Abstract
Non-alcoholic fatty liver disease (NAFLD), or metabolic (dysfunction)-associated fatty liver disease (MAFLD), is characterized by high global incidence and prevalence, a tight association with common metabolic comorbidities, and a substantial risk of progression and associated mortality. Despite the increasingly high medical and socioeconomic burden of NAFLD, the lack of approved pharmacotherapy regimens remains an unsolved issue. In this paper, we aimed to provide an update on the rapidly changing therapeutic landscape and highlight the major novel approaches to the treatment of this disease. In addition to describing the biomolecules and pathways identified as upcoming pharmacological targets for NAFLD, we reviewed the current status of drug discovery and development pipeline with a special focus on recent evidence from clinical trials.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, chronic liver disease, hepatoprotection, metabolic disorders
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) includes a range of chronic conditions characterized by excessive hepatic lipid accumulation, defined by the presence of steatosis in >5% of hepatocytes, in the absence of significant alcohol consumption or other causes of liver injury [1]. The global prevalence of NAFLD is currently estimated at 25% [2], and is projected to increase by 21% by 2030 [3]. About 20–25% of NAFLD cases are classified as non-alcoholic steatohepatitis (NASH), which has a substantially higher risk of progression to liver fibrosis, cirrhosis, and end-stage liver disease, and hepatocellular carcinoma (HCC) [3]. Considering the highly heterogeneous pathogenesis of metabolic liver diseases, the term ‘metabolic (dysfunction)-associated fatty liver disease’ (MAFLD) has recently been proposed as a broader alternative to the conventional ‘NAFLD’ [4].
Due to the increasingly high medical and socioeconomic burden of this disease as well as its strong association with obesity, metabolic and cardiovascular disorders, NAFLD pharmacotherapy has been the focus of latest research [3,5]. However, to date, no drug has been approved by neither the European Medicines Agency nor the United States Food and Drug Administration (FDA) for NAFLD [2,6].
In the present paper, we aimed to review the most recent data on major pharmacological targets in this disease, summarize the clinical evidence for novel investigational agents as well as currently marketed drugs, and highlight the latest advances in the drug development pipeline for NAFLD.
2. THRβ Agonists
Thyroid hormone receptor beta (THRβ) is a nuclear receptor and a transcription factor that mediates the genomic effects of thyroid hormones. THRβ1 is the predominant isoform expressed in the human liver, while other THRs are also found in the heart, brain, kidney, skeletal muscle, and other tissues. Upon activation by triiodothyronine or other agonist, THRβ displaces corepressor proteins from the deoxyribonucleic acid (DNA) and facilitates coactivator binding, thus allowing for gene transcription. THRβ can form heterodimers with other THR or nuclear receptor superfamily proteins, such as liver X receptor or peroxisome proliferator-activated receptors (PPAR) [7].
Hepatic THRβ upregulate free fatty acid (FA) uptake and oxidation, lipophagy and lipolysis, and promote mitochondrial biogenesis and respiration, leading to increased adenosine triphosphate (ATP) consumption and energy expenditure. THRβ have also been shown to induce transcriptional activation of bile acid (BA) synthesis, biliary lipid secretion, and cholesterol serum clearance, leading to a decrease in proatherogenic lipoprotein levels [8,9]. Additionally, THRβ stimulates normal hepatocyte proliferation, at the same time downregulating nuclear signaling pathways and inhibiting tumour growth and metastasis formation in HCC [10].
Selective THRβ agonists that are currently being developed for the treatment of NAFLD include resmetirom (MGL-3196), VK2809 (MB 07811), TERN501, ASC41, and MGL-3745. In a 36-week phase 2 randomized clinical trial (RCT) with an additional 36-week open label extension, resmetirom was safe, well tolerated, and significantly improved lipid profiles, liver steatosis (as indicated by magnetic resonance imaging/proton density fat fraction (MRI-PDFF)), liver stiffness (assessed by transient elastography), and N-terminal type III collagen pro-peptide (Pro-C3) levels in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH) [11], supporting its further evaluation in three ongoing phase 3 trials (NCT04197479, NCT04951219, NCT03900429). The lipid-lowering effect of resmetirom was accompanied by a significant improvement in alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (GGT) levels as well as a reduction in NAFLD activity (NAS) and enhanced liver fibrosis (ELF) scores [11].
VK2809 (MB07811) is a prodrug yielding an active metabolite via oxidation by hepatic CYP3A4 enzyme. A 12-week phase 2a RCT found VK2809 to possess significant antisteatotic activity in NAFLD patients [12]. ASC41, also a prodrug, improved serum lipids and liver histology in rats [13], and subsequently demonstrated a good safety profile and substantial hypolipidemic activity in healthy volunteers [14], advancing to phase 2 (NCT05118360). A newer THRβ agonist, TERN-501, reduced serum cholesterol levels and attenuated liver steatosis and fibrosis in rodent models of hyperlipidaemia and NASH [15], and has been recently approved for phase 1 clinical trials [16,17]. MGL-3745 is being evaluated in preclinical studies, and no data regarding its therapeutic activity are available yet. Two fixed-dose combinations (FDC), ASC43 and ASC45, containing ASC41 and either a FA synthase (FASN) inhibitor (ASC40) or a farnesoid X receptor (FXR) agonist (ASC42), are being evaluated in preclinical and phase 1 clinical trials, respectively [16].
3. Lipogenesis Inhibitors
Acetyl-CoA carboxylase (ACC) is a multi-subunit enzymatic complex that catalyzes the irreversible carboxylation of acetyl-coenzyme A (CoA) to produce malonyl-CoA. Acetyl- and malonyl-CoA are then utilized by FASN to synthesize palmitate, which is converted to stearate by the elongation of long-chain fatty acids family member 6. Stearoyl-CoA desaturase 1 (SCD1) converts stearoyl-CoA and palmitoyl-CoA into unsaturated fatty acids, which are later esterified with glycerol by several transferases, including diglyceride acyltransferase (DGAT), to produce triacylglycerides (TAG). Two DGAT isozymes have been identified so far: DGAT1 and DGAT2, which appear to be primarily responsible for esterifying exogenous and endogenous fatty acids, respectively [18]. ACC, FASN, SCD1, and DGAT catalyze the rate-limiting steps in the de novo lipogenesis, and have therefore been considered suitable targets for the treatment of NAFLD.
However, some animal evidence suggests that inhibition of TAG synthesis may worsen hepatic inflammation and fibrosis [19], and alter intestinal barrier function, leading to diarrhoea and steatorrhoea [20]. These findings might represent a challenge for the development of lipogenesis inhibitors and potentially limit their clinical application to early stages of liver steatosis not associated with inflammation and fibrogenesis [21].
3.1. ACC Inhibitors
Firsocostat (GS-0976) and clesacostat (PF-05221304) are selective ACC inhibitors currently under development for combination therapy of NASH. Since ACC is indirectly inhibited by FXR [22], firsocostat has been combined with the FXR agonist (see 4.1 FXR agonists) cilofexor in order to maximize the resulting effects. In the phase 2b ATLAS trial, 20 mg/d firsocostat + 30 mg/d cilofexor provided significant reductions in NAS scores, liver steatosis, lobular inflammation and hepatocellular ballooning (HCB), and improved liver biochemistry over 48 weeks in patients with septal fibrosis due to NASH [23]. Clesacostat (2–50 mg/d) has demonstrated good efficacy in terms of reducing liver steatosis, and is being developed in combination with the DGAT2 inhibitor ervogastat to address the frequently observed elevation of serum TAG, a known effect of ACC inhibitors [24]. Two phase 2 studies are ongoing to determine the optimal doses of both agents for NASH with and without liver fibrosis (NCT04399538, NCT04321031).
3.2. FASN Inhibitors
Known investigational FASN inhibitors include ASC40 (TVB-2640), FT-8225, and ASC44F, a fixed-dose combination containing ASC40 and ASC42 (a FXR agonist; see FXR agonists). ASC40 (50–150 mg/d) provided near complete (~90%) inhibition of de novo lipogenesis and reduced liver steatosis in obese subjects with substantial NAFLD risk [25]. In the phase 2 FASCINATE-1 trial, ASC40 at lower doses (25 or 50 mg/d) improved ALT and low-density lipoprotein (LDL) cholesterol levels, attenuated liver steatosis, fibrosis, lipotoxicity, dyslipidemia, and hepatic insulin resistance in obese NASH patients [26]. A subsequent phase 2 study, FASCINATE-2, has been initiated (NCT04906421). The anti-inflammatory and antifibrotic properties of ASC40 have been linked to inhibited proinflammatory cytokine production, T-cell differentiation, and repression of collagen synthesis [26]. FT-8225 and ASC44F have entered preclinical development [16,27]; no data regarding their efficacy is available yet.
3.3. SCD1 Inhibitors
Aramchol (C20-FABAC) is a synthetic conjugate of arachidic and cholic acids and the first-in-class inhibitor of SCD1. In addition to inhibiting de novo lipogenesis and hepatic stellate cell (HSC)-mediated fibrogenesis [28], aramchol is capable of reducing serum cholesterol levels and promoting gallstone dissolution via stimulation of macrophage cholesterol efflux and solubilization of cholesterol [29]. In the recently completed 52-week phase 2b ARREST trial, aramchol (600 mg/d) did not meet the primary endpoint (a significant decrease in hepatic TAG), but was well tolerated and improved liver histology and ALT levels [30]. The phase 3 ARMOR RCT has been designed to evaluate the safety and efficacy of 300 mg/d aramchol in patients with biopsy-confirmed liver fibrosis due to NASH (NCT04104321).
3.4. DGAT Inhibitors
Ervogastat (PF-06865571), a selective DGAT2 inhibitor, significantly reduced liver fat fraction in patients with mild NAFLD [31]. Two phase 2 RCT are underway to assess the safety and efficacy of ervogastat alone and in combination with clesacostat in NASH patients with and without liver fibrosis (NCT04399538, NCT04321031). ION224 (IONIS-DGAT2Rx) is a ligand-conjugated chimeric antisense oligonucleotide designed to suppress the biosynthesis of DGAT2. ION224 reduced total liver fat content and several fibrosis biomarker levels in a small-scale trial in patients with type 2 diabetes mellitus (T2DM) and NAFLD [32], and is planned to be evaluated in a longer phase 2 RCT in nondiabetic subjects (NCT04932512). Newest DGAT2 inhibitors include PF-07202954, and DGAT1 inhibitors, VK1430, SNP-610, and SNP-630; the latter two molecules are reported to have additional CYP2E1-inhibiting properties [33].
3.5. ω-3 PUFAs
ω-3 Polyunsaturated fatty acids (ω-3 PUFA) are long-chain FA characterized by the presence of a double bond three atoms away from the terminal methyl group in their chemical structure. The three most common biologically active ω-3 PUFA include α-linolenic (ALA) acid and its metabolites eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids. ω-3 PUFAs cause transcriptional repression of the key enzymes involved in hepatic glycolysis and de novo lipogenesis, such as ACC, FASN, and L-pyruvate kinase [34].
Increased ω-3 PUFA intake results in their increased incorporation into cell membrane phospholipids, corresponding to a positive shift in the ω-3/ω-6 ratio. This leads to decreased availability of the ω-6 arachidonic acid (AA), and the subsequent substrate-dependent inhibition of eicosanoid inflammatory mediator production by the leukocytes. Moreover, EPA and DHA have been found to suppress leukocyte chemotaxis and adhesion molecule expression, altogether providing a multimodal anti-inflammatory effect [35]. In addition, ω-3 PUFA are also known for their prominent antioxidant, regenerative, and antitumour properties [36].
A 6-week treatment with ω-3 PUFA (64% ALA + 21% EPA + 16% DHA) was associated with significant improvement of hepatic proteomic and plasma lipidomic markers of lipogenesis, lipotoxicity, oxidative stress, and mitochondrial respiration in patients with biopsy-confirmed NASH [37]. The increase in plasma ALA and DHA levels, and the subsequent decrease in AA levels correlated with the percentage of patients with improvements in NAS scores, lobular inflammation, and HCB. However, the overall liver histology and body weight were not significantly altered by ω-3 PUFA treatment [38].
A systematic review and a meta-analysis confirmed that DHA and EPA attenuate liver steatosis with no significant weight loss in adults [39], and a small-scale RCT reported the same effects for dietary DHA supplementation in children [40]. More recently, three meta-analyses, including up to 22 RCT and more than 1300 patients, found ω-3 PUFA to significantly decrease ALT, aspartate aminotransferase (AST), GGT levels, liver fat content and insulin resistance, having no significant effect on body weight in NAFLD [41,42,43].
Epeleuton (15-hydroxyeicosapentaenoic acid ethyl ester, DS102) is a second-generation synthetic EPA derivative. In a 16-week phase 2a RCT in obese subjects with NAFLD, epeleuton (2 g/d) improved circulating inflammatory markers, lipid profiles, and insulin resistance, but failed to reach either of the primary endpoints including reductions in ALT levels and liver stiffness [44]. Since a slight dose-dependent attenuation of liver steatosis was observed, further trials of longer duration are planned [45].
Icosabutate (NST-4016) is a structurally engineered ω-3 PUFA ether characterized to increased liver exposure due to direct absorption into the portal vein [46]. The 62-week phase 2b ICONA trial is underway to evaluate the efficacy of icosabutate (300 or 600 mg/d) in patients with biopsy-confirmed NASH (NCT04052516). Interim analysis data indicated significant dose-dependent decreases among both dosage groups in ALT, AST, GGT, and alkaline phosphatase (ALP) levels, while patients dosed with 600 mg/d icosabutate also had improvements in non-invasive fibrosis and inflammatory biomarker profiles [46].
4. Bile Acid Metabolism Modulators
4.1. FXR Agonists
Farnesoid X receptor (FXR), also known as bile acid receptor, is a nuclear receptor expressed at high levels in the liver and ileum. FXR acts as a BA sensor and governs a major negative feedback loop in the BA, glucose, cholesterol, and TAG metabolism [22]. Many of the effects of FXR are directly mediated by fibroblast growth factors (FGF) 19 and 21, downstream messengers whose functions are briefly described in Fibroblast growth factor analogues. Upon increased postprandial release of BA into the intestine, activated FXR induces the transcriptional repression of the rate-limiting enzyme cholesterol 7α-monooxygenase (CYP7A1) and several transporters involved in BA biosynthesis and liver uptake. CYP7A1 inhibition by FXR enhances the excretion of excessive cholesterol via the canalicular transporters into bile as well as directly into the intestinal lumen. FXR also upregulates the expression of bile salt export pump, multidrug resistance protein-3, and organic solute transporter α/β, which facilitate BA efflux from hepatocytes and maintain the enterohepatic circulation of bile [47]. FXR activation also favors bile acid conjugation and detoxification, and stimulates biliary phospholipid excretion.
FXR and its downstream targets repress sterol regulatory element-binding protein 1 SREBP-1c, the major transcription factor for lipogenic pathways, thereby inhibiting FASN, ACC, and SCD1. In addition, prandial glucose can increase the intestinal FXR activity via post-translational modification, shifting the equilibrium towards glycogen deposition and reducing blood glucose levels [22]. The multifaceted nature of FXR has made it one of the most attractive novel targets for NAFLD therapy. Current FXR-activating drug candidates include the sterol derivatives obeticholic acid (OCA), EDP-305, INT-767, and INT-787, and the non-steroidal compounds MET409, tropifexor, cilofexor, vonafexor, TERN-101, ASC42, EDP-297, HPG1860, and HPG7233.
OCA (Ocaliva®), the first-in-class FXR agonist, is approved by the FDA for non-cirrhotic primary biliary cholangitis (PBC) and is nearing approval for liver fibrosis due to NASH [48]. In phase 2 studies, OCA increased insulin sensitivity and reduced markers of liver damage in patients with T2DM and NAFLD [49], and reduced ALT levels, improved NAS scores, prevented and partially reversed fibrogenesis in non-diabetic, pre-cirrhotic NASH patients [50]. Currently, the efficacy and safety of OCA are being evaluated in two phase 3 trials, the REGENERATE trial in NASH/fibrosis patients (NCT02548351), and the REVERSE trial in patients with compensated cirrhosis due to NASH (NCT03439254).
OCA and especially its taurine and glycine conjugates are known to actively bind Takeda G-protein receptor 5 (TGR5), a cell membrane G protein-coupled receptor (GPCR) that has been largely implicated in BA-associated pruritus development [51] and gallstone formation [52]. Hence, several newer molecules, e.g., EDP-305 and INT-787, have been structurally optimized in an attempt to avoid TGR5 engagement and potential safety concerns [53]. However, PBC trials have shown that highly selective FXR agonists retain their adverse effects, at the same time losing in overall efficacy [54,55]. EDP-305 was later repurposed for use in NASH, and, along with its close analogue EDP-297, is now intended to be reserved for future combination regimens following mixed interim results of the phase 2b ARGON-2 study [56]. Since FXR and TGR5 seem to exert additive metabolic effects, dual FXR/TGR5 agonists with balanced activity towards both targets, such as INT-767 and BAR502, have been proposed for further development for NAFLD treatment [57,58].
MET409, a structurally optimized fexaramine-derived FXR agonist with greater affinity towards intestinal rather than hepatic FXR, is characterized by improved efficacy and a differentiated adverse effect (pruritus and increased LDL-cholesterol levels) profile compared to OCA [59], and is currently being evaluated in a phase 2a RCT alone and in combination with empagliflozin (NCT04702490).
Tropifexor (LJN452), cilofexor (GS-9674), and vonafexor (EYP001) represent a group of non-steroidal small molecules with structures different from both OCA and MET409, resulting in a differential pattern of FXR-related gene expression [60] and possibly improved anticholestatic activity. The parent compound for this group, turofexorate (WAY-362450), completed a phase 1 study, but its development was discontinued thereafter [61]. Tropifexor (200, but not 140 mcg/d) reduced ALT, GGT levels, body weight, and liver fat content, and attenuated liver fibrosis in patients with biopsy-confirmed NASH in the 48-week phase 2 FLIGHT-FXR RCT [62,63]. Two additional phase 2 trials are currently underway to investigate the efficacy of tropifexor combinations with licogliflozin (NCT04065841) and LYS006 (NCT04147195) in NASH/fibrosis and NAFLD/NASH, respectively.
