Abstract
The emergent issue of carbapenem-resistant Acinetobacter baumannii (A. baumannii) and Pseudomonas aeruginosa (P. aeruginosa) is a major problem in Thailand. The wide use of carbapenems can increase selective pressure of bacterial resistance. The objective of this study was to determine the relationship between carbapenem consumption and the susceptibility rates of A. baumannii and P. aeruginosa, including multi-drug resistance (MDR) strains. This was a retrospective study. Carbapenem consumption and susceptibility profiles were collected from 2007 to 2013 at the Her Royal Highness Princess Maha Chakri Sirindhorn Medical Center, Thailand. We found that the susceptibility rate of A. baumannii to imipenem and meropenem from the sputum and the bronchoalveolar lavage (BAL) specimens was significantly decreased during the study period, but no significant change was found in the P. aeruginosa data. The relationship between carbapenem consumption and the susceptibility rate of A. baumannii had a clear association with the use of ertapenem. We found a statistically significant negative correlation between ertapenem consumption and the susceptibility rate of A. baumannii to imipenem (r = −0.91; p = 0.004) and meropenem (r = −0.97; p = 0.000) in the data from the non-ICU wards. In addition, imipenem use had a moderate negative correlation with the MDR P. aeruginosa data but no statistical significance (r = −0.714; p > 0.05). In conclusion, our study suggested there is an association between carbapenem use and the susceptibility of A. baumannii and P. aeruginosa. Notwithstanding this, information on ecological factors should be considered for further study. These findings showed the need to optimize the carbapenem prescription policy. Avoiding carbapenem overuse and rethinking the appropriate initial therapy might decrease the rate of resistant organisms.
Keywords: carbapenem consumption, A. baumannii, P. aeruginosa, multidrug-resistant A. baumannii, multidrug-resistant P. aeruginosa
1. Introduction
Antimicrobial resistance (AMR) has become a global public health concern for the last two decades. The multidrug-resistant Gram-negative bacteria (MDRGN), including carbapenem-resistant Gram-negative bacteria, poses a major problem related to increased hospital length of stay, healthcare costs and mortality rates [1,2,3]. The high prevalence of MDRGN has been reported in the Southeast Asian region, including Thailand, especially for Acinetobacter baumannii (A. baumannii) and Pseudomonas aeruginosa (P. aeruginosa). In this region, carbapenem–resistant Acinetobacter baumannii (CRAB) was the most common pathogen associated with nosocomial infections, followed by carbapenem-resistant Pseudomonas aeruginosa (CRPA), which are concerning in the face of difficult-to-treat infections [4,5,6,7]. In Thailand, the prevalence of Gram-negative nosocomial infections has been increasing since 2006, especially in the university hospital setting [8,9]. A. baumannii has caused nosocomial outbreaks with multidrug or carbapenem resistance, and it has rapidly increased in all regions of Thailand since 2000 [8,9,10,11,12,13]. Moreover, the rates of the multidrug-resistant P. aeruginosa (MDR-P. aeruginosa) with carbapenem resistance were found to be 71.65% among the tertiary hospitals across Thailand in last decade [14]. The overuse of antibiotics has been associated with the development of AMR, accelerated by selective pressure on the bacteria [15,16,17,18].
Carbapenems, broad-spectrum antibiotics, have been widely used for empirical treatment of nosocomial infections caused by Gram-negative bacteria [19,20]. They are usually reserved for the treatment of infections caused by MDRGN. Resistance to carbapenems in A. baumannii and P. aeruginosa could be explained by several mechanisms: carbapenemase, efflux pumps and decreased outer membrane permeability [19,20,21,22]. A correlation between carbapenem consumption and the rate of CRAB and CRPA has been described in several studies [23,24,25,26]. An antimicrobial stewardship program was an important tool to prevent and control AMR [27,28,29,30,31]. However, there are limited data on the impact of carbapenem consumption that focuses on CRAB and CRPA in Thailand. Therefore, this study aimed to determine the relationship between carbapenem consumption and the susceptibility of A. baumannii and P. aeruginosa in a tertiary care hospital.
