Abstract
Simple Summary
The gastrointestinal microbiome of domestic cats can utilise dietary fibre to produce beneficial fermentation end products such as butyrate, an energy source for intestinal cells. However, domestic cats are obligate carnivores and therefore may not require plant derived dietary fibre. It has been hypothesised that in the wild, consumption of animal-derived substrates such as hair and cartilage may be fermented by the cat’s gastrointestinal microbiome, producing butyrate and other beneficial end products. Therefore, the aim of this study was to use a laboratory digestion and fermentation model (simulating the digestion process) to determine the concentrations of butyrate produced by various animal-derived substrates, including hydrolysed collagen, hair, and cartilage. Faecal samples were used in batch cultures to represent the microbes in the cat’s colon. Faecal samples came from cats fed either high protein or high carbohydrate diets. The type of faeces used was found to affect the fermentation profile produced, likely due to differing microbial community compositions, at the start of the batch cultures. Microbes present in high protein faeces fermented the hydrolysed collagen substrate, producing increased concentrations of butyrate. This finding has wider implications for use of animal-derived substrates in species-appropriate diet formulations.
Abstract
The gastrointestinal microbiome has a range of roles in the host, including the production of beneficial fermentation end products such as butyrate, which are typically associated with fermentation of plant fibres. However, domestic cats are obligate carnivores and do not require carbohydrates. It has been hypothesised that in the wild, collagenous parts of prey—the so-called animal-derived fermentable substrates (ADFS) such as tendons and cartilage—may be fermented by the cat’s gastrointestinal microbiome. However, little research has been conducted on ADFS in the domestic cat. Faecal inoculum was obtained from domestic cats either consuming a high carbohydrate (protein:fat:carbohydrate ratio of 35:20:28 (% dry matter basis)) or high protein (protein:fat:carbohydrate ratio of 75:19:1 (% dry matter basis)) diet. ADFS (hydrolysed collagen, cat hair, and cartilage) were used in a series of static in vitro digestions and fermentations. Concentrations of organic acids and ammonia were measured after 24 h of fermentation, and the culture community of microbes was characterised. The type of inoculum used affected the fermentation profile produced by the ADFS. Butyrate concentrations were highest when hydrolysed collagen was fermented with high protein inoculum (p < 0.05). In contrast, butyrate was not detectable when hydrolysed collagen was fermented in high carbohydrate inoculum (p < 0.05). The microbiome of the domestic cat may be able to ferment ADFS to provide beneficial concentrations of butyrate.
Keywords: in vitro, fermentation, butyrate, feline, collagen, faecal donor
1. Introduction
Domestic cats are obligate carnivores and do not require carbohydrates to fulfil their energy requirements [1]. However, consumption of dietary fibre may be important for their gastrointestinal microbiota, which has been associated with host health, nutrition, and overall wellbeing [2]. It has been hypothesised that in the wild, consumption of the collagenous parts of prey (such as skin, tendons, and cartilage) may act as a form of dietary fibre, termed herein as animal-derived fermentable substrates (ADFS).
The gastrointestinal microbiome of domestic cats is able to utilise dietary fibre (such as inulin) to produce beneficial fermentation end products, namely organic acids such as acetate, butyrate, and propionate (short chain fatty acids, SCFA) [3]. In human and rodent models, butyrate is an energy source for colonocytes and, therefore, greatly beneficial for the host [4,5]. Conversely, ammonia (from bacterial proteolysis) is typically thought to be detrimental to colonocytes if concentrations become too high in the intestinal lumen, as it inhibits mitochondrial oxygen consumption and SCFA oxidation [6,7]. Therefore, it is of interest to understand if the gastrointestinal microbiota can ferment ADFS in a similar manner to dietary fibre and produce similarly beneficial end products.
In vitro digestion and fermentation models are often used to replace or minimise the use of animal models. They have been used to assess fermentation characteristics of dietary fibre [8,9,10] by assessing factors such as gas production and fermentation end products. Research has shown that the source of the faecal inoculum used in in vitro experiments determines the fermentation end products produced because the microbiota present are pre-determined [11]. This is dictated predominantly by the diet consumed by the donor, which determines the microbiota that are able to utilise the substrates provided.
Depauw, et al. [12] assessed the in vitro fermentation of various ADFS (including hydrolysed collagen, rabbit skin, hair, and bone) using faecal inoculum from a cheetah (Acinonyx jubatus) that had consumed a whole rabbit diet. They observed that the microbiota present in the faecal inoculum were able to ferment collagen, producing similar amounts of organic acids per gram of organic matter (OM) to those resulting from the fermentation of fructo-oligosaccharide (FOS). This finding introduces the possibility that ADFS may fulfil the role of dietary fibre in obligate carnivores. A recent study by Deb-Choudhury, et al. [13] also showed that a wool hydrolysate could be fermented by the cat (Felis catus) in vivo. This suggests that a range of compounds could act as ADFS for the cat.
The aim of this study was to evaluate the fermentability of ADFS, namely, hydrolysed collagen, cartilage (fresh and freeze-dried), and cat hair (intact and chopped). This was carried out through assessment of organic acids produced by fermentation of the ADFS with faecal inoculum for a period of 24 h, which is the average transit time of digesta for young cats [14]. The ADFS were compared against inulin and cellulose as positive and negative controls, respectively. Two sources of faecal inoculum were used: one from donor cats consuming a high protein diet, and the other from donor cats consuming a high carbohydrate diet.
It was hypothesised that ADFS would be fermented by the microbiota present in the faecal inoculum. Furthermore, we hypothesised that microbiota present in the faeces of the cats fed a protein rich diet would be able to metabolise the protein-rich substrates readily, as opposed to the microbiota present in the faeces of cats fed a carbohydrate-rich diet.
2. Materials and Methods
This protocol was approved by the Massey University Animal Ethics Committee (MUAEC Protocol 18/08). Two cohorts of cats were used to provide the faecal inoculum for this study. All cats were housed at the Massey Centre for Feline Nutrition (Palmerston North, New Zealand) for the duration of the collection period.
2.1. Faecal Inoculum Collection
The cats providing the faecal inoculum were maintained on their nutritionally complete and balanced diets for a minimum of 21 days prior to faecal collection. They were healthy adult domestic short-haired cats of both male and female sexes, and aged between 2–8 years. The cohort (n = 8) providing the high-carbohydrate (CD) faecal inoculum was fed a diet with a protein:fat:carbohydrate ratio of 35:20:28 (% DM basis, Nutro™, MARS Petcare, Raglan, NSW, Australia). Cats providing the high protein inoculum (PD) were fed a diet with a protein:fat:carbohydrate ratio of 75:19:10 (% DM basis, Chef, Kraft Heinz Wattie’s, Hastings, New Zealand). Cats were housed in individual cages (80 cm × 80 cm × 110 cm) until defecation occurred. The faeces were collected within ten minutes of voiding, snap-frozen in liquid nitrogen, then stored at −80 °C before use in faecal fermentation studies.
