Abstract
Neurological disorders are important causes of morbidity and mortality around the world. The increasing prevalence of neurological disorders, associated with an aging population, has intensified the societal burden associated with these diseases, for which no effective treatment strategies currently exist. Therefore, the identification and development of novel therapeutic approaches, able to halt or reverse neuronal loss by targeting the underlying causal factors that lead to neurodegeneration and neuronal cell death, are urgently necessary. Plants and other natural products have been explored as sources of safe, naturally occurring secondary metabolites with potential neuroprotective properties. The secondary metabolites α- and β-asarone can be found in high levels in the rhizomes of the medicinal plant Acorus calamus (L.). α- and β-asarone exhibit multiple pharmacological properties including antioxidant, anti-inflammatory, antiapoptotic, anticancer, and neuroprotective effects. This paper aims to provide an overview of the current research on the therapeutic potential of α- and β-asarone in the treatment of neurological disorders, particularly neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), as well as cerebral ischemic disease, and epilepsy. Current research indicates that α- and β-asarone exert neuroprotective effects by mitigating oxidative stress, abnormal protein accumulation, neuroinflammation, neurotrophic factor deficit, and promoting neuronal cell survival, as well as activating various neuroprotective signalling pathways. Although the beneficial effects exerted by α- and β-asarone have been demonstrated through in vitro and in vivo animal studies, additional research is required to translate laboratory results into safe and effective therapies for patients with AD, PD, and other neurological and neurodegenerative diseases.
Keywords: α-asarone, β-asarone, neuroprotection, neuroinflammation, molecular role, therapeutic, neurological disorders
1. Introduction
The nervous system, a complex network of nerves and specialized cells, is responsible for the control of the body and communication among its parts. According to the World Health Organization (WHO), neurological disorders are defined as diseases of the central and peripheral nervous systems [1]. Neurological disorders can affect the brain, cranial nerves, peripheral nerves, and spinal cord and include neurotraumatic diseases, such as stroke and spinal cord injury; neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD); as well as neuropsychological disorders, such as depression and schizophrenia [2]. In general, neurological disorders are characterized by acute and progressive neuron degeneration, ultimately resulting in brain dysfunction and neuronal cell death [3]. The underlying molecular mechanisms of neurodegeneration include alterations in phospholipid metabolism, accumulation of lipid peroxides, mitochondrial dysfunction, protein misfolding, abnormal protein aggregation, diminished cellular energy levels, disturbed calcium (Ca2+) homeostasis, excitotoxicity, oxidative stress, neuroinflammation, dysregulated hormonal signalling, and apoptosis [4,5,6].
AD, one of the most common neurodegenerative diseases, is characterised by the progressive worsening of learning, memory, and other cognitive functions with age. At the cellular level, AD is associated with the formation of extracellular plaques consisting of amyloid-beta (Aβ) and neurofibrillary tangles that lead to extensive neuronal loss. By reducing cellular energy levels and increasing oxidative stress, inflammation, and apoptosis, these “senile” plaques and aggregates lead to neuronal cell death [7,8,9].
PD, the second most common neurodegenerative disease, is characterized pathologically by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) [10,11]. This nigral neuronal loss consequently results in dopamine (DA) deficiency in the striatum (ST), which is correlated with motor deficits such as tremors, rigidity, bradykinesia, postural instability, and gait impairment [12]. Dopaminergic cell death in PD is associated with the development of intracellular α-synuclein aggregates known as Lewy bodies [13].
Cerebral ischemic disease, a common form of stroke, is the fifth leading cause of death and disability impacting one million Americans every year [14]. It is caused by the blockage of blood vessels due to a thrombus or embolus [15]. At rest, the brain receives approximately 20% of the body’s total blood supply and is, therefore, highly sensitive to ischaemic events, and even short-lived ischaemia can result in significant cerebral damage [15]. During cerebral ischemia, part of the brain is deprived of oxygen and nutrients, which initiates a cascade of cellular and metabolic events that can result in severe brain damage. A considerable amount of evidence suggests that the release of excess glutamate during and after an ischemic insult leads to glutamate receptor hyperactivity, triggering harmful intracellular effects, including calcium overload and the generation of reactive oxygen species (ROS). This disruption of cellular homeostasis eventually leads to neurodegeneration [16,17].
Epilepsy is a neurological disorder characterized by temporary abnormal electrical activity in nerve cells [18]. In 2015, around 70 million people had been diagnosed with epilepsy worldwide, with 80% of these cases seen in developing countries [19]. Oxidative stress, glutamate excitotoxicity, and mitochondrial dysfunction, among others, have been implicated in the pathogenesis of epilepsy [20].
Several approaches have been proposed for the management of neuronal dysfunction and cell death associated with neurological disorders. However, current approaches primarily serve to reduce or manage symptoms, and no curative therapies have been introduced that can slow, prevent, or reverse disease development [21].
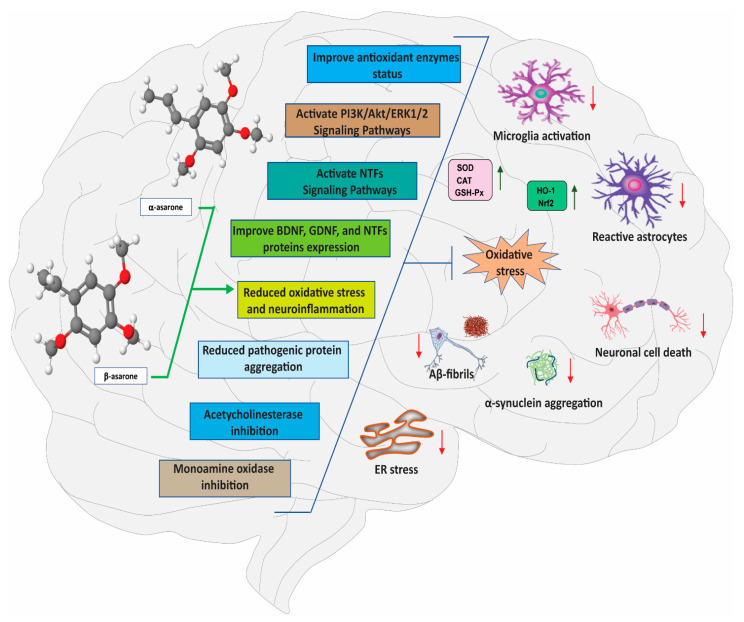
Medicinal plants, found throughout the natural environment, represent an immense source of bioactive compounds in the form of secondary metabolites and other bioactive constituents [22]. Secondary metabolites derived from medicinal plants have been shown to exert beneficial effects on the chemical balance in the brain by influencing the function of several neurotransmitter receptors, with positive outcomes on cognitive disorders [23]. Recently, our research has identified α- and β-asarone, found in high levels in the rhizomes of the medicinal plant Acorus calamus (L.), as important secondary metabolites with potential therapeutic benefits for the treatment of AD, PD, and other neurological disorders. The mechanisms underlying α- and β-asarone-mediated neuroprotection are multipronged, and include antioxidant, antiapoptotic, anti-neuroinflammatory effects, as well as the modulation of various cellular and molecular targets; these actions might ultimately contribute to the ability of α- and β-asarone to attenuate the severity of neurological disorders [24,25,26,27] (Figure 1). Some of the specific molecular targets involved in α- and β-asarone-mediated neuroprotection have recently started to be unveiled. For instance, α- and β-asarone have been reported to promote disintegration of protein aggregates (tau, Aβ, and α–synuclein) associated with neurodegenerative disorders [28,29], attenuate lipopolysaccharide (LPS)-mediated neuroinflammation, promote neuronal cell survival, improve motor and non-motor functions, and prevent the neurodegeneration of dopaminergic neurons in the brain [24,25]. Antidepressant-like effects of α- and β-asarone have also been described [30], while Pan et al. [31] showed that β-asarone protected cortical neurons and reduced the infarction volume in an experimental model of ischemic stroke.
Figure 1.
Molecular mechanism of neuroprotection by α- and β-asarone. The multi-target effects of α- and β-asarone in the brain include anti-oxidant, mitochondrial protecting, anti-apoptotic, anti-aggregation, anti-inflammatory, and the regulation of various neuroprotective signalling pathways. Red down-arrow (↓) and green up-arrow (↑) signs indicate inhibition and activation by α- and β-asarone treatment, respectively. BDNF, brain-derived neurotrophic factor; ERK, extracellular signal-regulated kinase; GDNF, glial cell-derived neurotrophic factor; PI3K/Akt, phosphatidylinositol-3-kinase; NTFs, neurotrophic factors.
Here, we review recent research on the mechanisms by which α- and β-asarone exert neuroprotective effects in vitro and in vivo, in an effort to clarify their pharmacological properties and critically evaluate their potential as therapeutics for the treatment of neurological diseases.
2. Occurrence, Bioavailability, and Pharmacokinetics of α- and β-Asarone
The secondary metabolites α- and β-asarone ((E)-/(Z)-1,2,4-trimethoxy-5-prop-1-enylbenzene) are highly concentrated in the rhizomes of Acorus calamus Linn., Acorus tatarinowii Schott, and Acorus gramineus Solander, which belongs to the Acoraceae plant family (commonly known as “sweet flag”) [32]. α-asarone as an active phytochemical is also present in the bark of the Mexican tree Guatteria gaumeri Greenman from the Annonaceae family [33]. A. calamus, either alone or in combination with other herbs, has been extensively cultivated in various tropical and subtropical regions worldwide [32,34,35] and widely used as a traditional medicine for centuries [32]. A. calamus contains several phytoconstituents, including alkaloids, volatile oils, tannins, glycosides (xanthone), essential oils, flavonoids, monoterpenes, steroids, lignin, sesquiterpenes, saponins, mucilage, and polyphenolic compounds [36,37]. In the Ayurvedic system, A. calamus is extensively used to treat numerous inflammatory disorders [36,38,39,40], and in China, traditional practitioners prescribe A. calamus to treat constipation, digestive problems, and other health issues [41]. A. calamus and its primary bioactive constituents have also been found to reduce stress-induced immunosuppression in rats, resulting in improved immune function [42]. Both α- and β-asarone are widely studied bioactive secondary metabolites, featuring a broad range of pharmacological properties, including antioxidant, anti-inflammatory, neuroprotective, antidiabetic, anticancer, antifungal, antimicrobial, anti-ulcer, anti-allergic, wound healing, pesticidal, insecticidal, and radioprotective properties, among others [36,43,44,45,46,47].
2.1. Bioavailability and Pharmacokinetics of α- and β-Asarone
Due to their lipophilic character, α- and β-asarone have limited oral bioavailability, which can be improved by increasing their stability and solubility [48,49]. The plasma half-lives of α-and β-asarone are relatively short due to the rapid distribution of these agents to vital organs, such as the liver, spleen, heart, kidney, lungs, and brain [25,48,50,51]. Oral administration of the essential oil (single dose of 200 mg/kg) from A. tatarinowii Schott containing 11% α-asarone and 74% β-asarone to rats revealed that the maximum plasma concentrations were 0.5 μg/mL (tmax = 11 min) for α-asarone and 2.5 μg/mL (tmax = 14 min) for β-asarone, with half-lives in plasma of approximately 1 h [52]. Importantly, α- and β-asarone are distributed extensively throughout the brain, indicating their ability to permeate the blood–brain barrier (BBB), which is often a limiting factor when developing treatments for neurological disorders, including neurodegenerative diseases [24,25]. Lu et al. [49] also reported the rapid absorption and permeation of the BBB by α-asarone in rats. In another study, oral administration of α-asarone at 80 mg/kg resulted in 34% bioavailability [53]. A recent pharmacokinetic study demonstrated that the intravenous (i.v.) administration of lipid nanoparticles loaded with α-asarone resulted in significantly increased α-asarone levels detected in murine plasma and brain parenchyma fractions, compared with free α-asarone, confirming the ability to establish and maintain a therapeutic concentration of α-asarone in plasma that can be rapidly transported across the BBB [54]. In another study, the intranasal delivery of α-asarone to the brain using lactoferrin-modified methoxy poly(ethylene glycol)-poly(lactide) copolymer (mPEG-PLA) nanoparticles showed better BBB permeability without poor bioavailability, compared with i.v. administration. Intranasal α-asarone delivery enhanced the brain-targeting efficiency and reduced liver accumulation [55]. Another study demonstrated that the absolute bioavailability, brain-targeting efficiency, and percentage of nasal-mediated brain delivery of nasally administered PLA-α-asarone nanoparticles were 74.2%, 142.24%, and 29.83%, respectively, and nasal administration decreased drug-induced hepatotoxicity [56]. In an in vitro BBB model, borneol and α-asarone, used as co-adjuvant agents, improved the brain delivery of the central nervous system (CNS) drugs puerarin and tetramethylpyrazine. Because this effect could be counteracted by inhibitors of adenosine receptors, the authors concluded that α-asarone may gain entry to the CNS through adenosine receptors (AR), which represent an important pathway for drug delivery. Additionally, the co-administration of borneol and α-asarone decreased the expression of zonula occludens 1 (ZO-1), an important BBB junction protein, but increased A1AR and A2AAR expression. An in vivo pharmacokinetic analysis confirmed that the co-administration of borneol and α-asarone significantly increased the concentration of puerarin and tetramethylpyrazine in the brain, suggesting that a low dose of α-asarone not only improved the oral bioavailability of puerarin and tetramethylpyrazine but also increased BBB permeability, with α-asarone exhibiting superior permeability enhancement than borneol [57].
An absorption, distribution, metabolism, and excretion (ADME) in silico analysis revealed that β-asarone had good oral bioavailability and binding affinity towards dopaminergic receptors [58]. Furthermore, in silico results indicated that β-asarone was likely to interact with various amino acid residues in both the D2 and D3 dopamine receptors through hydrogen bonds [58]. In the same study, the toxicity of β-asarone was predicted using Lazar and ProTox, computational tools used to forecast the toxic properties of molecules. Lazar predicted that β-asarone is carcinogenic in various rodent models. Computational analysis of acute toxicity using ProTox showed that β-asarone had a high LD50 value (418 mg/kg) and the probability of the compound being mutagenic in Salmonella typhimurium was found to be 0.573 [58]. Another study reported that the half-lives of β-asarone in the cerebellum, thalamus, brainstem, cortex, hippocampus, and blood were 8.149, 2.832, 7.142, 1.937, 1.300, and 1.380 h, respectively [59].
Preliminary results from pharmacokinetic studies indicate the rapid and significant brain permeability of α- and β-asarone, expected to satisfactorily induce significant neuroprotective actions necessary to produce a beneficial therapeutic effect. However, further in vivo studies remain necessary to draw definitive conclusions regarding the ADME properties and overall safety of α- and β-asarone.
