The definition of pregnancy-associated breast cancer (PABC) is inconsistently given as either breast cancer diagnosed exclusively during pregnancy, or combined with cases diagnosed within 6 months to 1 year after the birth.1 Although pregnancy and the postpartum period are intertwined, evolving evidence supports considering breast cancer that occurs during pregnancy (PrBC) as a separate and distinct entity from breast cancer that occurs during the postpartum period (PPBC)—which, according to newer data, can extend to 5–10 years after the birth—because each type has unique biological attributes and prognosis.
PrBC is diagnosed during pregnancy and represents about 4% of cases of breast cancer in women younger than 45 years.2 With an estimated incidence of 5·1 cases per 100 000 livebirths in young women, breast cancer is one of the most common cancer types occurring during pregnancy. The existing literature gives a mixed view of whether a breast cancer diagnosis during pregnancy confers a worse prognosis than breast cancer not diagnosed during pregnancy. Although genetic differences and reduced overall survival in PrBC have been described,1 large cohort studies of PrBC find that clinical tumour characteristics and prognosis are indistinguishable from breast cancers occurring in young, non-pregnant women.3,4
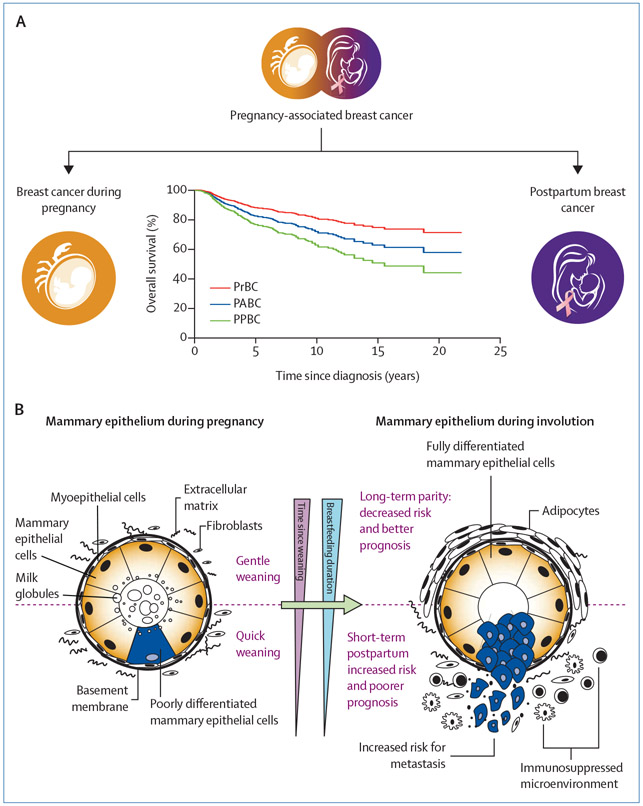
PPBC occurs within 5–10 years after pregnancy and is estimated to represent 35–55% of all cases of breast cancer in women younger than 45 years.5 PPBC is associated with worse survival rates and with a more than two times increased risk of metastasis than breast cancer diagnosed in young, premenopausal women during pregnancy or who have ever been pregnant (figure, A).1,4 Poor prognosis persists after adjustment for several clinicopatho-logical factors, including age at diagnosis, year of diagnosis, stage, grade, and hormone receptor status.6 Importantly, when the same data were grouped in the traditional PABC definition, as opposed to the biologically distinct proposed PrBC and PPBC definitions, this difference in outcomes was lost.2 Logically, if women at increased risk, such as women with PPBC, are included in a study as controls, the biological effect of previous childbirth is likely to go unidentified in any given analysis.
Figure: Schematic presentation of the cellular interactions causing the worse clinical outcome of postpartum breast cancer.
(A) The definition of PABC leads to conflicting clinical outcome data. When cases of PABC are differentiated between PrBC and PPBC, the worsened prognosis in this model is specifically true for PPBC. This distinction could explain the discrepancies in prognosis in published studies. (B) During pregnancy and lactation, proliferation and differentiation of the mammary gland that is purported to result in a long-term protective effect of pregnancy against breast cancer occurs. The short-term increased risk for PPBC is attributed to mammary gland involution, a process whereby mammary epithelial cells are subsequently removed by apoptosis to return the mammary gland to its prepregnant state (gentle weaning; upper panel). Involution shares numerous stromal attributes with protumour microenvironments, including immune suppression, which might lead to the formation of aggressive tumours with increased invasiveness (quick weaning; lower panel). PABC=pregnancy-associated breast cancer. PPBC=postpartum breast cancer. PrBC=breast cancer during pregnancy.
