Seoul | 2007–2016 | 129 | 78 | Coagulase negative staphylococci (15.9%) Staphylococcus aureus (12.1%) Pseudomonas aeruginosa (10.3%) |
| Liu (2019) | China | Taipei | 2007–2016 | 363 | 51 |
Pseudomonas species (44.7%) Nontuberculous Mycobacteria (7.5%) Propioebacterium species (6.8%) |
| Das (2019) | India | Hyderabad | 2007–2014 | 3981 | 29 |
Streptococcus pneumoniae (16.1%) Staphylococcus aureus (13.8%) Pseudomonas species (7.4%) |
| Khor (2020) | Malaysia | Sarawak | 2010–2016 | 221 | 30 |
Pseudomonas aeruginosa (33.6%) Staphylococcus aureus (3.4%) Corynebacterium species (1.7%) |
| Oceania | ||||||
| Hall (2004) | New Zealand | Christchurch | 1997–2001 | 87 | 59 | Coagulase negative staphylococci (19.3%) Moraxella species (19.3%) Coryebacterium species (16.0%) |
| Ly (2006) | Australia | Sydney | 2002–2003 | 112 | 42 | Coagulase negative staphylococci (38.0%) Pseudomonas aeruginosa (21.0%) Corynebacterium species and coryneform bacteria (15.0%) |
| Constantinou (2009) | Australia | Melbourne | 1998–2007 | 47 | 70 |
Pseudomonas aeruginosa (33.3%) Coagulase negative staphylococci (11.1%) Cutibacterium acnes (8.9%) |
| Pandita (2011) | New Zealand | Hamilton | 2007 | 265 | 65 | Coagulase negative staphylococci (40.8%) Staphylococcus aureus (11.5%) Streptococcus pneumonia (7.5%) |
| Watson (2019) | Australia | Sydney | 2016 | 224 | 75 | Coagulase negative staphylococci (47.8%) Staphylococcus aureus (9.6%) Pseudomonas aeruginosa (9.6%) |
| America | ||||||
| Alexandrakis (2000) | USA | Miami | 1990–1998 | 2920 | 50 |
Pseudomonas aeruginosa (25.7%) Staphylococcus aureus (19.4%) Serratia marcescens (7.6%) |
| Yeh (2006) | USA | Durham | 1997–2004 | 453 | 68 | Coagulase negative staphylococci (39.0%) Staphylococcus aureus (12.0%) Pseudomonas species (10.0%) |
| Afshari (2008) | USA | Boston | 1999–2000 | 485 | 66 | Coagulase negative staphylococci (45.5%) Staphylococcus aureus (15.2%) Diphtheroids (5.7%) |
| Lichtinger (2012) | Canada | Toronto | 2000–2010 | 1701 | 53 | Coagulase negative staphylococci (37.0%) Staphylococcus aureus (17.0%) Streptococcus species (17.0%) |
| Hernandez-Camarena (2015) | Mexico | Mexico City | 2002–2011 | 1638 | 33 |
Staphylococcus epidermidis (25%) Pseudomonas aeruginosa (12%) Coagulase negative staphylococci (10%) |
| Sand (2015) | USA | Los angeles | 2008–2012 | 476 | 62 | Coagulase negative staphylococci (51.4%) Pseudomonas aeruginosa (15.3%) Staphylococcus aureus (12.8%) |
| Rossetto (2017) | USA | Miami | 1992–2015 | 107 | 58 |
Pseudomonas aeruginosa (42.1%) Strenotrophomonas maltophilia (17.5%) Serratia marcescens (8.8%) |
| Tam (2017) | Canada | Toronto | 2000–2015 | 2330 | 49 | Coagulase negative staphylococci (37%) Staphylococcus aureus (15%) Streptococcus species (15%) |
| Jin (2017) | USA | Houston | 2011–2015 | 96 | 62 |
Pseudomonas aeruginosa (33.9%) Coagulase negative staphylococci (26.8%) Streptococcus pneumoniae (10.7%) |
| Peng (2018) | USA | San Francisco | 1996–2015 | 2203 | 24 |