2.2. Toxicology of α- and β-Asarone: Preclinical Studies
Toxicity studies examining the effects of low doses of α- and β-asarone in rodent models have not revealed severe adverse effects. For instance, Chen et al. [60] reported that sub-chronic treatment with α-asarone (50 and 100 mg/kg, per os [p.o.], for 28 days) did not result in overt behavioural changes (walking, rearing, and grooming) in a seizure model generated in Swiss albino mice. However, α-asarone administered at a higher dose (200 mg/kg, p.o., for 28 days) significantly diminished spontaneous locomotor activity, although no mortality was observed. An acute toxicity test revealed that the oral median lethal dose (LD50) for α-asarone in mice was greater than 1000 mg/kg, with no deaths reported in any test groups [60]. In another study, mice were treated with α-asarone (150, 200, 250, 300, and 350 mg/kg) and survival was recorded for 14 days after treatment. The LD50 of α-asarone was calculated to be 245.2 mg/kg, with 95% confidence limits of 209.2–287.4 mg/kg. Deaths occurred mostly within 24 h after injection, and piloerection, ptosis, dyspnea, and ataxia were the most frequent clinical signs observed [61]. An in vivo subacute toxicity study revealed that the oral administration of β-asarone (100 mg/kg, for five consecutive days) reduced body weight and food consumption without causing mortality in pre-weanling rats [62]. Moreover, the weights of the adrenal glands and heart increased, the thymus weight decreased, and increased single-cell degenerative changes were observed in the thymus following β-asarone treatment. However, no significant changes in haematology or enzyme levels indicating hepatotoxicity were detected [62]. In yet another study, a long-term safety evaluation examining the effects of oral administration of β-asarone at 10 and 20 mg/kg p.o. for 90 days in mice did not reveal significant changes in any haematological parameters; however, blood concentrations of total bilirubin (BIL-T) increased following treatment with 20 and 50 mg/kg of β-asarone p.o. for 90 days; K+ concentrations decreased following treatments with 20 mg/kg/day of β-asarone p.o. for 90 days; and Cl− concentrations decreased following treatments with 50 mg/kg of β-asarone p.o. for 90 days [63]. Following the oral administration of β-asarone at 200 µg/kg for 20 weeks in mice, no obvious toxicity was observed. During an LD50 toxicity study, treatment with β-asarone (500, 750, 1000, 1250, 1500, 1750, and 2000 mg/kg, i.v., for 24 h) did not result in marked behavioural changes and no obvious toxicity was observed [63]. Mice that died first appeared weak and less active, followed by gradual death, and the LD50 of β-asarone was calculated to be 1560 mg/kg [63]. Taken together, based on sub-acute toxicity tests, β-asarone at doses ≤100 mg/kg appears to be safe for clinical use, whereas the safety of doses > 100 mg/kg remains unclear. For further information on the toxicology of α- and β-asarone, we refer the reader to the following excellent reviews [27,64].
3. Neuroprotective Effects of α- and β-Asarone
In preclinical studies, α- and β-asarone show strong neuroprotective activities. At the molecular level, this neuroprotection has been attributed to antioxidant, anti-neuroinflammatory and antiapoptotic effects of α- and β-asarone, along with their ability to modulate various neuroprotective signalling pathways, such as the phosphatidylinositol-3-kinase (PI3K/Akt), cAMP-response element-binding protein (CREB), mitogen-activated protein kinase (MAPK), neurotrophic factors (NTFs), and Kelch-like ECH-associating protein 1 (Keap1)/nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/antioxidant responsive element (ARE) axes. The neuroprotective effects of α- and β-asarone are summarized in Table 1 and the different pathways by which α- and β-asarone exert these effects are examined below.
Table 1.
Pre-clinical evidence supporting the neuroprotective effects of α- and β-asarone.
| In Vitro/In Vivo | Study Model | Main Mechanism | Dose and Route of Administration | Reference |
|---|---|---|---|---|
| α-asarone | ||||
| BV-2 | LPS/Parkinson’s disease (100 ng for 24 h) |
↓ Microglial activation ↓ Neuroinflammation ↓ NF-κB activation |
α-asarone (10, 50 and 250 µM., for 24 h) | [25] |
| PC12 and cultured rat astrocytes | tBHP/Dementia (0–300 μM., for 3 h) |
↑ Neurotrophic factors ↑ Neurogenesis ↑ Akt activation ↑ Antioxidant response ↓ Oxidative stress ↓ PKA signalling ↓ Apoptosis |
α- and β-asarone (15, 30 and 50 µM., 48 h) |
[43,73,74] |
| Wistar rat | Noise stress/Stress model (100 dBA/4 h/d for 30 days) |
↓ Oxidative stress ↓ AChE activity ↓ HSP70mRNA expression |
α-asarone (9 mg/kg−1, i.p., 30 days) | [75] |
| ICR mouse | Scopolamine hydrochloride/Alzheimer’s disease (2 mg/kg, i.p., for 2 days) | ↑ Motor performance ↓ Oxidative stress ↓ AChE activity |
α-asarone (3, 10 and 30 mg/kg, p.o., 15 days) | [76] |
| C57BL/6 mice | Nicotine/Stress model (10–200 µg/mL for 40 days) |
↑ Motor performance ↑ Neurotrophic factors ↑ p-CREB protein expression ↓ Weight loss |
α-asarone (5, 10 and 20 mg/kg, i.p., 8 days) | [77] |
| APP/PS1 transgenic mice | Submicron emulsion injection/Alzheimer’s disease | ↑ Motor performance ↑ Neuronal morphology ↑ Neuronal cell survival ↓ Neuroinflammation ↓ Aβ and tau aggregation ↓ Autophagosomes |
α-asarone (30 and 60 mg/kg, i.p., 3 months) | [28] |
| Wistar rat | LPS/Neurotoxicity (30 µg/paw., for 6 h) |
↑ Motor performance ↑ Cognitive function ↑ Anti-inflammatory ↑ Anti-nociceptive action ↓ Neuroinflammation ↓ LPS toxicity |
α-asarone (3, 10 and 30 mg/kg, p.o., for 7 h) | [78] |
| C57BL/6 mice | MPTP/Parkinson’s disease (18 mg/kg, i.p., four injections at 2 h intervals for one day) |
↑ Motor performance ↑ DA levels ↑ Cognitive function ↑ Anti-inflammatory ↑ TH-positive cells ↓ Neuroinflammation ↓ NF-κB activation |
α-asarone (10 mg/kg, p.o., 15 days) | [25] |
| C57BL/6J mice | Ethanol/Dementia (Saline, i.p. + 2 g/kg ethanol, i.g., treatment duration not mentioned) |
↑ Motor performance ↑ Cognitive function ↓ NMDA receptors ↓ SYNI activity ↓ Glu levels |
α-asarone (7.5, 15 and 30 mg/kg, i.p., treatment duration not mentioned) | [79] |
| Wistar rat | Submicron emulsion injection/Alzheimer’s disease | ↑ Motor performance ↑ Cognitive function ↑ Hippocampal neurons ↓ Aβ deposits |
α-asarone (10 and 25 mg/kg, i.p., for 28 days) | [80] |
| β-asarone | ||||
| PC12 | H2O2/Neurotoxicity (400 µM for 24 h) |
↓ Oxidative stress ↓ ROS production ↑ Nrf2 and HO-1 activation |
β-asarone (15, 30 and 60 µg/mL, for 24 h) | [71] |
| SH-SY5Y | Aβ/Alzheimer’s disease (SH-SY5Y, 20 μM for 24 h) |
↓ Oxidative stress ↓ ROS production ↓ Apoptosis ↑ ASK1 siRNA activity |
β-asarone (10–100 µg/mL, for 24 h) | [81] |
| SH-SY5Y | Aβ25-35/Alzheimer’s disease (20 μM for 24 h) |
↓ Oxidative stress ↓ ROS production ↓ Neuroinflammation ↓ Apoptosis ↑ Autophagy efficiency ↑ Bcl2 protein expression |
β-asarone (10, 50 and 100 µM, for 24 h) | [82] |
| Wistar rat | Middle cerebral artery occlusion (MCAO)/Ischemia | ↑ Motor performance ↓ Oxidative stress |
β-asarone (10, 20 and 30 mg/kg, p.o., for 30 days) | [83] |
| Wistar rat PC12 cells |
MCAO/Ischemia OGD/R for 24 h |
↑ Cell viability ↑ Motor performance ↓ Brain infarct volume ↓ Apoptosis ↓ Neuronal cell injury ↓ Neuroinflammation |
α-asarone (10 and 20 mg/kg, i.v., for 24 h) α-asarone (12, 24, 48 μM for 24 h) |
[84] |
| APP/PS1 transgenic mice | Alzheimer’s disease | ↓ Senile plaques ↓ Aβ40 and Aβ42 aggregation ↓ Autophagosomes ↑ p62 expression |
β-asarone (10, 20 and 40 mg/kg, i.g., for 30 days) | [85] |
| Wistar rat | 6-OHDA/Parkinson’s disease (4 mg/mL, 6 µL in each rat for 30 days) |
↑ DA levels ↑ TH-positive cells ↑ HSP70 expression ↑ p62 expression ↑ Neuronal cell survival ↓ α-synuclein ↓ Macroautophagy ↓ Autophagosomes |
β-asarone (15 mg/kg, i.g., for 30 days) | [29] |
| C57BL/6 mice | MK-801/Schizophrenia (0.1 mg/kg, i.p., for 7 days) |
↑ Motor performance ↑ Body weight ↑ Cognitive function ↑ Synaptophysin ↑ Postsynaptic density ↑ Cognitive function ↑ Anti-inflammatory ↓ Neuroinflammation ↓ Microglia activation |
β-asarone (25 mg/kg, i.g., for 14 days) | [86] |
| Wistar rat | CUMS/Depression (CUMS for 21 days) |
↑ Motor performance ↑ Body weight ↑ Cognitive function ↑ Neurogenesis ↑ BrdU-positive cells ↑ ERK1/2 activation ↑ CREB activation |
β-asarone (25 mg/kg, i.g., for 28 days) | [87] |
| PC12 | Aβ (1–42)/Alzheimer’s disease (20 μM for 24 h) |
↑ Cell viability ↑ Bcl2 protein expression ↓ Apoptosis ↓ JNK signalling |
β-asarone (7.5, 15, and 30 μg/mL, for 24 h) | [88] |
| Wistar rat | 6-OHDA/Parkinson’s disease (4 µg/µL, 6 µL in each rat for 28 days) |
↑ Motor performance ↑ DA levels ↑ TH-positive cells ↑ p62 expression ↑ Bcl2 expression ↓ α-synuclein ↓ Apoptosis ↓ JNK signalling |
β-asarone (10, 20, 40 and 75 mg/kg, i.g., for 28 days) | [89] |
| AβPP/PS1 double-transgenic mice | Alzheimer’s disease | ↑ Motor performance ↑ Cognitive function ↑ CREB activation ↑ Bcl2 expression ↑ CaMKII-α-positive cells ↓ Neuronal apoptosis |
β-asarone (7 and 21 mg/kg, i.g., for 4 months) | [90] |
↑ = increase, ↓ = decrease. i.p., intraperitoneal route; p.o., oral route; i.g., intragastrically; NF-κB, nuclear factor kappa B; PKA, protein kinase A; AChE, acetylcholinesterase; HSP70, heat shock protein 70; CREB, cAMP-response element-binding protein; p-CREB, phosphorylated CREB; Aβ, amyloid beta; LPS, lipopolysaccharides; DA, dopamine; TH, tyrosine hydroxylase; NMDA, N-methyl D-aspartate; ROS, reactive oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; Bcl2, B-cell lymphoma 2; JNK, c-Jun N-terminal kinases; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; 6-OHDA, 6-hydroxydopamine; CUMS, Chronic unpredictable mild stress; MCAO, middle cerebral artery occlusion; H2O2, hydrogen peroxide.
3.1. Effects of α- and β-Asarone on Oxidative Stress
Oxidative stress results from an imbalance between the production of ROS and reactive nitrogen species (RNS) and the activity of antioxidant defence systems. Oxidative stress has been associated with the pathogenesis and progression of several neurodegenerative diseases, including AD and PD, and contributes to the damage associated with other neurological conditions (e.g., ischaemic stroke and schizophrenia) [65,66]. The antioxidant defence system neutralises the formation of excess free radicals in response to increased oxidative stress, preventing cellular damage. Various plant secondary metabolites with antioxidant properties exhibit demonstrable beneficial effects on brain function and overall health in humans [22,23]. Both in vitro and in vivo experiments have shown that α- and β-asarone exhibit antioxidant properties. The free-radical scavenging activities of α- and β-asarone have been demonstrated using various in vitro antioxidant assays, including the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals scavenging assay and the potassium ferricyanide reduction method, and by monitoring the levels of hydroxyl radicals, superoxide, and lipid peroxidation [67,68,69]. Interestingly, α- and β-asarone treatment significantly enhanced catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) activity levels and inhibited the excessive accumulation of malondialdehyde (MDA), lactate dehydrogenase (LDH), and ROS, suggesting that α- and β-asarone could improve enzymatic antioxidant defence systems [67,70,71]. Moreover, β-asarone pre-treatment was also found to activate the Nrf2 signalling pathway and its downstream target haemeoxygenase-1 (HO-1), which is involved in the quenching of ROS to mitigate oxidative stress [71]. When small interfering RNA (siRNA) was used to silence Nrf2, the protective effect of β-asarone was reduced, and H2O2-induced oxidative stress was enhanced in PC12 cells [71]. In addition, using an Aβ-stimulated PC12 cell model, Meng et al. observed that β-asarone pre-treatment could improve cell viability and mitigate cell damage and apoptosis. β-asarone could also decrease the level of ROS and MDA, increase the level of SOD, CAT, and GSH-PX, and promote the expression of Nrf2 and HO-1 [72] (Figure 2).
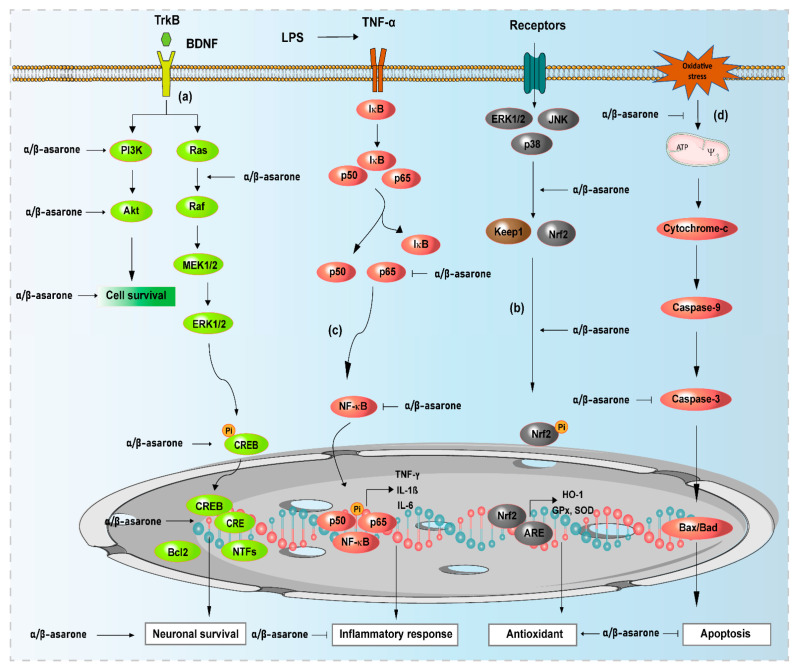
Figure 2.