Cilofexor reduced liver fat content (100 mg/d) and GGT, N-terminal type IV collagen pro-peptide, and serum primary BA levels (30 or 100 mg/d) over 24 weeks, but did not affect liver elasticity and ELF scores in NASH patients [64]. A phase 2 RCT has been initiated to evaluate the efficacy of a fixed-dosed combination of cilofexor and firsocostat, alone or in combination with semaglutide, for compensated cirrhosis due to NASH (NCT04971785). Vonafexor (100 mg/d for 12 weeks), a 2nd generation, highly selective non-steroidal FXR agonist, has recently been found to improve liver steatosis, the fibro-inflammation marker cT1 levels, and estimated glomerular filtration rate (eGFR) in NASH patients with normal or mildly decreased eGFR [65]. Nidufexor (LMB763), a non-steroidal partial FXR agonist, was being developed for NASH, liver fibrosis, and cholestatic liver disease, but appears to have been discontinued despite seemingly promising results from a phase 2 study in NASH subjects [66].
TERN-101 demonstrated a favorable safety profile and improved ALT levels and liver steatosis in the phase 2a LIFT RCT in patients with pre-cirrhotic NASH [67]. ASC42 treatment was associated with biochemical and histological improvements in animal NASH models [16], while no data are available yet for novel compounds HPG1860 and HPG7233 [68].
4.2. FGF Analogues
Fibroblast growth factors (FGF), including FGF19 and FGF21, are a family of hormone-like peptides with broad metabolic, transcriptional, and mitogenic activity. FGF19 is released by the ileal enterocytes into the enterohepatic circulation in response to postprandial FXR activation by BA. FGF21 is highly expressed in the liver and is released in response to PPARα, carbohydrate-response element-binding protein (ChREBP), and general control nonderepressible 2 kinase activation in the presence high serum glucose, high FFA and low amino acid levels [69]. FGF19 and FGF21 exert their physiological effects via activation of transmembrane complexes of the enzyme β-klotho (KLB) and its FGF co-receptors (FGFR) FGFR1c/2c/3c/4. FGF21 has the highest affinity for the FGFR1c/KLB complex, which is expressed in the adipose tissue and central nervous system, while FGF19 primarily targets FGFR4/KLB, which is found in hepatocytes [69].
Thus, FGF19 and FGF21 act as major downstream messengers in the FXR and PPARα signaling, and control the negative feedback loops to inhibit BA synthesis and lipolysis, respectively [69]. FGF19 represses CYP7A1, controls postprandial BA release into the intestinal lumen, inhibits hepatic gluconeogenesis, promotes glycogen deposition, and plays an important role in the regulation of hepatocyte proliferation and tumorigenesis. FGF21 lowers the preference for glucose intake, modulates energy expenditure, and promotes glucose and lipid uptake in the adipose tissue, which prevents ectopic lipid accumulation in liver and skeletal muscle [69].
The investigational FGF19 analogue aldafermin (NGM-282) (0.3–3 mg/d) failed to meet the primary endpoint of the 24-week phase 2b ALPINE 2/3 trial in NASH patients with stage 2 or 3 liver fibrosis, defined as a ≥1 NASH Clinical Research Network stage improvement in fibrosis with no worsening of NASH. However, the drug was well tolerated and was significantly superior to placebo in terms of NASH resolution, reduction of liver steatosis and non-invasive markers of liver injury [70]. The ALPINE 4 trial is ongoing to determine whether aldafermin could improve liver fibrosis and/or NASH in subjects with compensated cirrhosis (NCT04210245).
Current FGF21 analogues include efruxifermin (AKR-001), BIO89-100, NN9500, BFKB8488A, MK-3655, and GLP-1-Fc-FGF21 D1. Efruxifermin is a fusion protein of human immunoglobulin G1 (IgG1) Fc domain linked to a modified human FGF21 with balanced affinity towards FGFR1c/2c/3c. Efruxifermin (28–70 mg/d) improved lipoprotein profiles and glycaemic control in T2DM patients, significantly attenuated liver steatosis in the 16-week phase 2a BALANCED study [71], and is now being evaluated in three more phase 2 RCTs (NCT05039450, NCT04767529, NCT03976401). BIO89-100 is a specifically engineered glycolpolyethylene glycol (PEG)-ylated FGF21 analogue that led to clinically meaningful reductions in liver fat content, markers of inflammation and fibrosis in a phase 1b/2a proof-of-concept study in NASH patients [72]. Recently, the phase 2b ENLIVEN trial of BIO89-100 for stage 2/3 fibrosis due to NASH has been initiated (NCT04929483), while two phase 1 open-label studies are still underway (NCT05022693, NCT04048135). No preclinical data is available yet for the investigational compound NN9500 [73].
BFKB8488A and MK-3655 are humanized bispecific anti-FGFR1c/KLB agonist monoclonal antibodies (mAB). BFKB8488A was safe and adequately tolerated, reduced liver steatosis in a dose-dependent fashion, and improved markers of cardiometabolic and liver health in obese T2DM patients with NAFLD [74]. The phase 2b BANFF trial has been initiated to explore the efficacy and safety of this compound in non-alcoholic liver fibrosis (NCT04171765). MK-3655 (NMG313), an insulin-sensitizing anti-FGFR1c/KLB agonist mAB, was effective against liver steatosis in obese and insulin-resistant NAFLD patients, and has proceeded into a phase 2b study in pre-cirrhotic NASH with or without T2DM (NCT04583423).
GLP-1-Fc-FGF21 D1 is a novel fusion protein incorporating a KLB-binding FGF21 variant and a glucagon-like peptide 1 receptor (GLP1R) agonist. In murine models of T2DM and obesity, GLP-1-Fc-FGF21 D1 improved liver function, serum and hepatic lipid profiles, and reduced body weight and NAS scores with an efficacy superior to either FGF21 or GLP1R agonists alone [75]. The PEGylated human recombinant FGF21 pegbelfermin (ARX-618) has recently demonstrated suboptimal efficacy in compensated cirrhosis due to NASH in the FALCON 2 trial, resulting in pending discontinuation and/or possible repurposing for other indications [76].
5. Fibrogenesis Inhibitors
5.1. Galectin Antagonists
Galectins, formerly known as S-type lectins, are a family of carbohydrate-binding proteins that are selective towards β-galactoside-containing glycans. To date, 15 subtypes of galectins family have been identified in humans, of which galectin-1, -3, and -9 are the most implicated in liver disease. Numerous experimental and clinical study results suggest that galectins play important and diverse roles in fibrogenesis, cellular immunity and inflammatory response, cell cycle regulation, apoptosis, regeneration, and tumorigenesis [77,78].
Galectin-1 promotes the proliferation, migration and activation of HSC, and the subsequent fibrogenesis via stimulation of transforming growth factor β (TGFβ)/platelet-derived growth factor signaling and disruption of cell adhesion. In HCC, galectin-1 promotes the epithelial–mesenchymal transition, cell adhesion, metastasis, and immunosuppression. However, elevated galectin-1 expression has also been found beneficial for liver regeneration, liver allograft survival, and recovery after hepatic ischemia-reperfusion injury [77].
Galectin-3 has been identified as a pivotal regulator in the progression of hepatitis, hepatic fibrosis, cirrhosis, and HCC. It was found to promote autocrine and paracrine HSC activation and phagocytosis, mediate the TGFβ-dependent fibrogenesis, and upregulate the expression of certain profibrogenic cytokines such as interleukin (IL) 33. The exact role of galectin-3 for liver cirrhosis is not as clear, but its increased expression has been linked to accelerated cirrhosis development and deterioration of liver function [77]. However, galectin-3 might be protective against adipose tissue inflammation, diabetes, and atherosclerosis progression, most probably due to and its ability to scavenge the proinflammatory and proapoptotic advanced glycation end products, the increased activation of the NF-κB signaling pathway, and the downregulation of NLRP3 inflammasome and IL1β expression in immune cells [77].
A systematic review and meta-analysis by An et al. found that serum galectin-3/9 levels correlate with the risk of liver failure and cirrhosis, and high galectin-1/3 expression is associated with poor prognosis in HCC. However, the evidence for galectin involvement in chronic liver disease remains controversial, and the impact of galectin-3 levels on NAFLD/NASH is thought to be dependent upon the stage and severity of liver damage [78]. Hence, modern galectin-targeting drug candidates are intended for use in advanced NASH complicated by liver fibrosis and/or cirrhosis.
GM-CT-01 and belapectin (GR-MD-02) are semi-synthetic polysaccharides (galactomannan and galactoarabino-rhamnogalacturonan, respectively) having high affinity towards extracellular galectin-3 and, to a lesser extent, galectin-1. Both compounds promoted resolution of portal inflammation, HCB, liver fibrosis, and cirrhosis, and reduced portal pressure in a toxin-induced liver injury model in rats [79]; however, only belapectin seems to have advanced further into clinical development. In the 52-week phase 2b NASH-CX study in subjects with NASH, liver cirrhosis, and portal hypertension, belapectin (2 mg/kg/2 weeks) did not affect fibrosis or NAFLD activity, but reduced hepatic venous pressure gradient values and prevented the development of esophageal varices in a sub-group of patients [80]. The phase 2/3 NAVIGATE trial has been initiated to further evaluate belapectin in patients with liver cirrhosis due to NASH and clinical signs of portal hypertension but without esophageal varices at baseline (NCT04365868).
GB1211, an oral small-molecule selective galectin-3 antagonist, was effective in several preclinical fibrosis models, and well tolerated in human volunteers. A phase 1/2a trial in NASH/fibrosis patients was approved but subsequently placed on hold due to an undisclosed change in the clinical development strategy for this compound (NCT04607655).
5.2. TLR4 Antagonists
Toll-like receptor 4 (TLR4) belongs to the family of pattern recognition receptors that activate the innate immune system by recognizing their major ligand, lipopolysaccharide (LPS). In the liver, TLR4 are expressed in both parenchymal and non-parenchymal type cells. TLR4 activation results in the activation of NF-κB, mitogen-activated protein kinase and interferon regulatory factor-mediated pathways, and the subsequent inflammatory cytokine and interferon production [81].
TLR4 stimulate adhesion molecule expression and chemokine secretion by the HSC, which induces Kupffer cell migration and the recruitment of extrahepatic monocytes into the liver. TLR4 also downregulate HSC expression of the Bambi protein, an endogenous TGFβ receptor inhibitor, thus promoting profibrogenic TGFβ signaling. Additionally, TLR4 can inhibit miR-29 expression and increase fibronectin production in the HSC, further enhancing HSC activation and migration [82].
JKB-122 treatment (5 or 35 mg/d for 12 weeks) was well tolerated and significantly improved ALT and AST levels, liver steatosis, and serum lipid profiles in a phase 2 study in patients with NAFLD [83]. Another phase 2 RCT is planned to investigate whether JKB-122 could ameliorate liver fibrosis due to NASH (NCT04255069). Recently, eritoran, a synthetic bacterial lipid analogue, was found to significantly reduce ALT levels, lobular inflammation, intrahepatic neutrophil infiltration, and liver fibrosis, but not liver steatosis, in murine models of acute and chronic liver injury [81].
5.3. LOXL2 Inhibitors
Lysyl oxidase-like protein (LOXL) 2 is a histone modifier amine oxidase that has been identified as the primary enzyme facilitating covalent crosslinking of collagen and elastin fibers, thereby promoting collagen network formation and progression of liver fibrosis. LOXL2 inhibition following the onset of fibrosis has been demonstrated to augment and accelerate collagen degradation in rodent models [84]. Only a few studies have concerned the role of LOXL3, a less common isozyme of LOXL, in fibrotic diseases, and its value as a therapeutic target is somewhat controversial [85].
Simtuzumab (GS-6624), a humanized antagonist mAB, was one of the first LOXL2-targeting drug candidates, that was discontinued after showing a lack of efficacy regarding liver fibrosis and/or portal hypertension in two phase 2b trials in patients with bridging fibrosis or compensated cirrhosis associated with NASH [86].
Novel LOXL2 antagonists are represented by orally available small molecules. PXS-5153A, a dual LOXL2/LOXL3 inhibitor, reduced disease severity and improved liver function by diminishing collagen content and collagen crosslinks in two rodent models of liver fibrosis [87]. Its cognate PXS-5382A, selective towards LOXL2, showed a satisfactory pharmacokinetic profile in healthy volunteers (NCT04183517), and is expected to advance into phase 2 trials. Another LOXL2 inhibitor, GB2064, was initially researched for myelofibrosis, but appears to have had its potential indication list expanded to include other fibrotic diseases [88].
5.4. ATX Inhibitors
Autotaxin (ATX) is a glycoprotein enzyme that converts membrane-derived lysophospholipids into lysophosphatidic acid (LPA). LPA, in turn, acts as a multimodal signaling molecule and causes cytoskeleton remodelling, alters cell proliferation and migration, and promotes fibrogenesis as well as inflammatory reactions [89]. Non-competitive inhibition of autotaxin/LPA signaling by the indole derivative PAT-505 resulted in a significant attenuation of liver fibrosis in mouse models of NASH [90]. In addition, ATX induction has been implicated in BA-mediated pruritus development, suggesting potentially favorable safety profiles of ATX inhibitors. Two small-molecule ATX inhibitors, TJC0265 and TJC0316, have been identified as lead compounds and are currently undergoing optimization and preliminary in vivo testing [91].
6. Glucose Metabolism Modulators
6.1. PPAR Agonists
PPAR are a family of nuclear receptors that function as transcription factors and play a regulatory role in glucose homeostasis, lipid metabolism, inflammatory response, cell development and differentiation [92]. Up to now, three PPAR subtypes have been identified: (1) PPARα, expressed in the liver, adipose tissue, skeletal muscle, heart, and kidney; (2) PPARγ, expressed in the adipose tissue, colon, macrophages, pancreas, skeletal muscle, etc.; and (3) PPARδ(β), found ubiquitously. Upon ligand binding, PPAR induce the release of corepressors and recruitment of coactivators, allowing for gene transcription. Moreover, PPAR can regulate the mitogen-activated protein kinase pathways, and inhibit inflammatory reactions via transrepression of several proinflammatory transcription factors such as NF-κB [92].
Hepatic PPARα stimulate mitochondrial FA uptake, β-oxidation, ATP production, and ketogenesis. They upregulate glucose-sensing transcription factors ChREBP and sterol regulatory element-binding transcription factor 1, and promote FGF21 expression, which increases tissue insulin sensitivity and maintains glucose homeostasis. Recent experimental studies strongly suggest that decreased hepatic PPARα expression positively correlates with insulin resistance and NASH severity, while NASH resolution is associated with an upregulation of PPARα as well as its target genes [92]. However, the evidence for the use of selective PPARα agonists in NAFLD is limited.
Fenofibrate (200 mg/d) induced complete resolution of biochemical and ultrasonographic evidence of NAFLD in almost half of the patients in an open-label RCT [93], but had minimal efficacy regarding liver histology [94]. Gemfibrozil (600 mg/d for 4 weeks) reduced liver enzyme levels but also did not produce any meaningful changes in liver morphology [95], while clofibrate (2 g/d) failed to show any beneficial effect in NASH patients [96]. Pemafibrate (0.2 mg/d) improved biochemical markers of liver damage and steatohepatitis according to non-invasive measures in NAFLD subjects [97], and bezafibrate reduced liver steatosis in obese mice with metabolic syndrome [98].
PPARγ are critical positive regulators of adipocyte differentiation and lipogenesis, insulin sensitivity, and glucose uptake by skeletal muscle. Additionally, PPARγ may reduce inflammation via inhibition of macrophage activation and tumour necrosis factor α production [92]. Latest EASL-EASD-EASO [1] and AASLD [99] clinical practice guidelines support the use of pioglitazone, a selective PPARγ agonist, in progressive and/or high-risk, biopsy-proven NASH, due to its efficacy regarding liver histology in NASH patients with or without T2DM. More recently, lobeglitazone has been demonstrated to reduce intrahepatic fat content and biochemical markers of liver damage in T2DM patients with NAFLD [100]. An experimental study found nifedipine, an L-type calcium channel blocker, to exert protective action against diet-induced NASH in rats, most probably due PPARγ activation [101].
PPARδ is the less studied PPAR isotype despite its ubiquitous expression. Experimental studies have reported PPARδ to reduce very low-density lipoprotein cholesterol levels, inhibit adipocyte growth and lipid uptake, prevent the formation of reactive oxygen species, and modulate Kupffer cell activation [92]. A recent study found a correlation between severe, but not moderate, hepatic steatosis and decreased hepatic PPARδ expression [102].
Seladelpar (MBX-8025) is the only selective PPARδ agonist currently in the pipeline for the treatment of NAFLD. The interim analysis results of a 52-week phase 2b RCT of seladelpar (10–50 mg/d) found it to reduce ALT, GGT, and ALP levels, but only minimally influence liver steatosis at 12 weeks of treatment [103]. Further trials have so far focused more on the anticholestatic properties of seladelpar, of potential value in primary biliary cholangitis (NCT03301506).
Saroglitazar (Lipaglyn®), a dual PPARα/γ agonist, is approved in India for use in T2DM and precirrhotic NASH. In several RCT in NAFLD patients with and without T2DM, saroglitazar (4 mg/d) was shown to significantly improve liver biochemistry as well as hepatic steatosis by non-invasive measures over 16–24 weeks of treatment [104,105]. The group of experimental triple PPARα/γ/δ agonists is now represented by lanifibranor (IVA337) alone, after its predecessor elafibranor (GFT-505) was discontinued due to lack of efficacy in NAFLD in the phase 3 RESOLVE-IT trial (NCT02704403). Lanifibranor was well tolerated and reduced liver enzyme levels and markers of fibrosis in patients with precirrhotic, highly active NASH in the recently completed phase 2b study. The percentage of patients with meaningful improvements in steatosis, activity, and fibrosis scores was significantly higher in the lanifibranor-treated arms, indicating possible improvements in hepatitis activity as well as HCB [106]. Two more trials to evaluate the efficacy of lanifibranor in concomitant NAFLD and T2DM and in advanced fibrosis due to NASH are ongoing (NCT03459079, NCT04849728).