2.2. Substrates
A range of substrates was evaluated, including two forms of hydrolysed collagen: Peptan B (‘PHC’; Peptan B 2000 LD: Rosselott, New Zealand) and a hydrolysed bovine skin product (‘AHC’; ANZCO Foods, Christchurch, New Zealand). Individual rings of bovine tracheal cartilage were isolated from the trachea, connective tissue removed, and the cartilage sliced into ~40 mm × 10 mm fragments (‘fresh cartilage’). A subset of the cartilage was minced (Wolfking, Leingarten, Germany) before being freeze-dried (‘freeze-dried cartilage’). Hair obtained from domestic cats as part of normal grooming practices was collected from the Centre for Feline Nutrition (Massey University, Palmerston North). The hair was either left intact (‘intact cat hair’) or chopped up into ~0.5 cm length (‘chopped cat hair’). ‘inulin’ (Orafti Synergy 1®, Benuo, Belgium) and ‘cellulose’ (Avicel®, Hawkins Watts, New Zealand) were chosen as positive and negative controls, respectively, for fermentation.
2.3. In Vitro Digestion
In vitro digestion was undertaken using the static model method published by Minekus, et al. [15] which was modified to represent the physiology of the domestic cat; temperature was adjusted to 39 °C (domestic cat body temperature), the pH of simulated gastric fluid (SGF) was reduced to 2.5 (domestic cat stomach pH), and the oral phase was removed from the study, as cats lack salivary amylase [16].
A single batch was performed, including all substrates. A 5 g aliquot of substrate was added to 10 mL of SGF (Table A1) and 1 mL of 2% pepsin stock (20 mg/mL SGF) in a Schott bottle. Next, 300 mM CaCl2 was added, pH was corrected to 2.5 as required and the mixture vortexed for 60 s at 600 rpm to ensure thorough mixing. The bottles were then placed in a shaking incubator (65 rpm) and incubated at 39 °C for two hours. Simulated intestinal fluid (SIF: Table A1) (11 mL at pH 7) was then added along with 2.5 mL of 16 mM bile stock (Sigma-Aldrich, New Zealand), 40 µL of 300 mM CaCl2, 3 mL of reverse osmosis (RO) water and approximately 20–40 µL of 1 M sodium hydroxide (NaOH) until the pH was stabilised at 6.5. The mixture was then returned to the shaking incubator for a further 10 min. The bottles were subsequently removed and 5 mL of porcine pancreatin solution (800 U/mL in SIF solution) (Table A1) was added before they were returned to the shaking incubator for a further two hours. To deactivate the enzymes, following incubation, all Schott bottles were microwaved for 90 s, then put on ice.
Retentate was then dialysed as per manufacturer’s instructions (Spectra/Por Dialysis membrane, 100–500 D, Biotech CE Tubing: Pacific Laboratory Products Pty Ltd., Auckland, New Zealand). Briefly, dialysis tubes were then placed in RO water and stored in a cold room at 4 °C, with water changed three times in 24 h. The resulting retentate was removed from the dialysis tubing and stored at −20 °C before use in the in vitro fermentation.
2.4. In Vitro Fermentation
Each substrate was fermented in triplicate for 0, 4, 8, 12, and 24 h per faecal inoculum in autoclaved 5 mL Hungate tubes. Tubes were pre-warmed in a shaking incubator at 39 °C.
Defrosted retentate tubes were mixed thoroughly. An aliquot of phosphate buffer (pH 6.8) was added, mixed thoroughly, then bubbled with nitrogen for one minute to remove the dissolved oxygen present. The bottles were left for a further minute before 3% L-cysteine was added.
A 10% faecal solution was prepared from the PD and CD inocula using sodium phosphate buffer and were manually strained through a mesh bag (MicroAnalytix, Auckland, New Zealand). An aliquot of the inoculum to be tested was immediately pipetted into Eppendorf tubes kept on ice to represent the 0-h sample. For the other timepoints, PD or CD inoculum was added to Hungate tubes containing the allocated substrates. Each tube was subsequently topped with a layer of carbon dioxide, capped, and placed in a shaking incubator at 39 °C for either 4, 8, 12, or 24 h. Upon reaching the allocated time point, tubes were removed from the incubator, placed onto ice, vortexed, and centrifuged at 14,000× g for 15 min. The resulting supernatant and pellet were frozen immediately at −80 °C.
2.5. Laboratory Analysis
2.5.1. Macronutrient Profiles of Substrate
Moisture content of the substrate was determined using a convection oven at 105 °C, and ash residue at 550 °C (AOAC 930.15/925.10/942.10). Crude fat was analysed using the Soxtec 8000 meat extraction methodology (AOAC 991.36). Crude fibre was analysed using the non-enzymatic gravimetric method (AOAC 962.09/978.10). Total, soluble, and insoluble dietary fibre were calculated using the Megaenzyme assay (AOAC 991.43). Gross energy was measured using bomb calorimetry.
Nitrogen was measured using the Dumas method (AOAC 968.06). Typically, a conversion factor of 6.25 is applied to the nitrogen content of a sample to obtain the crude protein content. For the purposes of this study, the conversion factor of gelatin, 5.55, was applied to AHC, PHC (collectively denoted as hydrolysed collagen due to their similar compositions), and cat hair to account for differences in protein content [17] (Table 1). NFE were calculated by difference; 100 − (crude protein + crude fat + crude fibre + ash).
Table 1.
Nutrient composition of substrates used for in vitro digestion and fermentation.
| Substrates | |||||||
|---|---|---|---|---|---|---|---|
| Component | AHC | PHC | Freeze-Dried Cartilage | Fresh Cartilage | Cat Hair | Inulin | Cellulose |
| Gross energy (kJ/g) | 20.58 | 21.33 | 22.61 | 18.31 | 23.01 | 16.58 | 16.65 |
| Ash (% DM) | 6.11 | 0.98 | 5.08 | 9.15 | 1.08 | 0.1 | 0.11 |
| Crude Protein (% DM) | 89.70 ¶ | 97.48 ¶ | 75.21 | 69.92 | 82.20 ¶ | 0.21 | 0.21 |
| Crude Fat (% DM) | 0.54 | 0.44 | 13.28 | 1.69 | 5.59 | 2.52 | 0.11 |
| Crude Fibre (% DM) | 0.11 | 0.11 | 1.14 | 0.34 | 0.75 | 0.1 | 65.65 |
| NFE 1 (% DM) | 3.55 | 0.98 | 5.29 | 18.90 | 10.38 | 97.06 | 33.93 |
| Total Dietary Fibre (% DM) | ND | 0.11 | 12.34 | 22.03 | 3.23 | † | 100 |
| Insoluble Dietary Fibre (% DM) | ND | 0.11 | 5.08 | 2.37 | 3.23 | † | 100 |
| Soluble Dietary Fibre (% DM) | 0.86 | ND | 7.26 | 19.66 | ND | † | ND |
1 NFE—Nitrogen Free Extract, calculated by difference (100 − crude protein + crude fat + crude fibre + ash). ND—Not detected. † Not assayed. ¶ Substrates were converted from nitrogen to protein using the conversion factor 5.55.
2.5.2. Organic Acids and Ammonia
Supernatant was diluted 1:5 with PBS containing 2-ethylbutyric acid as an internal standard as previously described [18]. Briefly, aqueous extracts were acidified, phase separated into diethyl ether and stored at −80 °C until analysis. Organic acids were derivatised with N-tert-butyldimethylsilyl-N-methyl trifluoroacetamide plus 1% tert-butyldimethylchlorosilane (MTBSTFA + TBDMSCI, 99:1; Sigma-Aldrich, Auckland, New Zealand) and analysed on a Shimadzu capillary GC system (GC-2010 Plus, Tokyo, Japan) equipped with a flame ionization detector (FID) and fitted with a Restek column (SH-Rtx-1, 30 m × 0.25 mm ID × 0.25 µm) using helium as the carrier gas. The GC-FID was controlled, and data processed, using a Shimadzu GC Work Station LabSolutions Version 5.3, with sample organic acids quantified in reference to authentic standards. Ammonia was measured using the phenol-nitroprusside method [19].
