Abstract
Over the past two decades, significant advances have been made in the field of regenerative medicine. However, despite being of the utmost clinical urgency, there remains a paucity of therapeutic strategies for conditions with substantial neurodegeneration such as (progressive) multiple sclerosis (MS), spinal cord injury (SCI), Parkinson’s disease (PD) and Alzheimer’s disease (AD). Different cell types, such as mesenchymal stromal cells (MSC), neuronal stem cells (NSC), olfactory ensheathing cells (OEC), neurons and a variety of others, already demonstrated safety and regenerative or neuroprotective properties in the central nervous system during the preclinical phase. As a result of these promising findings, in recent years, these necessary types of cell therapies have been intensively tested in clinical trials to establish whether these results could be confirmed in patients. However, extensive research is still needed regarding elucidating the exact mechanism of action, possible immune rejection, functionality and survival of the administered cells, dose, frequency and administration route. To summarize the current state of knowledge, we conducted a systematic review with meta-analysis. A total of 27,043 records were reviewed by two independent assessors and 71 records were included in the final quantitative analysis. These results show that the overall frequency of serious adverse events was low: 0.03 (95% CI: 0.01–0.08). In addition, several trials in MS and SCI reported efficacy data, demonstrating some promising results on clinical outcomes. All randomized controlled studies were at a low risk of bias due to appropriate blinding of the treatment, including assessors and patients. In conclusion, cell-based therapies in neurodegenerative disease are safe and feasible while showing promising clinical improvements. Nevertheless, given their high heterogeneity, the results require a cautious approach. We advocate for the harmonization of study protocols of trials investigating cell-based therapies in neurodegenerative diseases, adverse event reporting and investigation of clinical outcomes.
Keywords: regeneration, cell-based therapy, neurodegenerative diseases
1. Introduction
Over the past two decades, significant advances have been made in the field of regenerative medicine, which focuses on developing methods to restore, regrow or replace damaged or dysfunctional cells, tissues and organs [1]. In 2009, Chondroselect (TiGenix N.V.) was the first regenerative cellular therapy that entered the European market, using autologous ex vivo expanded chondrocytes. Ever since, dozens of regenerative treatments have been successfully launched on the market targeting, amongst other things, cartilage defects, burn wounds, osteoarthritis, Crohn’s disease and ischemia [1]. Despite promising innovations in this medical field, treatment strategies to prevent neuronal loss in neurodegenerative diseases such as (progressive) multiple sclerosis (MS), spinal cord injury (SCI), Parkinson’s SP disease (PD) and Alzheimer’s SP disease (AD), which are characterized by a progressive loss of structure and function of neurons in the central nervous system, are currently lacking [2]. For these conditions, disease-modifying treatments or therapies, which alleviate symptoms but do not slow the disease progression, are primarily available. While substantial progress has been made with these drugs, efficacy often comes at a price, inducing damaging side-effects due to the generalized immune modulation of these therapies [3,4,5]. Therefore, new strategies to derive neuronal subtypes and to create environmental changes promoting neuroprotection and the reconstruction of a neuronal network are of utmost clinical urgency [6].
Different cell types, such as mesenchymal stromal cells (MSC) [7,8,9], bone-marrow mononuclear cells (BMMC) [10,11], olfactory mucosa auto grafts (OMA) [12], olfactory ensheathing cells (OEC) [13,14], neuronal stem cells (NSC) [15], Schwann cells [16] and macrophages [17], showed safety and promising regenerative properties. Several studies reported recovery in experimental autoimmune encephalomyelitis (EAE), which is an MS mouse model, including promotion of neuronal repair, neuroprotection and decreased demyelination [9,18]. Moreover, various cell types in SCI rat models showed recovery after complete SCI after transplantation. In addition to neuroprotective mechanisms, axon regeneration and remyelination were observed, leading to significant sensory and motor improvements [8,13,15,16]. In addition, different types of neuronal dopaminergic grafts showed survival and improved functionality in PD rat models [19,20,21]. As a result of these promising findings, in recent years, different types of cell therapies have been intensively investigated in clinical trials to establish whether these positive results could be confirmed in humans. In order to summarize the current state of knowledge, we conducted a systematic review and meta-analysis. In this article, we will discuss the most frequently used cell therapies for neurodegenerative diseases and examine whether the treatments are safe and exhibit improvement in clinical outcomes.
2. Methods
2.1. Literature Search
The protocol of the present systematic review was registered on PROSPERO (CRD42021288757).Two independent reviewers (JVdB and IW) systematically performed the literature search in the electronic databases PubMed, Web of Science and Clinicaltrials.gov. The final search was performed on the 2nd of December 2021. The search terms used for the search on PubMed were (“Nerve Regeneration”[MeSH Terms] OR “Nerve Regeneration”[Title/Abstract] OR “Biological Therapy”[MeSH Terms]) AND ((“multiple sclerosis”[Title/Abstract] OR “spinal cord injury”[Title/Abstract] OR “Parkinson disease”[Title/Abstract] OR “Alzheimer disease”[Title/Abstract]) OR (“Parkinsonian Disorders”[Mesh] OR “Multiple Sclerosis”[Mesh] OR “Spinal Cord Injuries”[Mesh] OR “Alzheimer Disease”[Mesh])). The search terms used for the search on clinicaltrails.gov were (Multiple sclerosis/Parkinson disease/Alzheimer disease /Spinal cord injury) AND (Regeneration/Remyelination/Biological therapy). The same terms were used for Web of Science, excluding ‘biological therapy’, due to the fact that mostly irrelevant search results were obtained. The following filters were applied in the databases PubMed and Web of Science in order to specify the search: (1) publication date between 1 January 2000 and 2 December 2021, (2) clinical trials, and (3) studies performed on subjects older than 18 years.
2.2. In- and Exclusion Criteria
Clinical trials reporting on treatment interventions in adult persons with neurodegenerative diseases including MS, AD, PD and SCI were included. The interventions studied were human cellular therapies inducing regeneration, including MSC, BMMC, peripheral blood derived stem cells (PBSCs), macrophages, NSC, neuron transplantations, OEC and OMA. Trials investigating chemical compounds, non-cellular biologicals and non-human cells as well as clinical trials primarily focusing on immunomodulatory effects were excluded. Since this is a quite recently emerging field, all types of studies including phase I, II and III, controlled and uncontrolled, randomized and non-randomized, blinded and unblinded studies were included.
2.3. Outcome Measures
The primary outcome was safety, including the proportion and number of patients with at least one serious adverse event (SAE) or at least one adverse event (AE) or with at least one SAE related to the intervention. The secondary outcome was efficacy. In studies with MS patients, efficacy was assessed by the proportion of patients with an improvement in the expanded disability status scale (EDSS). In studies with SCI patients, efficacy was assessed by the proportion of patients with an improvement in the American spinal injury association impairment scale (AIS). No analysis was performed for efficacy in studies for PD and AD, due to an inadequate number of studies with clinical data for these diseases. In addition, to the extent possible, a comparative analysis for efficacy was performed with respect to the control group. Finally, if possible, a comparative analysis was performed regarding administration route, including a comparison between intravenous (IV) administration and intrathecal (IT) administration.
2.4. Selection Process and Data Extraction
Two review authors (JVdB and IW) conducted the literature search separately, screened studies for eligibility and extracted the data. Disagreements between the two reviewers were clarified and solved by a consensus meeting. The initial selection was determined based on title, abstract, and keywords. Afterwards, the assessors checked for duplicates, using Doi and NCT number, which were subsequently excluded. A final selection of studies was made after reading the full text. When provided, the following information was extracted from the studies: (1) general information including Doi, NCT number, author, title and publication date; (2) study characteristics including type of neurodegenerative disease, cell type used, study design, duration follow-up and sample size; (3) participant characteristics including, gender, age, participants recruited and dropout rate; (4) information about the intervention including route of administration, dose and frequency and autologous versus allogenic administration; and (5) outcomes including the number of patients in the controlled/treated group, adverse events (causality and severity) and improvement in clinical outcomes such as AIS grade and EDSS.
2.5. Study Risk of Bias Assessment
Risk of bias assessment varied according to the study design. For randomized studies, the Risk of Bias (Rob2) tool from Cochrane was used [22]. For non-randomized controlled and uncontrolled studies, the ROBINS-I tool was used [23]. Assessments were performed at the study level. Risk of bias (RoB) was ranked as low risk, some concern or high risk and was visualized with the ROBVIS tool [24]. Disagreement between the two review authors (JVdB and IW) was resolved by a consensus meeting.
2.6. Data Synthesis and Statistical Analysis
The PRISMA guidelines were used in the implementation of the entire literature review process [25]. Figure 1 shows a flow diagram reporting the number of in- and excluded studies and the reason for exclusion. For the meta-analysis, categorical data were summarized as numbers or percentages. A random effects meta-analysis was used to calculate overall proportions and corresponding 95% confidence intervals. Studies with a control arm were included in a comparative random effects meta-analysis. Risk ratios and 95% confidence intervals are reported. For rare outcomes (such as serious adverse events), risk differences were used as effect measure instead, as risk ratios cannot be computed when no events are observed in the and control arm. All analyses were performed in R version 3.6 or higher.
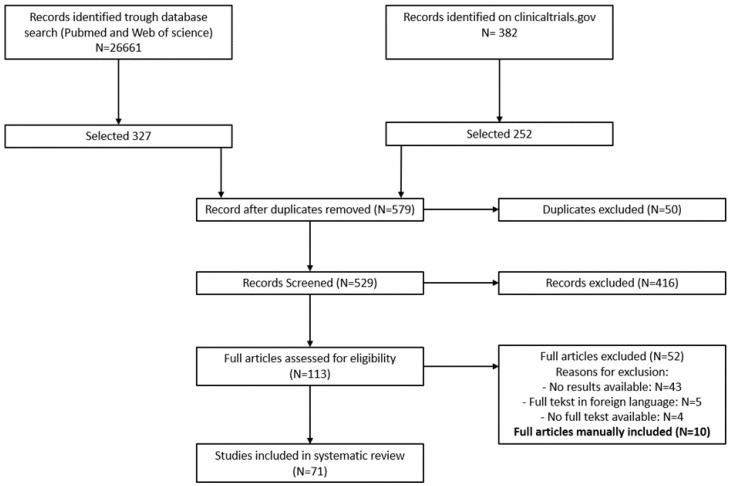
Figure 1.