Since the conception of the definition of PABC, more data on the cellular and molecular differences between PrBC and PPBC (including their respective tissue microenvironments [figure, B]) have become available. During pregnancy, the mammary gland epithelium undergoes proliferation and differentiation in preparation for lactation. After childbirth and in the absence of lactation, or during weaning, the gland remodels to a state that is morphologically and functionally similar to the prepregnancy state, via a process called involution. It is hypothesised that involution, in the presence of subclinical disease, increases the metastatic potential of PPBC (figure, B).7 Animal studies, supported by detailed studies of human breast involution, show wound healinglike alterations in the microenvironment of the involuting breast and an influx of immunosuppressive cells that promote the escape of tumour cells from the mammary gland.8,9 In patients with PPBC, patterns of altered immune infiltration, cytokine profiles, or both can persist in the primary tumour microenvironment for several years after delivery.10 As depicted in figure B, the interaction between subclinical disease, wound healing-like stromal remodelling, and infiltration of tolerant immune cells could explain the worse prognosis of PPBC compared with PrBC, as well as the conflicting results observed in studies that consider both entities under a single definition.
The association between reproductive factors and breast cancer is complex, making it hard to differentiate between mechanisms related to nulliparity, uniparity, multiparity, age at first birth, lactation, involution, hereditary factors, and the breast tumour itself. Nevertheless, it is becoming clear that a distinction should be made between breast cancers that occur during pregnancy and those that occur within 5–10 years postpartum. In clinical practice, this difference is obvious: the treatment of PrBC is individualised according to gestational age and taking foetal safety into consideration, whereas treatment decisions for PPBC do not need to account for these concerns. Moreover, there is a need for a better understanding of the importance of parity status as an independent prognostic factor for worse outcomes in premenopausal breast cancer. Only a separate investigation of both entities will improve our understanding of the biology of breast cancer during pregnancy, lactation, involution, and thereafter, and help to decipher the pathways underlying differences in tumour biology. Therefore, we recommend that the term PABC is no longer used, allowing investigators to focus specifically on breast cancer during pregnancy (PrBC) or during the postpartum period (PPBC), which could ultimately lead to optimised therapeutic modalities, particularly for PPBC with a poor prognosis.
Acknowledgments
ML reports an advisory role for Roche, Lilly, Novartis, and AstraZeneca; and speaker honoraria from Roche, Lilly, Novartis, Takeda, Sandoz, and Pfizer. FP reports personal fees from Ipsen and Roche. SL reports grants from AbbVie, Celgene, AstraZeneca, Amgen, Novartis, Pfizer, Daiichi-Sankyo, Immunomedics, and Roche; advisory board honoraria paid to the institution from AbbVie, Celgene, AstraZeneca, Amgen, Novartis, Pfizer, Lilly, Bristol Myers Squibb, Puma, Pierre Fabre, Merck Sharp and Dohme, and EirGenix; speaker honoraria paid to their institution from AbbVie, Celgene, AstraZeneca, Amgen, Novartis, Pfizer, and PriME/Medscape; personal fees from Chugai and Daiichi-Sankyo; honoraria for advisory board participation and speaking fees paid to the institution from Daiichi-Sankyo, Roche, and SeaGen; and has a patent pending (EP14153692.0).
Footnotes
All other authors declare no competing interests.
Contributor Information
Frédéric Amant, Department of Oncology, KU Leuven, Leuven, Belgium; Department of Surgery, Netherlands Cancer Institute, Amsterdam, Netherlands.
Hanne Lefrère, Department of Oncology, KU Leuven, Leuven, Belgium; Department of Surgery, Netherlands Cancer Institute, Amsterdam, Netherlands.
Virginia F Borges, Division of Medical Oncology, Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Elyce Cardonick, Cooper Maternal Fetal Medicine, MD Anderson Cancer Center at Cooper, Camden, NJ, USA.
Matteo Lambertini, University of Genova, Genoa, Italy; IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Sibylle Loibl, German Breast Group, Neu-Isenburg, Germany; Centre for Haematology and Oncology Bethanien, Frankfurt, Germany.
Fedro Peccatori, Gynecologic Oncology Programme, European Institute of Oncology IRCCS, Milan, Italy.
Ann Partridge, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
Pepper Schedin, Knight Cancer Institute, University of Oregon Health & Science University, Portland, OR, USA.
References
- 1.Lyons T, Schedin P, Borges V. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia 2009; 14: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callihan E, Gao D, Jindal S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat 2013; 138: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loibl S, Han SN, von Minckwitz G, et al. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol 2012; 13: 887–96. [DOI] [PubMed] [Google Scholar]
- 4.Amant F, Von Minckwitz G, Han S, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: Results from an international collaborative study. J Clin Oncol 2013; 31: 2532–39. [DOI] [PubMed] [Google Scholar]
- 5.Borges V, Elder A, Lyons T. Deciphering pro-lymphangiogenic programs during mammary involution and postpartum breast cancer. Front Oncol 2016; 6: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao C, Yu Z, Xiao J, et al. Prognosis of pregnancy-associated breast cancer: a meta-analysis. BMC Cancer 2020; 10: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schedin P Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006; 6: 281–91. [DOI] [PubMed] [Google Scholar]
- 8.Betts C, Pennock N, Caruso B, Ruffell B, Borges V, Schedin P. Mucosal immunity in the female murine mammary gland. J Immunol 2018; 201: 734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennock N, Martinson H, Guo Q, et al. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J Immunother Cancer 2018; 6: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons T, O’Brien J, Borges F, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nature Med 2011; 17: 1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]