2.5.3. 16S rRNA Amplicon Sequencing
Metagenomic DNA was extracted from the pellet using NucleoSpin Soil kits (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions, with modifications as follows. The DNA pellet was defrosted and centrifuged at 15,000× g for 3 min. Any remaining supernatant was removed and discarded. Lysis buffer and enhancer were added to the DNA pellet and gently mixed, then the mixture was transferred into a bead beating tube and mixed thoroughly using a Mini-Beadbeater-96 (BioSpec Products, Bartlesville, OK, USA) for four minutes.
Microbial profiles were determined by analysis of the V3 to V4 region of the bacterial 16S rRNA gene using Illumina MiSeq paired-end 2 × 250 base pair amplicon sequencing [20]. QIIME 1.8 was used to process sequences, with quality filtering of reads undertaken using the default settings. Forward and reverse reads were subsequently joined using the ‘join_paired_ends’ function [21]. The USEARCH method was used to align reads to the Greengenes database and identify chimeric sequences, which were subsequently removed from further analysis. UCLUST was used to cluster sequences at 97% similarity into OTUs, which were then assigned taxonomy using RDP classifier.
2.6. Statistical Analysis
All analyses were completed using R version 3.6.0 [22].
2.6.1. Organic Acids and Ammonia
To determine changes to organic acid and ammonia concentrations over time, triplicate samples were plotted according to substrate by faecal inoculum using the R package ‘ggplot2’. Samples from the 0-h time point were removed from this analysis as there were only six samples at this time point (only from the control group) and, therefore, could not be plotted for each substrate. Scatterplots and mean lines were used to visualise the data. Heptanoate, hexanoate isobutyrate, isovalerate, and valerate were below the limit of detection in all samples; therefore, they were excluded from any analyses.
Samples were split by faecal inoculum (high protein and high carbohydrate), and only the 24-h time point was assessed as the final endpoint of fermentation. Model-based predicted means, standard errors, and post hoc multiple comparisons were calculated using the ‘predictmeans’ package [23]. Multiple comparison p-values were adjusted using the “Tukey” method and are presented as letter-based comparisons. Assumptions of homogeneity and normality were met. Unless otherwise stated, data are presented as mean +/− standard error of the mean (SEM).
2.6.2. Bacterial Profile of In Vitro Fermentation
The 221 samples analysed had a mean sequencing depth of 21,206 reads. One sample was removed from the dataset (a 24-h intact cat hair CD) because of extremely low read numbers (<2500 reads), leaving n = 111 PD inoculum samples and n = 110 CD inoculum samples for analysis. To consolidate the data into phyla and genera, the R mixOmics package was used. Taxa of low relative abundance were removed (taxa present at <0.0005% relative abundance in six or more samples). At the phylum level, a total of 25 phyla were observed before filtering, and seven remained afterwards. Before filtering, a total of 482 bacterial taxa were observed at the genera level in these samples. After filtering rare taxa, 98 genera remained, which provided the dataset for all further analyses. Chao1 diversity index was used to assess alpha diversity with Kruskal–Wallis analysis to assess significant differences between the substrates.
Permutation ANOVA was used to determine changes by ‘Substrate’ and ‘Faecal Inoculum’ as covariates. Interaction between Substrate and Faecal Inoculum were then investigated. FDR < 0.05 was considered statistically significant. The top 30 most relatively abundant taxa present in each substrate at 24 h of in vitro fermentation were then visualised using bar plots in R using the ggplot2 package. Principal component analysis was also used to assess the beta diversity of bacterial genera.
3. Results
3.1. High Protein Faecal Inoculum (PD)
3.1.1. Organic Acid and Ammonia Profiles
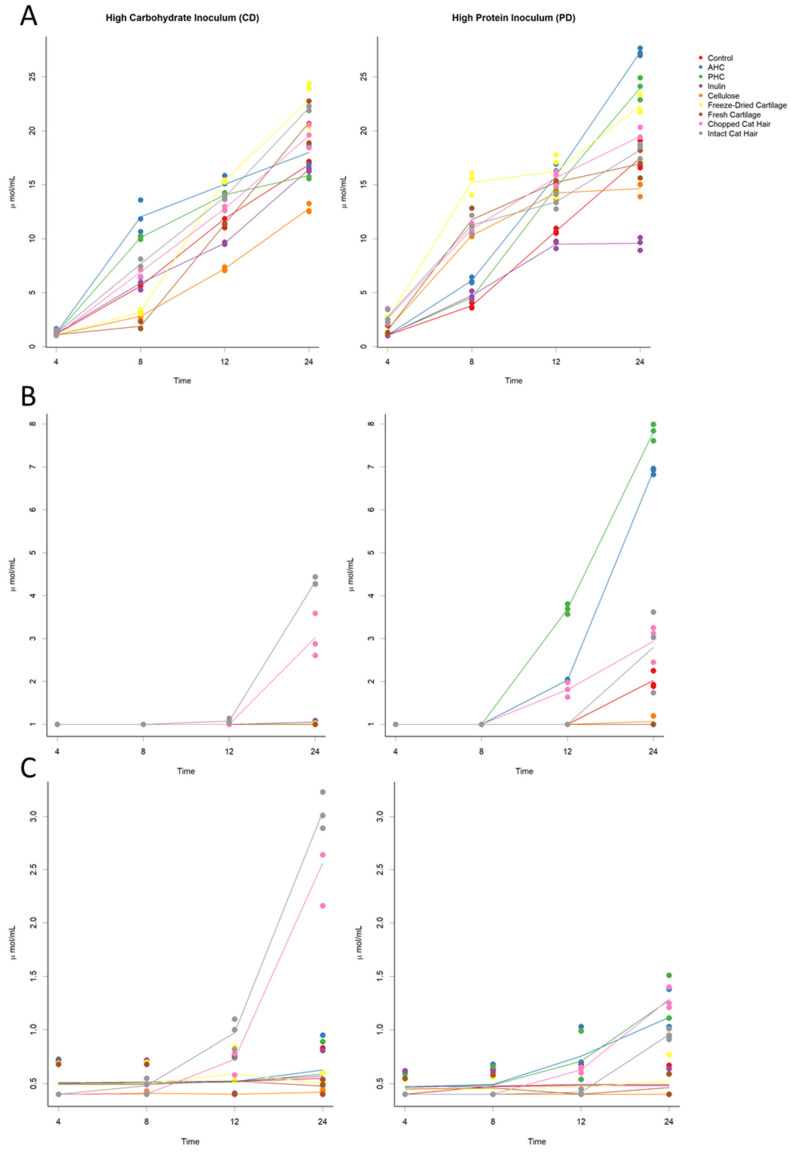
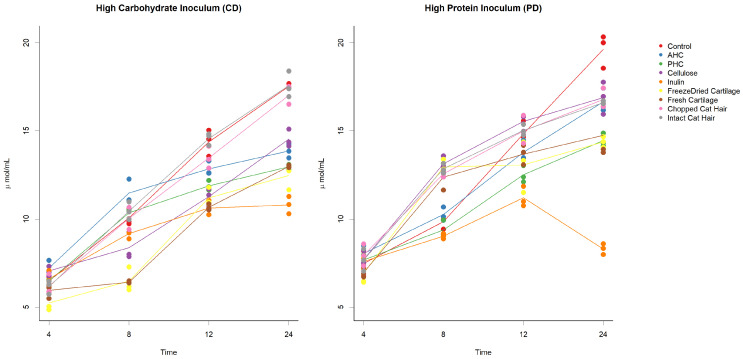
Organic acid profiles of each substrate across the 24-h sampling period of in vitro fermentation are shown in Figure 1A–C. The substrate being fermented affected the concentration of fermentation end products (Table 2). Fermentation of AHC and PHC for 24 h produced the greatest concentration of butyrate in comparison to the other substrates (p < 0.05) (Figure 1B). Fermentation of cat hair also produced significantly higher concentrations of butyrate and propionate (p < 0.05). In vitro fermentation in the control group produced the highest mean concentration of ammonia (p < 0.05); 19.6 ± 0.544 mM (Figure 2). The lowest mean concentration of ammonia was produced by the fermentation of inulin; 8.2 ± 0.178 mM (Table 2).