2. Results
2.1. Carbapenem Consumption
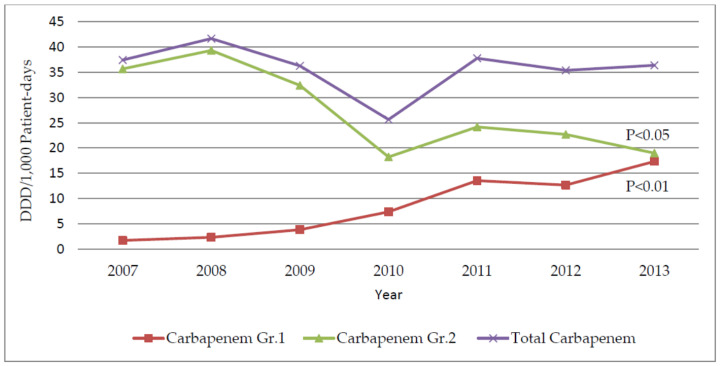
The DDD/1000 patient-days of group 1 carbapenems (ertapenem) was significantly increased over time from 1.75 to 17.36 DDD per 1000 patient-days (r = 0.97; p = 0.000) after introducing the carbapenem control program (CCP). In contrast, the use of group 2 carbapenems was significant decreased from 2007–2013 (r = −0.84; p = 0.018). The carbapenem consumption intensities are presented in Figure 1 and Table 1.
Figure 1.
The annual data of the group 1 carbapenems, group 2 carbapenems and total carbapenems consumption.
Table 1.
The annual consumption of carbapenems (DDD/1000 patient-days) from 2007–2013.
| Carbapenems Consumption (DDD/1000 Patient-Days) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Meropenem | Imipenem | Doripenem | Ertapenem | Gr1 | Gr2 | Total |
| 2007 # | 23.23 | 12.42 | 0 | 1.75 | 1.75 | 35.65 | 37.40 |
| 2008 | 37.19 | 1.81 | 0.29 | 2.35 | 2.35 | 39.29 | 41.64 |
| 2009 | 25.36 | 1.59 | 5.44 | 3.86 | 3.86 | 32.39 | 36.25 |
| 2010 | 13.56 | 3.21 | 1.47 | 7.39 | 7.39 | 18.24 | 25.63 |
| 2011 | 20.28 | 3.9 | 0 | 13.57 | 13.57 | 24.18 | 37.75 |
| 2012 | 19.67 | 3.02 | 0 | 12.67 | 12.67 | 22.69 | 35.36 |
| 2013 | 15.27 | 3.73 | 0 | 17.36 | 17.36 | 19.00 | 36.36 |
| r | −0.63 a | −0.44 b | −0.23 b | 0.97 a | 0.97 a | −0.84 a | −0.22 a |
| p value | 0.129 | 0.319 | 0.620 | 0.000 ** | 0.000 ** | 0.018 * | 0.631 |
# the carbapenem consumption was collected for 11 months; a, statistic calculation was based on the Pearson’s correlation coefficient; b, statistic calculation was based on the Spearman’s correlation coefficient; * Correlation is significant at the 0.05 level (2-tailed); ** Correlation is significant at the 0.01 level (2-tailed).
2.2. Microbiology and the Susceptibility Profiles of A. baumannii and P. aeruginosa
A total of 1352 non-duplicated A. baumannii isolates and a total of 1386 non-duplicated P. aeruginosa isolates were collected during the study period. We found that the susceptibility rates of A. baumannii and P. aeruginosa to imipenem and meropenem were different among the specimens and the wards. The result from the blood specimens showed that the susceptibility rate of A. baumannii did not significantly change over time. However, the data from the sputum and the bronchoalveolar lavage (BAL) specimens showed that the susceptibility of A. baumannii to meropenem was significant for a negative correlation over time in the data from all wards (r = −0.83; p = 0.021). The data from the non-ICU wards showed significantly decreased susceptibility of A. baumannii to imipenem (r = −0.92; p = 0.003) and meropenem (r = −0.97; p = 0.000). However, no significant change was found for the susceptibility rate of P. aeruginosa in all specimens. These susceptibility profiles are shown in Table 2 and Table 3.
Table 2.
The susceptibility rates of A. baumannii in different ward classification from 2007 to 2013 (n = 1352).