Staphylococcus aureus (25.1%) Coagulase negative staphylococci (20.5%) Streptococcus viridans (13%) |
| Termote (2018) | Canada | Vancouver | 2006–2011 | 281 | 75 | Coagulase negative staphylococci (25.6%) Streptococcus species (12.4%) Staphylococcus aureus (12.1%) |
2.2. Positive Rate of Culture
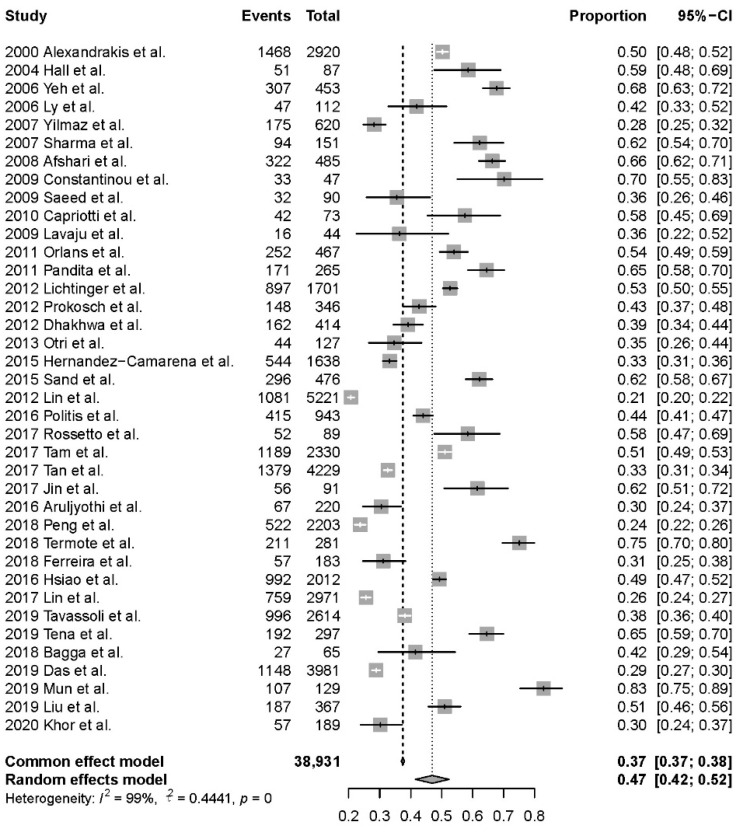
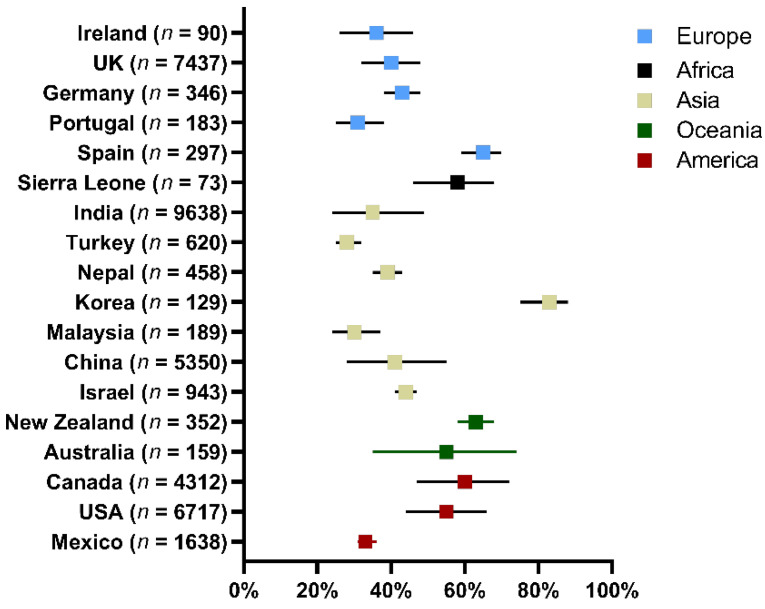
A total of 38,931 samples scraping from the cornea of BK patients were reviewed in the study. Among them, 14,596 samples were culture positive and positive rate of culture was 47% (95%CI, 42–52%) based on the 38 studies (Figure 2). The highest positive rate was 83% and the lowest positive rate was 21%. Data of positive rate was available among 21 countries (35 cities) in 5 continents. The highest and the lowest positive rate analyzed by counties were from Korea and from Turkey (83% vs. 28%, Figure 3, Table S1). There were no significant differences of positive culture rate among different countries (p = 0.464). Grouped by continents, the highest positive rate was 59% (95%CI, 48–68%), found in 4 articles performed in Oceania and the lowest was 40% (95%CI, 32–49%), found in 14 articles performed in Asia. However, there were no significant differences of positive culture rate between continents (p = 0.211).
Figure 2.
Analysis of positive rate of bacterial culture (38 studies).
Figure 3.
Positive rate of bacterial culture from corneal lesions in different regions.
2.3. Distribution of Bacteria Isolated from Corneal Lesions
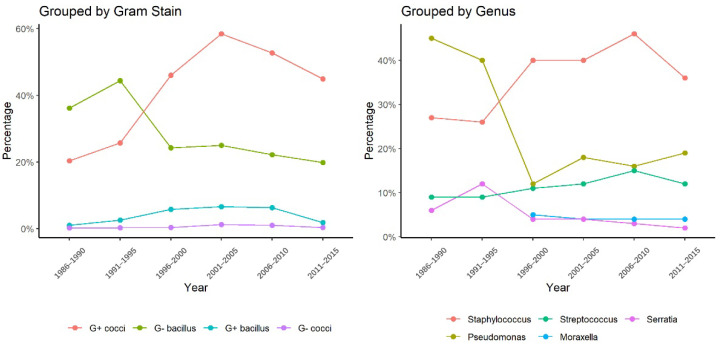
Within 14,596 samples reported from 38 studies, 15,350 bacterial strains isolated from corneas of BK cases were summarized (Table 2). Gram-positive cocci were the major type of bacteria (62%, 58–67%), followed by Gram-negative bacilli (30%, 26–33%), Gram-positive bacilli (5%, 4–7%), and Gram-negative cocci (5%, 4–7%). The five most common bacterial organism detected was Staphylococcus spp. (41.4%, 36.2–46.7%), Pseudomonas spp. (17.0%, 13.9–20.7%), Streptococcus spp. (13.1%, 10.9–15.7%), Corynebacterium spp. (6.6%, 5.3–8.3%) and Moraxella spp. (4.1%, 3.1–5.4%). Figure 4 presented the increasing trends of Gram-positive cocci and the decreasing trends of Gram-negative bacilli in 1996–2015. In 2000s, the proportion of Gram-positive cocci exceeded that of Gram-negative bacilli. The upward trend of Gram-positive cocci and the downward trend of Gram-negative bacilli were both significant (z = 1.71, p = 0.04; z = −1.88, p = 0.03). At the genus level, the percentage of Pseudomonas spp. declined from 39.9% (1990s) to 12.2% (2000s) and Staphylococcus spp. rose from 25.9% (1990s) to 39.5% (2000s) (Figure 4). The upward trend of Staphylococcus spp. was significant (z = 1.71, p = 0.04) and the downward trend of Pseudomonas spp. was not significant (z = −1.22, p = 0.22).
Table 2.
Distribution of bacteria isolated from corneal lesions of bacterial keratitis.