Figure 1.
(A–C). Scatter plots with mean lines of (A) acetate (B) butyrate and (C) propionate concentrations. Time (hours) is along the x-axis and organic acid concentration along the y-axis (µmol/mL). Each graph depicts both high protein faecal inoculum (PD) and high carbohydrate faecal inoculum (CD) and the changes that occurred over 24 h of fermentation. Each point (coloured circle) represents an individual replicate, and each line shows the mean for each substrate. Control samples are denoted by a red line, AHC (ANZCO hydrolysed collagen) by a blue line, PHC (Peptan hydrolysed collagen) by a green line, cellulose by an orange dashed line, inulin by a purple line, freeze-dried cartilage by a yellow line, fresh cartilage by a brown line, chopped cat hair by a pink line, and intact cat hair by a grey line.
Table 2.
Predicted means and associated standard error of the mean of organic acids and ammonia concentrations following in vitro fermentation of each substrate at the 24-h time point in the PD (high protein) faecal inoculum. Letter-based representation of pairwise comparisons is presented. Different letters indicate differences at significance level 0.05, adjusted using the Tukey method. AHC; ANZCO hydrolysed collagen. PHC; Peptan hydrolysed collagen.
| Acetate (µmol/mL) | Butyrate (µmol/mL) | Propionate (µmol/mL) | Total SCFA (µmol/mL) | |||||||||
| Substrate | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison |
| Control | 17.47 | 0.812 | C | 2.02 | 0.114 | BC | 0.49 | 0.090 | C | 30.82 | 0.362 | CD |
| AHC | 27.28 | 0.196 | A | 6.90 | 0.043 | A | 1.12 | 0.134 | A | 45.23 | 1.223 | A |
| PHC | 23.96 | 0.593 | B | 7.81 | 0.111 | A | 1.28 | 0.120 | A | 39.15 | 0.308 | B |
| Cellulose | 14.64 | 0.372 | D | ND | ND | C | ND | ND | C | 26.43 | 2.302 | D |
| Freeze-dried Cartilage | 22.33 | 0.513 | B | ND | ND | C | 0.52 | 0.123 | BC | 38.14 | 1.288 | B |
| Fresh Cartilage | 16.94 | 0.743 | CD | ND | ND | C | 0.46 | 0.063 | C | 31.77 | 0.638 | CD |
| Chopped cat hair | 19.48 | 0.469 | C | 2.94 | 0.248 | B | 1.29 | 0.058 | A | 33.46 | 0.980 | BC |
| Intact cat hair | 18.22 | 0.413 | C | 2.80 | 0.055 | B | 0.96 | 0.029 | AB | 31.53 | 1.326 | CD |
| Inulin | 9.56 | 0.343 | E | ND | ND | C | 0.48 | 0.080 | C | 20.09 | 0.664 | E |
| Formate (µmol/mL) | Lactate (µmol/mL) | Succinate (µmol/mL) | Ammonia (µmol/mL) | |||||||||
| Substrate | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison |
| Control | 10.71 | 0.681 | AB | 2.66 | 1.260 | B | 0.673 | 0.037 | C | 19.63 | 0.544 | A |
| AHC | 9.81 | 1.026 | ABC | 2.04 | 0.905 | B | 1.523 | 0.050 | B | 16.65 | 0.392 | B |
| PHC | 5.99 | 0.783 | C | 1.09 | 0.430 | B | 2.310 | 0.026 | A | 14.45 | 0.219 | CD |
| Cellulose | 10.22 | 1.895 | ABC | 2.15 | 0.162 | B | 0.533 | 0.123 | C | 16.89 | 0.525 | B |
| Freeze-dried Cartilage | 14.18 | 0.902 | A | 3.12 | 1.412 | B | 1.493 | 0.103 | B | 14.37 | 0.174 | D |
| Fresh Cartilage | 13.27 | 0.644 | AB | 2.07 | 0.886 | B | 1.300 | 0.072 | B | 14.75 | 0.892 | BCD |
| Chopped cat hair | 9.66 | 0.557 | ABC | 3.11 | 0.090 | B | 1.433 | 0.126 | B | 16.78 | 0.329 | B |
| Intact cat hair | 9.45 | 0.507 | BC | 1.71 | 0.448 | B | 1.180 | 0.120 | B | 16.60 | 0.049 | BC |
| Inulin | 8.94 | 0.762 | BC | 75.79 | 8.011 | A | 1.167 | 0.044 | B | 8.30 | 0.178 | E |
Figure 2.
Scatter plots with mean lines of ammonia concentrations. Time (hours) is along the x-axis and organic acid concentration along the y-axis (mM). Each graph depicts both high protein faecal inoculum (PD) and high carbohydrate faecal inoculum (CD) and the changes that occurred over 24 h of fermentation. Each point (coloured circle) represents an individual replicate, and each line shows the mean for each substrate. Control samples are denoted by a red line, AHC (ANZCO hydrolysed collagen) by a blue line, PHC (Peptan hydrolysed collagen) by a green line, cellulose by an orange dashed line, inulin by a purple line, freeze-dried cartilage by a yellow line, fresh cartilage by a brown line, chopped cat hair by a pink line, and intact cat hair by a grey line.