PRISMA flow diagram.
3. Results
3.1. Seacrch Results
The results of the search strategy are shown in a PRISMA flow diagram (Figure 1). In total, 27,043 records were screened and sixty-one were ultimately included in the quantitative analysis. Ten additional papers, fulfilling the inclusion criteria, were included in the analysis during the literature review and after consensus between the two assessors. The majority, namely 42 of the studies, evaluated the safety and/or efficacy of the administration of stem cells including MSC. Twelve studies regarding BMMCs, PBSCs and macrophages were included. The seventeen remaining studies investigated the administration or transplantation of neuronal cells including NSC, OMA, OEC, Schwann cells and neuron-like cell types (Table 1).
Table 1.
Overview of the 71 included studies. Abbreviations: mesenchymal stem cell (MSC), bone-marrow mononuclear cell (BMMC), olfactory mucosal autograft (OMA), neuronal stem cell (NSC), olfactory ensheathing cell (OEC), peripheral blood stem cell (PBSC), intravenous (IV), intrathecal (IT), intra-arterial (IA)relapse-remitting (RR), secondary-progressive (SP), primary-progressive (PP), relapsing-progressive (RP), acute (A), sub-acute (SA) chronic (C), varying subtypes (V), treatment (T), control (Co).
| Author + Year | Cell Type | Administration Route | Disease | #Patients | %Improved |
|---|---|---|---|---|---|
| Riordan NH. 2018 | MSC (UC) | IV | MS (RR,PP,SP) | T: 17 | NA |
| Fernández O. 2018 | MSC (Ad) | IV | MS (SP) | T: 19 Co: 10 |
T: NA Co: NA |
| Karussis D. 2010 | MSC (BM) | IT | MS (V) | T: 15 | 73 |
| Harris VK. 2018 | MSC (BM) | IT | MS (PP,SP) | T: 20 | 35 |
| Xiao Z. 2018 | MSC (UC) | Injury Site | SCI (A) | T: 2 | 100 |
| Ra JC. 2011 | MSC (Ad) | IV | SCI (C) | T: 8 | 13 |
| El-Kheir WA. 2014 | MSC (BM) | IT | SCI (C) | T: 50 Co: 20 |
T: 34 Co: 0 |
| Bonab MM. 2012 | MSC (BM) | IT | MS (SP,PR) | T: 22 | 18 |
| Dahbour S. 2017 | MSC (Ad) | IT | MS (SP,RR) | T: 10 | 20 |
| Lublin FD. 2014 | MSC (PD) | IV | MS (RR,SP) | T: 12 Co: 4 |
T: 42 Co: 25 |
| Llufriu S. 2014 | MSC (BM) | IV | MS (RR) | T: 8 | NA |
| Mendonça MV. 2014 | MSC (BM) | Injury Site | SCI (C) | T: 12 | 58 |
| Connick P. 2012 | MSC (BM) | IV | MS (SP) | T: 10 | NA |
| Cheng H. 2014 | MSC (UC) | IT | SCI (C) | T: 10 | NA |
| Li JF. 2014 | MSC (UC) | IV | MS (RR,SP) | T: 13 Co: 10 |
T: NA Co: NA |
| Satti HS. 2016 | MSC (BM) | IT | SCI (C) | T: 9 | NA |
| Bonab MM. 2007 | MSC (BM) | IT | MS (SP,PP) | T: 10 | 10 |
| Dai G. 2013 | MSC (BM) | Injury Site | SCI (C) | T: 20 Co: 20 |
45 0 |
| Karamouzian S. 2012 | MSC (BM) | IT | SCI (A,SA) | T: 11 Co: 20 |
T: 45 Co: 15 |
| Vaquero J. 2016 | MSC (BM) | IT + Injury site | SCI (C) | T: 12 | 33 |
| Vaquero J. 2017 | MSC (BM) | IT | SCI (C) | T: 10 | NA |
| Vaquero J. 2018 | MSC (BM) | IT | SCI (V) | T: 9 | 79 |
| Oh SK. 2015 | MSC (BM) | Injury site | SCI (C) | T: 16 | NA |
| Hur JW. 2016 | MSC (Ad) | IT | SCI (C) | T: 14 | NA |
| Yamout B. 2010 | MSC (BM) | IT | MS (SP,RR) | T: 7 | 57 |
| Saito F. 2012 | MSC (BM) | IT | SCI (A) | T: 5 | 40 |
| Venktataramana NK. 2010 | MSC (BM) | Surgery | PD | T: 7 | 43 |
| Pal R. 2009 | MSC (BM) | IT | SCI (C) | T: 25 | 0 |
| Saito F. 2008 | MSC (BM) | IT | SCI (A) | T: 1 | 0 |
| Duma C. 2019 | MSC (Ad) | CSF | SCI,MS,PD,AD | T: 31 | NA |
| Kim HJ. 2015 | MSC (UC) | Surgery | AD | T: 9 | 0 |
| Petrou P. 2020 | MSC (BM) | IT or IV | MS (SP,PP) | T: 32 Co: 16 |
T: NA Co: NA |
| Petrou P. 2021 | MSC (BM) | IT and/or IV | MS (SP,PP) | T: 24 | 42 |
| Uccelli A. 2021 | MSC (BM) | IV | MS (RR,SP,PP) | T: 69 Co: 75 |
T: 15 Co: 14 |
| Bhanot Y. 2011 | MSC (BM) | IT + Injury site | SCI (C) | T: 13 | 8 |
| Yazdani OS. 2016 | MSC (BM) + SC | IT | SCI (C) | T: 6 | 17 |
| Zhao Y. 2017 | MSC (UC) | Injury site | SCI (C) | T: 8 | 0 |
| Deng WS. 2020 | MSC (UC) | Injury site | SCI (A) | T: 20 Co: 20 |
T: 55 Co: 0 |
| Amr SM. 2014 | MSC (BM) | Injury site | SCI (C) | T: 14 | 100 |
| Rong L. 2020 | MSC (UC) | IT | SCI (C) | T: 24 | NA |
| Moviglia GA. 2006 | MSC (BM) | Injury site | SCI (C) | T: 2 | NA |
| Carstens M. 2020 | MSC (Ad) | Facial | PD | T: 2 | NA |
| Rice CM. 2010 | BMMC | IV | MS (RP) | T: 6 | 0 |
| Chhabra HS. 2016 | BMMC | IT + Injury site | SCI (A) | T: 14 Co: 7 |
NA NA |
| Kumar AA. 2009 | BMMC | IT | SCI (C) | T: 297 | 33 |
| Yoon SH. 2007 | BMMC | Injury site | SCI (V) | T: 35 Co: 13 |
T: 20 Co: 8 |
| Chernykh ER. 2007 | BMMC | IV + Injury site | SCI (C) | T: 18 Co: 18 |
NA NA |
| Syková E. 2006 | BMMC | IV + IA | SCI (V) | T: 20 | 15 |
| Park HC. 2005 | BMMC | Injury site | SCI (A) | T: 6 | 83 |
| Attar A. 2011 | BMMC | Injury site | SCI (A) | T: 4 | 75 |
| Lima C. 2006 | OMA | Injury site | SCI (C) | T: 7 | 29 |
| Lima C. 2009 | OMA | Injury site | SCI (C) | T: 20 | 30 |
| Curtis E. 2018 | NSC | Injury site | SCI (C) | T: 4 | NA |
| Levi AD. 2018 | NSC | Injury site | SCI (C) | T: 39 | NA |
| Levi AD. 2019 | NSC | Injury site | SCI (C) | T: 12 Co: 4 |
NA NA |
| Shin JC. 2015 | NSC | Injury site | SCI (V) | T: 19 Co: 15 |
T: 26 Co: 7 |
| Curt A. 2020 | NSC | Injury site | SCI (C) | T: 14 | 14 |
| Mackay-Sim A. 2008 | OEC | Injury site | SCI (C) | T: 3 Co: 3 |
T: 0 Co: 0 |
| Féron F. 2005 | OEC | Injury site | SCI (C) | T: 3 Co: 3 |
NA NA |
| Tabakow P. 2013 | OEC | Injury site | SCI (C) | T: 3 Co: 3 |
T: 67 Co: 0 |
| Wu J. 2012 | OEC | Injury site | SCI (C) | T: 11 | NA |
| Saberi H. 2008 | Schwann | Injury site | SCI (C) | T: 4 | 25 |
| Anderson KD. 2017 | Schwann | Injury Site | SCI (C) | T: 6 | 17 |
| Freed CR. 2001 | Neuron | Surgery | PD | T: 20 Co: 20 |
T: NA Co: NA |
| Minguez-Castellanos 2007 | Carotic Body | Surgery | PD | T: 12 | 83 |
| Brundin P. 2000 | Mesencephalic | Surgery | PD | T: 5 | 80 |
| Al-Zoubi A. 2014 | PBSC | IT + Injury site | SCI (C) | T: 19 | 37 |
| Christante AF. 2009 | PBSC | IA | SCI (C) | T: 39 | NA |
| Lammertse DP. 2012 | Macrophage | Injury site | SCI (A) | T: 19 Co: 14 |
T: 26 Co: 57 |
| Knoller N. 2005 | Macrophage | Injury site | SCI (A) | T: 8 | 38 |
| Bakay RAE. 2004 | Spheramine © | Surgery | PD | T: 6 | 100 |
3.2. Mesenchymal Stromal Cells Exert Neuroprotective and Immunomodulatory Properties after Transplantation
Currently, most of the studies that are being investigated in clinical trials are focusing on cell-based therapies using MSCs. These cells are characterized as pluripotent stem cells that can differentiate into osteoblasts, chondrocytes and adipocytes [26]. They can be easily extracted from diverse types of tissue, including bone marrow, adipose tissue, placenta and the umbilical cord [26,27,28,29], and are characterized by their adherent properties as well as their expression of CD44, CD73, CD90 and CD105, as well as lacking the expression of CD14, CD19, CD34, CD45 and HLA-class II [30,31]. MSCs are an ideal cell source for tissue regeneration owing to their interesting neuroprotective properties. They excrete growth factors, neurotrophins and cytokines inhibiting neuronal loss through anti-apoptotic effects and inducing neurogenesis and angiogenesis creating a favourable microenvironment for remyelination or regeneration [32,33]. Moreover, they exert immunomodulatory properties and more specifically create an anti-inflammatory environment by interacting with a variety of immune cells, such as the induction of inhibitory phenotypes in antigen presenting cells (APC), the downregulation of natural killer cells and cytotoxic T lymphocytes, driving the differentiation of naïve CD4+ lymphocytes into T helper (Th) cells and inducing an increase in T cells differentiating in CD4+ CD25+ regulatory T cells (Tregs) [34]. Altogether, these features make MSCs an interesting candidate for the treatment of neurodegenerative diseases.