Neuroprotective effects of α- and β-asarone and their functional mechanisms and targets. (a) α- and β-asarone bind to receptors of neurotrophic factors (tropomyocin-related kinase; TrkB) and other binding proteins, activate downstream kinases (PI3K/Akt, ERK1/2) and phosphorylate CREB protein to promote neuronal survival; (b) α- and β-asarone activate Keap1/Nrf2/ARE signaling pathways to increase the expression of antioxidant enzymes, preventing oxidative stress by reducing cellular ROS levels; (c) α- and β-asarone block NF-κB activation, preventing the production of proinflammatory cytokines and other inflammation mediators, inhibiting neuroinflammation; (d) α- and β-asarone inhibit cytochrome C release from the mitochondria, mitigating the activation of caspases and Bax expression, and increasing Bcl2 expression, thus preventing apoptosis. The block lines ( ) and arrows (→) indicate inhibition and activation by α- and β-asarone, respectively. ERK, extracellular signal-regulated kinase; Akt, protein kinase B; KEAP1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; ARE, antioxidant response element; CREB, cAMP-response element-binding protein; Bcl2, B-cell lymphoma 2; NF-κB, nuclear factor-kappa B; IκB, inhibitory kappa B; ERK1/2, extracellular signal-regulated kinases 1/2; NTFs, neurotrophic factors.
) and arrows (→) indicate inhibition and activation by α- and β-asarone, respectively. ERK, extracellular signal-regulated kinase; Akt, protein kinase B; KEAP1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; ARE, antioxidant response element; CREB, cAMP-response element-binding protein; Bcl2, B-cell lymphoma 2; NF-κB, nuclear factor-kappa B; IκB, inhibitory kappa B; ERK1/2, extracellular signal-regulated kinases 1/2; NTFs, neurotrophic factors.
Recently, Pages et al. [70] and Saki et al. [91] reported an increase in the expression levels of the endogenous antioxidant enzymes SOD and glutathione peroxidase (GPx) in the brains of mice and rats treated with α- and β-asarone compared with untreated animals. Furthermore, α-asarone treatment attenuated the oxidative stress response in the brains of a rat model of noise-induced stress, an effect mediated by the increased expression of SOD, GPx, and CAT, in addition to the upregulation of other endogenous non-enzymatic antioxidant molecules, such as vitamin C, vitamin E, and GSH [92]. Similarly, α-asarone treatment increased acetylcholinesterase (AChE) activity and normalised MDA and SOD levels in the hippocampus and cerebral cortex of AD-like scopolamine-induced amnesic mice [76]. Another study reported that α-asarone administration attenuated brain and kidney damage induced by γ-radiation exposure by restoring antioxidant levels, such as SOD, GPx, CAT, and GSH, and decreasing the lipid peroxidation levels [93]. Yang et al. [83] observed that β-asarone supplementation significantly restored GSH, glutathione reductase (GR), CAT, and glutathione S transferase (GST) levels and decreased lipid peroxidation levels in the hippocampus of a rat model of ischaemia induced by middle cerebral artery occlusion (MCAO). Wang et al. [94] showed that β-asarone treatment could effectively attenuate MDA damage and significantly increase CAT and SOD activities in a rat model of AD generated by the intracerebroventricular injection of Aβ₁₋₄₂ combined with ischemia. In a rat model of MCAO-induced ischemic stroke, β-asarone treatment activated Nrf2/ARE pathway-related proteins, an effect that was inhibited by an Nrf2 inhibitor [31]. These findings suggest that the antioxidant effects of α- and β-asarone could contribute to their therapeutic benefits in the treatment of neurological disorders.
3.2. Effects of α- and β-Asarone on Neuroprotective Signaling Pathways
A variety of pro-survival signalling pathways, such as PI3K/Akt and extracellular signal-regulated kinase (ERK)1/2, are activated by α- and β-asarone (Figure 2) [72,73]. These pathways play an important role in cellular function, synaptic plasticity, and memory by altering the phosphorylation condition of molecules and modulating gene expression [95,96,97]. Oxidative stress triggers MAPK cascades that result in the activation of pro-survival p38 MAPKs, c-Jun N-terminal kinase (JNK), and ERK signalling pathways. The activation of ERK signalling suppresses the death-inducing complex formation and boosts cell survival by upregulating the anti-apoptotic proteins Bcl-xL and Bcl-2 and inhibiting Fas-mediated apoptosis [98].
In vitro results collected over the years have shown that α- and β-asarone activate the PI3K/Akt/Nrf2 and protein kinase A (PKA) signalling pathways, which play crucial roles in protecting the cells against abnormal ROS levels and neuronal damage, as well as improving cell viability and neuroprotective function [43,72,73]. In Aβ-treated PC12 cells, pre-treatment with β-asarone increased cell viability and decreased cell apoptosis [88]. The protective effect of β-asarone against β-amyloid-induced neurotoxicity was partly mediated through the inhibition of Aβ-induced JNK activation. Additionally, β-asarone significantly attenuated the Aβ-induced downregulation of Bcl-w and Bcl-xL and inhibited mitochondrial cytochrome C release and the activation of caspase-3 [88]. In primary cultured rat astrocytes and SH-SY5Y cells, α- and β-asarone upregulated Akt signalling and protected cells from oxidative stress, an effect that was shown to be mediated by the scavenging of ROS formation and the stimulation of the Nrf2-ARE self-defence mechanism, and the subsequent triggering of the expression of antioxidant enzymes, and increased the levels of the anti-apoptotic protein Bcl-2 [73,82]. Another recent study showed that β-asarone protects cells from Aβ1–42-induced cytotoxicity and attenuates autophagy via activation of the Akt-mTOR signalling pathway, which may be involved in the neuroprotection of β-asarone against Aβ toxicity in PC12 cells [99]. α- and β-asarone activate not only PI3K/Akt but also ERK/CREB/BDNF pathways in vivo and in vitro, which help to enhance memory function, protect neurons, and recover behavioural changes including immobility time, locomotor activity, and escape latency [77,90,100,101].
3.3. Effects of α- and β-Asarone on Proteostasis, ER Stress, and Autophagy
The accumulation of misfolded intracellular and extracellular protein aggregates is a major hallmark of neurodegenerative disease pathogenesis [102,103,104]. In the brain, several native proteins (Aβ, tau, and α-synuclein) undergo conformational changes due to genetic and environmental factors [104]. Neurodegeneration has been associated with mutated and misfolded protein monomers, oligomers, and insoluble aggregates, formed through altered β-sheet interactions, which cause dysfunction in cellular proteolytic pathways [105,106]. Pathogenic misfolded protein aggregation exerts neurotoxic effects on the CNS, resulting in eventual neuronal cell death and the development of neurodegenerative diseases [3,107]. Many therapeutic approaches to neurodegenerative diseases have aimed to diminish the accumulation of toxic oligomers, fibril deposits, and aggregation intermediates [108,109]. Several studies have suggested that some phytochemicals can inhibit amyloidogenic monomer synthesis and fibrillar aggregate formation, enhancing the formation of nontoxic aggregates and stimulating the proteolytic system to target the neurotoxic pathogenic factors associated with neuronal loss in neurodegenerative disease [110,111].
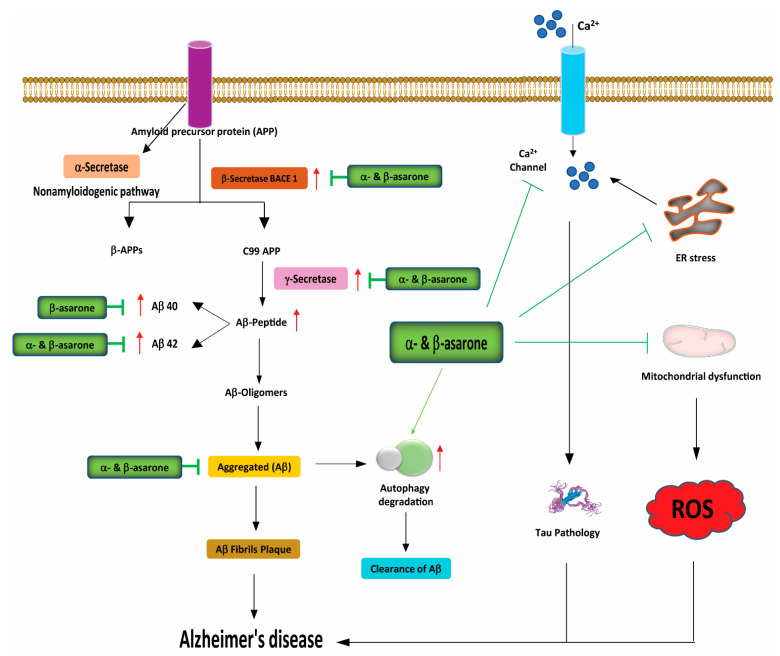
Amyloidogenic Aβ1–42 peptides are primarily generated by the cleavage of amyloid precursor protein (APP) by β- and γ-secretase [112]. Recent studies have found that α- and β-asarone suppress the expression of the β-secretase BACE1, improving cognitive and behavioural function in animal models of AD [113,114]. A very recent study found that α-asarone potentially targets the Aβ and tau pathology pathways by inhibiting Aβ42 aggregation, in addition to inhibiting tau phosphorylation, resulting in improved spatial learning memory in APP/presenilin-1 (PS1) transgenic mice [28] (Figure 3). Another study demonstrated that treatment with β-asarone reduced the number of senile plaques and decreased Aβ40, Aβ42, and APP expression levels in the hippocampus of APP/PS1 transgenic mice [85]. Moreover, β-asarone displayed a significant therapeutic effect against toxic protein deposition and increased the expression of the presynaptic protein synapsin 1 (SYN1), which should remove toxic superoxide anion radicals produced in cells [114]. A thioflavin T (ThT) fluorescence assay conducted in PC12 cells treated with α- and β-asarone showed an efficient and dose-dependent reduction in Aβ aggregation, protecting PC12 cells from Aβ aggregate-induced toxicity [115] (Figure 3).
Figure 3.
Neuroprotective effects of α- and β-asarone on the pathogenic mechanisms of Alzheimer’s disease (AD). The 99 amino acid C-terminal fragment of amyloid precursor protein APP-C99 (C99) is cleaved by γ-secretase to form the Aβ peptide, which plays a critical role in the etiology of AD. The 40-residue peptide Aβ (1–40) represents the most abundant Aβ isoform in the brain, while the 42-residue Aβ (1–42) shows a significant increase with certain forms of AD. α- and β-asarone block the formation of Aβ peptides by reducing γ-secretase, and lessen tau pathology by blocking intracellular calcium channels and inhibiting oxidative stress-mediated neuronal cell death by decreasing ROS release from the mitochondria and promoting the autophagic clearance of aggregate-prone proteins. Aβ, amyloid beta; APP, amyloid precursor protein; ROS, reactive oxygen species; ER stress, endoplasmic reticulum stress.
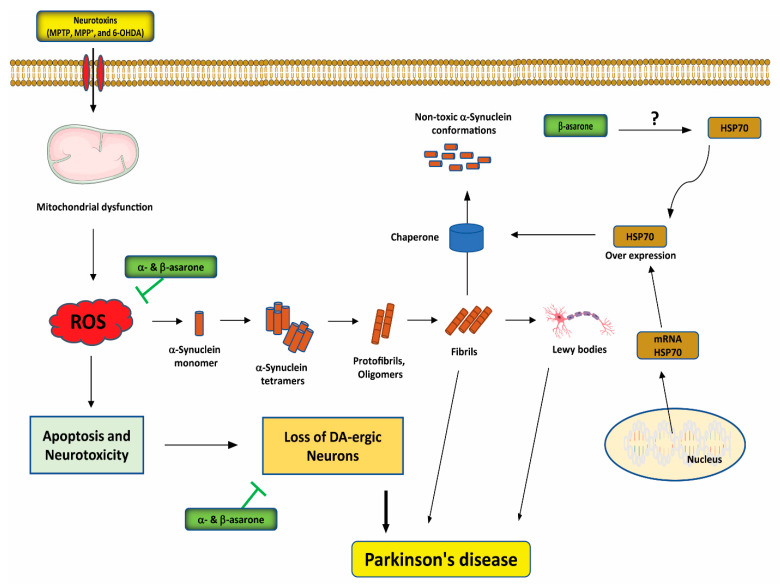
Presynaptic α-synuclein aggregation is considered the primary pathogenic factor in the development of α-synucleinopathies, such as PD [103]. Large α-synuclein aggregates, associated with the presence of missense mutations in the α-synuclein gene, including A30P, A53T, and E46K, may exert toxic effects through increased oxidative stress, mitochondrial dysfunction, altered patterns of phosphorylation, and interactions with the phospholipid membrane [116]. Moloney et al. [117] demonstrated that heat shock protein 70 (HSP70) protects dopaminergic neurons from protein aggregation and inhibits microglial activation, ultimately preventing apoptosis. The overexpression of HSP70 exerts a protective effect against early-onset α-synuclein–induced pathology, as demonstrated in an adeno-associated virus model of α-synuclein aggregation [118]. β-asarone treatment has also been found to alleviate dopaminergic cell death induced by 6-hydroxydopamine (6-OHDA) through the upregulation of HSP70 mRNA and protein expression levels and the downregulation of α-synuclein mRNA and protein expression levels [29] (Figure 4). In addition, in SH-SY5Y cells transfected with α-synuclein, β-asarone treatment protected against cell death induced by MPP+ [119]. In a mouse model, β-asarone treatment also exerted neuroprotective effects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD, preventing the typical decrease in tyrosine hydroxylase (TH)-positive cells and increasing α-synuclein expression levels, thus protecting dopaminergic neurons in the midbrains [119] (Figure 4).
Figure 4.
Neuroprotective effects of α- and β-asarone on the pathogenic mechanisms of Parkinson’s disease (PD). α- and β-asarone inhibit oxidative stress-mediated neuronal death and attenuate ROS release from the mitochondria. By inducing HSP70, β-asarone may potentially attenuate/or prevent protein misfolding, reducing the apoptosis of DA neurons. The question mark (?) indicates that the mechanisms by which α- and β-asarone interact with HSP70 to exert neuroprotective effects in PD are unclear. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPP+, 1-methyl-4-phenylpyridinium; 6-OHDA, 6-hydroxydopamine; PD, Parkinson’s disease; DA, dopamine; ROS, reactive oxygen species; HSP70, heat shock protein 70.
Pathogenic proteins associated with neurodegenerative diseases cause dysfunction in the cellular proteolytic systems, including the ubiquitin-proteasome system (UPS) and the autophagy–lysosome pathway [120]. Misfolded and unfolded proteins are identified by ubiquitination and targeted for degradation by the proteasome, and UPS dysfunction has been associated with the aggregation of misfolded proteins in PD, although the specific role played by the UPS in PD pathogenesis remains unclear [121]. Disease-specific protein aggregates that are too large for the UPS, including Aβ, tau, and α-synuclein aggregates, in addition to damaged organelles, such as mitochondria, are typically targeted for degradation by the autophagy–lysosome pathway [105].