3.1.2. Culture Community of High Protein Faecal Inoculum
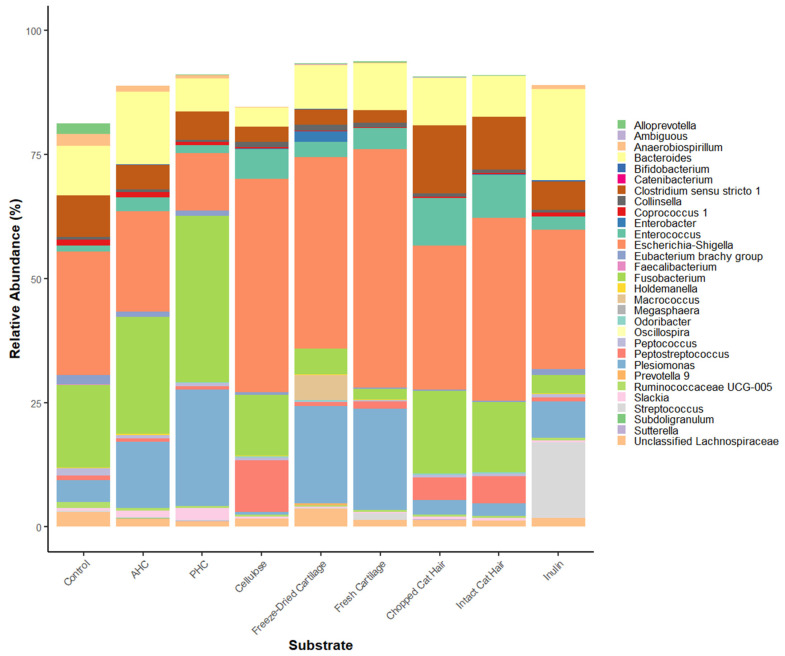
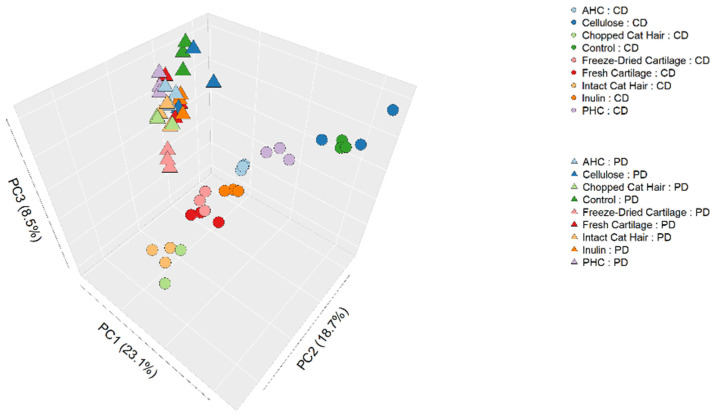
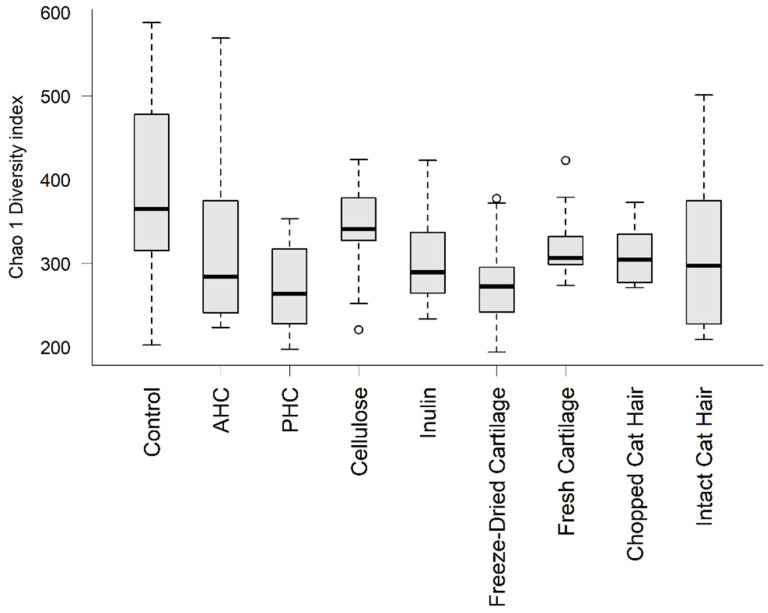
The effect of the substrate on the culture microbial community varied according to the source of the faecal inoculum (Figure A1). Two-way permutation ANOVA of 76 taxa with substrate and inoculum type as factors showed that both these factors had large effects on the microbial community with significant interactions between substrate and inoculum source (FDR < 0.05). Of these 76, taxa with relative abundance <1% across all substrates were filtered out for the purposes of displaying using bar plots, leaving 30 dominant taxa across all samples (Figure 3). Fusobacterium was the most dominant genera in AHC (23%) and PHC (33%) samples (Figure 3). Escherichia–Shigella was the most relatively abundant taxa (>20%) present in all other substrates (Figure 3). Chao1 alpha diversity assessment observed significant differences between the substrates (p = 0.012; Figure A2).
Figure 3.
Barplot of the relative abundance of the top 30 bacterial genera present at 24 h in the in vitro fermentation system of the high protein faecal inoculum (PD) according to substrate. Each colour in the bar plot represents a bacterial genus, with the size of the bar denoting their relative abundance.
3.2. High Carbohydrate Faecal Inoculum (CD)
3.2.1. Organic Acid and Ammonia Profiles
Organic acid profiles of each substrate across the 24-h sampling period of in vitro fermentation are shown in Figure 1A–C. The substrate being fermented affected the concentration of fermentation end products (Table 3). After 24 h, fermentation of chopped and intact cat hair produced the greatest (p < 0.05) concentration of butyrate and propionate when compared to the other substrates (Figure 1B,C). Fermentation of inulin produced the highest (p < 0.05) concentration of lactate, compared to the other substrates (Supplementary Figure S1; Table 3), although freeze-dried and fresh cartilage produced significantly greater amounts of lactate than the control group (p < 0.05).
Table 3.
Predicted means and associated standard error of the mean of organic acids and ammonia concentrations following in vitro fermentation of each substrate at the 24-h time point in the CD (high carbohydrate) faecal inoculum. Letter-based representation of pairwise comparisons is presented. Different letters indicate differences at significance level 0.05, adjusted using the Tukey method. AHC; ANZCO hydrolysed collagen. PHC; Peptan hydrolysed collagen.
| Acetate (µmol/mL) | Butyrate (µmol/mL) | Propionate (µmol/mL) | Total SCFA (µmol/mL) | |||||||||
| Substrate | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison |
| Control | 16.867 | 0.218276 | DE | ND | ND | C | 0.55 | 0.140 | B | 30.46 | 1.243959 | BCD |
| AHC | 17.99 | 0.555908 | CDE | ND | ND | C | 0.63 | 0.166 | B | 32.69 | 2.010075 | ABC |
| PHC | 15.85 | 0.20664 | EF | ND | ND | C | 0.59 | 0.152 | B | 28.1233 | 0.902244 | CD |
| Cellulose | 12.783 | 0.239188 | F | ND | ND | C | 0.42 | 0.012 | B | 24.24 | 0.574717 | D |
| Freeze-dried Cartilage | 22.873 | 1.264283 | A | ND | ND | C | 0.51 | 0.058 | B | 37.5167 | 1.573503 | A |
| Fresh Cartilage | 20.767 | 1.120897 | ABC | ND | ND | C | 0.48 | 0.041 | B | 36.7867 | 2.106255 | AB |
| Chopped cat hair | 19.54 | 0.62426 | BCD | 3.027 | 0.292252 | B | 2.56 | 0.214 | A | 30.4733 | 1.140663 | BCD |
| Intact cat hair | 22.127 | 0.139084 | AB | 4.33 | 0.555108 | A | 3.04 | 0.100 | A | 34.5367 | 0.398427 | ABC |
| Inulin | 16.417 | 0.096148 | DE | 1.053 | 0.027285 | C | 0.57 | 0.123 | B | 31.8 | 0.831164 | ABC |
| Formate (µmol/mL) | Lactate (µmol/mL) | Succinate (µmol/mL) | Ammonia (mM) | |||||||||
| Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison | Mean | SEM | Comparison | |
| Control | 11.933 | 1.281748 | AB | 0.783 | 0.269093 | C | 0.517 | 0.048 | D | 17.533 | 0.0805 | A |
| AHC | 12.963 | 1.413416 | AB | 0.563 | 0.165965 | C | 0.997 | 0.033 | C | 13.859 | 0.2324 | BC |
| PHC | 10.573 | 1.068431 | AB | 0.743 | 0.279543 | C | 1.320 | 0.032 | C | 12.958 | 0.0869 | C |
| Cellulose | 9.937 | 0.346041 | B | 1.04 | 0.280951 | C | 0.59 | 0.038 | D | 14.533 | 0.2932 | B |
| Freeze-dried Cartilage | 12.977 | 0.302067 | AB | 5.487 | 0.086859 | B | 2.2 | 0.040 | A | 12.463 | 0.41347 | C |
| Fresh Cartilage | 14.393 | 1.083349 | A | 5.183 | 1.036345 | B | 2.287 | 0.168 | A | 12.983 | 0.03876 | C |
| Chopped cat hair | 5.243 | 0.188532 | C | 2.373 | 0.315454 | C | 0.37 | 0.070 | D | 17.02 | 0.4105 | A |
| Intact cat hair | 4.937 | 0.127061 | C | 2.167 | 0.384765 | C | ND | ND | D | 17.578 | 0.43091 | A |
| Inulin | 13.65 | 0.700595 | AB | 22.593 | 0.929737 | A | 1.717 | 0.046 | B | 10.799 | 0.2829 | D |
In vitro fermentation in the control group and of cat hair substrates produced the highest mean concentration of ammonia (c.17 ± 0.081 mM; p < 0.05) (Figure 2). The lowest concentration of ammonia was produced by the fermentation of inulin; 10.8 ± 0.283 mM (p < 0.05) (Table 3; Figure 2).
