Abstract
Simple Summary
This review aims to provide practical information and viewpoints regarding fish germ cell transplantation for enhancing its commercial applications. We reviewed and summarized the data from more than 70 important studies and described the advantages, obstacles, recent advances, and future perspectives of fish germ cell transplantation. We concluded and proposed the critical factors for achieving better success and various options for germ cell transplantation with their pros and cons. Additionally, we discussed why this technology has not actively been utilized for commercial purposes, what barriers need to be overcome, and what potential solutions can advance its applications in aquaculture.
Abstract
Germ cell transplantation technology enables surrogate offspring production in fish. This technology has been expected to mitigate reproductive barriers, such as long generation time, limited fecundity, and complex broodstock management, enhancing seed production and productivity in aquaculture. Many studies of germ cell transplantation in various fish species have been reported over a few decades. So far, surrogate offspring production has been achieved in many commercial species. In addition, the knowledge of fish germ cell biology and the related technologies that can enhance transplantation efficiency and productivity has been developed. Nevertheless, the commercial application of this technology still seems to lag behind, indicating that the established models are neither beneficial nor cost-effective enough to attract potential commercial users of this technology. Furthermore, there are existing bottlenecks in practical aspects such as impractical shortening of generation time, shortage of donor cells with limited resources, low efficiency, and unsuccessful surrogate offspring production in some fish species. These obstacles need to be overcome through further technology developments. Thus, we thoroughly reviewed the studies on fish germ cell transplantation reported to date, focusing on the practicality, and proposed potential solutions and future perspectives.
Keywords: aquaculture, fish, germ cell transplantation, germline stem cells, surrogate propagation
1. Fish Germ Cell Transplantation in Aquaculture
Germ cell transplantation is the technology for surrogate production of donor-derived gametes. Donor cells possessing the ability of self-renewal and differentiation into gametes are injected into recipients that can support the survival, proliferation, and differentiation of donor cells so that recipients can produce donor-derived gametes. This technology has been spotlighted as an alternative to resolve critical reproduction obstacles of commercially important fish species. Although surrogate gamete production through this technology has been achieved academically in various species, including salmonids, cyprinids, and others considered commercially important [1,2], no industrial applications have been reported indicating that it is not commonly used in aquaculture. It may result from that established transplantation models have fewer advantages than their actual reproduction process, or surrogate gamete production technology has not been mature and successful in the critical species that do need this technology to solve their reproductive problems.
Thus, in this review, we discuss the advantages, important factors, obstacles, and recent advances regarding fish germ cell transplantation technology to understand its current status further and propose the solutions for its future commercial applications.
2. Germ Cell Transplantation Methods in Fish
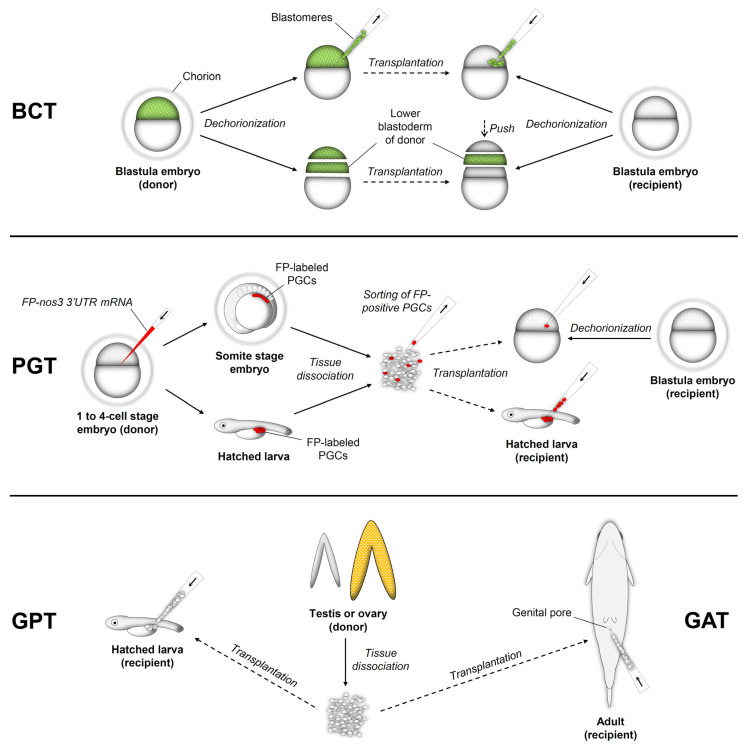
Fish germ cell transplantation can be classified into four different approaches based on the type of donors and recipients, including blastula cell transplantation (BCT), primordial germ cell transplantation (PGT), gonadal germ cell transplantation into the peritoneal cavity of larvae (GPT), and gonadal germ cell transplantation into adult recipients (GAT). In the following sections, the principle, results, and other aspects of each method are presented.
2.1. Blastula Cell Transplantation (BCT)
BCT is carried out by transplanting blastula cells that possess pluripotency into recipient blastula embryos. Fish blastula embryo consists of about 1000 cells, including primordial germ cells (PGCs) at various numbers depending on species [3]. PGCs migrate and incorporate into gonads during embryonic development through Sdf1a/Cxcr4b signal pathway [4].
Generally, less than 100 blastomeres or a lower part of the blastoderm are transplanted into each blastula embryo. Donor and recipient embryos are denuded by mechanical methods and/or treatments with proteases such as pronase or hatching enzyme to prepare donor cells or recipients [5,6,7]. When using dissociated blastomeres, donor cells are aspirated and transplanted into the blastoderm of blastula embryos with a fine needle [6,7]. In blastoderm transplantation, a lower part of the donor blastoderm is cut and placed onto the lower blastoderm part of a recipient embryo that has been removed half of the blastoderm, followed by pushing the upper half recipient blastoderm. Then, the donor and recipient blastoderms unite into one blastoderm [1,8] (Figure 1). The transplanted blastomeres or blastoderm integrate and mix with recipient cells. As the blastula cell population contains PGCs and somatic cells that can contribute to other lineages, chimerism can be observed throughout the organism that received blastula cells [9,10].
Figure 1.
Methods of fish germ cell transplantation. BCT: blastula cell transplantation, FP: fluorescent protein, GAT: gonadal (testicular/ovarian) germ cell transplantation into adult recipients, GPT: gonadal (testicular/ovarian) germ cell transplantation into the peritoneal cavity of larvae, PGC: primordial germ cell, PGT: primordial germ cell transplantation.
With BCT, the rates of germline chimera formation were variable and could reach 100% in some cases [10,11]. However, due to several manipulation steps such as removing chorion, low survival rates were noted in some recipients, such as rainbow trout (Oncorhynchus mykiss; 8.0%) [7] and zebrafish (Danio rerio; 26.1% or less) [6,8] (Appendix A, Table A1).
2.2. Primordial Germ Cell Transplantation (PGT)
PGCs are precursors of germline stem cells (GSCs) such as spermatogonia and oogonia. In fish, PGCs are specified by inheriting germplasm, maternally deposited in the oocytes, during early embryonic development [12]. In PGT, PGCs are generally harvested from embryos at somite stages by mechanical and enzymatic dissociation (Figure 1). When PGCs are labeled with fluorescence by microinjection of a fluorescent protein encoded mRNAs such as DsRed/GFP-nos3 3′ UTR [13], a fluorophore-conjugated dextran [14], or by transgenic fish expressing germ cell-specific fluorescent protein [15], they can be specifically collected under a fluorescence microscope [16], or by a fluorescence-activated cell sorting (FACS) [17,18]. For transplantation, purified PGCs are microinjected into the blastoderm of denuded blastula embryos [13] or the peritoneal cavity of anesthetized larvae [15]. Transplanted PGCs migrate and incorporate into developing recipient gonads, resulting in germline chimeras. Generally, 1–20 PGCs were transplanted into each recipient, and the germline chimera formation efficiencies were up to 89.5% [16] (Appendix A, Table A2).
2.3. Gonadal Germ Cell Transplantation into the Peritoneal Cavity of Larvae (GPT)
In addition to PGCs, larva recipients can also support the survival, proliferation, and differentiation of transplanted gonadal (testicular and ovarian) germ cells. Fish testes and ovaries carry GSCs called spermatogonia and oogonia, respectively, both of which possess the ability of self-renewal and differentiation into gametes. They can be harvested from donor testes or ovaries by mechanical and enzymatic dissociation. Like PGT, dissociated donor cells are microinjected into the peritoneal cavity of larvae (Figure 1). Of transplanted cells, spermatogonia and oogonia can colonize recipients’ gonads, forming germline chimeras.
Unlike PGT that used 1–20 PGCs for each recipient, more testicular or ovarian germ cells (up to 50,000 cells) were transplanted into each recipient (Appendix A, Table A3). The necessity of transplanting many cells may result from the relatively low proportion of GSCs in testicular or ovarian cell populations. For example, in immature rainbow trout (younger than 12 months old), the testicular and ovarian germ cells being vasa-positive accounted for less than 35.9% [19,20] and 11.9% [21] of total testicular and ovarian cell populations, respectively. Of the vasa-positive cells, only a small population can populate recipients’ gonads after transplantation. The cells expressing dead end (dnd) [22], nanos2 [23,24] and/or ly75 [25] have been considered to possess transplantability. The proportions of GSCs in the gonads may fluctuate according to the reproductive cycle and age of fish [22,26]. In the testes of immature fish such as 12 months old or younger rainbow trout [19] and 3 months old blue drum (Nibea mitsukurii) [27], all the germ cells were found to be spermatogonia. Therefore, immature fish have been preferred as donors due to the abundance of GSCs in their gonads (Appendix A, Table A3). Meanwhile, the rates of germline chimera formation were higher in testicular germ cell transplantation (up to 100%) rather than ovarian germ cell transplantation (34.5% or less; Appendix A, Table A3), which may be explained by the different proportions of GSCs between testicular and ovarian cells. On the other hand, regardless of the donor’s sex, both fish testicular and ovarian germ cells can contribute to either male or female germ lineages depending on the sex of recipients [21,28,29], indicating that testicular and ovarian germ cells possess the sexual plasticity and their fates are determined by recipients. Thus, both testicular and ovarian germ cells can be used to produce donor-derived sperm and eggs. This feature enables mono-sex seed production through germ cell transplantation technology (see Section 6.1).
2.4. Gonadal Germ Cell Transplantation into Adult Recipients (GAT)
Despite the mature immune system in adult recipients that can reject exogenous cells, transplanted gonadal (testicular and ovarian) germ cells can colonize adult recipients’ gonads and differentiate into functional gametes in fish (Appendix A, Table A4). It is thought that fish testis and ovary have the immune privilege provided by supporting cells such as Sertoli cells similar to mammalian testes [30]. Germ cells harvested from testes or ovaries with mechanical and enzymatic dissociation are transplanted into adult recipients by injecting cells directly into the gonads with a surgical incision or through the genital pore without incision (Figure 1). In general, a larger number of cells (2 × 106–1 × 107) were used for GAT since the gonad size of the adult recipients is significantly bigger than that of larva recipients (Appendix A, Table A4).
Since early embryos or larvae are used as recipients for BCT, PGT, and GPT, it takes a long time to obtain mature gametes through these technologies. Using adult recipients is an ideal solution to shorten the generation time of donor species because the recipients are already sexually mature. For example, in blue drum and Nile tilapia (Oreochromis niloticus), donor-derived sperm could be obtained at 7 and 9 weeks post-transplantation, respectively [31,32]. In addition, the survival rate of adult recipients was higher (greater than 75%) than those of other approaches. The rates of germline chimera formation in GAT were 5.0–33.3% (Appendix A, Table A4).
3. Advantages of Germ Cell Transplantation in Aquaculture
3.1. Shortening Generation Time
Selective breeding has been utilized to develop superior strains of commercial fish species that attain characteristics such as fancy appearance, improved growth performance, tolerance to environmental stressors, or disease resistance [33,34,35]. The processes require several generations to establish superior strains, which may take a long time depending on the target species. However, with germ cell transplantation using a recipient species with a shorter generation time, selective breeding can be achieved within a significantly reduced period. For example, in salmonids, Chinook salmon (Oncorhynchus tshawytscha) mature at 3 and 5 years in males and females, respectively [36], whereas rainbow trout reach sexual maturity at 1–2 years in males and 2–3 years in females [37]. By transplanting Chinook salmon’s germ cells into rainbow trout larvae, donor-derived sperm and eggs could be produced in rainbow trout recipients in 2 years [2,38]. Moreover, when this approach is applied to sturgeons with a much longer generation time, the breeding program might be significantly accelerated. Pšenička et al. [39] reported that GPT between Siberian sturgeon (Acipenser baerii; donors) and sterlet (Acipenser ruthenus; recipients) resulted in donor germ cells being incorporated into recipient gonads. Theoretically, if the recipient sterlet can produce Siberian sturgeon’s gametes, it is possible to obtain functional gametes of Siberian sturgeon 13–23 years earlier than the original sexual maturation timeline [39]. Furthermore, when germ cells are transplanted into the adult recipients (GAT), the generation time would be shortened more. For example, Nile tilapia testicular germ cells allotransplanted into adult males differentiated into functional sperm in 9 weeks, much shorter than the time usually needed to reach sexual maturity (around 6 months) [32].
3.2. Achieving Gamete Production of Semelparous Fish Recurrently through Multiple Seasons
Semelparous fish, such as most Pacific salmons, can only spawn once throughout their life cycle [40]. Although few populations of Chinook salmon survive after spawning and even repeat the spawning in the hatchery condition [41], most of them undergo deterioration and die soon following their first spawning season. Due to this characteristic, reusing the broodstock of these species reared for several years is impossible for the next round of seed production, meaning that farmers must repeatedly spend several years raising the new broodstock for only one-time use. This ineffectiveness can be mitigated by germ cell transplantation between semelparous fish (donors) and iteroparous fish (recipients). Indeed, semelparous Chinook salmon-derived sperm and eggs were produced by iteroparous recipient rainbow trout for multiple spawning seasons [2,38]. The surrogate production of semelparous germ cells in iteroparous recipients enables farmers to reduce the efforts and costs for broodstock management.
3.3. Solving Bottlenecks of Broodstock Maintenance
Effective broodstock maintenance and high-quality seed production are keys to successful aquaculture development. Of commercially important species, however, some fish are difficult or expensive to be domesticized to broodstocks that produce high-quality gametes. For example, Pacific bluefin tuna (Thunnus orientalis) and Southern bluefin tuna (Thunnus maccoyii) are highly popular and valuable marine fish that grow up to several hundred kilograms and require 3–5 years to reach sexual maturity [2]. Yet, due to their large body and long generation time, larger sea cages or land-based tanks for an extended period are needed for seed production of these species. In addition, as these fish are susceptible to light and noise, especially when they spawn, environmental conditions near the rearing facility should be controlled carefully [42,43]. All the above requirements translate into a considerable cost of maintaining the broodstocks. So far, only a few countries have achieved the reproduction of captive or reared bluefin tunas, despite their high demands and values [42,44], implying the difficulties in seed production of these species. To overcome these difficulties, xenotransplantation has been attempted with recipient species with advantages of a relatively smaller body, tolerance to environmental changes, shorter generation time, and well-established reproduction methodology, including blue drum [45], chub mackerel (Scomber japonicus) [22], Eastern little tuna (Euthynnus affinis) [46], yellowtail kingfish (Seriola lalandi) [47], or hybrid mackerel [48]. Although the successful production of gametes derived from the donor germ cells has not been reported, the incorporation of transplanted germ cells into recipients’ gonads was noted in these studies, indicating that these recipients could support the survival and migration of germ cells from bluefin tunas. Once mature bluefin tunas’ gametes are produced by one of these recipients, the vast rearing facility and rearing cost for an extended period would not be needed for seed production of bluefin tunas. The offspring would be produced cost-effectively with fewer risks, which can mitigate the limitation of seed availability and improve the productivity of bluefin tuna aquaculture. Besides bluefin tunas, this technology can also be applied to other fish species that encounter similar barriers described above.
3.4. Preservation and Restoration of Superior Strains Using Cryopreserved Cells
As the management of superior broodstocks developed by breeding programs or other approaches is very important for stable and high-quality seed productions, gamete preservation is highly recommended to prevent accidental losses of these valuable broodstocks [49]. Although the restoration of strains using cryopreserved sperm is possible by breeding through a few generations, it is only applicable to fish species that follow the male heterogamety sex-determination system (XX/XY), in which both X and Y chromosomes can be recovered from sperm. In cases of fish that have a female heterogamety sex-determination system (ZZ/ZW), the female’s genetic resource, the W chromosome, cannot be preserved by sperm (Z chromosome only) cryopreservation. While cryopreservation is successful in most fish sperm, it has not been achieved in fish eggs or embryos due to their low permeability, large volume, and yolk mass [50]. Unlike eggs or embryos, blastomeres [9,51], PGCs [52,53], spermatogonia [54,55], and oogonia [28,56] that can contribute to germ lineages have successfully been cryopreserved. Moreover, after transplanting these cryopreserved cells, the productions of donor-derived gametes have been achieved in many fish species [57]. Thus, the complete preservation and restoration of fish strains can be accomplished by cryopreservation and transplantation of blastomeres, PGCs, or ovarian germ cells, combined with sperm cryopreservation. This approach is imperative to preserve the valuable strains of several commercial fish that may have a ZZ/ZW sex-determination system, including flatfish [58], eels [59], and tilapias [60].
Notably, the production of germline chimeras was feasible by transplanting germ cells harvested from rainbow trout that were frozen with entire fish and kept in a deep-freezer (–80 °C) without using cryoprotectants. The viable germ cells could be harvested from frozen individuals of various sizes (18.8–203.9 g) [61]. These results imply that the preservation and restoration of fish lines can be achieved more practically even when standard cryopreservation methods are not available.
4. Factors Affecting the Success of Germ Cell Transplantation
Appendix A, Table A1, Table A2, Table A3 and Table A4 show that the transplantation success rates are highly variable among different studies. It is difficult to directly compare results from each study since most studies were conducted with various species and conditions by different researchers. Nevertheless, there are some factors noteworthy for achieving successful transplantation.
4.1. The Number of Donor Cells
The number of cells transplanted appears to have positive correlations with the success rates of transplanting testicular cells. In medaka (Oryzias latipes), larvae transplanted with 3000 or more unsorted testicular cells showed significantly higher colonization rates than those of recipients that received less than 3000 cells [55]. In rainbow trout and blue drum, similar patterns were observed, too. When 3000 or fewer unsorted testicular cells were transplanted into larvae, the colonization efficiency was in the range of 1.1–29.0% [19,20,62]. It increased up to 63.3% by transplanting more than 3000 testicular cells [63] (Appendix A, Table A3). While no comparative studies on the effect of donor cell number on colonization efficiency have been reported among adult recipients, transplanting 2 × 106–1 × 107 testicular or ovarian cells resulted in 37.5–100% colonization (Appendix A, Table A4).
The number of transplanted PGCs also affects the gonadal colonization efficiency in recipients. Li et al. [64] reported that when Dnd-overexpressed blastula embryos that contain more PGCs (~2.5 fold) employed as donors, higher germline chimerism after blastomere transplantation could be achieved, compared to regular blastula donors (81.2% vs. 47.2%). On the other hand, many donor cells do not seem to be necessary when transplanting blastomeres or PGCs. For example, 20–100 blastomeres were enough to generate germline chimeras in medaka [64], rainbow trout [7], and zebrafish [6]. For PGCs, germline chimeric goldfish and zebrafish could be obtained by transplanting a single PGC into blastula embryos [16,17,65]. In salmonids, 5–20 PGCs transplanted into larvae could colonize recipient gonads and undergo differentiation into functional gametes [15,66].
4.2. Purity of Donor Cells (Enrichment)
The purity of GSCs in donor cell populations can also affect the success rates of germ cell transplantation. Among the cells that constitute gonads, including germ cells at various stages, supporting cells, fibroblasts, and red blood cells, only GSCs, namely spermatogonia and oogonia, have the ability to colonize, self-renew, and differentiate into functional gametes in recipient gonads [21,67]. Thus, when an equal number of cells is transplanted, a higher success rate is expected if the enriched cells rather than crude cells are used. Several approaches have been attempted for the enrichment of GSCs, including Percoll density gradient centrifugation (PDGC), differential plating (DP), centrifugal elutriation (CE), FACS, magnetic-activated cell sorting (MACS) (Appendix A, Table A2, Table A3 and Table A4). In the following sections, the principle, enrichment effects, advantages, disadvantages, and applications of enrichment methods are presented.
4.2.1. Percoll Density Gradient Centrifugation (PDGC)
PDGC is a cell separating method based on cell density using Percoll solution that consists of colloidal silica particles [68]. The cell suspension from tissues is loaded onto the top of layered Percoll solutions with different densities. After centrifugation, desired cells harboring a specific range of densities, which form bands between Percoll layers, are harvested for transplantation. For example, in zebrafish and medaka, as GSCs were abundant in 25–35% density Percoll fractions, cells harvested from these fractions were subjected to transplantation [69,70].