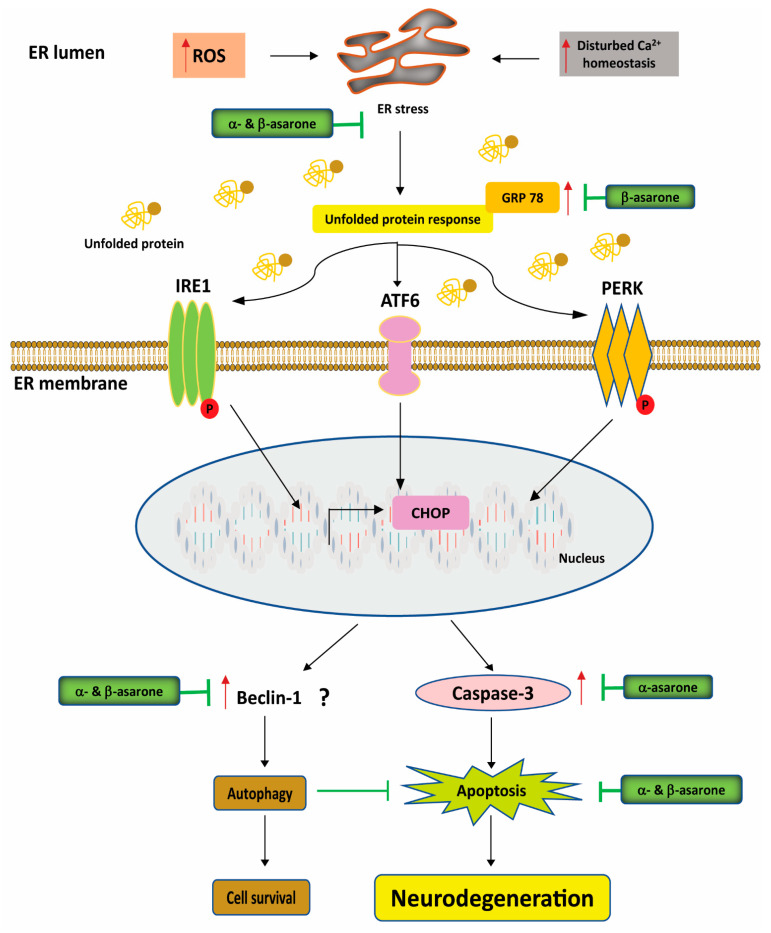
The endoplasmic reticulum (ER) plays a critical role in the proper folding of membrane-bound and non-cytoplasmic proteins [122,123,124]. The accumulation of misfolded or unfolded proteins in the ER causes cellular stress and triggers the unfolded protein response (UPR). Chronic or excessive UPR activation can eventually lead to cell death. UPR activation is mediated by three ER stress sensors: protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1 (IRE1), and activating transcription factor 6 (ATF6) [122,123,124]. The glucose-regulated protein GRP78 (also, known as BiP) primarily regulates the initiation of the UPR through its direct interactions with each signal-transducing sensor [122,125]. Like ATF6, ATF4 is a basic leucine zipper (bZIP) transcription factor important for maintaining intracellular homeostasis through the upregulation of UPR-target genes involved in efficient protein folding, the antioxidant response, and amino acid biosynthesis and transport. In addition to promoting an adaptive response, ATF4 upregulates the bZIP transcription factor C/EBP homologous protein (CHOP), which promotes cell death [122,126]. Collectively, the UPR pathways serve to halt protein biosynthesis, promote protein degradation, and generate chaperones to refold misfolded proteins.
ER stress has been identified in several experimental cellular models of PD [127], and the increased expression of wild-type α-synuclein is sufficient to cause ER stress [128]. ER stress is closely related to oxidative stress, which can also trigger UPR activation [129]. In a 6-OHDA-induced PD model, β-asarone treatment downregulated the mRNA levels of GRP78 and CHOP, resulting in the blockade of two of the three UPR activation pathways [130] (Figure 5). GRP78 preferentially binds unfolded or misfolded proteins in the ER, releasing its inhibitory hold on PERK, ATF6, and IRE1 [131]. Furthermore, β-asarone treatment was shown to reduce the expression of GRP78, phosphorylated PERK (p-PERK), and CHOP, regulating ER stress response and autophagy in a 6-OHDA-induced rat model of PD [132] (Figure 5). Interestingly, α-asarone treatment prevented 7-hydroxycholesterol-triggered macrophage apoptosis by alleviating ER stress-specific signalling, such as caspase induction and CHOP activation [133]. Furthermore, α-asarone treatment significantly reduced chronic constriction injury-induced ER stress in the spinal cord, in addition to decreasing microglial activation, and alleviating neuropathic pain [134]. In a recent in vitro study, pre-treatment with α-asarone protected hippocampal cells from oxidative and ER stress by decreasing ROS production and suppressing PERK signalling in glutamate- and tunicamycin-induced hippocampal HT22 cells [135].
Figure 5.
Neuroprotective effects of α- and β-asarone against neurological disease-associated ER stress. ER stress is detected by three UPR activator proteins, IRE1, PERK, and ATF. β-asarone inhibits the ER stress proteins GRP78 and CHOP, blocking two of the three UPR pathways. α- and β-asarone may also inhibit Beclin-1 expression, which promotes autophagy activation. The question mark (?) indicates that the mechanism by which α- and β-asarone affect Beclin-1 is still unclear. ROS, reactive oxygen species; ER stress, endoplasmic reticulum stress; GRP78, glucose-regulating protein 78; 6-OHDA, 6-hydroxydopamine; CHOP, C/EBP homologous binding protein; ATF6, activating transcription factor 6; IRE1, inositol-requiring enzyme 1; PERK, PERK phosphorylation.
Autophagy activation can upregulate the clearance of protein aggregates, prevent mitochondrial damage, control axon homeostasis and neurogenesis, ensure cell survival, and reduce growth factor deficiency and ER stress, with potential therapeutic benefits in slowing the pathological progression of AD, PD, and other neurodegenerative diseases [136,137]. Beclin-1, phosphorylated (p)-Akt, and mammalian target of rapamycin (mTOR) are known autophagy regulators, and some plant-derived secondary metabolites have been shown to exert neuroprotective effects via the stimulation of autophagy through both mTOR-dependent and independent mechanisms [138]. In a 6-OHDA-induced PD mouse model, β-asarone treatment significantly decreased the levels of both mRNA and protein of Beclin-1 and LC3B, and increased p62 expression, indicating autophagy activation [29]. Another study found that treatment with α-asarone enhanced autophagy in macrophages by upregulating autophagolysosomal formation [139]. A recent canonical correlation analysis revealed that β-asarone treatment can inhibit Aβ aggregation through the promotion of autophagy in a PC12 cell model of AD [114]. Another experiment demonstrated that α-asarone reduced the activation of eIF2α-CHOP by 7β-hydroxycholesterol, enhanced autophagy in macrophages through the upregulation of autophagolysosomal formation, increased phosphorylation of Bcl-2, and facilitated the entry of Beclin-1 into the autophagic process [139]. These findings suggest that α- and β-asarone may have the potential to reduce protein aggregation and ER stress through autophagy modulation, which can have regulatory effects on neurodegenerative disease progression.
3.4. Effects of α- and β-Asarone on Neuroinflammation
Microglia are located throughout the CNS and play important roles in the clearance of damaged neurons, infection prevention, foreign organism defence, and tissue repair [140]. Microglial activation is the main initiator of neuroinflammatory responses, and microglia can be activated by the presence of pathogens, tissue damage, infection, injury, or neurotoxins. Microglial activation leads to the release of various oxidants, including ROS and RNS, in addition to the induction of inflammatory factors, such as pro-inflammatory cytokines, chemokines, and neurotoxic molecules, triggering neuroinflammatory responses that contribute to neuronal cell death and promote the progression of neurodegeneration [141,142]. Consequently, the inhibition of pro-inflammatory cytokine release by microglial activation has been explored as an effective therapeutic intervention for attenuating neurological disorders [143,144,145].
Plant-derived secondary metabolites with strong antioxidant capacities can reduce the prevalence of age-related neurological disorders associated with increased inflammation [146,147]. In vitro and in vivo experiments have shown that, by attenuating the expression of pro-inflammatory cytokines and inflammatory mediators, including tumour necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, preventing inflammasome formation, or inducing the expression of neuroprotective anti-inflammatory and antioxidant molecules, α- and β-asarone can exert neuroprotective effects in various neurological disorders [78,82] (Figure 2). For instance, in the pilocarpine-induced rat model of epilepsy, α-asarone treatment attenuated cognitive deficits and reduced neuroinflammation and microglial activation via a reduction in NF-κB activation [148]. Treatment with α-asarone also suppressed pro-inflammatory cytokine production and attenuated the LPS-stimulated neuroinflammatory response in primary cultured microglial cells. Treatment with α-asarone further inhibited the activation of the inflammatory response by significantly reducing the degradation of the NF-κB inhibitors IκB-α and IκB-β and regulating NF-κB transcription in both in vitro primary cultured microglial cells and an in vivo pilocarpine-induced epileptic rat model [148] (Figure 2). In the MPTP-induced mouse model of PD, α-asarone treatment significantly reduced microglial activation and neuroinflammation in the brain [25]. In vitro studies have revealed that α-asarone treatment significantly attenuates the LPS-stimulated upregulation of neuroinflammatory responses, reduces pro-inflammatory cytokine production, and inhibits NF-κB activation by blocking the degradation of NF-κB inhibitor IκB-α in BV-2 microglial cells [25] (Figure 2). In the Aβ1-42-induced APP/PS1 transgenic mouse model of AD, the oral administration of α-asarone significantly decreased pro-inflammatory cytokine levels and reduced the expression of the microglial marker protein glial fibrillary acidic protein, alleviating neuroinflammation [28]. Similarly, in a rat model of a spinal cord injury, the oral administration of α-asarone significantly reduced the levels of IL-1β, IL-6, TNF-α, inducible nitric oxide synthase, monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 2, whereas the levels of anti-inflammatory mediators IL-4, IL-10, and arginase 1 were increased 24 h after spinal cord injury [149]. In addition, immunohistochemistry analysis revealed that α-asarone treatment effectively reduced neuroinflammation and reactive gliosis and increased the expression of M2 macrophage markers and angiogenesis [149].
In the LPS-stimulated BV-2 cell model, treatment with α-asarone decreased the mRNA and protein levels of MCP-1, modulated microglial morphological dynamics, and reduced the numbers of activated microglia and microglial process tips while promoting neurogenesis [150]. In a mouse model of chronic schizophrenia induced by MK-801, β-asarone treatment improved cognitive functions and synaptic plasticity, partly by suppressing microglial activation and downregulating the microglia-mediated inflammatory response [86]. Likewise, in an in vitro study, β-asarone treatment in Aβ1–42-induced PC12 cells reduced IL-1β and TNF-α levels, inhibited NF-κB activity, and downregulated the phosphorylation of extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) [100].
Taken together, a substantial amount of in vitro and in vivo data highlight the potential of α- and β-asarone as promising therapeutic agents against neurological disorders due to their antioxidant and anti-inflammatory properties, and their ability to downregulate the NF-κB pathway.
3.5. Effects of α- and β-Asarone on Neurogenesis, Neurotransmitter Metabolism, and Neuronal Cell Death
Neurotrophic factors (NTFs), such as the brain-derived neurotrophic factor (BDNF) and the glial-cell-derived neurotrophic factor (GDNF), play central roles in the survival, growth, functional development, and plasticity of glutamatergic and γ-aminobutyric acid (GABA)ergic synapses. NTFs modulate neuronal differentiation, influencing serotonergic and dopaminergic neurotransmission [151,152,153]. Accordingly, BDNF and GDNF have been identified as potential therapeutic targets in neurodegenerative diseases, and pre-clinical studies have reported the beneficial effects of the administration of BDNF and GDNF in PD models [154,155,156,157,158]. However, these essential NTFs cannot pass through the BBB, preventing NTFs directly administered to patients from reaching damaged neurons in the brain. Plant-derived secondary metabolites that are able to cross the BBB can directly target these same neurotrophic receptors, potentially activating the neuronal biogenesis of BDNF and GDNF [98,159].
β-asarone has been reported to enhance the levels of BDNF-targeted receptor-like tropomyosin-related kinase B (TrkB) and activate the ERK pathway, triggering antidepressant-like effects, enhancing the survival rate of cultured motor neurons, and significantly reducing the apoptosis rate of hippocampal neurons [160]. Interestingly, in cultured rat astrocytes and PC12 cells, α- and β-asarone treatment significantly promoted the expression and secretion of NTFs, such as nerve growth factor (NGF), BDNF, and GDNF, in a dose-dependent manner [43,74]. In addition, the application of the protein kinase A (PKA) inhibitor H89 to cultured astrocytes partially blocked α- and β-asarone-induced NTF expression, suggesting the involvement of PKA signalling in this effect [43] (Figure 2). Moreover, β-asarone treatment induced the expression of BDNF and GDNF and exerted neuroprotective activities via the ERK/cAMP signalling pathways in both in vitro and in vivo models [100,161]. Monoamine oxidase (MAO), an enzyme located in the outer mitochondrial membrane, is principally responsible for monoamine neurotransmitter homeostasis (epinephrine, norepinephrine, serotonin, and DA) in the brain, and regulates signalling pathways involved in neuron survival and death [162]. MAO exists in two forms, termed MAO-A and MAO-B. MAO-A inhibitors have anxiolytic and antidepressant activities, whereas MAO-B inhibitors, such as rasagiline and selegiline, activate neuroprotective pathways [163]. β-asarone and levodopa co-administration were found to decrease MAO-B activity, enhancing monoaminergic neurotransmitter levels [164].
The beneficial effects of α- and β-asarone on dopaminergic neuronal loss have been associated with increased DA levels, both in vitro and in vivo. In addition to the various potential mechanisms that contribute to the prevention of dopaminergic cell death, as described above, the co-administration of β-asarone and levodopa (L-DOPA) has been shown to increase the striatal and plasma levels of DA and DA metabolites, including 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxytryptamine (5-HT). Elevated DA metabolites were also observed in cortical and hippocampal regions [164,165,166]. Increased DA levels were associated with improved behavioural performance of 6-OHDA-induced rat models of PD [164]. Similarly, α-asarone treatment has been shown to increase the levels of the DA metabolite DOPAC in the striatum of an MPTP-induced mouse model of PD [25]. Treatment with β-asarone in 6-OHDA-induced rats resulted in significantly increased levels of DOPAC, HVA, and the serotonin metabolite 5-hydroxyindoleacetic acid in the striatum, accompanied by improved motor performance [89].
β-asarone treatment was also found to attenuate learning and memory impairments in both rat pups and adult rats in a chronic lead (Pb)-induced model of developmental delay, partially rescuing learning and memory abilities [167]. Interestingly, a previous study suggested that changes in the levels of amino acids in AD, particularly the imbalance between glutamate and GABA levels, are key factors contributing to neuronal injury and memory impairment [168,169]. Moreover, in an ethanol-induced dementia mouse model, the administration of α-asarone was able to maintain the equilibrium between glutamate and GABA in the hippocampus, enhancing learning and memory abilities [79]. As a result, in addition to potential disease-modifying properties, α- and β-asarone may also prove useful for symptom relief in neurological disorders.