| Isolates (n) | Wards | Antimicrobial Agents | Antimicrobials Susceptibility (%) by Year | Correlation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | r | p | |||
| Blood (154) |
All wards (154) |
Imipenem/cilastatin | 42.86 | 36.36 | 50.00 | 57.89 | 32.00 | 33.33 | 34.38 | −0.39 | 0.387 |
| Meropenem | 42.86 | 36.36 | 52.38 | 57.89 | 32.00 | 33.33 | 30.00 | −0.46 | 0.294 | ||
| MDR-AB | 57.14 | 61.54 | 42.31 | 42.86 | 72.00 | 72.73 | 63.64 | 0.44 | 0.318 | ||
| Non-ICU wards (84) |
Imipenem/cilastatin | 28.57 | 60.00 | 50.00 | 84.62 | 35.29 | 50.00 | 50.00 | 0.13 | 0.788 | |
| Meropenem | 28.57 | 60.00 | 54.55 | 84.62 | 35.29 | 50.00 | 47.06 | 0.07 | 0.884 | ||
| MDR-AB | 71.43 | 50.00 | 42.86 | 23.08 | 70.59 | 60.00 | 52.94 | −0.04 | 0.940 | ||
| ICU wards (70) |
Imipenem/cilastatin | 57.14 | 16.67 | 50.00 | 0.00 | 25.00 | 16.67 | 14.29 | −0.58 | 0.175 | |
| Meropenem | 57.14 | 16.67 | 50.00 | 0.00 | 25.00 | 16.67 | 7.69 | −0.63 | 0.131 | ||
| MDR-AB | 42.86 | 71.43 | 41.67 | 75.00 | 75.00 | 83.33 | 75.00 | 0.33 | 0.465 | ||
| Sputum and BAL (1198) |
All wards (1198) |
Imipenem/cilastatin | 25.25 | 20.00 | 18.40 | 19.55 | 19.31 | 20.43 | 14.90 | −0.74 | 0.059 |
| Meropenem | 24.49 | 20.69 | 18.01 | 19.55 | 17.59 | 19.46 | 13.56 | −0.83 * | 0.021 | ||
| MDR-AB | 49.36 | 78.23 | 83.95 | 81.54 | 39.59 | 75.51 | 84.76 | 0.07 | 0.879 | ||
| Non-ICU wards (694) |
Imipenem/cilastatin | 24.53 | 22.22 | 23.30 | 21.62 | 20.61 | 19.19 | 15.18 | −0.92 ** | 0.003 | |
| Meropenem | 25.00 | 23.33 | 22.33 | 21.62 | 18.60 | 18.18 | 13.68 | −0.97 ** | 0.000 | ||
| MDR-AB | 46.07 | 75.82 | 80.39 | 81.69 | 83.33 | 76.19 | 86.36 | 0.07 | 0.879 | ||
| ICU wards (504) |
Imipenem/cilastatin | 26.09 | 16.36 | 10.00 | 16.95 | 16.90 | 21.84 | 14.58 | −0.25 | 0.589 | |
| Meropenem | 23.91 | 16.36 | 10.34 | 16.95 | 15.71 | 20.93 | 13.41 | −0.29 | 0.528 | ||
| MDR-AB | 53.73 | 82.14 | 90.00 | 81.36 | 84.51 | 74.73 | 83.00 | −0.25 | 0.589 | ||
* Correlation is significant at the 0.05 level (2-tailed); ** Correlation is significant at the 0.01 level (2-tailed).
Table 3.
The susceptibility rates of P. aeruginosa in different ward classification from 2007 to 2013 (n = 1386).
| Isolates (n) | Wards | Antimicrobial Agents | Antimicrobials Susceptibility (%) by Year | Correlation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | r | p | |||
| Blood (98) |
All wards (98) |
Imipenem/cilastatin | 100.00 | 83.33 | 53.85 | 77.78 | 61.54 | 58.33 | 78.57 | −0.50 | 0.250 |
| Meropenem | 100.00 | 83.33 | 53.85 | 77.78 | 53.85 | 58.33 | 71.43 | −0.61 | 0.149 | ||
| MDR-PA | 16.67 | 23.08 | 53.58 | 18.75 | 46.15 | 29.41 | 28.57 | 0.22 | 0.629 | ||
| Non-ICU wards (72) |
Imipenem/cilastatin | 100.00 | 88.89 | 54.55 | 75.00 | 63.64 | 77.78 | 50.00 | −0.70 | 0.083 | |
| Meropenem | 100.00 | 88.89 | 54.55 | 75.00 | 63.64 | 77.78 | 50.00 | −0.70 | 0.083 | ||
| MDR-PA | 22.22 | 10.00 | 54.55 | 21.43 | 50.00 | 16.67 | 50.00 | 0.38 | 0.398 | ||
| ICU wards (26) |
Imipenem/cilastatin | 100.00 | 66.67 | 50.00 | 100.00 | 50.00 | 0.00 | 100.00 | −0.28 | 0.549 | |
| Meropenem | 100.00 | 66.67 | 50.00 | 100.00 | 0.00 | 0.00 | 85.71 | −0.39 | 0.383 | ||