6.2. MPC Inhibitors
In addition to their primary mechanism of action, pioglitazone and other thiazolidinediones are known to interact with the mitochondrial pyruvate carrier (MPC) to suppress pyruvate transport into the mitochondrial matrix. Since this direct, non-genomic effect is considered essential for the inhibition of hepatic gluconeogenesis by PPARγ ligands, novel MPC-inhibiting thiazolidinedione derivatives with minimal affinity towards PPARγ have been synthesized.
Azemiglitazone (MSDK-0602K) reduced liver enzyme levels in NASH/fibrosis patients regardless of T2DM presence [107], and was characterized by a markedly improved safety profile [108]. However, the drug did not demonstrate significant effects on any of the histological endpoints [107]. A phase 3 trial to evaluate azemiglitazone in diabetic or prediabetic patients with NAFLD/NASH has been initiated (NCT03970031). An alternative approach to minimizing the side effects of PPARγ agonists is represented by PXL065, the deuterium-stabilized R-isomer of pioglitazone [109], which is currently being assessed in a phase 2 RCT (NCT04321343).
6.3. Incretin Mimetics
Incretins are a small family of intestinal L-cell-derived peptide hormones that includes glucagon-like peptide 1 (GLP1), glucose-dependent insulinotropic polypeptide (GIP), and oxyntomodulin. The incretin axis mediates the physiological response to hyperglycaemia and couples glucose intake with pancreatic secretion [110]. GLP1 receptors (GLP1R) are expressed in the β-cells, hepatocytes, white adipose tissue, brain, and skeletal muscle [111]. GLP1R activation induces insulin secretion, decreases insulin resistance, inhibits glucagon release and lipogenesis, and suppresses appetite and gastrointestinal motility. Additionally, hepatic GLP1R stimulate FA β-oxidation, inhibit profibrogenic signaling pathways, and exert a mild anti-inflammatory effect by indirectly reducing CRP, proinflammatory cytokine, and chemokine production [112].
At the moment, semaglutide is the only GLP1R agonist being developed for the treatment of NASH in nondiabetic subjects. In the recently completed 72-week phase 2 trial, semaglutide treatment (0.4 mg/d) led to NASH resolution with no worsening of fibrosis in 59% vs. 17% in the placebo group, which is considered the highest response rate that a drug has ever achieved in a NASH trial up to now [113,114]. Currently, semaglutide is being evaluated in several phase 2 trials as monotherapy (NCT03884075, NCT04216589) as well as in combinations with empagliflozin (NCT04639414), and cilofexor/firsocostat (NCT04971785). A 5-year-long phase 3 study designed to include 1200 patients with precirrhotic NASH has been initiated (NCT04822181).
Exenatide, lixisenatide, liraglutide, and dulaglutide have all demonstrated significant antisteatotic activity, and (with the exception of lixisenatide) improved intrahepatic cholestasis in T2DM/NAFLD patients. Amelioration of cytolysis markers was reported for exenatide [115,116] and dulaglutide [117,118], and lixisenatide was effective against liver fibrosis and inflammation [119]. Liraglutide reduced hepatitis activity and liver fibrosis as well as attenuated HCB in NASH patients regardless of the presence of T2DM, as determined by the phase 2 LEAN study [120]. A recent meta-analysis by Ghosal et al., including 8 RCT and over 600 patients, found that GLP1R agonists in general improve liver function and histology by improving glycaemia, reducing body weight and hepatic fat content, which in turn might be beneficial for hepatic inflammation in NAFLD concomitant with T2DM [121].
While both GIP and GLP1 are potent insulin secretagogues, GIP has a more robust, dose-dependent secretory pattern, and appears to make a greater contribution than GLP1 to prandial insulin secretion in healthy subjects, while in T2M its activity is depleted. In addition, GIP, but not GLP1, stimulates glucagon secretion by the α-cells at low glycaemia under physiological conditions, and in a glucose-independent fashion in T2M [110]. GIP receptors (GIPR) upregulate lipogenesis, FA esterification and TAG accumulation in adipocytes, and inhibits prandial lipid absorption. Supraphysiological levels of GIP have been associated with increased systemic inflammatory response, and are considered a risk factor for the development of NASH [122].
Tirzepatide (LY3298176) is a synthetic injectable dual GLP1/GIP peptide agonist currently researched for NAFLD treatment. In T2DM subjects with NASH, tirzepatide (1–15 mg/week for 26 weeks) effectively reduced ALT, AST, cytokeratin 18, Pro-C3, and increased adiponectin levels [123]. Additionally, tirzepatide treatment led to greater improvements in liver fat content compared to titrated insulin degludec in T2DM, according to the phase 3 SURPASS-3 RCT results [124]. A phase 2 study, designated SYNERGY-NASH, has been initiated to evaluate the efficacy of tirzepatide in nondiabetic subjects with NASH (NCT04166773).
Oxyntomodulin shares sequence similarity with both and GLP1 and glucagon, and activates GLP1R and glucagon receptors (GCGR) under physiological conditions. Simultaneous GLP1R activation prevents hyperglycaemic response characteristic of glucagon, at the same time potentiating its catabolic effects and greatly intensifying hepatic glycolysis, glycogenolysis, and lipolysis [125]. Weight reduction, anorexigenic and hypoglycaemic effects have been linked to GLP1 activation, while GCGR activation is thought to contribute primarily to hepatic steatosis attenuation and improved mitochondrial respiration. The clinical utility of oxyntomodulin itself is limited by a short circulatory half-life due to rapid renal clearance and degradation by dipeptidyl peptidase 4 (DPP4) [126].
In contrast to the native hormone, synthetic oxyntomodulin mimetics are resistant to proteolytic cleavage and have prolonged pharmacological action. Cotadutide (MEDI0382) (100–300 μg/d) caused substantial improvements in liver enzyme levels and markers of liver fibrosis in concomitant obesity, T2DM, and NASH in a phase 2b study that included over 800 subjects [127]. Efinopegdutide (HM12525A, MK-6024), a PEGylated long-acting peptide agent, has demonstrated promising antihyperlipidaemic, antisteatotic, and anti-inflammatory activity in mice and hamsters [128], and is going to be evaluated in a phase 2 RCT in NASH with semaglutide as an active comparator (NCT04944992). Other dual GLP1/GCCR agonists intended for use in NAFLD include pemvidutide (ALT-801) (NCT05006885), danuglipron (PF-06882961) (in combination with ervogastat) [129], BI 456906 (NCT04771273), and HM14320 (a glucagon-containing combination) [130]. Finally, a novel triple GLP1R/GCGR/GIPR agonist, HM15211, induced significant reductions in liver steatosis, fibrosis, and inflammation in mice [131]; a phase 2 clinical trial is ongoing (NCT04505436).
GLP2, usually not considered an incretin, is prevalent in the gastrointestinal tract, where it promotes lipid absorption, regulates intestinal motility, mucosal morphology, function and integrity of the intestine [132]. Teduglutide, a selective GLP2R agonist, reduced liver steatosis and disease activity scores in rats, possibly by restoring normal intestinal permeability [133].
DPP4 inhibitors represent a group of indirect incretin mimetics as they prevent the proteolytic cleavage of GLP1, GIP, and oxyntomodulin. To the best of our knowledge, no DPP4 inhibitors are yet in the global pipeline for liver disease. However, a number of small-scale clinical trials have evaluated their potential efficacy in NAFLD in the presence or absence of concomitant T2DM. Among this group, only sitagliptin (100 mg/d) was found effective against hepatic steatosis and HCB irrespective of T2DM in a 1-year open-label RCT [134]. Vildagliptin (100 mg/d) [135], saxagliptin (5 mg/d) [136], omarigliptin (25 mg/week) [137], and teneligliptin (20 mg/d) [138] improved liver function and some non-invasive markers of NAFLD, and alogliptin (25 mg/d) was only moderately effective against NASH over 12 months of treatment in T2DM/NAFLD patients [139]. A recent meta-analysis by dos Santos et al. found the existing evidence for DPP4 inhibitors in NAFLD to be of poor quality and altogether not supportive of their clinical effectiveness [140]. Evogliptin [141], anagliptin [142,143], trelagliptin [144], gemigliptin [145], and linagliptin [146] have demonstrated beneficial effects in experimental rodent models, but their clinical value remains to be explored in future trials.
6.4. SGLT Inhibitors
Sodium/glucose cotransporter (SGLT) 2 inhibitors are a relatively novel class of oral antidiabetic agents that increase urinary glucose excretion by inhibiting glucose reabsorption by SGLT2 in the proximal tubules. Several trials have demonstrated the improvement of cardiovascular and renal outcomes by treatment with compounds of this class, namely, empagliflozin, canagliflozin, and dapagliflozin [147]. SGLT2 inhibitors are known for their multiple metabolic effects that are notably relevant to NAFLD pathophysiology, including the general shift towards increased ketogenesis, gluconeogenesis, glycogenolysis, and FA β-oxidation. They inhibit leptin production by adipocytes, leading to decreased food intake, increase adiponectin levels, provide mild insulin sensitization, suppress HSC activation and fibrogenesis. Additionally, SGLT2 inhibitors may indirectly suppress sympathetic innervation and increase the vagal tone, thereby preventing the activation of Kupffer cells and the associated inflammatory processes [148,149].
Recent evidence mostly supports the efficacy of the majority of SGLT2 inhibitors for improving liver dysfunction, steatosis and fibrosis in NAFLD concomitant with T2DM. Among this group, only dapagliflozin, empagliflozin, and canagliflozin treatment was associated with beneficial effects in nondiabetic NAFLD patients. Dapagliflozin (10 mg) significantly reduced ALT, AST, and GGT levels, according to a retrospective study [150], while empagliflozin (10 mg/d) also attenuated liver steatosis and liver stiffness, indicative of potential antifibrotic activity, in a small-scale RCT [151]. The phase 3 DEAN trial to evaluate dapagliflozin in biopsy-confirmed NASH patients has been initiated (NCT03723252). Canagliflozin (100 mg/d) improved liver enzyme levels and FIB-4 index values in an open-label, uncontrolled pilot study [152]. Additionally, empagliflozin had a beneficial effect on cognitive functions and reduced anxiety in an experimental NAFLD model [153].
Ipragliflozin (50 mg/d for 72 weeks) ameliorated liver fibrosis and enhanced NASH resolution [154], remogliflozin etabonate (50–1000 mg/d for 12 weeks) reduced FIB-4 and NAFLD-fibrosis scores [155], and ertugliflozin (5 or 15 mg/d for 52 weeks) reduced liver transaminase levels [156] in TD2M patients with different stages of NASH. Several pilot studies in T2DM/NAFLD subjects confirmed the antisteatotic properties of luseogliflozin and tofogliflozin [157,158,159].
The SGLT1 subtype plays a relatively smaller (10–20% and up to 40% when SGLT2 are blocked) role in the renal glucose reabsorption, but is more abundant in the small intestine along with the heart and lungs. Intestinal SGLT1 (iSGLT1) inhibition leads to substantially reduced glucose and galactose absorption from the intestinal lumen, and increased incretin (GLP1, peptide YY) release by enteroendocrine cells [160]. Currently, licogliflozin (LIK066) is the only SGLT1/2 inhibitor being evaluated in NASH independent of T2DM presence, alone and in combination with the FXR agonist tropifexor, in the ongoing phase 2 ELIVATE study (NCT04065841). Previously, licogliflozin (150 mg/d) reduced ALT, ALT, GGT levels and liver fat content over 12 weeks compared to placebo [161]. A novel compound, SGL5213, has been identified as a selective iSGLT1 inhibitor, and has demonstrated insulin-sensitizing, anti-inflammatory, and antifibrotic activity in a murine model of NAFLD [162].
6.5. α-Glucosidase Inhibitors
α-Glucosidase is a carbohydrate hydrolase located in the brush border of the small intestine that catalyzes the breakdown of dietary starch and disaccharides to yield glucose. α-Glucosidase inhibitors slow down carbohydrate digestion and absorption, thereby reducing postprandial hyperglycaemia. However, they are characterized by only modest overall antidiabetic activity, and are not too often used in clinical practice [163]. Despite some scientific interest concerning the use of this class of drugs for the treatment of liver diseases, data regarding their efficacy for NAFLD remain scarce. Acarbose (100 mg/d) improved AST, ALT levels and lipid profiles, albeit to a lesser extent than ezetimibe, in a 10-week small-scale RCT in non-diabetic NASH patients [164]. Miglitol treatment (150 mg/d for 12 months) was associated with significant improvements in steatosis, lobular and portal inflammation, and NAS scores, while fibrosis and hepatocyte ballooning remained unchanged [165]. Finally, voglibose prevented hepatic steatosis in obese rats, but was slightly inferior to empagliflozin [166].
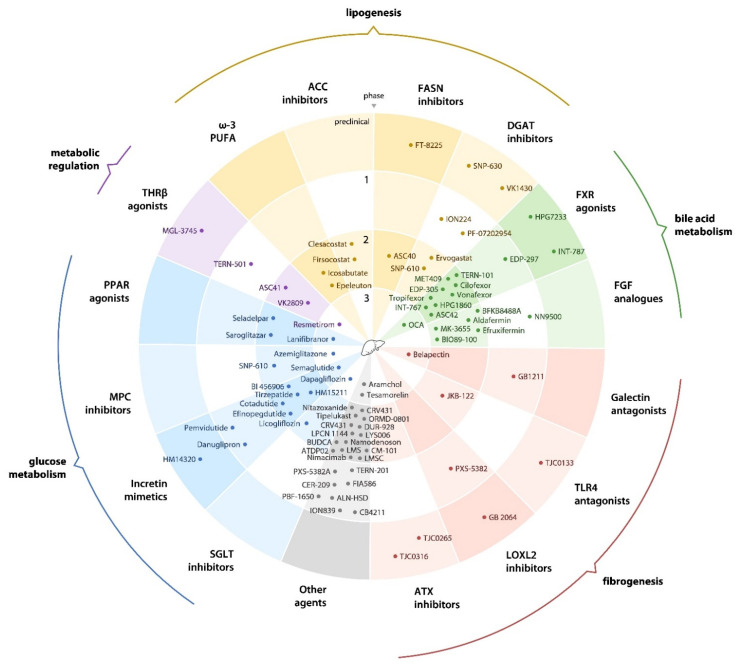
An overview of the current drug development pipeline for NAFLD is given in Figure 1.
Figure 1.
An overview of the current drug development pipeline for non-alcoholic fatty liver disease. FASN, fatty acid synthase; DGAT, diglyceride acyltransferase; FXR, farnesoid X receptor; FGF, fibroblast growth factor; TLR4, toll-like receptor 4; LOXL2, lysyl oxidase-like protein 2; ATX, autotaxin; SGLT, sodium/glucose contransporter; MPC, mitochondrial pyruvate carrier; PPAR, peroxisome proliferator-activated receptor; THRβ, thyroid hormone receptor β; PUFA, polyunsaturated fatty acid; ACC, acetyl-CoA carboxylase; BUDCA, berberine ursodeoxycholate; LMS, leucine-metformin-sildenafil; LMSC, liver-derived mesenchymal stromal cells.
7. Other Agents
7.1. Probiotics
According to recent evidence, NAFLD pathogenesis is tightly associated with intestinal bacterial overgrowth and an upset balance between Bacteroidetes and Firmicutes species, leading to alterations in the gut-derived metabolite and endotoxin production [167]. A number of small-scale RCT in paediatric and adult NAFLD patients have demonstrated the beneficial effects of probiotics containing various combinations of Bifidobacterium spp. [168], Lactobacillus spp. [169,170], Streptococcus thermophilus [171], Pediococcus pentosaceus [172], Lactococcus spp., Propionibacterium spp., and Acetobacter spp. [173] Two meta-analyses of 134 and 535 NAFLD patients from 4 and 9 RCTs, respectively, have confirmed that probiotics can improve insulin sensitivity, ameliorate dyslipidaemia and systemic inflammation, reduce intrahepatic fat content, and rescue impaired liver function, overall improving the clinical outcomes in NAFLD [174,175]. Additionally, probiotics based on Saccharomyces boulardii [176] and Clostridium butyricum [177] have demonstrated antisteatotic, anti-inflammatory, and antifibrotic properties in animal models of NAFLD. Clinical evidence for probiotic use in NAFLD is summarized in Table 1.
Table 1.
Probiotic formulations with evidence of hepatoprotective activity in non-alcoholic fatty liver disease.
| Composition | Liver-Related Effects | ||||||
|---|---|---|---|---|---|---|---|
| Cytolysis | Steatosis | HCB | Inflammation | Fibrosis | Cholestasis | References | |
| Bifidobacterium longum | + | + | ± | ± | [168] | ||
| Lactobacillus acidophilus | + | [178] | |||||
| L. acidophilus, B. lactis | + | [170] | |||||
| L. rhamnosus | + | [169] | |||||
| L. plantarum * | + | [179] | |||||
| L. paracasei * | + | + | [180] | ||||
| L. johnsonii * | + | + | [181] | ||||
| L. reuteri | + | [182] | |||||
| L. delbrueckii subsp. bulgaricus, Streptococcus thermophilus | + | [183] | |||||
| L. acidophilus, L. rhamnosus, B. bifidum, B. lactis | + | + | [184] | ||||
| L. acidophilus, L. rhamnosus, L. plantarum, L. delbrueckii subsp. bulgaricus, B. bifidum | + | + | [185] | ||||
| L. acidophilus, L. rhamnosus, L. paracasei, B. lactis, B. breve, Pediococcus pentosaceus | + | [172] | |||||
| L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, B. breve, B. longum, B. infantis | ± | [171] | |||||
| Lactobacillus spp., Bifidobacterium spp., Lactococcus spp., Propionibacterium spp., Acetobacter spp. | + | + | ± | + | [173] | ||
| L. acidophilus, L. plantarum, L. delbrueckii subsp. bulgaricus, L. casei, B. breve, B. longum, B. infantis, S. thermophilus | + | [186] | |||||
| Clostridium butyricum * | + | + | [177] | ||||
| Saccharomyces boulardii * | + | + | [176] | ||||
* Preclinical data given. +, definite positive effect; ±, possible positive effect; HCB, hepatocellular ballooning.