| Organism | Isolates | Percentage (%) | 95%CI (%) |
|---|---|---|---|
| Gram-positive cocci | 8786 | 62.3 | 57.9~66.5 |
| Staphylococcus | 5311 | 41.4 | 36.2~46.7 |
| Streptococcus | 1913 | 13.1 | 10.9~15.7 |
| Gemella | 18 | 3.8 | 2.4~6.0 |
| Micrococcus | 41 | 2.5 | 1.8~3.3 |
| Kocuria | 12 | 1.6 | 0.9~2.8 |
| Enterococcus | 10 | 1.3 | 0.7~2.4 |
| Aerococcus | 7 | 0.8 | 0.3~1.6 |
| Leuconostoc | 6 | 0.8 | 0.4~1.7 |
| Peptostreptococcus | 2 | 0.7 | 0.2~2.9 |
| Gram-negative bacilli | 3776 | 29.6 | 26.0~33.5 |
| Pseudomonas | 2331 | 17.0 | 13.9~20.7 |
| Moraxella | 311 | 4.1 | 3.1~5.4 |
| Serratia | 373 | 3.4 | 2.7~4.2 |
| Haemophilus | 64 | 2.2 | 1.8~2.8 |
| Proteus | 54 | 2.1 | 1.1~4.0 |
| Escherichia | 38 | 2.0 | 1.5~2.7 |
| Klebsiella | 17 | 1.8 | 1.1~2.8 |
| Achromobacter | 1 | 1.9 | 0.0~12.2 |
| Acinetobacter | 26 | 1.8 | 1.3~2.7 |
| Burkholderia | 18 | 1.8 | 1.2~2.9 |
| Enterobacter | 13 | 1.2 | 0.7~2.0 |
| Stenotrophomonas | 11 | 1.1 | 0.6~2.0 |
| Citrobacter | 2 | 1.0 | 0.3~3.9 |
| Morganella | 5 | 0.9 | 0.4~2.0 |
| Gram-positive bacilli | 871 | 5.2 | 3.9~6.8 |
| Corynebacterium | 284 | 6.6 | 5.2~8.3 |
| Nocardia | 96 | 3.9 | 2.4~6.0 |
| Cutibacterium | 243 | 3.3 | 1.7~6.0 |
| Bacilli | 184 | 2.6 | 0.7~8.5 |
| Sphingomonas | 2 | 2.6 | 0.7~8.5 |
| Brevibacterium | 2 | 2.6 | 0.7~8.5 |
| Clostridium | 2 | 1.0 | 0.3~3.9 |
| Mycobacterium | 10 | 0.8 | 0.4~1.5 |
| Aeromonas | 3 | 0.8 | 0.3~2.1 |
| Gram-negative cocci | 26 | 5.2 | 3.9~6.8 |
| Neisseria | 5 | 0.8 | 0.3~1.9 |
| not mentioned | 1891 | 11.9 | 9.3~15.1 |
Figure 4.
The changing trends of bacterial isolates from corneal lesions in 1990s–2020s.
2.4. Antibiotic Susceptibility of the Bacterial Strains Isolated from Corneal Lesions
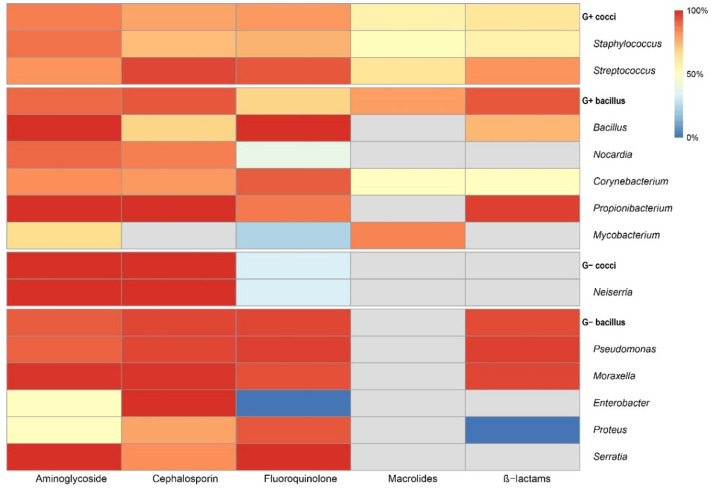
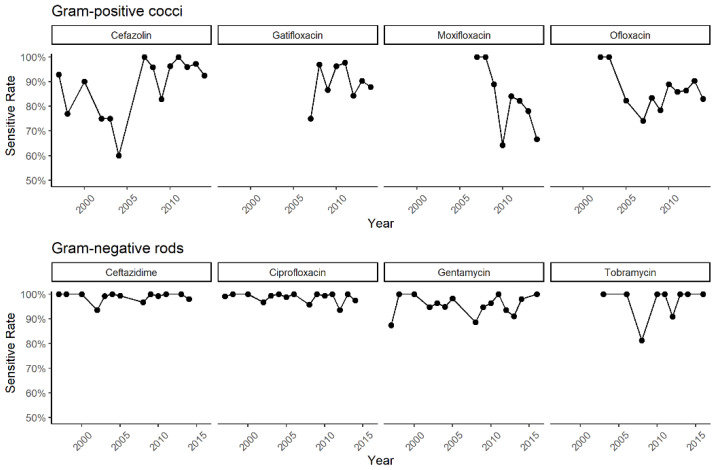
All results of drug susceptibility tests of the strains were summarized in Figure 5. Most Gram-positive cocci were susceptible to aminoglycoside (86%, 3916 sensitive in 4527), following cephalosporin and fluoroquinolone, 79% (1997 sensitive in 2515) and 81% (3921 sensitive in 4831), separately. A resistance of macrolides was observed (57%, 212 sensitive in 375). As for Gram-negative bacilli, most isolates were susceptible to cephalosporin and fluoroquinolone (cephalosporin: 96%, 1269 sensitive in 1328; fluoroquinolone: 96%, 2519 sensitive in 2611), followed by aminoglycoside (92%, 2547 sensitive in 2783). Figure 6 shown the changing trends of susceptibility of pathogens to common drugs. In Gram-positive cocci, the susceptibility to common antibiotics such as cefazolin, gatifloxacin, moxifloxacin and ofloxacin showed a decreasing trend since 2010. For Gram-negative bacilli, a susceptibility over 90% was maintained in all recommended antibiotics [24] in recent years.
Figure 5.
Results of drug susceptibility test of the strains isolated from corneal lesions.
Figure 6.
The changing trends of drug susceptibility in 1990s–2020s.
3. Discussion
Bacterial keratitis is the second most common cause of legal blindness worldwide [25]. Although broad-spectrum antibiotics, such as levofloxacin, have always been used to control BK, more targeted treatment was required to improve the clinical outcomes [26]. However, the spectrum of pathogenic bacteria and their susceptibility to antibiotics varied with the different regions. Kaye et al. showed that these variations were related to the latitude and the degree of urbanization of the population studied [26]. Therefore, local epidemiology of bacterial spectrum and its resistance to antibiotics should be paid attention and is mandatory to know. In this study, 30-year changing trends of microbiological profile of BK were reviewed.
From our results, the average rate of positive culture from the samples of BK was 47%. The large difference in positive culture rates (21~83%) between literatures may be related to the different indications of corneal scrapes in different medical institutions, the ability of microbiology laboratory, or to the fact that the study population had already received topical antibiotics treatment before corneal scraping.