PDGC is the most widely used method for fish GSCs enrichment as its procedure is quick and straightforward without the need for special techniques and equipment. However, high purity is not expected because it is challenging to separate GSCs from other cells having similar densities. In loach (Misgurnus anguillicaudatus), 30–36% density fraction consisted of about 60% of type A and early-type B spermatogonia [71]. In olive flounder (Paralichthys olivaceus), the proportion of vasa-positive ovarian cells increased from 37.8% to 83.6% after PDGC [72]. In Siberian sturgeon, cell populations containing vasa-positive testicular cells (79.4% of total) and ovarian cells (70.8% of total) were obtained by PDGC [39].
4.2.2. Differential Plating (DP)
DP is the cell separation method utilizing different adhesive characteristics among cells. After short-term incubation of cells on the substrata with culture medium, adherent cells can be separated from non-adherent cells by gentle pipetting or agitation. In general, as GSCs attach to substrata weakly, they can be separated from somatic cells such as fibroblasts that show relatively tight attachments [73,74]. In rainbow trout, vasa-positive cells were obtained at higher than 90% purity from immature testes by a series of DPs [75,76].
Since DP is simple and does not need special materials or devices like PDGC, it has also been widely used for cell enrichment. However, cell separation by DP may cause contamination with the cells harboring a similar adhesiveness to GSCs. Moreover, as DP takes up to several days, which is relatively longer than other methods, it raises a potential risk of spontaneous cell differentiation during in vitro culture [77,78]. On the other hand, DP would be a great tool when GSC-specific selective adhesive molecules become available. For example, in mice (Mus musculus), laminin was used for positive selection of spermatogonia, which showed 3–4-fold increased colonization efficiency compared to unsorted testicular cells [79].
4.2.3. Centrifugal Elutriation (CE)
CE enables researchers to separate cells based on their physical characteristics. Unlike other typical centrifugations, cells are exposed to an outward centrifugal force and an inward counter-flow in the CE separation chamber, which arranges cells according to their sedimentation velocity, depending on their sizes, shapes, and densities. Subsequently, cells are eluted in the collection chamber by adjusting outward centrifugal force and inward counter-flow [80]. Bellaiche et al. [23] obtained cell fractions containing higher than 90% purity of type A spermatogonia (ASG) from immature testes of rainbow trout by CE with pre-enrichment by PDGC.
CE requires special apparatus and well-developed adjustment conditions for effective enrichment [81]. As a result, it has only been applied to a few studies related to fish germ cell enrichment and transplantation.
4.2.4. Fluorescence-Activated Cell Sorting (FACS)
FACS facilitates cell separations based on light-scattering parameters of cells. During sorting, cells are exposed to a laser in a liquid stream, and their fluorescent characteristics are measured. Then, an electrical charge (positive or negative) is imposed on an individual cell, which can be sorted by an electrostatic deflection system [82]. Thus, desired cells that have specific light-scattering properties can be isolated by this method [83]. Especially, cells harboring fluorescence can readily be enriched by FACS due to their distinguishable light-scattering property, such as strong fluorescent intensity. Labeling germ cells with fluorescence can be achieved by microinjection of a fluorescent protein encoded mRNAs [13] or a fluorophore-conjugated dextran [14,84], with fluorophore-conjugated antibodies, or by transgenic fish expressing germ cell-specific fluorescent protein [15].
With transgenic rainbow trout (pvasa-GFP) carrying germ cells expressing GFP, PGCs and ASG could be enriched to 93.2% [85] and 93.2% purity [19], respectively. In non-transgenic fish, GFP-nos1 3′ UTR mRNAs were injected into 1 to 4-cell stage embryos to label PGCs, which resulted in reaching 100% purity after FACS [17]. Fluorophore-conjugated antibodies that were raised against fish ASG surface antigens led to 70.7–80.9% ASG purity after FACS in brown trout (Salmo trutta) [86], Pacific bluefin tuna [45], and rainbow trout [20]. Without fluorescence labeling, the enrichment of ASG was achieved by only the parameters such as forward scatter (FS; for cell size) and side scatter (SS; for granularity). 75.6–94.9% ASG purity could be achieved in blue drum, Japanese char (Salvelinus leucomaenis), masu salmon (Oncorhynchus masou), and sterlet [19,87]. With the optimized Hoechst 33342 staining condition, ASG could also be enriched using a side population [88].
Generally, a high level of enrichment is expected in cell populations sorted by FACS [89], especially when transgenic fish expressing GSC-specific fluorescent reporters or GSC-specific antibodies are employed. Of these, transgenic fish that carry GSCs expressing fluorescent proteins may encounter restrictions for commercial purposes [90]. Thus, employing the antibodies against specific surface proteins of GSCs seems one of the appropriate options to obtain highly enriched GSCs for aquaculture applications. However, the purities of fish GSCs after FACS with antibodies were 70.7–80.9% [20,45,86], which are lower than the purities (75.6–94.9%) achieved in FS and SS methods [19,87] and the purities (higher than 90%) enriched by FACS with antibodies in mammalian studies [91,92,93]. In addition, rainbow trout GSC antibodies (No. 80 and No. 95) also labeled a small population of somatic cells [20], indicating that developing more specific antibodies for fish GSCs is required to achieve higher purity by FACS. On the other hand, even if the specificity of an individual antibody is not great, high purity could be achieved by combining multiple antibodies since it enables sorting an overlap of cell populations labeled by multiple antibodies and/or excluding cell populations labeled with antibodies specific to undesired cell populations. In mammals, 98.8–99.8% purity of spermatogonia could be achieved by FACS using three combined antibodies against EPCAM, CD49E, and HLA-ABC [93]. Additionally, FS and SS methods can be another choice since they can achieve 75.6–94.9% GSC enrichment in fish [19,87], although it may require optimizing sorting conditions for each species.
As discussed above, FACS with GSC antibodies would be helpful to obtain highly enriched GSC populations from non-transgenic fish, which will be appropriate for commercial applications. However, FACS requires expensive apparatus, special materials, and trained personnel. Moreover, FACS sorts cells individually, which is inefficient for larger samples due to its low throughput (107 cells per hour) [62,89].
4.2.5. Magnetic-Activated Cell Sorting (MACS)
MACS separates cells with magnetic columns and magnetic nanoparticles-conjugated antibodies against specific cell surface antigens. Cells bound to the antibodies magnetically attach to the column so that these cells can be separated from unbound cells [94]. When the highly specific antibody is employed, MACS facilitates obtaining highly enriched cell populations. In fish, the GSC enrichment by MACS was reported by one study in salmonids, which employed the No. 172 antibody, resulting in 68.6 or 81.7% of sorted testicular cells and 54.8% of sorted ovarian cells being vasa-positive, respectively [62]. Compared to mammalian studies that achieved purity higher than 95% by MACS [95,96], the purity of fish GSC was lower, implying that the No. 172 antibody was not very specific to GSCs. Indeed, this antibody labeled differentiated spermatogenic cells even though it was developed against ASG [62].
Different from FACS, MACS cannot separate cells with gates customized by light-scattering properties. Therefore, more specific antibodies will be required to achieve a higher GSC purity. Additionally, combining antibodies would also be worth trying to improve purity [97]. Unlike FACS, MACS require less expensive equipment and skill settings [98]. In addition, MACS is applicable to large scales as it requires less than 15 min to sort magnetically labeled cells, regardless of the number of cells [62]. Thus, if the effective MACS condition is established, it will be valuable for the enrichment and transplantation of fish GSC in aquaculture operations.
4.2.6. Applications
As described above, each enrichment method has its advantages and disadvantages. The enrichment methods for fish GSCs are summarized in Table 1. Enrichment methods can be used solely or combinedly. By combining them, the limitation of each method can be mitigated, which would lead to higher purities than those of enrichment with a single method [70]. The improved purity of GSCs after enrichment resulted in an increase of colonization efficiencies (up to a 17.5-fold increase) compared to those of unsorted cells [19,20,62,70,73,86]. Improved colonization rates by enriching donor cells will contribute to efficient surrogate offspring production, reducing efforts for screening works. The highly effective enrichment method will be useful, especially when recipient fish or space is limited, but donor fish are abundant. By contrast, enrichment should be carried out cautiously when only limited donors are available since transplantable cells can partially be lost during enrichment [62,85].
Table 1.
Enrichment methods used for fish germline stem cells.
| Method | Principle | Advantage | Disadvantage | Enrichment of Fish GSCs |
|---|---|---|---|---|
| PDGC | Fractionating cells by their density with centrifugation | Simple procedure No need for special materials, techniques, and equipment |
Low purity | The most frequently used method 60–83.6% purity [39,71,72] |
| DP | Positive or negative selection based on adherence of cells by in vitro culture | Simple procedure No need for special materials, techniques, and equipment |
Low purity Relatively long procedure Potential risks of spontaneous differentiation during in vitro culture |
Frequently used >90% purity by serial DPs [73,75] No available commercial molecules for positive selection |
| CE | Aligning and eluting cells by their physical characteristics with centrifugation | High purity expected Not requiring TGs or ABs |
Requiring special sorting conditions and equipment | >90% purity by combining with PDGC [23] |
| FACS | Isolation of cells based on light-scattering properties | High purity expected Flexible sorting condition by customizing gates based on size, granularity, and fluorescent intensity of cells |
Requiring special skills and equipment Requiring specific ABs against the surface protein of target cells, TGs carrying germ cells expressing FPs, or other specific sorting conditions Not suitable for large scales |
Up to 100% purity of PGCs labeled with mRNA-nanos3 3′ UTR encoded FP [17] 93.2–99% purity with TGs [18,19,85] 70.7–80.9% purity using ABs [20,45,86] 75.6–94.9% purity without using TGs or ABs [19,87] Limitation of using TG fish for commercial application No available commercial fish ABs |
| MACS | Affinity based cell sorting with magnetic particles-conjugated ABs against cell surface proteins | High purity expected No need for special techniques or equipment Simpler than FACS Applicable to large scales |
Requiring specific ABs against the surface protein of target cells | 54.8–81.7% purity [62] No available commercial fish ABs |
AB: antibody, CE: centrifugal elutriation, DP: differential plating, FACS: fluorescence-activated cell sorting, FP: fluorescence protein, GSC: germline stem cell, MACS: magnetic-activated cell sorting, PDGC: Percoll density gradient centrifugation, PGC: primordial germ cell, TG: transgenic.
For the applications in aquaculture, extremely enriched donor cells do not seem necessary because improved success rates of transplantation are also achievable by using the immature gonads that contain a higher percentage of GSCs (see Section 2.3) or increasing the number of donor cells (see Section 4.1). In addition, enough recipients carrying donor-derived gametes can be secured by increasing the total number of recipients. Moreover, CE, FACS, and MACS, generally considered options to achieve high purity, require expensive apparatus, well-trained personnel, and/or special materials such as specific antibodies, which might result in increased costs. Thus, the reasonable and balanced points between the high-level enrichment and the investment should be determined with attention to practicality.
Together, to improve the efficiency of germ cell transplantation effectively, the enrichment of donor cells will need to be conducted depending on the availability of equipment, materials, techniques, donor/recipient fish, and rearing capacity, considering its pros and cons.
4.3. Age of Recipients
Among the factors, the age of the recipients can significantly affect the success rate of germ cell transplantation. Seki et al. [55] reported a significant decrease in colonization rates when testicular donor cells were transplanted into larvae older than 11 days post-fertilization (dpf) compared to larvae at 7 or 11 dpf in medaka. Meanwhile, in rainbow trout, transplantation of PGCs into recipients at 35 dpf showed relatively higher success rates, whereas the transplantation at earlier stages (younger than 35 dpf) or later stages (older than 40 dpf) resulted in significantly lower incorporation rates of PGCs into the recipient gonads [15]. In this regard, Yazawa et al. [99] reported that the developmental stage of larvae recipients is critical for a successful germ cell transplantation. In the xenotransplantation study with blue drum and chub mackerel, a high success rate could be obtained when transplantation was carried out with the recipient larvae at the stages when their germ cells were not completely surrounded by the gonadal somatic cells. In terms of adult recipients, there have been no comparative studies on the transplantation efficiency among different ages of them.
On the other hand, using younger recipients may require additional manipulations, such as dechorionization, resulting in low survivals [15,55]. By contrast, high survival rates (greater than 75%) of recipients were observed in adult recipients (Appendix A, Table A4). Even though wild-type adults were treated with high temperature and busulfan for eliminating endogenous germ cells before transplantation, it did not cause significant decreases in the survival of recipients when treated with the optimized conditions [72,100].
4.4. Prevention of Endogenous Germ Cell Development in Recipient Gonads
Prevention of endogenous germ cell development can give rise to efficient donor-derived gamete production. The recipients harboring endogenous germ cells can result in a high proportion of undesired offspring, introducing additional efforts for screening the desired offspring. Indeed, the low and/or variable proportions of donor-derived offspring were seen when fertile recipients were used in blue drum (2.2–89.2%) [63], rainbow trout (0.1–40.5%) [37], and zebrafish (3.9–7.3%) [101]. Thus, recipients’ endogenous germ cell development should be suppressed for the efficient surrogate production of donor-derived gametes. To prevent endogenous germ cell development in the recipients’ gonads, interspecific hybridization, triploidization, knockdown/knockout of dnd gene, high temperature/busulfan co-treatment, irradiation, etc. technology has been applied (Appendix A, Table A1, Table A2, Table A3 and Table A4). In the following sections, the principle, effects, pros and cons, other aspects, and applications of each sterilization method, which has frequently been used, are introduced.
4.4.1. Interspecific Hybridization
Interspecific hybridization has conventionally been utilized to induce sterility in fish by inhibiting normal meiosis through a mismatch of chromosomes [102]. Hybrid fish show various reproductive phenotypes, including unusual sex ratio, reduced fecundity, or sterility [48,69,103]. Some hybrid fish even do not carry germ cells in their gonads due to mitotic arrests of PGCs. No germ cells were detected in hybrids between male white croaker (Pennahia argentata) and female blue drum, eliminating the potential of endogenous gametogenesis [27]. Due to these characteristics, sterile hybrid fish have been utilized as recipients, achieving 100% of donor-derived offspring production [27,31,69,104].
Since interspecific hybridization does not require sophisticated techniques and special equipment, hybrids seem like a reasonable option for recipients, as long as their sterility and supporting capacity for donor germ cells are verified. The adult hybrid recipients that do not carry endogenous germ cells are considered the most effective option due to their sterility and a significantly shortened generation time after transplantation. With hybrids between male white croaker and female blue drum, 100% donor-derived gamete production could be achieved within 7 weeks after transplantation at the earliest [31]. Yet, due to the highly variable features regarding survival rates, gonadal development, and supporting capacity for donor germ cells among hybrids developed with the combination of distinct species and sex, preliminary studies need to be done before the employment. Currently, only a few hybrids that harbor germ cell-less gonads have been known [27]. Therefore, further investigations to find out germ cell-less hybrids will be required for the broader application to various donor species.
4.4.2. Triploidization
Triploid fish also do not undergo normal gametogenesis due to a mismatch of chromosomes [102]. Triploids can be generated by blocking the extrusion of the second polar body or breeding diploids with tetraploids which were produced by blocking the first mitosis [105,106]. Various techniques such as temperature shock (heat/cold), high pressure, chemicals, and electroporation have been developed to produce triploid fish in multiple species [107,108,109]. Although some triploid fish can produce a small amount of milt or eggs, their gametes may not have fertility, or their offspring cannot develop normally [110,111]. Moreover, in some species, triploid fish do not produce sperm or eggs at all [28,54,112]. They, as recipients, successfully produced donor-derived sperm or eggs in several germ cell transplantation studies (Appendix A, Table A3).
In fish, 100% triploidization was reported in species including Atlantic salmon (Salmo salar) [108], channel catfish (Ictalurus punctatus) [113], and rainbow trout [114] generated by simple temperature shocks. However, 100% triploidy is not always guaranteed, even with the same species and conditions that achieved 100% triploidy in other studies [63,115]. Tetraploid fish that can produce triploids by breeding with diploids are only available in limited species, including rainbow trout and loach [116,117]. In addition, tetraploids can potentially produce haploid or aneuploid sperm. Even diploid sperm, which is desired, may have low fertilization rates due to their head being larger than the micropyle of haploid eggs [118,119]. These indicate that using tetraploid fish to prepare triploid recipients would not widely be applicable. Besides, triploid recipients have the potential to produce their own endogenous gametes in some species. Indeed, triploid male grass puffer (Takifugu alboplumbeus) recipients produced 37.5% of total offspring derived from endogenous germ cells [120]. Thus, triploid recipients do not appear to be appropriate for allogeneic transplantation in various species. It might be acceptable if triploid endogenous gamete production is at the minimum level, and donor-derived offspring can easily be distinguished from the individuals derived from endogenous germ cells of the recipients.
4.4.3. Dnd-Knockdown
The dnd gene is essential for the migration and development of PGCs, and its role is conserved among fish [121]. Without an appropriate expression of Dnd, PGCs fail to migrate to the gonadal region, resulting in the formation of gonads without germ cells [122]. When utilizing antisense Morpholino oligomers-mediated Dnd-knockdown by microinjection [123,124] or immersion treatment using Vivo-conjugated Morpholino [125] with early-stage embryos, the sterility was induced efficiently. In several studies, these sterile recipients produced 100% donor-derived gametes after germ cell transplantation [123,124,126].
In most fish species, a single dnd Morpholino can successfully induce Dnd-knockdown, but multiple Morpholinos are needed when multiple dnd transcriptional variants generated by alternative splicing are present in the target species [127]. Moreover, as the embryo survival and knockdown efficiency can be significantly affected by the target sequence and concentration of Morpholino [127], identifying effective Morpholinos and optimizing treatment procedures are necessary for achieving high survival and sterility induction. Although Dnd-knockdown showed high sterilization efficiency, 100% sterility has not always been guaranteed [124,127]. Thus, screening desired embryos that do not carry germ cells in their gonads should be strictly conducted. This can be achieved by visualizing endogenous germ cells through labeling PGC via either transgenesis or co-injection of fluorescent protein-encoding mRNA stabilized by nanos3 or vasa 3′ UTR [127].
4.4.4. Dnd-Knockout
The development of the dnd-knockout line is another approach for producing sterile recipients. In zebrafish, dnd-knockout homozygous mutants were used as recipients. In the progeny test, the effectiveness of donor-derived offspring production was 100% in the sterile dnd mutant recipients and, in contrast, only 3.9–7.3% in the fertile wild-type recipients [101].
For generating dnd-knockout fish, the genetic information (dnd gene) and time (at least for two generations) are required to establish a dnd-knockout line. In addition, the screening of homozygous fish is necessary as only 25% of total offspring from heterozygous parents will be sterile homozygous fish. When the dnd-knockout line is developed based on transgenic fish expressing germ cell-specific fluorescent reporters, screening can be done efficiently by selecting fish that do not carry germ cells expressing fluorescent reporters. The drawbacks are the additional time needed to develop the transgenic lines and the regulatory hurdle of using them in a commercial-scale application [90].
4.4.5. Co-Treatment of High Temperature and Busulfan
Co-treatment of high temperature and busulfan has been applied to adult recipients for ablating endogenous germ cells (Appendix A, Table A4). High temperature arrests meiosis and induces massive apoptosis of spermatogenic cells in fish [32,72,128]. Busulfan is an alkylating agent that produces intrastrand or interstrand crosslinks of DNA, blocking DNA replication, cell proliferation, and differentiation [129]. As such, busulfan has been used to deplete fish testicular germ cells [128]. In zebrafish, co-treatment of high temperature and busulfan showed a synergetic effect in turning 88% of testicular tubules into the states without germ cells [128]. After transplantation, the adult recipients treated with this method showed 1.2–52.2% offspring derived from donor cells of total offspring (Appendix A, Table A4). Although a comparison of the transplantation success between untreated recipients and germ cell-depleted recipients has not been reported in fish, germ cell depletion by the co-treatment is expected to increase the proportion of donor-derived gametes, which has been shown in mammalian studies that 80% of donor-derived offspring production was achieved [130].
The advantage of co-treatment with high temperature and busulfan is the applicability to wild-type adult fish. The recipients can be prepared in a few weeks with this method without any special techniques or equipment. However, with this treatment, endogenous germ cells were not completely ablated, and the remaining GSCs could recover and repopulate gonads [128], impeding successful germ cell transplantation. In addition, as an inappropriate treatment can cause high mortality and inefficient germ cell ablation [72], optimizing procedures to achieve successful endogenous germ cell depletion is necessary.
On the other hand, long-term treatments (longer than 45 days) at a high temperature (37 °C) induced permanent sterilization in Nile tilapia and Mozambique tilapia (Oreochromis mossambicus) [131,132,133]. However, in another study, a similar treatment failed to induce sterility in male Nile tilapia since they recovered their fertility after returning to the normal condition [134]. The tolerance to heat treatment likely varies depending on the species or even among different strains of the same species. Thus, further studies are needed to verify its applicability to various species and strains.