7.2. Mesenchymal Stromal Cells
Lately, cell-based therapy has emerged as a feasible alternative for the treatment of different stages of NAFLD. In particular, experimental evidence supports the use of bone marrow- [187], umbilical cord- [188], and compact bone-derived mesenchymal stromal cells [189] as well as hepatocytes derived by differentiating induced pluripotent stem cells [190]. The crosstalk between hepatic stem cells and their possible therapeutic application for NAFLD are discussed in detail in a recent review by Overi et al. [191].
HepaStem® is a first-in-class allogeneic stem cell therapy product containing human adult liver-derived progenitor cells with potential indications including cirrhotic and precirrhotic NASH as well as acute-on-chronic liver failure (ACLF). HepaStem® cells, obtained from healthy donors, are expected to modulate the inflammatory response and inhibit HSC activation, thereby reducing liver fibrosis. A small-scale phase 2a RCT found HepaStem to be safe and well tolerated, and indicated potential efficacy for ACLF and/or decompensated liver cirrhosis [192].
7.3. Fraudulent Fatty Acids
Fraudulent, or abnormal fatty acids, represented by bempedoic acid (ETC-1002) and gemcabene (CI-1027), are molecules with structures similar to those of oleic or linolenic acid that regulate metabolic pathways in the liver, resulting in enhanced FA catabolism. After conversion into its active form, bempedoic acid acts as false substrate and inhibits hepatic adenosine triphosphate citrate (pro-S)-lyase, an enzyme upstream of 3-hydroxy-3-methylglutaryl-CoA reductase in the cholesterol synthesis pathway. This links fraudulent fatty acids to statins, whose possible beneficial effects for NAFLD are reviewed elsewhere [193].
Bempedoic acid is approved in the USA and EU as monotherapy and as a fixed-dose combination with ezetimibe for the treatment of hypercholesterolaemia [194]. In a high-fat diet-induced murine model of NASH, it caused significant reductions in ALT and AST levels, hepatic TAG accumulation, proinflammatory and profibrotic gene expression, resulting in improved NAFLD activity and liver fibrosis by histological analysis [195].
Gemcabene (PD-72953), a structurally optimized derivative of bempedoic acid, forms a CoA conjugate that inhibits ACC, and reduces apolipoprotein C-III expression [194]. In a mouse model of NASH/HCC, it diminished micro- and macrovesicular liver steatosis, HCB, inflammatory infiltration, and fibrosis, which corresponded to downregulated proinflammatory, lipogenesis, and profibrogenic marker expression [194]. Gemcabene was being developed for the treatment of paediatric NAFLD, but was discontinued and repurposed for another indication after a lack of efficacy was demonstrated in a phase 2a proof-of-concept study (NCT03436420).
7.4. Tesamorelin
Tesamorelin (TH9507) is a growth hormone (GH) releasing hormone analogue that is thought to stimulate lipolysis via increasing endogenous GH levels while maintaining feedback inhibition and limiting toxicity compared to native GH. Tesamorelin reduced liver fat content and visceral fat in a preliminary study in antiretroviral-treated patients with human immunodeficiency virus (HIV)-associated lipodystrophy [196]. A phase 2 trial to evaluate the effects of tesamorelin on liver steatosis and cardiovascular risk in obese NASH patients is recruiting (NCT03375788), and a phase 3 study in the general population with NAFLD including a HIV cohort has been planned [197].
7.5. Berberine Ursodeoxycholate
Berberine ursodeoxycholate (BUDCA, HTD1801) is an ionic salt of the isoquinoline alkaloid berberine and ursodeoxycholic acid (UDCA). According to a meta-analysis by Wei et al., berberine can significantly improve liver function, lipid profiles, and glycaemic control in patients with NAFLD [198] due to adenosine monophosphate-activated protein kinase (AMPK) activation, stimulation of glycolysis, and, possibly, inhibition of α-glucosidase [199]. UDCA, in turn, is a bile acid long used for the treatment of NASH and chronic cholestatic diseases, whose hepatoprotective effects are confirmed by several systematic reviews and meta-analyses [200,201]. In a phase 2 proof-of-concept RCT in T2DM patients with presumed NASH, BUDCA reduced liver enzyme levels and liver steatosis by MRI-PDFF [202].
7.6. Miricorilant
Miricorilant (CORT 118335) is an investigational glucocorticoid receptor agonist/antagonist and a mineralocorticoid receptor antagonist currently in development for NASH and antipsychotic-induced weight gain. Results of a phase 2a study in NASH patients demonstrated that miricorilant (600 mg/d) effectively ameliorated liver steatosis by radiological measures. However, miricorilant treatment was associated by transient yet significant increases in serum transaminases [203], and the trial was subsequently put on hold due to safety concerns (NCT03823703).
7.7. Nitazoxanide
Nitazoxanide (Alinia®) is an FDA-approved broad-spectrum thiazolide antiprotozoal and antiparasitic agent, lately reported to be a potent AMPK activator and inhibitor of HSC activation. In experimental studies in mice, nitazoxanide (100 mg/kg/d) attenuated dyslipidaemia, liver steatosis [204], fibrosis, inflammation, and HCB, demonstrating synergistic effects with the pan-PPAR agonist elafibranor [205]. Moreover, the anti-anaerobic activity of nitazoxanide may determine its use in preventing the recurrence of hepatic encephalopathy as a viable alternative to rifaximin (NCT04161053) [206].
7.8. Pirfenidone
Pirfenidone (Esbriet®) is a pyridine derivative with antifibrotic, anti-inflammatory, and antioxidant properties, the precise mechanisms of which are still unclear. In the liver, pirfenidone may decrease fibronectin, TGFβ, collagen production and attenuate fibrogenesis, hepatocyte necrosis, and necroinflammation [207,208]. In the phase 2 PROMETEO study, pirfenidone (1200 mg/d) markedly reduced transaminase levels and advanced liver fibrosis of predominantly nonalcoholic aetiology [209].
7.9. Miscellaneous
Other investigational drugs with potential therapeutic value in NAFLD include antileukotriene agents [210], GPCR modulators [211], anti-IL mABs [212], IL22 axis modulators [213], purinergic receptor agonists [214], antioxidants [215], antisense oligonucleotides [216], multitarget epigenetic regulators [217], and many more. A comprehensive list of drug candidates and experimental agents with evidence of hepatoprotective activity in NAFLD is given in Table 2.
Table 2.
Drug candidates and experimental agents with evidence of hepatoprotective activity in non-alcoholic fatty liver disease.
| Name | Mechanism of Action | Development Phase | Liver-Related Effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytolysis | Steatosis | HCB | Inflammation | Fibrosis | Cholestasis | References | |||
| Resmetirom | THRβ agonist | 3 | + | + | ± | ± | + | + | [11] |
| VK2809 | THRβ agonist | 2 | + | [12] | |||||
| ASC41 * | THRβ agonist | 2 | + | + | + | + | [13] | ||
| TERN-501 * | THRβ agonist | 1 | + | + | + | [15] | |||
| Firsocostat ** | ACC inhibitor | 2 | + | + | + | + | + | ± | [23] |
| Clesacostat ** | ACC inhibitor | 2 | + | [24,31] | |||||
| ASC40 | FASN inhibitor | 2 | + | + | + | + | [16] | ||
| Aramchol | SCD1 inhibitor | 3 | + | + | + | + | + | [30] | |
| Ervogastat | DGAT2 inhibitor | 2 | + | [31] | |||||
| ION224 | DGAT2 inhibitor | 1 | + | ± | [216] | ||||
| Docosahexaenoic acid | ω-3 PUFA | - | + | [40] | |||||
| Epeleuton | ω-3 PUFA | 2 | ± | [44] | |||||
| Icosabutate | ω-3 PUFA | 2 | + | + | ± | + | [46] | ||
| Eicosapentanoic acid * | ω-3 PUFA | - | + | + | + | [218] | |||
| Obeticholic acid | FXR agonist | 3 | + | + | + | + | + | + | [49,50] |
| EDP-305 | FXR agonist | 2 | + | + | + | + | [54,56] | ||
| Tropifexor | FXR agonist | 2 | + | + | + | + | [63] | ||
| Cilofexor | FXR agonist | 2 | + | + | + | [64] | |||
| Vonafexor | FXR agonist | 2 | + | + | + | [65] | |||
| MET409 | FXR agonist | 2 | + | + | [59] | ||||
| TERN-101 | FXR agonist | 2 | + | + | [67] | ||||
| ASC42 * | FXR agonist | 2 | + | + | + | [16] | |||
| INT-767 * | FXR agonist | 2 | + | + | + | + | [58] | ||
| EDP-297 * | FXR agonist | 1 | + | + | + | ± | [219] | ||
| BAR502 * | FXR agonist | - | + | + | + | [57] | |||
| GW4064 * | FXR agonist | - | + | + | [220] | ||||
| Aldafermin | FGF19 analogue | 2 | + | + | + | [70] | |||
| Efruxifermin | FGF21 analogue | 2 | + | + | ± | + | + | [71] | |
| BIO89-100 | FGF21 analogue | 2 | + | + | [72] | ||||
| BFKB8488A | FGFR1c/KLB agonist | 2 | + | [74] | |||||
| MK-3655 | FGFR1c/KLB agonist | 2 | + | [221] | |||||
| GLP-1-Fc-FGF21 D1 * | FGF21 analogue, GLP1R agonist | - | + | + | [75] | ||||
| GB1211 * | galectin-3 antagonist | 2 | + | [88] | |||||
| GM-CT-01 * | galectin-3/1 antagonist | - | + | + | + | + | [79] | ||
| JKB-122 | TLR4 antagonist | 2 | + | + | [83] | ||||
| Eritoran * | TLR4 antagonist | - | + | + | + | [81] | |||
| PXS-5153A * | LOXL2/3 inhibitor | - | + | + | [87] | ||||
| Bezafibrate * | PPARα agonist | - | + | + | ± | [98] | |||
| Pemafibrate | PPARα agonist | - | + | ± | + | [97] | |||
| Fenofibrate | PPARα agonist | - | + | ± | + | [93,94] | |||
| Gemfibrozil | PPARα agonist | + | [95] | ||||||
| Nifedipine * | PPARγ agonist | - | + | + | [101] | ||||
| Seladelpar | PPARδ agonist | 2 | + | + | [103] | ||||
| Saroglitazar | PPARα/γ agonist | 2 | + | + | + | + | [104,105] | ||
| Lanifibranor | PPARα/γ/δ agonist | 3 | + | + | + | + | + | [106] | |
| Pioglitazone | PPARγ agonist, MPC inhibitor | - | + | + | + | + | [222] | ||
| Lobeglitazone | PPARγ agonist, MPC inhibitor | - | + | + | [100] | ||||
| Azemiglitazone | MPC inhibitor | 3 | + | ± | ± | ± | [107] | ||
| PXL065 * | MPC inhibitor | 2 | + | + | + | [109] | |||
| Semaglutide | GLP1R agonist | 3 | + | + | [113] | ||||
| Exenatide | GLP1R agonist | - | + | + | + | [115,116] | |||
| Lixisenatide | GLP1R agonist | - | + | + | + | [119] | |||
| Liraglutide | GLP1R agonist | - | + | + | + | + | + | [120] | |
| Dulaglutide | GLP1R agonist | - | + | + | + | [117,118] | |||
| Teduglutide * | GLP2R agonist | - | + | ± | ± | [113] | |||
| Tirzepatide | GLP1R/GIPR agonist | 2 | + | + | [123,124] | ||||
| Cotadutide | GLP1R/GCGR agonist | 2 | + | + | ± | [127] | |||
| Efinopegdutide * | GLP1R/GCGR agonist | 2 | + | ± | ± | [128] | |||
| Pemvidutide | GLP1R/GCGR agonist | 1 | [223] | ||||||
| HM15211 * | GLP1R/GCGR/GIPR agonist | 2 | + | + | [131] | ||||
| Sitagliptin | DPP4 inhibitor | - | + | + | ± | [134] | |||
| Vildagliptin | DPP4 inhibitor | - | + | + | + | [135] | |||
| Saxagliptin | DPP4 inhibitor | - | + | + | ± | + | [136] | ||
| Alogliptin | DPP4 inhibitor | - | + | [139] | |||||
| Omarigliptin | DPP4 inhibitor | - | + | + | + | + | + | [137] | |
| Teneligliptin | DPP4 inhibitor | - | + | + | [138] | ||||
| Evogliptin * | DPP4 inhibitor | - | + | + | + | [141] | |||
| Anagliptin * | DPP4 inhibitor | - | + | + | + | [142,143] | |||
| Trelagliptin * | DPP4 inhibitor | - | + | + | + | ± | [144] | ||
| Gemigliptin * | DPP4 inhibitor | - | + | + | + | [145] | |||
| Linagliptin * | DPP4 inhibitor | - | + | + | [146] | ||||
| Dapagliflozin | SGLT2 inhibitor | - | + | + | ± | + | [150,224] | ||
| Empagliflozin | SGLT2 inhibitor | - | + | + | + | + | + | [151,225] | |
| Canagliflozin | SGLT2 inhibitor | - | + | + | + | + | [152] | ||
| Ipragliflozin | SGLT2 inhibitor | - | ± | + | + | [154,226] | |||
| Ertugliflozin | SGLT2 inhibitor | - | + | [156] | |||||
| Remogliflozin | SGLT2 inhibitor | - | + | + | [155] | ||||
| Luseogliflozin | SGLT2 inhibitor | - | + | + | + | [157,158] | |||
| Tofogliflozin | SGLT2 inhibitor | - | + | + | + | + | [159] | ||
| Licogliflozin ** | SGLT1/2 inhibitor | 2 | + | + | + | [160,161] | |||
| SGL5213 * | iSGLT1 inhibitor | - | + | + | ± | + | [162] | ||
| Miglitol | α-glucosidase inhibitor | - | + | + | ± | + | + | [165] | |
| Acarbose | α-glucosidase inhibitor | - | + | [164] | |||||
| Voglibose * | α-glucosidase inhibitor | - | + | [166] | |||||
| Liver-derived MSC | MSC | 2 | + | + | [192] | ||||
| Umbilical cord-derived MSC * | MSC | - | + | [188] | |||||
| Compact bone-derived MSC * | MSC | - | + | + | + | + | [189] | ||
| Tesamorelin | GHRH analogue | 3 | + | + | [196] | ||||
| Berberine ursodeoxycholate | multimodal metabolic | 2/1 | + | + | + | [202] | |||
| Miricorilant | GR agonist/antagonist, MR antagonist | 2 | + | [203] | |||||
| Nitazoxanide * | AMPK activator | 2 | + | + | [204,205] | ||||
| PXL770 | AMPK activator | 2 | + | [227] | |||||
| Leucine + metformin + sildenafil | AMPK activator, eNOS activator | 2 | + | [228] | |||||
| Pirfenidone * | multimodal antifibrotic | - | + | + | [209] | ||||
| PBI-4547 * | GPCR84 antagonist | - | + | + | ± | [229] | |||
| CpdA * | GPCR84 antagonist | - | + | + | + | + | [230] | ||
| CpdB * | GPCR84 antagonist | - | + | + | + | + | [230] | ||
| GPR120 agonist III * | GPCR120 agonist | - | + | ± | + | [231] | |||
| Metabolitin * | GPRC6A agonist | - | + | + | + | [232] | |||
| SCO-267 * | GPR40 agonist | - | + | + | + | [233] | |||
| Evolocumab | PCSK9 inhibitor | - | ± | [234] | |||||
| Alirocumab | PCSK9 inhibitor | - | ± | [234] | |||||
| X203 * | IL11 antagonist | - | + | + | + | + | [212] | ||
| X209 * | IL11RA antagonist | - | [212] | ||||||
| Ezetimibe | NPC1L1 inhibitor | - | + | ± | + | [235] | |||
| ORMD-0801 | oral insulin | 2 | + | [236] | |||||
| GalNAc-Stk25 ASO * | anti-STK25 ASO | - | + | + | + | + | [237] | ||
| Tipelukast * | LTR antagonist, PDE3/4 inhibitor, 5-LO/LT inhibitor | 2 | ± | + | + | + | [210] | ||
| CM-101 * | CCL24 antagonist | 2 | + | ± | ± | ± | + | ± | [238] |
| Namodenoson | A3AR agonist | 2 | + | [214,239] | |||||
| PLN-1474 * | αvβ1 antagonist | 1 | + | [240] | |||||
| CB4211 | MOTS-c analogue | 1 | + | + | [241] | ||||
| CER-209 * | P2Y13R agonist | 1 | + | + | [242] | ||||
| DUR-928 | multitarget epigenetic regulator | 2 | + | + | + | + | [217] | ||
| CRV431 | cyclophilin A/B/D inhibitor | 2 | + | [243] | |||||
| LPCN 1144 | androgen receptor agonist | 2 | + | + | + | + | [244] | ||
| Osteocalcin * | N/A | - | + | + | + | + | [245] | ||
* Preclinical data given; ** developed in combination; -, not in development. +, definite positive effect; ±, possible positive effect. HCB, hepatocellular ballooning; THRβ, thyroid hormone receptor β; ACC, acetyl-CoA carboxylase; FASN, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; DGAT2, diglyceride acyltransferase 2; PUFA, polyunsaturated fatty acid; FXR, farnesoid X receptor; FGF, fibroblast growth factor; FGFR1c/KLB, FGF receptor/β-klotho complex; TLR4, toll-like receptor 4; LOXL, lysyl oxidase-like protein; PPAR, peroxisome proliferator-activated receptor; MPC, mitochondrial pyruvate carrier; GLP1R, glucagon-like peptide 1 receptor; GIPR, glucose-dependent insulinotropic polypeptide receptor; GCGR, glucagon receptor; DPP4, dipeptidyl peptidase 4; SGLT, sodium/glucose cotransporter; iSGLT1, intestinal SGLT 1; MSC, mesenchymal stromal cells; GHRH, growth hormone releasing hormone; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; AMPK, adenosine monophosphate-activated protein kinase; eNOS, endothelial nitric oxide synthase; GPCR, G protein-coupled receptor; GPRC6A, G protein-coupled receptor family C group 6 member A; PCSK9, proprotein convertase subtilisin/kexin type 9; IL11, interleukin 11; IL11RA, IL11 receptor α subunit; NPC1L1, Niemann-Pick C1-like protein 1; STK25, serine/threonine kinase 25; ASO, antisense nucleotide; LTR, leukotriene receptor; PDE, phosphodiesterase; 5-LO/LT, 5-lipoxygenase/leukotriene pathway; CCL24, C-C motif chemokine ligand 24; A3AR, A3 adenosine receptor; MOTS-c, mitochondrial open reading frame of the twelve S ribosomal ribonucleic acid-c; P2Y13R, P2Y purinergic receptor 13.