| MDR-PA | 0.00 | 66.67 | 50.00 | 0.00 | 33.33 | 60.00 | 12.50 | 0.02 | 0.965 | ||
| Sputum and BAL (1288) |
All wards (1288) |
Imipenem/cilastatin | 65.81 | 87.17 | 74.58 | 80.84 | 67.25 | 61.39 | 70.44 | −0.39 | 0.394 |
| Meropenem | 66.67 | 80.34 | 74.43 | 83.23 | 70.06 | 63.70 | 69.85 | −0.30 | 0.507 | ||
| MDR-PA | 22.49 | 17.39 | 21.02 | 18.24 | 14.20 | 34.35 | 23.03 | 0.34 | 0.450 | ||
| Non-ICU wards (930) |
Imipenem/cilastatin | 72.00 | 86.21 | 75.37 | 81.48 | 67.18 | 62.34 | 73.79 | −0.49 | 0.270 | |
| Meropenem | 72.00 | 78.16 | 76.69 | 83.70 | 69.77 | 62.34 | 76.14 | −0.28 | 0.548 | ||
| MDR-PA | 17.86 | 20.00 | 19.55 | 19.69 | 12.30 | 33.62 | 18.49 | 0.07 | 0.879 | ||
| ICU wards (358) |
Imipenem/cilastatin | 54.76 | 90.00 | 72.09 | 78.13 | 67.50 | 61.05 | 64.29 | −0.22 | 0.630 | |
| Meropenem | 57.14 | 86.67 | 67.44 | 81.25 | 71.05 | 63.16 | 58.33 | −0.27 | 0.553 | ||
| MDR-PA | 31.58 | 10.00 | 23.26 | 12.50 | 20.00 | 36.08 | 32.20 | 0.39 | 0.391 | ||
2.3. Relationship between Carbapenems Consumption and the Susceptibility Rate of A. baumannii to Imipenem and Meropenem and MDR-A. baumannii Data
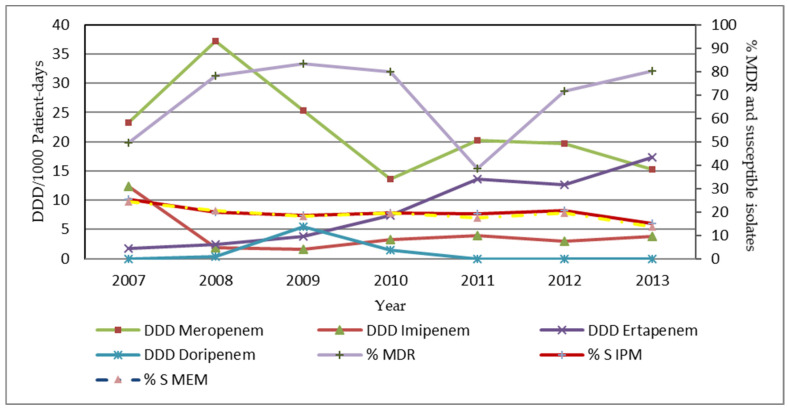
We found that the consumption of the group 1 carbapenems (ertapenem) had a negative correlation with the susceptibility rate of A. baumannii to imipenem and meropenem. There was a negative correlation between ertapenem consumption and the susceptibility rate of A. baumannii to meropenem (r = −0.79; p = 0.035) from the sputum and the BAL specimens from the data of all wards. Additionally, there was the strongest statistically significant negative correlation between ertapenem consumption and the susceptibility rate of A. baumannii to imipenem (r = −0.91; p = 0.004) and meropenem (r = −0.97; p = 0.000) in data from the non-ICU wards. The correlation is presented in Figure 2.
Figure 2.
The annual consumption of the individual carbapenem, the percentage of susceptibility and the MDR-A. baumannii data from the sputum and the BAL specimens from all wards. %S IPM = the percentage of susceptibility to imipenem; %S MEM = the percentage of susceptibility to meropenem.
2.4. Relationship between Carbapenem Consumption and the Susceptibility Rate of P. aeruginosa to Imipenem and Meropenem and MDR-P. aeruginosa Data
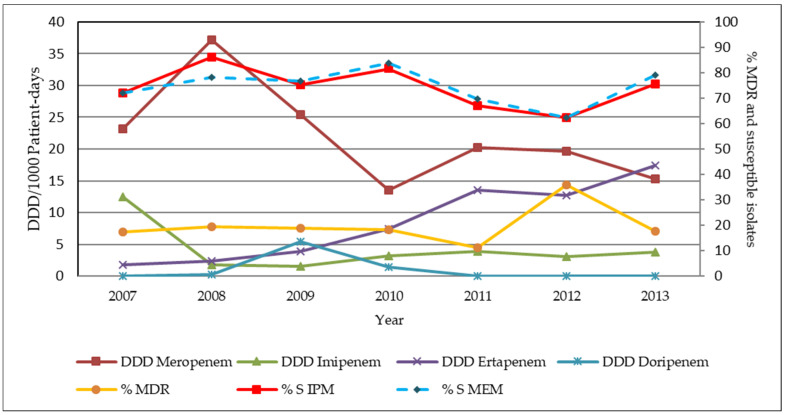
The result from the sputum and BAL specimens of all wards demonstrated that the susceptibility of P. Aeruginosa, including the MDR strains, to imipenem and meropenem was slowly decreased during the study period. However, when the CCP was initiated into the MSMC system, it did not have a significant impact on the susceptibility of P. aeruginosa, including the MDR strains, to imipenem and meropenem. We found that carbapenem consumption did not show a statistically significant change in the susceptibility rate of P. aeruginosa to imipenem and meropenem. The data from the sputum and BAL specimens of the non-ICU wards showed that imipenem use had a moderate negative statistical correlation with MDR-P. aeruginosa. However, this correlation was not statistically significant (r = −0.71, p > 0.05). The correlation is presented in Figure 3.
