Abstract
Objectives:
This study aimed to investigate the distribution of cognitive function in people with systemic lupus erythematosus (SLE) by objective and self-report measures and associations between cognition and participation among people with SLE.
Methods:
Fifty-five volunteers with SLE (age: 39.7 ± 12.7yrs, female: 92.7%) completed the Montreal Cognitive Assessment (MoCA) to measure cognitive ability objectively, the Cognitive Symptom Inventory (CSI) and PROMIS Cognitive Function 8a (CF) to assess self-reported everyday cognition, and PROMIS-43 Profile to assess self-reported ability to participate in social roles and activities (participation) and other disease-associated symptoms (e.g., depression, pain, fatigue).
Results:
The average MoCA score was 25.3 ± 3.1, with 47.3% of participants scoring <26, which is indicative of cognitive impairment. Group average CSI (35.8 ± 7.9), CF (T-score = 45.0 ± 8.5), and participation (T-score = 46.9 ± 11.2) scores suggest mildly impaired functional cognition and participation compared to normative data. Participation correlated with self-reported everyday cognition measures (r ≥ 0.56, p < 0.01) but not with MoCA (r = 0.25, p = 0.06). In hierarchical linear regression analysis, CSI, fatigue, and pain were each significant independent predictors of participation (R2 = 0.78, p < 0.01).
Conclusions:
We found that cognitive dysfunction is common among people with SLE. Along with pain and fatigue, reduced everyday cognitive function contributes to reduced participation in social, leisure, work, and family-related activities.
Keywords: Systemic lupus erythematosus, cognitive dysfunction, participation
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease that affects various organs, including skin, joints, kidneys, and central and peripheral nervous systems.1 Consequently, a wide spectrum of symptoms is associated with SLE.2 This includes neuropsychiatric (NP) manifestations such as cognitive dysfunction, mood disorders, psychosis, and seizures, which are experienced by up to 80% of people with SLE.3 While survival of people with SLE has improved over the past 60 years,4 people with SLE experience lower health-related quality of life compared to other populations with chronic disease such as congestive heart failure and hypertension.5
Cognitive impairment is one of the most common NP manifestations of SLE.6 A recent meta-analysis suggested significant SLE-related impairments in the cognitive domains of immediate visual memory, executive function (including fluency and visual reasoning), and visual attention span.7 The biologic mechanisms for cognitive dysfunction in SLE are unclear, but associated factors include antiphospholipid antibodies, matrix metalloproteinase 9, proinflammatory cytokines, vascular abnormalities, and neuropeptides.8
Cognitive function is considered a crucial factor that contributes to participation in daily life.9–11 Participation is defined as “involvement in life situations” by the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) and is a key indicator of health and well-being.12 Both cognition and participation in daily life are negatively affected by SLE;13,14 however, the associations between them are not well studied. Some studies have examined the association between cognition and employment status or work performance in SLE and found that executive function, attention, and memory are important predictors of unemployment in SLE.13,15 However, participation is a broad concept that also includes domains such as domestic life, interpersonal interactions and relationships, education, and community, social and civic life, all of which are important for overall health and well-being.12 To our knowledge, there is no evidence for how cognitive dysfunction in SLE affects people’s participation in these other areas of daily life. A better understanding of these relationships is necessary to inform the identification, development, and testing of interventions to address cognition and participation among people with SLE.
The purpose of this study was to investigate the distribution of cognitive function in SLE by objective and self-report measures and the association between cognition and participation in SLE. We used objective (Montreal Cognitive Assessment) and self-report (Cognitive Symptoms Inventory) measures of cognitive function previously recommended to determine the presence of cognitive impairment and its functional impact in SLE.16–18 We additionally used the Patient Reported Measurement Information System Cognitive Function scale, which has been extensively developed and validated to measure perceived cognitive deficits in everyday life in the general population and chronic conditions and has normative data for comparison. We hypothesize that cognitive dysfunction and everyday cognitive problems will be prevalent in our sample and that this cognitive dysfunction will relate to reduced participation.