In this study, the common bacterial isolates were Staphylococcus spp., Pseudomonas spp., Streptococcus spp., Corynebacterium spp. and Moraxella spp., which was consistent with studies in the USA [27], UK and Canada. Although the number of Moraxella strains was 311, lower than that in Serratia strains (373), Moraxella spp. were isolated from 7121 samples (4.4%, 16 studies) and 373 Serratia spp. were isolated from 10,431 samples (3.6%, 27 studies). Thus, we concluded that Moraxella spp. were more common than Serratia spp. Pseudomonas spp. was demonstrated to be the most common pathogen in Singapore and Malaysia, which may result from a local high frequency of using contact lenses. Keya et al. found the percentage of Enterobacterales was 15.3%, higher than the percentage of Staphylococcus aureus and Pseudomonas aeruginosa. In addition, the upward trend of Gram-positive cocci and the downward trend of Gram-negative bacilli were observed, especially for the upward trend of Staphylococcus spp. and the downward trend of Pseudomonas spp. at the genus level. Similar trends were also presented in the studies from the UK [6] and Iran [23]. CoNS, one of the most common strains of Staphylococcus spp., was the major bacteria of normal skin, including eye lid. It would have more opportunity to contaminate the cornea; in addition, the conduction of corneal scraping was also easily susceptible to its contamination. The decreasing percentage of Pseudomonas spp. may be attributed to the wide application of some antibiotics, such as tobramycin and fluoroquinolones, and to the improvement in health conditions. Though several studies [4,5,6] reported a significant increase of Moraxella keratitis in UK for the last two decades, the trend was not shown in this worldwide study. Perhaps this trend was limited to specific regions.
In Gram-positive cocci, most isolates were susceptible to aminoglycoside (86%, 3916 sensitive in 4527), and resistant to macrolides in more than half of drug susceptivity tests (57%, 212 sensitive in 375). In the other two classes of antibiotics commonly used in clinical practice against Gram-positive cocci, cephalosporin and fluoroquinolone, 79% (1997 sensitive in 2515) and 81% (3921 sensitive in 4831) isolates showed susceptivity. Since 2010, the susceptibility of Gram-positive cocci to common antibiotics such as cefazolin, gatifloxacin, moxifloxacin and ofloxacin has shown a decreasing trend. For Gram-negative bacilli, most isolates were susceptive to cephalosporin (96%, 1269/1328) and fluoroquinolones (96%, 2519/2611), followed by aminoglycoside (92%, 2547/2783). The trends of susceptibility seemed stable and maintained above 90%. Some studies had reported a group of Pseudomonas spp. with multiple drug resistance [28,29]. It is necessary to use targeted antibiotics in case of the development of resistant strains.
The meta-analysis revealed the trend and distribution of bacterial keratitis pathogens and their drug resistance pattern guiding ophthalmologists to diagnosis and to choosing antibiotics based on their local data. Our study also possessed some limitations. Original studies often reported their results via time period (e.g., 2000–2005 CoNS 30%), not specific year (e.g., 2000 CoNS 30%), which affected the accuracy of our study. Differences between original studies, such as culture methods, participants who received topical antibiotics treatment before corneal scraping and experience of clinics would increase the difficulty of pathogen distribution analysis. Our study could still provide useful guidance for clinics.
In conclusion, the worldwide average positive culture was 47% between 2000 to 2020. The percentage of Gram-positive cocci was increasing, and the percentage of Gram-negative bacilli was decreasing. The five most common bacterial organism were Staphylococcus spp., Pseudomonas spp., Streptococcus spp., Corynebacterium spp. and Moraxella spp. Increasing trends of Gram-positive cocci and the decreasing trends of Gram-negative bacilli were observed in 1996–2015. Most Gram-positive cocci were susceptive to aminoglycoside and were resistant to macrolides. Gram-negative bacilli were sensitive to cephalosporin, fluoroquinolone and aminoglycoside and maintained susceptibility above 90%. The decreasing trends of susceptibility were observed in Gram-positive cocci to most common antibiotics. Ophthalmologists should pay attention to the changing trends of pathogen distribution and their drug resistance patterns and modify the diagnosis and choose sensitive antibiotics based on the local data.
4. Materials and Methods
4.1. Databases and Search Strategy
Four databases, including Embase, Medline, Web of Science, and CINAHL, were searched, and publication date of articles were limited between January 2000 to December 2020. Main key words were “bacterial keratitis”, “culture results” and “drug resistance”. The whole search strategy was (“bacterial keratitis” OR “infectious keratitis” OR “microbial keratitis” OR “bacterial infections” OR “corneal ulcers” OR “bacterial infections of cornea”) AND (“organisms” OR “culture results” OR “isolates” OR “microbiology” OR “antibiotic susceptibility” OR “resistance pattern”). In addition, the document type was restricted to “article”, the language was restricted to “English”, and the subjects were restricted to “human”. More details of the search strategy could be found in Supplement Materials Table S1.
4.2. Literature Selection and Quality Assessment
The literature searched in the above databases was imported into the EndNote X 9 software library for merging and de-duplicating; then, the titles and abstracts were screened by two researchers (Z.Z., J.L.) according to the following inclusion criteria: (1) Purpose of the article should concentrate on reporting the distribution and resistance pattern of bacterial keratitis isolates; (2) Subjects of the literature must contain patients suspected of infective keratitis and confirmed by positive bacterial culture results; (3) Pathogens were isolating from corneal scrapes via culture and were identified at least at the genus level; (4) Drug susceptibility tests should be conducted for isolated strains via in vitro minimum inhibitory concentration testing (MIC) or the disk diffusion method. The homogeneity of our meta-analysis was controlled by exclusive criteria below: (1) Subjects with small sample size or specific risk factors; (2) Subjects already selected by authors would be excluded for positive rate analysis. (3) Multiple articles published using the same data would be deduplicated. After preliminary exclusion of unrelated references, we downloaded the full text of each citation; the literature quality was evaluated based on a methodological scoring system of “rate” [30]. The detailed quality criteria were as follows: (1) Whether there is a clear diagnostic basis for bacterial keratitis; (2) Whether the sample size (the number of bacterial culture specimens) is greater than 246 cases; (3) Whether there are clear criteria for positive bacterial culture; (4) Whether there are clear study parameters, such as positive rate or drug susceptibility results; (5) Whether the data is complete. Complete data should include the description of study populations, methods for the drug susceptibility test, and protocol for bacteria separating and identifying. Each criterion was given one score, and studies with a score of 4 or more were of high quality and included for analysis. All the steps of screening and quality assessment were carried out independently by two researchers (Z.W., X.X.). In case of disagreement over the inclusion of the literature, a third, more experienced researcher (Q.L.) would make the final decision.