4.4.6. Other Aspects
As described above, eliminating endogenous germ cells can effectively improve the efficiency of producing donor-derived offspring. Besides this, it has been considered that germ cell-ablated recipients would enhance the colonization of transplanted cells since less competition is anticipated between endogenous and transplanted germ cells. However, it is not conclusive that ablating endogenous germ cells always positively affects the colonization of donor cells in recipients. In some cases, the transplantation success rates were higher in recipient larvae injected with dnd-MO to ablate endogenous germ cells, compared to untreated control recipients or hybrid recipients between male pearl danio (Danio albolineatus) and female zebrafish [135,136]. Still, the frequency of recipients harboring donor germ cells was not significantly different between control and dnd-MO treated recipient larvae in puffers (40.0% vs. 40.5%) [127] and salmonids (63.2% vs. 70.0%) [124]. dnd-knockout zebrafish recipient larvae showed a colonization rate similar to that of wild-type recipients (5.0% vs. 6.7%) [101]. These might be caused by the unfitness of underdeveloped gonads that are germ cell-deficient. As discussed in Section 4.3, the age of recipients can affect the success rates of transplantation [15,99,137,138], and it has been known that the gonadal development of germ cell-deficient fish can be delayed compared to wild-type [8,139]. However, in those studies, recipients at the same age were used regardless of the recipient type, which probably created unexpectedly different gonadal environments, making the comparison between experimental groups difficult. Further studies will be needed to clarify the effects of ablating endogenous germ cells on the success rate of transplantation in the GPT model. The comparison study on the impacts of germ cell ablation on donor cell colonization in GAT approaches has not been reported yet. However, the positive impacts of ablating endogenous germ cells are expected, considering the higher colonization rate in treated recipients than untreated recipients in mice [140].
Another important aspect of sterile recipients is that some sterile fish have a male-biased sex ratio. For example, the germ cell-less hybrids between male white croaker and female blue drum have a phenotypical sex ratio of 98:2 (male:female) [27]. Moreover, all the germ cell-depleted zebrafish by Dnd-knockdown/knockout developed into males [101,141]. Germ cells appear to be a critical factor of sex-determination in some species [142,143]. Thus, when donor-derived eggs are required, the feminization treatment is needed for sterile recipients in which the male-biased sex ratio has been observed. Saito et al. [16] feminized Dnd-knockdown sterile zebrafish recipients with 17-β estradiol treatment to successfully obtain donor-derived eggs from 75.0% of treated recipients. Xu et al. [104] could obtain female adult recipients of hybrids between male white croaker and female blue drum by triploidization (26.3% female of total), which successfully produced donor-derived eggs.
4.4.7. Applications
For the application of germ cell transplantation in aquaculture, reducing labor-intensive works, costs, and the preparation period are vital aspects. Thus, when selecting a sterilization method, the simplicity, sterilization efficiency, and required time will need to be considered beforehand. When the decision is made with careful considerations on the pros and cons of each method, purpose of transplantation, and availability of technologies, it will lead to a successful outcome such as 100% donor-derived offspring production in a shortened period with fewer efforts and costs. The summarized information of methods for sterile recipient preparation is presented in Table 2.
Table 2.
The sterilization methods used to prepare recipients for germ cell transplantation in fish.
| Method | Principle | Advantage | Disadvantage | Recipient Case |
|---|---|---|---|---|
| Interspecific hybridization | Preventing normal meiosis by a chromosomal mismatch or arresting mitosis of PGCs | Simple preparation by breeding without any treatment Complete germ cell elimination (in germ cell-less hybrids) |
Unpredictable reproductive phenotypes depending on species/sex combination | ♂ D. albolineatus × ♀ D. rerio [69], O. curvinotus × O. latipes [144], ♂ P. argentata × ♀ N. mitsukurii [27,31,104] |
| Triploidization | Preventing normal meiosis by a chromosomal mismatch | Relatively simple procedure | Potential risks of endogenous germ cell-derived gamete production Unstable efficiency depending on species and method |
D. rerio [145], N. mitsukurii [63], O. masou [28,146], O. mykiss [21,56,147], O. latipes [55], P. olivaceus [148], T. alboplumbeus [120,138] |
| Dnd-knockdown | Disrupting PGC development by MO-mediated inhibition of Dnd expression | High efficiency expected Possibility of complete germ cell elimination |
Requiring special techniques, apparatus, or materials Not guaranteed 100% sterility |
C. auratus [29,65], D. rerio [16,123,126], O. masou [124], O. latipes [64], T. alboplumbeus [127] |
| Dnd-knockout | Disrupting germ cell development by blocking Dnd expression through gene editing | Guaranteed 100% sterility in homozygous mutants Complete germ cell elimination |
Requiring genetic knowledge, techniques, apparatus, and time to establish mutant lines Only 25% sterile fish of total |
D. rerio [101], O. mykiss [38] |
| High temperature + busulfan co-treatment | Arresting meiosis and inducing apoptosis of germ cells | Preparable in a few weeks with wild-type adults | Incomplete germ cell ablation Requiring optimized conditions to achieve low mortality and successful germ cell ablation |
O. hatcheri [100,149], O. niloticus [32], P. olivaceus [72] |
Dnd: Dead end, MO: Morpholino oligomer, PGC: primordial germ cell.
5. Obstacles and Potential Solutions of Germ Cell Transplantation in Aquaculture
5.1. Practical Acceleration of Selective Breeding Process
As described in Section 3.1, germ cell transplantation can reduce the time needed to obtain mature donor-derived gametes when recipients have a shorter generation time. Therefore, it would significantly accelerate the selective breeding process that requires multiple generations, especially for the species with a relatively long generation time, such as Chinook salmon and sturgeons.
On the contrary, xenotransplantations between species with a relatively short generation time do not seem to have that advantage. For instance, grass puffer was suggested as a recipient to reduce the generation time of tiger puffer (Takifugu rubripes). The expectation was that sperm and eggs of tiger puffer could be produced quickly by transplanting tiger puffer testicular cells into grass puffer larvae. However, donor germ cells were harvested from 1-year-old male tiger puffer, and the donor-derived sperm and eggs were obtained at 10 and 22 months post-transplantation, respectively [120,127,138]. Therefore, when using grass puffer larvae as recipients, it took 22 or 34 months to produce tiger puffer sperm or eggs, respectively. Given that 2-year-old male and 3-year-old female tiger puffer can reach sexual maturity [138], it is not convincing that grass puffer can make a significant difference in shortening tiger puffer reproduction. In salmonids, it was proposed that xenotransplantation using rainbow trout larvae recipients may shorten the generation time of Atlantic salmon [150]. Rainbow trout generally begin to produce sperm and eggs at the age of 2 and 3 years, respectively [150,151], which is only 1 year shorter than Atlantic salmon. In one study, donor sperm and eggs were produced 1 and 2 years post-transplantation with the donor cells harvested from 1-year-old male Atlantic salmon [150]. Similar to the xenotransplantation between tiger puffer and grass puffer, there was only minor improvement in shortening generation time. Thus, it is not anticipated that xenotransplantation can significantly reduce generation time in those species unless younger donors or matured recipients are employed.
Since selective breeding requires the phenotypic and/or genotypic information of each individual for selecting parents, it is very challenging to use donor cells harvested from the individuals at very early developmental stages that provide no phenotypic characteristics nor enough cells for transplantation. In addition, in the blastula transplantation approach, only 2.5% of total recipients produced donor-derived gametes in rainbow trout [7], indicating low efficiency. These imply a limitation of blastula transplantation in use for selective breeding. When it comes to larvae carrying PGCs, although genotyping is possible, it comes with a great risk of losing fish due to sampling procedures. As such, selecting donors by phenotypes is difficult, and the number of desired recipients would be very low. In salmonids, a rainbow trout larva at 30 dpf only carries 30–60 PGCs [152,153], which can be used only for limited recipient larvae that receive 5–20 PGCs per recipient [15,66,154]. Moreover, the germline transmission rates were lower than 20% (Appendix A, Table A2), which is not cost-effective, given the costs for genotyping and transplantation. Although pooled PGCs collected from dozens of larvae may enable securing more transplanted recipients, it is not suitable for the selective breeding program that requires maintaining the pure line of each broodstock strain.
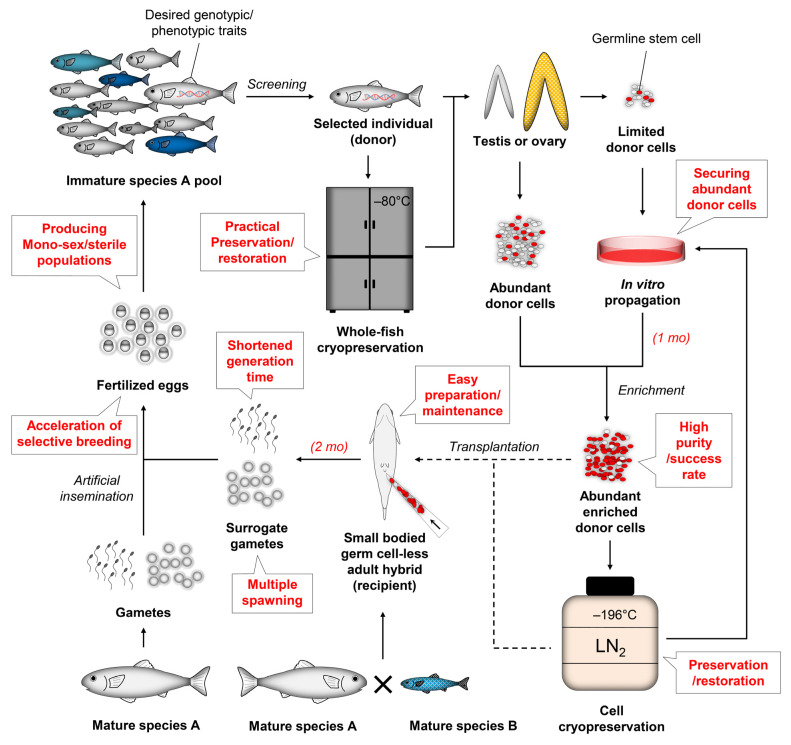
Using adult recipients with relatively higher survival rates can be an alternative approach to practically reduce the generation time since donor-derived gametes could be produced much quicker after transplantation [31,32] (Appendix A, Table A4). In this approach, what matters is securing enough donor cells. The individuals that can provide enough donor cells can be subjected to the acceleration of selective breeding through germ cell transplantation. For example, an immature yellowtail (Seriola quinqueradiata) male individual (10 months old) could provide about 3.8 × 108 testicular cells [155], which are enough at least for 30 adult recipients when 2 × 106–1 × 107 testicular cells are transplanted into each recipient. Once appropriate recipients are identified and available, this approach would be feasible. Moreover, unlike embryo or larva donors, grownup donors provide more phenotypic information for confirming their genotype, enabling a more accurate selection of the desired individuals. As such, the generation time would be shorter than larvae recipients, accelerating the selective breeding process.
5.2. Securing Enough Donor Cells for Transplantation via In Vivo or In Vitro Propagation
To obtain enough recipients with high success rates of transplantation, enough donor cells should be prepared to ensure that maximal cells can be transplanted into each recipient. As discussed in Section 4.1, transplanting more donor cells into larvae could result in higher colonization rates (Appendix A, Table A3). In particular, each adult recipient has generally been transplanted with 2 × 106–1 × 107 testicular or ovarian cells (Appendix A, Table A4), indicating that sufficient donor cells are necessary for this approach. However, the gonad size of the donors and its availability depends on species or other factors. Enough donor cells may not be obtained when suitable fish are rare and/or their gonads are too small, affecting the success rates of germ cell transplantation. As such, methods for germ cell propagation would provide solutions to increase the number of donor cells.
For in vivo germ cell propagation, the donor germ cells are transplanted into the recipient fish, which would provide an increased number of donor cells by proliferating in recipients. In zebrafish, the long-term maintenance and securing enough testicular germ cells could be achieved by subcutaneous transplantation of testicular tissue fragments or aggregates under the skin of recipient fish. The size of grafted testis fragments increased more than 20-fold over 3 months post-transplantation. Through a series of transplantations, the grafted tissues could be maintained for longer than 3 years, providing enough cells, including spermatogonia and viable sperm derived from donor cells [156]. This approach was also attempted in rainbow trout, and the functional sperm derived from donor cells was produced in autografted recipients within 5 months post-transplantation [157]. So far, this technique has been successful only when aggregates were made with the mixture of donor testicular cells and cells harvested from testes of inbred recipient strains [158], when immune-deficient recipients (rag1 mutant) were employed [156], or when autografts were carried out [157]. The recipients allografted with donor testicular fragments showed no donor germ cells in where tissue fragments were transplanted after 9 weeks, indicating that donor cells were rejected by the host immune system [157]. Immunosuppressants such as tacrolimus and cyclosporine have failed to suppress the immune rejection toward allogeneic donor testicular cells [159]. Further studies are required for the successful protection of donor tissues from the host immune system since the grafted tissues were degraded even in the immunosuppressants treated recipients. On the other hand, in vivo propagation of donor cells may also be achieved by transplantation into recipients at an early developmental stage when the immune system is not mature yet [57]. When serial transplantations of limited donor cells into larvae that can support the survival and proliferation of donor germ cells are carried out, it would propagate and increase the desired donor germ cells. For this approach, the contamination with the recipient’s germ cells will need to be prevented by employing germ cell-deficient larvae such as germ cell-less hybrids or Dnd-knockdown/knockout fish.
In vitro propagation of germ cells has been attempted in various species. PGCs, spermatogonia, and oogonia could be maintained for several months in vitro, showing colony formation and proliferation. The cultured germ cells could also colonize and differentiate into gametes in the recipient’s gonads after transplantation [160]. However, only a few studies showed a significantly increased number of GSCs after culture. A 3-fold increase of spermatogonia was observed in zebrafish after 20 days of culture, using the medium with supplements including 2-mercaptoethanol, embryonic extract, mammalian growth factors, and serum [158]. Notably, in rainbow trout, a 37.8-fold increase in the number of cells was achieved by in vitro culture of spermatogonia together with rainbow trout Sertoli cell feeders in TS medium-3 for 28 days. During in vitro culture, the characteristics of spermatogonia were maintained, demonstrated by transplantation assay [147]. Considering that germ cells from other salmonids could be nursed in the microenvironmental conditions supplied by rainbow trout recipients after transplantation [2,19,62,86,150], this culture system is probably able to apply to other salmonids or extend to other fish to propagate donor germ cells. Further studies on enhancing proliferation, maintaining GSC characteristics, and prolonging the culture period without causing GSC differentiation would enable a sufficient supply of donor cells for germ cell transplantation.
Collectively, the in vivo or in vitro germ cell propagation will facilitate securing abundant donor cells for transplantation and obtaining more recipients successfully carrying donor-derived gametes, which would be beneficial for surrogate offspring production as well as the selective breeding with donors that can provide only limited donor cells.
5.3. Enhancing Transplantation Efficiency with Vulnerable Recipients during Early Development
Some fish species have low survival and high susceptibility to physical stresses during early development, which can cause low success rates after embryo manipulation and germ cell transplantation. Thus, considerable efforts will be required to obtain enough recipients carrying donor-derived gametes in these species. Given the circumstances, adult recipients can be an alternative option instead of larva recipients. Zhou et al. [148] reported that transplanted spermatogonial stem cells from olive flounder, summer flounder (Paralichthys dentatus), or turbot (Scophthalmus maximus) could colonize recipient gonads and differentiate into functional sperm when triploid olive flounder larvae were used as recipients. Even though at least 500 recipients were transplanted with donor germ cells of each species, only limited recipients (2.2 to 6.8%; estimated) carried donor-derived gametes at 2 to 3 years after transplantation due to their low survival during early development. By contrast, when allogeneic transplantation was conducted using adult male olive flounder co-treated with high temperature and busulfan, all recipients survived, and 11.7% of total recipients produced donor-derived functional sperms within 10 months after transplantation [72]. Because GAT had higher success and survival rates and a shorter generation time than GPT, GAT would be a superb option in these species and other species that encounter the same challenges of low survival rate during early development. Moreover, with the optimized transplantation conditions and appropriate sterile recipients, it is expected that enhanced efficiency and a higher proportion of donor-derived offspring would be achieved.
5.4. Choice of Compatible Recipient Species for Xenotransplantation
The appropriate recipients with extraordinary abilities to support the survival, proliferation, and maturation of donor cells and improve productivity by reducing rearing costs for broodstock management are critical for applying xenotransplantation in aquaculture. In salmonids, the frequently used recipient species are rainbow trout and masu salmon, in which sperm and eggs of Atlantic salmon, rainbow trout, or Chinook salmon were successfully produced [38,146,150]. Goldfish (Carassius auratus) are also considered to have the capability to produce gametes of Cyprinid donor fish [29].
Besides those successful cases, xenotransplantations have been attempted in many other species. Of those, testicular germ cells from Pacific bluefin tuna or Southern bluefin tuna were transplanted into the blue drum, chub mackerel, Eastern little tuna, yellowtail kingfish, or hybrid mackerel successfully colonized recipients’ gonads. In sturgeons, testicular or ovarian germ cells obtained from American peddle fish (Polyodon spathula), Chinese sturgeon (Acipenser sinensis), Dabry’s sturgeon (Acipenser dabryanus), or Siberian sturgeon were able to colonize gonads of Dabry’s sturgeon or sterlet recipients after transplantation. Yet, the production of donor-derived gametes has not been reported in those species (Appendix A, Table A3). Meanwhile, in xenotransplantations between fish with far phylogenetic distances (greater than 200 million years), PGCs from Japanese eel (Anguilla japonica) and sturgeon could be incorporated into zebrafish and goldfish gonads, respectively. However, the production of donor-derived gametes was not reported [161,162]. These results may imply a well-conserved PGC migration mechanism and pathway but incompatible gonadal microenvironments among fish, making it challenging in surrogate offspring production through xenotransplantation among the fish with a far phylogenetic distance.
On the other hand, germ cells from goldfish and loach could proliferate and differentiate into functional sperm in zebrafish recipients with more than 100 million years of phylogenetic distance (MYD) from donors. In contrast, the ovaries of recipients did not mature and produce eggs [1,16]. Productions of donor-derived sperm have been achieved by xenotransplantation within the same genus, family, or order [1]. The spermatogenesis of transplanted Jundia catfish (Rhamdia quelen; Order Siluriformes) germ cells was observed even in the gonads of Nile tilapia (Order Cichliformes) that has 229.9 MYD from Jundia catfish [1,163]. Different from male recipients, the evolutionary distance seems strongly associated with the supporting capacity of female recipients. The most distant species that produced donor eggs through xenotransplantation were goldfish and common carp (Cyprinus carpio), with only 34 MYD from each other [29]. Thus, the successful spermatogenesis of donor germ cells in male recipients does not guarantee functional oogenesis in female recipients of the same species, indicating that a more compatible ovarian microenvironment may be required for successful female xenotransplantation.
In addition, the property of eggs produced by xenogeneic recipients was more close to the egg property of the recipients rather than that of the donors. The size of pearl daino’s eggs produced by zebrafish recipients (705.6 μm) resembled that of zebrafish eggs (699.3 μm), while pearl danio showed the egg diameter of 815.9 μm [16]. In puffers, the eggs of tiger puffer produced by grass puffer had clear chorions and a diameter of 8.8 mm, which were similar to that of grass puffer’s eggs but much different from tiger puffer’s eggs that have milky chorions and a diameter of 12.6 mm [120,127]. More importantly, regardless of recipients (triploid or Dnd-knockdown treated), the eggs produced by most female grass puffer recipients showed poor hatching rates (lower than 25%), implying the challenges of maintaining egg quality in recipients’ ovaries. Considering that eggs produced by xenotransplantation between goldfish and common carp (34 MYD difference), and rainbow trout and masu salmon (14.2 MYD difference) showed comparable hatching rates with that of control [29,124], the low hatching rates from the xenotransplantation between tiger puffer and grass puffer (4.2 MYD difference) suggests that the egg quality produced by xenogeneic recipients is associated with maternal contribution process such as vitellogenesis and choriogenesis rather than the phylogenetic distance.
Thus, unless planning to use a verified combination of donors and recipients, the preliminary investigation of supporting capacity is necessary prior to selecting recipient species, especially when surrogate egg production is crucial. Meanwhile, body size, generation time, spawning frequency, susceptibility to stress, and other physiological and economic characteristics of recipients should also be considered to have benefits of germ cell transplantation.
6. Advanced Applications of Germ Cell Transplantation
6.1. Production of Mono-Sex Populations
Due to characteristic differences between male and female fish, the mono-sex population is beneficial for commercial aspects in various species. For example, the female seedlings of Atlantic salmon [164], olive flounder [165], and rainbow trout [166] are preferred in aquaculture as they either grow faster than males, or males generally reach sexual maturity earlier than females, resulting in the loss of growth potential and flesh quality [167]. In sturgeons, females are more valuable owing to caviar in their gonads. In contrast, some male-biased populations are favorable because of their growth performance or fancy appearances, such as Nile tilapia or ornamental fish [168,169]. Accordingly, diverse methods have been attempted to produce mono-sex populations in various species.
Rearing conditions, including high temperature, hypoxia, low pH, or stocking density, affected male or female-biased populations [170]. Manipulating rearing conditions is considered an ecologically friendly approach to producing mono-sex populations on a commercial-scale when environmental factors are critical for the sex-determination of fish. However, the sensitivity to environmental factors varies among fish species depending on their sex-determination mechanism. Regulating rearing conditions are less-effective for controlling the sex ratio for the species whose sex is mainly determined by genotype, such as rainbow trout [171]. For instance, in rainbow trout, high temperature affected sex ratio, but the treated populations were male- or female-biased depending on broodstock [172]. In addition, stressful conditions such as high temperature and high density compromise animal welfare [173].