Figure 3.
The annual consumption of the individual carbapenem, the percentage of susceptibility and the MDR-P. aeruginosa data from the sputum and the BAL specimens from the non-ICU wards. %S IPM = the percentage of susceptibility to imipenem. %S MEM = the percentage of susceptibility to meropenem.
3. Discussion
This study assessed the relationship between carbapenem consumption (Group 1 and Group 2) and the susceptibility patterns of the nosocomial infections caused by A. baumannii and P. aeruginosa. During the study period, Group 2 carbapenem consumption significantly decreased over time (r = −0.84, p = 0.018). Meropenem was the main carbapenem used, which was the most decreased carbapenem consumption among the group 2 carbapenems, especially in the period of the initiation of the CCP (2009–2010). On the other hand, ertapenem consumption significantly increased over time (r = 0.97, p = 0.000). Similarly, after replacement of the Group 2 carbapenems with ertapenem under the CCP, ertapenem consumption increased (p < 0.0001), while the group 2 carbapenem consumption significantly decreased over time (p = 0.028) [29].
The susceptibility rate of A. baumannii to imipenem and meropenem from the blood specimens decreased in the data from all wards (r = −0.39; p = 0.387 and r = −0.46; p = 0.294, respectively) and the ICU wards (r = −0.58; p = 0.175 and r = −0.63; p = 0.131, respectively) but showed no significant change during the years from 2007–2013. This finding was consistent with the result from Lee et al., which reported a significant decrease in the susceptibility rate of A. baumannii to imipenem and meropenem during their 7-year study period [29]. However, the increased susceptibility rate of A. baumannii was the most significant during the years from 2009–2010 (the period of the CCP initiation). Moreover, the data from the non-ICU wards showed the most increased susceptibility rates. While the highest increased susceptibility rate was identified in the year 2010, the MDR-A. baumannii rate was also increased. This situation could be from the outbreak of the MDR-A. baumannii in May and June 2010. According to the data from the sputum and the BAL specimens, the data from the non-ICU wards showed a statistically significant decrease in the susceptibility rate of A. baumannii to imipenem and meropenem over time. This finding could be explained by comparing the more severe patients in the ICU wards to the less severe patients in the non-ICU wards, leading to the higher carbapenem consumption and the higher resistance rate in the ICU wards.
The susceptibility rate of P. aeruginosa to imipenem and meropenem in all specimens from the data of all wards showed that the non-ICU wards and the ICU wards were slowly decreased during the study period. The susceptibility rates were increased the most during 2010, while the incidence rate of the MDR-P. aeruginosa was decreased the most after the initiation of the CCP. Moreover, as for the result from the blood isolates of the ICU wards, the rate of the susceptibility for P. aeruginosa was the highest in the year 2010, which was 100%, and the incidence rate of the MDR-P. aeruginosa was 0%. This data was only from two isolates; therefore, the incidence rate of the MDR-P. aeruginosa in the ICU wards might not represent for the trend of the MDR-P. aeruginosa in that year’s data.
Our study demonstrated that the ertapenem consumption had a significantly negative correlation with the susceptibility rate of A. baumannii to imipenem and meropenem. The data was obtained from the sputum and the BAL specimens from all wards and the non-ICU wards during the study period. Similar to the data from Lee et al., there were significantly negative correlations between the use of ertapenem and the susceptibility rate of A. baumannii to imipenem and meropenem [29]. However, the ertapenem use had no impact on the susceptibility rate of A. baumannii to imipenem, as reported by Sousa et al. [30]. In addition, there was no relationship between the proportion of CRAB isolates obtained from the infected patients and the intense use of ertapenem, as reported by Yoon et al. [31]. Therefore, the increased use of ertapenem might have selective pressure with the resistance to A. baumannii. In addition, CRAB could occur from multifactorial causes, including long-term use of broad-spectrum antimicrobials, consumption of antimicrobials, under/sub therapeutic dosage of the antimicrobials, prolonged stay in a hospital or long-term care facilities, ICU admission, underlying diseases, catheter indwelling and contamination by healthcare personnel [32,33,34]. Nevertheless, carbapenem consumption did not have a statistically significant correlation among P. aeruginosa data. We identified imipenem to have a statistically moderate negative correlation with MDR-P. aeruginosa but was statistically insignificant.