8. Future Directions
Other biomolecules and pathways most recently identified as potential therapeutic targets in NAFLD have been highlighted in many comprehensive review articles [246,247,248,249]. Of those, the sirtuins, a family of highly conserved histone and protein deacetylases, can be considered of special interest regarding future therapeutic concepts for NAFLD. Sirtuins act as NAD+-sensing signaling proteins to facilitate stress response [250], maintain homeostasis during acute and chronic inflammatory response [251], and partake in the regulation of energy metabolism, redox balance, cell cycle, and suppression of tumorigenesis [252].
Sirtuin 1 (SIRT1), the SIRT1/NF-κB axis, SIRT3, and SIRT4 play a major role in regulating hepatic lipid metabolism, controlling oxidative stress, and mediating chronic inflammation in NAFLD and alcoholic fatty liver disease [253,254,255]. Accordingly, SIRT1 activation by the polyphenol resveratrol and several small molecules have been shown to provide protection against NAFLD and T2DM in rodent models [253,256], and may be beneficial in human metabolic disease [257]. SIRT2, SIRT3, and SIRT4 upregulation induced by an investigational molecule also prevented the progression of hepatic steatosis and fibrosis in obese rats [258]. While still at early research and development stages, selective sirtuin activators and inhibitors are considered a promising group of drug candidate molecules with primarily anti-inflammatory mode of action [251,254,257].
9. Conclusions
Being the most prevalent cause of chronic liver disease worldwide, NAFLD is at the same time one of the greatest areas of unmet medical need in terms of availability and adequacy of pharmacotherapeutic options. The modern pipeline for NAFLD includes a plethora of drug candidates with diverse and innovative mechanisms of action, although reported evidence on their clinical effectiveness has so far been limited. Upcoming treatment approaches to different stages of NAFLD include the modulation of nuclear receptor activity in order to maintain lipid and glucose homeostasis, stimulation of bile acid metabolism, and direct inhibition of fibrogenesis, along with a few less explored but highly promising options.
Despite several investigational agents being seemingly close to regulatory approval as monotherapies, novel therapeutic strategies for NAFLD will likely involve the use of multitarget drugs or rational drug combinations. Given the exceptionally complex pathophysiology and the multifaceted nature of this disease, NAFLD pharmacotherapy can be expected to remain a priority for biomedical research in the nearest future.
Author Contributions
Data analysis, writing—original draft preparation, visualization, V.A.P.; Conceptualization, writing—review and editing, N.N.B. and S.V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Analytics and Devices Ltd. (St. Petersburg, Russia).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Part of the data analyzed in this study are openly available at ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/home (accessed on 10 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Monelli F., Venturelli F., Bonilauri L., Manicardi E., Manicardi V., Giorgi Rossi P., Massari M., Ligabue G., Riva N., Schianchi S., et al. Systematic review of existing guidelines for NAFLD assessment. Hepatoma Res. 2021;7:25. doi: 10.20517/2394-5079.2021.03. [DOI] [PubMed] [Google Scholar]
- 3.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 5.Attia S.L., Softic S., Mouzaki M. Evolving Role for Pharmacotherapy in NAFLD/NASH. Clin. Transl. Sci. 2021;14:11–19. doi: 10.1111/cts.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoneda M., Honda Y., Saito S., Nakajima A. What considerations are there for the pharmacotherapeutic management of nonalcoholic steatohepatitis? Expert Opin. Pharmacother. 2021;22:1217–1220. doi: 10.1080/14656566.2021.1912014. [DOI] [PubMed] [Google Scholar]
- 7.Ortiga-Carvalho T.M., Sidhaye A.R., Wondisford F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014;10:582–591. doi: 10.1038/nrendo.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pramfalk C., Pedrelli M., Parini P. Role of thyroid receptor β in lipid metabolism. Biochim. Biophys. Acta. 2011;1812:929–937. doi: 10.1016/j.bbadis.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Dawson P.A., Parini P. Hepatic thyroid hormone receptor β1 agonism: Good for lipids, good for bile? J. Lipid Res. 2018;59:1551–1553. doi: 10.1194/jlr.C088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gionfra F., De Vito P., Pallottini V., Lin H.Y., Davis P.J., Pedersen J.Z., Incerpi S. The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer. Front. Endocrinol. 2019;10:532. doi: 10.3389/fendo.2019.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison S.A., Bashir M., Moussa S.E., McCarty K., Frias J.P., Taub R., Alkhouri N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients with NASH. Hepatol. Commun. 2021;5:573–588. doi: 10.1002/hep4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loomba R., Neutel J., Mohseni R., Bernard D., Severance R., Dao M., Saini S., Margaritescu C., Homer K., Tran B., et al. VK2809, a Novel Liver-Directed Thyroid Receptor Beta Agonist, Significantly Reduces Liver Fat with Both Low and High Doses in Patients with Non-Alcoholic Fatty Liver Disease: A Phase 2 Randomized, Placebo-Controlled Trial. J. Hepatol. 2019;70:e141–e382. doi: 10.1016/S0618-8278(19)30266-X. [DOI] [Google Scholar]
- 13.Li X., He H., Sho E., Yang B., Wu J.J. Significant Improvement of NAFLD Activity Scores and Liver Fibrosis by ASC41, a Selective THR-β Agonist, in High Fat Diet Induced NASH SD Rats; Proceedings of the Digital International Liver Congress 2021; Virtual. 23–26 June 2021. [Google Scholar]
- 14.Wu J., He H., Palmer M., Yan Y. A Phase Ib Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Asc41, A THR-Β Agonist, for 28-Days in Overweight and Obese Subjects with Elevated LDL-C, a Population Characteristic of NAFLD; Proceedings of the Liver Meeting 2021; Virtual. 12–15 November 2021. [Google Scholar]
- 15.Kirschberg T., Jones C., Xu Y., Wang Y., Fenaux M., Klucher K. TERN-501, a Potent and Selective Agonist of Thyroid Hormone Receptor Beta, Strongly Reduces Histological Features and Biomarkers of Non-Alcoholic Steatohepatitis Associated Pathology in Rodent Models; Proceedings of the Digital International Liver Congress 2020; Virtual. 27–29 August 2020. [Google Scholar]
- 16.Ascletis Pharma Inc. [(accessed on 10 December 2021)]. Available online: https://ascletis.com/
- 17.Terns Pharmaceuticals. [(accessed on 10 December 2021)]. Available online: https://www.ternspharma.com/
- 18.Qi J., Lang W., Geisler J.G., Wang P., Petrounia I., Mai S., Smith C., Askari H., Struble G.T., Williams R., et al. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J. Lipid Res. 2012;53:1106–1116. doi: 10.1194/jlr.M020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K., Bhanot S., Monia B.P., Li Y.X., Diehl A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 20.Takemoto K., Fukasaka Y., Yoshimoto R., Nambu H., Yukioka H. Diacylglycerol acyltransferase 1/2 inhibition induces dysregulation of fatty acid metabolism and leads to intestinal barrier failure and diarrhea in mice. Physiol. Rep. 2020;8:e14542. doi: 10.14814/phy2.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parlati L., Régnier M., Guillou H., Postic C. New targets for NAFLD. JHEP Rep. 2021;3:100346. doi: 10.1016/j.jhepr.2021.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panzitt K., Wagner M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166133. doi: 10.1016/j.bbadis.2021.166133. [DOI] [PubMed] [Google Scholar]
- 23.Loomba R., Noureddin M., Kowdley K.V., Kohli A., Sheikh A., Neff G., Bhandari B.R., Gunn N., Caldwell S.H., Goodman Z., et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology. 2021;73:625–643. doi: 10.1002/hep.31622. [DOI] [PubMed] [Google Scholar]
- 24.Calle R.A., Amin N.B., Carvajal-Gonzalez S., Ross T.T., Bergman A., Aggarwal S., Crowley C., Rinaldi A., Mancuso J., Aggarwal N., et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 2021;27:1836–1848. doi: 10.1038/s41591-021-01489-1. [DOI] [PubMed] [Google Scholar]
- 25.Syed-Abdul M.M., Parks E.J., Gaballah A.H., Bingham K., Hammoud G.M., Kemble G., Buckley D., McCulloch W., Manrique-Acevedo C. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males with Metabolic Abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 26.Loomba R., Rinella M., Harrison S.A., Wong V.W.-S., Ratziu V., Mohseni R., Lucas J., Gutierrez J.A., Rahimi R., Trotter J., et al. Novel, first-in-class, fatty acid synthase inhibitor, TVB-2640 versus placebo demonstrates clinically significant reduction in liver fat by MRI-PDFF in NASH; Proceedings of the Digital International Liver Congress 2020; Virtual. 27–29 August 2020. [Google Scholar]
- 27.Forma Therapeutics. [(accessed on 10 December 2021)]. Available online: https://www.formatherapeutics.com/
- 28.Bhattacharya D., Basta B., Mato J.M., Craig A., Fernández-Ramos D., Lopitz-Otsoa F., Tsvirkun D., Hayardeny L., Chandar V., Schwartz R.E., et al. Aramchol downregulates stearoyl CoA-desaturase 1 in hepatic stellate cells to attenuate cellular fibrogenesis. JHEP Rep. 2021;3:100237. doi: 10.1016/j.jhepr.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leikin-Frenkel A., Gonen A., Shaish A., Goldiner I., Leikin-Gobbi D., Konikoff F.M., Harats D., Gilat T. Fatty acid bile acid conjugate inhibits hepatic stearoyl coenzyme A desaturase and is non-atherogenic. Arch. Med. Res. 2010;41:397–404. doi: 10.1016/j.arcmed.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V., de Guevara L., Safadi R., Poordad F., Fuster F., Flores-Figueroa J., Arrese M., Fracanzani A.L., Ben Bashat D., Lackner K., et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021;27:1825–1835. doi: 10.1038/s41591-021-01495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calle R., Aggarwal S., Mancuso J., Bergman A., Somayaji V., Ross T., Esler W. Co-administration of PF-05221304 and PF-06865571 delivers robust whole liver fat reduction and mitigation of acetyl-coa carboxilase inhibitor induced hypertriglyceridemia in patients with NAFLD. J. Hepatol. 2020;73:S401–S652. doi: 10.1016/S0168-8278(20)31392-1. [DOI] [Google Scholar]
- 32.Loomba R., Morgan E., Watts L., Xia S., Hannan L.A., Geary R.S., Baker B.F., Bhanot S. Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: A multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2020;5:829–838. doi: 10.1016/S2468-1253(20)30186-2. [DOI] [PubMed] [Google Scholar]
- 33.Sinew Pharma Inc. [(accessed on 10 December 2021)]. Available online: https://www.sinewpharma.com/en/
- 34.Dentin R., Benhamed F., Pégorier J.P., Foufelle F., Viollet B., Vaulont S., Girard J., Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 36.Siriwardhana N., Kalupahana N.S., Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 37.Okada L.S.D.R.R., Oliveira C.P., Stefano J.T., Nogueira M.A., Silva I.D.C.G.D., Cordeiro F.B., Alves V.A.F., Torrinhas R.S., Carrilho F.J., Puri P., et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH-Proteomic and lipidomic insight. Clin. Nutr. 2018;37:1474–1484. doi: 10.1016/j.clnu.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Nogueira M.A., Oliveira C.P., Ferreira Alves V.A., Stefano J.T., Rodrigues L.S., Torrinhas R.S., Cogliati B., Barbeiro H., Carrilho F.J., Waitzberg D.L. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2016;35:578–586. doi: 10.1016/j.clnu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Parker H.M., Johnson N.A., Burdon C.A., Cohn J.S., O’Connor H.T., George J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Nobili V., Bedogni G., Alisi A., Pietrobattista A., Risé P., Galli C., Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double-blind randomised controlled clinical trial. Arch. Dis. Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 41.He X.X., Wu X.L., Chen R.P., Chen C., Liu X.G., Wu B.J., Huang Z.M. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2016;11:e0162368. doi: 10.1371/journal.pone.0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan J.H., Guan B.J., Gao H.Y., Peng X.E. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine. 2018;97:e12271. doi: 10.1097/MD.0000000000012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C.H., Fu Y., Yang S.J., Chi C.C. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients. 2020;12:2769. doi: 10.3390/nu12092769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Climax J., Newsome P.N., Hamza M., Weissbach M., Coughlan D., Sattar N., McGuire D.K., Bhatt D.L. Effects of Epeleuton, a Novel Synthetic Second-Generation n-3 Fatty Acid, on Non-Alcoholic Fatty Liver Disease, Triglycerides, Glycemic Control, and Cardiometabolic and Inflammatory Markers. J. Am. Heart Assoc. 2020;9:e016334. doi: 10.1161/JAHA.119.016334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Affimune. [(accessed on 10 December 2021)]. Available online: https://www.afimmune.com/
- 46.Harrison S., Gunn N.T., Sheikh M.Y., Rudraraju M., Kohli A., Neff G., Round P., Fraser D.A., Beysen C., Rossi S., et al. Icosabutate, a novel structurally engineered fatty acid, significantly reduces relevant markers of NASH and fibrosis in 16 weeks: Interim analysis results of the ICONA trial. J. Hepatol. 2021;75:S294–S803. [Google Scholar]
- 47.Jiang L., Zhang H., Xiao D., Wei H., Chen Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021;19:2148–2159. doi: 10.1016/j.csbj.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Intercept Pharmaceuticals. [(accessed on 10 December 2021)]. Available online: https://www.interceptpharma.com/homepage-non-usa/
- 49.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 50.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alemi F., Kwon E., Poole D.P., Lieu T., Lyo V., Cattaruzza F., Cevikbas F., Steinhoff M., Nassini R., Materazzi S., et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Investig. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An P., Wei G., Huang P., Li W., Qi X., Lin Y., Vaid K.A., Wang J., Zhang S., Li Y., et al. A novel non-bile acid FXR agonist EDP-305 potently suppresses liver injury and fibrosis without worsening of ductular reaction. Liver Int. 2020;40:1655–1669. doi: 10.1111/liv.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chau M., Li Y., Roqueta-Rivera M., Garlick K., Shen R., Wang G., Or Y.S., Jiang L.-J. Characterization of EDP-305, a Highly Potent and Selective Farnesoid X Receptor Agonist, for the Treatment of Non-alcoholic Steatohepatitis. Int. J. Gastroenterol. 2019;3:4–16. doi: 10.11648/j.ijg.20190301.12. [DOI] [Google Scholar]
- 54.Enanta Pharmaceuticals, Inc. Enanta Announces Results of INTREPID Study of EDP-305 for the Treatment of Primary Biliary Cholangitis: News Release; 2020. [(accessed on 10 December 2021)]. Available online: https://www.enanta.com/investors/news-releases/press-release/2020/Enanta-Announces-Results-of-INTREPID-Study-of-EDP-305-for-the-Treatment-of-Primary-Biliary-Cholangitis/default.aspx.
- 55.Biagioli M., Fiorucci S. Bile acid activated receptors: Integrating immune and metabolic regulation in non-alcoholic fatty liver disease. Liver Res. 2021;5:119–141. doi: 10.1016/j.livres.2021.08.003. [DOI] [Google Scholar]
- 56.Enanta Pharmaceuticals, Inc. Enanta Pharmaceuticals Provides Update on NASH FXR Agonist Programs: News Release; 2021. [(accessed on 10 December 2021)]. Available online: https://www.enanta.com/investors/news-releases/press-release/2021/Enanta-Pharmaceuticals-Provides-Update-on-NASH-FXR-Agonist-Programs/
- 57.Carino A., Cipriani S., Marchianò S., Biagioli M., Santorelli C., Donini A., Zampella A., Monti M.C., Fiorucci S. BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Sci. Rep. 2017;7:42801. doi: 10.1038/srep42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roth J.D., Feigh M., Veidal S.S., Fensholdt L.K., Rigbolt K.T., Hansen H.H., Chen L.C., Petitjean M., Friley W., Vrang N., et al. INT-767 improves histopathological features in a diet-induced ob/ob mouse model of biopsy-confirmed non-alcoholic steatohepatitis. World J. Gastroenterol. 2018;24:195–210. doi: 10.3748/wjg.v24.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison S.A., Bashir M.R., Lee K.J., Shim-Lopez J., Lee J., Wagner B., Smith N.D., Chen H.C., Lawitz E.J. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J. Hepatol. 2021;75:25–33. doi: 10.1016/j.jhep.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 60.Kremoser C. FXR agonists for NASH: How are they different and what difference do they make? J. Hepatol. 2021;75:12–15. doi: 10.1016/j.jhep.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Schumacher J.D., Guo G.L. Pharmacologic Modulation of Bile Acid-FXR-FGF15/FGF19 Pathway for the Treatment of Nonalcoholic Steatohepatitis. Handb. Exp. Pharmacol. 2019;256:325–357. doi: 10.1007/164_2019_228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucas K.J., Lopez P., Lawitz E., Sheikh A., Aizenberg D., Hsia S., Boon Bee G.G., Vierling J., Frias J., White J., et al. Tropifexor, a highly potent FXR agonist, produces robust and dose-dependent reductions in hepatic fat and serum alanine aminotransferase in patients with fibrotic NASH after 12 weeks of therapy: FLIGHT-FXR Part C interim results. Dig. Liver Dis. 2020;52:e19–e45. doi: 10.1016/j.dld.2019.12.129. [DOI] [Google Scholar]
- 63.Novartis A Randomized, Double-Blind, Placebo Controlled, 3-Part, Adaptive Design, Multicenter Study to Assess Safety, Tolerability and Efficacy of Tropifexor (LJN452) in Patients with Non-Alcoholic Steatohepatitis (NASH): FLIGHT-FXR, 2020. [(accessed on 10 December 2021)]. Available online: https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=17816.