Presently, 42 clinical trials have investigated the safety and/or efficacy of bone marrow-derived MSC (BMMSC), adipose-derived MSC (ADMSC), umbilical cord-derived MSC (UC-MSC) and placenta-derived MSC (PD-MSC) in MS, SCI, PD and AD. Autologous BMMSCs and ADMSCs are the most commonly used. Interestingly, ADMSCs expose superior characteristics for clinical use, as the isolation is less painful and invasive, they reach a higher yield, have better proliferative properties and demonstrate a superiority in the production of cytokines and neuroprotective factors, as compared to BMMSCs [35,36,37,38,39,40]. In addition, allogenic MSCs such as UC-MSCs and PD-MSC are also emerging in the clinical field [41,42,43,44,45]. Besides their non-invasive harvesting method, these cells possess an extremely high proliferative ability and improved therapeutic properties in comparison with ADMSCs [39,46]. The administration routes vary widely among the different clinical trials. Most protocols use IT injection, even though this technique is mildly invasive and is associated with some side effects such as transient headaches, mild fever and very exceptionally meningitis [37,47,48,49] or cerebrospinal fluid (CSF) leak [50]. However, because of a better migration to the lesion site in the central nervous system (CNS) via the CSF and a superior neuroprotective effect in preclinical models, IT injection is preferably used over IV administration [9,47,51,52,53]. In addition, other methods are used in SCI, PD or AD such as administration into the injury site and administration into the brain by surgery [43,50,54,55]. Finally, four of these 42 studies used biomaterial scaffolds in combination with UC-MSCs or BMMSCs in patients with SCI [56,57,58,59]. These scaffolds are used to bridge the lesion gap and guide axonal growth across the injury site. In addition, they can be used to deliver stem cells and functional biomolecules, such as brain-derived neurotrophic factor (BDNF) [60], optimizing the microenvironment to promote survival of the administered cells and inducing regeneration at the injury site [61,62,63]. Interestingly, no adverse events related to the surgical administration of scaffolds in combination with MSCs were reported. The study by Zhao et al. [58] showed only sensory improvements, in contrast to the other studies which showed motor and sensory improvements in the majority [56,57] or even all the SCI patients [59]. To date, there are no universal dosage guidelines for MSCs in cell-based therapy. Therefore, the number of administrations and the number of cells administered varies greatly among the different clinical trials. Nevertheless, several studies showed superior improvements in EDSS status and AIS for repeated administration of BMMSC over a single dose [9,64,65].
3.3. Bone-Marrow and Peripheral Blood Stem Cells Showed Clinical Improvement in Some Patients
Eight studies, seven in SCI and one in MS, using BMMC were included. This cell fraction, usually aspirated from the iliac crest, comprises a heterogeneous population of cells with a variety of functions including hematopoietic stem cells (HSCs), endothelial progenitor cells, MSCs, monocytes, and lymphocytes [66]. Several of these cells have shown neuroprotective and regenerative properties in preclinical studies, although the exact mechanisms involved remain to be defined [10,11,67,68]. BMMCs have the advantage that they do not require any isolation, cultivation or expansion steps, minimizing the risk of contamination and functional or genetic instability [69]. On the other hand, BMMCs may contain inflammatory components such as activated macrophages and lymphocytes that could inhibit the regeneration process. Nevertheless, none of these studies reported a (serious) AE suggesting this [69,70,71,72,73,74,75,76]. No universal administration and dosing guidelines are available, and therefore these parameters varied significantly between the studies. Clinical trials performed by Rice et al. and Syková et al. involving IV administration did not show clinical improvement in both MS and SCI patients [69,74]. In contrast, studies administering the cells close to the injury site including IT or intra-arterial (IA) administration and injection into the injured spinal cord showed marked clinical improvements in several SCI patients. [71,72,75,76]. These findings were confirmed in the study by Syková et al. comparing IV and IA administration near to the injury site in SCI [74]. Interestingly, Park et al. and Yoon et al. administered granulocyte macrophage-colony stimulating factor (GM-CSF) in addition to bone marrow cell transplantation [72,75]. This resulted in more stem cell mobilization, enhanced survival and an improved neuroprotective and regenerative effect by inducing cytokine production. There were no other AEs observed than fever due to the GM-CSF administration [72,75,77]. Finally, four studies investigated the safety and efficacy of autologous obtained stem cells of the PBSCs and macrophages in SCI patients [78,79,80,81]. PBSCs show similar properties to the BMMCs and were administered IT, IA or by injection into the spinal cord, whereas macrophages have immunomodulatory and wound healing properties possibly leading to neurologic restoration. No issues regarding safety after PBSC administration were reported and several patients showed an improvement in AIS grade [79]. In patients who received autologous macrophages, no (serious) AEs were reported related to the cellular product. Indeed, seven SAEs related to the surgical procedure, microinjections into the injured spinal cord, serve as an indication for the invasive character of this method. Both studies showed preliminary efficacy as several patients’ AIS grade improved.
3.4. Transplantation of Neural Cells Induces Reorganisation and Repair of the Neural Network
Over the past few years, several clinical trials investigated the application of neural transplantation to induce regeneration. Five studies used NSCs derived from the foetal brain or spinal cord [82,83,84,85,86], as these multipotent cells have the potential to self-renew and differentiate in a site-specific manner into NSC cell types including astrocytes, oligodendrocytes and neurons [87]. Despite the allogeneic origin of the NSCs, they were well-tolerated and safe under the administration of immunosuppressive drugs to prevent immunologic rejection of the stem cells which was observed in earlier clinical trials [88,89]. No tumours or abnormalities were detected after 1 year of follow-up [82,83,85,86]. One study reported safety up to 6 years after administration [84]. Nevertheless, the six SAEs related to the surgical procedure, including three CSF-leaks, one pseudo-meningocele and two Staph. epidermis infections, underline the invasive nature of the procedure. The number of administered cells, injected at the injury site in the spinal cord, ranged between 20 and 100 × 106 cells. Clinical improvements were observed in two studies including the placebo-controlled study of Shin et al. [84,85], whereas only sensory changes [86] or no improvements at all were reported in the other studies [82,83].
Two studies, from the group of Lima et al. investigated the regenerative properties of OMA in SCI [90,91]. The olfactory mucosa is an interesting target for cellular therapy as it exhibits the fastest rate of neurogenesis in adults and consists of two distinct cell populations, namely the OEC and NSC. OEC are specialized glial cells who myelinate axons both in the CNS and peripheral nervous system (PNS) and exhibit a mixture of astrocyte-specific and Schwann cell-specific characteristics [92]. Preclinical studies showed potential to myelinate and promote axonal growth in the injured spinal cord [14]. This can, in combination with the properties of the previously described NSCs lead to the formation of new neuronal networks [13,14,90,91,93,94]. In addition, the olfactory mucosa is an ideal graft because it can be acquired autologously with minimal invasive techniques, allowing NSCs to integrate in a controlled manner without rejection [90]. The autografts were administered, after removal of the scar tissue, into the cavity of the injury. No AEs related to the cell therapy were observed. Nevertheless, due to the invasive nature of the administration method, one SAE directly related to the procedure was reported, namely meningitis [90]. Lima et al. reported several patients with improvements in the AIS scale as well as in motor, sensory and urodynamic parameters [90,91]. Four studies investigated the safety and/or efficacy of autologous or allogenic OEC in patients with SCI which were administered into the injured spinal cord after laminectomy [95,96,97,98]. Safety and feasibility were reported in all trials [95,97,98], and one study reported safety during a period up to three years post transplantation [96]. The small placebo-controlled study from Tabakow et al. reported modest improvements in sensory and motor function in comparison to the control group [97], whereas the study of Wu et al. only showed slight sensory improvements and the absence of motor improvements, and the study of Mackay-Sim et al. showed no clinical improvement [96,98]. Two studies investigated autologous Schwann-cell administration which has comparable properties to OEC and a similar administration procedure. Both studies reported safety and some clinical improvements in two patients with SCI [99,100]. Finally, four studies investigated the survival, safety and or efficacy of the transplantation of different cell types in patients with PD including embryonic dopamine neurons, embryonic caudate and putamen grafts, autologous carotid body glomus cells and human retinal pigment epithelial cells attached to micro carriers [89,101,102,103]. The cell products of grafts were implanted by an invasive surgical procedure into the putamen. All the clinical trials reported the survival of the transplants; however, three SAEs related to the surgical procedure were observed in the study of Minguez-Castellanos et al. [101]. In addition, improvements in the Unified Parkinson’s Disease Rating Scale (UDPRS) scores were noted in some patients. Noteworthily, in the sham-surgery controlled study of Freed et al., clinical deterioration was observed between 6 and 12 months post transplantation and was probably caused by graft-induced dyskinesia (GID) [89]. This raised serious concerns and led to the termination of implanting dopamine neurons into the brain of PD patients.