Cell death by apoptosis is an important mechanism of cell loss in neurodegenerative diseases. Apoptosis occurs in response to a variety of biochemical and morphological alterations, including cell shrinkage, nucleosomal degradation, and chromatin condensation. Apoptosis signalling by mitochondria is considered a promising therapeutic target for the treatment of neurodegenerative disease [170,171]. The first evidence of α- and β-asarone-mediated protection against neuronal cell death was deduced from in vitro studies using NMDA-treated primary cultured rat cortical cells and Aβ-treated PC-12 cells as models of neurodegeneration [172,173]. The oral administration of α-asarone to an MCAO-induced mouse model of ischaemic stroke significantly increased the number of neuronal nuclei (NeuN)-immunoreactive cells and decreased the number of S100 calcium-binding protein β (S100β)-immunoreactive cells, resulting in greater-than-additive effects on sensorimotor function and motor balance, based on analyses of corner and rotarod behavioural tests [26]. A similar study showed that α-asarone at a dose of 10 and 20 mg/kg treatment could decrease brain infarct volume, improve neurological function, and reduce the incidence of post-stroke epilepsy [84]. These neuroprotective effects were attributed to improved glial activation and autophagy, indicating that α-asarone is a very promising drug candidate for cerebral ischemia stroke [84]. In an MPTP-injected mouse model of PD, α-asarone treatment suppressed glial activation, protected dopaminergic TH-positive neurons in the substantia nigra and TH-positive fibres in the striatum, and attenuated PD-like behavioural impairments, as assessed by the Y-maze and pole tests [25]. Another experiment showed that α-asarone significantly improved behavioural performance and learning and memory in C57BL/6J mice treated with ethanol to induce cognitive impairment [79]. Moreover, treatment with β-asarone partially reversed chronic unpredictable mild stress (CUMS)-induced depression-like behaviours, as assessed by both the sucrose preference and forced swim tests, which was associated with increased hippocampal neurogenesis, as indicated by bromodeoxyuridine (BrdU) immunoreactivity [87]. α-asarone treatment significantly attenuated the immobility time of mice subjected to nicotine withdrawal, as assessed by the forced swim test, indicating an antidepressant effect of α-asarone, with no apparent effect on locomotor activity [77]. In immunostaining and microscopy analyses following chronic α-asarone treatment, both the size and morphology of hippocampal neurons returned to a relatively normal state [80]. In aged rats, treatment with α-asarone also enhanced cognitive functions, an effect that was correlated with a reduction in neuronal loss and decreased toxic Aβ production [80].
An increasing body of evidence has shown that Bcl-2, along with Bax and caspase proteins, play major roles in the apoptotic pathways associated with the pathogenesis of neurodegenerative diseases [174,175,176]. The downregulation of the anti-apoptotic regulators Bcl-2, Bcl-xL and Bcl-w results in the inhibition of JNK phosphorylation, the release of cytochrome c from the mitochondria, and caspase-3 activation, ultimately leading to apoptosis. β-asarone treatment prevented the downregulation of these anti-apoptotic proteins, reducing apoptosis in neuronal PC-12 cells and ameliorating Aβ-induced cognitive impairments in the hippocampus of an Aβ-induced rat model of AD [88,177]. A recent study showed that β-asarone treatment protected against 6-OHDA-induced dopaminergic neurodegeneration in a rat model of PD through the downregulation of JNK and p-JNK expression, resulting in the indirect increase in Bcl-2, accompanied by improved behavioural performance in the open-field and rotarod tests, as well as improved movement initiation and stepping time [89]. Recent in vitro and in vivo studies have also demonstrated that β-asarone treatment can protect PC12 cells and cortical neurons by inhibiting neuronal apoptosis through the activation of the calcium/calmodulin-dependent protein kinase II (CaMKII-α)/CREB/Bcl-2 pathway [90]. An in vivo study also indicated that β-asarone treatment improves cognitive function, reduces neuronal apoptosis, and significantly increases CaMKII/CREB/Bcl-2 expression in the cortices of APP/PS1 mice [90].
Myocyte-enhancing factor 2D (MEF2D) is a transcription factor that plays important roles in synaptic plasticity, neuronal development, and neuronal survival, and the dysregulation of MEF2D has been implicated in the pathogenesis of PD [178]. Increased MEF2D activity protected dopaminergic neurons from stress signals in rodent models of PD [179,180]. Enzyme-linked immunosorbent assays and immunohistochemistry analyses revealed that the β-asarone-mediated activation of MEF2D reduced the loss of TH-positive neurons in the mesencephalon, restoring nigrostriatal neuronal morphology in 6-OHDA-induced mouse models of PD [29], indicating that β-asarone can stimulate the activity of MEF2D via chaperone-mediated autophagy.
The growing body of evidence from both in vitro and in vivo studies indicates that α- and β-asarone exert neuroprotective effects by inducing NTF expression and/or potentiating NTF functions, besides their anti-apoptotic, and anti-inflammatory activities, and thus might have potential in the treatment of neurodegenerative diseases, such as AD and PD. However, additional preclinical and clinical studies are required to better understand these effects. Thorough in vivo studies examining the therapeutic potential of α- and β-asarone in other animal models of neuronal diseases, such as Huntington’s disease, cerebral ischaemia, schizophrenia, and other neurological disorders, are also still missing.
4. Neuroprotective Effects of α- and β-Asarone on Other Neurological Disorders
According to the WHO, approximately 10% of the global population suffer from depression and anxiety disorders [181]. Mitogen-activated protein kinases (MAPK) have been implicated in the pathophysiology of depression and addiction. A previous study reported that MAPK phosphatase-1 (MKP-1) is upregulated in CUMS-induced mice [182]. MKP-1 is not only expressed in response to stress but is also a key negative regulator of the ERK signalling pathway [183], contributing to depressive behaviour [184]. In the chronic CUMS-induced rat model, β-asarone treatment produced significant antidepressive effects [183]. Rats treated with β-asarone presented with significantly increased scores for horizontal and vertical activity in the open-field test and significantly increased total consumption of sucrose solution in the sucrose preference test, likely mediated by the inhibition of stress-induced hippocampal cell death. Furthermore, immunohistochemical and mRNA analyses indicated that β-asarone treatment protected hippocampal neurons from CUMS-induced cell death through the downregulation of MKP-1 [185].
In a study designed to investigate the antidepressant properties of essential oil and asarones from the rhizomes of A. tatarinowii, treatment with α- and β-asarone produced antidepressant-like activity, as measured by the forced swim test and tail suspension test [30]. Interestingly, α-asarone treatment was associated with a biphasic effect in the tail suspension test in mice, inducing an antidepressant effect at lower doses (15 and 20 mg/kg, i.p.) and a depressive-like effect at higher doses (≥50 mg/kg, i.p.) [186]. The antidepressant effect of α-asarone was mediated by its interaction with the noradrenergic (α1 and α2 adrenoceptors) and serotonergic (5-HT1A receptors) systems, whereas the depressive-like effects observed following the administration of high levels of α-asarone may be mediated by interactions with the GABAergic system [186]. In another study, α-asarone showed an anxiolytic effect in animal models of anxiety, as assessed by the elevated plus-maze, the light–dark transition test, the novel food consumption test, and the marble-burying test [187]. Treatment with α-asarone attenuated exogenous corticosterone-induced anxiety in rats, as assessed using the elevated plus-maze, hole-board, and open-field tests, by modulating the corticotrophin-releasing factor and neuroprotective cell signalling pathways [188].
The anticonvulsant effects of α-asaronol ((E)-3’-hydroxyasarone), an active constituent derived from α-asarone, were assessed in an in vivo anticonvulsant screening assay using the three most commonly employed mouse models of seizure, including 3-mercaptopropionic-acid (3-MP)-induced seizures, maximal-electroshock seizures (MES), and subcutaneous-injection-pentylenetetrazole (PTZ)-induced seizures [189]. The oral administration of α-asaronol resulted in a broad spectrum of anticonvulsant activity and displayed better protective indices (PI = 11.11 in MES mice and PI = 8.68 in PTZ mice) and lower acute toxicity (LD50 = 2940 mg/kg) than its metabolic parent compound (α-asarone). Additionally, α-asaronol displayed a prominent anticonvulsant profile, with a 50% effective dose (ED50) of 62.02 mg/kg in the MES model and an ED50 of 79.45 mg/kg in the PTZ model, whereas the control substance stiripentol displayed ED50 values of 240 mg/kg and 115 mg/kg, respectively [189].
5. Challenges to the Translation of the Neuroprotective Effects of α- and β-Asarone from Bench to Bedside
The pharmacological potential of α- and β-asarone has mostly been assessed pre-clinically. In fact, no clinical trial has yet been conducted to investigate the potential therapeutic benefits of α- and β-asarone in the treatment of neurological disorders.
One of the major issues limiting the clinical use of α- and β-asarone is related to their poor bioavailability. The oral bioavailability of α- and β-asarone is especially low because of their highly lipophilic character and thus poor water solubility. Pharmaceutical formulations of α- and β-asarone injection solutions typically require the use of solubility promoters such as Tween-80 or propylene glycol. However, these agents can cause allergic reactions [190,191]. To address this problem, alternative delivery systems have been developed. Drug delivery systems using liposomes and nanoparticles as carriers have been used successfully to encapsulate α- and β-asarone and increase their bioavailability [54,192]. Interestingly, the recent development of a novel α-asarone-embedded lipid nanoformulation has led to enhanced BBB transmigration and significantly increased α-asarone levels in murine plasma and inside the brain [54]. Nose-to-brain delivery via olfactory pathways is another unconventional method by which phytochemicals and other agents could enter the brain bypassing the BBB [193]. In this context, the intranasal delivery of α-asarone to the brain using mPEG-PLA and PLA nanoparticles has been shown to be a better brain-targeting strategy compared to i.v. administration. In addition, nasal-mediated brain delivery reduced liver accumulation and decreased drug-induced hepatotoxicity in rats [55,56]. Therefore, novel pharmaceutical delivery strategies may overcome the bioavailability issues of α-asarone, enhancing its potential effectiveness in humans. In the case of β-asarone, further studies on the most effective delivery systems need to be performed, not only to validate their efficacy but also to address potential safety issues.
Another important factor to consider is the dose-dependency of the pharmacological effects of α- and β-asarone. While α- and β-asarone at lower doses (<50 mg/kg) exhibit a wide range of therapeutic activities such as antidepressant, antianxiety, anti-Alzheimer, and anti-Parkinson effects, higher doses (≥50 mg/kg) result in hypomotility (decreased locomotor activity), and impaired motor coordination [27]. Therefore, further animal studies are required for understanding the toxicology of asarones in neurodegenerative disease models in order to establish a theoretical basis for developing safe α- and β-asarone-based therapeutics.
Importantly, toxicological studies have revealed that both α- and β-asarone can cause hepatomas and might possess mutagenic, genotoxic, carcinogenic, and teratogenic effects [27,64]. According to the European Commission [62] and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [194], β-asarone is not recommended for clinical use because of its dose-dependent toxicity, and thus the inability to establish a safe exposure limit. The development of more efficient systems to deliver α- and β-asarone to their biological targets, particularly the brain, might allow for effective localized biological action while minimizing toxicity. We strongly suggest that the bioavailability, biodistribution, tolerances, safety, and effectiveness of α- and β-asarone and their vehicles should be carefully addressed and taken into account when designing clinical trials, as these are common causes of trial failure.
The most important issue when targeting neurodegenerative diseases is bioavailability, particularly the ability to cross the BBB. Efficacy data provided by a number of preliminary studies have placed inappropriate emphasis on α- and β-asarone properties and provided little information about their bioavailability. More detailed studies are necessary to ensure that pre-clinical findings are more closely scrutinised in order to increase the likelihood of success of eventual clinical trials.
6. Conclusions and Future Prospects
α- and β-asarone display a range of pharmacological attributes that contribute to their protective effects against multiple neurotoxic stimuli. The neuroprotective effects of α- and β-asarone have been attributed to several potential mechanisms of action, including: (1) antioxidant properties; (2) the regulation of various neuroprotective signalling pathways; (3) the reduction of aggregate formation and promotion of the clearance of pathogenic protein aggregates; (4) anti-inflammatory properties; (5) the inhibition of microglial activation; (6) the activation of NTFs-mediated neuroprotection; and (7) the modulation of neurotransmitter levels associated with behavioural functions and neuronal cell survival. These neuroprotective features make α- and β-asarone potentially promising therapeutic agents for the treatment of various neurological disorders, including AD, PD, cerebral ischemia, and epilepsy. However, despite promising pre-clinical evidence, the successful translation of results from the bench to the clinic has yet to be achieved, due to the potential toxicity of α- and β-asarone and their low bioavailability. The development of new delivery systems might reduce α- and β-asarone toxicity and ultimately bring tangible therapeutic benefits for patients.
Abbreviations
AChE, acetylcholinesterase; AD, Alzheimer’s disease; ADME, absorption, distribution, metabolism, and excretion; Aβ, Amyloid beta; APP, amyloid precursor protein; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; CAT, catalase; COMT, catechol-O-methyltransferase; COX-2, cyclooxygenase-2; CREB, cAMP-response element-binding protein; CUMS, chronic unpredictable mild stress; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; DPPH, α-diphenyl-β-picrylhydrazyl; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; GDNF, glial cell-derived neurotrophic factor; GR, glutathione reductase; GSH, glutathione; HD, Huntington’s disease; HPLC, High Performance Liquid Chromatography; HO-1, heme oxygenase-1; HSP70, heat-shock protein 70; HVA, homovanillic acid; IL-1β, interleukin-1β; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LB, Lewy bodies; LDH, lactate dehydrogenase; MAO, monoamine oxidase; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NGF, nerve growth factor; NMDA, N-methyl-D-aspartate; Nrf2, nuclear factor-erythroid factor 2-related factor 2; NTFs, neurotrophic factors; NF-κB, nuclear factor kappa B; PD, Parkinson’s disease; PI3K/Akt, phosphatidylinositol-3-kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species; SNpc, substantia nigra pars compacta; SOD, superoxide dismutase; TNF-α, tumour necrosis factor; TrkB, tropomyosin-related kinase B; UPR, unfolded protein response; UPS, ubiquitin-proteasome system; WHO, World Health Organization; 5-HT, 5-hydroxytryptamine; 6-OHDA, 6-hydroxydopamine.
Author Contributions
Design, organization, writing—original draft preparation, interpretation of the figures, R.B.; table preparation, D.-Y.C. and S.-H.S.; validation, I.-S.K.; supervision of the review and approval of the final draft, D.-K.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neurological Disorders: Public Health Challenges. [(accessed on 5 October 2021)]. Available online: https://www.who.int/publications/i/item/9789241563369.