Unlike environmental factors, the treatment of sex reversal chemicals, including sex steroid hormones, inhibitors, and antagonists, can effectively drive fish to male- or female-biased populations regardless of their sex-determination mechanism [174]. For instance, in rainbow trout, nearly 100% sex-reversed male population was obtained by treating 17α-methyltestosterone [175]. However, since increasing concerns associated with the adverse effects of sex steroids on the environment and humans, the regulations regarding sex steroids in aquaculture have become strict. For example, in the US, the use of 17α-methyltestosterone has been controlled by the Food and Drug Administration Investigational New Animal Drug (INAD) program (INAD #11-236 and #8557), which allows the use of this hormone only for limited species such as Nile tilapia, Atlantic salmon, and rainbow trout. Therefore, alternative approaches to producing a mono-sex population are needed for sustainable aquaculture.
Germ cell transplantation is one of the potential alternatives. By transplanting ovarian germ cells that carry XX chromosomes into sterile male recipients, milt containing only X sperm can be obtained and used to produce all-female fish [28,72]. On the other hand, one-quarter of YY males can be obtained from fertilizing normal X or Y sperm with X or Y eggs produced by female recipients that received testicular germ cells. Accordingly, all-male populations can be produced by mating YY males with XX females [176,177]. In fish that possess ZZ/ZW sex-determination system, the all-male or -female populations can be generated by conducting the above procedures reversely. In particular, when using sterile recipients (see Section 4.4), efficient production of 100% donor-derived all-male or -female offspring would be possible without using stressful rearing conditions or sex reversal chemicals, contributing to sustainable aquaculture.
6.2. Production of Sterile Fish through Transplantation of Germ Cells Carrying Mutated Somatic Genes
Reproductively sterile fish have advantages for aquaculture operations. First, sterility prevents sexual maturation that hinders growth and diminishes product value. Fish devote energy and deposit nutrition into gonads during sexual maturation, leading to ineffective feed conversion [178]. In addition, sexual maturation may hinder growth potential, resulting in smaller sizes [179,180] and ruin flesh quality and flavor [181,182]. Therefore, the prevention of sexual maturation is crucial in some commercial fish species, such as Arctic charr (Salvelinus alpinus) [183] and Atlantic salmon [180]. Second, genetic containment can be accomplished with sterile fish that prevent polluting wild populations’ genetic pool when selectively bred or even genetically modified farmed fish escape. Especially, sterilization is vital for containing transgenics and genome-edited mutants for their commercial applications [184,185]. Third, sterility is a means for producers to protect valuable strains from unauthorized propagation and unexpected technological leakages.
Sterility can be induced in various ways, including interspecific hybridization [27,69,186], triploidization [108,110,112,115], irradiation [187,188], dnd-knockout [101,189], or Dnd-knockdown by dnd-MO immersion [125]. Interspecific hybridization may not be practical when hybrids themselves are not commercially valuable. Additionally, hybrids’ reproductive phenotypes are highly variable depending on the combination of species/sex (see Section 4.4.1), limiting their applicability in aquaculture. For triploidization, the induction efficiency depends on species and methods. Triploid fish may produce functional gametes in some species (see Section 4.4.2), raising the risks of contaminating the genetic pool of the wild populations or unexpected leakages of valuable strains. Moreover, triploid fish may have low early survival, high deformity, and different behavioral and physiological characteristics from diploids [190], which might not be preferred in some cases. Although irradiation and dnd-MO immersion are useful methods to produce sterile diploids, avoiding undesired phenotypes of hybrids or triploids, the optimized condition is required to achieve 100% sterility [125,188]. Sterility can also be induced by breeding mutants such as dnd-knockout fish (see Section 4.4.4). Still, the limitation of this approach is that only 25% of sterile offspring can be obtained from heterozygous parents [101,189].
On the contrary, 100% sterile offspring can be obtained through transplantation technology in cases of sterility caused by mutated somatic genes essential for gonad development. In this approach, sterile wild-type recipients are employed to support mutant germ cell development. For example, sterile female homozygous follicle-stimulating hormone receptor (fshr) mutants could continuously be produced by germ cell transplantation in medaka. Donor cells carrying XX chromosomes were collected from homozygous fshr mutants and transplanted into sterile hybrid recipients. The resultant female recipients carrying mutant donor X eggs were mated with sex-reversed homozygous mutant fertile neo-male medaka, producing all-female sterile mutants [144]. As the mutation of fshr could induce sterility in other fish species [191], the fshr mutant model can potentially be useful for producing sterile fish in other species with male or female heterogamety sex-determination system. Besides the fshr knockout model, infertility was observed in male or female fish that harbor other mutated genes, including gonadal soma-derived factor [192], estrogen receptors [193], luteinizing hormone [194], or luteinizing hormone receptor [191], suggesting a broader potential of developing additional models for producing sterile fish. Together, germ cell transplantation with sterile wild-type or hybrid recipients would help the continuous production of homozygous sterile fish of these mutants for commercial purposes.
7. Future Perspectives
Since the first report of germline chimeric fish made by blastomere transplantation [6], many methods have been developed to generate germline chimeras in various species with diverse transplantation techniques. While germ cell transplantation technology has a promising potential to enhance aquaculture productivity and mitigate reproductive bottlenecks, its procedures involve many steps, including donor cell isolation, recipient preparation, and transplantation that require special apparatus, skills, and labor, which translate into substantial costs in its commercial applications. Therefore, its application needs to have unique and more significant benefits than the general breeding process can offer to gain cost-effective advantages. Notably, the commercially valuable species have apparent difficulties in broodstock management and/or seed production, such as bluefin tunas and sturgeons. Once the appropriate and effective recipients harboring significant benefits in reproducing desired donor species have been identified and established for each valuable species, the surrogate production model would be in demand by the aquaculture industry. Moreover, when combined with approaches for producing mono-sex populations or sterile fish, germ cell transplantation technology will provide a more efficient and ecologically friendly way to produce valuable seedlings. In particular, since the generation of 100% sterile mono-sex fish harboring mutated somatic genes is only achievable through germ cell transplantation, it is a unique opportunity to demonstrate this technology in commercial operations. Furthermore, with cryopreservation, transplantation technology would enable more practical and secure broodstock management.
To maximize the effectiveness of transplantation, adult recipients seem superior to embryo or larva recipients because it can significantly increase recipient survival rates and reduce the generation time of target species. Thus, as long as sufficient donor cells and sterile adult recipients possessing a supporting capacity for donor germ cells are available, adult recipients will need to be prioritized. Additionally, developing in vitro culture systems for germ cells would be one of the critical factors that can enhance the applicability of germ cell transplantation in adult recipients. In vitro propagation of germ cells will facilitate acquiring abundant donor cells, thereby obtaining enough recipients with higher transplantation success rates and reduced animal sacrifice. Mainly, this will be valuable for accelerating the selective breeding process with individuals carrying fewer germ cells.
As discussed in Section 4, transplantation success rates are highly variable, and many factors are involved in accomplishing successful transplantation. Therefore, further studies for optimizing transplantation procedures will be required to achieve a stable and improved efficiency for aquaculture application. A suggested model to enhance the advantages and practicality of fish germ cell transplantation is illustrated in Figure 2. Taken together, germ cell transplantation technology can be utilized for various purposes in aquaculture. With suitable recipients, optimized transplantation conditions, and related advanced technologies, it will solve several reproductive problems and improve productivity across multiple valuable commercial species.
Figure 2.
A suggested model to enhance the advantages and practicality of fish germ cell transplantation. Red bolds represent the benefits of the step or output. Red italics in brackets indicate the anticipated time for the step.
Appendix A
Table A1.
Blastula cell transplantation in fish.
| Donor | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells |
% of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) |
% of Donor-Derived Offspring |
Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Cells | Species | Fish | Total | Male | Female | ||||
| C. auratus | All-female (XX) | A lower part of BD | Hybrid | ♂ C. carpio × ♀ C. auratus, all-male | N/A | N/A | 100 at SM | N/A | Con: 79.9 TP: 72.3 at 4 d |
N/A | [11] |
| WT | BMs | C. auratus langsdorfii | 3N | 3.1 at 4 d | N/A | N/A | N/A | Con: 65.8 CP + TP: 35.6 at 4 d |
N/A | [9] | |
| WT | 50–100 BMs | D. rerio | WT | 7.3 at 24 h | N/A | N/A | N/A | 73.3 at 24 h | N/A | [13] | |
| C. auratus langsdorfii | 3N | A lower part of BD | C. auratus | WT | N/A | N/A | N/A | 100 at 3–4 y | Con: 91.4 TP: 98.4 at 3 d |
3.1–89.3 | [10] |
| D. albolineatus | WT | 50–100 BMs | D. rerio | WT | 6.6 at 24 h | N/A | N/A | N/A | 96.8 at 24 h | N/A | [13] |
| D. rerio | WT or TG:pRSV-LacZ (LacZ/-) | 20–100 BMs | D. rerio | Albino | N/A | 17.9 at 2–3 mo | N/A | N/A | 16.7 at SM | 1.0–40.0 | [6] |
| WT | 50–100 BMs | D. rerio | WT | 9.0 at 24 h | N/A | N/A | N/A | 86.7 at 24 h | N/A | [13] | |
| TG:pvasa-DsRed2-vasa;pβact-EGFP | A whole BD | D. rerio | Dnd-KD | 94.7 at prim-5 St | N/A | N/A | N/A | Con: 62.9 Dnd-KD: 50.3 TP: 26.1 at adult |
N/A | [8] | |
| M. anguillicaudatus | WT | 50–100 BMs | D. rerio | WT | 1.5 at 24 h | N/A | N/A | N/A | 75.8 at 24 h | N/A | [13] |
| O. mykiss | WT, mid-blastula (2.5 d) | ~80 BMs | O. mykiss | WT, early blastula (1.5 d) |
N/A | 31.6 at 2 y | 55.6 at 2 y | 10.0 at 2 y | 8.0 at 2 y | 0.3–14.6 | [7] |
| O. latipes | TG:pLFABP-rfp;pvasa-GFP | 30 BMs | O. latipes | WT | 47.2 at St 18–23 | N/A | N/A | N/A | N/A | 4.3 | [64] |
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | WT | 81.2 at St 18–23 | N/A | N/A | N/A | N/A | 31.4 | ||||
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | Dnd-KD | 81.1 at St 18–23 | 91.4 at SM | N/A | N/A | N/A | 96.5 | ||||
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | γ-irradiated | 79.2 at St 18–23 | N/A | N/A | N/A | N/A | N/A | ||||
3N: triploid, BD: blastoderm, BM: blastomere, Con: control, CP: cryopreserved, Dnd: dead end, KD: knockdown, N/A: not available, SM: sexual maturity, St: stage, TG: transgenic, TP: transplantation, WT: wild-type.
Table A2.
Primordial germ cell transplantation in fish.
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells |
% of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring |
Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age | Cells | Method | Purity | Species | Fish | Age | Total | Male | Female | ||||
| A. japonica | WT | Somito-genesis | 1 PGC | MC | N/A | D. rerio | WT | Blastula | 42.7 at 2 d | N/A | N/A | N/A | Con: 92.7 TP: 92.6 at 2 d |
N/A | [161] |
| C. auratus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | N/A | N/A | Y at 10–12 mo | N | N/A | N/A | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 63.5 at 2 d | N/A | N/A | N/A | 69.0 at 2 d | N/A | [13] | |
| Albino | 10–15 somite St | 1 PGC | MC | N/A | C. auratus | Dnd-KD | Blastula | 42.7 at 3 d | 42.9 at 5 mo–2 y | 62.5 at 5 mo–2 y | 30.1 at 5 mo–2 y | Dnd-KD: 88.3 TP: 84.9 |
92.5–100 | [65] | |
| D. albolineatus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 45.1 at 2 d | 89.5 at SM | 93.8 at SM | 66.7 at SM | Dnd-KD: 59.2 TP: 74.7 at 2 d |
100 | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 40.2 at 2 d | N/A | N/A | N/A | 76.8 at 2 d | N/A | [13] | |
| D. rerio | WT | 10–15 somite St | 1 PGC | FACS | 87.8–100 | D. rerio | Dnd-KD | Blastula | 31.5 at 1 dpt | N/A | N/A | N/A | Dnd-KD: 92.9 Dnd-KD + TP: 81.4 at 1 dpt |
N/A | [17] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 30.0 at 2 d | N/A | N/A | N/A | 89.7 at 2 d | N/A | [13] | |
| 21–25 somite St | 10.1 at 2 d | N/A | N/A | N/A | 82.4 at 2 d | N/A | |||||||||
| Prim-5 St | 5.3 at 2 d | N/A | N/A | N/A | 78.4 at 2 d | N/A | |||||||||
| Prim-15 St | 4.5 at 2 d | N/A | N/A | N/A | 87.3 at 2 d | N/A | |||||||||
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 20.7 at prim-5 St | N/A | N/A | N/A | Con: 74.1 Dnd-KD: 59.4 TP: 66.7 |
N/A | [8] | |
| M. anguillicaudatus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | N/A | N/A | Y at 10–12 mo | N | N/A | N/A | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 43.1 at 2 d | N/A | N/A | N/A | 85.7 at 2 d | N/A | [13] | |
| O. masou | WT | 40 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 24.4 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] |
| O. mykiss | TG:pvasa-GFP | 35 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | 2.5 d | 0 at 30 dpt | N/A | N/A | N/A | 26 at 30 dpt | ♂: 3.9 ♀: 4.2–6.1 |
[15] |
| 35 d | 6 d | 0 at 30 dpt | N/A | N/A | N/A | 62 at 30 dpt | |||||||||
| 35 d | 35 d | 21.6 at 30 dpt | 15.4 at 1–2 y | 16.7 at 1 y | 14.3 at 2 y | 94 at 30 dpt | |||||||||
| 35 d | 35 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 35 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 35 d | 0–10 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 35 d | 40 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 40 d | 0–10 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 40 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 35 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| TG:pvasa-GFP | 35 d | 20 PGCs | MC | N/A | O. masou | WT | Newly hatched | 16.7 at 30 dpt | N/A | 13.5 at 1 y | Y at 17 mo | N/A | ♂: 0.4 | [154] | |
| WT | 32 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 12.3 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] | |
| TG:pvasa-GFP | 30 dpf | 15–20 PGCs | MC | N/A | O. mykiss | WT | 32–34 dpf | Fresh: 12.5 CP (d1): 10.1 at 30 dpt |
Fresh: 10.9 CP (d1): 8.3 at 3 y |
Fresh: 10.3 CP (d1): 7.8 at 3 y |
Fresh: 12.1 CP (d1): 9.1 at 3 y |
Fresh: 98.5 CP (d1): 86.5 at 30 dpt |
Fresh ♂: 0.2–16.3 Fresh ♀: 0.2–7.6 CP (d1) ♂: 2.0–13.5 CP (d1) ♀: 0.1–3.3 |
[53] | |
| S. trutta | WT | 40 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 9.4 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] |
| Hybrid |
H. huso × A. ruthenus |
Late-neural St | 1 PGC | MC | N/A | C. auratus | WT | Blastula | 9.1 at 3 d | N/A | N/A | N/A | 95.7 at 3 d | N/A | [162] |
Asterisk: estimated average value from the related graphs (no numerics presented), Con: control, CP: cryopreserved, Dnd: dead end, dpt: day post-transplantation, FACS: fluorescence-activated cell sorting, KD: knockdown, MC: manually collected under a fluorescence microscope, N: no (failed, no numerical data presented), N/A: not available, PGC: primordial germ cell, SM: sexual maturity, St: stage, TG: transgenic, TP: transplantation, Y: yes (succeeded, no numerical data presented), WT: wild-type.
Table A3.
Gonadal germ cell transplantation into the peritoneal cavity of larvae in fish.