3.5. Quality Assessment Risk of Bias
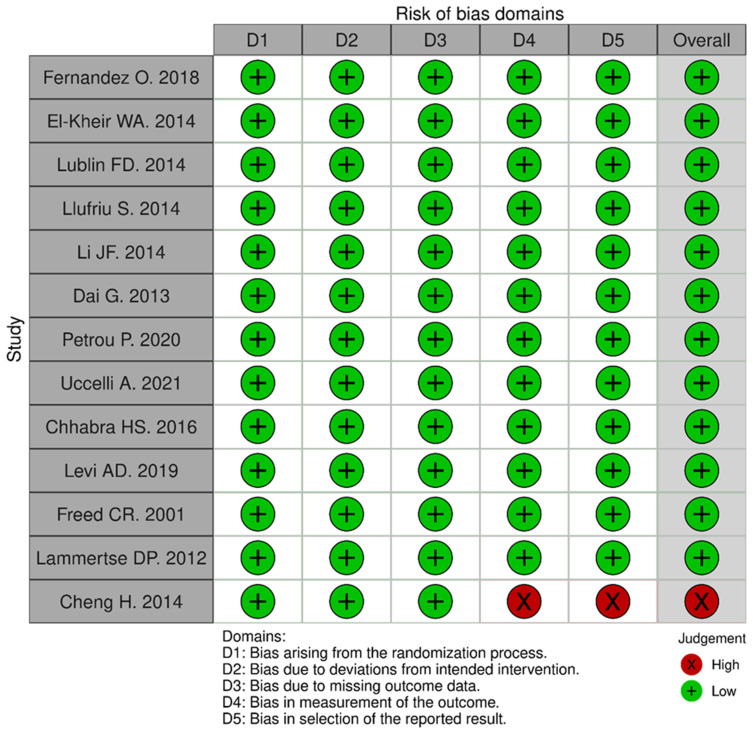
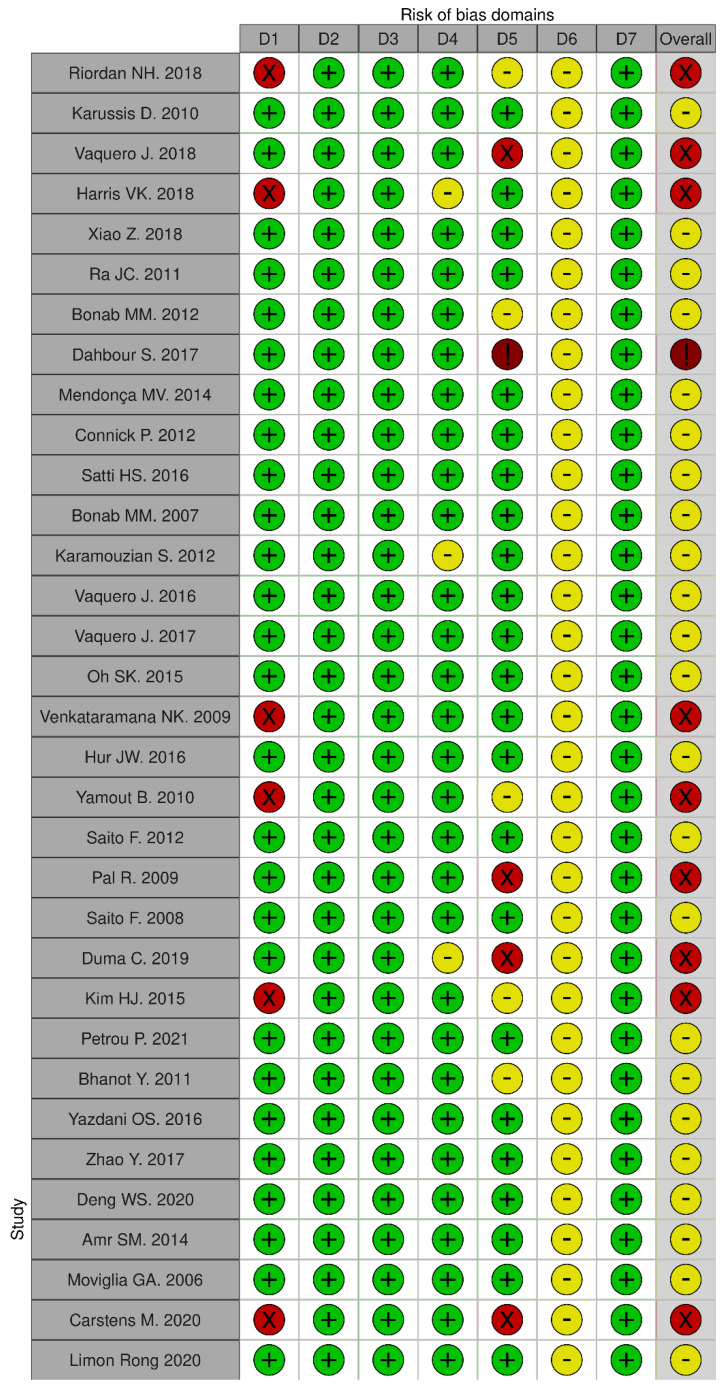
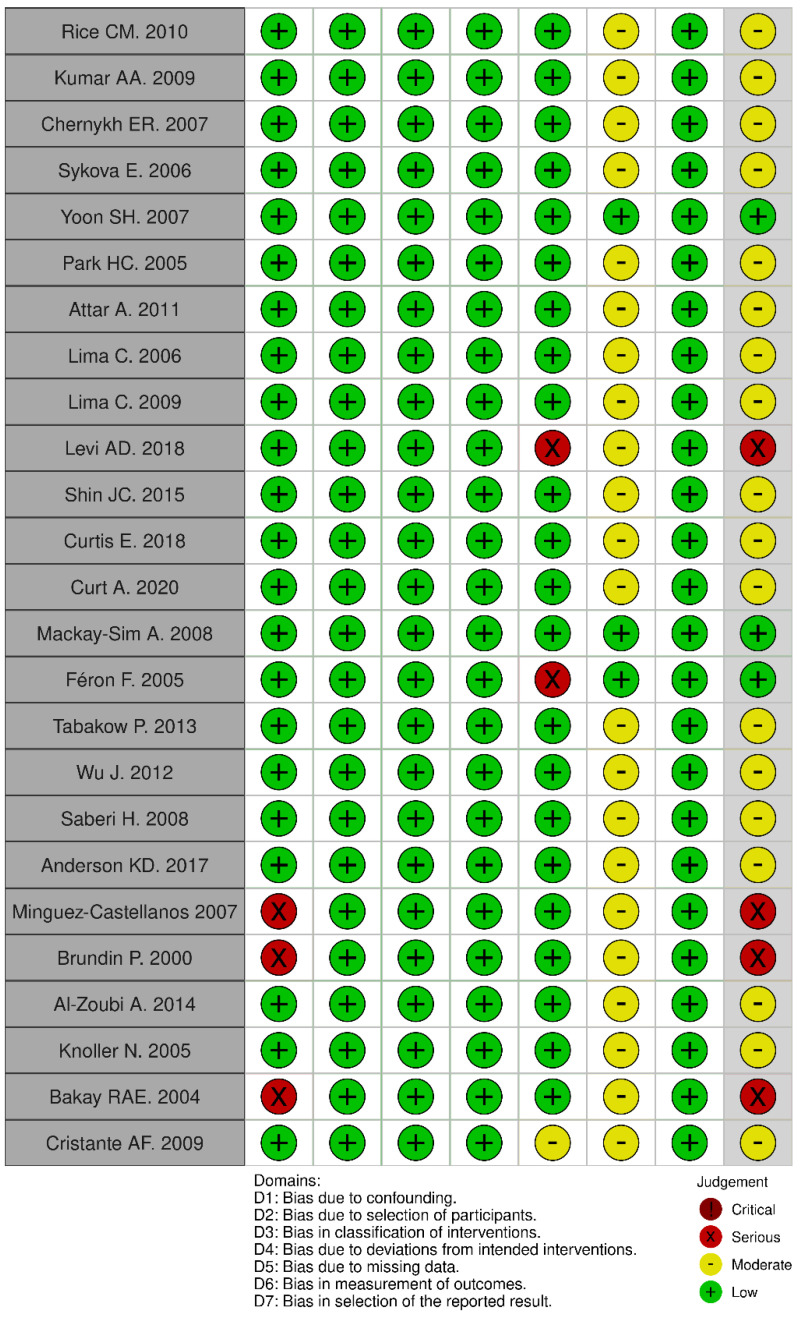
Twelve out of thirteen randomized and placebo controlled-clinical trials were at a low risk of bias as assessed with the RoB2 tool, and only one study was at high risk due to bias in the measurement of outcomes and reporting of the data (Appendix A) [22]. Most of the non-randomized studies were at medium risk of bias using the ROBINS-I tool, which was associated with the open label treatment without masking assessors and/or patients to the intervention (Appendix B) [23]. Nine of these non-randomized studies were at high risk. Indeed, the combination of the cell-based treatment and immunosuppressive therapies in MS and Levodopa in PD can lead to a potential source of confounding. Finally, one study was at critical risk of bias due to the exclusion of more than 30% in the data reporting [104].
