Abstract
Hepatitis G virus (HGV) isolates obtained from 20 Myanmarese and 10 Vietnamese subjects were analyzed. A cluster of isolates not belonging to any known genotype of HGV was found in five Myanmarese subjects and three Vietnamese subjects by phylogenetic analysis, and we classified this new genotype as type 4. These results revealed that the HGV genome can be classified into at least four major genotypes.
The hepatitis G virus (HGV) or GB virus C (GBV-C) is a newly identified human RNA virus (8, 9). This virus belongs to the Flaviviridae family, which includes the hepatitis C virus (HCV), as it has some molecular similarity with members of this family. Database screening indicates that the encoded polyprotein sequences of HGV and GBV-C have 95% amino acid sequence similarity (85% similarity at the nucleotide level) (23). Based on this high degree of sequence identity, HGV and GBV-C appear to be independent isolates of the same virus. HGV/GBV-C is mainly transmitted via blood. Although the disease-inducing activity of HGV/GBV-C is not yet clear, viral RNA is detected more frequently in patients with liver diseases than in control subjects (1). Epidemiological investigation demonstrated that HGV/GBV-C infection is present at a frequency of about 1.4% in healthy populations in Japan and the United States, 20% of patients who have received multiple transfusions, and 30% of intravenous drug users (1, 10). The genome of HGV/GBV-C exhibits a sequence variation among different isolates. On the basis of this variation, it has been proposed that HGV can be classified into three major genotypes, some of which may be divided further into a few subtypes (10, 11, 14, 18). The prevalence of HGV genotypes differs in different geographic regions. For example, HGV type 1 is most prevalent in West Africa, including Ghana, type 2 is prevalent in the United States and Europe, and type 3 is prevalent in Asia (14, 18). In the present study, we analyzed the 5′ untranslated regions (UTR) of HGV isolates obtained from Myanmarese and Vietnamese subjects to determine the prevalence of the genotypes in these countries.
We collected serum samples from 204 individuals residing in Myanmar (mainly in Yangon) and from 62 human immunodeficiency virus-infected patients residing in Vietnam (in Ho Chi Minh City and An Gian). In both countries these sera were sampled between 1996 and 1998 and stored at −20°C or lower until use. Nucleic acid was extracted from 100-μl serum samples by using an RNA extraction kit (SepaGene RV-R; Sanko Junyaku Co., Ltd., Tokyo, Japan). The resulting pellet was resuspended in RNase-free water and was then subjected to nested reverse transcription (RT)-PCR by a one-step method as previously described (1). The PCR primers for screening were designed to start from the 5′ UTR of HGV PNF2161 isolate. The sequences of the HGV-specific primers were 5′-GGTCGTAAATCCCGGTCACC-3′ (HG1, sense primer; nucleotides [nt] 139 to 158) and 5′-CCCACTGGTCCTTGTCAACT-3′ (HG1R, antisense primer; nt 381 to 400) for the outer primer pairs (262 bases) and 5′-TAGCCACTATAGGTGGGTCT-3′ (HG2, sense primer; nt 163 to 182) and 5′-ATTGAAGGGCGACGTGGACC-3′ (HG2R, antisense primer; nt 331 to 350) for the inner primer pairs (188 bases). We chose this 5′ UTR of 188 bases in length, because it contains not only several blocks of well-conserved sequences that can be useful in screening for HGV PCR assay but also genotype-specific sequences, as reported previously (18). The first PCR reaction was combined with the RT step (one-step method) in a single tube containing 40 μl of a reaction buffer with the following composition: 10 U of RNase inhibitor (Promega, Madison, Wis.), 100 U of Moloney murine leukemia virus reverse transcriptase (Promega), 50 ng of each outer primer, 200 μM concentration of each of the four deoxynucleotides, 1 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.), and 1× PCR buffer containing 1.5 mM MgCl2. We used AmpliTaq Gold DNA polymerase to obtain an automatic hot-start reaction. Each thermocycler (GeneAmp PCR systems 9600 and 9700; Perkin-Elmer) was programmed to first incubate the samples for 50 min at 37°C for the initial RT step and then to carry out preheating at 95°C for 10-min activation of AmpliTaq Gold DNA polymerase, followed by 50 cycles consisting of 94°C for 20 s, 55°C for 20 s, and 72°C for 40 s. For the second reaction, 2 μl of the first PCR product was added to a tube containing the second set of inner primers, deoxynucleotides, AmpliTaq Gold DNA polymerase, and PCR buffer as in the first reaction but without reverse transcriptase and omitting the initial 50-min incubation at 37°C. The PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and evaluated under UV light. PCR products for the 5′ UTR of the HGV genome were also sequenced and then subjected to phylogenetic analysis for HGV genotype. PCR products of HGV were separated by 2% agarose gel electrophoresis and purified by using the QIAquick gel extraction kit (Qiagen Inc., Chatsworth, Calif.). Recovered PCR products were subjected to direct sequencing from both directions by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer). Sequences of amplified cDNA were determined by using a sequencer (ABI model 373A; Applied Biosystems, Foster City, Calif.).