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells |
% of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age (Size) | Cells | Method | Purity | Species | Fish | Age (Size) | Total | Male | Female | ||||
| A. baerii | WT | 4 y | TCs | PDGC | 79.4 | A. ruthenus | WT | 1 wph | 60.0 at 90 dpt | N/A | N/A | N/A | Con: 96.7 TP: 95.8 |
N/A | [39] |
| WT | 4 y | TCs | N/C | N/A | A. ruthenus | WT | Newly hatched | Fresh: 55.0 CP: 65.0 at 90 dpt |
N/A | N/A | N/A | N/A | N/A | [195] | |
| WT | 4 y | OCs | PDGC | 70.8 | A. ruthenus | WT | 1 wph | 60.0 at 90 dpt | N/A | N/A | N/A | Con: 96.7 TP: 95.8 |
N/A | [39] | |
| WT | 4 y | OCs | N/C | N/A | A. ruthenus | WT | Newly hatched | Fresh: 70.0 CP: 55.0 at 90 dpt |
N/A | N/A | N/A | N/A | N/A | [195] | |
| A. dabryanus | WT | 2 y | 50,000 TCs | N/C | N/A | A. dabryanus | WT | 7–8 dph | 70.0 at 51 dpt | N/A | N/A | N/A | Con: 86.2 TP: 76.7 at 51 dpt |
N/A | [196] |
| A. sinensis | WT | 11.5 y | 50,000 OCs | N/C | N/A | A. dabryanus | WT | 7–8 dph | 20.0 at 51 dpt | N/A | N/A | N/A | Con: 86.2 TP: 68.6 at 51 dpt |
N/A | [196] |
| C. carpio | WT | 2 y | 30,000–50,000 TCs | PDGC | N/A | C. auratus | Dnd-KD | 7 d | 62.5 at 1 mo | 43.7 at 3 y | 32.4 at 3 y | 11.2 at 3 y | Con: 74 Dnd-KD: 79 Dnd-KD + TP: 72 at 18 mpf |
100 | [29] |
| D. rerio | TG:ppiwil1-DsRed | 2–3 mo | 10–60 SG | PDGC | N/A | D. rerio | Dnd-KD | 2 w | N/A | N/A | Cult (3 w): 19 Cult (6 w): 18 at SM |
N/A | Cult (3 w): 72.2 Cult (6 w): 64.9 at SM |
100 | [126] |
| TG:pvasa-EGFP | Adult | 3000 GFP+ TCs | N/C | N/A | D. rerio | WT | 9–10 d | 6.7 at 50 dpt | N/A | 6.7 at SM | N/A | N/A | 3.9–7.3 | [101] | |
| Dnd-KO | 5.0 at 50 dpt | N/A | 5.0 at SM | N/A | N/A | 100 | |||||||||
| TG:pvasa-EGFP | 2 mo | 3000–5000 TCs | N/C | N/A | D. rerio | 3N | 7 d | 53.2 at adult | N/A | 43.5 at adult | N/A | 2N Con: 83.3 3N Con: 75.6 3N TP: 68.9 at adult |
100 | [145] | |
| TG:pvasa-EGFP or TG:pβact-YFP |
3–6 mo | 3000 GFP+ TCs | N/C | N/A | D. rerio | WT | 7 d | Fresh: 31.1 CP: 24.4 VF: 22.2 at 50 dpt |
N/A | N/A | N/A | Contro: 80 TP: 85 at 50 dpt |
N/A | [135] | |
| Dnd-KD | Fresh:58.3 CP: 47.4 VF: 50.0 at 50 dpt |
N/A | 43 at 6 mo | N/A | N/A | 100 | |||||||||
| TG:pvasa-DsRed2- vasa;pβact-EGFP |
3 mo | OCs | PDGC | N/A | Hybrid | ♂ D. albolineatus × ♀ D. rerio |
2 w | 20.0 at 8 w | 17.9 at 6 mo | 17.9 at 6 mo | 0 at 6 mo | 52.7 at 6 mo | 100 | [69] | |
| TG:pziwi-Neo;pziwi-DsRed | 10–12 w | 20–40 FGSCs | PDGC | N/A | D. rerio | Dnd-KD | 2 w | N/A | N/A | Cult (3 w): 20.0 Cult (6 w): 16.0 at SM |
N/A | Cult (3 w): 73.5 Cult (6 w): 71.7 at SM |
100 | [123] | |
| TG:pvasa-EGFP | 2 mo | 500 OCs | N/C | N/A | D. rerio | 3N | 7 d | 43.6 at adult | N/A | 34.5 at adult | N/A | 2N Con: 83.3 3N Con: 75.6 3N TP: 61.1 at adult |
100 | [145] | |
| N. mitsukurii | WT | 3 mo | 10,000 TCs | N/C | N/A | S. japonicus | WT | 5 d | 33.5 at 21 dpt | N/A | N/A | N/A | 20.3 at 21 dpt | N/A | [99] |
| 7 d | 70.0 at 21 dpt | N/A | N/A | N/A | 20.6 at 21 dpt | N/A | |||||||||
| 9 d | 4.4 at 21 dpt | N/A | N/A | N/A | 27.6 at 21 dpt | N/A | |||||||||
| WT | 3 mo | 3000 TCs | FACS | Unsort: 18.8 Sort: 94.0 |
N. mitsukurii | WT | 12 d | Unsort: 7.6 Sort: 60.0 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [19] | |
| TG:pHSC-GFP | 3 mo | 10,000 TCs | N/C | N/A | N. mitsukurii | 3N (<100% incidence) | 12 dph | 2N: 63.3 3N: 56.0 at 18 dpt |
2N: 6.1 3N: 31.4 at 6 mo |
2N: 5.3 3N: 36.8 at 6 mo |
2N: 6.8 3N: 28.9 at 6 mo |
41.1 at 18 dpt | 2N ♂: 6.8–89.2 3N ♂: 100 2N ♀: 2.2–25.0 3N ♀: 100 |
[63] | |
| TG:pHSC-GFP | 3 mo | 10,000 TCs | N/C | N/A | N. mitsukurii | WT | 12 d | 58.3 at 2 wpt | N/A | N/A | N/A | 68.1 at 2 wpt | N/A | [27] | |
| Hybrid | ♂ P. argentata × ♀ N. mitsukurii, 3N | 12 d | 58.6 at 2 wpt | 34.4 at 6 mo | 34.4 at 6 mo | 0 at 6 mo | 34.7 at 2 wpt | ♂: 100 | |||||||
| O. masou | WT | 12–13 mo | 3000 TCs | FACS | Unsort: 16.4 Sort: 75.6 |
O. mykiss | WT | Newly hatched | Unsort: 0–25 * Sort: 80.0 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [19] |
| WT, black | 8 mo | 50,000 TCs | N/C | N/A | O. masou | 3N | 40–42 d | Fresh: 50–60 * CP: 58.2 at 50 dpt |
CP: 39.1 at 2 y | CP: 43.5 at 2 y | CP: 34.8 at 2 y | N/A | 100 | [28] | |
| WT, black | 8 mo | 50,000 OCs | N/C | N/A | O. masou | 3N | 40–42 d | Fresh: 40–60 * CP: 41.2 at 50 dpt |
CP: 25.8 at 2 y | CP: 29.3 at 2 y | CP: 23.1 at 2 y | N/A | 100 | [28] | |
| O. mykiss | Albino, TG:pvasa-GFP | 9 mo | 18,000 TCs (10,000 GFP+ cells) | FACS | N/A | O. mykiss | WT | Newly hatched | ♂: 38.8 ♀: 57.8 at 2 mpt |
N/A | 50 at 1 y | 40 at 2 y | N/A | ♂: 5.5 (0.2–40.5) ♀: 2.1 (0.1–9.9) |
[37] |
| TG:pvasa-GFP | Adult | TCs | N/C | N/A | O. masou | 3N | Newly hatched | N/A | N/A | 34.5 at 2 y | 50.0 at 17 mo; 10.0 at 2–3 y | N/A | ♂: 100 ♀: 100 |
[146] | |
| TG:pvasa-GFP | 8–10 mo | 10,000 TCs | DP | 95–99 | O. mykiss | WT | Newly hatched | Sort: 77.7 Sort + Cult (1 mo): 52.2 at 30 dpt |
N/A | N/A | N/A | N/A | N/A | [75] | |
| TG:pvasa-GFP | 16 mo | 3000 TCs | FACS | N/A | O. mykiss | WT | Newly hatched | Sort-A: 51.0 Sort-B: 0 at 60 dpt |
N/A | N/A | N/A | N/A | N/A | [67] | |
| 7 mo | 3000 ASG | N/C | N/A | 53.6 at 60 dpt | N/A | N/A | N/A | N/A | N/A | ||||||
| WT | 8–12 mo | 3000 TCs | FACS | N/A | O. mykiss | WT | Newly hatched | Unsort: 23.0 Sort: 78.3 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [19] | |
| TG:pvasa-GFP | 8–12 mo | Unsort: 35.9 Sort: 93.2 |
Unsort: 29.0 Sort: 82.0 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | |||||||
| TG:pvasa-GFP | 16 or 24 mo | Sort: 90.0–96.2 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| TG:pvasa-GFP | 11–12 mo | 5000 TCs | DP | >90 | O. mykiss | WT | 25–27 d | Fresh: 59.7 Cult + Sort: 94.5 Cult + Sort + Tryp-2 h: 43.9 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [73] | |
| TG:pMlc2-GFP | 6 mo | 10,000 ASG | PDGC + CE | >90 | O. mykiss | WT | Peri-hatching | 90 at 6 mo | N/A | 90 at 1–2 y | N/A | N/A | 1–56 | [23] | |
| 1 y | >90 | 78 at 6 mo | N/A | N/A | N/A | N/A | N/A | ||||||||
| Mature | >70 | 42 at 6 mo | N/A | N/A | N/A | N/A | N/A | ||||||||
| TG:pvasa-GFP | 11 mo | 500 GFP+ TCs | N/C | N/A | O. mykiss | 3N | 32 d | Fresh: 30–40 * CP (F371): 30–40 * at 50 dpt |
Fresh: 32.4 CP (F371): 28.6 at 2 y |
Fresh: 35.0 CP (F371): 40.0 at 2 y |
Fresh: 29.4 CP (F371): 15.4 at 2 y |
N/A | 100 | [61] | |
| 18 mo | O. mykiss | 32 d | Fresh: 40–60 * CP (F371): 40–60 * at 30 dpt |
N/A | N/A | N/A | N/A | N/A | |||||||
| 11 mo | O. masou | 37 d | Fresh: 20–30 * CP (F371): 20–30 * at 50 dpt |
Fresh: 27.6 CP (F371): 24.0 at 2 y |
Fresh: 33.3 CP (F371): 30.8 at 2 y |
Fresh: 23.5 CP (F371): 16.7 at 2 y |
N/A | 100 | |||||||
| TG:pvasa-GFP | 8 mo | 30,000 TCs | N/C | N/A | O. masou | WT | 38–40 d | 63.2 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [124] | |
| Dnd-KD | 70 at 20 dpt | 54.5 at 2 y | 46.7 at 2 y | 71.4 at 2 y | N/A | ♂: 100 (6 Inds); 72.3 (1 Ind) ♀: 100 (2 Inds); 21.1 (3 Inds) |
|||||||||
| TG:pvasa-GFP | 9–11 mo | 1000 TCs | FACS (AB No. 80 or No. 95) | Unsort: <20 * Sort (No. 80): 70.7 Sort (No. 95): 80.9 |
O. mykiss | WT | Newly hatched | Unsort: 1.1 Sort (No. 80): 19.2 Sort (No. 95): 18.4 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [20] | |
| TG:pvasa-GFP | 13–14 mo | <2000 TCs | MACS (AB No. 172) | Unsort: 49.8 Sort: 81.7 |
O. mykiss | WT | Newly hatched | Unsort: 10–20 * Sort: 30–40 * at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [62] | |
| TG:pvasa-GFP | 12–15 mo | 15,000 SG | DP | N/A | O. mykiss | 3N | 30 d | Fresh: 60–80 * Cult: 60–80 * at 20 dpt |
Fresh: 89.2 Cult: 65.0 at 1 y |
Fresh: 88.0 Cult: 77.6 at 1 y |
Fresh: 73.3 Cult: 36.8 at 2 y |
N/A | ♂: 100 ♀: 100 |
[147] | |
| TG:pvasa-GFP | 6–9 mo | 15,000 OCs | N/C | N/A | O. mykiss | 3N | 25 d | ♂: 50.0 ♀: 47.1 at 5 mo |
10.0 at 2 y | 15.0 at 2 y | 5.0 at 2 y | N/A | ♂: 100 ♀: 100 |
[21] | |
| TG:pvasa-GFP | 9 mo | <10,000 GPF+ OCs | N/C | N/A | O. mykiss | 3N | 33 d | Fresh: 60–80 * CP: 57.1 at 50 dpt |
Fresh: 25.0 CP: 24.0 at 2.5 y |
Fresh: 30.8 CP: 28.0 at 2.5 y |
Fresh: 18.2 CP: 20.0 at 2.5 y |
N/A | Fresh: 100 CP: 100 |
[56] | |
| TG:pvasa-GFP | 12–14 mo | <10,000 OCs | MACS (AB No. 172) | Unsort: 5.2 Sort: 54.8 |
O. mykiss | WT | Newly hatched | Unsort: 0–20 * Sort: 60–80 * at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [62] | |
| O. nerka | WT | 15 mo | <5000 TCs | MACS (AB No. 172) | Unsort: 16.4 Sort: 68.6 |
O. mykiss | WT | Newly hatched | Unsort: 0–20 * Sort: 40–60 * at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [62] |
| O. niloticus | TG:pβact-EGFP | 5–12 mo | 20,000 TCs | N/C | N/A | O. niloticus | WT | 6 d | 0 at 5 mo | N/A | N/A | N/A | N/A | N/A | [137] |
| 7 d | 6.7 at 5 mo | 37.5 at 1 y | 40.0 at 1 y | 33.3 at 1 y | N/A | 2.4 | |||||||||
| 8 d | 0 at 5 mo | N/A | N/A | N/A | N/A | N/A | |||||||||
| O. latipes | TG:pvasa-GFP | 2–4 mo | 15,000 TCs | N/C | N/A | O. latipes | WT | 7 d | >50 * at 1 mpt | N/A | N/A | N/A | 20.0 at 1 mpt | N/A | [55] |
| 15,000 TCs | WT | 11 d | >50 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 14 d | <50 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 19 d | 0 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 23 d | 0 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 300 TCs | WT | 11 d | 10 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 1000 TCs | WT | 11 d | 10 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 3000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 10,000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 30,000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 15,000 TCs | 3N | 11 d | N/A | Fresh: 63.6 CP: 70.0 at 2–3 mo |
N/A | N/A | N/A | 100 | |||||||
| Wnt4b (-/-) mutant | - | 15,000 TCs | N/C | N/A | O. latipes | 3N | 11 d | N/A | CP: 83.3 at 2–3 mo | N/A | N/A | N/A | 100 | [55] | |
| Tokyo-medaka (endangered) | 1 y | TG:pvasa-GFP, 3N | N/A | CP: 63.9 at 2–3 mo | N/A | N/A | N/A | 100 | |||||||
| Kaga (inbred) | - | TG:pvasa-GFP, 3N | N/A | CP: 58.6 at 2–3 mo | N/A | N/A | N/A | 100 | |||||||
| Fshr mutant (-/-), XX male | 3 mo | 3000–9000 TCs | N/C | N/A | Hybrid | ♂ O. curvinotus × ♀ O. latipes or ♂ O. latipes × ♀ O. curvinotus |
Newly hatched | N/A | N/A | N/A | 35.3 at 3 mo | N/A | 100 | [144] | |
| TG:pvasa-GFP | 2 mo | 12,000 TCs | N/A | N/A | N/A | 48.3 at 3 mo | N/A | N/A | |||||||
| WT | Adult | 1000 OCs | PDGC + DP | N/A | O. latipes | WT | 11 d | [Cult (10 d)] Con: 15.0 On pDA: 25.0 On pDA/PLL: 0 at 20 d |
N/A | N/A | N/A | 50.0–80.0 at 20 d | N/A | [197] | |
| WT | Adult | 1000 OCs | PDGC + DP | N/A | O. latipes | WT | 11 d | Con: 14.3 Sort: 32.1 at 20 d |
N/A | N/A | N/A | 57.1–72.4 at 20 d | N/A | [70] | |
| P. dentatus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 17 dph | 100 at 14 dpt | N/A | Merged: 100 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 4.0 at 2 y |
Merged: Y | [148] |
| 20 dph | 90.0 at 14 dpt | N/A | N/A | ||||||||||||
| P. olivaceus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 16 dph | 100 at 14 dpt | N/A | Merged: 100 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 6.8 at 2 y |
Merged: Y | [148] |
| 19 dph | 100 at 14 dpt | N/A | N/A | ||||||||||||
| 22 dph | 100 at 14 dpt | N/A | N/A | ||||||||||||
| P. spathula | WT | 2.5 y | 50,000 TCs | PDGC | N/A | A. dabryanus | WT | 7–8 dph | 80.6 at 2 mo | N/A | N/A | N/A | Con: 76.5 TP: 68.5 at 2 mo |
N/A | [198] |
| S. salar | WT | 1 y | 2000–5000 TCs | N/C | N/A | O. mykiss | 3N (Shasta/ Shasta/albino) |
Newly hatched | 61.1 at 4 wpt | 11.0 at 1–2 y | 10.0 at 1–2 y | 12.1 at 2 y | N/A | ♂: 100 ♀: N/A |
[150] |
| S. trutta | WT | 9–12 mo | 4000–5000 TCs | FACS (AB No. 95) | Unsort: 21.2 Sort: 80.6 |
O. mykiss | WT | Newly hatched | Con: 15.8 Sort: 34.7 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [86] |
| S. leucomaenis | WT | 12–13 mo | 3000 TCs | FACS | Unsort: 10.1 Sort: 79.9 |
O. mykiss | WT | Newly hatched | Unsort: 0 Sort: 48.8 at 20 dpt |
N/A | N/A | N/A | N/A | N/A | [19] |
| S. maximus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 15 dph | 60.0 at 14 dpt | N/A | Merged: 40.0 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 10.0 at 2 y |
Merged: Y | [148] |
| 18 dph | 40.0 at 14 dpt | N/A | N/A | ||||||||||||
| 21 dph | 30.0 at 14 dpt | N/A | N/A | ||||||||||||
| S. quinqueradiata | WT | 10 mo | 20,000 TCs | N/C | N/A | S. quinqueradiata | WT | 6 dph | 100 at 28 dpt | N/A | N/A | N/A | 8.0 at 28 dpt | N/A | [155] |
| 8 dph | 100 at 28 dpt | N/A | N/A | N/A | 10.9 at 28 dpt | N/A | |||||||||
| 10 dph | 85.7 at 28 dpt | N/A | N/A | N/A | 15.9 at 28 dpt | N/A | |||||||||
| 12 dph | 85.7 at 28 dpt | N/A | N/A | N/A | 29.6 at 28 dpt | N/A | |||||||||
| Merged | N/A | N/A | 100 at 1.5–2.5 y | 100 at 2.5 y | 5.08 at SM | ♂: 66.6 (19.6–98.8) ♀: 63.2 (17.0–97.5) |
|||||||||
| T. rubripes | WT | 1 y | 4000–6000 TCs | N/C | N/A | T. alboplumbeus | 3N (<100% incidence) | 1 dph | 50 at 4 wpt | N/A | 38.3 at 11 mo | 31.3 at 2 y | 47 at 72 wpt | ♂: 100 ♀: 5–95 |
[138] |
| 5 dph | 33.3 at 4 wpt | N/A | 23.7 at 11 mo | 23.1 at 2 y | 41.4 at 72 wpt | ||||||||||
| WT | 10–12 mo | 5000 TCs | N/C | N/A | T. alboplumbeus | 3N (<100% incidence) | 1 dph | Fresh: 39.1 CP: 38.9 at 40 dph |
N/A | [CP] 2N: 36.4 3N: 64.2 at 10 mph |
[CP] 2N: 0 3N: 1.6 at 2 y |
Con: 41.4 Fresh: 21.8 CP: 14.5 at 40 dph |
2N ♂: 3.2–87.5 (3 Inds) 2N ♀: 0 (10 Inds) 3N ♂: 100 (11 Inds); 62.5 (1 Ind) 3N ♀: 100 (1 Ind) |
[120] | |
| WT | 1 y | 5000 TCs | N/C | N/A | T. alboplumbeus | WT | 1 dph | 40.0 at 40 dph | N/A | N/A | N/A | Con: 23.8 at 40 dph; 45.3 at 12 mph TP: 14.8 at 40 dph |
N/A | [127] | |
| Dnd-KD | 40.5 at 40 dph | N/A | 91.7 at 10 mo | 26.7 at 2 y | Dnd-KD: 20.3 and 35.0 Dnd-KD + TP: 15.8 and 16.9 at 40 dph and 12 mph |
♂: 100 (7 Inds); 12.5–87.5 (2 Inds) ♀: 100 (3 Inds) |
|||||||||
| T. maccoyii | WT | 57.0 kg | TCs | PDGC | N/A | S. lalandi | WT | 6–7 dph | 33.3 at 18 dpt | N/A | N/A | N/A | 9.1 at 64 dph | N/A | [47] |
| 9–10 dph | 37.5 at 18 dpt | N/A | N/A | N/A | 9.2 at 64 dph | N/A | |||||||||
| T. orientalis | WT | 0 y | TCs | N/C | N/A | S. japonicus | WT | 7 d | 0 at 21 dpt | N/A | N/A | N/A | N/A | N/A | [22] |
| 1 y | 5.4 at 21 dpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| 3 y | 0 at 21 dpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| WT | 2–4 y | >10,000 TCs | FACS (AB No. 152) | Unsort: 16.6 Sort: 77.3 |
N. mitsukurii | WT | 12 dph | PDGC Sort (PKH26 labled): 33.3 PDGC Sort (No. 152 AB labeled): 63.3 at 14 dpt |
N/A | N/A | N/A | N/A | N/A | [45] | |
| WT | 3 y | >10,000 TCs | PDGC | N/A | Hybrid | ♂ S. japonicus × ♀ S. australasicus | 10 d | 100 at 14 dpt | N/A | N/A | N/A | N/A | N/A | [48] | |
| WT | 2 y | >30,000 TCs | N/C | N/A | S. japonicus | WT | 7 dph | Y at 14 dpt and 4 mpt | N/A | N/A | N/A | N/A | N/A | [46] | |
| E. affinis | WT | 10 dph | Y at 14 dpt and 3 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
2N: diploid, 3N: triploid, AB: antibody, ASG: type A spermatogonia, Asterisk: estimated average value from the related graphs (no numerics presented), CE: centrifugal elutriation, Con: control, CP: cryopreserved, Cult: cultured, Dnd: dead end, DP: differential plating, dph: day post-hatching, dpt: day post-transplantation, FACS: fluorescence-activated cell sorting, FGSC: female germline stem cell, Ind: individual, KD: knockdown, KO: knockout, MACS: magnetic-activated cell sorting, mpt: month post-transplantation, N/A: not available, N/C: not carried out, OC: ovarian cell, PDGC: Percoll density gradient centrifugation, SG: spermatogonia, SM: sexual maturity, Sort: sorted, TC: testicular cell, TG: transgenic, TP: transplantation, Trip: trypsinized, Unsort: unsorted, VF: vitrified, wph: week post-hatching, wpt: week post-transplantation, WT: wild-type, Y: yes (succeed, no numerical data presented).
Table A4.
Gonadal germ cell transplantation into adult recipients in fish.
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells |
% of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) |
% of Donor-Derived Offspring |
Ref | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age (Size) | Cells | Method | Purity | Species | Fish | Sex | Age (Size) | Total | Male | Female | ||||
| D. rerio | TG:pvasa -EGFP |
Adult | TCs | FACS | Unsort: 10–20 * Sort: 60–70 * |
D. rerio | WT, HT + B treated | ♂ | Adult | 30 at 3 wpt | N/A | N/A | N/A | N/A | N/A | [128] |
| ♀ | Adult | Y at 3 wpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| I. furcatus | WT | 2 y | 2 × 104–1.43 × 106 TCs | PDGC | N/A | I. punctatus | 3N | ♂ | 2 y | 87.5 at 10 mpt |
N/A | Y at 2 ypt | N/A | N/A | Y | [199] |
| N. mitsukurii | TG:pHSC -GFP |
3 mo | 2 × 106 TCs | N/C | N/A | Hybrid | ♂ P. argentata × ♀ N. mitsukurii | ♂ | 6 mo | 37.5 at 6 wpt |
N/A | 10 at 25 wpt | N/A | 77.0 at 1 dpt |
100 | [31] |
| TG:pHSC -GFP |
3 mo | 2 × 106 OCs | N/C | N/A | Hybrid | ♂ P. argentata × ♀ N. mitsukurii, 3N | ♀ | 6 mo | N/A | 33.3 at 9 mpt |
16.6 at 9 mpt | 16.6 at 9 mpt |
75 at mpt |
100 | [104] | |
| O. bonariensis | WT | 4–5 mo | 4 × 104 TCs (for each lobe, surgery) | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♂ | 1 y | 33.3 at 6 wpt |
N/A | 20.0 at 6 mpt | N/A | N/A | 1.2–13.3 | [149] |
| WT | 4–5 mo | 3.75 × 106 TCs | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♂ | 1 y | 80.0 at 8 wpt |
N/A | 17.6 at 7 mpt | N/A | N/A | 12.6–39.7 | [100] | |
| WT | 4–5 mo | 3.75 × 106 Ocs | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♀ | 1 y | 60.0 at 8 wpt |
N/A | N/A | 5.0 at 7 mpt |
N/A | 39.7–52.2 | [100] | |
| O. niloticus | WT | Adult | 1 × 107 TCs | PDGC + DP | N/A | O. niloticus | WT, HT + B treated | ♂ | Adult | 89.5 at 8–9 wpt |
N/A | Y at 9 wpt | N/A | N/A | 6.3 | [32] |
| P. olivaceus | WT | 1 y | OCs | PDGC | Unsort: 37.8 Sort: 83.6 |
P. olivaceus | WT, HT + B treated | ♂ | 2 y | Y at 123 dpt |
N/A | 11.7 at 10 mpt |
N/A | 100 at 10 mpt |
43.0–45.5 | [72] |
| R. quelen | WT | 289 g | 1 × 107 TCs | PDGC + DP | N/A | O. niloticus | WT, HT + B treated | ♂ | 208 g | 100 at 2 mpt |
N/A | N/A | N/A | N/A | N/A | [163] |
3N: triploid, Asterisk: estimated average value from the related graphs (no numerics presented), B: busulfan, DP: differential plating, dpt: day post-transplantation, FACS: fluorescence-activated cell sorting, HT: high temperature, mpt: month post-transplantation, N/A: not available, N/C: not carried out, OC: ovarian cell, PDGC: Percoll density gradient centrifugation, Sort: sorted, TC: testicular cell, Unsort: unsorted, wpt: week post-transplantation, WT: wild-type, Y: yes (succeed, no numerical data presented), ypt: year post-transplantation.