A study by Neves et al. showed that imipenem was independently related to the incidence of MDR strains (r = 0.67, p = 0.01) [35]. However, that study did not separate the data to each ward as in our study data. The reason might be explained by the imipenem resistance to P. aeruginosa, considered to be associated with a loss of the porin OprD combined with the activity of the chromosomal beta-lactamase (AmpC), while the overexpression of multidrug efflux pumps was considered to confer the meropenem resistance [36].
Our study had some limitations. First, we collected information on antimicrobial consumption, excluding information on ecological factors, which also influence AMR. It depicts association but not causal relations. More works need to be performed on finding different causation. Second, the exclusion of duplicated isolates may result in the under/overestimation of antimicrobial resistance. Finally, a lack of generalization may be concerning due to the single center being analyzed. Nevertheless, our findings are valuable in understanding the relationship of carbapenem consumption and the resistance for the implementation of a carbapenems stewardship program.
4. Materials and Methods
4.1. Study Design and Data Collection
This research was designed as a retrospective study at the Her Royal Highness Princess Maha Chakri Sirindhorn Medical Center (MSMC), which is a 360-bed university hospital with 12 medical wards and 5 intensive care units (ICU). There were 4 carbapenems, including imipenem, meropenem, doripenem and ertapenem in this study, which were divided into group 1 carbapenems (ertapenem) and group 2 carbapenems (imipenem, meropenem and doripenem). Antimicrobial consumption and the susceptibility pattern of A. baumannii and P. aeruginosa were analyzed. We collected the amounts of carbapenem consumption in grams from January 2007 to December 2013 from the MSMC database system described in yearly consumption. The amounts of the antimicrobial consumption were converted into “Define Daily Doses” (DDD). According to definitions from the Anatomical Therapeutic Chemical (ATC) Classification System and the World Health Organization (WHO), the DDD was expressed as days of therapy per 1000 patient days (DDD/1000 patient-days). The susceptibility rates were determined in all wards (17 wards), the non-ICU wards (12 medical wards) and the ICU wards (5 ICU wards), respectively.
Microbiological data and the susceptibility were collected from the database of the MSMC microbiological laboratory from January 2007 to December 2013. The susceptibilities were tested by disk diffusion, according to the Clinical and Laboratory Standards Institute (CLSI) standards during that period. The non-duplicated isolate was defined as “the first isolates of a species/patient/analysis period”. The research specified the period to exclude the duplicated isolates from the median patient-days in the nosocomial infected patients since the infections were identified until the discharge date. Therefore, the duplicated isolates were excluded from the study as of 18-day interval.
4.2. Statistical Analysis
The relationship between carbapenem consumption and the susceptibility were analyzed by using either the parametric Pearson’s or the non-parametric Spearman’s correlation coefficient. The p value of less than 0.05 was considered statistically significant. The Statistical Package for Social Science (SPSS) program, version 17, was used for all analyses.
5. Conclusions
We have identified an association between carbapenem use and the susceptibility of A. baumannii and P. aeruginosa. Our results indicate that carbapenem consumption is one of the contributing factors associated with the carbapenem-resistant rate. However, molecular analysis studies should be performed to elucidate the effect of carbapenem consumption on the susceptibilities of A. baumannii and P. aeruginosa. Avoiding carbapenem overuse and implementing an appropriate initial therapy might decrease the rate of resistant organisms. Further multicenter studies on other determinants that impact carbapenem resistance with more relevant data are needed.
Acknowledgments
The authors would like to thank all participations in the MSMC for supportive data in this study.