Nine full-genome sequences and four near-full-genome sequences covering the entire open reading frame of HGV/GBV-C isolates with sequences obtained from GenBank databases were used for comparison of the sequences of the isolates examined in the present study. The isolate designations and accession numbers (references cited provide the reported sequences) were as follows: HGV PNF2161 and R10291, from the United States (U44402 and U45966, respectively) (9); GBV-C from West Africa (U36380) (8); HGV GA128, from Ghana (AB013500) (17); GBV-C-EA, from East Africa (U63715) (5); HGV-Iw, from Japan (D87255) (20); GT110 and GT230, from Japan (D90600 and D90601, respectively) (13); GSI93, from Japan (D87263) (12); HGV IM71, from Japan (AB008342) (7); BGHC, CG01BD, CG07BD, and G13HC, from Japan (AB003288, AB003289, AB003290, and AB003293, respectively) (21). Nucleotide sequences were multiply aligned by using CLUSTAL X, version 1.5. The distance matrix of the nucleotide differences among the isolates was constructed by the eight-parameter method (16), and phylogenetic trees were constructed by the neighbor-joining method (19) from the matrix. These procedures were computed by using Phylo_win, version 1.2 (6), on a DEC alpha 2000 server, and the trees were drawn by TreeView, version 1.3 (15). The reliability and topology of each tree branch were tested by bootstrap analysis (4) of the data for 100 bootstrap resamplings of the columns in alignment of the HGV 5′ UTR sequence.
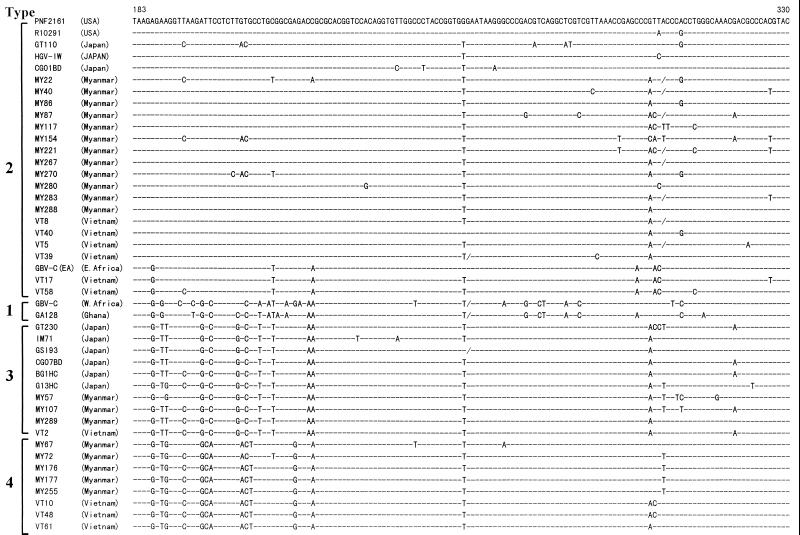
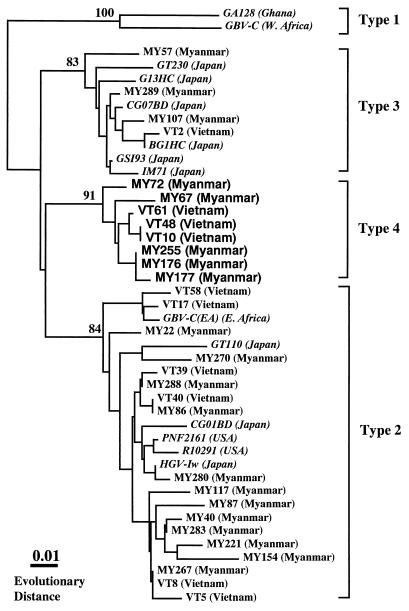
HGV RNA was detected in 22 (11%) of 204 Myanmarese and 19 (31%) of 62 Vietnamese subjects. As shown in Fig. 1, the alignment of the representative 5′ UTR of HGV isolates in the present study revealed that there was a consensus sequence at the same nucleotide positions present in different isolates from each country. On the basis of this determined sequence, a phylogenetic tree was constructed to determine the relationship among these different isolates and genotypes of HGV. The results showed that in addition to the three known reported HGV genotypes, a cluster of isolates not belonging to any known genotype of HGV was found in five Myanmarese subjects and three Vietnamese subjects in the present study (Fig. 2). In addition, these unclassified HGV isolates were compared with reported HGV/GBV-C isolates for which partial sequences of the 5′ UTR were obtained from databases. The result revealed that none of these isolates was classifiable into any known genotype of HGV. Based on these analyses, we designated this new genotype type 4. The mean values of evolutionary distance between nucleotide sequences within each genotype were less than 0.042 (Table 1). In comparisons between genotypes, the values for distance ranged from 0.084 (for the comparison between types 3 and 4) to 0.161 (for the comparison between types 1 and 4). Bootstrap analysis showed that HGV type 4 was more closely related to type 3 than to types 1 and 2. Of the total of 30 HGV isolates collected from the Myanmarese and Vietnamese subjects, 18 (53%) were type 2, 8 (27%) were type 4, and 4 (13%) were type 3 (Table 2). No type 1 isolate was seen in the present study.