Author Contributions
Conceptualization, J.H.R. and T.-T.W.; reference curation, J.H.R.; writing, J.H.R. and T.-T.W.; review and editing, L.X. and T.-T.W. All authors have read and approved the final manuscript.
Funding
This article was supported by the National Research Foundation of Korea (project number 2019R1A6A3A03033401), China Scholarship Council: Scholarship to support L.X., USDA National Institute of Food and Agriculture Biotechnology Risk Assessment Grant Program competitive grant (project number 2015-33522-24279), and the University of Maryland Baltimore County (project number STRT7TEN-1113).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goto R., Saito T. A State-of-the-Art Review of Surrogate Propagation in Fish. Theriogenology. 2019;133:216–227. doi: 10.1016/j.theriogenology.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizaki G., Yazawa R. Application of Surrogate Broodstock Technology in Aquaculture. Fish. Sci. 2019;85:429–437. doi: 10.1007/s12562-019-01299-y. [DOI] [Google Scholar]
- 3.Braat A.K., Speksnijder J.E., Zivkovic D. Germ Line Development in Fishes. Int. J. Dev. Biol. 1999;43:745–760. [PubMed] [Google Scholar]
- 4.Raz E. Primordial Germ-Cell Development: The Zebrafish Perspective. Nat. Rev. Genet. 2003;4:690–700. doi: 10.1038/nrg1154. [DOI] [PubMed] [Google Scholar]
- 5.Joly J.-S., Kress C., Vandeputte M., Bourrat F., Chourrout D. Irradiation of Fish Embryos Prior to Blastomere Transfer Boosts the Colonisation of Their Gonads by Donor-Derived Gametes. Mol. Reprod. Dev. 1999;53:394–397. doi: 10.1002/(SICI)1098-2795(199908)53:4<394::AID-MRD4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Lin S., Long W., Chen J., Hopkins N. Production of Germ-Line Chimeras in Zebrafish by Cell Transplants from Genetically Pigmented to Albino Embryos. Proc. Natl. Acad. Sci. USA. 1992;89:4519–4523. doi: 10.1073/pnas.89.10.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi Y., Yoshizaki G., Takeuchi T. Production of Germ-Line Chimeras in Rainbow Trout by Blastomere Transplantation. Mol. Reprod. Dev. 2001;59:380–389. doi: 10.1002/mrd.1044. [DOI] [PubMed] [Google Scholar]
- 8.Tzung K.W., Goto R., Saju J.M., Sreenivasan R., Saito T., Arai K., Yamaha E., Hossain M.S., Calvert M.E.K., Orbán L. Early Depletion of Primordial Germ Cells in Zebrafish Promotes Testis Formation. Stem Cell Rep. 2015;4:61–73. doi: 10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusuda S., Teranishi T., Koide N., Nagai T., Arai K., Yamaha E. Pluripotency of Cryopreserved Blastomeres of the Goldfish. J. Exp. Zool. 2004;301A:131–138. doi: 10.1002/jez.a.20017. [DOI] [PubMed] [Google Scholar]
- 10.Yamaha E., Kazama-Wakabayashi M., Otani S., Fujimoto T., Arai K. Germ-Line Chimera by Lower-Part Blastoderm Transplantation between Diploid Goldfish and Triploid Crucian Carp. Genetica. 2001;111:227–236. doi: 10.1023/A:1013780423986. [DOI] [PubMed] [Google Scholar]
- 11.Yamaha E., Murakami M., Hada K., Otani S., Fujimoto T., Tanaka M., Sakao S., Kimura S., Sato S., Arai K. Recovery of Fertility in Male Hybrids of a Cross between Goldfish and Common Carp by Transplantation of PGC (Primordial Germ Cell)-Containing Graft. Genetica. 2003;119:121–131. doi: 10.1023/A:1026061828744. [DOI] [PubMed] [Google Scholar]
- 12.Robles V., Riesco M.F., Psenicka M., Saito T., Valcarce D.G., Cabrita E., Herráez P. Biology of Teleost Primordial Germ Cells (PGCs) and Spermatogonia: Biotechnological Applications. Aquaculture. 2017;472:4–20. doi: 10.1016/j.aquaculture.2016.03.004. [DOI] [Google Scholar]
- 13.Saito T., Goto-Kazeto R., Fujimoto T., Kawakami Y., Arai K., Yamaha E. Inter-Species Transplantation and Migration of Primordial Germ Cells in Cyprinid Fish. Int. J. Dev. Biol. 2010;54:1481–1486. doi: 10.1387/ijdb.103111ts. [DOI] [PubMed] [Google Scholar]
- 14.Saito T., Psenicka M. Novel Technique for Visualizing Primordial Germ Cells in Sturgeons (Acipenser ruthenus, A. gueldenstaedtii, A. baerii, and Huso huso) Biol. Reprod. 2015;93:1–7. doi: 10.1095/biolreprod.115.128314. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi Y., Yoshizaki G., Takeuchi T. Generation of Live Fry from Intraperitoneally Transplanted Primordial Germ Cells in Rainbow Trout. Biol. Reprod. 2003;69:1142–1149. doi: 10.1095/biolreprod.103.017624. [DOI] [PubMed] [Google Scholar]
- 16.Saito T., Goto-Kazeto R., Arai K., Yamaha E. Xenogenesis in Teleost Fish through Generation of Germ-Line Chimeras by Single Primordial Germ Cell Transplantation. Biol. Reprod. 2008;78:159–166. doi: 10.1095/biolreprod.107.060038. [DOI] [PubMed] [Google Scholar]
- 17.Goto-Kazeto R., Saito T., Takagi M., Arai K., Yamaha E. Isolation of Teleost Primordial Germ Cells Using Flow Cytometry. Int. J. Dev. Biol. 2010;54:1487–1492. doi: 10.1387/ijdb.092914rg. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi Y., Yoshizaki G., Kobayashi T., Takeuchi T. Mass Isolation of Primordial Germ Cells from Transgenic Rainbow Trout Carrying the Green Fluorescent Protein Gene Driven by the vasa Gene Promoter. Biol. Reprod. 2002;67:1087–1092. doi: 10.1095/biolreprod67.4.1087. [DOI] [PubMed] [Google Scholar]
- 19.Kise K., Yoshikawa H., Sato M., Tashiro M., Yazawa R., Nagasaka Y., Takeuchi Y., Yoshizaki G. Flow-Cytometric Isolation and Enrichment of Teleost Type A Spermatogonia Based on Light-Scattering Properties. Biol. Reprod. 2012;86:1–12. doi: 10.1095/biolreprod.111.093161. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M., Ichida K., Sadaie S., Miwa M., Fujihara R., Nagasaka Y., Yoshizaki G. Establishment of Novel Monoclonal Antibodies for Identification of Type A Spermatogonia in Teleosts. Biol. Reprod. 2019;101:478–491. doi: 10.1093/biolre/ioz080. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizaki G., Ichikawa M., Hayashi M., Iwasaki Y., Miwa M., Shikina S., Okutsu T. Sexual Plasticity of Ovarian Germ Cells in Rainbow Trout. Development. 2010;137:1227–1230. doi: 10.1242/dev.044982. [DOI] [PubMed] [Google Scholar]
- 22.Yazawa R., Takeuchi Y., Morita T., Ishida M., Yoshizaki G. The Pacific Bluefin Tuna (Thunnus orientalis) dead end Gene Is Suitable as a Specific Molecular Marker of Type A Spermatogonia. Mol. Reprod. Dev. 2013;80:871–880. doi: 10.1002/mrd.22224. [DOI] [PubMed] [Google Scholar]
- 23.Bellaiche J., Lareyre J.J., Cauty C., Yano A., Allemand I., Le Gac F. Spermatogonial Stem Cell Quest: nanos2, Marker of a Subpopulation of Undifferentiated a Spermatogonia in Trout Testis. Biol. Reprod. 2014;90:1–14. doi: 10.1095/biolreprod.113.116392. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S., Kobayashi K., Nishimura T., Higashijima S.I., Tanaka M. Identification of Germline Stem Cells in the Ovary of the Teleost Medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa K., Shikina S., Takeuchi Y., Yoshizaki G. Lymphocyte Antigen 75 (Ly75/CD205) Is a Surface Marker on Mitotic Germ Cells in Rainbow Trout. Biol. Reprod. 2010;83:597–606. doi: 10.1095/biolreprod.109.082081. [DOI] [PubMed] [Google Scholar]
- 26.Sato M., Hayashi M., Yoshizaki G. Stem Cell Activity of Type A Spermatogonia Is Seasonally Regulated in Rainbow Trout. Biol. Reprod. 2017;96:1303–1316. doi: 10.1093/biolre/iox049. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa H., Xu D., Ino Y., Yoshino T., Hayashida T., Wang J., Yazawa R., Yoshizaki G., Takeuchi Y. Hybrid Sterility in Fish Caused by Mitotic Arrest of Primordial Germ Cells. Genetics. 2018;209:507–521. doi: 10.1534/genetics.118.300777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S., Bang W.Y., Yang H.S., Lee D.S., Song H.Y. Production of Juvenile Masu Salmon (Oncorhynchus masou) from Spermatogonia-Derived Sperm and Oogonia-Derived Eggs via Intraperitoneal Transplantation of Immature Germ Cells. Biochem. Biophys. Res. Commun. 2021;535:6–11. doi: 10.1016/j.bbrc.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Franěk R., Kašpar V., Shah M.A., Gela D., Pšenička M. Production of Common Carp Donor-Derived Offspring from Goldfish Surrogate Broodstock. Aquaculture. 2021;534:736252. doi: 10.1016/j.aquaculture.2020.736252. [DOI] [Google Scholar]
- 30.Schulz R.W., de França L.R., Lareyre J.J., LeGac F., Chiarini-Garcia H., Nobrega R.H., Miura T. Spermatogenesis in Fish. Gen. Comp. Endocrinol. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Xu D., Yoshino T., Konishi J., Yoshikawa H., Ino Y., Yazawa R., Dos Santos Nassif Lacerda S.M., De França L.R., Takeuchi Y. Germ Cell-Less Hybrid Fish: Ideal Recipient for Spermatogonial Transplantation for the Rapid Production of Donor-Derived Sperm. Biol. Reprod. 2019;101:492–500. doi: 10.1093/biolre/ioz045. [DOI] [PubMed] [Google Scholar]
- 32.Lacerda S.M.S.N., Batlouni S.R., Costa G.M.J., Segatelli T.M., Quirino B.R., Queiroz B.M., Kalapothakis E., França L.R. A New and Fast Technique to Generate Offspring after Germ Cells Transplantation in Adult Fish: The Nile Tilapia (Oreochromis niloticus) Model. PLoS ONE. 2010;5:e10740. doi: 10.1371/journal.pone.0010740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandeputte M. Selective Breeding of Quantitative Traits in the Common Carp (Cyprinus carpio): A Review. Aquat. Living Resour. 2003;16:399–407. doi: 10.1016/S0990-7440(03)00056-1. [DOI] [Google Scholar]
- 34.Janssen K., Chavanne H., Berentsen P., Komen H. Impact of Selective Breeding on European Aquaculture. Aquaculture. 2017;472:8–16. doi: 10.1016/j.aquaculture.2016.03.012. [DOI] [Google Scholar]
- 35.Stevens C.H., Croft D.P., Paull G.C., Tyler C.R. Stress and Welfare in Ornamental Fishes: What Can Be Learned from Aquaculture? J. Fish Biol. 2017;91:409–428. doi: 10.1111/jfb.13377. [DOI] [PubMed] [Google Scholar]
- 36.Tattam I.A., Ruzycki J.R., McCormick J.L., Carmichael R.W. Length and Condition of Wild Chinook Salmon Smolts Influence Age at Maturity. Trans. Am. Fish. Soc. 2015;144:1237–1248. doi: 10.1080/00028487.2015.1082503. [DOI] [Google Scholar]
- 37.Okutsu T., Suzuki K., Takeuchi Y., Takeuchi T., Yoshizaki G. Testicular Germ Cells Can Colonize Sexually Undifferentiated Embryonic Gonad and Produce Functional Eggs in Fish. Proc. Natl. Acad. Sci. USA. 2006;103:2725–2729. doi: 10.1073/pnas.0509218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshizaki G. Germ Cell Transplantation in Fish: Mutant dnd Rainbow Trout Can Produce Chinook Salmon Gametes; Proceedings of the 2021 Exotic Species Webinar Series Recordings; 10 February 2021; Reston, VA, USA: Society for the Study of Reproduction; 2021. [Google Scholar]
- 39.Pšenička M., Saito T., Linhartová Z., Gazo I. Isolation and Transplantation of Sturgeon Early-Stage Germ Cells. Theriogenology. 2015;83:1085–1092. doi: 10.1016/j.theriogenology.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Cederholm C.J., Kunze M.D., Murota T., Sibatani A. Pacific Salmon Carcasses: Essential Contributions of Nutrients and Energy for Aquatic and Terrestrial Ecosystems. Fisheries. 1999;24:6–15. doi: 10.1577/1548-8446(1999)024<0006:PSC>2.0.CO;2. [DOI] [Google Scholar]
- 41.Unwin M.J., Kinnison M.T., Quinn T.P. Exceptions to Semelparity: Postmaturation Survival, Morphology, and Energetics of Male Chinook Salmon (Oncorhynchus tshawytscha) Can. J. Fish. Aquat. 1999;56:1172–1181. doi: 10.1139/f99-045. [DOI] [Google Scholar]
- 42.Zohar Y., Mylonas C.C., Rosenfeld H., de la Gándara F., Corriero A. Chapter 7—Reproduction, Broodstock Management, and Spawning in Captive Atlantic Bluefin Tuna. In: Benetti D.D., Partridge G.J., Buentello A., editors. Advances in Tuna Aquaculture. Academic Press; San Diego, CA, USA: 2016. pp. 159–188. [Google Scholar]
- 43.Bluefin Tuna Fish Farming. [(accessed on 6 January 2022)]. Available online: https://factsanddetails.com/world/cat53/sub340/item2188.html.
- 44.Sawada Y., Okada T., Miyashita S., Murata O., Kumai H. Completion of the Pacific Bluefin Tuna Thunnus orientalis (Temminck et Schlegel) Life Cycle. Aquac. Res. 2005;36:413–421. doi: 10.1111/j.1365-2109.2005.01222.x. [DOI] [Google Scholar]
- 45.Ichida K., Kawamura W., Miwa M., Iwasaki Y., Kubokawa T., Hayashi M., Yazawa R., Yoshizaki G. Specific Visualization of Live Type A Spermatogonia of Pacific Bluefin Tuna Using Fluorescent Dye-Conjugated Antibodies. Biol. Reprod. 2019;100:1637–1647. doi: 10.1093/biolre/ioz047. [DOI] [PubMed] [Google Scholar]
- 46.Yazawa R., Kubokawa T., Ichida K., Kawamura W., Tani R., Kamio S., Morita T., Yoshizaki G. Establishment of a Tracing Technique for Transplanted Bluefin Tuna Germ Cells in Recipient’s Gonads Using Monoclonal Antibodies Specifically Recognizing Bluefin Tuna Spermatogenic Cells. Fish. Sci. 2021;87:105–112. doi: 10.1007/s12562-020-01486-2. [DOI] [Google Scholar]
- 47.Bar I., Smith A., Bubner E., Yoshizaki G., Takeuchi Y., Yazawa R., Chen B.N., Cummins S., Elizur A. Assessment of Yellowtail Kingfish (Seriola lalandi) as a Surrogate Host for the Production of Southern Bluefin Tuna (Thunnus maccoyii) Seed via Spermatogonial Germ Cell Transplantation. Reprod. Fertil. Dev. 2016;28:2051–2064. doi: 10.1071/RD15136. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura W., Tani R., Yahagi H., Kamio S., Morita T., Takeuchi Y., Yazawa R., Yoshizaki G. Suitability of Hybrid Mackerel (Scomber australasicus × S. japonicus) with Germ Cell-Less Sterile Gonads as a Recipient for Transplantation of Bluefin Tuna Germ Cells. Gen. Comp. Endocrinol. 2020;295:113525. doi: 10.1016/j.ygcen.2020.113525. [DOI] [PubMed] [Google Scholar]
- 49.Cabrita E., Sarasquete C., Martínez-Páramo S., Robles V., Beirão J., Pérez-Cerezales S., Herráez M.P. Cryopreservation of Fish Sperm: Applications and Perspectives. J. Appl. Ichthyol. 2010;26:623–635. doi: 10.1111/j.1439-0426.2010.01556.x. [DOI] [Google Scholar]
- 50.Chao N.H., Liao I.C. Cryopreservation of Finfish and Shellfish Gametes and Embryos. Aquaculture. 2001;197:161–189. doi: 10.1016/S0044-8486(01)00586-5. [DOI] [Google Scholar]
- 51.Leveroni Calvi S., Maisse G. Cryopreservation of Rainbow Trout (Oncorhynchus mykiss) Blastomeres: Influence of Embryo Stage on Postthaw Survival Rate. Cryobiology. 1998;36:255–262. doi: 10.1006/cryo.1998.2084. [DOI] [PubMed] [Google Scholar]
- 52.Kawakami Y., Goto-Kazeto R., Saito T., Fujimoto T., Higaki S., Takahashi Y., Arai K., Yamaha E. Generation of Germ-Line Chimera Zebrafish Using Primordial Germ Cells Isolated from Cultured Blastomeres and Cryopreserved Embryoids. Int. J. Dev. Biol. 2010;54:1491–1499. doi: 10.1387/ijdb.093059yk. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T., Takeuchi Y., Takeuchi T., Yoshizaki G. Generation of Viable Fish from Cryopreserved Primordial Germ Cells. Mol. Reprod. Dev. 2007;74:207–213. doi: 10.1002/mrd.20577. [DOI] [PubMed] [Google Scholar]
- 54.Lee S., Iwasaki Y., Shikina S., Yoshizaki G. Generation of Functional Eggs and Sperm from Cryopreserved Whole Testes. Proc. Natl. Acad. Sci. USA. 2013;110:1640–1645. doi: 10.1073/pnas.1218468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seki S., Kusano K., Lee S., Iwasaki Y., Yagisawa M., Ishida M., Hiratsuka T., Sasado T., Naruse K., Yoshizaki G. Production of the Medaka Derived from Vitrified Whole Testes by Germ Cell Transplantation. Sci. Rep. 2017;7:43185. doi: 10.1038/srep43185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S., Katayama N., Yoshizaki G. Generation of Juvenile Rainbow Trout Derived from Cryopreserved Whole Ovaries by Intraperitoneal Transplantation of Ovarian Germ Cells. Biochem. Biophys. Res. Commun. 2016;478:1478–1483. doi: 10.1016/j.bbrc.2016.08.156. [DOI] [PubMed] [Google Scholar]
- 57.Yoshizaki G., Lee S. Production of Live Fish Derived from Frozen Germ Cells via Germ Cell Transplantation. Stem Cell Res. 2018;29:103–110. doi: 10.1016/j.scr.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Chen S.L., Tian Y.S., Yang J.F., Shao C.W., Ji X.S., Zhai J.M., Liao X.L., Zhuang Z.M., Su P.Z., Xu J.Y., et al. Artificial Gynogenesis and Sex Determination in Half-Smooth Tongue Sole (Cynoglossus semilaevis) Mar. Biotechnol. 2009;11:243–251. doi: 10.1007/s10126-008-9139-0. [DOI] [PubMed] [Google Scholar]
- 59.Geffroy B., Bardonnet A. Sex Differentiation and Sex Determination in Eels: Consequences for Management. Fish Fish. 2016;17:375–398. doi: 10.1111/faf.12113. [DOI] [Google Scholar]
- 60.Baroiller J.F., D’Cotta H., Bezault E., Wessels S., Hoerstgen-Schwark G. Tilapia sex determination: Where temperature and genetics meet. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;153:30–38. doi: 10.1016/j.cbpa.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Lee S., Seki S., Katayama N., Yoshizaki G. Production of Viable Trout Offspring Derived from Frozen Whole Fish. Sci. Rep. 2015;5:16045. doi: 10.1038/srep16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ichida K., Hayashi M., Miwa M., Kitada R., Takahashi M., Fujihara R., Boonanuntanasarn S., Yoshizaki G. Enrichment of Transplantable Germ Cells in Salmonids Using a Novel Monoclonal Antibody by Magnetic-Activated Cell Sorting. Mol. Reprod. Dev. 2019;86:1810–1821. doi: 10.1002/mrd.23275. [DOI] [PubMed] [Google Scholar]
- 63.Yoshikawa H., Takeuchi Y., Ino Y., Wang J., Iwata G., Kabeya N., Yazawa R., Yoshizaki G. Efficient Production of Donor-Derived Gametes from Triploid Recipients Following Intra-Peritoneal Germ Cell Transplantation into a Marine Teleost, Nibe Croaker (Nibea mitsukurii) Aquaculture. 2017;478:35–47. doi: 10.1016/j.aquaculture.2016.05.011. [DOI] [Google Scholar]