Methods
Research design and participants
This cross-sectional study enrolled 58 participants with the diagnosis of SLE who met the 1997 ACR and/or the 2012 Systemic Lupus International Collaborating Clinics classification criteria for SLE.19,20 Exclusion criteria included diagnosis of a neurological condition aside from SLE (e.g., stroke, traumatic brain injury, Parkinson’s disease), diagnosis of dementia (determined by review of medical history and chart), developmental cognitive defects, or other barriers to participation (e.g., non-fluent in English). Patients who met eligibility criteria were recruited from the Washington University Lupus Clinic (St. Louis, MO, USA) during their routine clinic visits from July 2018-January 2019. The university’s institutional review board approved all recruitment and experimental procedures (protocol no. 201902149, initially approved 22 March 2019, last approved 05 May 2019). All participants provided written informed consent upon enrollment.
Measures
Participants completed the primary measures of interest (described below) in conjunction with their regular clinical appointment. An objective test and two self-report measures were used to assess cognition, and a self-report measure was used to assess participation and other disease-associated symptoms. Demographic characteristics were obtained from medical records.
The Montreal Cognitive Assessment (MoCA) was used as an objective measure of global cognitive function. While it was originally designed to detect mild cognitive impairment in the elderly, it is now used as a screening tool for cognitive impairment in a variety of clinical populations.21 Recent studies suggest it is effective for detecting cognitive impairment in people with SLE.17,18 The MoCA assesses the cognitive domains of visuo-spatial and executive functions, naming, memory, attention, language, abstraction, and orientation. It is scored from 0 to 30 points with higher scores indicating better cognitive functioning. To adjust for education level, if years of education is 12 or less (and the total MoCA score is less than 30), 1 point is added to the total score.19 A score below 26 implies cognitive impairment,21 and a score below 21 is indicative of dementia in the context of aging or other neurodegenerative diseases such as Alzheimer’s disease and Parkinson disease.21,22
The Cognitive Symptom Inventory (CSI) was used as a subjective measure of everyday cognitive function, specifically cognitive problems in everyday activities. It consists of 21 questions that examine an individual’s perceived problems with everyday activities that require cognition, such as managing money and remembering to take medications.23 The ACR recommends the CSI be included in evaluations of cognitive function in SLE.16 The CSI is scored from 0 to 84 points, with higher scores indicating more perceived problems in everyday activities.
The Patient Reported Measurement Information System (PROMIS) Cognitive Function v2.0 8-item Short Form (CF) was used as a subjective measure of everyday cognitive function, specifically perceived cognitive deficits and the extent to which they interfere with daily functioning. It includes eight items that assess perceived cognitive deficits during the past seven days, such as, “I have had trouble concentrating” and “I have had trouble shifting back and forth between different activities that require thinking”.24 Items are rated on a 5-point scale (1 = Very often (several times a day), 5 = Never), with higher scores indicating better perceived cognitive function. Raw scores were converted to T-scores for interpretation, with scores >45 in the normal range, 40–45 indicating mild impairment, 30–40 indicating moderate impairment, and <30 indicating severe impairment.
PROMIS-43 Profile v2.1 was used as a self-report measure of participation and other disease-associated symptoms. It assesses perceived function in seven domains: depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities (hereafter referred to as “participation”).25 The participation domain, used as a dependent variable in these analyses, assesses perceived trouble doing leisure, family, work, and social activities such as “I have trouble doing all of the family activities that I want to do,” “I have trouble doing all of my usual work (include work at home),” and “I have to limit the things I do for fun with others”. Each domain is assessed by six items with 5-point response scales. Raw scores were converted to T-scores for interpretation. For participation and physical function, higher scores indicate better function in that domain, with scores >45 in the normal range, 40–45 indicating mild impairment, 30–40 indicating moderate impairment, and <30 indicating severe impairment. For anxiety, depression, fatigue, sleep disturbance, and pain interference, higher scores indicate worse function in that domain, with scores <55 in the normal range, 55–60 indicating mild impairment, 60–70 indicating moderate impairment, and >70 indicating severe impairment.