4.3. Data Extraction
According to the purpose of this study, the data extraction scale of the literature was developed. For each included article, four aspects of information would be extracted. The first is the basic information of the literature and the institution of the author, including the publication year, time that the research was conducted, author, title, medical institution, etc. Next, the necessary data of positive rate of culture, bacterial strains distribution and drug susceptibility were extracted. The positive rate of culture was defined as the proportion of BK patients with positive culture results in all culture-treated patients suspected of infective keratitis. The parameter necessary for analyzing bacterial strain distribution contained the total number of strains isolated, number classified by Gram staining and number of strains as accurate as possible to species. Parameters related to drug susceptibility included the number of susceptible or resistant species performed on a certain species of bacterium to a certain drug. All above data were extracted into Microsoft Excel software.
4.4. Statistical Analysis
Data were analyzed with SPSS software (SPSS for windows, version 16.0, SPSS, Chicago, IL, USA). The meta-analysis was conducted using R program (V.4.0, R Foundation for Statistical Computing, Vienna, Austria) with meta package. All effect sizes were transformed into a single common metric, event rate with its 95% confidence interval, which indicated the number of participants in each sample endorsing bacterial keratitis. Either a random effects model or a fixed effects model was used to perform meta-analysis, which was determined by I2 statistic; I2 > 50% indicates a large heterogeneity among included studies and, correspondingly, a random effects model would be used; otherwise, a fixed effects model would be used.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11020238/s1, Table S1: Positive rate of bacterial culture from corneal lesions in different regions, References [5,7,8,11,12,16,18,21,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] are cited in the Supplementary Materials.
Appendix A
Search Strategy
Search terms
Database: Embase, Medline, Web of Science and CINAHL
Epub Ahead of Print, in Process & other Non-Indexed Citation, Daily, and Versions from 1 January 2000 to 30 November 2020
The search limits were from 2000 to 2020 and English language, Human.
-
3.Search Strategies
- bacterial keratitis
- infectious keratitis
- microbial keratitis
- bacterial infections
- corneal ulcers
- bacterial infections of cornea
- 1 or 2 or 3 or 4 or 5 or 6
- organisms
- culture results
- isolates
- microbiology
- antibiotic susceptibility
- drug resistance
- resistance pattern
- 8 or 9 or 10 or 11 or 12 or 13 or 14
- 7 and 15
Appendix B
Appendix B.1. Search Result
Appendix B.1.1. Summary
The whole search process was shown in Table A1.
Table A1.
Databases searched in our study.
| Database Name | Endnote Importer Order | Number of References before Deduplication | Number of References after Deduplication (Removed) |
|---|---|---|---|
| Ovid Embase | 1 | 829 | 822 |
| Ovid Medline(R) | 2 | 1697 | 1241 |
| Web of Science | 3 | 2107 | 1515 |
| CINAHL | 4 | 101 | 0 |
| TOTAL | 3578 |
Appendix B.1.2. Ovid Embase
Search information was shown in Table A2.
Table A2.
Search information in Ovid Embase.
| Database name | Embase |
| Database platform | Ovid |
| Date of database coverage | 1974 to 6 December 2021 |
| Date searched | 12/07/2021 |
| Searched by | ZJ Zhang |
| Number of hits | 829 |
bacterial keratitis.mp. (1048)
infectious keratitis.mp. (854)
microbial keratitis.mp. (968)
corneal ulcer.mp. (1187)
bacterial infection of cornea.mp. (1)
1 or 2 or 3 or 4 or 5 (3260)
organism.mp. (43790)
culture result.mp. (2317)
isolates.mp. (80983)
microbiology.mp. (100066)
antibiotic susceptibility.mp. (6595)
drug resistance.mp. (152927)
resistance pattern.mp. (1971)
7 or 8 or 9 or 10 or 11 or 12 or 13 (347062)
6 and 14 (829)
Appendix B.1.3. Ovid Medline(R)
Search information was shown in Table A3.
Table A3.
Search information in Ovid Medline(R).
| Database name | Medline(R) |
| Database platform | Ovid |
| Date of database coverage | 1946 to November Week 4 2021 |
| Date searched | 12/07/2021 |
| Searched by | ZJ Zhang |
| Number of hits | 1697 |
bacterial keratitis (448)
infectious keratitis (608)
microbial keratitis (615)
corneal ulcer (2134)
bacterial infection of cornea (1)
1 or 2 or 3 or 4 or 5 (3084)
organism (30665)
culture result (425)
isolates (78696)
microbiology (298577)
antibiotic susceptibility (4540)
drug resistance (148045)
resistance pattern (1054)
7 or 8 or 9 or 10 or 11 or 12 or 13 (452932)
6 and 14 (1697)
Appendix B.1.4. Web of Science
Search information was shown in Table A4.
Table A4.
Search information in Web of Science.
| Database name | Web of Science Core |
| Database platform | Web of Science |
| Date of database coverage | 1985 to 2021 |
| Date searched | 12/07/2021 |
| Searched by | ZJ Zhang |
| Number of hits | 2107 |
ALL = (bacterial keratitis) (2247)
ALL = (infectious keratitis) (2275)
ALL = (microbial keratitis) (1859)
ALL = (corneal ulcer) (2299)
ALL = (bacterial infection of cornea) (721)
1 or 2 or 3 or 4 or 5 (6332)
ALL = (organism) (289051)
ALL = (culture result) (611251)
ALL = (isolates) (920667)
ALL = (microbiology) (305542)
ALL = (antibiotic susceptibility) (31902)
ALL = (drug resistance) (188954)
ALL = (resistance pattern) (69975)
7 or 8 or 9 or 10 or 11 or 12 or 13 (2090362)
(6 and 14) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article) (2107)
Appendix B.1.5. EBSCO CLNAHL
Search information was shown in Table A5.
Table A5.