- 64.Patel K., Harrison S.A., Elkhashab M., Trotter J.F., Herring R., Rojter S.E., Kayali Z., Wong V.W., Greenbloom S., Jayakumar S., et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 65.Harrison S.A., Ratziu V., White A., Reiss G.M., Bowman W.K., Gunn N., Loustaud Ratti V., Bureau C., Lawitz E.J., Alkhouri N., et al. Vonafexor, s FXR Agonist, Induced Hepatic snd Renal Improvement in the Randomized, Double-Blind, Placebocontrolled LIVIFYNASH Trial; Proceedings of the Liver Meeting 2021; Virtual. 12–15 November 2021. [Google Scholar]
- 66.Novartis A randomized, Patient and Investigator Blinded, Placebo-Controlled, Multicenter Study to Assess the Safety, Tolerability, Pharmacokinetics and Efficacy of LMB763 in Patients with Non-Alcoholic Steatohepatitis (NASH), 2020. [(accessed on 10 December 2021)]. Available online: https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=17527.
- 67.Loomba R., Kowdley K.V., Vuppalanchi R., Hassanein T., Rojter S.E., Sheikh M.Y., Moussa S., Chung D., Eng C., Marmon T., et al. Liver-Distributed FXR Agonist TERN-101 Demonstrates Favorable Safety and Efficacy Profile in NASH Phase 2a LIFT Study. Hepatology. 2021;74:97A–98A. [Google Scholar]
- 68.Hepagene Therapeutics, Inc. [(accessed on 10 December 2021)]. Available online: http://www.hepagene.com/#/m_about2.
- 69.Henriksson E., Andersen B. FGF19 and FGF21 for the Treatment of NASH-Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 from Mice to Human. Front. Endocrinol. 2020;11:601349. doi: 10.3389/fendo.2020.601349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison S.A., Abdelmalek M.F., Neff G.W., Gunn N., Guy C.D., Alkhouri N., Bashir M., Freilich B., Almeda J., Knapple W., et al. Topline Results from the ALPINE 2/3 Study: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase 2b Trial Evaluating 3 Doses of the FGF19 Analogue Aldafermin on Liver Histology in Patients with Nonalcoholic Steatohepatitis and Stage 2 or 3 Fibrosis. Hepatology. 2021;74:5A. [Google Scholar]
- 71.Harrison S.A., Ruane P.J., Freilich B.L., Neff G., Patil R., Behling C.A., Hu C., Fong E., de Temple B., Tillman E.J., et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021;27:1262–1271. doi: 10.1038/s41591-021-01425-3. [DOI] [PubMed] [Google Scholar]
- 72.Frias J.P., Lawitz E.J., Ortiz-LaSanta G., Franey B., Morrow L., Chen C.-Y., Tseng L., Charlton R.W., Mansbach H., Margalit M., et al. BIO89-100 Demonstrated Robust Reductions in Liver Fat and Liver Fat Volume (LFV) by MRI-PDFF, Favorable Tolerability and Potential for Weekly (QW) or Every 2 Weeks (Q2W) Dosing in a Phase 1b/2a Placebo-Controlled, Double-Blind, Multiple Ascending Dose Study in NASH. J. Endocr. Soc. 2021;5:A5–A6. doi: 10.1210/jendso/bvab048.010. [DOI] [Google Scholar]
- 73.Novo Nordisk. [(accessed on 10 December 2021)]. Available online: https://www.novonordisk.com/
- 74.Baruch A., Wong C., Chinn L.W., Vaze A., Sonoda J., Gelzleichter T., Chen S., Lewin-Koh N., Morrow L., Dheerendra S., et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl. Acad. Sci. USA. 2020;117:28992–29000. doi: 10.1073/pnas.2012073117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan Q., Lin S., Li Y., Liu L., Li X., Gao X., Yan J., Gu B., Chen X., Li W., et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine. 2021;63:103202. doi: 10.1016/j.ebiom.2020.103202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelmalek M.F., Sanyal A.J., Nakajima A., Neuschwander-Tetri B.A., Goodman Z., Lawitz E.J., Harrison S.A., Jacobson I.M., Imajo K., Gunn N., et al. Efficacy and Safety of Pegbelfermin in Patients with Nonalcoholic Steatohepatitis and Compensated Cirrhosis: Results from the Phase 2b, Randomized, Double-Blind, Placebo-Controlled Falcon 2 Study; Proceedings of the Liver Meeting 2021; Virtual. 12–15 November 2021. [Google Scholar]
- 77.Sun M.J., Cao Z.Q., Leng P. The roles of galectins in hepatic diseases. J. Mol. Histol. 2020;51:473–484. doi: 10.1007/s10735-020-09898-1. [DOI] [PubMed] [Google Scholar]
- 78.An Y., Xu S., Liu Y., Xu X., Philips C.A., Chen J., Méndez-Sánchez N., Guo X., Qi X. Role of Galectins in the Liver Diseases: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:744518. doi: 10.3389/fmed.2021.744518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Traber P.G., Chou H., Zomer E., Hong F., Klyosov A., Fiel M.I., Friedman S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M., Noureddin M., Pyko M., Shiffman M., Sanyal A., et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients with Nonalcoholic Steatohepatitis with Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334–1345.e5. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 81.Hsieh Y.C., Lee K.C., Wu P.S., Huo T.I., Huang Y.H., Hou M.C., Lin H.C. Eritoran Attenuates Hepatic Inflammation and Fibrosis in Mice with Chronic Liver Injury. Cells. 2021;10:1562. doi: 10.3390/cells10061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang L., Seki E. Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 2012;3:138. doi: 10.3389/fphys.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang K.C., Chen M.Y., Lin C.L., Yang S.S., Hsu C.W., Wang C.C., Huang Y.H., Chang C.C., Shih Y.C., Liu S.H. JKB-111 in Patients with Non-Alcoholic Fatty Liver: A Phase 2 Randomized Double-Blind Placebo-Control Study; Proceedings of the Digital International Liver Congress 2020; Virtual. 27–29 August 2020. [Google Scholar]
- 84.Klepfish M., Gross T., Vugman M., Afratis N.A., Havusha-Laufer S., Brazowski E., Solomonov I., Varol C., Sagi I. LOXL2 Inhibition Paves the Way for Macrophage-Mediated Collagen Degradation in Liver Fibrosis. Front. Immunol. 2020;11:480. doi: 10.3389/fimmu.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen W., Yang A., Jia J., Popov Y.V., Schuppan D., You H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology. 2020;72:729–741. doi: 10.1002/hep.31236. [DOI] [PubMed] [Google Scholar]
- 86.Harrison S.A., Abdelmalek M.F., Caldwell S., Shiffman M.L., Diehl A.M., Ghalib R., Lawitz E.J., Rockey D.C., Schall R.A., Jia C., et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Schilter H., Findlay A.D., Perryman L., Yow T.T., Moses J., Zahoor A., Turner C.I., Deodhar M., Foot J.S., Zhou W., et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J. Cell. Mol. Med. 2019;23:1759–1770. doi: 10.1111/jcmm.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galecto, Inc. [(accessed on 10 December 2021)]. Available online: https://galecto.com/
- 89.Geraldo L.H.M., Spohr T.C.L.S., Amaral R.F.D., Fonseca A.C.C.D., Garcia C., Mendes F.A., Freitas C., dos Santos M.F., Lima F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021;6:45. doi: 10.1038/s41392-020-00367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bain G., Shannon K.E., Huang F., Darlington J., Goulet L., Prodanovich P., Ma G.L., Santini A.M., Stein A.J., Lonergan D., et al. Selective Inhibition of Autotaxin Is Efficacious in Mouse Models of Liver Fibrosis. J. Pharmacol. Exp. Ther. 2017;360:1–13. doi: 10.1124/jpet.116.237156. [DOI] [PubMed] [Google Scholar]
- 91.TaiwanJ Pharmaceuticals. [(accessed on 10 December 2021)]. Available online: https://www.taiwanj.com/pages/page_index_en.
- 92.Boeckmans J., Natale A., Rombaut M., Buyl K., Rogiers V., De Kock J., Vanhaecke T., Rodrigues R.M. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells. 2019;9:37. doi: 10.3390/cells9010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Athyros V.G., Mikhailidis D.P., Didangelos T.P., Giouleme O.I., Liberopoulos E.N., Karagiannis A., Kakafika A.I., Tziomalos K., Burroughs A.K., Elisaf M.S. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: A randomised study. Curr. Med. Res. Opin. 2006;22:873–883. doi: 10.1185/030079906X104696. [DOI] [PubMed] [Google Scholar]
- 94.Fernández-Miranda C., Pérez-Carreras M., Colina F., López-Alonso G., Vargas C., Solís-Herruzo J.A. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig. Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Basaranoglu M., Acbay O., Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J. Hepatol. 1999;31:384. doi: 10.1016/S0168-8278(99)80243-8. [DOI] [PubMed] [Google Scholar]
- 96.Laurin J., Lindor K.D., Crippin J.S., Gossard A., Gores G.J., Ludwig J., Rakela J., McGill D.B. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: A pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 97.Hatanaka T., Kosone T., Saito N., Takakusagi S., Tojima H., Naganuma A., Takagi H., Uraoka T., Kakizaki S. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open. 2021;5:1183–1189. doi: 10.1002/jgh3.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sasaki Y., Shimada T., Iizuka S., Suzuki W., Makihara H., Teraoka R., Tsuneyama K., Hokao R., Aburada M. Effects of bezafibrate in nonalcoholic steatohepatitis model mice with monosodium glutamate-induced metabolic syndrome. Eur. J. Pharmacol. 2011;662:1–8. doi: 10.1016/j.ejphar.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 99.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 100.Lee Y.H., Kim J.H., Kim S.R., Jin H.Y., Rhee E.J., Cho Y.M., Lee B.W. Lobeglitazone, a Novel Thiazolidinedione, Improves Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: Its Efficacy and Predictive Factors Related to Responsiveness. J. Korean Med. Sci. 2017;32:60–69. doi: 10.3346/jkms.2017.32.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakagami H., Shimamura M., Miyake T., Shimosato T., Minobe N., Moritani T., Kiomy Osako M., Nakagami F., Koriyama H., Kyutoku M., et al. Nifedipine prevents hepatic fibrosis in a non-alcoholic steatohepatitis model induced by an L-methionine-and choline-deficient diet. Mol. Med. Rep. 2012;5:37–40. doi: 10.3892/mmr.2011.594. [DOI] [PubMed] [Google Scholar]
- 102.Zarei M., Barroso E., Palomer X., Dai J., Rada P., Quesada-López T., Escolà-Gil J.C., Cedó L., Zali M.R., Molaei M., et al. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol. Metab. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.CymaBay Therapeutics CymaBay Therapeutics Reports Topline 12-Week Data from an Ongoing Phase 2b Study of Seladelpar in Patients with Nonalcoholic Steatohepatitis; 2019. [(accessed on 10 December 2021)]. Available online: https://www.globenewswire.com/news-release/2019/06/11/1866763/0/en/CymaBay-Therapeutics-Reports-Topline-12-Week-Data-from-an-Ongoing-Phase-2b-Study-of-Seladelpar-in-Patients-with-Nonalcoholic-Steatohepatitis.html.
- 104.Goyal O., Nohria S., Goyal P., Kaur J., Sharma S., Sood A., Chhina R.S. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: A prospective, observational, real world study. Sci. Rep. 2020;10:21117. doi: 10.1038/s41598-020-78342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gawrieh S., Noureddin M., Loo N., Mohseni R., Awasty V., Cusi K., Kowdley K.V., Lai M., Schiff E., Parmar D., et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology. 2021;74:1809–1824. doi: 10.1002/hep.31843. [DOI] [PubMed] [Google Scholar]
- 106.Francque S.M., Bedossa P., Ratziu V., Anstee Q.M., Bugianesi E., Sanyal A.J., Loomba R., Harrison S.A., Balabanska R., Mateva L., et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 107.Harrison S.A., Alkhouri N., Davison B.A., Sanyal A., Edwards C., Colca J.R., Lee B.H., Loomba R., Cusi K., Kolterman O., et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J Hepatol. 2020;72:613–626. doi: 10.1016/j.jhep.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 108.Fukunaga T., Zou W., Rohatgi N., Colca J.R., Teitelbaum S.L. An insulin-sensitizing thiazolidinedione, which minimally activates PPARγ, does not cause bone loss. J. Bone Miner. Res. 2015;30:481–488. doi: 10.1002/jbmr.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacques V., Bolze S., Hallakou-Bozec S., Czarnik A.W., Divakaruni A.S., Fouqueray P., Murphy A.N., Van der Ploeg L.H.T., DeWitt S. Deuterium-Stabilized (R)-Pioglitazone (PXL065) Is Responsible for Pioglitazone Efficacy in NASH yet Exhibits Little to No PPARγ Activity. Hepatol. Commun. 2021;5:1412–1425. doi: 10.1002/hep4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El K., Gray S.M., Capozzi M.E., Knuth E.R., Jin E., Svendsen B., Clifford A., Brown J.L., Encisco S.E., Chazotte B.M., et al. GIP mediates the incretin effect and glucose tolerance by dual actions on α cells and β cells. Sci. Adv. 2021;7:eabf1948. doi: 10.1126/sciadv.abf1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X.C., Gusdon A.M., Liu H., Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J. Gastroenterol. 2014;20:14821–14830. doi: 10.3748/wjg.v20.i40.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samson S.L., Sathyanarayana P., Jogi M., Gonzalez E.V., Gutierrez A., Krishnamurthy R., Muthupillai R., Chan L., Bajaj M. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia. 2011;54:3093–3100. doi: 10.1007/s00125-011-2317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., Sanyal A.J., Sejling A.S., Harrison S.A., NN9931-4296 Investigators A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 114.Liava C., Sinakos E. Semaglutide for nonalcoholic steatohepatitis: Closer to a solution? Hepatobiliary Surg. Nutr. 2021;10:541–544. doi: 10.21037/hbsn-21-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dutour A., Abdesselam I., Ancel P., Kober F., Mrad G., Darmon P., Ronsin O., Pradel V., Lesavre N., Martin J.C., et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016;18:882–891. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 116.Sofogianni A., Filippidis A., Chrysavgis L., Tziomalos K., Cholongitas E. Glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: An update. World J. Hepatol. 2020;12:493–505. doi: 10.4254/wjh.v12.i8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cusi K., Sattar N., García-Pérez L.E., Pavo I., Yu M., Robertson K.E., Karanikas C.A., Haupt A. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: A post hoc analysis of the AWARD programme. Diabet. Med. 2018;35:1434–1439. doi: 10.1111/dme.13697. [DOI] [PubMed] [Google Scholar]
- 118.Kuchay M.S., Krishan S., Mishra S.K., Choudhary N.S., Singh M.K., Wasir J.S., Kaur P., Gill H.K., Bano T., Farooqui K.J., et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial) Diabetologia. 2020;63:2434–2445. doi: 10.1007/s00125-020-05265-7. [DOI] [PubMed] [Google Scholar]
- 119.Koutsovasilis A., Sotiropoulos A., Pappa M., Apostolou O., Binikos I., Kordinas V., Papadaki D., Tamvakos I., Bousboulas S. Effectiveness of lixisenatide in nonalcoholic fatty liver disease in patients with type 2 diabetes after an acute coronary syndrome compared to sitagliptin and pioglitazone; Proceedings of the EASD Virtual Meeting 2016; Munich, Germany. 14 September 2016. [Google Scholar]
- 120.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., LEAN Trial Team. Abouda G., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 121.Ghosal S., Datta D., Sinha B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D) Sci. Rep. 2021;11:22063. doi: 10.1038/s41598-021-01663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thondam S.K., Cuthbertson D.J., Wilding J.P.H. The influence of Glucose-dependent Insulinotropic Polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD) Peptides. 2020;125:170208. doi: 10.1016/j.peptides.2019.170208. [DOI] [PubMed] [Google Scholar]
- 123.Hartman M.L., Sanyal A.J., Loomba R., Wilson J.M., Nikooienejad A., Bray R., Karanikas C.A., Duffin K.L., Robins D.A., Haupt A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes. Diabetes Care. 2020;43:1352–1355. doi: 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gastaldelli A., Cusi K., Landó F., Bray R., Brouwers B., Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in patients with type 2 diabetes (SURPASS-3 MRI); Proceedings of the EASD Virtual Meeting 2021; Virtual. 30 September 2021. [Google Scholar]
- 125.Pocai A. Action and therapeutic potential of oxyntomodulin. Mol. Metab. 2013;3:241–251. doi: 10.1016/j.molmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boland M.L., Laker R.C., Mather K., Nawrocki A., Oldham S., Boland B.B., Lewis H., Conway J., Naylor J., Guionaud S., et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat. Metab. 2020;2:413–431. doi: 10.1038/s42255-020-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nahra R., Wang T., Gadde K.M., Oscarsson J., Stumvoll M., Jermutus L., Hirshberg B., Ambery P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults with Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021;44:1433–1442. doi: 10.2337/dc20-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jung S.Y., Park Y.J., Lee J.S., Kim E.J., Lee Y.M., Kim Y.H., Trautmann M., Kwon S.C. Potent Cholesterol Lowering Effect by HM12525A, A Novel Long-acting GLP-1/Glucagon Dual Receptor Agonist; Proceedings of the American Diabetes Association’s (ADA) 76th Scientific Sessions; New Orleans, LA, USA. 10–14 June 2016. [Google Scholar]
- 129.Pfizer Inc. [(accessed on 10 December 2021)]. Available online: https://www.pfizer.com/
- 130.Hanmi Pharmaceutical Co., Ltd. [(accessed on 10 December 2021)]. Available online: http://www.hanmipharm.com/ehanmi/handler/Home-Start.