Data analysis
Data were stored and managed using REDCap electronic data capture tools hosted at Washington University26 and analyzed with SPSS, version 25. Descriptive statistics were calculated for all variables. For the PROMIS scales, raw scores were transformed into T-scores using published criteria based on reference samples from the US general population.27 Bivariate relationships between the cognitive measures, disease-associated symptoms, and participation were calculated using Pearson r correlations. Then a hierarchical linear regression analysis was conducted to determine the independent relationships between cognition and participation after accounting for other disease-associated symptoms. Disease-associated symptoms with significant bivariate relationships with participation were entered in the first block (with the exception of physical function, which consists of items that overlap considerably with the concept of participation), MoCA was entered in the second block, and CF and CSI were entered in the third block. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Participant characteristics
The final analyzed sample consisted of 55 participants (three participants were missing PROMIS data). Their demographic characteristics are presented in Table 1. About 93% were female and 51% were African American, which is consistent with published epidemiology data.28
Table 1.
Participants characteristics (N = 55).
| Mean | SD | Min–Max | |
|---|---|---|---|
| Age, yr | 39.7 | 12.7 | 19–67 |
| N | % | ||
| Gender | |||
| Female | 51 | 92.7 | |
| Male | 4 | 7.3 | |
| Ethnicity | |||
| Hispanic or Latino | 1 | 1.8 | |
| NOT Hispanic of Latino | 53 | 96.4 | |
| Unknown/Not Reported | 1 | 1.8 | |
| Race | |||
| Asian | 3 | 5.5 | |
| Black or African American | 28 | 50.9 | |
| White | 22 | 40.0 | |
| Unknown/Not Reported | 1 | 1.8 | |
| Other | 1 | 1.8 | |
| Marital Status | |||
| Unknown | 5 | 11.6 | |
| Single | 29 | 53.5 | |
| Married | 17 | 30.2 | |
| Divorced/Separated | 3 | 4.7 | |
| Widowed | 1 | 1.8 | |
| Education | |||
| Unknown | 4 | 7.2 | |
| Some high school | 1 | 1.8 | |
| GED | 2 | 3.6 | |
| High school graduate | 7 | 12.7 | |
| Some college | 27 | 49.1 | |
| Completed 4 yr undergraduate degree | 7 | 12.7 | |
| Completed post-graduate degree | 7 | 12.7 |
Cognition dysfunction and reduced participation are prevalent
Table 2 shows the descriptive statistics for the cognitive measures (MoCA, CF, and CSI), participation, and the other disease-associated symptoms. Figure 1 shows the distributions of scores on the MoCA, CSI, CF and Participation. For the MoCA, 52.7% of participants scored in the normal range (≥26) and 47.3% scored in the cognitive impairment range (<26).21 Among people in the cognitive impairment range, three participants (5.5% of the entire sample) had scores <21, which is used as the cutoff for potential dementia in the context of aging or neurodegenerative disease.21,22 The sample’s average CF T-score indicated mildly impaired cognitive function, with 45.5% of participants in the normal range, 21.8% mildly impaired, and 32.7% moderately impaired. There are no published cutoffs or normative data for the CSI, but the sample’s average score was similar to that of an SLE sample from a prior study (33.6 ± 10.5), which was significantly higher (indicating worse everyday cognitive function) than a comparison sample of people with rheumatoid arthritis (29.4 ± 6.8).29
Table 2.