3.2.2. Culture Community of High Carbohydrate Faecal Inoculum
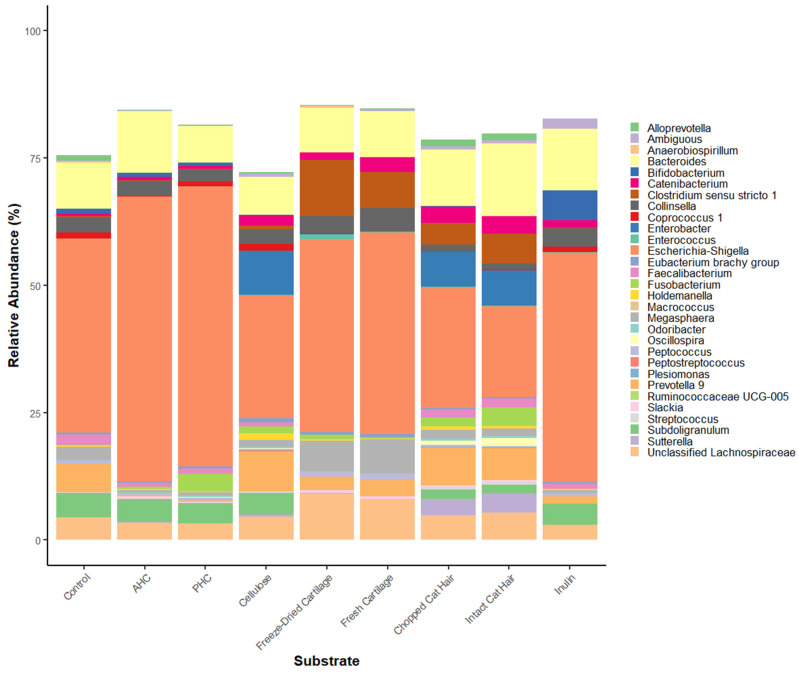
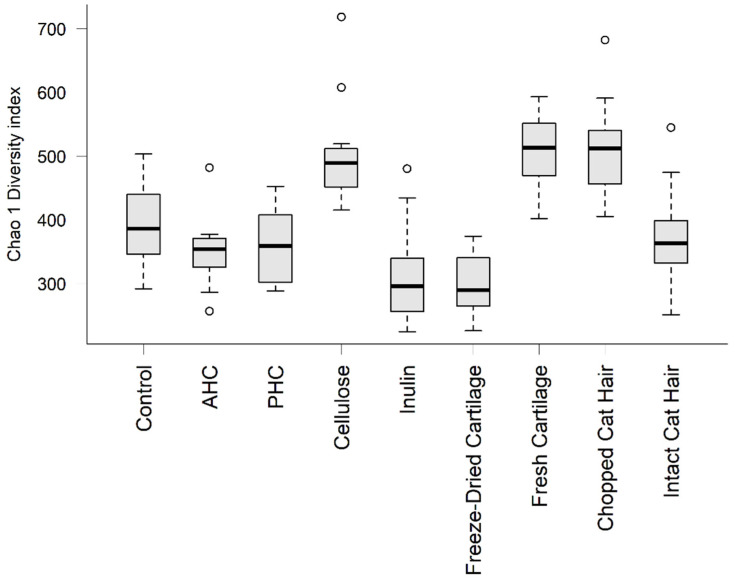
At 24 h, Escherichia–Shigella was the taxon with the greatest relative abundance ranging from 17% of sequence reads in the intact cat hair samples, to 55% in the AHC and PHC samples (Figure 4). The next most relatively abundant taxon in the AHC and PHC samples was Bacteroides (12% and 7%, respectively). Bacteroides also had the second-greatest relative abundance in the chopped and intact cat hair samples (11% and 14%, respectively) followed by Prevotella 9 (c.7%) (Figure 4). Chao1 alpha diversity assessment observed significant differences between the substrates (p < 0.05; Figure A3).
Figure 4.
Barplot of the relative abundance of the top 30 bacterial genera present at 24 h in the in vitro fermentation system of the high carbohydrate faecal inoculum (CD) according to substrate. Each colour in the bar plot represents a bacterial genus, with the size of the bar denoting their relative abundance.
4. Discussion
This study aimed to assess a variety of ADFS to determine their fermentation end products, and subsequently identify which of them may confer a beneficial organic acid profile (i.e., increased butyrate and decreased ammonia). All substrates studied, except cellulose, were readily fermented by the feline inoculum. However, as expected, the type of faecal inoculum (PD or CD) had a major impact on the culture communities and fermentation profiles observed. The fermentation of hydrolysed collagen (both AHC and PHC) produced the highest butyrate concentrations, but only when they were fermented with the PD inoculum. In contrast, in the CD inoculum, fermentation of chopped and intact cat hair substrates produced the greatest concentrations of butyrate.
4.1. High Protein Faecal Inoculum (PD)
Fermentation of both AHC and PHC substrates significantly increased concentrations of acetate, butyrate, and propionate. Butyrate production was greatest from the fermentation of hydrolysed collagen. This may be because these substrates have a high crude protein content (97% DM in AHC and PHC vs. 90% DM for the other substrates) that can be utilised by Fusobacterium [24] and Escherichia–Shigella [25] present in high relative abundances in this model. Previous studies have shown that PHC (when fermented in cheetah inoculum) also results in relatively high butyrate concentrations [12].
Both substrate and faecal donor are factors that have previously been shown to affect ammonia production [26]. An in vitro study by Pinna, et al. [27], showed that protein content in the fermentation system was positively correlated with ammonia production. Production of ammonia by Escherichia–Shigella [26], Bacteroides, and Clostridium [28], all of which were enriched in these samples, can occur via the utilisation of both peptides and amino acids; however, peptides have been found to yield greater ammonia concentrations [26,29]. Surprisingly, despite the higher protein content of the AHC and PHC substrates, ammonia production was similar between all substrates that were assessed.
Other markers of protein fermentation (e.g., branched chain fatty acids) were below the limit of detection in this study. This may be due to the low level of valine, leucine, and isoleucine in the ADFS studied [30]. However, other methodological factors such as sampling times or cross-feeding within the system may also explain these results.