- 131.Kim J.K., Lee J.S., Park E., Lee J., Bae S., Kim D., Choi I.C. HM15211, a Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist, Exhibits Anti-inflammatory and Fibrotic Effects in AMLN/TAA–Induced Liver Inflammation and Fibrosis Mice. Diabetes. 2020;69:1804-P. doi: 10.2337/db20-1804-P. [DOI] [Google Scholar]
- 132.Janssen P., Rotondo A., Mulé F., Tack J. Review article: A comparison of glucagon-like peptides 1 and 2. Aliment. Pharmacol. Ther. 2013;37:18–36. doi: 10.1111/apt.12092. [DOI] [PubMed] [Google Scholar]
- 133.Hu Y.-X. Therapeutic effect of teduglutide on non-alcoholic fatty liver disease in rats. World Chin. J. Dig. 2016;24:1009. doi: 10.11569/wcjd.v24.i7.1009. [DOI] [Google Scholar]
- 134.Alam S., Ghosh J., Mustafa G., Kamal M., Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: A 1-year randomized control trial. Hepat. Med. 2018;10:23–31. doi: 10.2147/HMER.S158053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aktaş A., Ozan Z.T. The efficacy and safety of vildagliptin treatment for nonalcoholic fatty liver disease in type 2 diabetes mellitus. Cumhur. Med. J. 2020;42:491–499. doi: 10.7197/cmj.835387. [DOI] [Google Scholar]
- 136.Li J.J., Zhang P., Fan B., Guo X.L., Zheng Z.S. The efficacy of saxagliptin in T2DM patients with non-alcoholic fatty liver disease: Preliminary data. Rev. Assoc. Med. Bras. 2019;65:33–37. doi: 10.1590/1806-9282.65.1.33. [DOI] [PubMed] [Google Scholar]
- 137.Hattori S., Nomoto K., Suzuki T., Hayashi S. Beneficial effect of omarigliptin on diabetic patients with non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. Diabetol. Metab. Syndr. 2021;13:28. doi: 10.1186/s13098-021-00644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta V.K. Teneligliptin Significantly Reduces Liver Fat Content (LFC) and Delays Progression of NASH in Type 2 Diabetes Mellitus Patients. Diabetes. 2019;68:1029-P. doi: 10.2337/db19-1029-P. [DOI] [Google Scholar]
- 139.Mashitani T., Noguchi R., Okura Y., Namisaki T., Mitoro A., Ishii H., Nakatani T., Kikuchi E., Moriyasu H., Matsumoto M., et al. Efficacy of alogliptin in preventing non-alcoholic fatty liver disease progression in patients with type 2 diabetes. Biomed. Rep. 2016;4:183–187. doi: 10.3892/br.2016.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dos Santos L.R., Duarte M.L., Peccin M.S., Gagliardi A.R.T., Melnik T. Dipeptidyl Peptidase IV Inhibitors for Nonalcoholic Fatty Liver Disease—Systematic Review and Metanalysis. Curr. Diabetes Rev. 2021;17:e101120187811. doi: 10.2174/1573399816999201110195634. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z., Park H., Bae E.J. Efficacy of evogliptin and cenicriviroc against nonalcoholic steatohepatitis in mice: A comparative study. Korean J. Physiol. Pharmacol. 2019;23:459–466. doi: 10.4196/kjpp.2019.23.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sakai Y., Nagashimada M., Nagata N., Ni Y., Kaneko S., Ota T. Dipeptidyl peptidase-4 inhibitor anagliptin alleviates lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice; Proceedings of the EASD Virtual Meeting 2015; Stockholm, Sweden. 15 September 2015. [Google Scholar]
- 143.Kawakubo M., Tanaka M., Ochi K., Watanabe A., Saka-Tanaka M., Kanamori Y., Yoshioka N., Yamashita S., Goto M., Itoh M., et al. Dipeptidyl peptidase-4 inhibition prevents nonalcoholic steatohepatitis-associated liver fibrosis and tumor development in mice independently of its anti-diabetic effects. Sci. Rep. 2020;10:983. doi: 10.1038/s41598-020-57935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang G., Wu B., Zhang L., Jin X., Wang K., Xu W., Zhang B., Wang H. The protective effects of trelagliptin on high-fat diet-induced nonalcoholic fatty liver disease in mice. J. Biochem. Mol. Toxicol. 2021;35:e22696. doi: 10.1002/jbt.22696. [DOI] [PubMed] [Google Scholar]
- 145.Choi S.H., Leem J., Park S., Lee C.K., Park K.G., Lee I.K. Gemigliptin ameliorates Western-diet-induced metabolic syndrome in mice. Can. J. Physiol. Pharmacol. 2017;95:129–139. doi: 10.1139/cjpp-2016-0026. [DOI] [PubMed] [Google Scholar]
- 146.Klein T., Fujii M., Sandel J., Shibazaki Y., Wakamatsu K., Mark M., Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med. Mol. Morphol. 2014;47:137–149. doi: 10.1007/s00795-013-0053-9. [DOI] [PubMed] [Google Scholar]
- 147.Saisho Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases. 2020;8:14. doi: 10.3390/diseases8020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu P., Wen W., Li J., Xu J., Zhao M., Chen H., Sun J. Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm. Metab. Res. 2019;51:487–494. doi: 10.1055/a-0958-2441. [DOI] [PubMed] [Google Scholar]
- 149.Sumida Y., Yoneda M., Tokushige K., Kawanaka M., Fujii H., Yoneda M., Imajo K., Takahashi H., Ono M., Nozaki Y., et al. Hepatoprotective Effect of SGLT2 Inhibitor on Nonalcoholic Fatty Liver Disease. Diabetes Res. Open Access. 2020;2:17–25. doi: 10.36502/2020/droa.6159. [DOI] [Google Scholar]
- 150.Ribeiro Dos Santos L., Baer Filho R. Treatment of nonalcoholic fatty liver disease with dapagliflozin in non-diabetic patients. Metabol. Open. 2020;5:100028. doi: 10.1016/j.metop.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taheri H., Malek M., Ismail-Beigi F., Zamani F., Sohrabi M., Reza Babaei M., Khamseh M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020;37:4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Itani T., Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: A prospective cohort study. Obes. Sci. Pract. 2018;4:477–482. doi: 10.1002/osp4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prikhodko V.A., Sysoev Y.I., Poveryaeva M.A., Bunyat A.V., Karev V.E., Ivkin D.Y., Sukhanov D.S., Shustov E.B., Okovityi S.V. Effects of Empagliflozin and L-Ornithine L-Aspartate on Behavior, Cognitive Functions, and Physical Performance in Mice with Experimentally Induced Steatohepatitis. Bull. RSMU. 2020;3:49–57. doi: 10.24075/brsmu.2020.034. [DOI] [Google Scholar]
- 154.Takahashi H., Kessoku T., Kawanaka M., Nonaka M., Hyogo H., Fujii H., Nakajima T., Imajo K., Tanaka K., Kubotsu Y., et al. Ipragliflozin Improves the Hepatic Outcomes of Patients with Diabetes with NAFLD. Hepatol. Commun. 2021. online ahead of print . [DOI] [PMC free article] [PubMed]
- 155.Wilkison B., Cheatham B., Walker S. Remogliflozin Etabonate Reduces FIB-4 and NAFLD Fibrosis Scores in Type 2 Diabetic Subjects. Hepatology. 2016;64:548A. [Google Scholar]
- 156.Gallo S., Calle R.A., Terra S.G., Pong A., Tarasenko L., Raji A. Effects of Ertugliflozin on Liver Enzymes in Patients with Type 2 Diabetes: A Post-Hoc Pooled Analysis of Phase 3 Trials. Diabetes Ther. 2020;11:1849–1860. doi: 10.1007/s13300-020-00867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shibuya T., Fushimi N., Kawai M., Yoshida Y., Hachiya H., Ito S., Kawai H., Ohashi N., Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes. Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
- 158.Sumida Y., Murotani K., Saito M., Tamasawa A., Osonoi Y., Yoneda M., Osonoi T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial) Hepatol. Res. 2019;49:64–71. doi: 10.1111/hepr.13236. [DOI] [PubMed] [Google Scholar]
- 159.Yoneda M., Honda Y., Ogawa Y., Kessoku T., Kobayashi T., Imajo K., Ozaki A., Nogami A., Taguri M., Yamanaka T., et al. Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): A randomized prospective open-label controlled trial. BMJ Open Diabetes Res. Care. 2021;9:e001990. doi: 10.1136/bmjdrc-2020-001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He Y.L., Haynes W., Meyers C.D., Amer A., Zhang Y., Mahling P., Mendonza A.E., Ma S., Chutkow W., Bachman E. The effects of licogliflozin, a dual SGLT1/2 inhibitor, on body weight in obese patients with or without diabetes. Diabetes Obes. Metab. 2019;21:1311–1321. doi: 10.1111/dom.13654. [DOI] [PubMed] [Google Scholar]
- 161.Harrison S.A., Manghi F.P., Smith W.B., Alpenidze D., Aizenberg D., Burggraaf K., Chen C.-Y., Zuckerman E., Ravussin E., Charatcharoenwitthaya P., et al. LIK066 (Licogliflozin), an SGLT1/2 Inhibitor, Robustly Decreases ALT and Improves Markers of Hepatic and Metabolic Health in Patients with Non-Alcoholic Fatty Liver Disease: Interim Analysis of a 12-Week, Randomized, Placebo-Controlled, Phase 2a Study; Proceedings of the Liver Meeting 2019; Boston, MA, USA. 8–12 November 2019. [Google Scholar]
- 162.Honda Y., Ozaki A., Iwaki M., Kobayashi T., Nogami A., Kessoku T., Ogawa Y., Tomeno W., Imajo K., Yoneda M., et al. Protective effect of SGL5213, a potent intestinal sodium-glucose cotransporter 1 inhibitor, in nonalcoholic fatty liver disease in mice. J. Pharmacol. Sci. 2021;147:176–183. doi: 10.1016/j.jphs.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Iogna Prat L., Tsochatzis E.A. The effect of antidiabetic medications on non-alcoholic fatty liver disease (NAFLD) Hormones. 2018;17:219–229. doi: 10.1007/s42000-018-0021-9. [DOI] [PubMed] [Google Scholar]
- 164.Hajiaghamohammadi A.A., Miroliaee A., Samimi R., Alborzi F., Ziaee A. A Comparison of Ezetimibe and Acarbose in Decreasing Liver Transaminase in Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. Govaresh. 2013;18:186–190. [Google Scholar]
- 165.Komatsu M., Tanaka N., Kimura T., Fujimori N., Sano K., Horiuchi A., Sugiura A., Yamazaki T., Shibata S., Joshita S., et al. Miglitol attenuates non-alcoholic steatohepatitis in diabetic patients. Hepatol. Res. 2018;48:1092–1098. doi: 10.1111/hepr.13223. [DOI] [PubMed] [Google Scholar]
- 166.Kim J.W., Lee Y.J., You Y.H., Moon M.K., Yoon K.H., Ahn Y.B., Ko S.H. Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and α-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes. J. Cell. Biochem. 2018. online ahead of print . [DOI] [PubMed]
- 167.Meroni M., Longo M., Dongiovanni P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malaguarnera M., Vacante M., Antic T., Giordano M., Chisari G., Acquaviva R., Mastrojeni S., Malaguarnera G., Mistretta A., Li Volti G., et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 169.Vajro P., Mandato C., Licenziati M.R., Franzese A., Vitale D.F., Lenta S., Caropreso M., Vallone G., Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 170.Nabavi S., Rafraf M., Somi M.H., Homayouni-Rad A., Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 171.Román E., Nieto J.C., Gely C., Vidal S., Pozuelo M., Poca M., Juárez C., Guarner C., Manichanh C., Soriano G. Effect of a Multistrain Probiotic on Cognitive Function and Risk of Falls in Patients with Cirrhosis: A Randomized Trial. Hepatol. Commun. 2019;3:632–645. doi: 10.1002/hep4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahn S.B., Jun D.W., Kang B.K., Lim J.H., Lim S., Chung M.J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019;9:5688. doi: 10.1038/s41598-019-42059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kobyliak N., Abenavoli L., Mykhalchyshyn G., Kononenko L., Boccuto L., Kyriienko D., Dynnyk O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin Liver Dis. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- 174.Ma Y.Y., Li L., Yu C.H., Shen Z., Chen L.H., Li Y.M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao X., Zhu Y., Wen Y., Liu G., Wan C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: A meta-analysis of randomized controlled trials. Hepatol. Res. 2016;46:1226–1233. doi: 10.1111/hepr.12671. [DOI] [PubMed] [Google Scholar]
- 176.Liu Y.T., Li Y.Q., Wang Y.Z. Protective effect of Saccharomyces boulardii against intestinal mucosal barrier injury in rats with nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2016;24:921–926. doi: 10.3760/cma.j.issn.1007-3418.2016.12.009. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 177.Yamauchi R., Takedatsu H., Yokoyama K., Yamauchi E., Kawashima M., Tsuchiya N., Takata K., Tanaka T., Morihara D., Takeyama Y., et al. Synergistic effect of clostridium butyricum miyairi on rifaximin in mice model of non-alcoholic steatohepatitis by methionine choline-deficient diet. J. Hepatol. 2020;73:S401–S652. doi: 10.1016/S0168-8278(20)31389-1. [DOI] [Google Scholar]
- 178.Abdel Monem S.M. Probiotic Therapy in Patients with Nonalcoholic Steatohepatitis in Zagazig University Hospitals. Euroasian J. Hepatogastroenterol. 2017;7:101–106. doi: 10.5005/jp-journals-10018-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li C., Nie S.P., Zhu K.X., Ding Q., Li C., Xiong T., Xie M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014;5:3216–3223. doi: 10.1039/C4FO00549J. [DOI] [PubMed] [Google Scholar]
- 180.Yao F., Jia R., Huang H., Yu Y., Mei L., Bai L., Ding Y., Zheng P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch. Med. Sci. 2019;15:1336–1344. doi: 10.5114/aoms.2019.86611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xin J., Zeng D., Wang H., Ni X., Yi D., Pan K., Jing B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014;98:6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 182.Ting W.J., Kuo W.W., Hsieh D.J., Yeh Y.L., Day C.H., Chen Y.H., Chen R.J., Padma V.V., Chen Y.H., Huang C.Y. Heat Killed Lactobacillus reuteri GMNL-263 Reduces Fibrosis Effects on the Liver and Heart in High Fat Diet-Hamsters via TGF-β Suppression. Int. J. Mol. Sci. 2015;16:25881–25896. doi: 10.3390/ijms161025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aller R., De Luis D.A., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., De La Fuente B., Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 184.Famouri F., Shariat Z., Hashemipour M., Keikha M., Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017;64:413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 185.Wong V.W., Won G.L., Chim A.M., Chu W.C., Yeung D.K., Li K.C., Chan H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013;12:256–262. doi: 10.1016/S1665-2681(19)31364-X. [DOI] [PubMed] [Google Scholar]
- 186.Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., Giammaria P., Reali L., Anania F., Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ezquer M., Ezquer F., Ricca M., Allers C., Conget P. Intravenous administration of multipotent stromal cells prevents the onset of non-alcoholic steatohepatitis in obese mice with metabolic syndrome. J. Hepatol. 2011;55:1112–1120. doi: 10.1016/j.jhep.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 188.Hsu M.J., Karkossa I., Schäfer I., Christ M., Kühne H., Schubert K., Rolle-Kampczyk U.E., Kalkhof S., Nickel S., Seibel P., et al. Mitochondrial Transfer by Human Mesenchymal Stromal Cells Ameliorates Hepatocyte Lipid Load in a Mouse Model of NASH. Biomedicines. 2020;8:350. doi: 10.3390/biomedicines8090350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang H., Wang D., Yang L., Wang Y., Jia J., Na D., Chen H., Luo Y., Liu C. Compact bone-derived mesenchymal stem cells attenuate nonalcoholic steatohepatitis in a mouse model by modulation of CD4 cells differentiation. Int. Immunopharmacol. 2017;42:67–73. doi: 10.1016/j.intimp.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 190.Chien Y., Huang C.S., Lin H.C., Lu K.H., Tsai P.H., Lai Y.H., Chen K.H., Lee S.D., Huang Y.H., Wang C.Y. Improvement of non-alcoholic steatohepatitis by hepatocyte-like cells generated from iPSCs with Oct4/Sox2/Klf4/Parp1. Oncotarget. 2018;9:18594–18606. doi: 10.18632/oncotarget.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Overi D., Carpino G., Franchitto A., Onori P., Gaudio E. Hepatocyte Injury and Hepatic Stem Cell Niche in the Progression of Non-Alcoholic Steatohepatitis. Cells. 2020;9:590. doi: 10.3390/cells9030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nevens F., Gustot T., Laterre P.F., Lasser L.L., Haralampiev L.E., Vargas V., Lyubomirova D., Albillos A., Najimi M., Michel S., et al. A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation. JHEP Rep. 2021;3:100291. doi: 10.1016/j.jhepr.2021.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Iqbal U., Perumpail B.J., John N., Sallam S., Shah N.D., Kwong W., Cholankeril G., Kim D., Ahmed A. Judicious Use of Lipid Lowering Agents in the Management of NAFLD. Diseases. 2018;6:87. doi: 10.3390/diseases6040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oniciu D.C., Hashiguchi T., Shibazaki Y., Bisgaier C.L. Gemcabene downregulates inflammatory, lipid-altering and cell-signaling genes in the STAM™ model of NASH. PLoS ONE. 2018;13:e0194568. doi: 10.1371/journal.pone.0194568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sanjay K.V., Vishwakarma S., Zope B.R., Mane V.S., Mohire S., Dhakshinamoorthy S. ATP citrate lyase inhibitor Bempedoic Acid alleviate long term HFD induced NASH through improvement in glycemic control, reduction of hepatic triglycerides & total cholesterol, modulation of inflammatory & fibrotic genes and improvement in NAS score. Curr. Res. Pharmacol. Drug Discov. 2021;2:100051. doi: 10.1016/j.crphar.2021.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stanley T.L., Feldpausch M.N., Oh J., Branch K.L., Lee H., Torriani M., Grinspoon S.K. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: A randomized clinical trial. JAMA. 2014;312:380–389. doi: 10.1001/jama.2014.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Theratechnologies Inc. [(accessed on 10 December 2021)]. Available online: https://www.theratech.com/