- 64.Li M., Hong N., Xu H., Song J., Hong Y. Germline Replacement by Blastula Cell Transplantation in the Fish Medaka. Sci. Rep. 2016;6:29658. doi: 10.1038/srep29658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goto R., Saito T., Takeda T., Fujimoto T., Takagi M., Arai K., Yamaha E. Germ Cells Are Not the Primary Factor for Sexual Fate Determination in Goldfish. Dev. Biol. 2012;370:98–109. doi: 10.1016/j.ydbio.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Yoshizaki G., Tago Y., Takeuchi Y., Sawatari E., Kobayashi T., Takeuchi T. Green Fluorescent Protein Labeling of Primordial Germ Cells Using a Nontransgenic Method and Its Application for Germ Cell Transplantation in Salmonidae. Biol. Reprod. 2005;73:88–93. doi: 10.1095/biolreprod.104.034249. [DOI] [PubMed] [Google Scholar]
- 67.Yano A., Suzuki K., Yoshizaki G. Flow-Cytometric Isolation of Testicular Germ Cells from Rainbow Trout (Oncorhynchus mykiss) Carrying the Green Fluorescent Protein Gene Driven by Trout vasa Regulatory Regions. Biol. Reprod. 2008;78:151–158. doi: 10.1095/biolreprod.107.064667. [DOI] [PubMed] [Google Scholar]
- 68.Pertoft H., Laurent T.C., Låås T., Kågedal L. Density Gradients Prepared from Colloidal Silica Particles Coated by Polyvinylpyrrolidone (Percoll) Anal. Biochem. 1978;88:271–282. doi: 10.1016/0003-2697(78)90419-0. [DOI] [PubMed] [Google Scholar]
- 69.Wong T.T., Saito T., Crodian J., Collodi P. Zebrafish Germline Chimeras Produced by Transplantation of Ovarian Germ Cells into Sterile Host Larvae. Biol. Reprod. 2011;84:1190–1197. doi: 10.1095/biolreprod.110.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryu J.H., Gong S.P. Enhanced Enrichment of Medaka Ovarian Germline Stem Cells by a Combination of Density Gradient Centrifugation and Differential Plating. Biomolecules. 2020;10:1477. doi: 10.3390/biom10111477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshikawa H., Morishima K., Fujimoto T., Saito T., Kobayashi T., Yamaha E., Arai K. Chromosome Doubling in Early Spermatogonia Produces Diploid Spermatozoa in a Natural Clonal Fish. Biol. Reprod. 2009;80:973–979. doi: 10.1095/biolreprod.108.075150. [DOI] [PubMed] [Google Scholar]
- 72.Ren Y., Sun Z., Wang Y., Yu Q., Wang G., He Z., Liu Y., Jiang X., Kang X., Hou J. Production of Donor-Derived Offsprings by Allogeneic Transplantation of Oogonia in the Adult Japanese Flounder (Paralichthys olivaceus) Aquaculture. 2021;543:736977. doi: 10.1016/j.aquaculture.2021.736977. [DOI] [Google Scholar]
- 73.Shikina S., Nagasawa K., Hayashi M., Furuya M., Iwasaki Y., Yoshizaki G. Short-Term in Vitro Culturing Improves Transplantability of Type A Spermatogonia in Rainbow Trout (Oncorhynchus mykiss) Mol. Reprod. Dev. 2013;80:763–773. doi: 10.1002/mrd.22208. [DOI] [PubMed] [Google Scholar]
- 74.Sato T., Katagiri K., Kubota Y., Ogawa T. In Vitro Sperm Production from Mouse Spermatogonial Stem Cell Lines Using an Organ Culture Method. Nat. Protoc. 2013;8:2098–2104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 75.Shikina S., Ihara S., Yoshizaki G. Culture Conditions for Maintaining the Survival and Mitotic Activity of Rainbow Trout Transplantable Type A Spermatogonia. Mol. Reprod. Dev. 2008;75:529–537. doi: 10.1002/mrd.20771. [DOI] [PubMed] [Google Scholar]
- 76.Shikina S., Yoshizaki G. Improved In Vitro Culture Conditions to Enhance the Survival, Mitotic Activity, and Transplantability of Rainbow Trout Type A Spermatogonia. Biol. Reprod. 2010;83:268–276. doi: 10.1095/biolreprod.109.082123. [DOI] [PubMed] [Google Scholar]
- 77.Hong N., Li Z., Hong Y. Fish Stem Cell Cultures. Int. J. Biol. Sci. 2011;7:392–402. doi: 10.7150/ijbs.7.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nasiri Z., Hosseini S.M., Hajian M., Abedi P., Bahadorani M., Baharvand H., Nasr-Esfahani M.H. Effects of Different Feeder Layers on Short-Term Culture of Prepubertal Bovine Testicular Germ Cells In-Vitro. Theriogenology. 2012;77:1519–1528. doi: 10.1016/j.theriogenology.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 79.Shinohara T., Avarbock M.R., Brinster R.L. β1- and α6-Integrin Are Surface Markers on Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marraccino R.L., Keng P.C. Centrifugal Elutriation. In: Pagano M., editor. Cell Cycle—Materials and Methods. Springer; Berlin/Heidelberg, Germany: 1996. pp. 53–62. [Google Scholar]
- 81.Liu Y., Nan B., Niu J., Kapler G.M., Gao S. An Optimized and Versatile Counter-Flow Centrifugal Elutriation Workflow to Obtain Synchronized Eukaryotic Cells. Front. Cell Dev. Biol. 2021;9:905. doi: 10.3389/fcell.2021.664418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu P., Zhang W., Xin H., Deng G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016;4:116. doi: 10.3389/fcell.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adan A., Alizada G., Kiraz Y., Baran Y., Nalbant A. Flow Cytometry: Basic Principles and Applications. Crit. Rev. Biotechnol. 2017;37:163–176. doi: 10.3109/07388551.2015.1128876. [DOI] [PubMed] [Google Scholar]
- 84.Pšenička M., Saito T. Chapter 16 Specificity of Germ Cell Technologies in Sturgeons. In: Yoshida M., Asturiano J.F., editors. Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology. Springer; Singapore: 2020. pp. 335–356. [Google Scholar]
- 85.Kobayashi T., Yoshizaki G., Takeuchi Y., Takeuchi T. Isolation of Highly Pure and Viable Primordial Germ Cells from Rainbow Trout by GFP-Dependent Flow Cytometry. Mol. Reprod. Dev. 2004;67:91–100. doi: 10.1002/mrd.20003. [DOI] [PubMed] [Google Scholar]
- 86.Ichida K., Matsushita Y., Amano Y., Miwa M., Nagasawa K., Hayashi M., Mizutani H., Takahashi M., Boonanuntanasarn S., Yoshizaki G. Visualization and Tracking of Live Type a Spermatogonia Using a Fluorescence-Conjugated Antibody in Salmo Species. Aquaculture. 2021;533:736096. doi: 10.1016/j.aquaculture.2020.736096. [DOI] [Google Scholar]
- 87.Xie X., Tichopád T., Kislik G., Langerová L., Abaffy P., Šindelka R., Franěk R., Fučíková M., Steinbach C., Shah M.A., et al. Isolation and Characterization of Highly Pure Type A Spermatogonia From Sterlet (Acipenser ruthenus) Using Flow-Cytometric Cell Sorting. Front. Cell Dev. Biol. 2021;9:772625. doi: 10.3389/fcell.2021.772625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayashi M., Sato M., Nagasaka Y., Sadaie S., Kobayashi S., Yoshizaki G. Enrichment of Spermatogonial Stem Cells Using Side Population in Teleost1. Biol. Reprod. 2014;91:1–8. doi: 10.1095/biolreprod.113.114140. [DOI] [PubMed] [Google Scholar]
- 89.Plouffe B.D., Murthy S.K., Lewis L.H. Fundamentals and Application of Magnetic Particles in Cell Isolation and Enrichment: A Review. Rep. Prog. Phys. 2015;78:016601. doi: 10.1088/0034-4885/78/1/016601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osmond A.T.Y., Colombo S.M. The Future of Genetic Engineering to Provide Essential Dietary Nutrients and Improve Growth Performance in Aquaculture: Advantages and Challenges. J. World Aquac. Soc. 2019;50:490–509. doi: 10.1111/jwas.12595. [DOI] [Google Scholar]
- 91.Altman E., Yango P., Moustafa R., Smith J.F., Klatsky P.C., Tran N.D. Characterization of Human Spermatogonial Stem Cell Markers in Fetal, Pediatric, and Adult Testicular Tissues. Reproduction. 2014;148:417–427. doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falciatori I., Borsellino G., Haliassos N., Boitani C., Corallini S., Battistini L., Bernardi G., Stefanini M., Vicini E. Identification and Enrichment of Spermatogonial Stem Cells Displaying Side-Population Phenotype in Immature Mouse Testis. FASEB J. 2004;18:376–378. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- 93.Dovey S.L., Valli H., Hermann B.P., Sukhwani M., Donohue J., Castro C.A., Chu T., Sanfilippo J.S., Orwig K.E. Eliminating Malignant Contamination from Therapeutic Human Spermatogonial Stem Cells. J. Clin. Investig. 2013;123:1833–1843. doi: 10.1172/JCI65822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan Y., Du X., Zhao F., Xu B. Magnetic Nanoparticles for the Manipulation of Proteins and Cells. Chem. Soc. Rev. 2012;41:2912–2942. doi: 10.1039/c2cs15315g. [DOI] [PubMed] [Google Scholar]
- 95.Kokkinaki M., Lee T.L., He Z., Jiang J., Golestaneh N., Hofmann M.C., Chan W.Y., Dym M. The Molecular Signature of Spermatogonial Stem/Progenitor Cells in the 6-Day-Old Mouse Testis. Biol. Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He Z., Kokkinaki M., Jiang J., Dobrinski I., Dym M. Isolation, Characterization, and Culture of Human Spermatogonia. Biol. Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee M.Y., Lufkin T. Development of the “Three-Step Macs”: A Novel Strategy for Isolating Rare Cell Populations in the Absence of Known Cell Surface Markers from Complex Animal Tissue. J. Biomol. Tech. 2012;23:69–77. doi: 10.7171/jbt.12-2302-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu B., Murthy S.K. Stem Cell Separation Technologies. Curr. Opin. Chem. Eng. 2013;2:3–7. doi: 10.1016/j.coche.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yazawa R., Takeuchi Y., Higuchi K., Yatabe T., Kabeya N., Yoshizaki G. Chub Mackerel Gonads Support Colonization, Survival, and Proliferation of Intraperitoneally Transplanted Xenogenic Germ Cells. Biol. Reprod. 2010;82:896–904. doi: 10.1095/biolreprod.109.081281. [DOI] [PubMed] [Google Scholar]
- 100.Majhi S.K., Hattori R.S., Rahman S.M., Strüssmann C.A. Surrogate Production of Eggs and Sperm by Intrapapillary Transplantation of Germ Cells in Cytoablated Adult Fish. PLoS ONE. 2014;9:e95294. doi: 10.1371/journal.pone.0095294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q., Fujii W., Naito K., Yoshizaki G. Application of dead end-Knockout Zebrafish as Recipients of Germ Cell Transplantation. Mol. Reprod. Dev. 2017;84:1100–1111. doi: 10.1002/mrd.22870. [DOI] [PubMed] [Google Scholar]
- 102.Wong T.T., Zohar Y. Production of Reproductively Sterile Fish: A Mini-Review of Germ Cell Elimination Technologies. Gen. Comp. Endocrinol. 2015;221:3–8. doi: 10.1016/j.ygcen.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu Y., Shibata N., Sakaizumi M., Yamashita M. Production of Diploid Eggs through Premeiotic Endomitosis in the Hybrid Medaka between Oryzias latipes and O. curvinotus. Zool. Sci. 2000;17:951–958. doi: 10.2108/zsj.17.951. [DOI] [Google Scholar]
- 104.Xu D., Yoshino T., De Bello Cioffi M., Yoshikawa H., Ino Y., Yazawa R., Lacerda S.M.D.S.N., Takeuchi Y. Production of Donor-Derived Eggs after Ovarian Germ Cell Transplantation into the Gonads of Adult, Germ Cell-Less, Triploid Hybrid Fish. Biol. Reprod. 2020;103:1289–1299. doi: 10.1093/biolre/ioaa168. [DOI] [PubMed] [Google Scholar]
- 105.Golpour A., Siddique M.A.M., Siqueira-Silva D.H., Pšenička M. Induced Sterility in Fish and Its Potential and Challenges for Aquaculture and Germ Cell Transplantation Technology: A Review. Biologia. 2016;71:853–864. doi: 10.1515/biolog-2016-0118. [DOI] [Google Scholar]
- 106.Nam Y.K., Choi G.C., Park D.J., Kim D.S. Survival and Growth of Induced Tetraploid Mud Loach. Aquac. Int. 2001;9:61–71. doi: 10.1023/A:1012540024333. [DOI] [Google Scholar]
- 107.Refstie T., Vassvik V., Gjedrem T. Induction of Polyploidy in Salmonids by Cytochalasin B. Aquaculture. 1977;10:65–74. doi: 10.1016/0044-8486(77)90033-3. [DOI] [Google Scholar]
- 108.Benfey T.J., Sutterlin A.M. Triploidy Induced by Heat Shock and Hydrostatic Pressure in Landlocked Atlantic Salmon (Salmo salar L.) Aquaculture. 1984;36:359–367. doi: 10.1016/0044-8486(84)90328-4. [DOI] [Google Scholar]
- 109.Okomoda V.T., Aminath L., Oladimeji S.A., Abol-Munafi A.B., Korede A.I., Ikhwanuddin M., Umaru J.A., Hassan A., Martins C.O., Shahreza S.M. First Report on Successful Triploidy Induction in Clarias gariepinus (Burchell, 1822) Using Electroporation. Sci. Rep. 2020;10:2425. doi: 10.1038/s41598-020-59389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamasaki M., Takeuchi Y., Miyaki K., Yoshizaki G. Gonadal Development and Fertility of Triploid Grass Puffer Takifugu niphobles Induced by Cold Shock Treatment. Mar. Biotechnol. 2013;15:133–144. doi: 10.1007/s10126-012-9470-3. [DOI] [PubMed] [Google Scholar]
- 111.Tiwary B.K., Kirubagaran R., Ray A.K. The Biology of Triploid Fish. Rev. Fish Biol. Fish. 2004;14:391–402. doi: 10.1007/s11160-004-8361-8. [DOI] [Google Scholar]
- 112.Wolters W.R., Libey G.S., Chrisman C.L. Effect of Triploidy on Growth and Gonad Development of Channel Catfish. Trans. Am. Fish. Soc. 1982;111:102–105. doi: 10.1577/1548-8659(1982)111<102:EOTOGA>2.0.CO;2. [DOI] [Google Scholar]
- 113.Wolters W.R., Libey G.S., Chrisman C.L. Induction of Triploidy in Channel Catfish. Trans. Am. Fish. Soc. 1981;110:310–312. doi: 10.1577/1548-8659(1981)110<310:IOTICC>2.0.CO;2. [DOI] [Google Scholar]
- 114.Lincoln R.F., Scott A.P. Production of All-Female Triploid Rainbow Trout. Aquaculture. 1983;30:375–380. doi: 10.1016/0044-8486(83)90179-5. [DOI] [Google Scholar]
- 115.Otterå H., Thorsen A., Karlsen Ø., Fjelldal P.G., Morton H.C., Taranger G.L. Performance of Triploid Atlantic Cod (Gadus morhua L.) in Commercial Aquaculture. Aquaculture. 2016;464:699–709. doi: 10.1016/j.aquaculture.2016.08.018. [DOI] [Google Scholar]
- 116.Chourrout D., Chevassus B., Krieg F., Happe A., Burger G., Renard P. Production of Second Generation Triploid and Tetraploid Rainbow Trout by Mating Tetraploid Males and Diploid Females—Potential of Tetraploid Fish. Theor. Appl. Genet. 1986;72:193–206. doi: 10.1007/BF00266992. [DOI] [PubMed] [Google Scholar]
- 117.Arai K., Mukaino M. Clonal Nature of Gynogenetically Induced Progeny of Triploid (Diploid x Tetraploid) Loach, Misgurnus anguillicaudatus (Pisces: Cobitididae) J. Exp. Zool. 1997;278:193–206. doi: 10.1002/(SICI)1097-010X(19970815)278:6<412::AID-JEZ9>3.0.CO;2-R. [DOI] [Google Scholar]
- 118.Yoshikawa H., Morishima K., Kusuda S., Yamaha E., Arai K. Diploid Sperm Produced by Artificially Sex-Reversed Clone Loaches. J. Exp. Zool. 2007;307A:75–83. doi: 10.1002/jez.a.337. [DOI] [PubMed] [Google Scholar]
- 119.Nam Y.K., Kim D.S. Ploidy Status of Progeny from the Crosses between Tetraploid Males and Diploid Females in Mud Loach (Misgurnus mizolepis) Aquaculture. 2004;236:575–582. doi: 10.1016/j.aquaculture.2003.12.026. [DOI] [Google Scholar]
- 120.Yoshikawa H., Ino Y., Shigenaga K., Katayama T., Kuroyanagi M., Yoshiura Y. Production of Tiger Puffer Takifugu rubripes from Cryopreserved Testicular Germ Cells Using Surrogate Broodstock Technology. Aquaculture. 2018;493:302–313. doi: 10.1016/j.aquaculture.2018.05.016. [DOI] [Google Scholar]
- 121.Baloch A.R., Franěk R., Saito T., Pšenička M. Dead-end (Dnd) Protein in Fish—A Review. Fish Physiol. Biochem. 2021;47:777–784. doi: 10.1007/s10695-018-0606-x. [DOI] [PubMed] [Google Scholar]
- 122.Weidinger G., Stebler J., Slanchev K., Dumstrei K., Wise C., Lovell-Badge R., Thisse C., Thisse B., Raz E. Dead end, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival. Curr. Biol. 2003;13:1429–1434. doi: 10.1016/S0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- 123.Wong T.T., Tesfamichael A., Collodi P. Production of Zebrafish Offspring from Cultured Female Germline Stem Cells. PLoS ONE. 2013;8:e62660. doi: 10.1371/journal.pone.0062660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshizaki G., Takashiba K., Shimamori S., Fujinuma K., Shikina S., Okutsu T., Kume S., Hayashi M. Production of Germ Cell-Deficient Salmonids by dead end Gene Knockdown, and Their Use as Recipients for Germ Cell Transplantation. Mol. Reprod. Dev. 2016;83:298–311. doi: 10.1002/mrd.22625. [DOI] [PubMed] [Google Scholar]
- 125.Wong T.T., Zohar Y. Production of Reproductively Sterile Fish by a Non-Transgenic Gene Silencing Technology. Sci. Rep. 2015;5:15822. doi: 10.1038/srep15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong T.T., Collodi P. Dorsomorphin Promotes Survival and Germline Competence of Zebrafish Spermatogonial Stem Cells in Culture. PLoS ONE. 2013;8:e71332. doi: 10.1371/journal.pone.0071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoshikawa H., Ino Y., Kishimoto K., Koyakumaru H., Saito T., Kinoshita M., Yoshiura Y. Induction of Germ Cell-Deficiency in Grass Puffer by dead end 1 Gene Knockdown for Use as a Recipient in Surrogate Production of Tiger Puffer. Aquaculture. 2020;526:735385. doi: 10.1016/j.aquaculture.2020.735385. [DOI] [Google Scholar]
- 128.Nóbrega R.H., Greebe C.D., van de Kant H., Bogerd J., de França L.R., Schulz R.W. Spermatogonial Stem Cell Niche and Spermatogonial Stem Cell Transplantation in Zebrafish. PLoS ONE. 2010;5:e12808. doi: 10.1371/journal.pone.0012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iwamoto T., Hiraku Y., Oikawa S., Mizutani H., Kojima M., Kawanishi S. DNA Intrastrand Cross-Link at the 5′-GA-3′ Sequence Formed by Busulfan and Its Role in the Cytotoxic Effect. Cancer Sci. 2004;95:454–458. doi: 10.1111/j.1349-7006.2004.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brinster R.L., Avarbock M.R. Germline Transmission of Donor Haplotype Following Spermatogonial Transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nozu R., Nakamura M. Influence of Prolonged Cultivation on Sexual Characteristics of Sterilized Female Tilapia, Oreochromis mossambicus, Induced by High-Temperature Treatment. Aquaculture. 2020;524:735245. doi: 10.1016/j.aquaculture.2020.735245. [DOI] [Google Scholar]
- 132.Nakamura M., Nozu R., Ijiri S., Kobayashi T., Hirai T., Yamaguchi Y., Seale A., Lerner D.T., Grau G.E. Sexual Characteristics of High-Temperature Sterilized Male Mozambique Tilapia, Oreochromis mossambicus. Zool. Lett. 2015;1:21. doi: 10.1186/s40851-015-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pandit N.P., Bhandari R.K., Kobayashi Y., Nakamura M. High Temperature-Induced Sterility in the Female Nile Tilapia, Oreochromis niloticus. Gen. Comp. Endocrinol. 2015;213:110–117. doi: 10.1016/j.ygcen.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 134.Jin Y.H., Davie A., Migaud H. Temperature-Induced Testicular Germ Cell Loss and Recovery in Nile Tilapia Oreochromis niloticus. Gen. Comp. Endocrinol. 2019;283:113227. doi: 10.1016/j.ygcen.2019.113227. [DOI] [PubMed] [Google Scholar]