4.2. High Carbohydrate FAECAL Inoculum (CD)
In this in vitro system, chopped and intact hair were the substrates that produced the highest butyrate concentrations, but this was not observed by Depauw et al. (2012) [12], when fermenting rabbit hair, despite both hair types being structurally similar [31]. Cat hair is primarily composed of keratin, which is not typically readily fermentable, but the addition of the in vitro digestion step in the current study may have altered the keratin structure, allowing its utilisation by bacteria present in the high-carbohydrate faecal inoculum. Therefore, the increased production of butyrate may be due to this step in the experiment, or bacterial cross-feeding in the in vitro system.
4.3. Model Considerations
This study provides further insight into the benefits and limitations of a static in vitro model for companion animal research. In vitro models can provide important information and act as a screening process to test and build hypotheses, but it must be emphasised that they do not truly represent the complexity of the cat’s colonic environment. A limitation of in vitro models is the inability to account for absorption of metabolites that would occur in the colon, and the impact of the starter faecal inoculum on in vitro fermentation patterns [11]. While the latter was assessed in the current study, other factors may also influence the results observed, including the time of sampling or the number of biological replicates of donor faeces.
In this study, the 24-h time point was chosen for substrate comparison, given that the total transit time of young cats is approximately 26 h [14]. Previous in vitro studies investigated longer incubation periods (up to 72 h) but observed no further increase in gas production after 24 h, indicating that fermentation had reached capacity and all substrates were fully fermented [12]. In the current study, the substrates may still have been fermenting, but the corresponding microbial profiles indicated an abundance of Escherichia–Shigella. Therefore, continuing the fermentation for a longer period (>24 h) may not have provided any further clarification as to which substrate would confer the most beneficial effects in vivo.
As the triplicate measurements at each sampling time point were taken from the same faecal inoculum, they are pseudoreplicates from the same inoculum rather than true biological replicates. However, this is a common limitation that in principle applies to most culture experiments [32]. To better represent the true population, more faeces from more cats would be required. However, previous in vitro studies also used a pooling technique from a limited number of animals (2–4 faecal samples) [9,27,33,34]. More recently, Bosch, et al., [35] studied the impacts of the number of faecal samples and donors, and observed that the degree of inter-individual variation was dependent on the complexity of the substrate.
Nevertheless, a major advantage of an in vitro model is the ability to screen and document changes over time for multiple substrates simultaneously. By determining changes to organic acid and ammonia concentrations in this time period, comparative information can be generated which would not be possible in vivo. This model successfully screened substrates according to their fermentative properties, identifying a substrate which may confer beneficial effects when used in conjunction with a complete and balanced raw meat diet in vivo. Additionally, the findings in this study highlight the potential use of ADFS in species-appropriate diet formulations for domestic cats that do not have a carbohydrate requirement.
5. Conclusions
This study used an in vitro model to determine changes to the fermentation end products and bacterial profiles from a variety of ADFS. ADFS were indeed fermented, but the starting faecal inoculum had the greatest effect on the fermentation profiles observed. The faecal inoculum from cats fed with high protein diets was able to readily ferment high protein substrates due to the bacteria present in the culture community. This finding has wider implications for the use of ADFS in species-appropriate diet formulations.
Acknowledgments
Massey University Centre for Feline nutrition staff Karin Weidgraaf, Rachael Richardson, and Claire Sweeney for their assistance with the cats. Nicola Schreurs (Massey University) for her assistance with the substrate preparation. Jane Mullaney (AgResearch Ltd.) for her assistance with the in vitro digestion and fermentation protocol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12040498/s1, Figure S1: Scatter plot and means of lactate concentrations. Time (hours) is along the x-axis and organic acid concentration along the y-axis (mM). Both high protein faecal inoculum (PD) and high carbohydrate faecal inoculum (CD) and the changes that occurred over 24 h of fermentation are shown. Each point (coloured circle) represents an individual replicate, and each line shows the mean for each substrate. Control samples are denoted by a black solid line, AHC (ANZCO hydrolysed collagen) by a red dashed line, PHC (Peptan hydrolysed collagen) by a blue dashed line, cellulose by a green dashed line, inulin by an orange dotted line, freeze-dried cartilage by a brown dot-dashed line, fresh cartilage by a purple large dashed line, chopped cat hair by a pink dot-dashed line, and intact cat hair by a light blue dot double-dashed line.
Appendix A
Table A1.
Simulated Gastric Fluid (SGF) and Simulated Intestinal Fluid (SIF) stock solutions used in the in vitro digestion step of each experiment. Volume of each compound required and the final concentration in a 500 mL stock solution are noted (adapted from Minekus, et al. [15]).
| SGF | SIF | |||
|---|---|---|---|---|
| Volume of Stock | Concentration in SGF | Volume of Stock | Concentration in SIF | |
| mL | mmol/L | mL | mmol/L | |
| KCl | 6.9 | 6.9 | 6.8 | 6.8 |
| KH2PO4 | 0.9 | 0.9 | 0.8 | 0.8 |
| NaHCO3 | 12.5 | 25 | 42.5 | 85 |
| NaCl | 11.8 | 47.2 | 9.6 | 38.4 |
| MgCl2(H2O)6 | 0.4 | 0.1 | 1.1 | 0.33 |
| (NH4)2CO3 | 0.5 | 0.5 | - | - |
| RO water | 367 | 339.2 | ||
| Final Volume | 400 | 400 | ||
Figure A1.
Principal components analysis of genera present in samples following in vitro fermentation of each substrate at the 24-h time point in either high protein (PD) or high carbohydrate (CD) faecal inoculum. AHC; ANZCO hydrolysed collagen. PHC; Peptan hydrolysed collagen.
Figure A2.
Assessment of alpha diversity using the Chao1 diversity index from samples following in vitro fermentation of substrates at the 24-h time point using high protein faecal inoculum (PD). Small circles denote outliers.
Figure A3.
Assessment of alpha diversity using the Chao1 diversity index from samples following in vitro fermentation of substrates at the 24-h time point using high carbohydrate faecal inoculum (CD). Small circles denote outliers.
