Abstract
Cellular redox homeostasis is crucial for normal plant growth and development. Each developmental stage of plants has a specific redox mode and is maintained by various environmental cues, oxidants, and antioxidants. Reactive oxygen species (ROS) and reactive nitrogen species are the chief oxidants in plant cells and participate in cell signal transduction and redox balance. The production and removal of oxidants are in a dynamic balance, which is necessary for plant growth. Especially during reproductive development, pollen development depends on ROS-mediated tapetal programmed cell death to provide nutrients and other essential substances. The deviation of the redox state in any period will lead to microspore abortion and pollen sterility. Meanwhile, pollens are highly sensitive to environmental stress, in particular to cell oxidative burst due to its peculiar structure and function. In this regard, plants have evolved a series of complex mechanisms to deal with redox imbalance and oxidative stress damage. This review summarizes the functions of the main redox components in different stages of pollen development, and highlights various redox protection mechanisms of pollen in response to environmental stimuli. In continuation, we also discuss the potential applications of plant growth regulators and antioxidants for improving pollen vigor and fertility in sustaining better agriculture practices.
Keywords: environmental stress, oxidants, pollen, redox signaling, ROS
1. Introduction
As sessile organisms, plants are more susceptible to environmental influences than animals. In order to survive and reproduce, they need to constantly adjust their physiology and morphology to adapt to various environmental challenges, which often cause cellular oxidative damage. Therefore, plants have evolved complex oxidative stress regulation systems, such as Heat Shock Protein (HSP) networks, endoplasmic reticulum-unfolded protein response (ER-UPR), cytosolic protein response (CPR), Ca2+ signaling, and redox regulation systems [1,2]. Among them, cellular redox regulation plays an indispensable role. Redox biology results from the modification of target proteins by reactive oxygen species (ROS) and other oxidants, which, in turn, affect protein structure, function, and signal transduction [3]. Plants possess a large network of ROS producing/scavenging pathways involving more than 100 genes to strictly maintain the appropriate cellular redox status [4]. Various antioxidants in plant cells keep oxidants at non-toxic levels, and any change in this balance could serve as a redox signal [5]. As long as the concentration of ROS in plant cells remains within the normal range, the normal cellular metabolic activities can carry on.
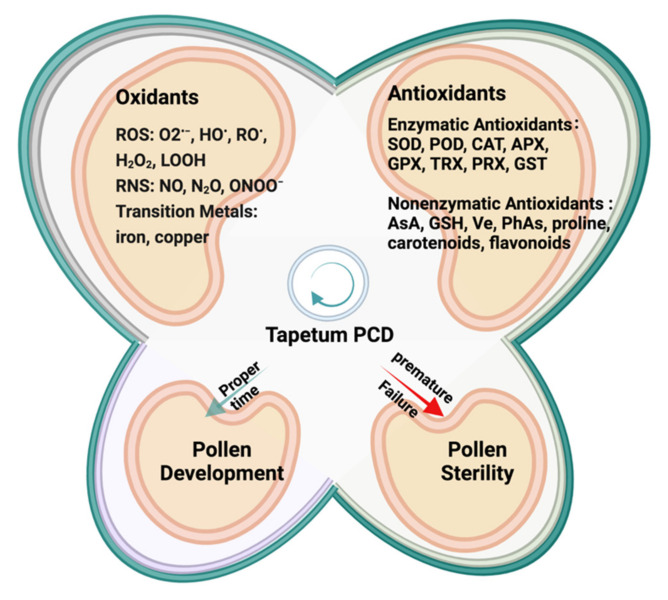
Pollen plays a key role in the life cycle of flowering plants and is directly related to plant fertility and crop productivity. However, pollen development is very susceptible to environmental influences, so plants have evolved intricate mechanisms to ensure the smooth completion of this process. Studies on reproductive development have uncovered a clear link between redox processes and pollen development [6,7]. Pollen development is completed in anthers and is generally divided into three stages: microsporogenesis, male gametophyte formation, and pollen maturation [8]. The coordinated development of anthers and pollen is necessary for the successful reproduction of plants. Tapetum is a layer of cells in anther, which maintains high metabolic activity during microsporogenesis. Tapetum cells secrete proteins, lipids, carbohydrates, and secondary metabolites through programmed cell death (PCD) to provide nutrients and other essential substances for pollen development [9,10,11]. The distortion of tapetum morphology and the change in tapetum degradation time usually cause pollen deficiency, which further affects the number and morphology of pollen, the structure of pollen wall and pollen fertility [1]. Numerous studies have shown that tapetal PCD is induced by the ROS homeostasis. Transient peaks of ROS production have been observed in the developing anthers of rice (Oryza sativa), tomato (Solanum lycopersicum), and tobacco (Nicotiana tabacum) [12,13]. However, excessive accumulation of ROS indiscriminately damages cellular constituents, including proteins, DNAs, and lipids, and leads to atypical tapetal PCD and defective pollen wall formation [3]. As we have seen, redox regulation and signaling have come into sight as crucial mechanisms that regulate the sexual reproduction of plants, in which ROS, nitric oxide (NO), and other classical antioxidants and biomolecules are closely involved [14]. These redox components (Figure 1) are essential for maintaining redox homeostasis and ensuring normal anther development and pollen germination [15,16].
Figure 1.
Redox biomolecules involved in pollen development. The normal development of pollen requires timely degradation of the tapetum, and this process is very susceptible to the disturbance of redox homeostasis. Premature or failure of tapetum degradation will lead to microspore abortion and pollen sterility. In the development and regulation of pollen and anthers, the main oxidants contain reactive oxygen species (ROS) and reactive nitrogen species (RNS), and the antioxidants can be divided into enzymes and non-enzymatic antioxidants.
Abiotic stresses such as heat, cold, and drought can induce oxidative stress in plant cells, resulting in the imbalance of cellular redox system. In most plants, meiosis to microspore development seems to be the most sensitive process to environmental stress conditions [17]. The stresses during this period often lead to microspore abortion, reduced number of pollen grains during flowering, and a reduction in the proportion of fertile pollen [18]. In the process of evolution, pollen has developed a different and complicated mechanism in response to environmental oxidative stresses. On the one hand, it shares the similar redox regulation mechanisms with other vegetative and reproductive processes. Excessive accumulation of oxidants such as ROS can cause irreversible cell damage, but can also act as signals to trigger a variety of stress responses [19,20], including increasing the expression of antioxidant genes and the production of enzymatic/non-enzymatic antioxidants, strengthening the repair and recovery of denatured proteins, and ultimately enhancing tolerance in plants [1,17]. On the other hand, pollen has its specific redox mechanisms. Specific alterations in phospholipid saturation and accumulation of chaperonin HSPs, sugars, and nonprotein amino acids (such as proline) are identified to regulate pollen oxidative stresses [1,21].
2. Redox Biology in Pollen Development
2.1. Role of Oxidants in Pollen Development
The major endogenous sources of oxidants in plants (Figure 1) are various intermediates of oxygen, nitrogen, and carbon metabolism including ROS, reactive nitrogen species (RNS), reactive carbonyl species (RCS), and transition metals such as iron and copper [22]. ROS and RNS are the most common oxidants and are key regulators of plant development [7].
ROS are a collective term that includes superoxide (O2•−), the hydroxyl radical (HO•), lipid alkoxy (RO•) radicals, hydrogen peroxide (H2O2), and lipid peroxides (LOOH) [23]. ROS levels are dynamic in anther development. They begin to accumulate in the early stage of meiosis, gradually increase in the meiotic stage, and reach their highest level in the late stage of microspores. However, their signals decrease from the mitotic stage to the anther dehiscence in different plant species, such as Arabidopsis (Arabidopsis thaliana), tomato, tobacco, and wheat (Triticum aestivum) [13,24,25].
The production of ROS is precisely regulated during pollen development. ROS, which are produced by NADPH oxidases, in plants known as Respiratory Burst Oxidase Homologs (RBOHs), are essential for PCD during tapetum degradation in Arabidopsis, rice, tomato, and tobacco [12,13,24,26]. Failure or premature PCD of tapetum frequently leads to male sterility [27,28]. Therefore, a complex transcriptional network has been found to be responsible for fine-tuning the expression of RBOHs, generating ROS signals for the tapetal PCD to ensure pollen development [24,29]. The genome of Arabidopsis encodes a minimum of 10 RBOHs, and they have distinct expression profiles in the different organs and at various developmental stages [30]. For example, RBOHE overexpression leads to precocious tapetum degeneration, while RBOHE deficiency shows delayed tapetum degradation, both of which lead to pollen fertility. The ROS levels in rbohe mutants are generally reduced, while the increase in ROS levels in RBOHE overexpression plants only exists in the early developmental stage of pollen, indicating the importance of temporal and spatial regulation of ROS levels for pollen development [24]. Moreover, RBOHC plays a redundant role in tapetal degeneration and pollen development compared with RBOHE [24]. In tomato, Brassinazole Resistant 1 (BZR1) regulates RBOH1 transcription and RBOH1-mediated ROS directly trigger PCD and the degradation of tapetum cells, which, in turn, plays an important role in pollen and seed development [31].
