Abstract
We report a case of sepsis caused by Bifidobacterium longum in a 19-year-old male who had developed high fever, jaundice, and hepatomegaly after acupuncture therapy with small gold needles. Anaerobic, non-spore-forming, gram-positive bacilli were isolated from his blood and finally identified as B. longum. He recovered completely after treatment with ticarcillin and metronidazole. To our knowledge, this is the first report of incidental sepsis caused by B. longum.
CASE REPORT
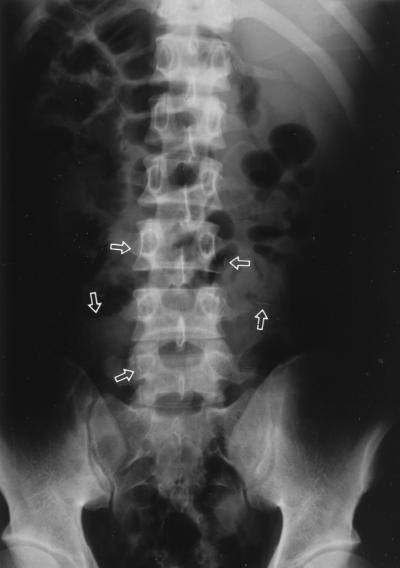
A 19-year-old male was admitted to the hospital due to high fever, jaundice, and hepatomegaly. A month prior to admission, he had developed a herniated intervertebral disk of the lumbar spine. He had a partial laminectomy and began receiving acupuncture therapy in a local clinic practicing oriental medicine. Due to continuing lumbar pain, several 1-cm-long gold needles had been inserted into the lumbar area 10 days prior to admission; 5 days later, the patient started to develop chills, fever, nausea, vomiting, and diarrhea. Upon presentation, hepatic enlargement was observed by ultrasonography and several gold needles were located on X-ray film near the lumbar area (Fig. 1). The blood culture grew anaerobic, non-spore-forming, gram-positive bacilli. A clinical diagnosis of sepsis by anaerobic bacilli was made, and empiric treatment with ticarcillin and metronidazole was begun. The patient was fully recovered after 10 days, and a checkup 1 week later showed a normal range of hepatic function.
FIG. 1.
Radiological findings of the simple abdomen showing many small needles (arrows).
Microbiological investigation.
Three blood samples, which were taken from three different sites at 30-min intervals, inoculated into both tryptic soy broth and thioglycollate medium, and incubated at 37°C for 2 days, yielded gram-positive bacilli. The three cultures in thioglycollate grew much better than those in tryptic soy broth, suggesting that the bacilli were anaerobic. The gram-positive bacilli appeared to be very pleomorphic and produced no endospores. When the Dongguk isolate was subcultured on 5% sheep blood agar at 37°C for 2 days, the anaerobically grown colonies were 0.5 to 1 mm in diameter and appeared whitish, raised, mucoid, and nonhemolytic, while aerobically grown colonies were hardly visible even after 5 days. The isolate was inoculated onto thioglycollate medium without dextrose or indicator (Difco, Detroit, Mich.) and tested by conventional biochemical means according to Holdeman et al. (5). These tests were repeated twice. Gas-liquid chromatography (GLC) with a Capco instrument (Clinical Analysis Products, Sunnyvale, Calif.) was performed to analyze the metabolic end products. Acetic acid and lactic acid were detected as volatile and nonvolatile fatty acids, respectively. The isolate showed the biochemical characteristics and GLC profile of Bifidobacterium longum, as described by Holdeman et al. (5) (Table 1).
TABLE 1.
Culture and biochemical characteristics of the B. longum type strain and the Dongguk isolate
| Characteristic or test | B. longuma | Dongguk isolate |
|---|---|---|
| Acid from: | ||
| Arabinose | + | + |
| Cellobiose | v | − |
| Erythritol | − | − |
| Esculin | v | − |
| Fructose | + | + |
| Glucose | + | + |
| Inositol | − | − |
| Lactose | + | + |
| Maltose | + | + |
| Mannitol | − | − |
| Mannose | v | + |
| Melezitose | + | + |
| Melibiose | + | + |
| Raffinose | + | + |
| Rhamnose | −, w | − |
| Ribose | + | + |
| Salicin | − | − |
| Sorbitol | − | − |
| Sucrose | + | + |
| Trehalose | v | − |
| Xylose | + | + |
| Adonitol | − | − |
| Dulcitol | − | − |
| Glycerol | − | − |
| Inulin | w | + |
| Esculin hydrolysis | v | − |
| Indole production | − | − |
| Nitrate reduction | − | − |
| Catalase | − | − |
| Bile growth | 43 | + |
| Hemolysis | − | − |
| Motility | − | − |
| Gas | 2− | − |
| GLC | Acetic acid | Acetic acid |
| Lactic acid | Lactic acid |
Data are from reference 5. Symbols and abbreviations: +, positive; −, negative; v, variable; w, weak acid; 43, most strains show good growth on bile, while some strains show moderate growth; 2−, most strains produce gas, while some strains do not.