Author Contributions
Conceptualization, C.F.B., D.G.T., N.J.C., E.N.B. and W.Y.; Formal analysis, C.F.B., S.-Y.H. and H.M.S.; Funding acquisition, E.N.B.; Methodology, C.F.B., N.J.C., D.I.R. and W.Y.; Writing—original draft, C.F.B.; Writing—review & editing, C.F.B., D.G.T., N.J.C., E.N.B., S.-Y.H., H.M.S. and W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the New Zealand government (Ministry of Business, Innovation and Employment: C10X1501), awarded to ENB and co-funded by the New Zealand Premium Petfood Alliance (K9 Natural, Ziwi and The Real Petfood company). Additional support to CFB was provide by AgResearch (SSIF contract A26482). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Massey University Animal Ethics Committee, MUAEC protocol 18/08.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study can be found in NCBI BioProject ID PRJNA767450.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.AAFCO . 2021 Official Publication. AAFCO Publications; Champaign, IL, USA: 2021. [Google Scholar]
- 2.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Amp. Hepatol. 2012;9:577. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 3.Butowski C.F., Thomas D.G., Young W., Cave N.J., McKenzie C.M., Rosendale D.I., Bermingham E.N. Addition of plant dietary fibre to a raw red meat high protein, high fat diet, alters the faecal bacteriome and organic acid profiles of the domestic cat (Felis catus) PLoS ONE. 2019;14:e0216072. doi: 10.1371/journal.pone.0216072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming S.E., Fitch M.D., DeVries S., Liu M.L., Kight C. Nutrient utilization by cells isolated from rat jejunum, cecum and colon. J. Nutr. 1991;121:869–878. doi: 10.1093/jn/121.6.869. [DOI] [PubMed] [Google Scholar]
- 5.Clausen M.R., Mortensen P.B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37:684–689. doi: 10.1136/gut.37.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darcy-Vrillon B., Cherbuy C., Morel M.T., Durand M., Duee P.H. Short chain fatty acid and glucose metabolism in isolated pig colonocytes: Modulation by NH4+ Mol. Cell. Biochem. 1996;156:145–151. doi: 10.1007/BF00426337. [DOI] [PubMed] [Google Scholar]
- 7.Davila A.M., Blachier F., Gotteland M., Andriamihaja M., Benetti P.H., Sanz Y., Tome D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013;68:95–107. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Sunvold G.D., Fahey G.C., Jr., Merchen N.R., Bourquin L.D., Titgemeyer E.C., Bauer L.L., Reinhart G.A. Dietary fiber for cats: In vitro fermentation of selected fiber sources by cat fecal inoculum and in vivo utilization of diets containing selected fiber sources and their blends. J. Anim. Sci. 1995;73:2329–2339. doi: 10.2527/1995.7382329x. [DOI] [PubMed] [Google Scholar]
- 9.Cutrignelli M.I., Bovera F., Tudisco R., D’Urso S., Marono S., Piccolo G., Calabrò S. In vitro fermentation characteristics of different carbohydrate sources in two dog breeds (German shepherd and Neapolitan mastiff) J. Anim. Physiol. Anim. Nutr. 2009;93:305–312. doi: 10.1111/j.1439-0396.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- 10.De Godoy M.R., Mitsuhashi Y., Bauer L.L., Fahey G.C., Jr., Buff P.R., Swanson K.S. In vitro fermentation characteristics of novel fibers, coconut endosperm fiber and chicory pulp, using canine fecal inoculum. J. Anim. Sci. 2015;93:370–376. doi: 10.2527/jas.2014-7962. [DOI] [PubMed] [Google Scholar]
- 11.Brahma S., Martínez I., Walter J., Clarke J., Gonzalez T., Menon R., Rose D.J. Impact of dietary pattern of the fecal donor on in vitro fermentation properties of whole grains and brans. J. Funct. Foods. 2017;29:281–289. doi: 10.1016/j.jff.2016.12.042. [DOI] [Google Scholar]
- 12.Depauw S., Bosch G., Hesta M., Whitehouse-Tedd K., Hendriks W.H., Kaandorp J., Janssens G.P. Fermentation of animal components in strict carnivores: A comparative study with cheetah fecal inoculum. J. Anim. Sci. 2012;90:2540–2548. doi: 10.2527/jas.2011-4377. [DOI] [PubMed] [Google Scholar]
- 13.Deb-Choudhury S., Bermingham E.N., Young W., Barnett M.P.G., Knowles S.O., Harland D., Clerens S., Dyer J.M. The effects of a wool hydrolysate on short-chain fatty acid production and fecal microbial composition in the domestic cat (Felis catus) Food Funct. 2018;9:4107–4121. doi: 10.1039/C7FO02004J. [DOI] [PubMed] [Google Scholar]
- 14.Peachey S.E., Dawson J.M., Harper E.J. Gastrointestinal transit times in young and old cats. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000;126:85–90. doi: 10.1016/S1095-6433(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 15.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carriere F., Boutrou R., Corredig M., Dupont D., et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 16.Kienzle E. Carbohydrate metabolism of the cat 1. Activity of amylase in the gastrointestinal tract of the cat. J. Anim. Physiol. Anim. Nutr. 1993;69:92–101. doi: 10.1111/j.1439-0396.1993.tb00793.x. [DOI] [Google Scholar]
- 17.Mariotti F., Tomé D., Mirand P.P. Converting nitrogen into protein—beyond 6.25 and jones’ factors. Crit. Rev. Food Sci. Nutr. 2008;48:177–184. doi: 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- 18.Richardson A.J., Calder A.G., Stewart C.S., Smith A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 1989;9:5–8. doi: 10.1111/j.1472-765X.1989.tb00278.x. [DOI] [Google Scholar]
- 19.Weatherburn M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967;39:971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- 20.Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R.M., Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 23.Luo D., Ganesh S., Koolaard J. Predictmeans: Calculate Predicted Means for Linear Models; R Package Version 1.0.1. 2018. [(accessed on 11 February 2022)]. Available online: https://CRAN.R-project.org/package=predictmeans.
- 24.Anand S., Kaur H., Mande S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016;7:1945. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gschaedler A., Boudrant J. Amino acid utilization during batch and continuous cultures of Escherichia coli on a semi-synthetic medium. J. Biotechnol. 1994;37:235–251. doi: 10.1016/0168-1656(94)90131-7. [DOI] [Google Scholar]
- 26.Richardson A.J., McKain N., Wallace R.J. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13:6. doi: 10.1186/1471-2180-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinna C., Stefanelli C., Biagi G. In vitro effect of dietary protein level and nondigestible oligosaccharides on feline fecal microbiota. J. Anim. Sci. 2014;92:5593–5602. doi: 10.2527/jas.2013-7459. [DOI] [PubMed] [Google Scholar]
- 28.Vince A.J., Burridge S.M. Ammonia production by intestinal bacteria: The effects of lactose, lactulose and glucose. J. Med. Microbiol. 1980;13:177–191. doi: 10.1099/00222615-13-2-177. [DOI] [PubMed] [Google Scholar]
- 29.Smith E.A., Macfarlane G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: Effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol. 1998;25:355–368. doi: 10.1111/j.1574-6941.1998.tb00487.x. [DOI] [Google Scholar]
- 30.Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deedrick D., Koch S. Microscopy of hair part II: A practical guide and manual for animal hairs. Forensic Sci. Commun. 2004;6:3. [Google Scholar]
- 32.Lazic S.E., Clarke-Williams C.J., Munafò M.R. What exactly is ‘N’ in cell culture and animal experiments? PLoS Biol. 2018;16:e2005282. doi: 10.1371/journal.pbio.2005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosch G., Pellikaan W.F., Rutten P.G., van der Poel A.F., Verstegen M.W., Hendriks W.H. Comparative in vitro fermentation activity in the canine distal gastrointestinal tract and fermentation kinetics of fiber sources. J. Anim. Sci. 2008;86:2979–2989. doi: 10.2527/jas.2007-0819. [DOI] [PubMed] [Google Scholar]
- 34.Bosch G., Wrigglesworth D.J., Cone J.W., Pellikaan W.F., Hendriks W.H. Effects of preservation conditions of canine feces on in vitro gas production kinetics and fermentation end products. J. Anim. Sci. 2013;91:259–267. doi: 10.2527/jas.2012-5262. [DOI] [PubMed] [Google Scholar]
- 35.Bosch G., Heesen L., de Melo Santos K., Cone J.W., Pellikaan W.F., Hendriks W.H. Evaluation of an in vitro fibre fermentation method using feline faecal inocula: Inter-individual variation. J. Nutr. Sci. 2017;6:e24. doi: 10.1017/jns.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in NCBI BioProject ID PRJNA767450.