Author Contributions
Conceptualization, P.M. and W.T.; data collection, P.T. and W.T.; analysis, P.T., S.G., T.P., and P.M.; writing—original draft preparation, T.P.; finalized manuscript, W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Human Research Ethics Committee of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University, which received approval on 26 February 2014 (MU-DT/PY-IRB 2014/010.2602) and the Human Research Ethics Committee of the MSMC, Faculty of Medicine, Srinakharinwirot University, which received approval on 28 June 2014 (SWUEC/X-040/2557).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the finding of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pumart P., Phodha T., Thamlikitkul V., Riewpaiboon A., Prakongsai P., Limwattananon S. Health and economic impacts of antimicrobial resistance in Thailand. J. Health Syst. Res. 2012;6:352–360. [Google Scholar]
- 2.Christian G.G., Dominique L.M., Otto C., Yehuda C. Clinical and Economic Impact of Common Multidrug-Resistant Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2008;52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. World Health Organization; Geneva, Switzerland: 2017. [(accessed on 11 November 2021)]. Available online: https://apps.who.int/iris/handle/10665/259462. [PubMed] [Google Scholar]
- 4.Molton J.S., Tambyah P.A., Ang B.S., Ling M.L., Fisher D.A. The global spread of healthcare-associated multidrug-resistant bacteria: A perspective from Asia. Clin. Infect. Dis. 2013;56:1310–1318. doi: 10.1093/cid/cit020. [DOI] [PubMed] [Google Scholar]
- 5.Mendes R.E., Mendoza M., Banga Singh K.K., Castanheira M., Bell J.M., Turnidge J.D., Lin S.S., Jones R.N. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011) Antimicrob. Agents Chemother. 2013;57:5721–5726. doi: 10.1128/AAC.01121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suwantarat N., Carroll K.C. Epidemiology and molecular characterization of multidrug-resistant Gram-negative bacteria in Southeast Asia. Antimicrob. Resist. Infect. Control. 2016;5:15. doi: 10.1186/s13756-016-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiratisin P., Chongthaleong A., Tan T.Y., Lagamayo E., Roberts S., Garcia J., Davies T. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: Results of Asia-Pacific surveillance from the COMPACT II study. Int. J. Antimicrob. Agents. 2012;39:311–316. doi: 10.1016/j.ijantimicag.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Danchaivijitr S., Judaeng T., Sripalakij S., Naksawas K., Plipat T. Prevalence of nosocomial infection in Thailand 2006. J. Med. Assoc. Thai. 2007;90:1524–1529. [PubMed] [Google Scholar]
- 9.Werarak P., Waiwarawut J., Tharavichitkul P., Pothirat C., Rungruanghiranya S., Geater S.L., Chongthaleong A., Sittipunt C., Horsin P., Chalermskulrat W., et al. Acinetobacter baumannii nosocomial pneumonia in tertiary care hospitals in Thailand. J. Med. Assoc. Thai. 2012;95:S23–S33. [PubMed] [Google Scholar]
- 10.Dejsirilert S., Tiengrim S., Sawanpanyalert P., Aswapokee N., Malathum K. Antimicrobial resistance of Acinetobacter baumannii: Six years of National Antimicrobial Resistance Surveillance Thailand (NARST) surveillance. J. Med. Assoc. Thai. 2009;92:S34–S45. [PubMed] [Google Scholar]
- 11.Hsu L.Y., Apisarnthanarak A., Khan E., Suwantarat N., Ghafur A., Tambyah P.A. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2017;30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thirapanmethee K., Srisiri-a-nun T., Houngsaitong J., Montakantikul P., Khuntayaporn P., Chomnawang M.T. Prevalence of OXA-Type β-Lactamase Genes among Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates in Thailand. Antibiotics. 2020;9:864. doi: 10.3390/antibiotics9120864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paiboonvong T., Rodjun V., Houngsaitong J., Chomnawang M., Montakantikul P., Chulavatnatol S. Comparative in vitro activity of sitafloxacin against multidrug-resistant and carbapenem-resistant Acinetobacter baumannii clinical isolates in Thailand. Pharm. Sci. Asia. 2020;47:37–42. doi: 10.29090/psa.2020.01.019.0012. [DOI] [Google Scholar]
- 14.Khuntayaporn P., Montakantikul P., Mootsikapun P., Thamlikitkul V., Chomnawang M.T. Prevalence and genotypic relatedness of carbapenem resistance among multidrug-resistant P. aeruginosa in tertiary hospitals across Thailand. Ann. Clin. Microbiol. Antimicrob. 2012;11:25. doi: 10.1186/1476-0711-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell B.G., Schellevis F., Stobberingh E., Goossens H., Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arepyeva M.A., Kolbin A.S., Sidorenko S.V., Lawson R., Kurylev A.A., Balykina Y.E., Mukhina N.V., Spiridonova A.A. A mathematical model for predicting the development of bacterial resistance based on the relationship between the level of antimicrobial resistance and the volume of antibiotic consumption. J. Glob. Antimicrob. Resist. 2017;8:148–156. doi: 10.1016/j.jgar.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Aremu T.O., Oluwole O.E., Adeyinka K.O. An understanding of the drivers of infectious diseases in the modern world can aid early control of future pandemics. Pharmacy. 2021;9:181. doi: 10.3390/pharmacy9040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papp-Wallace K.M., Endimiani A., Taracila M.A., Bonomo R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codjoe F.S., Donkor E.S. Carbapenem Resistance: A Review. Med. Sci. 2017;6:1. doi: 10.3390/medsci6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichenberger E.M., Thaden J.T. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics. 2019;8:37. doi: 10.3390/antibiotics8020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavascki A.P., Carvalhaes C.G., Picao R.C., Gales A.C. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: Resistance mechanisms and implications for therapy. Expert Rev. Anti. Infect. Ther. 2010;8:71–93. doi: 10.1586/eri.09.108. [DOI] [PubMed] [Google Scholar]