- 2.MacDonald B.K., Cockerell O.C., Sander J.W.A.S., Shorvon S.D. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Pt 4Brain. 2000;123:665–676. doi: 10.1093/brain/123.4.665. [DOI] [PubMed] [Google Scholar]
- 3.Gorman A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008;12:2263–2280. doi: 10.1111/j.1582-4934.2008.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iuchi K., Takai T., Hisatomi H. Cell Death via Lipid Peroxidation and Protein Aggregation Diseases. Biology. 2021;10:399. doi: 10.3390/biology10050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jha S.K., Jha N.K., Kumar D., Ambasta R.K., Kumar P. Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neurodegeneration. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2017;1863:1132–1146. doi: 10.1016/j.bbadis.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Jurcau A. Insights into the Pathogenesis of Neurodegenerative Diseases: Focus on Mitochondrial Dysfunction and Oxidative Stress. Int. J. Mol. Sci. 2021;22:11847. doi: 10.3390/ijms222111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deture M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G.F., Xu T.H., Yan Y., Zhou Y.R., Jiang Y., Melcher K., Xu H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanabria-Castro A., Alvarado-Echeverría I., Monge-Bonilla C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Ann. Neurosci. 2017;24:46. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouli A., Torsney K.M., Kuan W.-L. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Codon Publications; Brisbane, Australia: 2018. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; pp. 3–26. [PubMed] [Google Scholar]
- 11.Maiti P., Manna J., Dunbar G.L., Maiti P., Dunbar G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017;6:28. doi: 10.1186/s40035-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel P.P., Hirsch E.C., Hunot S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron. 2016;90:675–691. doi: 10.1016/j.neuron.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Benito M., Granado N., García-Sanz P., Michel A., Dumoulin M., Moratalla R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front. Pharmacol. 2020;11:356. doi: 10.3389/fphar.2020.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musuka T.D., Wilton S.B., Traboulsi M., Hill M.D. Diagnosis and management of acute ischemic stroke: Speed is critical. Can. Med. Assoc. J. 2015;187:887–893. doi: 10.1503/cmaj.140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Numis A.L., Fox C.K. Arterial ischemic stroke in children: Risk factors and etiologies. Curr. Neurol. Neurosci. Rep. 2014;14:422. doi: 10.1007/s11910-013-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y., Li P., Guo Q., Shang K., Yan D., Du S., Lu Y. Pathophysiology and Biomarkers in Acute Ischemic Stroke—A Review. Trop. J. Pharm. Res. 2014;12:1097–1105. doi: 10.4314/tjpr.v12i6.35. [DOI] [Google Scholar]
- 17.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:E344–E418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 18.England M.J., Liverman C.T., Schultz A.M., Strawbridge L.M. Epilepsy across the spectrum: Promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25:266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhigwa A., Preux P.M., Gérard D., Marin B., Boumediène F., Ntamwira C., Tsai C.H. Comorbidities of epilepsy in low and middle-income countries: Systematic review and meta-analysis. Sci. Rep. 2020;10:9015. doi: 10.1038/s41598-020-65768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson-Smith J.N., Patel M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017;18:2635. doi: 10.3390/ijms18112365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain R., Zubair H., Pursell S., Shahab M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018;8:177. doi: 10.3390/brainsci8090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorbanpour M., Varma A. Medicinal Plants and Environmental Challenges. Springer; Cham, Switzerland: 2017. Medicinal plants and environmental challenges; pp. 1–413. [Google Scholar]
- 23.Balakrishnan R., Azam S., Cho D.Y., Su-Kim I., Choi D.K. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives. Oxid. Med. Cell. Longev. 2021;2021:6680935. doi: 10.1155/2021/6680935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim H.W., Kumar H., Kim B.W., More S.V., Kim I.W., Park J.I., Park S.Y., Kim S.K., Choi D.K. β-Asarone (cis-2,4,5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory mediators by inhibiting NF-κB signaling and the JNK pathway in LPS activated BV-2 microglia cells. Food Chem. Toxicol. 2014;72:265–272. doi: 10.1016/j.fct.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Kim B.W., Koppula S., Kumar H., Park J.Y., Kim I.W., More S.V., Kim I.S., Han S.D., Kim S.K., Yoon S.H., et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology. 2015;97:46–57. doi: 10.1016/j.neuropharm.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Lee H.J., Ahn S.M., Pak M.E., Jung D.H., Lee S.Y., Shin H.K., Choi B.T. Positive effects of α-asarone on transplanted neural progenitor cells in a murine model of ischemic stroke. Phytomedicine. 2018;51:151–161. doi: 10.1016/j.phymed.2018.09.230. [DOI] [PubMed] [Google Scholar]
- 27.Chellian R., Pandy V., Mohamed Z. Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicine. 2017;32:41–58. doi: 10.1016/j.phymed.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Zeng L., Zhang D., Liu Q., Zhang J., Mu K., Gao X., Zhang K., Li H., Wang Q., Zheng Y. Alpha-asarone Improves Cognitive Function of APP/PS1 Mice and Reducing Aβ 42, P-tau and Neuroinflammation, and Promoting Neuron Survival in the Hippocampus. Neuroscience. 2021;458:141–152. doi: 10.1016/j.neuroscience.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Huang L., Deng M., He Y., Lu S., Liu S., Fang Y. β-asarone increases MEF2D and TH levels and reduces α-synuclein level in 6-OHDA-induced rats via regulating the HSP70/MAPK/MEF2D/Beclin-1 pathway: Chaperone-mediated autophagy activation, macroautophagy inhibition and HSP70 up-expression. Behav. Brain Res. 2016;313:370–379. doi: 10.1016/j.bbr.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Han T., Han P., Peng W., Wang X.R. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm. Biol. 2013;51:589–594. doi: 10.3109/13880209.2012.751616. [DOI] [PubMed] [Google Scholar]
- 31.Pa H., Xu Y., Cai Q., Wu M., Din M. Effects of β-Asarone on Ischemic Stroke in Middle Cerebral Artery Occlusion Rats by an Nrf2-Antioxidant Response Elements (ARE) Pathway-Dependent Mechanism. Med. Sci. Monit. 2021;27:e931884. doi: 10.12659/MSM.931884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajput S.B., Tonge M.B., Karuppayil S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine. 2014;21:268–276. doi: 10.1016/j.phymed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Chamorro G., Salazar M., Salazar S., Mendoza T. Pharmacology and toxicology of Guatteria gaumeri and alpha-asarone. Rev. Investig. Clin. 1993;45:597–604. (In Spanish) [PubMed] [Google Scholar]
- 34.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acorus calamus: Scientific Validation of Ayurvedic Tradition from Natural Resources. Pharm. Biol. 2007;45:651–666. doi: 10.1080/13880200701538724. [DOI] [Google Scholar]
- 35.Sharma V., Singh I., Chaudhary P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014;28:1454–1466. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 36.Sharma V., Sharma R., Gautam D., Kuca K., Nepovimova E., Martins N. Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 2020;9:1176. doi: 10.3390/jcm9041176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthuraman A., Singh N. Acute and sub-acute oral toxicity profile of Acorus calamus (Sweet flag) in rodents. Asian Pac. J. Trop. Biomed. 2012;2:S1017–S1023. doi: 10.1016/S2221-1691(12)60354-2. [DOI] [Google Scholar]
- 38.Muthuraman A., Singh N. Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complement. Altern. Med. 2011;11:24. doi: 10.1186/1472-6882-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain N., Jain R., Jain A., Jain D.K., Chandel H.S. Evaluation of wound-healing activity of Acorus calamus Linn. Nat. Prod. Res. 2010;24:534–541. doi: 10.1080/14786410802531782. [DOI] [PubMed] [Google Scholar]
- 40.Kim H., Han T.H., Lee S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009;122:149–156. doi: 10.1016/j.jep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Shu H., Zhang S., Lei Q., Zhou J., Ji Y., Luo B., Hong L., Li F., Liu B., Long C. Ethnobotany of Acorus in China. Acta Soc. Bot. Pol. 2018;87:3585. doi: 10.5586/asbp.3585. [DOI] [Google Scholar]
- 42.Sarjan H.N., Divyashree S., Yajurvedi H.N. The protective effect of the Vacha rhizome extract on chronic stress-induced immunodeficiency in rat. Pharm. Biol. 2017;55:1358. doi: 10.1080/13880209.2017.1301495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam K.Y.C., Wu Q.Y., Hu W.H., Yao P., Wang H.Y., Dong T.T.X., Tsim K.W.K. Asarones from Acori tatarinowii Rhizoma stimulate expression and secretion of neurotrophic factors in cultured astrocytes. Neurosci. Lett. 2019;707:134308. doi: 10.1016/j.neulet.2019.134308. [DOI] [PubMed] [Google Scholar]
- 44.Das B.K., Choukimath S.M., Gadad P.C. Asarone and metformin delays experimentally induced hepatocellular carcinoma in diabetic milieu. Life Sci. 2019;230:10–18. doi: 10.1016/j.lfs.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 45.Das B.K., Swamy A.V., Koti B.C., Gadad P.C. Experimental evidence for use of Acorus calamus (asarone) for cancer chemoprevention. Heliyon. 2019;5:e01585. doi: 10.1016/j.heliyon.2019.e01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.Y., Lee J.Y., Yun B.S., Hwang B.K. Antifungal Activity of β-Asarone from Rhizomes of Acorus gramineus. J. Agric. Food Chem. 2004;52:776–780. doi: 10.1021/jf035204o. [DOI] [PubMed] [Google Scholar]
- 47.Wang N., Han Y., Luo L., Zhang Q., Ning B., Fang Y. β-asarone induces cell apoptosis, inhibits cell proliferation and decreases migration and invasion of glioma cells. Biomed. Pharmacother. 2018;106:655–664. doi: 10.1016/j.biopha.2018.06.169. [DOI] [PubMed] [Google Scholar]
- 48.Wu H.B., Fang Y.Q. Pharmacokinetics of β-asarone in rats. Acta Pharm. Sin. 2004;39:836–838. [PubMed] [Google Scholar]
- 49.Lu J., Fu T., Qian Y., Zhang Q., Zhu H., Pan L., Guo L., Zhang M. Distribution of α-asarone in brain following three different routes of administration in rats. Eur. J. Pharm. Sci. 2014;63:63–70. doi: 10.1016/j.ejps.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Ren C., Gong T., Sun X., Zhang Z., Zhang Y. Alpha-Asarone incorporated in mixed micelles suitable for intravenous administration: Formulation, in-vivo distribution and anaphylaxis study. Pharmazie. 2011;66:875–880. [PubMed] [Google Scholar]
- 51.Liu L., Fang Y.Q. Analysis of the distribution of β-asarone in rat hippocampus, brainstem, cortex and cerebellum with gas chromatography-mass spectrometry (GC-MS) J. Med. Plants Res. 2011;5:1728–1734. [Google Scholar]
- 52.Meng X., Zhao X., Wang S., Jia P., Bai Y., Liao S., Zheng X. Simultaneous Determination of Volatile Constituents from Acorus tatarinowii Schott in Rat Plasma by Gas Chromatography-Mass Spectrometry with Selective Ion Monitoring and Application in Pharmacokinetic Study. J. Anal. Methods Chem. 2013;2013:949830. doi: 10.1155/2013/949830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian Y.Y., Lu J., Zhang L.H., Shi F.Y., Fu T.M., Guo L.W. Pharmacokinetic study on dry powder inhalation administration of α-asarone in rats. China J. Chin. Mater. Med. 2015;40:739–743. [PubMed] [Google Scholar]
- 54.Ramalingam P., Ganesan P., Prabakaran D.S., Gupta P.K., Jonnalagadda S., Govindarajan K., Vishnu R., Sivalingam K., Sodha S., Choi D.K., et al. Lipid Nanoparticles Improve the Uptake of α-Asarone Into the Brain Parenchyma: Formulation, Characterization, In Vivo Pharmacokinetics, and Brain Delivery. AAPS PharmSciTech. 2020;21:299. doi: 10.1208/s12249-020-01832-8. [DOI] [PubMed] [Google Scholar]
- 55.Pan L., Zhou J., Ju F., Zhu H. Intranasal delivery of α-asarone to the brain with lactoferrin-modified mPEG-PLA nanoparticles prepared by premix membrane emulsification. Drug Deliv. Transl. Res. 2018;8:83–96. doi: 10.1007/s13346-017-0438-8. [DOI] [PubMed] [Google Scholar]
- 56.Lu J., Guo L.W., Fu T.M., Zhu G.L., Dai Z.N., Zhan G.J., Chen L.L. Pharmacokinetics of α-asarone after intranasal and intravenous administration with PLA-α-asarone nanoparticles. China J. Chin. Mater. Med. 2017;42:2366–2372. doi: 10.19540/j.cnki.cjcmm.20170307.009. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 57.Wu J.Y., Li Y.J., Yang L., Hu Y.Y., Hu X.B., Tang T.T., Wang J.M., Liu X.Y., Xiang D.X. Borneol and a-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018;25:1858–1864. doi: 10.1080/10717544.2018.1516005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta M., Kant K., Sharma R., Kumar A. Evaluation of In Silico Anti-parkinson Potential of β-asarone. Cent. Nerv. Syst. Agents Med. Chem. 2018;18:128–135. doi: 10.2174/1871524918666180416153742. [DOI] [PubMed] [Google Scholar]
- 59.Fang Y.Q., Shi C., Liu L., Fang R.M. Pharmacokinetics of β-asarone in rabbit blood, hippocampus, cortex, brain stem, thalamus and cerebellum. Pharmazie. 2012;67:120–123. [PubMed] [Google Scholar]
- 60.Chen Q.X., Miao J.K., Li C., Li X.W., Wu X.M., Zhang X.P. Anticonvulsant activity of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013;36:23–30. doi: 10.1248/bpb.b12-00376. [DOI] [PubMed] [Google Scholar]
- 61.Morales-Ramírez P., Madrigal-Bujaidar E., Mercader-Martínez J., Cassani M., González G., Chamorro-Cevallos G., Salazar-Jacobo M. Sister-chromatid exchange induction produced by in vivo and in vitro exposure to alpha-asarone. Mutat. Res. 1992;279:269–273. doi: 10.1016/0165-1218(92)90243-S. [DOI] [PubMed] [Google Scholar]
- 62.European Commision . Opinion of the Scientific Committee on Food on the Presence of β-Asarone in Flavourings and Other Food Ingredients with Flavouring Properties. European Commision; Brussels, Belgium: 2002. pp. 1–15. [Google Scholar]
- 63.Liu L., Wang J., Shi L., Zhang W., Du X., Wang Z., Zhang Y. β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine. 2013;20:512–520. doi: 10.1016/j.phymed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Uebel T., Hermes L., Haupenthal S., Müller L., Esselen M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021;41:1166–1179. doi: 10.1002/jat.4112. [DOI] [PubMed] [Google Scholar]
- 65.Manzanero S., Santro T., Arumugam T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Murray A.J., Rogers J.C., Katshu M.Z.U.H., Liddle P.F., Upthegrove R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry. 2021;12:703452. doi: 10.3389/fpsyt.2021.703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asha Devi S., Mali A.L., Rahee M.A., Belinda E.D.S. Antioxidant properties of Alpha asarone. Asian J. Biochem. 2014;9:107–113. doi: 10.3923/ajb.2014.107.113. [DOI] [Google Scholar]