FIG. 1.
Alignment of nucleotide sequences of the HGV 5′ UTR isolated from Myanmarese and Vietnamese subjects. Sources of all isolates with the accession numbers are described in the text. The nucleotide positions are based on those of the HGV PNF2161 isolate. Dashes indicate nucleotides that are identical to the top sequence (HGV PNF2161), and deletions of nucleotides are indicated by slashes. USA, United States.
FIG. 2.
Phylograms generated by neighbor-joining analysis of genetic distances in the 5′ UTR of HGV/GBV-C isolates. Database-derived HGV/GBV-C isolates are presented in italics. The numbers are percentages of bootstrap replicates supporting these branches.
TABLE 1.
Mean values for nucleotide evolutionary distance within and between HGV/GBV-C genotypes in 5′ UTR
| Genotype | na | Genotype
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 1 (West Africa) | 4 | 0.002 | 0.152 | 0.126 | 0.161 |
| 2 (United States/Europe) | 44 | 0.032 | 0.091 | 0.089 | |
| 3 (Asia) | 11 | 0.042 | 0.084 | ||
| 4 (Southeast Asia) | 4 | 0.012 | |||
Number of sequences analyzed.
TABLE 2.
Genotypic distribution of HGV in southeast Asia
| Country | na | No. (%) of HGV isolates with genotype
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Myanmar | 20 | 0 | 12 (60) | 3 (15) | 5 (25) |
| Vietnam | 10 | 0 | 6 (40) | 1 (10) | 3 (30) |
| Total | 30 | 0 | 18 (53) | 4 (13) | 8 (27) |
Number of sequences analyzed.
The investigation of diversity among the sequences of different isolates of a virus is important because variants may differ in such characteristics as their patterns of serologic reactivity, pathogenicity, virulence, and responses to therapy. Although HGV/GBV-C RNAs have been detected in healthy individuals and patients with liver disease, the role of HGV/GBV-C infection in liver diseases is not well understood. It appears that persistent infections with these viruses are common, but they do not seem to be associated with significant liver injuries (2, 3). HGV/GBV-C has also been seen in 7 to 50% of patients with non-A-E acute or fulminant hepatitis (1, 3, 22), but a causal role has not been established. On the other hand, HGV/GBV-C has genetic variation which corresponds to the geographic distribution, and it has been proposed that HGV/GBV-C can be classified into three major genotypes. In the present study, we characterized the 5′ UTR sequences of HGV isolated from serum specimens collected in Myanmar and Vietnam. Although we examined only 148-base sequences in the HGV 5′ UTR, bootstrap resampling and the nucleotide evolutionary distance values provided strong support for the concept that four HGV genotypes exist. This result was also confirmed by phylogenetic analysis by using the full-length sequence of the HGV type 4 genome (data not shown). We intend to report the characterization of the entire sequence of HGV type 4 isolates after further study. These findings suggest that phylogenetic analysis of a 148-base length of the 5′ UTR as used in our investigation is sufficient to perform HGV genotyping.