- 135.Marinović Z., Li Q., Lujić J., Iwasaki Y., Csenki Z., Urbányi B., Yoshizaki G., Horváth Á. Preservation of Zebrafish Genetic Resources through Testis Cryopreservation and Spermatogonia Transplantation. Sci. Rep. 2019;9:13861. doi: 10.1038/s41598-019-50169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Franěk R., Cheng Y., Fučíková M., Kašpar V., Xie X., Shah M.A., Linhart O., Šauman I., Pšenička M. Who Is the Best Surrogate for Germ Stem Cell Transplantation in Fish? Aquaculture. 2022;549:737759. doi: 10.1016/j.aquaculture.2021.737759. [DOI] [Google Scholar]
- 137.Farlora R., Hattori-Ihara S., Takeuchi Y., Hayashi M., Octavera A., Alimuddin, Yoshizaki G. Intraperitoneal Germ Cell Transplantation in the Nile Tilapia Oreochromis niloticus. Mar. Biotechnol. 2014;16:309–320. doi: 10.1007/s10126-013-9551-y. [DOI] [PubMed] [Google Scholar]
- 138.Hamasaki M., Takeuchi Y., Yazawa R., Yoshikawa S., Kadomura K., Yamada T., Miyaki K., Kikuchi K., Yoshizaki G. Production of Tiger Puffer Takifugu rubripes Offspring from Triploid Grass Puffer Takifugu niphobles Parents. Mar. Biotechnol. 2017;19:579–591. doi: 10.1007/s10126-017-9777-1. [DOI] [PubMed] [Google Scholar]
- 139.Pradhan A., Olsson P.E. Germ Cell Depletion in Zebrafish Leads to Incomplete Masculinization of the Brain. Gen. Comp. Endocrinol. 2018;265:15–21. doi: 10.1016/j.ygcen.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 140.Brinster R.L., Zimmermann J.W. Spermatogenesis Following Male Germ-Cell Transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong T.T., Tesfamichael A., Collodi P. Identification of Promoter Elements Responsible for Gonad-Specific Expression of Zebrafish Deadend and Its Application to Ovarian Germ Cell Derivation. Int. J. Dev. Biol. 2013;57:767–772. doi: 10.1387/ijdb.120234tw. [DOI] [PubMed] [Google Scholar]
- 142.Kurokawa H., Saito D., Nakamura S., Katoh-Fukui Y., Ohta K., Baba T., Morohashi K.I., Tanaka M. Germ Cells Are Essential for Sexual Dimorphism in the Medaka Gonad. Proc. Natl. Acad. Sci. USA. 2007;104:16958–16963. doi: 10.1073/pnas.0609932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siegfried K.R., Nüsslein-Volhard C. Germ Line Control of Female Sex Determination in Zebrafish. Dev. Biol. 2008;324:277–287. doi: 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 144.Nagasawa K., Ishida M., Octavera A., Kusano K., Kezuka F., Kitano T., Yoshiura Y., Yoshizaki G. Novel Method for Mass Producing Genetically Sterile Fish from Surrogate Broodstock via Spermatogonial Transplantation. Biol. Reprod. 2019;100:535–546. doi: 10.1093/biolre/ioy204. [DOI] [PubMed] [Google Scholar]
- 145.Franěk R., Tichopád T., Fučíková M., Steinbach C., Pšenička M. Production and Use of Triploid Zebrafish for Surrogate Reproduction. Theriogenology. 2019;140:33–43. doi: 10.1016/j.theriogenology.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 146.Okutsu T., Shikina S., Kanno M., Takeuchi Y., Yoshizaki G. Production of Trout Offspring from Triploid Salmon Parents. Science. 2007;317:1517. doi: 10.1126/science.1145626. [DOI] [PubMed] [Google Scholar]
- 147.Iwasaki-Takahashi Y., Shikina S., Watanabe M., Banba A., Yagisawa M., Takahashi K., Fujihara R., Okabe T., Valdez D.M., Yamauchi A., et al. Production of Functional Eggs and Sperm from in Vitro-Expanded Type A Spermatogonia in Rainbow Trout. Commun. Biol. 2020;3:308. doi: 10.1038/s42003-020-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou L., Wang X., Liu Q., Yang J., Xu S., Wu Z., Wang Y., You F., Song Z., Li J. Successful Spermatogonial Stem Cells Transplantation within Pleuronectiformes: First Breakthrough at inter-family Level in Marine Fish. Int. J. Biol. Sci. 2021;17:4426–4441. doi: 10.7150/ijbs.63266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Majhi S.K., Hattori R.S., Yokota M., Watanabe S., Strüssmann C.A. Germ Cell Transplantation Using Sexually Competent Fish: An Approach for Rapid Propagation of Endangered and Valuable Germlines. PLoS ONE. 2009;4:e6132. doi: 10.1371/journal.pone.0006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hattori R.S., Yoshinaga T.T., Katayama N., Hattori-Ihara S., Tsukamoto R.Y., Takahashi N.S., Tabata Y.A. Surrogate Production of Salmo salar Oocytes and Sperm in Triploid Oncorhynchus mykiss by Germ Cell Transplantation Technology. Aquaculture. 2019;506:238–245. doi: 10.1016/j.aquaculture.2019.03.037. [DOI] [Google Scholar]
- 151.OECD . Safety Assessment of Transgenic Organisms in the Environment. Volume 7. OECD Publishing; Paris, France: 2017. Atlantic salmon (Salmo salar) [DOI] [Google Scholar]
- 152.Moore G.A. The Germ Cells of the Trout (Salmo irideus Gibbons) Trans. Am. Microsc. Soc. 1937;56:105–112. doi: 10.2307/3222728. [DOI] [Google Scholar]
- 153.Yoshizaki G., Sakatani S., Tominaga H., Takeuchi T. Cloning and Characterization of a vasa-like Gene in Rainbow Trout and Its Expression in the Germ Cell Lineage. Mol. Reprod. Dev. 2000;55:364–371. doi: 10.1002/(SICI)1098-2795(200004)55:4<364::AID-MRD2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 154.Takeuchi Y., Yoshizaki G., Takeuchi T. Biotechnology: Surrogate Broodstock Produces Salmonids. Nature. 2004;430:629–630. doi: 10.1038/430629a. [DOI] [PubMed] [Google Scholar]
- 155.Morita T., Kumakura N., Morishima K., Mitsuboshi T., Ishida M., Hara T., Kudo S., Miwa M., Ihara S., Higuchi K., et al. Production of Donor-Derived Offspring by Allogeneic Transplantation of Spermatogonia in the Yellowtail (Seriola quinqueradiata) Biol. Reprod. 2012;86:176. doi: 10.1095/biolreprod.111.097873. [DOI] [PubMed] [Google Scholar]
- 156.Kawasaki T., Siegfried K.R., Sakai N. Differentiation of Zebrafish Spermatogonial Stem Cells to Functional Sperm in Culture. Development. 2016;143:566–574. doi: 10.1242/dev.129643. [DOI] [PubMed] [Google Scholar]
- 157.Hayashi M., Sakuma D., Yoshizaki G. Production of Functional Sperm by Subcutaneous Auto-Grafting of Immature Testes in Rainbow Trout. Mol. Reprod. Dev. 2018;85:155–162. doi: 10.1002/mrd.22949. [DOI] [PubMed] [Google Scholar]
- 158.Kawasaki T., Saito K., Sakai C., Shinya M., Sakai N. Production of Zebrafish Offspring from Cultured Spermatogonial Stem Cells. Genes Cells. 2012;17:316–325. doi: 10.1111/j.1365-2443.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 159.Yoshinaga T.T., Júnior J.R.K., Butzge A.J., Olio R.L., Hernandez-Blazquez F.J., Carreira A.C.O., Gomes C.D.O.M.S., Bianchi P.K.F.D.C., Tabata Y.A., Hattori R.S. Testicular Subcutaneous Allografting Followed by Immunosuppressive Treatment Promotes Maintenance of Spermatogonial Cells in Rainbow Trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2021;112:108–115. doi: 10.1016/j.fsi.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 160.Xie X., Nóbrega R., Pšenička M. Spermatogonial Stem Cells in Fish: Characterization, Isolation, Enrichment, and Recent Advances of In Vitro Culture Systems. Biomolecules. 2020;10:644. doi: 10.3390/biom10040644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saito T., Goto-Kazeto R., Kawakami Y., Nomura K., Tanaka H., Adachi S., Arai K., Yamaha E. The Mechanism for Primordial Germ-Cell Migration Is Conserved between Japanese Eel and Zebrafish. PLoS ONE. 2011;6:e24460. doi: 10.1371/journal.pone.0024460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saito T., Psěnička M., Goto R., Adachi S., Inoue K., Arai K., Yamaha E. The Origin and Migration of Primordial Germ Cells in Sturgeons. PLoS ONE. 2014;9:e86861. doi: 10.1371/journal.pone.0086861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silva M.A., Costa G.M.J., Lacerda S.M.S.N., Brandão-Dias P.F.P., Kalapothakis E., Silva Júnior A.F., Alvarenga E.R., França L.R. Successful Xenogeneic Germ Cell Transplantation from Jundia Catfish (Rhamdia quelen) into Adult Nile Tilapia (Oreochromis niloticus) Testes. Gen. Comp. Endocrinol. 2016;230–231:48–56. doi: 10.1016/j.ygcen.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 164.Crouse C., Davidson J., May T., Summerfelt S., Good C. Production of Market-Size European Strain Atlantic Salmon (Salmo salar) in Land-Based Freshwater Closed Containment Aquaculture Systems. Aquac. Eng. 2021;92:102138. doi: 10.1016/j.aquaeng.2020.102138. [DOI] [Google Scholar]
- 165.Yamamoto E. Studies on Sex-Manipulation and Production of Cloned Populations in Hirame, Paralichthys olivaceus (Temminck et Schlegel) Aquaculture. 1999;173:235–246. doi: 10.1016/S0044-8486(98)00448-7. [DOI] [Google Scholar]
- 166.Sheehan R.J., Shasteen S.P., Suresh A.V., Kapuscinski A.R., Seeb J.E. Better Growth in All-Female Diploid and Triploid Rainbow Trout. Trans. Am. Fish. Soc. 1999;128:491–498. doi: 10.1577/1548-8659(1999)128<0491:BGIAFD>2.0.CO;2. [DOI] [Google Scholar]
- 167.Bye V.J., Lincoln R.F. Commercial Methods for the Control of Sexual Maturation in Rainbow Trout (Salmo gairdneri R.) Aquaculture. 1986;57:299–309. doi: 10.1016/0044-8486(86)90208-5. [DOI] [Google Scholar]
- 168.Beardmore J.A., Mair G.C., Lewis R.I. Monosex Male Production in Finfish as Exemplified by Tilapia: Applications, Problems, and Prospects. Aquaculture. 2001;197:283–301. doi: 10.1016/S0044-8486(01)00590-7. [DOI] [Google Scholar]
- 169.Piferrer F., Lim L.C. Application of Sex Reversal Technology in Ornamental Fish Culture. Aquar. Sci. Conserv. 1997;1:113–118. doi: 10.1023/A:1018391702814. [DOI] [Google Scholar]
- 170.Shen Z.G., Wang H.P. Sex Control in Aquaculture. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2018. Environmental Sex Determination and Sex Differentiation in Teleosts—How Sex Is Established; pp. 85–115. [Google Scholar]
- 171.Baroiller J.F., D’Cotta H., Saillant E. Environmental Effects on Fish Sex Determination and Differentiation. Sex. Dev. 2009;3:118–135. doi: 10.1159/000223077. [DOI] [PubMed] [Google Scholar]
- 172.Magerhans A., Müller-Belecke A., Hörstgen-Schwark G. Effect of Rearing Temperatures Post Hatching on Sex Ratios of Rainbow Trout (Oncorhynchus mykiss) Populations. Aquaculture. 2009;294:25–29. doi: 10.1016/j.aquaculture.2009.05.001. [DOI] [Google Scholar]
- 173.Maria Poli B. Farmed Fish Welfare-Suffering Assessment and Impact on Product Quality. Ital. J. Anim. Sci. 2009;8:139–160. doi: 10.4081/ijas.2009.s1.139. [DOI] [Google Scholar]
- 174.Wang H.P., Shen Z.G. Sex Control in Aquaculture. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2018. Sex Control in Aquaculture; pp. 1–34. [Google Scholar]
- 175.Weber G.M., Leeds T.D., Schneider R.P. Sex Reversal of Female Rainbow Trout by Immersion in 17α-Methyltestosterone. Aquaculture. 2020;528:735535. doi: 10.1016/j.aquaculture.2020.735535. [DOI] [Google Scholar]
- 176.Okutsu T., Shikina S., Sakamoto T., Mochizuki M., Yoshizaki G. Successful Production of Functional Y Eggs Derived from Spermatogonia Transplanted into Female Recipients and Subsequent Production of YY Supermales in Rainbow Trout, Oncorhynchus mykiss. Aquaculture. 2015;446:298–302. doi: 10.1016/j.aquaculture.2015.05.020. [DOI] [Google Scholar]
- 177.Takeuchi Y., Yazawa R., Yoshizaki G. Chapter 17 Intraperitoneal Germ Cell Transplantation Technique in Marine Teleosts. In: Yoshida M., Asturiano J.F., editors. Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology. Springer; Singapore: 2020. pp. 357–379. [Google Scholar]
- 178.Weber G.M., Hostuttler M.A., Cleveland B.M., Leeds T.D. Growth Performance Comparison of Intercross-Triploid, Induced Triploid, and Diploid Rainbow Trout. Aquaculture. 2014;433:85–93. doi: 10.1016/j.aquaculture.2014.06.003. [DOI] [Google Scholar]
- 179.Norberg B., Weltzien F.A., Karlsen Ø., Holm J.C. Effects of Photoperiod on Sexual Maturation and Somatic Growth in Male Atlantic Halibut (Hippoglossus hippoglossus L.) Comp. Biochem. Physiol. 2001;129B:357–365. doi: 10.1016/S1096-4959(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 180.Good C., Davidson J. A Review of Factors Influencing Maturation of Atlantic Salmon, Salmo salar, with Focus on Water Recirculation Aquaculture System Environments. J. World Aquac. Soc. 2016;47:605–632. doi: 10.1111/jwas.12342. [DOI] [Google Scholar]
- 181.Oppedal F., Taranger G.L., Hansen T. Growth Performance and Sexual Maturation in Diploid and Triploid Atlantic Salmon (Salmo salar L.) in Seawater Tanks Exposed to Continuous Light or Simulated Natural Photoperiod. Aquaculture. 2003;215:145–162. doi: 10.1016/S0044-8486(02)00223-5. [DOI] [Google Scholar]
- 182.Iversen M., Myhr A.I., Wargelius A. Approaches for Delaying Sexual Maturation in Salmon and Their Possible Ecological and Ethical Implications. J. Appl. Aquac. 2016;28:330–369. doi: 10.1080/10454438.2016.1212756. [DOI] [Google Scholar]
- 183.Liu Q., Duston J. Preventing Sexual Maturation in Arctic Charr by 24 h Light Overwinter and Suppressing Somatic Growth. Aquaculture. 2016;464:537–544. doi: 10.1016/j.aquaculture.2016.07.038. [DOI] [Google Scholar]
- 184.Bailey C. Transgenic Salmon: Science, Politics, and Flawed Policy. Soc. Nat. Resour. 2015;28:1249–1260. doi: 10.1080/08941920.2015.1089610. [DOI] [Google Scholar]
- 185.Okoli A.S., Blix T., Myhr A.I., Xu W., Xu X. Sustainable Use of CRISPR/Cas in Fish Aquaculture: The Biosafety Perspective. Transgenic Res. 2021 doi: 10.1007/s11248-021-00274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Scheerer P.D., Thorgaard G.H. Increased Survival in Salmonid Hybrids by Induced Triploidy. Can. J. Fish. Aquat. 1983;40:2040–2044. doi: 10.1139/f83-235. [DOI] [Google Scholar]
- 187.Egami N., Hama-Furukawa A. Late Effects of Continuous Gamma-Irradiation of the Developmental Stage on the Gonads in Oryzias Latipes. In: Egami N., editor. Radiation Effects on Aquatic Organisms. Japan Scientific Societies Press; Tokyo, Japan: 1980. pp. 105–117. [Google Scholar]
- 188.Saito T., Güralp H., Iegorova V., Rodina M., Pšenička M. Elimination of Primordial Germ Cells in Sturgeon Embryos by Ultraviolet Irradiation†. Biol. Reprod. 2018;99:556–564. doi: 10.1093/biolre/ioy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wargelius A., Leininger S., Skaftnesmo K.O., Kleppe L., Andersson E., Taranger G.L., Schulz R.W., Edvardsen R.B. Dnd Knockout Ablates Germ Cells and Demonstrates Germ Cell Independent Sex Differentiation in Atlantic Salmon. Sci. Rep. 2016;6:21284. doi: 10.1038/srep21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Piferrer F., Beaumont A., Falguière J.-C., Flajšhans M., Haffray P., Colombo L. Polyploid Fish and Shellfish: Production, Biology and Applications to Aquaculture for Performance Improvement and Genetic Containment. Aquaculture. 2009;293:125–156. doi: 10.1016/j.aquaculture.2009.04.036. [DOI] [Google Scholar]
- 191.Zhang Z., Lau S.W., Zhang L., Ge W. Disruption of Zebrafish Follicle-Stimulating Hormone Receptor (fshr) but Not Luteinizing Hormone Receptor (lhcgr) Gene by TALEN Leads to Failed Follicle Activation in Females Followed by Sexual Reversal to Males. Endocrinology. 2015;156:3747–3762. doi: 10.1210/en.2015-1039. [DOI] [PubMed] [Google Scholar]
- 192.Yan Y.L., Desvignes T., Bremiller R., Wilson C., Dillon D., High S., Draper B., Buck C.L., Postlethwait J. Gonadal Soma Controls Ovarian Follicle Proliferation through Gsdf in Zebrafish. Dev. Dyn. 2017;246:925–945. doi: 10.1002/dvdy.24579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu H., Cui Y., Jiang L., Ge W. Functional Analysis of Nuclear Estrogen Receptors in Zebrafish Reproduction by Genome Editing Approach. Endocrinology. 2017;158:2292–2308. doi: 10.1210/en.2017-00215. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Z., Zhu B., Ge W. Genetic Analysis of Zebrafish Gonadotropin (FSH and LH) Functions by TALEN-Mediated Gene Disruption. Mol. Endocrinol. 2015;29:76–98. doi: 10.1210/me.2014-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pšenička M., Saito T., Rodina M., Dzyuba B. Cryopreservation of Early Stage Siberian Sturgeon Acipenser baerii Germ Cells, Comparison of Whole Tissue and Dissociated Cells. Cryobiology. 2016;72:119–122. doi: 10.1016/j.cryobiol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 196.Ye H., Li C.J., Yue H.M., Du H., Yang X.G., Yoshino T., Hayashida T., Takeuchi Y., Wei Q.W. Establishment of Intraperitoneal Germ Cell Transplantation for Critically Endangered Chinese Sturgeon Acipenser sinensis. Theriogenology. 2017;94:37–47. doi: 10.1016/j.theriogenology.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 197.Jeong Y., Ryu J.H., Nam Y.K., Gong S.P., Kang S.M. Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating. Mar. Drugs. 2019;17:11. doi: 10.3390/md17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ye H., Takeuchi Y., Wu M., Yue H., Ruan R., Du H., Zhou C., Xiang H., Li C., Wei Q. Assessment of Yangtze Sturgeon as Recipient for the Production of American Paddlefish Gametes through Spermatogonia Transplantation. Theriogenology. 2020;158:168–179. doi: 10.1016/j.theriogenology.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 199.Perera D.A., Alsaqufi A., Shang M., Wade D.C., Su B., Elaswad A., Fobes M., Beam R., Garcia G., Dunn D.A., et al. Xenogenesis-Production of Channel Catfish × Blue Catfish Hybrid Progeny by Fertilization of Channel Catfish Eggs with Sperm from Triploid Channel Catfish Males with Transplanted Blue Catfish Germ Cells. N. Am. J. Aquac. 2017;79:61–74. doi: 10.1080/15222055.2016.1221008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.