Descriptive statistics from the cognitive, disease-associated symptoms, and participation measures.
| Assessment | Mean | SD | Min–Max |
|---|---|---|---|
| MoCA | 25.3 | 3.1 | 17–30 |
| CSI | 35.8 | 7.9 | 21–56 |
| PROMIS Cognitive Function 8a (T scores) | 45.0 | 8.5 | 30.1–63.5 |
| PROMIS-43 Profile (T scores) | |||
| Physical function | 42.6 | 9.1 | 21.6–58.7 |
| Anxiety | 52.6 | 11.6 | 39.1–74.1 |
| Depression | 50.2 | 11.3 | 38.4–76.9 |
| Fatigue | 58.2 | 10.1 | 33.4–76.8 |
| Sleep disturbance | 56.8 | 10.1 | 31.7–76.1 |
| Participation | 46.9 | 11.3 | 26.7–65.5 |
| Pain interference | 56.7 | 10.7 | 41.1–76.3 |
MoCA: Montreal Cognitive Assessment; CSI: Cognitive Symptom Inventory; PROMIS: Patient Reported Outcome Measures Information System; Participation: ability to participate in social roles and activities.
Figure 1.
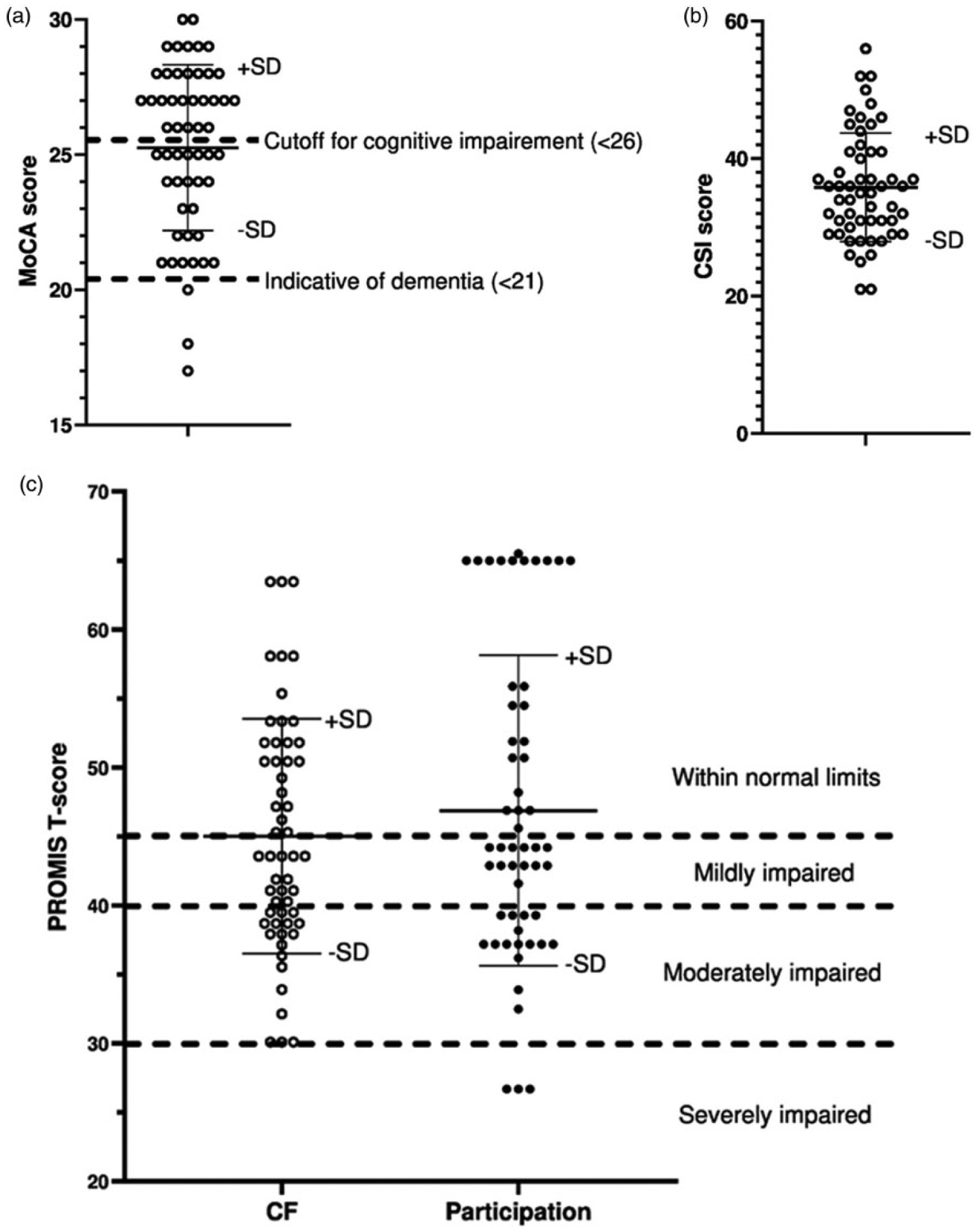
Distribution of (a) MoCA, (b) CSI, and (c) PROMIS CF and participation scores.