In conclusion, we identified a novel genotype of HGV that is present in Myanmarese and Vietnamese subjects. It seems likely that HGV type 4 is rather common in the southeastern parts of the Asian continent. Additional studies, including analyses of sequences from other geographic regions, are required for the further classification of HGV/GBV-C. An investigation of worldwide HGV seroepidemiology, including that of Asia, is in progress in our laboratory, and we intend to report the results of our further study in the future.
Nucleotide sequence accession numbers.
The HGV nucleotide sequence data reported in this paper have been submitted to the DDBJ, EMBL, and GenBank databases under accession no. AB013187 for VT61, AB013188 for VT48, AB013189 for VT10, AB013193 for VT2, AB013201 for VT17, AB013203 for VT58, AB013212 for VT40, AB013220 for VT39, AB013232 for VT8, AB013233 for VT5, AB018646 for MY40, AB018647 for MY22, AB018648 for MY72, AB018649 for MY117, AB018650 for MY57, AB018651 for MY67, AB018652 for MY86, AB018653 for MY87, AB018654 for MY107, AB018655 for MY154, AB018656 for MY176, AB018657 for MY177, AB018658 for MY221, AB018659 for MY255, AB018660 for MY267, AB018661 for MY270, AB018662 for MY280, AB018663 for MY283, AB018664 for MY288, and AB018665 for MY289.
Acknowledgments
We thank Takeshi Kurata, National Institute of Infectious Diseases, for his continuous encouragement during this study and Yutaka Takebe, AIDS Research Center at National Institute of Infectious Diseases, for providing valuable serum samples.
This study was supported in part by Grants-in-Aid for Science Research of the Ministry of Education, Science and Culture of Japan and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Abe K, Moriyama M, Hayashi S, Nakai K, Miyauchi I, Edamoto Y, Saito T, Fukushima S, Shimizu T, Matsumura H, Arakawa Y. Prevalence of hepatitis G virus infection among patients with liver diseases in Japan. Int Hepatol Commun. 1997;6:239–248. [Google Scholar]
- 2.Alter H J, Nakatsuji Y, Melpolder J, Wages J, Wesley R, Shih W-K, Kim J P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- 3.Alter M J, Gallagher M, Morris T T, Moyer L A, Meeks E L, Krawczynski K, Kim J P, Margolis H S. Acute non-A-E hepatitis in the United States and the role of hepatitis G virus infection. N Engl J Med. 1997;336:741–746. doi: 10.1056/NEJM199703133361101. [DOI] [PubMed] [Google Scholar]
- 4.Billis D M, Bull J J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol. 1993;42:182–192. [Google Scholar]
- 5.Erker J C, Simons J N, Muerhoff A S, Leary T P, Chalmers M L, Desai S M, Mushahwar I K. Molecular cloning and characterization of a GB virus C isolate from a patient with non-A-E hepatitis. J Gen Virol. 1996;77:2713–2720. doi: 10.1099/0022-1317-77-11-2713. [DOI] [PubMed] [Google Scholar]
- 6.Galtier N, Gouy M, Gautier C. SeaView and Phylo_win, two graphic tools for sequence. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko T, Hayashi S, Arakawa Y, Abe K. Molecular cloning of full-length sequence of hepatitis G virus genome isolated from a Japanese patient with liver disease. Hepatol Res. 1998;12:207–216. [Google Scholar]