- 68.Parki A., Chaubey P., Prakash O., Kumar R., Pant A.K. Seasonal Variation in Essential Oil Compositions and Antioxidant Properties of Acorus calamus L. Accessions. Medicines. 2017;4:81. doi: 10.3390/medicines4040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loying R., Gogoi R., Sarma N., Borah A., Munda S., Pandey S.K., Lal M. Chemical Compositions, In-vitro Antioxidant, Anti-microbial, Anti-inflammatory and Cytotoxic Activities of Essential Oil of Acorus calamus L. Rhizome from North-East India. J. Essent. Oil Bear. Plants. 2019;22:1299–1312. doi: 10.1080/0972060X.2019.1696236. [DOI] [Google Scholar]
- 70.Pages N., Maurois P., Delplanque B., Bac P., Stables J.P., Tamariz J., Chamorro G., Vamecq J. Activities of α-asarone in various animal seizure models and in biochemical assays might be essentially accounted for by antioxidant properties. Neurosci. Res. 2010;68:337–344. doi: 10.1016/j.neures.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Hei X., Xie M., Xu J., Li J., Liu T. β-Asarone Exerts Antioxidative Effects on H2O2-Stimulated PC12 Cells by Activating Nrf2/HO-1 Pathway. Neurochem. Res. 2020;45:1953–1961. doi: 10.1007/s11064-020-03060-9. [DOI] [PubMed] [Google Scholar]
- 72.Meng M., Zhang L., AI D., Wu H., Peng W. β-Asarone Ameliorates β-Amyloid–Induced Neurotoxicity in PC12 Cells by Activating P13K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2021;12:1079. doi: 10.3389/fphar.2021.659955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lam K.Y.C., Yao P., Wang H., Duan R., Dong T.T.X., Tsim K.W.K. Asarone from Acori tatarinowii Rhizome prevents oxidative stress-induced cell injury in cultured astrocytes: A signaling triggered by Akt activation. PLoS ONE. 2017;12:e0179077. doi: 10.1371/journal.pone.0179077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lam K.Y.C., Chen J., Lam C.T.W., Wu Q., Yao P., Dong T.T.X., Lin H., Tsim K.W.K. Asarone from Acori tatarinowii Rhizoma Potentiates the Nerve Growth Factor-Induced Neuronal Differentiation in Cultured PC12 Cells: A Signaling Mediated by Protein Kinase A. PLoS ONE. 2016;11:e0163337. doi: 10.1371/journal.pone.0163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sundaramahalingam M., Ramasundaram S., Rathinasamy S.D., Natarajan R.P., Somasundaram T. Role of Acorus calamus and alpha-asarone on hippocampal dependent memory in noise stress exposed rats. Pak. J. Biol. Sci. 2013;16:770–778. doi: 10.3923/pjbs.2013.770.778. [DOI] [PubMed] [Google Scholar]
- 76.Kumar H., Kim B.W., Song S.Y., Kim J.S., Kim I.S., Kwon Y.S., Koppula S., Choi D.K. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012;76:1518–1522. doi: 10.1271/bbb.120247. [DOI] [PubMed] [Google Scholar]
- 77.Chellian R., Pandy V., Mohamed Z. Alpha-asarone attenuates depression-like behavior in nicotine-withdrawn mice: Evidence for the modulation of hippocampal pCREB levels during nicotine-withdrawal. Eur. J. Pharmacol. 2018;818:10–16. doi: 10.1016/j.ejphar.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 78.Saldanha A.A., Vieira L., de Oliveira F.M., Lopes D.d.O., Ribeiro R.I.M.d.A., Thomé R.G., dos Santos H.B., Silva D.B., Carollo C.A., de Siqueira J.M., et al. Anti-inflammatory and central and peripheral anti-nociceptive activities of α-asarone through the inhibition of TNF-α production, leukocyte recruitment and iNOS expression, and participation of the adenosinergic and opioidergic systems. Inflammopharmacology. 2020;28:1039–1052. doi: 10.1007/s10787-019-00679-1. [DOI] [PubMed] [Google Scholar]
- 79.Li Q., Xu F., Zhang Q., Li X., Guo M., Zhang Y., Wang Z., Wang J., Zhao J., Tian Y., et al. Effect of α-asarone on ethanol-induced learning and memory impairment in mice and its underlying mechanism. Life Sci. 2019;238:116898. doi: 10.1016/j.lfs.2019.116898. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y., Gao X., Liu Q., Zeng L., Zhang K., Mu K., Zhang D., Zou H., Wu N., Ou J., et al. Alpha-asarone improves cognitive function of aged rats by alleviating neuronal excitotoxicity via GABA A receptors. Neuropharmacology. 2020;162:107843. doi: 10.1016/j.neuropharm.2019.107843. [DOI] [PubMed] [Google Scholar]
- 81.Zou D.J., Wang G., Liu J.C., Dong M.X., Li X.M., Zhang C., Zhou L., Wang R., Niu Y.C. Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Pharmazie. 2011;66:44–51. [PubMed] [Google Scholar]
- 82.Chang W., Teng J. β-asarone prevents Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y cells: Down expression Beclin-1, LC3B and up expression Bcl-2. Int. J. Clin. Exp. Med. 2015;8:20658. [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y.X., Chen Y.T., Zhou X.J., Hong C.L., Li C.Y., Guo J.Y. Beta-asarone, a major component of Acorus tatarinowii Schott, attenuates focal cerebral ischemia induced by middle cerebral artery occlusion in rats. BMC Complement. Altern. Med. 2013;13:236. doi: 10.1186/1472-6882-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang K., Liu Q., Luo L., Feng X., Hu Q., Fan X., Mao S. Neuroprotective Effect of Alpha-asarone on the Rats Model of Cerebral Ischemia-Reperfusion Stroke via Ameliorating Glial Activation and Autophagy. Neuroscience. 2021;473:130–141. doi: 10.1016/j.neuroscience.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 85.Deng M., Huang L., Zhong X. β-asarone modulates Beclin-1, LC3 and p62 expression to attenuate Aβ40 and Aβ42 levels in APP/PS1 transgenic mice with Alzheimer’s disease. Mol. Med. Rep. 2020;21:2095–2102. doi: 10.3892/mmr.2020.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao X., Xu X., Li F., Xie G., Zhang T. Anti-inflammatory treatment with β-asarone improves impairments in social interaction and cognition in MK-801 treated mice. Brain Res. Bull. 2019;150:150–159. doi: 10.1016/j.brainresbull.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 87.Dong H., Gao Z., Rong H., Jin M., Zhang X. β-asarone reverses chronic unpredictable mild stress-induced depression-like behavior and promotes hippocampal neurogenesis in rats. Molecules. 2014;19:5634–5649. doi: 10.3390/molecules19055634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li C., Xing G., Dong M., Zhou L., Li J., Wang G., Zou D., Wang R., Liu J., Niu Y. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 2010;635:96–102. doi: 10.1016/j.ejphar.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 89.Zhang S., Gui X.H., Huang L.P., Deng M.Z., Fang R.M., Ke X.H., He Y.P., Li L., Fang Y.Q. Neuroprotective Effects of β-Asarone Against 6-Hydroxy Dopamine-Induced Parkinsonism via JNK/Bcl-2/Beclin-1 Pathway. Mol. Neurobiol. 2016;53:83–94. doi: 10.1007/s12035-014-8950-z. [DOI] [PubMed] [Google Scholar]
- 90.Wei G., Chen Y.B., Chen D.F., Lai X.P., Liu D.H., Deng R.D., Zhou J.H., Zhang S.X., Li Y.W., Lii H., et al. β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. J. Alzheimer’s Dis. 2013;33:863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- 91.Saki G., Eidi A., Mortazavi P., Panahi N., Vahdati A. Effect of β-asarone in normal and β-amyloid-induced Alzheimeric rats. Arch. Med. Sci. 2020;16:699–706. doi: 10.5114/aoms.2020.94659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manikandan S., Devi R.S. Antioxidant property of alpha-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol. Res. 2005;52:467–474. doi: 10.1016/j.phrs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Sandeep D., Nair C.K.K. Radioprotection by α-asarone: Prevention of genotoxicity and hematopoietic injury in mammalian organism. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;722:62–68. doi: 10.1016/j.mrgentox.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 94.Wang B.L., Xuan L., Dai S.J., Ji L.T., Li C.Y., Yang Y.X. Protective effect of β-asarone on AD rat model induced by intracerebroventricular injection of Aβ₁₋₄₂ combined 2-VO and its mechanism. China J. Chin. Mater. Med. 2017;42:4847–4854. doi: 10.19540/j.cnki.cjcmm.20171218.004. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 95.Sánchez-Alegría K., Flores-León M., Avila-Muñoz E., Rodríguez-Corona N., Arias C. PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. Int. J. Mol. Sci. 2018;19:3725. doi: 10.3390/ijms19123725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long H.Z., Cheng Y., Zhou Z.W., Luo H.Y., Wen D.D., Gao L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021;12:648636. doi: 10.3389/fphar.2021.648636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun J., Nan G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target (Review) Int. J. Mol. Med. 2017;39:1338–1346. doi: 10.3892/ijmm.2017.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naoi M., Shamoto-Nagai M., Maruyama W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019;14:FNL9. doi: 10.2217/fnl-2018-0028. [DOI] [Google Scholar]
- 99.Xue Z., Guo Y., Zhang S., Huang L., He Y., Fang R., Fang Y. Beta-asarone attenuates amyloid beta-induced autophagy via Akt/mTOR pathway in PC12 cells. Eur. J. Pharmacol. 2014;741:195–204. doi: 10.1016/j.ejphar.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 100.He Y., He J.N., Fu J., Bao Y.T., Li C.Y., Yang Y.X. Protective effect of β-asarone on PC12 cells injury induced by Aβ₁₋₄₂ astrocytic activation. China J. Chin. Mater. Med. 2016;41:1282–1288. doi: 10.4268/cjcmm20160720. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 101.Mao J., Huang S., Liu S., Feng X.L., Yu M., Liu J., Sun Y.E., Chen G., Yu Y., Zhao J., et al. A herbal medicine for Alzheimer’s disease and its active constituents promote neural progenitor proliferation. Aging Cell. 2015;14:784–796. doi: 10.1111/acel.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soto C., Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018;21:1332–1340. doi: 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vasili E., Dominguez-Meijide A., Outeiro T.F. Spreading of α-synuclein and tau: A systematic comparison of the mechanisms involved. Front. Mol. Neurosci. 2019;12:107. doi: 10.3389/fnmol.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar D., Kumar P. Aβ, Tau, and α-Synuclein aggregation and integrated role of PARK2 in the regulation and clearance of toxic peptides. Neuropeptides. 2019;78:101971. doi: 10.1016/j.npep.2019.101971. [DOI] [PubMed] [Google Scholar]
- 105.Ciechanover A., Kwon Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi M.L., Gandhi S. Crucial role of protein oligomerization in the pathogenesis of Alzheimer’s and Parkinson’s diseases. FEBS J. 2018;285:3631–3644. doi: 10.1111/febs.14587. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura T., Lipton S.A. Cell death: Protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- 108.Sweeney P., Park H., Baumann M., Dunlop J., Frydman J., Kopito R., McCampbell A., Leblanc G., Venkateswaran A., Nurmi A. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017;6:6. doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pietrobono D., Giacomelli C., Trincavelli M.L., Daniele S., Martini C. Inhibitors of protein aggregates as novel drugs in neurodegenerative diseases. Glob. Drugs Ther. 2017;2:1–5. [Google Scholar]
- 110.Bieschke J. Natural Compounds May Open New Routes to Treatment of Amyloid Diseases. Neurotherapeutics. 2013;10:429–439. doi: 10.1007/s13311-013-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Javed H., Nagoor Meeran M.F., Azimullah S., Adem A., Sadek B., Ojha S.K. Plant Extracts and Phytochemicals Targeting α-Synuclein Aggregation in Parkinson’s Disease Models. Front. Pharmacol. 2019;9:1555. doi: 10.3389/fphar.2018.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kabir M.T., Uddin M.S., Begum M.M., Thangapandiyan S., Rahman M.S., Aleya L., Mathew B., Ahmed M., Barreto G.E., Ashraf G.M. Cholinesterase Inhibitors for Alzheimer’s Disease: Multitargeting Strategy Based on Anti-Alzheimer’s Drugs Repositioning. Curr. Pharm. Des. 2019;25:3519–3535. doi: 10.2174/1381612825666191008103141. [DOI] [PubMed] [Google Scholar]
- 113.Shin J.W., Cheong Y.J., Koo Y.M., Kim S., Noh C.K., Son Y.H., Kang C., Sohn N.W. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomol. Ther. 2014;22:17–26. doi: 10.4062/biomolther.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang N., Wang H., Li L., Li Y., Zhang R. β-Asarone Inhibits Amyloid-β by Promoting Autophagy in a Cell Model of Alzheimer’s Disease. Front. Pharmacol. 2020;10:1529. doi: 10.3389/fphar.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J.E., Kim N., Yeo J.Y., Seo D.G., Kim S., Lee J.S., Hwang K.W., Park S.Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules. 2019;24:4297. doi: 10.3390/molecules24234297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Risiglione P., Zinghirino F., Di Rosa M.C., Magrì A., Messina A. Alpha-Synuclein and Mitochondrial Dysfunction in Parkinson’s Disease: The Emerging Role of VDAC. Biomolecules. 2021;11:718. doi: 10.3390/biom11050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moloney T.C., Hyland R., O’Toole D., Paucard A., Kirik D., O’Doherty A., Gorman A.M., Dowd E. Heat shock protein 70 reduces α-synuclein-induced predegenerative neuronal dystrophy in the α-synuclein viral gene transfer rat model of Parkinson’s disease. CNS Neurosci. Ther. 2014;20:50–58. doi: 10.1111/cns.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong Z., Wolfer D.P., Lipp H.P., Büeler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol. Ther. 2005;1:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Q.S., Wang Z.H., Zhang J.L., Duan Y.L., Li G.F., Zheng D.L. Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting α-synuclein protein expression. Biomed. Pharmacother. 2016;83:153–159. doi: 10.1016/j.biopha.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 120.Lehman N.L. The ubiquitin proteasome system in neuropathology. Acta Neuropathol. 2009;118:329–347. doi: 10.1007/s00401-009-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cook C., Stetler C., Petrucelli L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:a009423. doi: 10.1101/cshperspect.a009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sprenkle N.T., Sims S.G., Sánchez C.L., Meares G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017;12:42. doi: 10.1186/s13024-017-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rao R.V., Bredesen D.E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Høyer-Hansen M., Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 126.Yang H., Niemeijer M., van de Water B., Beltman J.B. ATF6 Is a Critical Determinant of CHOP Dynamics during the Unfolded Protein Response. iScience. 2020;23:100860. doi: 10.1016/j.isci.2020.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ryu E.J., Harding H.P., Angelastro J.M., Vitolo O.V., Ron D., Greene L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang P., Gan M., Ebrahim A.S., Lin W.L., Melrose H.L., Yen S.H.C. ER stress response plays an important role in aggregation of α-synuclein. Mol. Neurodegener. 2010;5:56. doi: 10.1186/1750-1326-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holtz W.A., Turetzky J.M., Jong Y.J.I., O’Malley K.L. Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death evoked by parkinsonian mimetics. J. Neurochem. 2006;99:54–69. doi: 10.1111/j.1471-4159.2006.04025.x. [DOI] [PubMed] [Google Scholar]