Discussion.
Anaerobic bacterial sepsis is often caused by organisms found in the gastrointestinal tract, skin, urogenital tract, or oral cavity. Bacteroides fragilis and Clostridium perfringens are the anaerobic agents isolated most frequently from infections (3, 7). Bifidobacterium spp. colonize the intestinal tract, the mouth, and, in some instances, the vagina in humans (1) and are rarely isolated from clinical specimens, with the exception of Bifidobacterium dentium as one of the causative agents of dental caries and related diseases (2, 6). It is known to be difficult to identify Bifidobacterium spp. due not only to variability in aerotolerance, colony morphology, and stainability on Gram staining but also to the difficulty in distinguishing the organisms from other gram-positive, non-spore-forming, anaerobic bacilli by conventional biochemical tests (1). Definitive identification of the genus Bifidobacterium requires analysis of metabolic products, volatile and nonvolatile fatty acids, in broth media by GLC (4, 8).
We encountered a case of sepsis due to B. longum, an anaerobic, non-spore-forming, gram-positive bacillus which has been widely regarded as being of benefit to the host by preventing other pathogens from overgrowing in the intestinal tract (1). Since there were no obvious predisposing conditions preceding anaerobic infection in the young male patient other than acupuncture therapy, it is speculated that the organism was introduced to the blood circulation either from improperly sterilized acupuncture needles or from the colon via minute perforations caused by those needles. This case emphasizes the potential for serious infections caused by normally harmless gastrointestinal tract flora when invasive acupuncture therapy is improperly provided.
REFERENCES
- 1.Baron E J, Peterson L R, Finegold S M. Anaerobic gram-positive bacilli. In: Shanahan J F, editor. Bailey & Scott’s diagnostic microbiology. 9th ed. St. Louis, Mo: Mosby; 1994. pp. 504–523. [Google Scholar]
- 2.Crociani F, Biavati B, Allessandrini A, Chiarini C, Scardovi V. Bifidobacterium inopinatum sp. nov. and Bifidobacterium denticolens sp. nov., two new species isolated from human dental caries. Int J Syst Bacteriol. 1996;46:564–571. doi: 10.1099/00207713-46-2-564. [DOI] [PubMed] [Google Scholar]
- 3.Dorsher C W, Rosenblatt J E, Wilson W R, Ilstrup D M. Anaerobic bacteremia: decreasing rate over a 15-year period. Rev Infect Dis. 1991;13:633–636. doi: 10.1093/clinids/13.4.633. [DOI] [PubMed] [Google Scholar]
- 4.Engelkirk P G, Duben-Engelkirk J, Dowell V R., Jr . Definitive identifications. In: Engelkirk P, et al., editors. Principles and practice of clinical anaerobic bacteriology. Belmont, Calif: Star Publishing Co.; 1992. pp. 181–232. [Google Scholar]
- 5.Holdeman L V, Cato E P, Moore W E C. Anaerobe laboratory manual. 4th ed. Blacksburg: Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- 6.Scardovi V, Crociani F. Bifidobacterium catenulatum, Bifidobacterium dentium, and Bifidobacterium angulatum: three new species and their deoxyribonucleic acid homology relationships. Int J Syst Bacteriol. 1974;24:6–20. [Google Scholar]
- 7.Vazquez F, Mendez F J, Perez F, Mendoza M C. Anaerobic bacteremia in a general hospital: retrospective five-year analysis. Rev Infect Dis. 1987;9:1038–1043. doi: 10.1093/clinids/9.5.1038. [DOI] [PubMed] [Google Scholar]
- 8.Wüst J. Presumptive diagnosis of anaerobic bacteremia by gas-liquid chromatography of blood cultures. J Clin Microbiol. 1977;6:586–590. doi: 10.1128/jcm.6.6.586-590.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]