In addition to the direct influence of RBOH proteins, many regulatory genes have been identified to be responsible for anther cell differentiation and pollen formation in Arabidopsis [8]. Anther Dehiscence Repressor (ADR), which is involved in regulating male sterility in Arabidopsis, is N-myristoylated and targeted to the peroxisome during the early stages of flower development to negatively regulate anther dehiscence by suppressing ROS accumulation [32]. Tomato Inflorescence Deficient in Abscission (SlIDA) encodes a 14-mer EPIP peptide, which plays an essential role in pollen development and pollen tube elongation by regulating ROS homeostasis. Slida mutants assume decreased fertility, PCD defects in tapetum and septum, and failure of anther dehiscence. Exogenous use of synthetic 14-mer EPIP peptide rescues this defect and enhances ROS-signaling [33]. Rice ARGONAUTE 2 (OsAGO2) is highly expressed in anthers and regulates anther development by regulating the methylation levels of the promoter region of Hexokinase 1 (OsHXK1), which controls the appropriate production of ROS and the proper timing of tapetal PCD [34].
The fine-tuning of ROS involves many other molecules and antioxidants. For instance, Metallothioneins (MTs) are Cys-rich proteins that can scavenge HO• and singlet oxygen. OsMT2b, a ROS scavenger during rice male reproduction, interacts with Defective tapetum cell death 1 (DTC1) and its activity is inhibited by DTC1, which can regulate tapetum PCD process and the normal development of pollen in rice [35]. OsMADS3 can directly up-regulate the expression of MT-1-4b to modulate ROS levels in rice male reproductive development [12].
In addition, fatty acids (FAs) are also involved in the process of pollen development and are closely related to the redox regulation [36,37,38]. In maize (Zea mays), lipid biosynthesis mediated by male sterility 33 (ZmMS33) is critical to the normal structure and function of chloroplasts in tapetum cells and the late proliferation and expansion of anther cells. The loss of function of ZmMS33 leads to the accumulation of H2O2 in the anthers, early and excessive autophagy, premature PCD of tapetum cells, and it affects the late development of anthers [28]. In Arabidopsis, the loss of Fatty Acid Export 1(FAX1) function breaks FA/lipid homeostasis and ROS homeostasis in the cells, suppresses transcriptional activation of anther developmental genetic networks, and damages tapetum development and pollen wall formation, leading to male sterility [38]. Consequently, the production of ROS is regulated at different levels at different stages, so as to maintain the balance of redox and the normal development of pollen.
RNS includes NO, nitrous oxide (N2O), peroxynitrite (ONOO−), and other types of nitrogen-containing reactive free radicals [22]. Gaseous NO is a major RNS, which acts as an oxidation-reduction signaling molecule with diverse physiological functions in plants. The half-life of NO in biological tissues is longer than that of ROS and is considered to be a relatively stable free radical [14]. NO was first proposed to participate in growth and diversion through its negative chemotaxis on lily (Lilium longiflorum) pollen tube growth [39]. Later, the pollen of several plants was discovered to produce NO [40]. In olive (Olea europaea), ROS and NO are produced in reproductive organs in a phased and tissue-specific manner. Stigma surface, anther tissue, pollen grains, and pollen tube are the tissues that accumulate the most ROS and NO [41]. During the receiving period, pollen grains and pollen tubes have an increased ability to produce NO. When NO is actively produced, the presence of ROS decreases [41]. These studies suggest that NO may evolve for intercellular communication tasks during the programming stage of sexual reproduction. There have been many studies on the role of RNS, especially NO, on the interaction of pollen and pistil, pollen tube growth, pollen tube rupture to release sperm, and fertilization [14,42,43]. For example, the arrival of Arabidopsis pollen tubes to the ovule triggers NO accumulation in filamentous organs. This process, which is FERONIA (FER)-dependent and mediated by de-esterified pectin, prevents multiple pollen tubes from penetrating the female gametophyte and averts polyspermia [43]. S-nitrosoglutathione (GSNO), the main biologically active form of RNS, is the major donor of NO. GSNO reductase (GSNOR) has been shown to metabolize GSNO and play a key role in NO signaling [22,44]. Knockout of the GSNOR gene in tomato increased the level of endogenous NO and S-nitrosylation, resulting in a decrease in total pollen, and a decrease in germination and vigor [45].
2.2. Role of Enzymatic Antioxidants in Pollen Development
In order to maintain the redox balance, plants have evolved a complete antioxidant mechanism. Many antioxidants are involved in the process of pollen development (Table 1), which can be divided into two categories: enzymatic antioxidants and non-enzymatic antioxidants [3]. Previous studies have shown that these plant antioxidants have precise temporal and spatial expression patterns and play crucial roles in the redox homeostasis and development of male reproductive tissues [15].
Table 1.
Role of various antioxidants during flower development in different plant species.
| Plant Species | Related Antioxidants | Functional Period | References |
|---|---|---|---|
| Arabidopsis | CAT | Pollen development | [46,47] |
| TRX, GSH | Development of flower buds | [68,79,80] | |
| GSH, GSSG | Flower development and pollen vigor | [66] | |
| CC-type GRX | Petal and anther development | [52,53,54,55] | |
| Tomato | TRX, PRX | Microspore development, anther secondary cell wall thickening and anther dehiscence | [57,58,59,61] |
| Flavonols | Stamen development, pollen wall formation and late flower development | [73,75,81] | |
| Proline | Pollen development | [70,76,77] | |
| ASA | Development of anthers and pollen | [69] | |
| Maize | CAT, SOD | Pollen development | [82] |
| Wheat | SOD, CAT, POD, APX, GPX | Pollen development | [25,49] |
| Soybean | CAT, POD; GSSG, Flavonols | Pollen development and germination | [63] |
| Kiwifruit | PAs | Pollen development and germination | [71] |
The main enzymatic antioxidants (Figure 1) include superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutaredoxin (GRX), glutathione S-transferase (GST), thioredoxin (TRX), and peroxiredoxin (PRX) [3,22].
Among them, SOD, CAT, POD, and APX are considered to be classic and important antioxidant enzymes. The deletion of CAT1/2/3 genes in Arabidopsis will not only cause severe vegetative growth defects, but also minor reproductive defects [46]. Maize CAT1 is the only CAT expressed in mature pollen and the immature milky endosperm [47]. When compared to other tissues of the plant, pollen has a relatively low POD activity as well as low isoenzyme polymorphism [48]. However, POD may play a role in the interaction between pistil and pollen [48]. Many sterile line anthers show abnormal tapetal PCD and organelles, which may be due to abnormal transcription levels of antioxidant enzyme genes and excessive ROS that disrupt the balance of the antioxidant system [25,49]. In the early development of wheat sterile line pollen, the expression levels of SOD, CAT, POD, APX, and GPX are significantly up-regulated, and the enzyme activity increases rapidly [25].
GRXs and TRXs have been described as the main protein families responsible for the redox status of protein cysteine (Cys) residues within the cells, and these Cys residues are usually maintained in their reduced state within the cells [15,50,51]. ROXY proteins are conserved plant-specific GRXs. In several species such as Arabidopsis, wheat, and rice, ROXY proteins have been shown to play critical roles in regulating cell division, cell size, and early anther differentiation [52,53,54]. Roxy proteins regulate anther development by activating the transcription of TGACG (TGA) motif-binding factors (TGA9 and TGA10) through cysteine modification. The double mutants tga9/tga10 and roxy1/roxy2 have fairly similar defects in the development of male gametes in Arabidopsis [55]. GhTRXL3-2 can interact with FLOWERING LOCUS T (GhFT) in cotton (Gossypium hirsutum), and heterologous overexpression of GhTRXL3-2 in Arabidopsis results in early flowering [56]. Cystathionine β-synthase (CBS) domain-containing proteins are ubiquitous redox regulators in plants. In the Arabidopsis anthers, CBSXs, TRXs, and PRXs proteins are involved in anther dehiscence and pollen release via the control of H2O2 [57,58,59]. CBSX1 regulates TRX and lignin polymerization in the anther endothecium [57]. In addition, CBSX2 directly modulates TRX in chloroplasts, which affects the level of H2O2, thereby, affecting the expression of genes related to secondary cell wall thickening in anther development [58]. Recent study has further confirmed that deficient lignin deposition-mediated anther indehiscence is caused by insufficient ROS accumulation in Arabidopsis CBSX3 RNAi plants [59].
PRXs are involved in various aspects of plant development and environmental response, and are usually related to lignin modification [60]. In Arabidopsis, PRX9 and PRX40 encode expansin peroxidase and cross-link extensins to contribute to the integrity of tapetum and microspore cell wall during anther development [61].
2.3. Role of Non-Enzymatic Antioxidants in Pollen Development
Non-enzymatic antioxidants (Figure 1) refer to small molecular compounds with antioxidant effects, including ascorbate (ASA), glutathione (GSH), proline, α-tocopherol (Ve), carotenoids, flavonoids, and phenolic acids (PhAs) [3,22].