The sample’s average participation T-score indicated low normal participation, with 43.6% of participants in the normal range, 23.6% mildly impaired, 27.3% moderately impaired, and 5.5% severely impaired.27
Cognitive dysfunction is associated with reduced participation
The MoCA did not correlate with either of the self-report measures of everyday cognition (CSI: r = –0.13, p = 0.37; CF: r = 0.23; p = 0.10), but the CSI and CF scores correlated with each other (r = −0.68, p < 0.001). Participation correlated with both self-report measures of everyday cognition (r ≥ 0.56, p < 0.001) but not significantly with MoCA (r = 0.25, p = 0.06), such that lower participation was associated with poorer perceived cognition. In addition, participation correlated with the other disease-associated symptoms (r ≥ 0.49, p < 0.001), such that lower participation was associated with lower functioning or higher symptom burden in those domains.
Coefficients for the hierarchical regression model are in Table 3. The disease-associated symptoms accounted for an initial 67.7% of the variance in participation, F (5, 49) = 20.52, p < 0.001, the MoCA accounted for an additional 0.6% of the variance, FΔ(1, 48) = 0.89, p = 0.35, and then the CSI and CF together accounted for an additional 10.1% of the variance, FΔ(2, 46) = 10.67, p < 0.001, resulting in a significant model, R2 = 0.78, F(2, 46) = 10.67, p < 0.001. CSI, fatigue, and pain were each significant independent predictors of participation.
Table 3.
Multiple linear regression model examining independent predictors of participation.*
| St | Independent variables | B | SE B | β | t | p |
|---|---|---|---|---|---|---|
| 1 | Disease-associated symptoms (PROMIS-43 Profilea) | |||||
| Anxiety | −.031 | .147 | −.027 | −.210 | .835 | |
| Depression | .210 | .150 | .180 | 1.403 | .167 | |
| Fatigue | −.483 | .105 | −.439 | −4.608 | .000 | |
| Sleep Disturbance | .019 | .103 | .017 | .181 | .857 | |
| Pain Interference | −.449 | .092 | −.471 | −4.875 | .000 | |
| 2 | Objective cognition | |||||
| MoCA | .155 | .207 | .065 | .751 | .457 | |
| 3 | Self-reported everyday cognition | |||||
| CSI | −.332 | .090 | −.356 | −3.666 | .001 | |
| PROMIS Cognitive Function 8a | .025 | .105 | .027 | .241 | .811 |
PROMIS: Patient Reported Outcome Measures Information System; MoCA: Montreal Cognitive Assessment; CSI: Cognitive Symptom Inventory.
The bold and italics indicate significant independent predictors.
Discussion
Cognitive impairment in SLE is well documented, but its impact on day-to-day functioning has not been well studied. Our purpose was to investigate cognition and its relevance to participation among people with SLE. To this end, we used an objective assessment of cognitive ability and self-report measures of everyday cognitive function, other potentially influential disease-associated symptoms, and participation. We found that both perceived cognitive dysfunction and reduced participation are prevalent among people with SLE, and that perceived cognitive dysfunction is associated with reduced participation even after accounting for the effects of other common disease-associated symptoms.