Search information in EBSCO CLNAHL.
| Database name | CINAHL |
| Database platform | EBSCO |
| Date of database coverage | 1961 to present |
| Date searched | 12/07/2021 |
| Searched by | ZJ Zhang |
| Number of hits | 101 |
TX bacterial keratitis (26)
TX infectious keratitis (32)
TX microbial keratitis (48)
TX corneal ulcer (113)
TX bacterial infection of cornea (0)
S1 or S2 or S3 or S4 or S5 (173)
TX organism (3856)
TX culture result (1861)
TX isolates (6443)
TX microbiology (29341)
TX antibiotic susceptibility (653)
TX drug resistance (11158)
TX resistance pattern (592)
S7 or S8 or S9 or S10 or S11 or S12 or S13 (41951)
S6 and S14 (101)
Author Contributions
Conceptualization, Z.Z. and Q.L.; methodology, K.C.; software, X.X.; formal analysis, K.C.; investigation, Z.W.; writing—original draft preparation, Z.Z.; writing—review and editing, Q.L.; visualization, J.L.; supervision, Q.L.; project administration, Q.L.; funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2301000); the National Natural Science Foundation of China, grant number 81970765.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitcher J.P., Srinivasan M., Upadhyay M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Petrillo F., Folliero V., Santella B., Franci G., Foglia F., Trotta M.C., Della Rocca M.T., Avitabile T., Gagliano C., Galdiero M. Prevalence and Antibiotic Resistance Patterns of Ocular Bacterial Strains Isolated from Pediatric Patients in University Hospital of Campania “Luigi Vanvitelli”, Naples, Italy. Int. J. Microbiol. 2020;2020:8847812. doi: 10.1155/2020/8847812. [DOI] [Google Scholar]
- 3.Petrillo F., Pignataro D., Lella F.M.D., Reibaldi M., Fallico M., Castellino N., Parisi G., Trotta M.C., D’Amico M., Santella B. Antimicrobial Susceptibility Patterns and Resistance Trends of Staphylococcus aureus and Coagulase-Negative Staphylococci Strains Isolated from Ocular Infections. Antibiotics. 2021;10:527. doi: 10.3390/antibiotics10050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting D.S.J., Ho C.S., Cairns J., Elsahn A., Al-Aqaba M., Boswell T., Said D.G., Dua H.S. A 12-year analysis of incidence, microbiological profiles, and in vitro antimicrobial susceptibility of infectious keratitis: The nottingham eye study. Investig. Ophthalmol. Vis. Sci. Conf. 2020;61:328–333. doi: 10.1136/bjophthalmol-2020-316128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan S., Walkden A., Au L., Fullwood C., Hamilton A., Qamruddin A., Armstrong M., Brahma A., Carley F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye. 2017;31:1229–1236. doi: 10.1038/eye.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ting D.S.J., Settle C., Morgan S.J., Baylis O., Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England Study. Eye. 2018;32:1416–1417. doi: 10.1038/s41433-018-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavassoli S., Nayar G., Darcy K., Grzeda M., Luck J., Williams O.M., Tole D. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye. 2019;33:1619–1625. doi: 10.1038/s41433-019-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam A.L., Côté E., Saldanha M., Lichtinger A., Slomovic A.R. Bacterial Keratitis in Toronto: A 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea. 2017;36:1528–1534. doi: 10.1097/ICO.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 9.Cariello A.J., Passos R.M., Yu M.C.Z., Hofling-Lima A.L. Microbial keratitis at a referral center in Brazil. Int. Ophthalmol. 2011;31:197–204. doi: 10.1007/s10792-011-9441-0. [DOI] [PubMed] [Google Scholar]
- 10.Marujo F.I., Hirai F.E., Yu M.C.Z., Hofling-Lima A.L., Freitas D.d., Sato E.H. Distribution of infectious keratitis in a tertiary hospital in Brazil. Arq. Bras. Oftalmol. 2013;76:370–373. doi: 10.1590/S0004-27492013000600011. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Camarena J.C., Graue-Hernandez E.O., Ortiz-Casas M., Ramirez-Miranda A., Navas A., Pedro-Aguilar L., Lopez-Espinosa N.L., Gaona-Juarez C., Bautista-Hernandez L.A., Bautista-de Lucio V.M. Trends in Microbiological and Antibiotic Sensitivity Patterns in Infectious Keratitis: 10-Year Experience in Mexico City. Cornea. 2015;34:778–785. doi: 10.1097/ICO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 12.Politis M., Wajnsztajn D., Rosin B., Block C., Solomon A. Trends of Bacterial Keratitis Culture Isolates in Jerusalem: A 13-Years Analysis. PLoS ONE. 2016;11:e0165223. doi: 10.1371/journal.pone.0165223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green M., Carnt N., Apel A., Stapleton F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019;103:1481–1486. doi: 10.1136/bjophthalmol-2018-312881. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera-Aguas M., Khoo P., George C.R., Lahra M.M., Watson S.L. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin. Exp. Ophthalmol. 2020;48:183–191. doi: 10.1111/ceo.13672. [DOI] [PubMed] [Google Scholar]
- 15.Green M., Apel A., Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao C.-H., Sun C.-C., Yeh L.-K., Ma D.H., Chen P.Y., Lin H.-C., Tan H.-Y., Chen H.-C., Chen S.-Y., Huang Y.-C. Shifting Trends in Bacterial Keratitis in Taiwan: A 10-Year Review in a Tertiary-Care Hospital. Cornea. 2016;35:313–317. doi: 10.1097/ICO.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 17.Ormerod L.D., Hertzmark E., Gomez D.S., Stabiner R.G., Schanzlin D.J., Smith R.E. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology. 1987;94:1322–1333. doi: 10.1016/S0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrakis G., Alfonso E.C., Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. doi: 10.1016/S0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 19.Houang E., Lam D., Fan D., Seal D. Microbial keratitis in Hong Kong: Relationship to climate, environment and contact-lens disinfection. Trans. R. Soc. Trop. Med. Hyg. 2001;95:361–367. doi: 10.1016/S0035-9203(01)90180-4. [DOI] [PubMed] [Google Scholar]