The AsA-GSH cycle includes two interdependent redox pairs: AsA/dehydroascorbate (DHA) and GSH/glutathione disulfide (GSSG). They regulate cellular redox status and plant resistance [50,62,63]. APX oxidizes AsA to DHA, and GSH is used as an electron donor by dehydroascorbate reductase (DHAR) to convert DHA to the reduced form of AsA. GSSG can be recycled into GSH by glutathione reductase (GR) and GSH can also interact with ROS and is oxidized to GSSG [50,63]. GSH has a high concentration in plant cells while GSSG are usually kept at very low levels and can be rapidly sequestered in the vacuole [3,64,65]. The metabolically active high-ROS pollen would require high levels of antioxidant activity, particularly high activities of enzymes such as GR in order to maintain high GSH/GSSG ratios [66]. GSH accumulation is required for the initiation of the floral meristem and has a strong association with flowering time [67,68]. Metabolic analysis of soybean sterile line pollen has found that the ROS scavenging system is defective, and GSSG is the most decreased among amino acid derivatives [63]. The flowers of glutathione-deficient cad2-1 mutants, which contain a low GSH/GSSG ratio, cause a high degree of oxidation of anthers, pollen grains, and pollen tubes in Arabidopsis [66]. However, an excess of antioxidants also affects pollen development. The accumulation of AsA in tomato plants is related to damage to the structure of floral organs, especially the development of anthers and pollen, leading to male sterility [69].
Metabolic studies on some sterile lines and specific mutants have shown that the decrease in the levels of specific metabolites, such as flavonols, proline, and polyamines (PAs), is related to the decrease in pollen fertility [70,71,72,73]. Flavonols are catalyzed and synthesized by flavonol synthase (FLS). FLS1 is highly expressed in Arabidopsis flowers, and also exhibits flavonol accumulation [74]. Studies have shown that many MYB family transcription factors are closely related to the production and function of flavonols, and play vital roles in the development of stamens. There are two FLS genes involved in the spatiotemporal biosynthesis of flavonols in Freesia hybrida flowers, which are controlled by differential phylogenetic MYB regulators [73]. The accumulation of excessive ROS in Arabidopsis stamens of myb21 mutants leads to defects in stamen development, which can be partially rescued by treatment with ROS inhibitors or overexpression of FLS1 [75]. The proline needed for pollen development and reproduction is mainly synthesized in developing microspores and mature pollen grains [72]. The interruption of proline synthesis in Arabidopsis can lead to abortion and sterility during gametophyte development [70,76]. Prolyl Aminopeptidase 1 (PAP1) can release N-terminal proline. Loss of PAP1 function in plants reduces pollen fertility and sensitivity to osmotic stress [77]. PAs mainly exists in free form in higher plants, including putrescine (Put), spermidine (Spd), and spermine (Spm) [78]. S-adenosylmethionine decarboxylase (SAMDC) is a key enzyme for the synthesis of PAs. High levels of soluble SAMDC are found in male fertile anthers from the late stage of microspores, and there are no soluble SAMDC in male sterile anthers [71]. PAs, especially Spd, maintain high levels throughout the pollen development of kiwifruit (Actinidia deliciosa), and decline in the final stage of sterile pollen development [71].
3. The Protective Mechanism of Antioxidant in Pollen Development under Capricious Environment
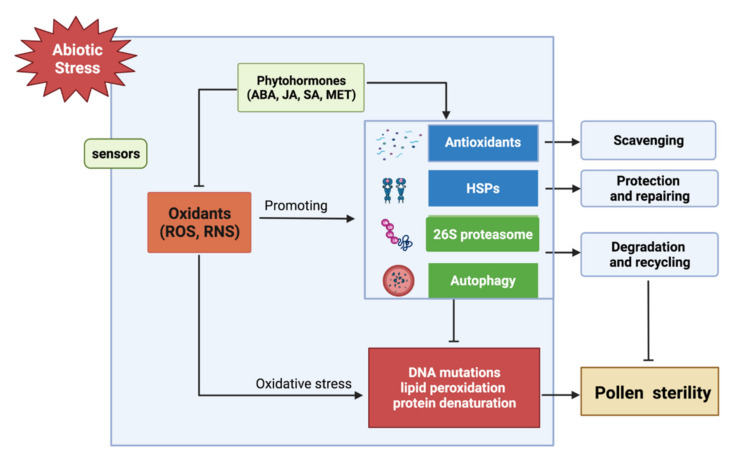
Abiotic stresses such as high temperature, drought, and cold have negative impacts on the reproductive development of plants, and pollen is particularly sensitive to environmental stimuli during the entire development process. Oxidative stress induced by abiotic stress can trigger excessive accumulation of ROS and cause serious damage to cells, including protein denaturation, lipid peroxidation, and DNA mutations, even PCD [17]. It ultimately affects the quantity and morphology of pollen, cell wall structure, and importantly, pollen metabolism, leading to pollen abortion and male sterility during anther development [18,83]. Plants have integrated antioxidant protection systems in response to environmental stimuli (Figure 2). The antioxidant system, protein degradation and regeneration system, and phytohormones and peptides all participate in the adaptation of pollen to abiotic stress by mediating a wide range of adaptive responses [2,17,84].
Figure 2.
Regulation of redox balance in response to abiotic stresses. Abiotic stresses can induce reactive oxygen species (ROS) bursts, causing damage to cell structure (membrane lipids) and biomolecules (DNA, proteins), leading to pollen sterility. At the same time, different signal transduction and regulatory networks are induced, thereby improving the ability to scavenging oxidants, enhancing the protection and repair capabilities of Heat Shock Proteins (HSPs), and promoting the degradation of denatured proteins mediated by 26S proteasome and autophagy, ultimately achieving the goal of reducing the damage of oxidative stress to cells and improving the fertility of pollen.
3.1. Regulation of Redox Balance
The activity/content of antioxidants and specific metabolites increases to detoxify ROS and protect anther development under different abiotic stresses (Table 2). Among all antioxidant enzymes, CAT exhibits one of the highest turnover rates to catalyze H2O2 to H2O in an energy-efficient manner [85]. Therefore, CAT plays an indispensable role in the detoxification of excess ROS produced under abiotic stresses [86]. During meiosis, the APX and GR activities in rice anthers are relatively less sensitive to high temperature than the CAT and SOD activities in anthers [87]. Under drought stress, the antioxidant enzyme activities of drought-resistant wheat varieties, including SOD, POD, APX, GR, and CAT, were higher than those of drought-sensitive varieties, thereby removing excess ROS and protecting anther development [88]. Non-enzymatic antioxidant molecules also participate in the oxidative protection of pollen. PAs is a regulator of redox homeostasis, which can increase the activities of antioxidant enzymes and is also one of the sources of ROS [78]. Flavonols are considered to be powerful antioxidants for the detoxification of ROS [89,90]. The accumulation of pollen-specific flavonols promotes drought-induced male fertility by eliminating ROS in Arabidopsis [75]. The accumulation of flavonols in tomato plants under heat stress is much higher than that under the combination of salinity and heat [91,92]. Under cold stress, treatment with Spd can regulate Ca2+, ROS homeostasis, and cell wall deposition to reorganize the growth pattern of pollen tubes and significantly increase pollen germination rate and pollen tube length [93].
Table 2.
Regulatory function of antioxidants in pollen development under different stresses.
| Stress | Plant Species | Related Antioxidants | Stress Damage | References |
|---|---|---|---|---|
| Heat | Rice | CAT, SOD, POD | Decline in pollen viability and spikelet fertility | [87,94] |
| Rice | ASA | Spikelet-opening impairment | [100] | |
| Tomato | GST, APX, Flavonoids, PAs | Microspore abortion and low pollen germination rate | [92,102,103] | |
| Drought | Wheat | SOD, POD, APX, CAT | Male fertility and grain number reduction | [88] |
| Rice | SOD, POD | Tapetal defects and reduced pollen fertility | [95,98] | |
| Arabidopsis | Flavonols | Stamen defects and reduced fertility | [75] | |
| Rice | SOD, POD | Severe tapetal defects and reduced pollen fertility | [96] | |
| Camellia | Spd | Pollen germination rate and pollen tube length | [93] | |
| Cold | Chickpea | Proline, GPX, GSH | Disruption of gamete development and pollen sterility | [104] |
In addition to changes in metabolic activity and substances, antioxidant-related transcription levels and protein levels are also induced by stresses. Cu/Zn-SODa is sensitive to heat stress and is closely related to the down-regulation of cCu/Zn-SOD1 expression, thus having a great influence on the total SOD activity. [94]. High temperature exposure during meiosis significantly affects the expression and activity of SOD, CAT, and POD in developing anthers, but has no significant effect on APX and GR [87]. Under heat stress, the decrease in CAT activity in rice anthers is mainly due to the rapid inhibition of OsCATB transcripts, which is one of the factors leading to the increase in ROS accumulation in anthers and the decrease in pollen vigor [87]. Transcription factor MYB Important for Drought Response 1 (MID1) is expressed in rice anthers and is further induced by drought stress. In MID1 overexpression plants, the expression of POD and SOD is increased, which causes an increase in ROS scavenging capacity to protect the tapetum cells and enhance drought tolerance [95]. Cold stress causes ROS accumulation, which leads to tapetum degradation and pollen abortion. A point mutation of the Low Temperature Tolerance 1 (LTT1) gene prevents ROS overproduction, allowing for correct pollen development, thereby improving the cold tolerance and seed setting ability of rice [96].