Our findings are consistent with the high rate of cognitive impairment among people with SLE previously reported in the literature. Almost half of the participants in this sample had cognitive impairment according to the MoCA cognitive screening tool, which is within the prevalence range reported in other studies in SLE that have used the MoCA (e.g., 35–59%).17,30 Additionally, our results highlight the severity of cognitive impairment in this population. Whereas the average MoCA score of this sample was at the cutoff for cognitive impairment, several participants had scores that are considered indicative of dementia (<21) in the context of aging or other conditions such as Alzheimer’s disease and Parkinson disease.18,22 This degree of cognitive impairment is noteworthy given the young age of the sample and the fact that people with neurological or other conditions known to affect cognition were excluded. Although our data regarding the distribution of cognitive impairment should be interpreted with caution since this was not an epidemiological study, these findings reinforce the notion that cognitive impairment is a frequent and sometimes severe sequela of SLE.
Results from the self-report cognitive measures suggest deficits in everyday cognitive functioning among people with SLE as well. More than half of the participants reported some degree of everyday cognitive impairment compared to the general US population on the PROMIS CF, with approximately a third of them scoring in the moderate impairment range. To our knowledge, there are no published cutoff criteria for the CSI; however, our sample had a similar CSI score to that of an SLE sample from another study, which was significantly higher (worse) than a comparison sample of participants with rheumatoid arthritis.21 Taken together, these findings demonstrate that people with SLE are experiencing noticeable cognitive problems in their daily lives.
Objective cognitive function did not correlate with self-reported cognition or participation, but self-reported cognition did correlate to participation. These results may be related to the method of assessment, or they could imply that asking people about their cognition in everyday life is more informative of overall daily function than performance on tests of isolated cognitive skills. Either way, they suggest that paying attention to self-reported in addition to objectively measured cognition will enhance researchers’ and health professionals’ understanding of both cognition and daily function among people with SLE.
Providing further support for the functional relevance of cognitive dysfunction in SLE, cognition accounted for a substantial portion of the variance in participation after accounting for the effects of a range of other disease-associated factors such as pain, sleep problems and depressive symptoms. Specifically, increased perceived problems in everyday cognitive activities as measured by the CSI was a significant independent predictor of reduced participation. These findings corroborate recommendations that the CSI should be included in evaluations of cognitive function in SLE as an indicator of functional capabilities associated with cognition.23,29 However, further work is required to determine the meaning of different CSI scores and to establish clinically- and functionally-relevant cutoff scores.
Our results provide new insight into cognitive dysfunction and its importance for daily function among people with SLE. Previous studies have shown that cognitive dysfunction contributes to poor employment-related outcomes in SLE, which is one aspect of participation.13,15,16 We have additionally shown that perceived cognitive dysfunction interferes with social, leisure, work, and family aspects of participation among people with SLE, which are essential for health and well-being.31 These findings highlight the broad effects of SLE-associated cognitive dysfunction. In addition, our cohort has a unique composition compared to prior studies of cognition in SLE, in that it has a higher proportion of African American participants (51%).28 The prevalence and severity of SLE is higher in African Americans compared to Caucasians, so our results may be more representative of the dysfunction in both cognition and participation experienced by African Americans with SLE compared to existing work.17,28