3.6. Meta-Analysis
3.6.1. Serious Adverse Events
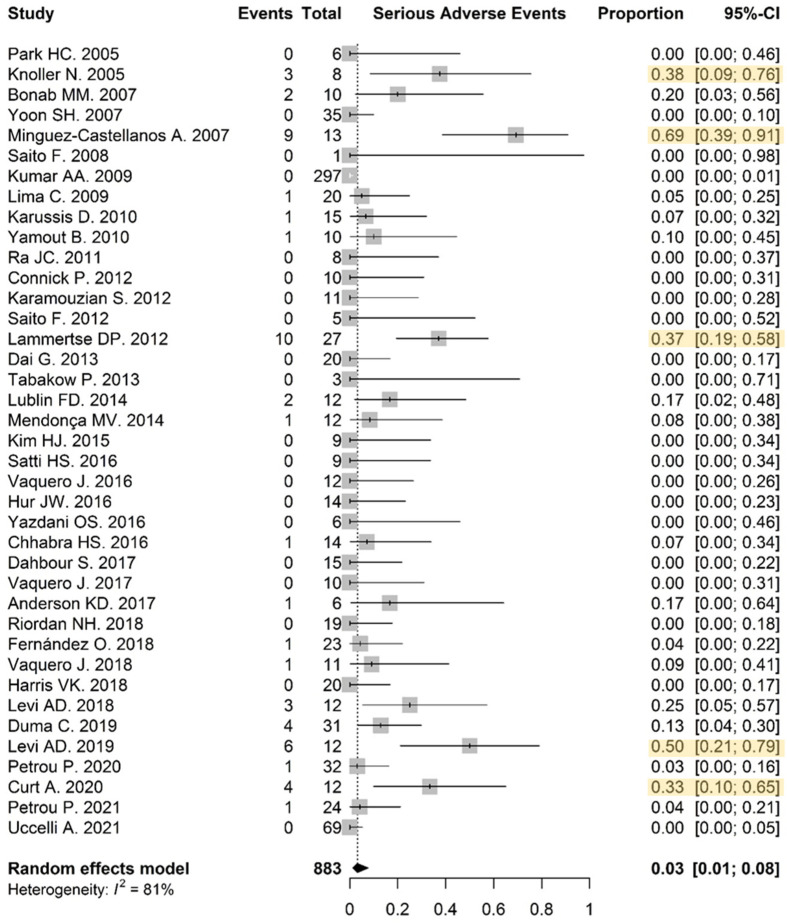
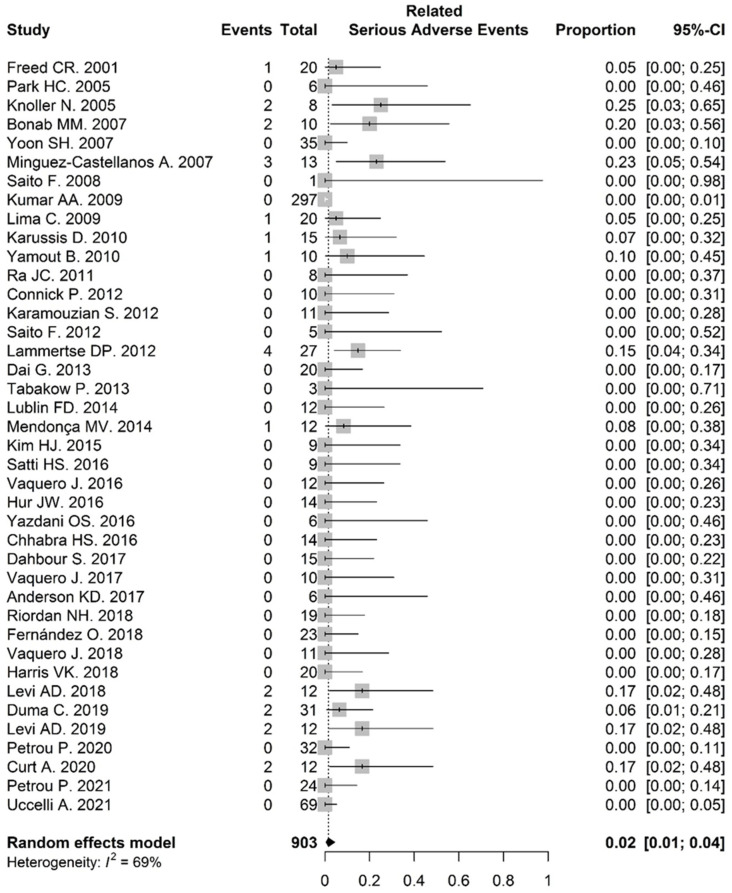
Out of the 70 studies, 39, including 10 controlled trials, reported adequate safety data involving the proportion of patients experiencing SAEs. In these, 53/883 patients on the experimental arms encountered SAEs, in 19 out of 39 studies. The overall frequency of SAEs was low: 0.03 (95% CI: 0.01–0.08). Five studies showed a significantly higher proportion of patients experiencing SAEs in comparison to the global effect of the studies (Figure 2). Forty studies were included in the calculation of the proportion of patients experiencing an SAE related to the cellular therapy or administration procedure. The overall incidence of related SAEs was low—0.02 (95% CI: 0.01–0.04)—and only one study by Minguez-Castellanos et al. [101] showed a significantly higher proportion of SAEs related to the administration regimen and/or cell product in comparison to the global effect of the studies (Appendix C).
Figure 2.
Safety—patients experiencing SAEs in the intervention group: the proportion was visualized for each study by the middle of the grey boxes including the 95% confidence interval indicated by the horizontal lines. The overall frequency of SAE was low: 0.03 (95% CI: 0.01–0.08). Five studies, marked in yellow, showed a significantly higher incidence of SAE.
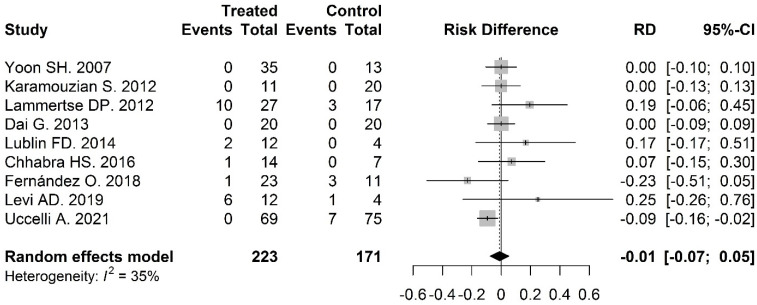
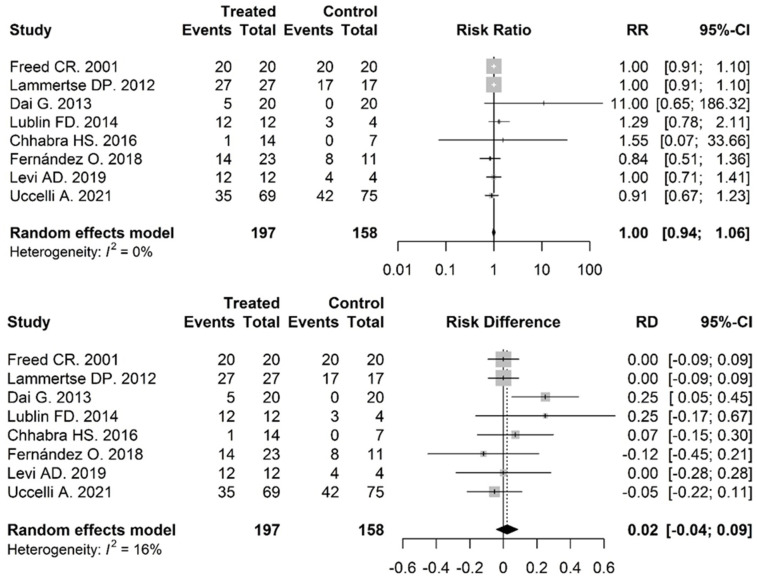
The incidence of SAEs was compared between intervention and control groups in nine of the studies and was 0.03 (95% CI: 0.01–0.20) and 0.06 (95% CI: 0.02–0.19), respectively. The risk difference for SAEs in intervention groups versus control groups was −0.01 (95% CI: −0.07–0.05) and was not significant p = 0.73 (Figure 3).
Figure 3.
Safety—risk difference in SAE incidence between intervention and control arm: the risk difference for SAE in intervention groups versus control groups was −0.01 (95% CI: −0.07–0.05, p = 0.73).
3.6.2. Adverse Events
Thirty-three studies, including eight controlled trials, reported adequate data regarding the proportion of patients experiencing AEs. Overall, 331/511 patients in the experimental groups experienced an AE in 32 out of 33 studies. The overall frequency of adverse events was high: 0.857 (95% CI: 0.66–0.95). The incidence of AEs between the intervention and control groups in eight studies was compared by calculating the risk-difference and risk ratio. The calculated risk difference was 0.02 (95% CI: −0.04–0.09, p = 0.49), and the risk ratio for AEs in intervention groups versus control groups was 1.00 (95% CI: 0.94–1.06, p = 0.96) (Appendix D).
3.6.3. Clinical Response in MS Patients
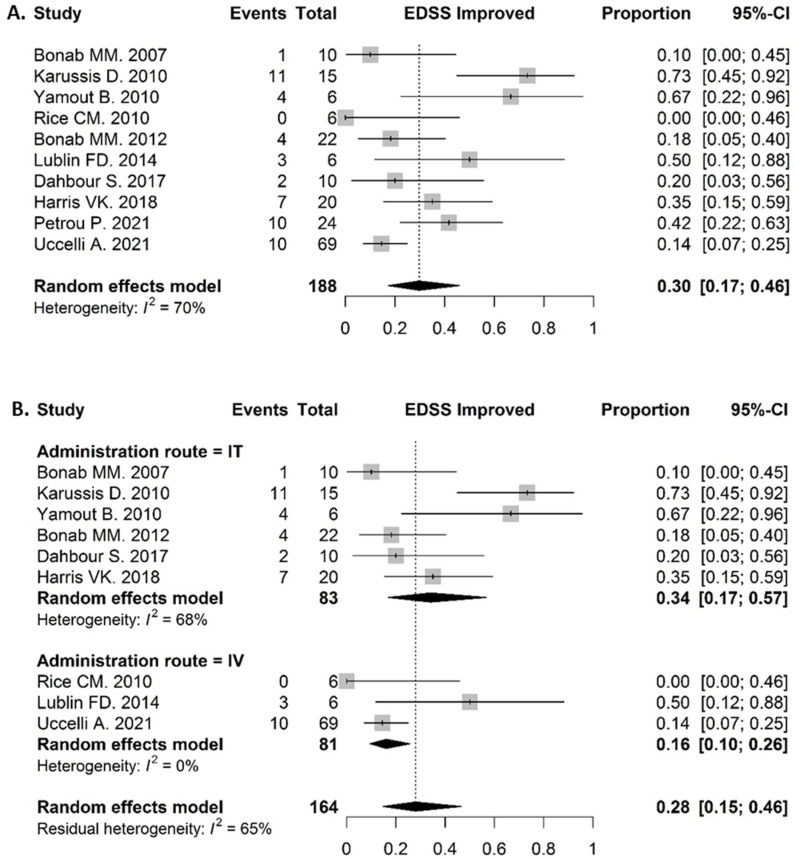
Ten studies reported adequate results of the EDSS status in MS patients. Out of 188 patients, 52 improved their EDSS in the experimental arm. The proportion of clinically improved patients after a 12-month follow up was 0.30 (95% CI: 0.17–0.46) (Figure 4A). No comparative analysis was done because only two studies were included and the analysis would therefore have no added value. The efficacy of two different administration routes, namely IT and IV, was compared by means of a subgroup difference test. The proportion of patients who improved their EDSS status in the IT group was 0.34 (95% CI: 0.17–0.57), whereas this proportion was lower in the IV group—0.16 (95% CI: 0.10–0.26). However, the result for the subgroup differences was not significant, χ2 = 3.11 and p = 0.078 (Figure 4B).
Figure 4.