As important signal molecules, phytohormones also participate in the regulation of redox state in anther and pollen under abiotic stresses (Figure 2). Heat stress at the meiotic stage of pollen disturbs ROS and sugar homeostasis, leading to pollen sterility in rice, which can be reversed by abscisic acid (ABA) [97]. Rice Drought-Induced LTP (OsDIL) is mainly expressed in anthers and responds to drought and ABA. OsDIL regulates the drought resistance of rice during reproductive development by up-regulating the expression of SOD, POD, and ABA synthesis gene Zeaxanthin Epoxidase 1 (ZEP1) [98]. Auxin reduces the membrane lipid peroxidation and ROS accumulation, as well as affecting the underlying antioxidant enzyme activities in rice spikelets under drought stress [99]. Jasmonic acid (JA) actively regulates ROS production, the antioxidant system, and amylase activity in the spikelets of sterile rice to alleviate the spikelet opening obstacles during the flowering period under heat stress [100]. Salicylic acid (SA) is known as a phenolic compound and hormone-like substance. Under heat stress, SA can enhance the antioxidant ability of rice plants to maintain the redox state by scavenging excess ROS, and prevent the degradation of the tapetum [101].
3.2. Maintenance of Protein Homeostasis
Proteomics studies have shown that mature pollen pre-synthesizes a large number of proteins with predefined functions, such as cell wall metabolism, energy metabolism, signal transduction, etc. [105]. Oxidative stress caused by abiotic stresses usually changes protein stability, membrane fluidity, the cytoskeleton, and metabolic balance in anther and pollen development. Therefore, maintaining the stability of functional proteins is very important for pollen development.
HSPs function by re-stabilizing protein conformation under stress conditions (Figure 2). HSPs and ROS detoxification systems participate in the crosstalk between different stress signaling transduction networks to enhance plant resistance under abiotic stresses [106,107]. After short-term heat stress in many species, different types of HSPs, such as small HSPs, HSP70, HSP90, and HSP100, are accumulated in the developing anthers and pollen grains [105,108]. Melatonin (MET), a novel signaling molecule in plant response to stresses, promotes the high levels of HSPs and Autophagy-Related Genes (ATGs) transcripts and induces autophagic signaling in tomato anthers, and ultimately increases pollen viability and germination under heat stress [109].
Oxidative stress results in massive protein denaturation and cytoplasmic organelle damage. The removal of denatured proteins and organelles is essential for cell survival [110]. The ubiquitin–proteasome system and autophagy (Figure 2) are the two main proteolytic systems involved in degradation of misfolded and damaged proteins and organelles [111,112]. Loss of 26S proteasome function leads to a series of plant development defects. For example, loss function of RPT2 subunit, a component of the 26S proteasome, impairs plant growth and reproductive development in Arabidopsis [113]. Studies have reported that many genes encoding ubiquitin–conjugating enzyme (E2) and ubiquitin ligase (E3) are stress-induced and can regulate the tolerance of plant reproductive organs to a variety of abiotic stresses [114,115,116]. The RING-type E3 ligase High Expression of Osmotically Responsive Genes1 (HOS1) can ubiquitinate flowering-promoting transcription factor CONSTANS (CO), which is then degraded by the proteasome [117,118]. Exposure to low temperature promotes the degradation of CO by the HOS1-dependent proteasome and leads to FT inhibition, thereby delaying flowering [117,118]. Autophagy is also required in the tapetal PCD during the development and maturation of pollen, and participates in plant responses to abiotic stresses [119,120]. Moreover, autophagy is also involved in the breakdown of liposomes and the transfer of lipids from tapetum cells to the surface of microspores in rice [121]. Autophagy-deficient mutants in Arabidopsis and rice are reported to be hypersensitive to oxidative stress. Therefore, autophagy, which is induced during tapetal PCD and pollen development, plays critical roles in organelle quality control and environmental stress response [121,122]. In short, the protein quality control mediated by 26S proteasome and autophagy plays a decisive role in pollen development and stress resistance. There are many overlaps between these two protein regulatory mechanisms, and their different roles in specific conditions need to be further studied.
4. Potential Agricultural Application of Antioxidants for Normal Pollen Development under Stresses
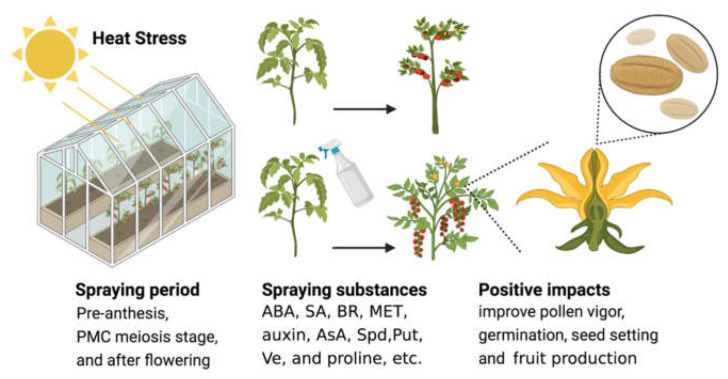
In agricultural production, exogenous spraying of plant growth regulators (PGRs) such as auxin, ABA, SA, BR, and MET can increase plant resistance under biotic and abiotic stresses, reduce damage to the tapetum layer, and improve pollen vigor and fertility (Figure 3) [97,109,123,124,125,126,127,128]. In addition to PGRs, there are other antioxidant sources such as proline, Put, Spd, Ve, and AsA that also play important roles in plant growth, development, and stress resistance [43,72,129,130,131,132,133,134,135,136,137].
Figure 3.
Regulation of redox balance in response to different abiotic stresses. In many plants (tomato, rice, wheat, and soybean), exogenous spraying of many antioxidant regulators at different stages of flower development can effectively enhance the resistance of plants and pollen under abiotic stresses, improve pollen vigor, germination rate, fruit setting rate, and yield.
Exogenous application of auxin improves the tolerance of developing pollen to continuous mild heat stress in Arabidopsis and barley (Hordeum Vulgare L.) [123]. ABA spraying on the leaf surface during the meiotic phase of pollen mother cell significantly reduces the pollen sterility caused by heat stress in rice [97]. Pretreatment with SA could alleviate damage of the spikelet differentiation during the floret differentiation stage of rice under heat stress [124]. The application of ethylene releaser before the short-term heat stress treatment improves tomato pollen thermotolerance, whereas application of an ethylene inhibitor reduced this [125]. Exogenous application of 24-epibrassinolide (EBR) and ecdysone analogs (7,8-dihydro-8α-20-hydroxyecdysone; DHECD) can effectively improve pollen vigor and germination, and reduce pollen bursting in rice [126]. BRs also promote spikelet development through increasing antioxidant system levels and energy charge during panicle development in rice [127]. Recent studies have found that exogenous MET alleviates ROS production and induces activity of antioxidant enzymes under heat stress in tomato [109]. The application of exogenous MET significantly improves the translocation of carbon assimilates to drought-stressed anthers in cotton, which helps to produce more ATP for reproductive activities, finally improving male fertility [128].
Proline is extensively involved in the response to various abiotic stresses [129,130]. Supplementing proline to mung bean (Vigna radiata) plants significantly increases its endogenous concentration in leaves, anthers, pollen grains, and ovules, and significantly improves reproductive functions under heat stress [131]. Studies have also shown that excessive production of proline in Arabidopsis pollen may increase the fertility of pollen and increase crop yields under unfavorable conditions [70,72]. Therefore, inhibiting the biosynthesis of proline in pollen can be used to create male sterility [70,72]. Exogenous application of Spd apparently alleviates heat stress-induced tapetum damage in rice [132,133,134]. In addition, exogenous Spd and Put increase their endogenous levels and improve antioxidant capacity and membrane stability, the spikelet fertility, seed setting rate, and ear yield in rice under heat stress [43,134,135]. Pre-anthesis ASA treatment before heat stress enhances the thermotolerance capacity of wheat pollens [137]. Exogenous application of Ve, glycine betaine, and SA also increases the antioxidant levels, prevents oxidative damage to plant membranes, and significantly improves the pollen germination and spikelet fertility of rice [136]. Anyway, PGRs and substances can play a regulatory role in a more refined and efficient manner; thereby, increasing plant resistance and yield; however, only a few have been promoted in actual production. Therefore, it is very necessary to develop more broad-spectrum and practical formulas and to increase the scale of practical applications in production.