In terms of other disease-associated symptoms, we found that fatigue and pain were also strong independent predictors of participation. These results dovetail with qualitative work in which people with SLE report pain and fatigue as among the most frequent or problematic aspects of the disease and describe their negative impact on social, leisure and family participation.32,33 Interestingly, other common manifestations of SLE that have been found to affect activities of daily living and health-related quality of life, such as depressive symptoms, anxiety and sleep problems,14 were not independently related to participation in our sample. These factors are known to be related to cognition, fatigue, and pain and to each other in SLE,33 so it is possible that their contributions to participation were masked. While multicollinearity statistics indicated this was not a problem in our models, future studies with larger samples should aim to disentangle the relationships among the various disease-associated symptoms and their effects on participation in people with SLE. In particular, the effects of emotional difficulties, such as depressed mood and anxiety, on perceived cognitive dysfunction and participation warrant further investigation since these problems are prevalent and often coexist in people with SLE,16 and previous studies have shown that they are associated with perceived cognitive dysfunction and participation at work.9,13,29
This study has several limitations. The sample was relatively small with potentially limited power, so we did not account for other demographic or disease-associated factors that could influence cognition, participation, or their relationship (e.g., age, disease activity, medications). Given that people with SLE experience wide fluctuations in their symptoms9 and this was a cross-sectional study, our results could have been influenced by the timing of evaluation or differing response timeframes of the measures, or it may not have captured the full effect of SLE on people’s lives. In addition, many of our outcomes relied on the self-report of participants, which might be subject to bias from physical condition, cognitive status, or mood. Finally, we used a global cognitive screening tool, whereas future studies should employ comprehensive neuropsychological assessment to determine the relative effects of deficits in specific cognitive domains on daily function in SLE.
In summary, we found that cognitive dysfunction is common and, in some cases, severe among people with SLE. Furthermore, along with pain and fatigue, reduced perceived everyday cognitive function relates to reduced participation in social, leisure, work and family-related activities. This study represents an initial step in understanding the implications of SLE-associated cognitive dysfunction for everyday life. Future longitudinal studies in larger, more diverse samples are needed to draw firm conclusions about the profile and pattern of cognitive dysfunction in SLE and the nature of the relationships among cognition, other disease-associated factors, and participation in this population. This will lead to improved detection of functionally relevant cognitive deficits among people with SLE and cognitive rehabilitation strategies to help individuals continue to participation in their important and meaningful activities and roles.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kuhn A, Bonsmann G, Anders H-J, Herzer P, Tenbrock K and Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Arztebl Int 2015; 112: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladman DD. Prognosis and treatment of systemic lupus erythematosus. Curr Opin Rheumatol 1996; 8: 430–437. [DOI] [PubMed] [Google Scholar]
- 3.Liang MH, Corzillius M, Bae SC, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed] [Google Scholar]
- 4.Mak A, Cheung MW-L, Chiew HJ, Liu Y and Chun-Man Ho R. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum 2012; 41: 830–839. [DOI] [PubMed] [Google Scholar]
- 5.Jolly M How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005; 32: 1706–1708. [PubMed] [Google Scholar]
- 6.Tani C, Moraes-Fontes M, Carli L, et al. Neuropsychiatric questionnaires in systemic lupus erythematosus. Clin Exp Rheumatol 2014; 32: S59–S64. [PubMed] [Google Scholar]
- 7.Leslie B and Crowe S. Cognitive functioning in systemic lupus erythematosus: a meta-analysis. Lupus 2018; 27: 920–929. [DOI] [PubMed] [Google Scholar]
- 8.Kozora E, Hanly JG, Lapteva L and Filley CM. Cognitive dysfunction in systemic lupus erythematosus: past, present, and future. Arthritis Rheum 2008; 58: 3286–3298. [DOI] [PubMed] [Google Scholar]