Efficacy. (A) Clinical response in MS patients: Proportion of patients with improved EDSS status: 0.30 (95% CI: 0.17–0.46). (B) Comparison in clinical response by administration route in MS patients: the proportion of patients who improved their EDSS status in the IT group was 0.34 (95% CI: 0.17–0.57), in the IV group 0.16 (95% CI: 0.10–0.26). The subgroup differences were not significant, χ2 = 3.11 and p = 0.078.
3.6.4. Clinical Response in SCI Patients
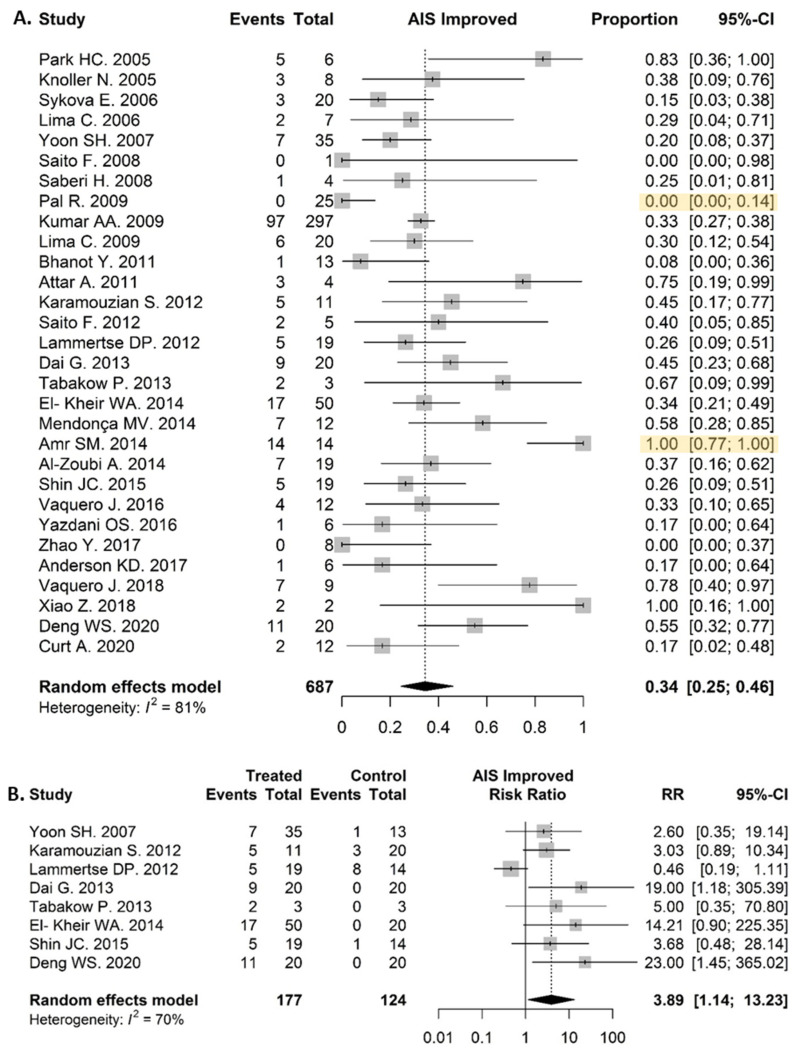
Thirty clinical trials with SCI patients were included in this analysis evaluating the proportion of patients who improved their AIS grade. Out of 687 SCI patients, 229 improved their AIS grade in the experimental arm, resulting in a proportion of clinical improved patients after a 6–12 month follow up of 0.35 (95% CI: 0.25–0.46) (Figure 5A). One study by Amr et al. showed a significantly higher proportion of improvement in comparison to the global effect of the studies, as all 14 patients changed their AIS grade [59]. On the other hand, one study showed significantly lower improvement in AIS grade, as 0 out of 25 patients showed clinical changes [105]. In addition, a risk-ratio analysis in eight studies, comparing the intervention and control group, was performed. The proportion of AIS-improved patients in the experimental group was remarkably higher (0.35 (95% CI: 0.27–0.44)) than in the control group (0.04 (95% CI: 0.01–0.22)). The calculated risk ratio was 3.89 (95% CI: 1.14–13.23, p = 0.030) (Figure 5B).
Figure 5.
Efficacy. (A) Clinical response in SCI patients: 229/687 patients improved their SCI grade, resulting in a proportion of 0.35 (95% CI: 0.25–0.46). (B) Comparison of clinical improvement in SCI patients between treatment and control group: the proportion in the experimental group was remarkably higher (0.35 (95% CI: 0.27–0.44)) than in the control group (0.04 (95% CI: 0.01–0.22)). The calculated risk ratio was 3.89 (95% CI: 1.14–13.23) and p = 0.030.
4. Discussion
This systematic review with meta-analysis aimed to evaluate the safety and efficacy of regenerative cell-based therapies in neurodegenerative diseases including MS, SCI, PD and AD. First, the majority of the studies reported that the application of cell therapy is safe and feasible in these patients. In particular, the results demonstrate that the frequency of SAE after administration of a cell-based treatment was low (0.03 (95% CI: 0.01–0.20)). In addition, no significant differences in SAE occurrence were observed between the control and treatment groups. Second, studies in MS and SCI patients demonstrated efficacy of cell-based therapies, evaluating clinical outcomes via EDSS and AIS grade, respectively. Noteworthily, the proportion of MS patients that improved their EDSS status during one year of follow up was 0.30 (95% CI: 0.17–0.46), whereas the proportion of SCI patients with a AIS grade improvement was 0.35 (95% CI: 0.25–0.46) during a 6–12-month follow up. All results should be interpreted with caution and will be discussed below.
4.1. Cell-Based Therapy in Neurodegenerative Diseases Showed Safety and Feasibility
In general, most of the studies reported safety and feasibility of the treatment based on the incidence of SAEs. In particular, the results demonstrate that the frequency of SAE after administration of a cell-based treatment was low (0.03 (95% CI: 0.01–0.20)). Nevertheless, five studies showed a significant higher incidence of SAEs which were related to the administration regimen rather than the use of the cell product itself. Furthermore, the incidence of AE was high (0.86 (95% CI: 0.66–0.95)) both in control and treatment groups and showed no difference in the comparative analysis. Such high incidence might be attributed to the severe clinical condition of the patients and the associated medical complications such as urinary tract infections which occur at a rate of more than two times a year in patients with SCI and advanced MS [106,107].
4.2. Modest Clinical Improvements Were Observed in MS Patients’ EDSS Scores
The EDSS of Kurtzke was used as an outcome to assess efficacy of the cell-based treatments, as it is the most frequently used and best-known instrument to monitor disease progression in MS [108,109]. Our analysis reported a proportion of 0.30 (95% CI: 0.17–0.46) MS patients that improved their EDSS status during a one year follow up. This represents a remarkably high proportion for a disease with gradually worsening clinical manifestation, indicating some promising efficacy. However, the included population is composed of subjects who have varying subtypes of MS and different EDSS scores at baseline, which might have an impact on the course of the disease [110]. Noteworthily, whereas most trials had an experimental design with a small sample size, Uccelli et al. included 134 (predominantly RR) MS patients in a randomized, double blinded, placebo-controlled clinical trial, administering BMMSC intravenously. However, they did not show any difference in EDSS improvement between the treatment and control groups [111].
4.3. AIS Grade Improved in Several SCI Patients after Cell-Based Therapy
American Spinal Injury Association Impairment Scale (AIS) was used to assess efficacy of the cell-based treatments in patients with SCI. The AIS classification has a tremendous prognostic value and defines precisely the level and degree of a patient’s deficit both on motor and sensory level [112]. Our analysis showed that the proportion of patients that improved their AIS grade was 0.35 (95% CI: 0.25–0.46) in the experimental group. In addition, a comparative risk ratio analysis showed significant higher clinical improvement in the treatment group versus control. However, these results must be assessed with caution. Apart from the heterogeneity in study design, dose, administration route and cell type, there are additional factors influencing the recovery rate, including the AIS-grade, injury-level and the time that has elapsed since injury [113]. The time elapsed since injury has a major influence on the difference in recovery rate. Acute AIS-A patients have about a 20% chance of spontaneously improving their grade [113,114], whereas only 5.6% of patients who remain AIS-A after one year improve their grade in the period up to five years after the injury [114]. The majority of patients that were included were classified with chronic and complete injury (AIS-A grade), as these patients showed the lowest rate of spontaneous recovery, allowing for better determination of the exact impact of the treatment [112]. In addition, the risk of conducting further damage as a result of the surgical procedure or the cell product was minimised.
4.4. Further Optimisation of Administration Regimen and Dosing Is Necessary
Despite promising results regarding efficacy in various clinical trials and proven safety and feasibility, many questions remain unanswered. The administration route, dose and frequency vary widely between the individual studies. Even among studies for the same disease and using the same cell type, there is large variability between these parameters. IV and IT administration are the least invasive procedures to administer cell products and are generally considered safe. However, two encephalopathies were observed after IT administration of BMMSCs in patients with MS, probably due to a secondary reaction after lysis of the administered cells in the CSF [47,48]. In addition, two cases of meningitis were reported in MS patients immediately after IT administration of MSCs [49]. Therefore, it is important to optimize the administration regimen in order to minimize the risk of side effects. Several preclinical studies, both in MS and SCI models, have already shown that IT administration shows better efficacy and migration than the IV route [9,47,53,115]. In addition, a comparative study of Petrou et al. showed superior clinical improvements after BMMSC administration in progressive MS patients for the IT route [116]. However, our comparative analysis in MS patients did not show significant clinical improvement for IT over IV administration, although the p-value (0.078) did suggest a tendency towards an increased improvement in the patients who received the cellular therapy IT. However, the number of studies in the IV group was limited (n = 3 versus n = 7 in the IT group) and the study by Uccelli et al. [111] had a substantial weight, meaning that we have to approach these results carefully.
Several clinical and preclinical studies [9] reported superior results after multiple doses and a peak in clinical improvement in patients after 1–3 months [117,118,119]. Repeated doses, as reported in the study by Vaquero et al. [120], would therefore be recommended to achieve a more beneficial clinical effect. Furthermore, in SCI, PD and AD patients, the cells were administered mainly at the site of injury to allow them to exert an optimal neuroregenerative effect. This often involved invasive and high-risk surgical procedures, including durotomy and laminectomy, together with the inevitable complications. Consequently, repeated doses are in this case a very high risk and may not balance out the benefits.