5. Conclusions and Prospective
In the process of growth and development, plants are constantly stimulated by various environmental cues, which interfere with their redox homeostasis. In order to perform normal developmental activities, plants have evolved complex and sophisticated redox regulation systems. These redox processes are continuously happening inside the plant cells within certain variable range. Any deviation in redox balance within the adjustable range can trigger plant signaling cascades through various biomolecules. These redox signals (ROS/RNS) regulate diverse aspects of male gametophyte development through their crosstalk with phytohormones and other signal transduction pathways. When subjected to abiotic stresses such as high temperature, the balance between oxidants and antioxidants becomes disturbed and this results in oxidative stress. Depending on the dosage of oxidants, the cellular structure and metabolism will be damaged to varying degrees, and eventually this will lead to microspore abortion and pollen sterility. By studying the redox system during stamen development under stressful conditions, the importance of their action/interaction may be clarified. This further improves our understanding on vital prospects of the redox signaling cascade in plant growth and development. In this review, we have gathered the recent information related to redox signaling in pollen development and stress response; however, there are still many questions/areas needing to be explored thoroughly. For example, (i) how the pollen development process and tapetal PCD are precisely regulated by the redox system; (ii) what other genes or biomolecules are involved, and how the crosstalk between different signal networks is unified in the process of pollen development; and (iii) are there any other special mechanisms for the regulation of pollen resistance under abiotic stresses. Moreover, many studies have shown that PGRs and antioxidants can help improve the plants’ resistance towards stresses and regulate flowers and fruit-setting rate. However, due to the complexity of actual production conditions, the abundance of crops, and the long period of field trials and extensions, their application in agricultural production is relatively limited. Clarifying these mechanisms can help us in developing more efficient, targeted, or universally adaptable regulatory substances to support sustainable agricultural production.
Abbreviations
| 24-epibrassinolide | EBR |
| 7,8-dihydro-8α-20-hydroxyecdysone | DHECD |
| abscisic acid | ABA |
| Anther Dehiscence Gene | ADR |
| ARGONAUTE 2 | AGO2 |
| ascorbate | AsA |
| ascorbate peroxidase | APX |
| Autophagy-Related Genes | ATGs |
| Brassinazole Resistant 1 | BZR1 |
| catalase | CAT |
| CONSTANS | CO |
| cytosolic protein response | CPR |
| cysteine Cys Cystathionine β-synthase | CBS |
| Defective tapetum cell death 1 | DTC1 |
| dehydroascorbate | DHA |
| dehydroascorbate reductase | DHAR |
| Drought-Induced LTP | DIL |
| endoplasmic reticulum-unfolded protein response | ER-UPR |
| Fatty Acid Export 1 | FAX1 |
| fatty acids | FAs |
| FERONIA | FER |
| Flavonol Synthase | FLS |
| FLOWERING LOCUS T | FT |
| glutathione | GSH |
| glutathione disulfide | GSSG |
| glutathione peroxidase | GPX |
| glutathione reductase | GR |
| glutathione S-transferase | GST |
| GSNO reductase | GSNOR |
| Heat Shock Protein | HSP |
| High Expression of Osmotically Responsive Genes1 | HOS1 |
| Hexokinase 1 | HXK1 |
| hydrogen peroxide | H2O2 |
| hydroxyl radical | HO• |
| superoxide | O2•− |
| Inflorescence Deficient in Abscission | IDA |
| jasmonic acid | JA |
| lipid alkoxy | RO• |
| lipid peroxides | LOOH |
| Low Temperature Tolerance 1 | LTT1 |
| melatonin | MET |
| metallothioneins | MTs |
| male sterility 33 | MS33 |
| MYB Important for Drought Response1 | MID1 |
| nitric oxide | NO |
| nitrous oxide | N2O |
| peroxidase | POD |
| peroxiredoxin | PRX |
| peroxynitrite | ONOO− |
| phenolic acids | PhAs |
| plant growth regulators | PGRs |
| polyamines | PAs |
| programmed cell death | PCD |
| Prolyl Aminopeptidase 1 | PAP1 |
| putrescine | Put |
| reactive carbonyl species | RCS |
| reactive nitrogen species | RNS |
| reactive oxygen species | ROS |
| Respiratory Burst Oxidase Homologs | RBOHs |
| S-adenosylmethionine decarboxylase | SAMDC |
| salicylic acid | SA |
| S-nitrosoglutathione | GSNO |
| GSNO reductase | GSNOR |
| spermidine | Spd |
| spermine | Spm |
| superoxide dismutase | SOD |
| TGACG | TGA |
| thioredoxin | TRX |
| α-tocopherol | Ve |
| ubiquitin-conjugating enzyme | E2 |
| ubiquitin ligase | E3 |
| Zeaxanthin epoxidase 1 | ZEP1 |
Author Contributions
Conceptualization, J.Z.; writing—original draft preparation, D.-L.X. and J.Z.; writing—review and editing, D.-L.X., X.-L.Z., C.-Y.Z., M.K.K. and J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFD1000800), the National Natural Science Foundation of China (31922078 and 31872089), and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-0011).
Conflicts of Interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rieu I., Twell D., Firon N. Pollen Development at High Temperature: From Acclimation to Collapse. Plant Physiol. 2017;173:1967–1976. doi: 10.1104/pp.16.01644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh M.B., Lohani N., Bhalla P.L. The Role of Endoplasmic Reticulum Stress Response in Pollen Development and Heat Stress Tolerance. Front Plant Sci. 2021;12:661062. doi: 10.3389/fpls.2021.661062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Miller G., Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. ROS signaling: The new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Sankaranarayanan S., Ju Y., Kessler S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Front Plant Sci. 2020;11:1199. doi: 10.3389/fpls.2020.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Considine M.J., Foyer C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014;21:1305–1326. doi: 10.1089/ars.2013.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez J.F., Talle B., Wilson Z.A. Anther and pollen development: A conserved developmental pathway. J. Integr. Plant Biol. 2015;57:876–891. doi: 10.1111/jipb.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Q., Zhu W., Li L., Zhang S., Yin Y., Ma H., Wang X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA. 2010;107:6100–6105. doi: 10.1073/pnas.0912333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson Z.A., Song J., Taylor B., Yang C. The final split: The regulation of anther dehiscence. J. Exp. Bot. 2011;62:1633–1649. doi: 10.1093/jxb/err014. [DOI] [PubMed] [Google Scholar]
- 11.Sueldo D.J., van der Hoorn R.A.L. Plant life needs cell death, but does plant cell death need Cys proteases? FEBS J. 2017;284:1577–1585. doi: 10.1111/febs.14034. [DOI] [PubMed] [Google Scholar]
- 12.Hu L., Liang W., Yin C., Cui X., Zong J., Wang X., Hu J., Zhang D. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell. 2011;23:515–533. doi: 10.1105/tpc.110.074369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S.X., Feng Q.N., Xie H.T., Li S., Zhang Y. Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biol. 2017;17:76. doi: 10.1186/s12870-017-1025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domingos P., Prado A.M., Wong A., Gehring C., Feijo J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant. 2015;8:506–520. doi: 10.1016/j.molp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Traverso J.A., Pulido A., Rodriguez-Garcia M.I., Alche J.D. Thiol-based redox regulation in sexual plant reproduction: New insights and perspectives. Front Plant Sci. 2013;4:465. doi: 10.3389/fpls.2013.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schippers J.H., Foyer C.H., van Dongen J.T. Redox regulation in shoot growth, SAM maintenance and flowering. Curr. Opin. Plant Biol. 2016;29:121–128. doi: 10.1016/j.pbi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi P., Wiese A.J., Ghatak A., Zaveska Drabkova L., Weckwerth W., Honys D. Heat stress response mechanisms in pollen development. New Phytol. 2021;231:571–585. doi: 10.1111/nph.17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinta G., Khan A., AbdElgawad H., Verma V., Srivastava A.K. Unveiling the Redox Control of Plant Reproductive Development during Abiotic Stress. Front Plant Sci. 2016;7:700. doi: 10.3389/fpls.2016.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bose J., Rodrigo-Moreno A., Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014;65:1241–1257. doi: 10.1093/jxb/ert430. [DOI] [PubMed] [Google Scholar]