- 9.Baker K and Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology 2009; 48: 281–284. [DOI] [PubMed] [Google Scholar]
- 10.Ben Ari E, Johansson S, Ytterberg C, Bergström J and von Koch L. How are cognitive impairment, fatigue and signs of depression related to participation in daily life among persons with multiple sclerosis? Disabil Rehabil 2014; 36: 2012–2018. [DOI] [PubMed] [Google Scholar]
- 11.Harrison M, Morris K, Horton R, et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology 2005; 65: 1325–1327. [DOI] [PubMed] [Google Scholar]
- 12.International classification of functioning, disability and health: ICF. Geneva: World Health Organization, 2001. [Google Scholar]
- 13.Appenzeller S, Cendes F and Costallat LT. Cognitive impairment and employment status in systemic lupus erythematosus: a prospective longitudinal study. Arthritis Rheum 2009; 61: 680–687. [DOI] [PubMed] [Google Scholar]
- 14.Robinson D Jr, Aguilar D, Schoenwetter M, et al. Impact of systemic lupus erythematosus on health, family, and work: the patient perspective. Arthritis Care Res 2010; 62: 266–273. [DOI] [PubMed] [Google Scholar]
- 15.Covey TJ, Shucard JL, Shucard DW, Stegen S and Benedict RHB. Comparison of neuropsychological impairment and vocational outcomes in systemic lupus erythematosus and multiple sclerosis patients. J Int Neuropsychol Soc 2012; 18: 530–540. [DOI] [PubMed] [Google Scholar]
- 16.Mikdashi JA, Esdaile JM, Alarcón GS, et al. ; Ad Hoc Committee on Lupus Response Criteria: Cognition Sub-committee. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus 2007; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 17.Nantes SG, Su J, Dhaliwal A, Colosimo K and Touma Z. Performance of screening tests for cognitive impairment in systemic lupus erythematosus. J Rheumatol 2017; 44: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 18.Paez-Venegas N, Jordan-Estrada B, Chavarria-Avila E, et al. The Montreal cognitive assessment test: a useful tool in screening of cognitive impairment in patients with systemic lupus erythematosus. J Clin Rheumatol 2019; 25: 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725–1725. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, B edirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 22.Dalrymple-Alford J, MacAskill M, Nakas C, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010; 75: 1717–1725. [DOI] [PubMed] [Google Scholar]
- 23.Alarcón GS, Cianfrini L, Bradley LA, et al. ; Lumina Study Group. Systemic lupus erythematosus in three ethnic groups. X. Measuring cognitive impairment with the cognitive symptoms inventory. Arthritis Rheum 2002; 47: 310–319. [DOI] [PubMed] [Google Scholar]
- 24.Lai JS, Wagner LI, Jacobsen PB and Cella D. Self-reported cognitive concerns and abilities: two sides of one coin.? Psychooncology 2014; 23: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010; 63: 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and work-flow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Cella D, Gershon R, et al. Representativeness of the patient-reported outcomes measurement information system internet panel. J Clin Epidemiol 2010; 63: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojan G and Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol 2018; 30: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanly JG, Su L, Omisade A, Farewell VT and Fisk JD. Screening for cognitive impairment in systemic lupus erythematosus. J Rheumatol 2012; 39: 1371–1377. [DOI] [PubMed] [Google Scholar]
- 30.Kanapathy A, Nik Jaafar N, Shaharir S, Chan LF, Rozita M and Ch’ng SS. Prevalence of cognitive impairment using the Montreal Cognitive Assessment questionnaire among patients with systemic lupus erythematosus: a cross-sectional study at two tertiary centres in Malaysia. Lupus 2019; 28: 854–861. [DOI] [PubMed] [Google Scholar]
- 31.Utset TO, Fink J and Doninger NA. Prevalence of neurocognitive dysfunction and other clinical manifestations in disabled patients with systemic lupus erythematosus. J Rheumatol 2006; 33: 531–538. [PubMed] [Google Scholar]
- 32.Babaoglu H, Li J, Goldman D, Magder LS and Petri M. Predictors of predominant Lupus Low Disease Activity State (LLDAS-50). Lupus 2019; 28: 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McElhone K, Abbott J, Gray J, Williams A and Teh LS. Patient perspective of systemic lupus erythematosus in relation to health-related quality of life concepts: a qualitative study. Lupus 2010; 19: 1640–1647. [DOI] [PubMed] [Google Scholar]