- 198.Wei X., Wang C., Hao S., Song H., Yang L. The Therapeutic Effect of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Evid.-Based Complementary Altern. Med. 2016;2016:3593951. doi: 10.1155/2016/3593951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yin J., Ye J., Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B. 2012;2:327–334. doi: 10.1016/j.apsb.2012.06.003. [DOI] [Google Scholar]
- 200.Simental-Mendía M., Sánchez-García A., Simental-Mendía L.E. Effect of ursodeoxycholic acid on liver markers: A systematic review and meta-analysis of randomized placebo-controlled clinical trials. Br. J. Clin. Pharmacol. 2020;86:1476–1488. doi: 10.1111/bcp.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang W., Tang Y., Huang J., Hu H. Efficacy of ursodeoxycholic acid in nonalcoholic fatty liver disease: An updated meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2020;29:696–705. doi: 10.6133/apjcn.202012_29(4).0004. [DOI] [PubMed] [Google Scholar]
- 202.Harrison S.A., Gunn N., Neff G.W., Kohli A., Liu L., Flyer A., Goldkind L., Di Bisceglie A.M. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat. Commun. 2021;12:5503. doi: 10.1038/s41467-021-25701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kowdley K.V., Butler P., Cubberley S., Hand A.L., Jenders R.A., Kroon J., Leibowitz M., Moore A.C., Guyer B. Miricorilant, a Selective GR Modulator, Induced a Rapid and Significant Reduction in Liver Fat Content in a Randomized, Placebo-Controlled Phase 2a Study in Patients with Non-Alcoholic Steatohepatitis; Proceedings of the Liver Meeting 2021; Virtual. 12–15 November 2021. [Google Scholar]
- 204.Li F., Jiang M., Ma M., Chen X., Zhang Y., Zhang Y., Yu Y., Cui Y., Chen J., Zhao H., et al. Anthelmintics nitazoxanide protects against experimental hyperlipidemia and hepatic steatosis in hamsters and mice. Acta Pharm. Sin. B 2021, in press, corrected proof. [DOI] [PMC free article] [PubMed]
- 205.Walczak R., Carole B., Benoit N., Descamps E., Nathalie D., Meghien S., Hum D., Staels B., Friedman S., Loomba R., et al. Elafibranor and nitazoxanide synergize to reduce fibrosis in a NASH model. J. Hepatol. 2018;68:S105–S364. doi: 10.1016/S0168-8278(18)30928-0. [DOI] [Google Scholar]
- 206.Glal K.A.M., Abd-Elsalam S.M., Mostafa T.M. Nitazoxanide versus rifaximin in preventing the recurrence of hepatic encephalopathy: A randomized double-blind controlled trial. J. Hepato-Biliary-Pancreat. Sci. 2021;28:812–824. doi: 10.1002/jhbp.947. [DOI] [PubMed] [Google Scholar]
- 207.Flores-Contreras L., Sandoval-Rodríguez A.S., Mena-Enriquez M.G., Lucano-Landeros S., Arellano-Olivera I., Alvarez-Álvarez A., Sanchez-Parada M.G., Armendáriz-Borunda J. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C. BMC Gastroenterol. 2014;14:131. doi: 10.1186/1471-230X-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cui Y., Zhang M., Leng C., Blokzijl T., Jansen B.H., Dijkstra G., Faber K.N. Pirfenidone Inhibits Cell Proliferation and Collagen I Production of Primary Human Intestinal Fibroblasts. Cells. 2020;9:775. doi: 10.3390/cells9030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Poo J.L., Torre A., Aguilar-Ramírez J.R., Cruz M., Mejía-Cuán L., Cerda E., Velázquez A., Patiño A., Ramírez-Castillo C., Cisneros L., et al. Benefits of prolonged-release pirfenidone plus standard of care treatment in patients with advanced liver fibrosis: PROMETEO study. Hepatol. Int. 2020;14:817–827. doi: 10.1007/s12072-020-10069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matsuda K., Iwaki Y. MN-001 (tipelukast), a novel, orally bioavailable drug, reduces fibrosis and inflammation and down-regulates TIMP-1, collagen Type 1 and LOXL2 mRNA overexpression in an advanced NASH (non-alcoholic steatohepatitis) model; Proceedings of the Liver Meeting 2014; Boston, MA, USA. 7–11 November 2014. [Google Scholar]
- 211.Yang M., Zhang C.Y. G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment. World J. Gastroenterol. 2021;27:677–691. doi: 10.3748/wjg.v27.i8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Widjaja A.A., Singh B.K., Adami E., Viswanathan S., Dong J., D’Agostino G.A., Ng B., Lim W.W., Tan J., Paleja B.S., et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology. 2019;157:777–792.e14. doi: 10.1053/j.gastro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 213.Zai W., Chen W., Liu H., Ju D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines. 2021;9:1912. doi: 10.3390/biomedicines9121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stemmer S.M., Manojlovic N.S., Marinca M.V., Petrov P., Cherciu N., Ganea D., Ciuleanu T.E., Pusca I.A., Beg M.S., Purcell W.T., et al. Namodenoson in Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial. Cancers. 2021;13:187. doi: 10.3390/cancers13020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ferramosca A., Di Giacomo M., Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017;23:4146–4157. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ionis Pharmaceuticals, Inc. [(accessed on 10 December 2021)]. Available online: https://www.ionispharma.com/
- 217.Lawitz E., Hassanein T., Denham D., Waters M., Borg B., Mille G., Scott D., Miksztal A., Culwell J., Ellis D., et al. Efficacy Signals of 4-Week Oral DUR-928 in NASH Subjects; Proceedings of the Digital International Liver Congress 2021; Virtual. 23–26 June 2021. [Google Scholar]
- 218.Konuma K., Itoh M., Suganami T., Kanai S., Nakagawa N., Sakai T., Kawano H., Hara M., Kojima S., Izumi Y., et al. Eicosapentaenoic acid ameliorates non-alcoholic steatohepatitis in a novel mouse model using melanocortin 4 receptor-deficient mice. PLoS ONE. 2015;10:e0121528. doi: 10.1371/journal.pone.0121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sojoodi M., Wang Y., Erstad D.J., Caravan P., Lanuti M., Qadan M., Fuchs B.C., Hoang K., Or Y.S., Jiang L., et al. EDP-297, a novel and potent fxr agonist, exhibit robust antifibrotic effects with significant liver function in a rat model of non-alcoholic steatohepatitis. J. Hepatol. 2020;73:S401–S652. doi: 10.1016/S0168-8278(20)31387-8. [DOI] [Google Scholar]
- 220.Ma Y., Huang Y., Yan L., Gao M., Liu D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm. Res. 2013;30:1447–1457. doi: 10.1007/s11095-013-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fraile J.M., Palliyil S., Barelle C., Porter A.J., Kovaleva M. Non-Alcoholic Steatohepatitis (NASH)—A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des. Dev. Ther. 2021;15:3997–4009. doi: 10.2147/DDDT.S315724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., Austin A.S., Freeman J.G., Morgan L., Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 223.Altimmune, Inc. Pemvidutide (ALT-801): Phase 1 12-Week Results. [(accessed on 10 December 2021)]. Available online: https://ir.altimmune.com/static-files/d3319658-9809-44ad-a729-05dd6a6bf4c5.
- 224.Tobita H., Sato S., Miyake T., Ishihara S., Kinoshita Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr. Ther. Res. Clin. Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lai L.L., Vethakkan S.R., Nik Mustapha N.R., Mahadeva S., Chan W.K. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig. Dis. Sci. 2020;65:623–631. doi: 10.1007/s10620-019-5477-1. [DOI] [PubMed] [Google Scholar]
- 226.Seko Y., Sumida Y., Tanaka S., Mori K., Taketani H., Ishiba H., Hara T., Okajima A., Umemura A., Nishikawa T., et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017;47:1072–1078. doi: 10.1111/hepr.12834. [DOI] [PubMed] [Google Scholar]
- 227.Cusi K., Alkhouri N., Harrison S.A., Fouqueray P., Moller D.E., Hallakou-Bozec S., Bolze S., Grouin J.M., Jeannin Megnien S., Dubourg J., et al. Efficacy and safety of PXL770, a direct AMP kinase activator, for the treatment of non-alcoholic fatty liver disease (STAMP-NAFLD): A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Gastroenterol. Hepatol. 2021;6:889–902. doi: 10.1016/S2468-1253(21)00300-9. [DOI] [PubMed] [Google Scholar]
- 228.Chalasani N., Vuppalanchi R., Rinella M., Middleton M.S., Siddiqui M.S., Barritt A.S., IV, Kolterman O., Flores O., Alonso C., Iruarrizaga-Lejarreta M., et al. Randomised clinical trial: A leucine-metformin-sildenafil combination (NS-0200) vs placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2018;47:1639–1651. doi: 10.1111/apt.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Simard J.C., Thibodeau J.F., Leduc M., Tremblay M., Laverdure A., Sarra-Bournet F., Gagnon W., Ouboudinar J., Gervais L., Felton A., et al. Fatty acid mimetic PBI-4547 restores metabolic homeostasis via GPR84 in mice with non-alcoholic fatty liver disease. Sci Rep. 2020;10:12778. doi: 10.1038/s41598-020-69675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Puengel T., De Vos S., Hundertmark J., Kohlhepp M., Guldiken N., Pujuguet P., Auberval M., Marsais F., Shoji K.F., Saniere L., et al. The Medium-Chain Fatty Acid Receptor GPR84 Mediates Myeloid Cell Infiltration Promoting Steatohepatitis and Fibrosis. J. Clin. Med. 2020;9:1140. doi: 10.3390/jcm9041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen X., Liu C., Ruan L. G-Protein-Coupled Receptors 120 Agonist III Improves Hepatic Inflammation and ER Stress in Steatohepatitis. Dig. Dis. Sci. 2021;66:1090–1096. doi: 10.1007/s10620-020-06280-9. [DOI] [PubMed] [Google Scholar]
- 232.Teng B., Huang C., Cheng C.L., Udduttula A., Yu X.F., Liu C., Li J., Yao Z.Y., Long J., Miao L.F., et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J. Hepatol. 2020;73:383–393. doi: 10.1016/j.jhep.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 233.Ookawara M., Matsuda K., Watanabe M., Moritoh Y. The GPR40 Full Agonist SCO-267 Improves Liver Parameters in a Mouse Model of Nonalcoholic Fatty Liver Disease without Affecting Glucose or Body Weight. J. Pharmacol. Exp. Ther. 2020;375:21–27. doi: 10.1124/jpet.120.000046. [DOI] [PubMed] [Google Scholar]
- 234.Shafiq M., Walmann T., Nutalapati V., Gibson C., Zafar Y. Effects of proprotein convertase subtilisin/kexin type-9 inhibitors on fatty liver. World J. Hepatol. 2020;12:1258–1266. doi: 10.4254/wjh.v12.i12.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jun B.G., Cheon G.J. The utility of ezetimibe therapy in nonalcoholic fatty liver disease. Korean J. Intern. Med. 2019;34:284–285. doi: 10.3904/kjim.2019.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kidron M., Perles S., Kaloti R., Ghantous R., Sandouka S.F., Malaabi Y., Safadi R. Oral Insulin–Induced Reduction in Liver Fat Content in T2DM Patients with Nonalcoholic Steatohepatitis. Diabetes. 2020;69:115-LB. doi: 10.2337/db20-115-LB. [DOI] [Google Scholar]
- 237.Cansby E., Nuñez-Durán E., Magnusson E., Amrutkar M., Booten S.L., Kulkarni N.M., Svensson L.T., Borén J., Marschall H.U., Aghajan M., et al. Targeted Delivery of Stk25 Antisense Oligonucleotides to Hepatocytes Protects Mice Against Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2019;7:597–618. doi: 10.1016/j.jcmgh.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Segal-Salto M., Barashi N., Katav A., Edelshtein V., Aharon A., Hashmueli S., George J., Maor Y., Pinzani M., Haberman D., et al. A blocking monoclonal antibody to CCL24 alleviates liver fibrosis and inflammation in experimental models of liver damage. JHEP Rep. 2020;2:100064. doi: 10.1016/j.jhepr.2019.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Safadi R., Braun M., Francis A., Milgrom Y., Massarwa M., Hakimian D., Hazou W., Issachar A., Harpaz Z., Farbstein M., et al. Randomised clinical trial: A phase 2 double-blind study of namodenoson in non-alcoholic fatty liver disease and steatohepatitis. Aliment. Pharmacol. Ther. 2021;54:1405–1415. doi: 10.1111/apt.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pliant Therapeutics. [(accessed on 10 December 2021)]. Available online: https://pliantrx.com/
- 241.CohBar, Inc. [(accessed on 10 December 2021)]. Available online: https://www.cohbar.com/
- 242.Cerenis Therapeutics Results of the Phase I Study of Repeated and Increasing Doses to Assess CER-209 in NASH/NAFLD: Press Release; 2018. [(accessed on 10 December 2021)]. Available online: https://www.abionyx.com/images/pdfs/images/pr_cerenis_CER_209_ENG_final_e06d4.pdf.
- 243.Hepion Pharmaceuticals. [(accessed on 10 December 2021)]. Available online: https://hepionpharma.com/
- 244.Lipocine. [(accessed on 10 December 2021)]. Available online: https://www.lipocine.com/
- 245.Gupte A.A., Sabek O.M., Fraga D., Minze L.J., Nishimoto S.K., Liu J.Z., Afshar S., Gaber L., Lyon C.J., Gaber O., et al. Osteocalcin protects against non-alcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2020;155:4697–4705. doi: 10.1210/en.2014-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sookoian S., Pirola C.J., Valenti L., Davidson N.O. Genetic Pathways in Nonalcoholic Fatty Liver Disease: Insights from Systems Biology. Hepatology. 2020;72:330–346. doi: 10.1002/hep.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kumar V., Xin X., Ma J., Tan C., Osna N., Mahato R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021;176:113888. doi: 10.1016/j.addr.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fang Z., Dou G., Wang L. MicroRNAs in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2021;17:1851–1863. doi: 10.7150/ijbs.59588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wu P., Zhang M., Webster N.J.G. Alternative RNA Splicing in Fatty Liver Disease. Front. Endocrinol. 2021;12:613213. doi: 10.3389/fendo.2021.613213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bosch-Presegué L., Vaquero A. Sirtuins in stress response: Guardians of the genome. Oncogene. 2014;33:3764–3775. doi: 10.1038/onc.2013.344. [DOI] [PubMed] [Google Scholar]
- 251.Vachharajani V., McCall C.E. Sirtuins: Potential therapeutic targets for regulating acute inflammatory response? Expert Opin. Ther. Targets. 2020;24:489–497. doi: 10.1080/14728222.2020.1743268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu Y., Yan Y., Gius D.R., Vassilopoulos A. Metabolic regulation of Sirtuins upon fasting and the implication for cancer. Curr. Opin. Oncol. 2013;25:630–636. doi: 10.1097/01.cco.0000432527.49984.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Colak Y., Ozturk O., Senates E., Tuncer I., Yorulmaz E., Adali G., Doganay L., Enc F.Y. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med. Sci. Monit. 2011;17:HY5–HY9. doi: 10.12659/MSM.881749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Luci C., Bourinet M., Leclère P.S., Anty R., Gual P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front. Endocrinol. 2020;11:597648. doi: 10.3389/fendo.2020.597648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.de Gregorio E., Colell A., Morales A., Marí M. Relevance of SIRT1-NF-κB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int. J. Mol. Sci. 2020;21:3858. doi: 10.3390/ijms21113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Milne J.C., Lambert P.D., Schenk S., Carney D.P., Smith J.J., Gagne D.J., Jin L., Boss O., Perni R.B., Vu C.B., et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nassir F., Ibdah J.A. Sirtuins and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016;22:10084–10092. doi: 10.3748/wjg.v22.i46.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kundu A., Dey P., Park J.H., Kim I.S., Kwack S.J., Kim H.S. EX-527 Prevents the Progression of High-Fat Diet-Induced Hepatic Steatosis and Fibrosis by Upregulating SIRT4 in Zucker Rats. Cells. 2020;9:1101. doi: 10.3390/cells9051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Part of the data analyzed in this study are openly available at ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/home (accessed on 10 December 2021).