- 130.Ning B., Deng M., Zhang Q., Wang N., Fang Y. β-Asarone Inhibits IRE1/XBP1 Endoplasmic Reticulum Stress Pathway in 6-OHDA-Induced Parkinsonian Rats. Neurochem. Res. 2016;41:2097–2101. doi: 10.1007/s11064-016-1922-0. [DOI] [PubMed] [Google Scholar]
- 131.Hotamisligil G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ning B., Zhang Q., Wang N., Deng M., Fang Y. β-Asarone Regulates ER Stress and Autophagy Via Inhibition of the PERK/CHOP/Bcl-2/Beclin-1 Pathway in 6-OHDA-Induced Parkinsonian Rats. Neurochem. Res. 2019;44:1159–1166. doi: 10.1007/s11064-019-02757-w. [DOI] [PubMed] [Google Scholar]
- 133.Park S.H., Kang M.K., Choi Y.J., Kim Y.H., Antika L.D., Lim S.S., Kang Y.H. Dietary compound α-asarone alleviates ER stress-mediated apoptosis in 7β-hydroxycholesterol-challenged macrophages. Mol. Nutr. Food Res. 2016;60:1033–1047. doi: 10.1002/mnfr.201500750. [DOI] [PubMed] [Google Scholar]
- 134.Gui Y., Li A., Zhang J., Li G., Ruan X., Guo Q., Zou W. α-Asarone alleviated chronic constriction injury–induced neuropathic pain through inhibition of spinal endoplasmic reticulum stress in an liver X receptor–dependent manner. Anesth. Analg. 2018;127:775–783. doi: 10.1213/ANE.0000000000002792. [DOI] [PubMed] [Google Scholar]
- 135.Mikami M., Takuya O., Yoshino Y., Nakamura S., Ito K., Kojima H., Takahashi T., Iddamalgoda A., Inoue S., Shimazawa M. Acorus calamus extract and its component α-asarone attenuate murine hippocampal neuronal cell death induced by l-glutamate and tunicamycin. Biosci. Biotechnol. Biochem. 2021;85:493–501. doi: 10.1093/bbb/zbaa071. [DOI] [PubMed] [Google Scholar]
- 136.Park H., Kang J.H., Lee S. Autophagy in Neurodegenerative Diseases: A Hunter for Aggregates. Int. J. Mol. Sci. 2020;21:3369. doi: 10.3390/ijms21093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rahman M.A., Rahman M.R., Zaman T., Uddin M.S., Islam R., Abdel-Daim M.M., Rhim H. Emerging Potential of Naturally Occurring Autophagy Modulators Against Neurodegeneration. Curr. Pharm. Des. 2020;26:772–779. doi: 10.2174/1381612826666200107142541. [DOI] [PubMed] [Google Scholar]
- 138.Ahsan A., Liu M., Zheng Y., Yan W., Pan L., Li Y., Ma S., Zhang X., Cao M., Wu Z., et al. Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm. Sin. B. 2021;11:1708–1720. doi: 10.1016/j.apsb.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park S.H., Kang M.K., Choi Y.J., Kim Y.H., Antika L.D., Kim D.Y., Lee E.J., Lim S.S., Kang Y.H. α-Asarone blocks 7β-hydroxycholesterol-exposed macrophage injury through blocking elF2α phosphorylation and prompting beclin-1-dependent autophagy. Oncotarget. 2017;8:7370–7383. doi: 10.18632/oncotarget.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harry G.J., Kraft A.D. Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology. 2012;33:191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jeong H.-K., Ji K., Min K., Joe E.-H. Brain inflammation and microglia: Facts and misconceptions. Exp. Neurobiol. 2013;22:59–67. doi: 10.5607/en.2013.22.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lyman M., Lloyd D.G., Ji X., Vizcaychipi M.P., Ma D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 143.Liu C.Y., Wang X., Liu C., Zhang H.L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell. Neurosci. 2019;13:514. doi: 10.3389/fncel.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bachiller S., Jiménez-Ferrer I., Paulus A., Yang Y., Swanberg M., Deierborg T., Boza-Serrano A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fatoba O., Itokazu T., Yamashita T. Microglia as therapeutic target in central nervous system disorders. J. Pharmacol. Sci. 2020;144:102–118. doi: 10.1016/j.jphs.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Moussa C., Hebron M., Huang X., Ahn J., Rissman R.A., Aisen P.S., Turner R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017;14:61. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaur N., Chugh H., Sakharkar M.K., Dhawan U., Chidambaram S.B., Chandra R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020;11:3707–3731. doi: 10.1021/acschemneuro.0c00427. [DOI] [PubMed] [Google Scholar]
- 148.Liu H.J., Lai X., Xu Y., Miao J.K., Li C., Liu J.Y., Hua Y.Y., Ma Q., Chen Q. α-Asarone Attenuates Cognitive Deficit in a Pilocarpine-Induced Status Epilepticus Rat Model via a Decrease in the Nuclear Factor-κB Activation and Reduction in Microglia Neuroinflammation. Front. Neurol. 2017;8:661. doi: 10.3389/fneur.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jo M.J., Kumar H., Joshi H.P., Choi H., Ko W.K., Kim J.M., Hwang S.S.S., Park S.Y., Sohn S., Bello A.B., et al. Oral Administration of α-Asarone Promotes Functional Recovery in Rats With Spinal Cord Injury. Front. Pharmacol. 2018;9:445. doi: 10.3389/fphar.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cai Q., Li Y., Mao J., Pei G. Neurogenesis-promoting natural product α-asarone modulates morphological dynamics of activated microglia. Front. Cell. Neurosci. 2016;10:280. doi: 10.3389/fncel.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006;361:1545. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang H., Ozbay F., Lappalainen J., Kranzler H.R., Van Dyck C.H., Charney D.S., Price L.H., Southwick S., Yang B.Z., Rasmussen A., et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006;141:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumar Gupta V., Sharma B. Role of Phytochemicals in Neurotrophins Mediated Regulation of Alzheimer’s Disease. Int. J. Complement. Altern. Med. 2017;7:00231. [Google Scholar]
- 154.Maswood N., Grondin R., Zhang Z., Stanford J.A., Surgener S.P., Gash D.M., Gerhardt G.A. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol. Aging. 2002;23:881–889. doi: 10.1016/S0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 155.Grondin R., Zhang Z., Yi A., Cass W.A., Maswood N., Andersen A.H., Elsberry D.D., Klein M.C., Gerhardt G.A., Gash D.M. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 156.Grondin R., Cass W.A., Zhang Z., Stanford J.A., Gash D.M., Gerhardt G.A. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stahl K., Mylonakou M.N., Stahl K., Amiry-Moghaddam M., Torp R. Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res. 2011;1378:105–118. doi: 10.1016/j.brainres.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 158.Takeda M. Intrathecal infusion of brain-derived neurotrophic factor protects nigral dopaminergic neurons from degenerative changes in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced monkey parkinsonian model. Hokkaido J. Med. Sci. 1995;70:829–838. [PubMed] [Google Scholar]
- 159.Cui X., Lin Q., Liang Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020;12:209. doi: 10.3389/fnagi.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dong H., Cong W., Guo X., Wang Y., Tong S., Li Q., Li C. β-asarone relieves chronic unpredictable mild stress induced depression by regulating the extracellular signal-regulated kinase signaling pathway. Exp. Ther. Med. 2019;18:3767–3774. doi: 10.3892/etm.2019.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee B., Sur B., Cho S.G., Yeom M., Shim I., Lee H., Hahm D.H. Effect of beta-asarone on impairment of spatial working memory and apoptosis in the hippocampus of rats exposed to chronic corticosterone administration. Biomol. Ther. 2015;23:571–581. doi: 10.4062/biomolther.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Naoi M., Maruyama W., Shamoto-Nagai M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural Transm. 2018;125:53–66. doi: 10.1007/s00702-017-1709-8. [DOI] [PubMed] [Google Scholar]
- 163.Finberg J.P.M., Rabey J.M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016;7:340. doi: 10.3389/fphar.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang L., Deng M., Fang Y., Li L. Dynamic changes of five neurotransmitters and their related enzymes in various rat tissues following β-asarone and levodopa co-administration. Exp. Ther. Med. 2015;10:1566–1572. doi: 10.3892/etm.2015.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang L., Deng M., Zhang S., Fang Y., Li L. Coadministration of β-asarone and levodopa increases dopamine in rat brain by accelerating transformation of levodopa: A different mechanism from Madopar. Clin. Exp. Pharmacol. Physiol. 2014;41:685–690. doi: 10.1111/1440-1681.12270. [DOI] [PubMed] [Google Scholar]
- 166.Huang L., Deng M., Zhang S., Lu S., Gui X., Fang Y. β-asarone and levodopa coadministration increases striatal levels of dopamine and levodopa and improves behavioral competence in Parkinson’s rat by enhancing dopa decarboxylase activity. Biomed. Pharmacother. 2017;94:666–678. doi: 10.1016/j.biopha.2017.07.125. [DOI] [PubMed] [Google Scholar]
- 167.Yang Q.Q., Xue W.Z., Zou R.X., Xu Y., Du Y., Wang S., Xu L., Chen Y.Z., Wang H.L., Chen X.T. β-Asarone Rescues Pb-Induced Impairments of Spatial Memory and Synaptogenesis in Rats. PLoS ONE. 2016;11:e0167401. doi: 10.1371/journal.pone.0167401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiménez-Balado J., Eich T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021;116:146–159. doi: 10.1016/j.semcdb.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jakaria M., Park S.Y., Haque M., Karthivashan G., Kim I.S., Ganesan P., Choi D.K. Neurotoxic agent-induced injury in neurodegenerative disease model: Focus on involvement of glutamate receptors. Front. Mol. Neurosci. 2018;11:307. doi: 10.3389/fnmol.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martin L.J. Mitochondrial and Cell Death Mechanisms in Neurodegenerative Diseases. Pharmaceuticals. 2010;3:839–915. doi: 10.3390/ph3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu Y., Chen M., Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. doi: 10.1016/j.mito.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 172.Cho J., Ho Kim Y., Kong J.Y., Ha Yang C., Gook Park C. Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci. 2002;71:591–599. doi: 10.1016/S0024-3205(02)01729-0. [DOI] [PubMed] [Google Scholar]
- 173.Irie Y., Keung W.M. Rhizoma Acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-beta peptide. Brain Res. 2003;963:282–289. doi: 10.1016/S0006-8993(02)04050-7. [DOI] [PubMed] [Google Scholar]
- 174.Tatton W.G., Olanow C.W. Apoptosis in neurodegenerative diseases: The role of mitochondria. Biochim. Biophys. Acta BBA—Bioenerg. 1999;1410:195–213. doi: 10.1016/S0005-2728(98)00167-4. [DOI] [PubMed] [Google Scholar]
- 175.KA J. Cell death mechanisms in neurodegeneration. J. Cell. Mol. Med. 2001;5:1–17. doi: 10.1111/j.1582-4934.2001.tb00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan J., Dawson T.M., Dawson V.L. Cell Death Mechanisms of Neurodegeneration. Adv. Neurobiol. 2017;15:403–425. doi: 10.1007/978-3-319-57193-5_16. [DOI] [PubMed] [Google Scholar]
- 177.Geng Y., Li C., Liu J., Xing G., Zhou L., Dong M., Li X., Niu Y. Beta-asarone improves cognitive function by suppressing neuronal apoptosis in the beta-amyloid hippocampus injection rats. Biol. Pharm. Bull. 2010;33:836–843. doi: 10.1248/bpb.33.836. [DOI] [PubMed] [Google Scholar]
- 178.Yin Y., She H., Li W., Yang Q., Guo S., Mao Z. Modulation of Neuronal Survival Factor MEF2 by Kinases in Parkinson’s Disease. Front. Physiol. 2012;3:171. doi: 10.3389/fphys.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.She H., Yang Q., Shepherd K., Smith Y., Miller G., Testa C., Mao Z. Direct regulation of complex I by mitochondrial MEF2D is disrupted in a mouse model of Parkinson disease and in human patients. J. Clin. Investig. 2011;121:930–940. doi: 10.1172/JCI43871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smith P.D., Mount M.P., Shree R., Callaghan S., Slack R.S., Anisman H., Vincent I., Wang X., Mao Z., Park D.S. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yi Z., Li Z., Yu S., Yuan C., Hong W., Wang Z., Cui J., Shi T., Fang Y. Blood-Based Gene Expression Profiles Models for Classification of Subsyndromal Symptomatic Depression and Major Depressive Disorder. PLoS ONE. 2012;7:e31283. doi: 10.1371/journal.pone.0031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Qi X., Lin W., Li J., Pan Y., Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav. Brain Res. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 183.Vogt A., Tamewitz A., Skoko J., Sikorski R.P., Giuliano K.A., Lazo J.S. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
- 184.Duric V., Banasr M., Licznerski P., Schmidt H.D., Stockmeier C.A., Simen A.A., Newton S.S., Duman R.S. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun Y.R., Wang W., Li S.S., Dong H.Y., Zhang X.J. β-asarone from Acorus gramineus alleviates depression by modulating MKP-1. Genet. Mol. Res. 2015;14:4495–4504. doi: 10.4238/2015.May.4.7. [DOI] [PubMed] [Google Scholar]
- 186.Chellian R., Pandy V., Mohamed Z. Biphasic Effects of α-Asarone on Immobility in the Tail Suspension Test: Evidence for the Involvement of the Noradrenergic and Serotonergic Systems in Its Antidepressant-Like Activity. Front. Pharmacol. 2016;7:72. doi: 10.3389/fphar.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu S., Chen S.W., Xu N., Liu X.H., Zhang H., Wang Y.Z., Xu X.D. Anxiolytic-like effect of α-asarone in mice. Phytother. Res. 2012;26:1476–1481. doi: 10.1002/ptr.4596. [DOI] [PubMed] [Google Scholar]
- 188.Lee B., Sur B., Yeom M., Shim I., Lee H., Hahm D.H. Alpha-Asarone, a Major Component of Acorus gramineus, Attenuates Corticosterone-Induced Anxiety-Like Behaviours via Modulating TrkB Signaling Process. Korean J. Physiol. Pharmacol. 2014;18:191–200. doi: 10.4196/kjpp.2014.18.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He X., Bai Y., Zeng M., Zhao Z., Zhang Q., Xu N., Qin F., Wei X., Zhao M., Wu N., et al. Anticonvulsant activities of α-asaronol ((E)-3′-hydroxyasarone), an active constituent derived from α-asarone. Pharmacol. Rep. 2018;70:69–74. doi: 10.1016/j.pharep.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 190.Ma W.C., Zhang Q., Li H., Chu T., Shi H.Y., Mao S.J. Literature review and cause analysis of adverse reactions caused by asarone injection. Chin. J. Pharmacovigil. 2010;7:243–246. [Google Scholar]
- 191.Yang H.Y., Deng Y.P. Literature analysis of 122 adverse drug reactions induced by asarone injection. Chin. J. Ethnomed. Ethnopharm. 2012;6:36–39. [Google Scholar]
- 192.Guan Y.M., Liu J., Zhang J.L., Chen L.H., Zhu W.F., Zang Z.Z., Jin C., Wu L. Preparation and evaluation of four kinds of mixed essential oil liposomes in Jieyu Anshen Formula. China J. Chin. Mater. Med. 2019;44:1363–1370. doi: 10.19540/j.cnki.cjcmm.20190115.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 193.Lungare S., Hallam K., Badhan R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016;513:280–293. doi: 10.1016/j.ijpharm.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 194.FAO . Joint FAO/WHO Expert Committee on Food Additives (JECFA) FAO; Rome, Italy: 1987. [Google Scholar]