- 20.Gopinathan U., Sharma S., Garg P., Rao G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthalmol. 2009;57:273–279. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prokosch V., Gatzioufas Z., Thanos S., Stupp T. Microbiological findings and predisposing risk factors in corneal ulcers. Graefes Arch. Clin. Exp. Ophthalmol. 2012;250:369–374. doi: 10.1007/s00417-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 22.Termote K., Joe A.W., Butler A.L., McCarthy M., Blondeau J.M., Iovieno A., Holland S.P., Yeung S.N. Epidemiology of bacterial corneal ulcers at tertiary centres in Vancouver, BC. Can. J. Ophthalmol. 2018;53:330–336. doi: 10.1016/j.jcjo.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Termote K., Joe A.W., Butler A.L., McCarthy M., Blondeau J.M., Iovieno A., Holland S.P., Yeung S.N. Ocular Pathogens and Antibiotic Sensitivity in Bacterial Keratitis Isolates at King Khaled Eye Specialist Hospital, 2011 to 2014. Cornea. 2016;35:789–794. doi: 10.1097/ICO.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 24.Hooi S.H., Hooi S.T. Culture-proven bacterial keratitis in a Malaysian general hospital. Med. J. Malaysia. 2005;60:614–623. [PubMed] [Google Scholar]
- 25.Soleimani M., Tabatabaei S.A., Masoumi A., Mirshahi R., Ghahvechian H., Tayebi F., Momenaei B., Mahdizad Z., Mohammadi S.S. Infectious keratitis: Trends in microbiological and antibiotic sensitivity patterns. Eye. 2021;35:3110–3115. doi: 10.1038/s41433-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye R., Kaye A., Sueke H., Neal T., Winstanley C., Horsburgh M., Kaye S. Recurrent bacterial keratitis. Investig. Ophthalmol. Vis. Sci. 2013;54:4136–4139. doi: 10.1167/iovs.13-12130. [DOI] [PubMed] [Google Scholar]
- 27.Lin A., Rhee M.K., Akpek E.K., Amescua G., Farid M., Garcia-Ferrer F.J., Varu D.M., Musch D.C., Dunn S.P., Mah F.S. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P1–P55. doi: 10.1016/j.ophtha.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Al-Mujaini A., Al-Kharusi N., Thakral A., Wali U.K. Bacterial keratitis: Perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ. Med. J. 2009;9:184–195. [PMC free article] [PubMed] [Google Scholar]
- 29.Kaye S., Tuft S., Neal T., Tole D., Leeming J., Figueiredo F., Armstrong M., McDonnell P., Tullo A., Parry C. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Investig. Ophthalmol. Vis. Sci. 2010;51:362–368. doi: 10.1167/iovs.09-3933. [DOI] [PubMed] [Google Scholar]
- 30.Jeng B.H., Gritz D.C., Kumar A.B., Holsclaw D.S., Porco T.C., Smith S.D., Whitcher J.P., Margolis T.P., Wong I.G. Epidemiology of ulcerative keratitis in Northern California. Arch. Ophthalmol. 2010;128:1022–1028. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 31.Dhakwa K., Sharma M., Bajimaya S., Dwivedi A., Rai S.K. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal J. Ophthalmol. 2012;4:119–127. doi: 10.3126/nepjoph.v4i1.5863. [DOI] [PubMed] [Google Scholar]
- 32.Das S., Samantaray R., Mallick A., Sahu S.K., Sharma S. Types of organisms and in-vitro susceptibility of bacterial isolates from patients with microbial keratitis: A trend analysis of 8 years. Indian J. Ophthalmol. 2019;67:49–53. doi: 10.4103/ijo.IJO_500_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson W.S., Wilson M.C., Guyatt G.H., Cook D.J., Nishikawa J., Evidence-Based Medicine Working Group Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. JAMA. 1999;281:1214–1219. doi: 10.1001/jama.281.13.1214. [DOI] [PubMed] [Google Scholar]
- 34.Afshari N.A., Ma J.J., Duncan S.M., Pineda R., Starr C.E., DeCroos F.C., Johnson C.S., Adelman R.A. Trends in resistance to ciprofloxacin, cefazolin, and gentamicin in the treatment of bacterial keratitis. J. Ocul. Pharmacol. Ther. 2008;24:217–223. doi: 10.1089/jop.2007.0085. [DOI] [PubMed] [Google Scholar]
- 35.Aruljyothi L., Radhakrishnan N., Prajna V.N., Lalitha P. Clinical and microbiological study of paediatric infectious keratitis in South India: A 3-year study (2011–2013) Br. J. Ophthalmol. 2016;100:1719–1723. doi: 10.1136/bjophthalmol-2015-307631. [DOI] [PubMed] [Google Scholar]
- 36.Capriotti J., Pelletier J., Shah M., Caivano D., Turay P., Ritterband D. The etiology of infectious corneal ulceration in Sierra Leone. Int. Ophthalmol. 2010;30:637–640. doi: 10.1007/s10792-010-9348-1. [DOI] [PubMed] [Google Scholar]
- 37.Constantinou M., Jhanji V., Tao L.W., Vajpayee R.B. Clinical review of corneal ulcers resulting in evisceration and enucleation in elderly population. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:1389–1393. doi: 10.1007/s00417-009-1111-9. [DOI] [PubMed] [Google Scholar]
- 38.Feilmeier M.R., Sivaraman K.R., Oliva M., Tabin G.C., Gurung R. Etiologic diagnosis of corneal ulceration at a tertiary eye center in Kathmandu, Nepal. Cornea. 2010;29:1380–1385. doi: 10.1097/ICO.0b013e3181d92881. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira C.S., Figueira L., Moreira-Gonçalves N., Moreira R., Torrão L., Falcão-Reis F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center-Where Are We in 2015? Eye Contact Lens. 2018;44:15–20. doi: 10.1097/ICL.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 40.Fong C.F., Hu F.R., Tseng C.H., Wang I.J., Chen W.L., Hou Y.C. Antibiotic Susceptibility of Bacterial Isolates from Bacterial Keratitis Cases in a University Hospital in Taiwan. Am. J. Ophthalmol. 2007;144:682–689. doi: 10.1016/j.ajo.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 41.Hall R.C., McKellar M.J. Bacterial keratitis in Christchurch, New Zealand, 1997–2001. Clin. Exp. Ophthalmol. 2004;32:478–481. doi: 10.1111/j.1442-9071.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 42.Jin H., Parker W.T., Law N.W., Clarke C.L., Gisseman J.D., Pflugfelder S.C., Wang L., Al-Mohtaseb Z.N. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br. J. Ophthalmol. 2017;101:1483–1487. doi: 10.1136/bjophthalmol-2016-310026. [DOI] [PubMed] [Google Scholar]