4.5. Several Hurdles Remain to Be Surpassed in Order to Develop a Successful Neuroregenerative Cell-Based Therapy
Indeed, all results should be interpreted with caution. Besides the already mentioned wide heterogeneity between the studies regarding cell type, administration regimen, dose and the disease status in the patient population, it should be noted that the majority of the studies compromise early-stage clinical trials which have a small sample size and a lack of blinding. In particular, the latter might induce some challenges. In case the cell product has to be administered via an invasive surgical procedure, ideally a sham-surgical control would be preferred. Nevertheless, the risk associated with these surgical procedures raises significant ethical issues, highlighting the hurdles to achieve an optimal trial design versus what is ethically correct. Furthermore, the results of many of the records found on clinical trials.gov remain unpublished, which may point towards a publication bias. In addition, it has already been shown in the studies of the NECTAR program that promising results in preclinical studies and early human clinical trials for cellular therapies including ventral mesencephalic (VM) transplants [21,121,122,123] do not necessarily guarantee further treatment success. Indeed, after performing two placebo-controlled clinical trials [88,89], transplanted neurons showed survival in the brain of the patient and some variable clinical improvements in younger patients in the first months after transplantation. The clinical trial performed by Freed et al. did not use immunosuppressive therapy, and therefore immune rejection of the grafts was able to occur, leading to the development of GID [89]. This was confirmed by the study of Olanow et al. in which patients improved in the first six months while immunosuppressants were administered, but deteriorated after the termination of the immunosuppressive therapy. These findings were further supported by the analysis of post mortem tissue showing the presence of activated immune cells such as microglia in and around the graft deposits [88]. In addition, Lewy bodies, which are abnormal deposits of the alpha-synuclein protein and PD pathology, were observed in several post mortem analyses, also pointing to biological restrictions of direct cell transplantation into the brain [124,125]. These findings highlight that several hurdles remain to be faced regarding biological restrictions on the road to regenerative cell-based therapies. Therefore, extensive research will have to be conducted in the future including elucidating the exact mechanism of action, possible immune rejection, functionality and the survival of the administered cells to draw adequate conclusions. In doing so, controlled or comparative and blinded studies with a large sample size and longer follow-up should be performed using the same cell type. In addition, post mortem analysis of the engrafted tissue that was administered at the injury site needs to be conducted to investigate eventual biological restrictions such as immune rejection.
4.6. Future Perspectives
Ongoing and future studies will help to define the exact mechanism of action, functionality and survival of the administered cells, the dose, treatment schedule and route of administration of cell-based therapies in neurodegenerative diseases as well as the exact mechanism of action which leads to clinical improvement and the survival and engraftment in the host tissue in patients with neurodegenerative diseases. Furthermore, research into innovative and novel therapeutic strategies needs to be pursued. Indeed, in the last few years, several trials have investigated the combination of cell administration with biomaterials such as scaffolds in patients with SCI [56,57,58,59]. These trials showed encouraging efficacy results, underlining the need to conduct research into new therapeutic strategies to enhance the microenvironment, which is a crucial factor to successfully induce the regeneration process [126,127]. In addition, the inflammatory microenvironment which is often observed in MS and SCI is unfavourable for CNS repair and can be adapted by the administration of anti-inflammatory cells such as regulatory T cells, which are currently extensively tested in the clinic [128,129,130]. These cells can be easily engineered to overexpress neuroprotective factors such as neurotrophins, thereby improving their neuroregenerative and neuroprotective properties [131,132]. Optimizing and combining different therapeutic strategies will possibly bring a solution for neurodegenerative diseases in the future.
5. Conclusions
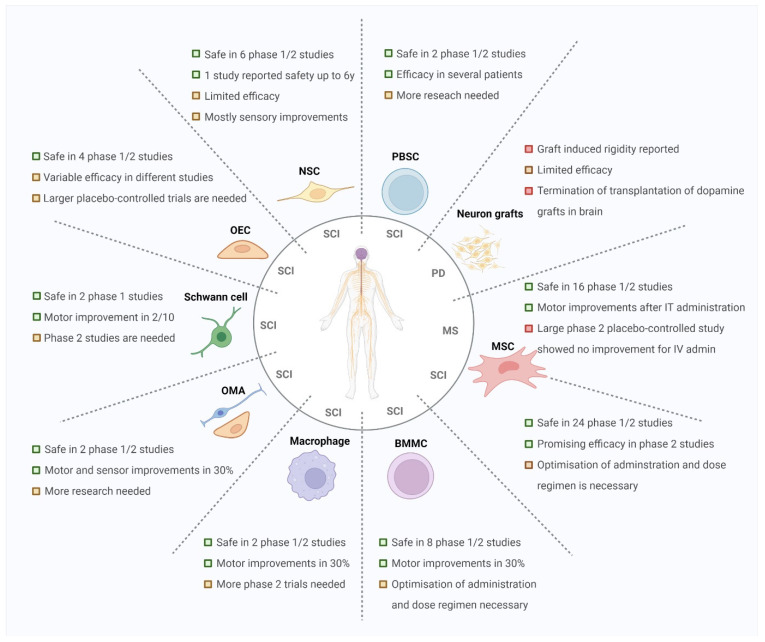
Our analyses showed that cellular-based therapies are safe and feasible and showed promising clinical improvements in several patients with MS and SCI (Figure 6). However, taking into account the failure of NECTAR studies in PD patients in the past, extensive research into the exact mechanism of action, functionality, and survival of the administered cells, combinations of different therapeutic strategies, administration routes and dose regimens is still needed to successfully develop a regenerative cell-based therapy for neurodegenerative diseases.
Figure 6.
Overview of the current status of the different types of cell-based therapies in the clinic and their most common use in the different neurodegenerative diseases. Abbreviations: spinal cord injury (SCI), multiple sclerosis (MS), Parkinson’s disease (PD), intrathecal (IT), intravenous (IV), mesenchymal stromal cell (MSC), bone-marrow mononuclear cells (BMMC), olfactory mucosal autograft (OMA), olfactory ensheathing cell (OEC), neuronal stem cell (NSC), peripheral blood stem cell (PBSC).
Appendix A
Figure A1.
Visualisation of the results of the RoB2 tool for randomized trials.
Appendix B
Figure A2.
Visualisation of the ROBINS-I tool for non-randomized trials.
Appendix C
Figure A3.
Forest plot of SAE related to the treatment in the experimental group.
Appendix D
Figure A4.
Forest plot of proportion of AEs in experimental group vs. control analysed by the risk ratio and risk difference.
Author Contributions
Conceptualization, J.V.d.B., I.W. and N.C.; software, K.W.; formal analysis, K.W. and J.V.d.B.; investigation, J.V.d.B., I.W. and Y.E.O.; data curation, J.V.d.B. and I.W.; writing—original draft preparation, J.V.d.B.; writing—review and editing, I.W. and N.C.; supervision, I.W.; funding acquisition, J.V.d.B. and I.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors of this work received research funding from the Belgian Charcot Foundation and Roche Belgium. In addition Jasper Van den Bos holds a PhD fellowship from the Belgian Charcot Foundation (FCS-2020-JVDB7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. In addition, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golchin A., Shams F., Kangari P., Azari A., Hosseinzadeh S. Regenerative Medicine: Injectable Cell-Based Therapeutics and Approved Products. Cell Biol. Transl. Med. 2019;1237:75–95. doi: 10.1007/5584_2019_412. [DOI] [PubMed] [Google Scholar]
- 2.De Gioia R., Biella F., Citterio G., Rizzo F., Abati E., Nizzardo M., Bresolin N., Comi G.P., Corti S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:3103. doi: 10.3390/ijms21093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne N., Siatskas C., Barnard A., Bernard C.C.A. The Prospect of Stem Cells as Multi-Faceted Purveyors of Immune Modulation, Repair and Regeneration in Multiple Sclerosis. Curr. Stem Cell Res. Ther. 2011;6:50–62. doi: 10.2174/157488811794480735. [DOI] [PubMed] [Google Scholar]
- 4.Shao A., Tu S., Lu J., Zhang J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019;10:1–13. doi: 10.1186/s13287-019-1357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestrino R., Schapira A.H.V. Parkinson disease. Eur. J. Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 6.Sivandzade F., Cucullo L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int. J. Mol. Sci. 2021;22:2153. doi: 10.3390/ijms22042153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta M. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Menezes K., Nascimento M.A., Gonçalves J.P., Cruz A.S., Lopes D.V., Curzio B., Bonamino M., De Menezes J.R.L., Borojevic R., Rossi M.I.D., et al. Human Mesenchymal Cells from Adipose Tissue Deposit Laminin and Promote Regeneration of Injured Spinal Cord in Rats. PLoS ONE. 2014;9:e96020. doi: 10.1371/journal.pone.0096020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris V.K., Yan Q.J., Vyshkina T., Sahabi S., Liu X., Sadiq S.A. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J. Neurol. Sci. 2011;313:167–177. doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama Y., Radtke C., Honmou O., Kocsis J.D. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki M., Honmou O., Akiyama Y., Uede T., Hashi K., Kocsis J.D. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwatsuki K., Yoshimine T., Kishima H., Aoki M., Yoshimura K., Ishihara M., Ohnishi Y., Lima C. Transplantation of olfactory mucosa following spinal cord injury promotes recovery in rats. NeuroReport. 2008;19:1249–1252. doi: 10.1097/WNR.0b013e328305b70b. [DOI] [PubMed] [Google Scholar]