- 8.Leary T P, Muerhoff A S, Simons J N, Pilot-Matias T J, Erker J C, Chalmers M L, Schlauder G G, Dawson G J, Desai S M, Mushahwar I K. Sequence and genomic organization of GBV-C: a novel member of the Flaviviridae associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Linnen J, Wages J, Zhang-Keck Z-Y, Fry K, Krawczynski K, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shin J W K, Young L, Piatak M, Hoover C, Fernandez J, Chen S, Zou J-C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 10.Muerhoff A S, Simons J N, Leary T P, Erker J C, Chalmers M L, Pilot-Matias T J, Dawson G J, Desai S M, Mushahwar I K. Sequence heterogeneity within the 5′-terminal region of the hepatitis GB virus C genome and evidence for genotypes. J Hepatol. 1996;25:379–384. doi: 10.1016/s0168-8278(96)80125-5. [DOI] [PubMed] [Google Scholar]
- 11.Muerhoff A S, Smith D B, Leary T P, Erker J C, Desai S M, Mushahwar I K. Identification of GB virus C variants by phylogenetic analysis of 5′-untranslated and coding region sequence. J Virol. 1997;71:6501–6508. doi: 10.1128/jvi.71.9.6501-6508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakao H, Okamoto H, Fukuda M, Tsuda F, Mitsui T, Masuko K, Iizuka H, Miyakawa Y, Mayumi M. Mutation rate of GB virus C/hepatitis G virus over the entire genome and in subgenomic regions. Virology. 1997;233:43–50. doi: 10.1006/viro.1997.8615. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto H, Nakao H, Inoue T, Fukuda M, Kishimoto J, Iizuka H, Tsuda F, Miyakawa Y, Mishiro M. The entire nucleotide sequence of two GB virus C/hepatitis G virus isolates of distinct genotypes from Japan. J Gen Virol. 1997;78:737–745. doi: 10.1099/0022-1317-78-4-737. [DOI] [PubMed] [Google Scholar]
- 14.Orito E, Mizokami M, Nakano T, Wu R R, Cao K, Ohba K, Ueda R, Mukaide M, Hikiji K, Matsumoto Y, Iino S. GB virus C/hepatitis G virus infection among Japanese patients with chronic liver diseases and blood donors. Virus Res. 1996;46:89–93. doi: 10.1016/s0168-1702(96)01379-2. [DOI] [PubMed] [Google Scholar]
- 15.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 16.Rzhetsky A N. Tests of applicability of several substitution models for DNA sequence data. Mol Biol Evol. 1995;12:131–151. doi: 10.1093/oxfordjournals.molbev.a040182. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Ishikawa K, Osei-Kwasi M, Kaneko T, Brandful J A M, Nuvor V, Aidoo S, Ampofo W, Apeagyei F A, Ansah J E, Adu-Sarkodie Y, Nkrumah F K, Abe K. Prevalence of hepatitis G virus and characterization of viral genome in Ghana. Hepatol Res. 1999;13:221–231. [Google Scholar]
- 18.Saito T, Shiino T, Arakawa Y, Hayashi S, Abe K. Geographical characterization of hepatitis G virus genome: evidence for HGV genotypes based on phylogenetic analysis. Hepatol Res. 1998;10:121–130. [Google Scholar]
- 19.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–423. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 20.Shao L, Shinzawa H, Ishikawa K, Zhang X, Ishibashi M, Misawa H, Yamada N, Togashi H, Takahashi T. Sequence of hepatitis G virus genome isolated from a Japanese patient with non-A-E hepatitis: amplification and cloning by long reverse transcription-PCR. Biochem Biophys Res Commun. 1996;228:785–791. doi: 10.1006/bbrc.1996.1732. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Hijikata M, Hino K, Mishiro S. Entire polyprotein-ORF sequence of Japanese GBV-C/HGV isolates: implications for new genotypes. Hepatol Res. 1997;8:139–148. [Google Scholar]
- 22.Yoshiba M, Okamoto H, Mishiro S. Detection of the GBV-C hepatitis virus genome in serum from patients with fulminant hepatitis of unknown aetiology. Lancet. 1995;346:1131–1132. doi: 10.1016/s0140-6736(95)91802-7. [DOI] [PubMed] [Google Scholar]
- 23.Zuckerman A J. Alphabet of hepatitis viruses. Lancet. 1996;347:558–559. doi: 10.1016/s0140-6736(96)91267-2. [DOI] [PubMed] [Google Scholar]