- 43.Khor H.G., Cho I., Lee K.R.C.K., Chieng L.L. Spectrum of Microbial Keratitis Encountered in the Tropics. Eye Contact Lens. 2020;46:17–23. doi: 10.1097/ICL.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 44.Lavaju P., Arya S., Khanal B., Amatya R., Patel S. Demograhic pattern, clinical features and treatment outcome of patients with infective keratitis in the eastern region of Nepal. Nepal J. Ophthalmol. 2009;1:101–106. doi: 10.3126/nepjoph.v1i2.3683. [DOI] [PubMed] [Google Scholar]
- 45.Lichtinger A., Yeung S.N., Kim P., Amiran M.D., Iovieno A., Elbaz U., Ku J.Y., Wolff R., Rootman D.S., Slomovic A.R. Shifting trends in bacterial keratitis in Toronto: An 11-year review. Ophthalmology. 2012;119:1785–1790. doi: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 46.Lin C.C., Prajna L., Srinivasan M., Prajna N.V., McLeod S.D., Acharya N.R., Lietman T.M., Porco T.C. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123–1127. doi: 10.1097/ICO.0b013e31825694d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L., Lan W., Lou B., Ke H., Yang Y., Lin X., Liang L. Genus Distribution of Bacteria and Fungi Associated with Keratitis in a Large Eye Center Located in Southern China. Ophthalmic Epidemiol. 2017;24:90–96. doi: 10.1080/09286586.2016.1254250. [DOI] [PubMed] [Google Scholar]
- 48.Liu H.-Y., Chu H.-S., Wang I.-J., Chen W.-L., Hu F.-R. Microbial Keratitis in Taiwan: A 20-Year Update. Am. J. Ophthalmol. 2019;205:74–81. doi: 10.1016/j.ajo.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Ly C.N., Pham J.N., Badenoch P.R., Bell S.M., Hawkins G., Rafferty D.L., McClellan K.A. Bacteria commonly isolated from keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin. Clin. Exp. Ophthalmol. 2006;34:44–50. doi: 10.1111/j.1442-9071.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 50.Mun Y., Kim M.K., Oh J.Y. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS ONE. 2019;14:e0213103. doi: 10.1371/journal.pone.0213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orlans H.O., Hornby S.J., Bowler I.C. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: A 10-year review. Eye. 2011;25:489–493. doi: 10.1038/eye.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otri A.M., Fares U., Al-Aqaba M.A., Miri A., Faraj L.A., Said D.G., Maharajan S., Dua H.S. Profile of sight-threatening infectious keratitis: A prospective study. Acta Ophthalmol. 2013;91:643–651. doi: 10.1111/j.1755-3768.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 53.Pandita A., Murphy C. Microbial keratitis in Waikato, New Zealand. Clin. Exp. Ophthalmol. 2011;39:393–397. doi: 10.1111/j.1442-9071.2010.02480.x. [DOI] [PubMed] [Google Scholar]
- 54.Peng M.Y., Cevallos V., McLeod S.D., Lietman T.M., Rose-Nussbaumer J. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea. 2018;37:84–87. doi: 10.1097/ICO.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossetto J.D., Cavuoto K.M., Osigian C.J., Chang T.C.P., Miller D., Capo H., Spierer O. Paediatric infectious keratitis: A case series of 107 children presenting to a tertiary referral centre. Br. J. Ophthalmol. 2017;101:1488–1492. doi: 10.1136/bjophthalmol-2016-310119. [DOI] [PubMed] [Google Scholar]
- 56.Saeed A., D’Arcy F., Stack J., Collum L.M., Power W., Beatty S. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in ireland. Cornea. 2009;28:285–292. doi: 10.1097/ICO.0b013e3181877a52. [DOI] [PubMed] [Google Scholar]
- 57.Sand D., She R., Shulman I.A., Chen D.S., Schur M., Hsu H.Y. Microbial keratitis in los angeles: The doheny eye institute and the los angeles county hospital experience. Ophthalmology. 2015;122:918–924. doi: 10.1016/j.ophtha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 58.Schaefer F., Bruttin O., Zografos L., Guex-Crosier Y. Bacterial keratitis: A prospective clinical and microbiological study. Br. J. Ophthalmol. 2001;85:842–847. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma N., Venugopal R., Singhal D., Maharana P.K., Sangwan S., Satpathy G. Microbial Keratitis in Stevens-Johnson Syndrome: A Prospective Study. Cornea. 2019;38:938–942. doi: 10.1097/ICO.0000000000001960. [DOI] [PubMed] [Google Scholar]
- 60.Sharma S., Taneja M., Gupta R., Upponi A., Gopinathan U., Nutheti R., Garg P. Comparison of clinical and microbiological profiles in smear-positive and smear-negative cases of suspected microbial keratitis. Indian J. Ophthalmol. 2007;55:21–25. doi: 10.4103/0301-4738.29490. [DOI] [PubMed] [Google Scholar]
- 61.Tena D., Rodríguez N., Toribio L., González-Praetorius A. Infectious Keratitis: Microbiological Review of 297 Cases. Jpn. J. Infect. Dis. 2019;72:121–123. doi: 10.7883/yoken.JJID.2018.269. [DOI] [PubMed] [Google Scholar]
- 62.Watson S., Cabrera-Aguas M., Khoo P., Pratama R., Gatus B.J., Gulholm T., El-Nasser J., Lahra M.M. Keratitis Antimicrobial Resistance Surveillance Program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019;47:20–25. doi: 10.1111/ceo.13364. [DOI] [PubMed] [Google Scholar]
- 63.Yeh D.L., Stinnett S.S., Afshari N.A. Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am. J. Ophthalmol. 2006;142:1066–1068. doi: 10.1016/j.ajo.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 64.Yilmaz S., Ozturk I., Maden A. Microbial keratitis in West Anatolia, Turkey: A retrospective review. Int. Ophthalmol. 2007;27:261–268. doi: 10.1007/s10792-007-9069-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.