- 20.Frederickson Matika D.E., Loake G.J. Redox regulation in plant immune function. Antioxid. Redox Signal. 2014;21:1373–1388. doi: 10.1089/ars.2013.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basha E., O’Neill H., Vierling E. Small heat shock proteins and alpha-crystallins: Dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B., Sun C., Lin X., Busch W. The Emerging Role of GSNOR in Oxidative Stress Regulation. Trends Plant Sci. 2021;26:156–168. doi: 10.1016/j.tplants.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013;48:332–356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H.T., Wan Z.Y., Li S., Zhang Y. Spatiotemporal Production of Reactive Oxygen Species by NADPH Oxidase Is Critical for Tapetal Programmed Cell Death and Pollen Development in Arabidopsis. Plant Cell. 2014;26:2007–2023. doi: 10.1105/tpc.114.125427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z., Shi X., Li S., Zhang L., Song X. Oxidative Stress and Aberrant Programmed Cell Death Are Associated With Pollen Abortion in Isonuclear Alloplasmic Male-Sterile Wheat. Front. Plant Sci. 2018;9:595. doi: 10.3389/fpls.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura S., Waszczak C., Hunter K., Wrzaczek M. Bound by Fate: The Role of Reactive Oxygen Species in Receptor-Like Kinase Signaling. Plant Cell. 2017;29:638–654. doi: 10.1105/tpc.16.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurusu T., Kuchitsu K. Autophagy, programmed cell death and reactive oxygen species in sexual reproduction in plants. J. Plant Res. 2017;130:491–499. doi: 10.1007/s10265-017-0934-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhu T., Li Z., An X., Long Y., Xue X., Xie K., Ma B., Zhang D., Guan Y., Niu C., et al. Normal Structure and Function of Endothecium Chloroplasts Maintained by ZmMs33-Mediated Lipid Biosynthesis in Tapetal Cells Are Critical for Anther Development in Maize. Mol. Plant. 2020;13:1624–1643. doi: 10.1016/j.molp.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Mhamdi A., Van Breusegem F. Reactive oxygen species in plant development. Development. 2018;145:dev164376. doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- 30.Gilroy S., Bialasek M., Suzuki N., Gorecka M., Devireddy A.R., Karpinski S., Mittler R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016;171:1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan M.Y., Xie D.L., Cao J.J., Xia X.J., Shi K., Zhou Y.H., Zhou J., Foyer C.H., Yu J.Q. Brassinosteroid-mediated reactive oxygen species are essential for tapetum degradation and pollen fertility in tomato. Plant J. 2020;102:931–947. doi: 10.1111/tpj.14672. [DOI] [PubMed] [Google Scholar]
- 32.Dai S.Y., Hsu W.H., Yang C.H. The Gene Anther Dehiscence Repressor (ADR) Controls Male Fertility by Suppressing the ROS Accumulation and Anther Cell Wall Thickening in Arabidopsis. Sci. Rep. 2019;9:5112. doi: 10.1038/s41598-019-41382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R., Shi C., Wang X., Li R., Meng Y., Cheng L., Qi M., Xu T., Li T. Tomato SlIDA has a critical role in tomato fertilization by modifying reactive oxygen species homeostasis. Plant J. 2020;103:2100–2118. doi: 10.1111/tpj.14886. [DOI] [PubMed] [Google Scholar]
- 34.Zheng S., Li J., Ma L., Wang H., Zhou H., Ni E., Jiang D., Liu Z., Zhuang C. OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proc. Natl. Acad. Sci. USA. 2019;116:7549–7558. doi: 10.1073/pnas.1817675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi J., Moon S., Lee Y.S., Zhu L., Liang W., Zhang D., Jung K.H., An G. Defective Tapetum Cell Death 1 (DTC1) Regulates ROS Levels by Binding to Metallothionein during Tapetum Degeneration. Plant Physiol. 2016;170:1611–1623. doi: 10.1104/pp.15.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J., Cui M., Yang L., Kim Y.J., Zhang D. Genetic and Biochemical Mechanisms of Pollen Wall Development. Trends Plant Sci. 2015;20:741–753. doi: 10.1016/j.tplants.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Ding Z., Vizcay-Barrena G., Shi J., Liang W., Yuan Z., Werck-Reichhart D., Schreiber L., Wilson Z.A., Zhang D. Aborted microspores Acts as a Master Regulator of Pollen Wall Formation in Arabidopsis. Plant Cell. 2014;26:1544–1556. doi: 10.1105/tpc.114.122986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu L., He S., Liu Y., Shi J., Xu J. Arabidopsis FAX1 mediated fatty acid export is required for the transcriptional regulation of anther development and pollen wall formation. Plant Mol. Biol. 2020;104:187–201. doi: 10.1007/s11103-020-01036-5. [DOI] [PubMed] [Google Scholar]
- 39.Feijo J.A., Costa S.S., Prado A.M., Becker J.D., Certal A.C. Signalling by tips. Curr. Opin. Plant Biol. 2004;7:589–598. doi: 10.1016/j.pbi.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Bright J., Hiscock S.J., James P.E., Hancock J.T. Pollen generates nitric oxide and nitrite: A possible link to pollen-induced allergic responses. Plant Physiol. Biochem. 2009;47:49–55. doi: 10.1016/j.plaphy.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Zafra A., Rodriguez-Garcia M.I., Alche Jde D. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 2010;10:36. doi: 10.1186/1471-2229-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prado A.M., Colaco R., Moreno N., Silva A.C., Feijo J.A. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Mol. Plant. 2008;1:703–714. doi: 10.1093/mp/ssn034. [DOI] [PubMed] [Google Scholar]
- 43.Duan Q., Liu M.J., Kita D., Jordan S.S., Yeh F.J., Yvon R., Carpenter H., Federico A.N., Garcia-Valencia L.E., Eyles S.J., et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature. 2020;579:561–566. doi: 10.1038/s41586-020-2106-2. [DOI] [PubMed] [Google Scholar]
- 44.Airaki M., Sanchez-Moreno L., Leterrier M., Barroso J.B., Palma J.M., Corpas F.J. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant Cell Physiol. 2011;52:2006–2015. doi: 10.1093/pcp/pcr133. [DOI] [PubMed] [Google Scholar]
- 45.Gong B., Yan Y., Zhang L., Cheng F., Liu Z., Shi Q. Unravelling GSNOR-Mediated S-Nitrosylation and Multiple Developmental Programs in Tomato Plants. Plant Cell Physiol. 2019;60:2523–2537. doi: 10.1093/pcp/pcz143. [DOI] [PubMed] [Google Scholar]
- 46.Su T., Wang P., Li H., Zhao Y., Lu Y., Dai P., Ren T., Wang X., Li X., Shao Q., et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018;60:591–607. doi: 10.1111/jipb.12649. [DOI] [PubMed] [Google Scholar]
- 47.Guan L.M., Scandalios J.G. Catalase transcript accumulation in response to dehydration and osmotic stress in leaves of maize viviparous mutants. Redox Rep. 2000;5:377–383. doi: 10.1179/135100000101535951. [DOI] [PubMed] [Google Scholar]
- 48.Bredemeijer G. The role of peroxidases in pistil-pollen interactions. Theor. Appl. Genet. 1984;68:193–206. doi: 10.1007/BF00266889. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z., Shi X., Li S., Hu G., Zhang L., Song X. Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat. Int. J. Mol. Sci. 2018;19:1708. doi: 10.3390/ijms19061708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer Y., Belin C., Delorme-Hinoux V., Reichheld J.P., Riondet C. Thioredoxin and glutaredoxin systems in plants: Molecular mechanisms, crosstalks, and functional significance. Antioxid. Redox Signal. 2012;17:1124–1160. doi: 10.1089/ars.2011.4327. [DOI] [PubMed] [Google Scholar]
- 51.Kelliher T., Walbot V. Hypoxia triggers meiotic fate acquisition in maize. Science. 2012;337:345–348. doi: 10.1126/science.1220080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing S., Rosso M.G., Zachgo S. ROXY1, a member of the plant glutaredoxin family, is required for petal development in Arabidopsis thaliana. Development. 2005;132:1555–1565. doi: 10.1242/dev.01725. [DOI] [PubMed] [Google Scholar]
- 53.Hong L., Tang D., Zhu K., Wang K., Li M., Cheng Z. Somatic and reproductive cell development in rice anther is regulated by a putative glutaredoxin. Plant Cell. 2012;24:577–588. doi: 10.1105/tpc.111.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing S., Zachgo S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008;53:790–801. doi: 10.1111/j.1365-313X.2007.03375.x. [DOI] [PubMed] [Google Scholar]
- 55.Murmu J., Bush M.J., DeLong C., Li S., Xu M., Khan M., Malcolmson C., Fobert P.R., Zachgo S., Hepworth S.R. Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol. 2010;154:1492–1504. doi: 10.1104/pp.110.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H., Li Y., Huang X. Genome-Wide Analysis of the Thioredoxin Gene Family in Gossypium hirsutum L. and the Role of the Atypical Thioredoxin Gene GhTRXL3-2 in Flowering. J. Plant Biol. 2021;64:461–473. doi: 10.1007/s12374-021-09318-1. [DOI] [Google Scholar]
- 57.Yoo K.S., Ok S.H., Jeong B.C., Jung K.W., Cui M.H., Hyoung S., Lee M.R., Song H.K., Shin J.S. Single cystathionine beta-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell. 2011;23:3577–3594. doi: 10.1105/tpc.111.089847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung K.W., Kim Y.Y., Yoo K.S., Ok S.H., Cui M.H., Jeong B.C., Yoo S.D., Jeung J.U., Shin J.S. A cystathionine-beta-synthase domain-containing protein, CBSX2, regulates endothecial secondary cell wall thickening in anther development. Plant Cell Physiol. 2013;54:195–208. doi: 10.1093/pcp/pcs166. [DOI] [PubMed] [Google Scholar]
- 59.Shin J.S., So W.M., Kim S.Y., Noh M., Hyoung S., Yoo K.S., Shin J.S. CBSX3-Trxo-2 regulates ROS generation of mitochondrial complex II (Succinate dehydrogenase) in Arabidopsis. Plant Sci. 2020;294:110458. doi: 10.1016/j.plantsci.2020.110458. [DOI] [PubMed] [Google Scholar]
- 60.Shigeto J., Tsutsumi Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016;209:1395–1402. doi: 10.1111/nph.13738. [DOI] [PubMed] [Google Scholar]