- 23.Mascarello M., Simonetti O., Knezevich A. Correlation between antibiotic consumption and resistance of bloodstream bacteria in a University Hospital in North Eastern Italy, 2008–2014. Infection. 2017;45:459–467. doi: 10.1007/s15010-017-0998-z. [DOI] [PubMed] [Google Scholar]
- 24.Cao J., Song W., Gu B., Mei Y.N., Tang J.P., Meng L., Yang C.Q., Wang H., Zhou H. Correlation between carbapenem consumption and antimicrobial resistance rates of Acinetobacter baumannii in a university-affiliated hospital in China. J. Clin. Pharmacol. 2013;53:96–102. doi: 10.1177/0091270011435988. [DOI] [PubMed] [Google Scholar]
- 25.Tan C.K., Tang H.J., Lai C.C., Chen Y.Y., Chang P.C., Liu W.L. Correlation between antibiotic consumption and carbapenem-resistant Acinetobacter baumannii causing health care associated infections at a hospital from 2005 to 2010. J. Microbiol. Immunol. Infect. 2015;48:540–544. doi: 10.1016/j.jmii.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Yang P., Chen Y., Jiang S., Shen P., Lu X., Xiao Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob. Resist. Infect. Control. 2018;7:137. doi: 10.1186/s13756-018-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolau D.P., Carmeli Y., Crank C.W., Goff D.A., Graber C.J., Lima A.L., Goldstein E.J. Carbapenem stewardship: Does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of the evidence. Int. J. Antimicrob. Agents. 2012;39:11–15. doi: 10.1016/j.ijantimicag.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Abdallah M., Badawi M., Amirah M.F., Rasheed A., Mady A.F., Alodat M., Alharthy A. Impact of carbapenem restriction on the antimicrobial susceptibility pattern of Pseudomonas aeruginosa isolates in the ICU. J. Antimicrob. Chemother. 2017;72:3187–3190. doi: 10.1093/jac/dkx273. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.M., Lai C.C., Wang Y.Y., Lee M.C., Hsueh P.R. Impact of susceptibility profiles of Gram-negative bacteria before and after the introduction of ertapenem at a medical center in northern Taiwan from 2004 to 2010. Diagn. Microbiol. Infect Dis. 2013;75:94–100. doi: 10.1016/j.diagmicrobio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Sousa D., Castelo-Corral L., Gutiérrez-Urbón J.M., Molina F., López-Calviño B., Bou G., Llinares P. Impact of ertapenem use on Pseudomonas aeruginosa and Acinetobacter baumannii imipenem susceptibility rates: Collateral damage or positive effect on hospital ecology? J. Antimicrob. Chemother. 2013;68:1917–1925. doi: 10.1093/jac/dkt091. [DOI] [PubMed] [Google Scholar]
- 31.Yoon Y.K., Yang K.S., Lee S.E., Kim H.J., Sohn J.W., Kim M.J. Effects of Group 1 versus Group 2 carbapenems on the susceptibility of Acinetobacter baumannii to carbapenems: A before and after intervention study of carbapenem-use stewardship. PLoS ONE. 2014;9:e99101. doi: 10.1371/journal.pone.0099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Hello S., Falcot V., Lacassin F., Mikulski M., Baumann F. Risk factors for carbapenem-resistant Acinetobacter baumannii infections at a tertiary care hospital in New Caledonia. Scand. J. Infect. Dis. 2010;42:821–826. doi: 10.3109/00365548.2010.496087. [DOI] [PubMed] [Google Scholar]
- 33.Sheng W.H., Liao C.H., Lauderdale T.L., Ko W.C., Chen Y.S., Liu J.W., Lau Y.J., Wang L.H., Liu K.S., Tsai T.Y., et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int. J. Infect. Dis. 2010;14:e764–e769. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y.L., Wan Y.F., Zhou L.Y., Ye M.L., Liu S., Xu C.Q., He Y.Q., Chen J.H. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am. J. Infect. Control. 2013;41:e59–e63. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Neves M.T.d, Lorenzo M.E.P.d, Almeida R.A.M.B., Fortaleza C.M.C.B. Antimicrobial use and incidence of multidrug-resistant Pseudomonas aeruginosa in a teaching hospital: An ecological approach. Rev. Soc. Bras. Med. Trop. 2010;43:629–632. doi: 10.1590/S0037-86822010000600005. [DOI] [PubMed] [Google Scholar]
- 36.El Amin N., Giske C.G., Jalal S., Keijser B., Kronvall G., Wretlind B. Carbapenem resistance mechanisms in Pseudomonas aeruginosa: Alterations of porin OprD and efflux proteins do not fully explain resistance patterns observed in clinical isolates. APMIS. 2005;113:187–196. doi: 10.1111/j.1600-0463.2005.apm1130306.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of this study are available on request from the corresponding author.