- 61.Jacobowitz J.R., Doyle W.C., Weng J.K. PRX9 and PRX40 Are Extensin Peroxidases Essential for Maintaining Tapetum and Microspore Cell Wall Integrity during Arabidopsis Anther Development. Plant Cell. 2019;31:848–861. doi: 10.1105/tpc.18.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chin D.C., Hsieh C.C., Lin H.Y., Yeh K.W. A Low Glutathione Redox State Couples with a Decreased Ascorbate Redox Ratio to Accelerate Flowering in Oncidium Orchid. Plant Cell Physiol. 2016;57:423–436. doi: 10.1093/pcp/pcv206. [DOI] [PubMed] [Google Scholar]
- 63.Ding X., Wang X., Li Q., Yu L., Song Q., Gai J., Yang S. Metabolomics Studies on Cytoplasmic Male Sterility during Flower Bud Development in Soybean. Int. J. Mol. Sci. 2019;20:2869. doi: 10.3390/ijms20122869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noctor G., Mhamdi A., Queval G., Foyer C.H. Regulating the redox gatekeeper: Vacuolar sequestration puts glutathione disulfide in its place. Plant Physiol. 2013;163:665–671. doi: 10.1104/pp.113.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foyer C.H., Noctor G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016;39:951–964. doi: 10.1111/pce.12621. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Quiros E., Alche J.D., Karpinska B., Foyer C.H. Glutathione redox state plays a key role in flower development and pollen vigour. J. Exp. Bot. 2020;71:730–741. doi: 10.1093/jxb/erz376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kocsy G., Tari I., Vankova R., Zechmann B., Gulyas Z., Poor P., Galiba G. Redox control of plant growth and development. Plant Sci. 2013;211:77–91. doi: 10.1016/j.plantsci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Bashandy T., Guilleminot J., Vernoux T., Caparros-Ruiz D., Ljung K., Meyer Y., Reichheld J.P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell. 2010;22:376–391. doi: 10.1105/tpc.109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deslous P., Bournonville C., Decros G., Okabe Y., Mauxion J.P., Jorly J., Gadin S., Bres C., Mori K., Ferrand C., et al. Overproduction of ascorbic acid impairs pollen fertility in tomato. J. Exp. Bot. 2021;72:3091–3107. doi: 10.1093/jxb/erab040. [DOI] [PubMed] [Google Scholar]
- 70.Mattioli R., Biancucci M., Lonoce C., Costantino P., Trovato M. Proline is required for male gametophyte development in Arabidopsis. BMC Plant Biol. 2012;12:236. doi: 10.1186/1471-2229-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falasca G., Franceschetti M., Bagni N., Altamura M.M., Biasi R. Polyamine biosynthesis and control of the development of functional pollen in kiwifruit. Plant Physiol. Biochem. 2010;48:565–573. doi: 10.1016/j.plaphy.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Mattioli R., Biancucci M., El Shall A., Mosca L., Costantino P., Funck D., Trovato M. Proline synthesis in developing microspores is required for pollen development and fertility. BMC Plant Biol. 2018;18:356. doi: 10.1186/s12870-018-1571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shan X., Li Y., Yang S., Yang Z., Qiu M., Gao R., Han T., Meng X., Xu Z., Wang L., et al. The spatio-temporal biosynthesis of floral flavonols is controlled by differential phylogenetic MYB regulators in Freesia hybrida. New Phytol. 2020;228:1864–1879. doi: 10.1111/nph.16818. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen N.H., Kim J.H., Kwon J., Jeong C.Y., Lee W., Lee D., Hong S.W., Lee H. Characterization of Arabidopsis thaliana Flavonol Synthase 1 (FLS1)—Overexpression plants in response to abiotic stress. Plant Physiol. Biochem. 2016;103:133–142. doi: 10.1016/j.plaphy.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X., He Y., Li L., Liu H., Hong G. Involvement of the R2R3-MYB transcription factor MYB21 and its homologs in regulating flavonol accumulation in Arabidopsis stamen. J. Exp. Bot. 2021;72:4319–4332. doi: 10.1093/jxb/erab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biancucci M., Mattioli R., Forlani G., Funck D., Costantino P., Trovato M. Role of proline and GABA in sexual reproduction of angiosperms. Front Plant Sci. 2015;6:680. doi: 10.3389/fpls.2015.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghifari A.S., Teixeira P.F., Kmiec B., Singh N., Glaser E., Murcha M.W. The dual-targeted prolyl aminopeptidase PAP1 is involved in proline accumulation in response to stress and during pollen development. J. Exp. Bot. 2021;73:78–93. doi: 10.1093/jxb/erab397. [DOI] [PubMed] [Google Scholar]
- 78.Chen D., Shao Q., Yin L., Younis A., Zheng B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front Plant Sci. 2018;9:1945. doi: 10.3389/fpls.2018.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reichheld J.P., Khafif M., Riondet C., Droux M., Bonnard G., Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell. 2007;19:1851–1865. doi: 10.1105/tpc.107.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marty L., Siala W., Schwarzlander M., Fricker M.D., Wirtz M., Sweetlove L.J., Meyer Y., Meyer A.J., Reichheld J.P., Hell R. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:9109–9114. doi: 10.1073/pnas.0900206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Battat M., Eitan A., Rogachev I., Hanhineva K., Fernie A., Tohge T., Beekwilder J., Aharoni A. A MYB Triad Controls Primary and Phenylpropanoid Metabolites for Pollen Coat Patterning. Plant Physiol. 2019;180:87–108. doi: 10.1104/pp.19.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alberto Acevedo J.G.S. Catalase and superoxide dismutase gene expression and distribution during stem development in maize. Dev. Genet. 1991;12:423–430. doi: 10.1002/dvg.1020120607. [DOI] [Google Scholar]
- 83.De Storme N., Geelen D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014;37:1–18. doi: 10.1111/pce.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma K.D., Nayyar H. Regulatory Networks in Pollen Development under Cold Stress. Front Plant Sci. 2016;7:402. doi: 10.3389/fpls.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Mhamdi A., Queval G., Chaouch S., Vanderauwera S., Van Breusegem F., Noctor G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010;61:4197–4220. doi: 10.1093/jxb/erq282. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Q., Zhou L., Liu J., Cao Z., Du X., Huang F., Pan G., Cheng F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018;37:741–757. doi: 10.1007/s00299-018-2264-y. [DOI] [PubMed] [Google Scholar]
- 88.Dong B., Zheng X., Liu H., Able J.A., Yang H., Zhao H., Zhang M., Qiao Y., Wang Y., Liu M. Effects of Drought Stress on Pollen Sterility, Grain Yield, Abscisic Acid and Protective Enzymes in Two Winter Wheat Cultivars. Front Plant Sci. 2017;8:1008. doi: 10.3389/fpls.2017.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Brunetti C., Di Ferdinando M., Fini A., Pollastri S., Tattini M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013;14:3540–3555. doi: 10.3390/ijms14023540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez V., Mestre T.C., Rubio F., Girones-Vilaplana A., Moreno D.A., Mittler R., Rivero R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front Plant Sci. 2016;7:838. doi: 10.3389/fpls.2016.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paupiere M.J., Muller F., Li H., Rieu I., Tikunov Y.M., Visser R.G.F., Bovy A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017;30:81–94. doi: 10.1007/s00497-017-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cetinbas-Genc A., Cai G., Del Duca S. Treatment with spermidine alleviates the effects of concomitantly applied cold stress by modulating Ca(2+), pH and ROS homeostasis, actin filament organization and cell wall deposition in pollen tubes of Camellia sinensis. Plant Physiol. Biochem. 2020;156:578–590. doi: 10.1016/j.plaphy.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Q., Zhou L., Liu J., Du X., Asad M.A., Huang F., Pan G., Cheng F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol. Biochem. 2018;122:90–101. doi: 10.1016/j.plaphy.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Guo C., Yao L., You C., Wang S., Cui J., Ge X., Ma H. MID1 plays an important role in response to drought stress during reproductive development. Plant. J. 2016;88:280–293. doi: 10.1111/tpj.13250. [DOI] [PubMed] [Google Scholar]
- 96.Xu Y., Wang R., Wang Y., Zhang L., Yao S. A point mutation in LTT1 enhances cold tolerance at the booting stage in rice. Plant Cell Environ. 2020;43:992–1007. doi: 10.1111/pce.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rezaul I.M., Baohua F., Tingting C., Weimeng F., Caixia Z., Longxing T., Guanfu F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019;165:644–663. doi: 10.1111/ppl.12759. [DOI] [PubMed] [Google Scholar]
- 98.Guo C., Ge X., Ma H. The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol. Biol. 2013;82:239–253. doi: 10.1007/s11103-013-0057-9. [DOI] [PubMed] [Google Scholar]
- 99.Sharma L., Dalal M., Verma R.K., Kumar S.V.V., Yadav S.K., Pushkar S., Kushwaha S.R., Bhowmik A., Chinnusamy V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018;150:9–24. doi: 10.1016/j.envexpbot.2018.02.013. [DOI] [Google Scholar]
- 100.Yang J., Fei K., Chen J., Wang Z., Zhang W., Zhang J. Jasmonates alleviate spikelet-opening impairment caused by high temperature stress during anthesis of photo-thermo-sensitive genic male sterile rice lines. Food Energy Secur. 2020;9:e233. doi: 10.1002/fes3.233. [DOI] [Google Scholar]
- 101.Feng B., Zhang C., Chen T., Zhang X., Tao L., Fu G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018;18:245. doi: 10.1186/s12870-018-1472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank G., Pressman E., Ophir R., Althan L., Shaked R., Freedman M., Shen S., Firon N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bita C.E., Zenoni S., Vriezen W.H., Mariani C., Pezzotti M., Gerats T. Temperature stress differentially modulates transcription in meiotic anthers of heat-tolerant and heat-sensitive tomato plants. BMC Genet. 2011;12:384. doi: 10.1186/1471-2164-12-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiran A., Sharma P.N., Awasthi R., Nayyar H., Seth R., Chandel S.S., Siddique K.H.M., Zinta G., Sharma K.D. Disruption of carbohydrate and proline metabolism in anthers under low temperature causes pollen sterility in chickpea. Environ. Exp. Bot. 2021;188:104500. doi: 10.1016/j.envexpbot.2021.104500. [DOI] [Google Scholar]
- 105.Chaturvedi P., Doerfler H., Jegadeesan S., Ghatak A., Pressman E., Castillejo M.A., Wienkoop S., Egelhofer V., Firon N., Weckwerth W. Heat-Treatment-Responsive Proteins in Different Developmental Stages of Tomato Pollen Detected by Targeted Mass Accuracy Precursor Alignment (tMAPA) J. Proteome Res. 2015;14:4463–4471. doi: 10.1021/pr501240n. [DOI] [PubMed] [Google Scholar]
- 106.Guo M., Liu J.H., Ma X., Luo D.X., Gong Z.H., Lu M.H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front Plant. Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacob P., Hirt H., Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017;15:405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X., Xiong H., Liu A., Zhou X., Peng Y., Li Z., Luo G., Tian X., Chen X. Microarray data uncover the genome-wide gene expression patterns in response to heat stress in rice post-meiosis panicle. J. Plant Biol. 2014;57:327–336. doi: 10.1007/s12374-014-0177-z. [DOI] [Google Scholar]
- 109.Qi Z.Y., Wang K.X., Yan M.Y., Kanwar M.K., Li D.Y., Wijaya L., Alyemeni M.N., Ahmad P., Zhou J. Melatonin Alleviates High Temperature-Induced Pollen Abortion in Solanum lycopersicum. Molecules. 2018;23:386. doi: 10.3390/molecules23020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minibayeva F., Dmitrieva S., Ponomareva A., Ryabovol V. Oxidative stress-induced autophagy in plants: The role of mitochondria. Plant Physiol. Biochem. 2012;59:11–19. doi: 10.1016/j.plaphy.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 111.Smalle J., Vierstra R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 112.Lamming D.W., Bar-Peled L. Lysosome: The metabolic signaling hub. Traffic. 2019;20:27–38. doi: 10.1111/tra.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee K.H., Minami A., Marshall R.S., Book A.J., Farmer L.M., Walker J.M., Vierstra R.D. The RPT2 subunit of the 26S proteasome directs complex assembly, histone dynamics, and gametophyte and sporophyte development in Arabidopsis. Plant Cell. 2011;23:4298–4317. doi: 10.1105/tpc.111.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu F.Q., Xue H.W. The ubiquitin-proteasome system in plant responses to environments. Plant Cell Environ. 2019;42:2931–2944. doi: 10.1111/pce.13633. [DOI] [PubMed] [Google Scholar]
- 115.Shu K., Yang W. E3 Ubiquitin Ligases: Ubiquitous Actors in Plant Development and Abiotic Stress Responses. Plant Cell Physiol. 2017;58:1461–1476. doi: 10.1093/pcp/pcx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stone S.L. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front Plant Sci. 2014;5:135. doi: 10.3389/fpls.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazaro A., Valverde F., Pineiro M., Jarillo J.A. The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell. 2012;24:982–999. doi: 10.1105/tpc.110.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung J.H., Seo P.J., Park C.M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating constans degradation under cold stress. J. Biol. Chem. 2012;287:43277–43287. doi: 10.1074/jbc.M112.394338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S., Yan H., Mei W.M., Tse Y.C., Wang H. Boosting autophagy in sexual reproduction: A plant perspective. New Phytol. 2020;226:679–689. doi: 10.1111/nph.16414. [DOI] [PubMed] [Google Scholar]
- 120.Norizuki T., Minamino N., Ueda T. Role of Autophagy in Male Reproductive Processes in Land Plants. Front. Plant Sci. 2020;11:756. doi: 10.3389/fpls.2020.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanamata S., Kurusu T., Kuchitsu K. Roles of autophagy in male reproductive development in plants. Front Plant Sci. 2014;5:457. doi: 10.3389/fpls.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barany I., Berenguer E., Solis M.T., Perez-Perez Y., Santamaria M.E., Crespo J.L., Risueno M.C., Diaz I., Testillano P.S. Autophagy is activated and involved in cell death with participation of cathepsins during stress-induced microspore embryogenesis in barley. J. Exp. Bot. 2018;69:1387–1402. doi: 10.1093/jxb/erx455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakata T., Oshino T., Miura S., Tomabechi M., Tsunaga Y., Higashitani N., Miyazawa Y., Takahashi H., Watanabe M., Higashitani A. Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. USA. 2010;107:8569–8574. doi: 10.1073/pnas.1000869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang C.X., Feng B.H., Chen T.T., Zhang X.F., Tao L.X., Fu G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017;83:313–323. doi: 10.1007/s10725-017-0296-x. [DOI] [Google Scholar]
- 125.Firon N., Pressman E., Meir S., Khoury R., Altahan L. Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat-stress conditions. AoB Plants. 2012;2012:pls024. doi: 10.1093/aobpla/pls024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thussagunpanit J., Jutamanee K., Kaveeta L., Chai-arree W., Pankean P., Suksamrarn A. Effects of a brassinosteroid and an ecdysone analogue on pollen germination of rice under heat stress. J. Pestic. Sci. 2013;38:105–111. doi: 10.1584/jpestics.D13-029. [DOI] [Google Scholar]
- 127.Zhang W., Zhu K., Wang Z., Zhang H., Gu J., Liu L., Yang J., Zhang J. Brassinosteroids function in spikelet differentiation and degeneration in rice. J. Integr. Plant Biol. 2019;61:943–963. doi: 10.1111/jipb.12722. [DOI] [PubMed] [Google Scholar]
- 128.Hu W., Cao Y., Loka D.A., Harris-Shultz K.R., Reiter R.J., Ali S., Liu Y., Zhou Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Biochem. 2020;151:579–588. doi: 10.1016/j.plaphy.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 129.Liang X., Zhang L., Natarajan S.K., Becker D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013;19:998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Freitas P.A.F., de Souza Miranda R., Marques E.C., Prisco J.T., Gomes-Filho E. Salt Tolerance Induced by Exogenous Proline in Maize Is Related to Low Oxidative Damage and Favorable Ionic Homeostasis. J. Plant Growth Regul. 2018;37:911–924. doi: 10.1007/s00344-018-9787-x. [DOI] [Google Scholar]
- 131.Priya M., Sharma L., Singh I., Bains T.S., Siddique K.H.M., H B., Nair R.M., Nayyar H. Securing reproductive function in mungbean grown under high temperature environment with exogenous application of proline. Plant Physiol. Biochem. 2019;140:136–150. doi: 10.1016/j.plaphy.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Endo M., Tsuchiya T., Hamada K., Kawamura S., Yano K., Ohshima M., Higashitani A., Watanabe M., Kawagishi-Kobayashi M. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 2009;50:1911–1922. doi: 10.1093/pcp/pcp135. [DOI] [PubMed] [Google Scholar]
- 133.Zhou R., Hu Q., Pu Q., Chen M., Zhu X., Gao C., Zhou G., Liu L., Wang Z., Yang J., et al. Spermidine Enhanced Free Polyamine Levels and Expression of Polyamine Biosynthesis Enzyme Gene in Rice Spikelets under Heat Tolerance before Heading. Sci. Rep. 2020;10:8976. doi: 10.1038/s41598-020-64978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang S., Zhang H., Li L., Liu X., Chen L., Chen W., Ding Y. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018;45:911–921. doi: 10.1071/FP17149. [DOI] [PubMed] [Google Scholar]
- 135.Das A., Karwa S., Taunk J., Bahuguna R.N., Chaturvedi A.K., Kumar P., Chinnusamy V., Pal M. Putrescine exogenous application alleviates oxidative stress in reproductive tissue under high temperature in rice. Plant Physiol. Rep. 2021;26:381–391. doi: 10.1007/s40502-021-00590-4. [DOI] [Google Scholar]
- 136.Mohammed A.R., Tarpley L. High nighttime temperatures affect rice productivity through altered pollen germination and spikelet fertility. Agric. For. Meteorol. 2009;149:999–1008. doi: 10.1016/j.agrformet.2008.12.003. [DOI] [Google Scholar]
- 137.Kumar R.R., Goswami S., Gadpayle K.A., Singh K., Sharma S.K., Singh G.P., Pathak H., Rai R.D. Ascorbic acid at pre-anthesis modulate the thermotolerance level of wheat (Triticum aestivum) pollen under heat stress. J. Plant Biochem. Biotechnol. 2013;23:293–306. doi: 10.1007/s13562-013-0214-x. [DOI] [Google Scholar]