- 13.Lu J., Féron F., Mackay-Sim A., Waite P.M.E. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- 14.Ramón-Cueto A., Cordero M.I., Santos-Benito F.F., Avila J. Functional Recovery of Paraplegic Rats and Motor Axon Regeneration in Their Spinal Cords by Olfactory Ensheathing Glia. Neuron. 2000;25:425–435. doi: 10.1016/S0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 15.Cummings B.J., Uchida N., Tamaki S.J., Salazar D.L., Hooshmand M., Summers R., Gage F.H., Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearse D.D., Sanchez A.R., Pereira F.C., Andrade C.M., Puzis R., Pressman Y., Golden K., Kitay B.M., Blits B., Wood P.M., et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 17.Bomstein Y., Marder J.B., Vitner K., Smirnov I., Lisaey G., Butovsky O., Fulga V., Yoles E. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J. Neuroimmunol. 2003;142:10–16. doi: 10.1016/S0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 18.Bai L., Lennon D.P., Caplan A.I., DeChant A., Hecker J., Kranso J., Zaremba A., Miller R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo-Aral J.J., Méndez-Ferrer S., Pardal R., Echevarría M., López-Barneo J. Trophic Restoration of the Nigrostriatal Dopaminergic Pathway in Long-Term Carotid Body-Grafted Parkinsonian Rats. J. Neurosci. 2003;23:141–148. doi: 10.1523/JNEUROSCI.23-01-00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espejo E.F., Montoro R.J., Armengol J.A., López-Barneo J. Cellular and Functional Recovery of Parkinsonian Rats after Intrastriatal Transplantation of Carotid Body Cell Aggregates. Neuron. 1998;20:197–206. doi: 10.1016/S0896-6273(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 21.Björklund A., Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 22.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 28.Miao Z., Jin J., Chen L., Zhu J., Huang W., Zhao J., Qian H., Zhang X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Broxmeyer H.E., Douglas G.W., Hangoc G., Cooper S., Bard J., English D., Arny M., Thomas L., Boyse E.A. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl. Acad. Sci. USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrzejewska A., Lukomska B., Janowski M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells. 2019;37:855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin H.J., Bae Y.K., Kim M., Kwon S.-J., Jeon H.B., Choi S.J., Kim S.W., Yang Y.S., Oh W., Chang J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013;14:17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uccelli A., Benvenuto F., Laroni A., Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol. 2011;24:59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Ghannam S., Pène J., Torcy-Moquet G., Jorgensen C., Yssel H. Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype. J. Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 35.Ra J.C., Shin I.S., Kim S.H., Kang S.K., Kang B.C., Lee H.Y., Kim Y.J., Jo J.Y., Yoon E.J., Choi H.J., et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z., Chen Y., Zhang H., Min S., Yu B., He B., Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15:434–448. doi: 10.1016/j.jcyt.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Duma C., Kopyov O., Kopyov A., Berman M., Lander E., Elam M., Arata M., Weiland D., Cannell R., Caraway C., et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: Results of a 3-year phase 1 study of 113 injections in 31 patients. Mol. Biol. Rep. 2019;46:5257–5272. doi: 10.1007/s11033-019-04983-5. [DOI] [PubMed] [Google Scholar]
- 38.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 39.Madrigal M., Rao K.S., Riordan N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014;12:1–14. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazini L., Rochette L., Amine M., Malka G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs) Int. J. Mol. Sci. 2019;20:2523. doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riordan N.H., Morales I., Fernández G., Allen N., Fearnot N.E., Leckrone M.E., Markovich D.J., Mansfield D., Avila D., Patel A.N., et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 2018;16:57. doi: 10.1186/s12967-018-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J.-F., Zhang D.-J., Geng T., Chen L., Huang H., Yin H.-L., Zhang Y.-Z., Lou J.-Y., Cao B., Wang Y.-L. The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis. Cell Transplant. 2014;23:113–122. doi: 10.3727/096368914X685005. [DOI] [PubMed] [Google Scholar]
- 43.Kim H.J., Seo S.W., Chang J.W., Lee J.I., Kim C.H., Chin J., Choi S.J., Kwon H., Yun H.J., Lee J.M., et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2015;1:95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H., Liu X., Hua R., Dai G., Wang X., Gao J., An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lublin F.D., Bowen J.D., Huddlestone J., Kremenchutzky M., Carpenter A., Corboy J.R., Freedman M.S., Krupp L., Paulo C., Hariri R.J., et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 2014;3:696–704. doi: 10.1016/j.msard.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Talwadekar M.D., Kale V.P., Limaye L.S. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts–a paired sample study. Sci. Rep. 2015;5:15784. doi: 10.1038/srep15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., Bulte J.W.M., Petrou P., Ben-Hur T., Abramsky O., et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients With Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamout B., Hourani R., Salti H., Barada W., El-Hajj T., Al-Kutoubi A., Herlopian A., Baz E.K., Mahfouz R., Khalil-Hamdan R., et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Bonab M.M., Yazdanbakhsh S., Lotfi J., Alimoghaddom K., Talebian F., Hooshmand F., Ghavamzadeh A., Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran. J. Immunol. IJI. 2007;4:50–57. [PubMed] [Google Scholar]
- 50.Mendonça M.V.P., Larocca T.F., Souza B.S.D.F., Villarreal C.F., Silva L.F.M., Matos A.C., Novaes M.A., Bahia C.M.P., Martinez A.C.D.O.M., Kaneto C.M., et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonab M.M., Sahraian M.A., Aghsaie A., Karvigh S.A., Hosseinian S.M., Nikbin B., Lotfi J., Khorramnia S., Motamed M.R., Togha M., et al. Autologous Mesenchymal Stem Cell Therapy in Progressive Multiple Sclerosis: An Open Label Study. Curr. Stem Cell Res. Ther. 2012;7:407–414. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- 52.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 53.Paul C., Samdani A.F., Betz R.R., Fischer I., Neuhuber B. Grafting of Human Bone Marrow Stromal Cells Into Spinal Cord Injury. Spine. 2009;34:328–334. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai G., Liu X., Zhang Z., Yang Z., Dai Y., Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Venkataramana N.K., Kumar S.K., Balaraju S., Radhakrishnan R.C., Bansal A., Dixit A., Rao D.K., Das M., Jan M., Gupta P.K., et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Deng W.-S., Ma K., Liang B., Liu X.-Y., Xu H.-Y., Zhang J., Shi H.-Y., Sun H.-T., Chen X.-Y., Zhang S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020;15:1686. doi: 10.4103/1673-5374.276340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Z., Tang F., Zhao Y., Han G., Yin N., Li X., Chen B., Han S., Jiang X., Yun C., et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Tang F., Xiao Z., Han G., Wang N., Yin N., Chen B., Jiang X., Yun C., Han W., et al. Clinical Study of NeuroRegen Scaffold Combined with Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017;26:891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amr S.M., Gouda A., Koptan W.T., Galal A.A., Abdel-Fattah D.S., Rashed L.A., Atta H.M., Abdel-Aziz M.T. Bridging defects in chronic spinal cord injury using peripheral nerve grafts combined with a chitosan-laminin scaffold and enhancing regeneration through them by co-transplantation with bone-marrow-derived mesenchymal stem cells: Case series of 14 patients. J. Spinal Cord Med. 2013;37:54–71. doi: 10.1179/2045772312Y.0000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han S., Wang B., Jin W., Xiao Z., Chen B., Xiao H., Ding W., Cao J., Ma F., Li X., et al. The collagen scaffold with collagen binding BDNF enhances functional recovery by facilitating peripheral nerve infiltrating and ingrowth in canine complete spinal cord transection. Spinal Cord. 2014;52:867–873. doi: 10.1038/sc.2014.173. [DOI] [PubMed] [Google Scholar]
- 61.Ashammakhi N., Kim H.-J., Ehsanipour A., Bierman R.D., Kaarela O., Xue C., Khademhosseini A., Seidlits S.K. Regenerative Therapies for Spinal Cord Injury. Tissue Eng. Part B Rev. 2019;25:471–491. doi: 10.1089/ten.teb.2019.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu B., Zhao Y., Xiao Z., Wang B., Liang H., Li X., Fang Y., Han S., Li X., Fan C., et al. A Dual Functional Scaffold Tethered with EGFR Antibody Promotes Neural Stem Cell Retention and Neuronal Differentiation for Spinal Cord Injury Repair. Adv. Healthc. Mater. 2017;6:1601279. doi: 10.1002/adhm.201601279. [DOI] [PubMed] [Google Scholar]
- 63.Han S., Xiao Z., Li X., Zhao H., Wang B., Qiu Z., Mei X., Xu B., Fan C., Chen B., et al. Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine. Sci. China Life Sci. 2017;61:2–13. doi: 10.1007/s11427-016-9002-6. [DOI] [PubMed] [Google Scholar]
- 64.Vaquero J., Zurita M., Rico M.A., Bonilla C., Aguayo C., Montilla J., Bustamante S., Carballido J., Marin E., Martinez F., et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18:1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Petrou P., Kassis I., Ginzberg A., Halimi M., Yaghmour N., Abramsky O., Karussis D. Long-Term Clinical and Immunological Effects of Repeated Mesenchymal Stem Cell Injections in Patients with Progressive Forms of Multiple Sclerosis. Front. Neurol. 2021;12:737. doi: 10.3389/fneur.2021.639315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesentier-Louro L.A., Zaverucha-Do-Valle C., de Castro P.H.R., Silva-Junior A.J., Pimentel-Coelho P.M., Mendez-Otero R., Santiago M.F. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016;2016:5078619. doi: 10.1155/2016/5078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawada H., Takizawa S., Takanashi T., Morita Y., Fujita J., Fukuda K., Takagi S., Okano H., Ando K., Hotta T. Administration of Hematopoietic Cytokines in the Subacute Phase After Cerebral Infarction Is Effective for Functional Recovery Facilitating Proliferation of Intrinsic Neural Stem/Progenitor Cells and Transition of Bone Marrow-Derived Neuronal Cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 68.Koshizuka S., Okada S., Okawa A., Koda M., Murasawa M., Hashimoto M., Kamada T., Yoshinaga K., Murakami M., Moriya H., et al. Transplanted Hematopoietic Stem Cells from Bone Marrow Differentiate into Neural Lineage Cells and Promote Functional Recovery after Spinal Cord Injury in Mice. J. Neuropathol. Exp. Neurol. 2004;63:64–72. doi: 10.1093/jnen/63.1.64. [DOI] [PubMed] [Google Scholar]
- 69.Rice C.M., Mallam E.A., Whone A.L., Walsh P., Brooks D.J., Kane N., Butler S.R., Marks D.I., Scolding N.J. Safety and Feasibility of Autologous Bone Marrow Cellular Therapy in Relapsing-Progressive Multiple Sclerosis. Clin. Pharmacol. Ther. 2010;87:679–685. doi: 10.1038/clpt.2010.44. [DOI] [PubMed] [Google Scholar]
- 70.Chhabra H.S., Sarda K., Arora M., Sharawat R., Singh V., Nanda A., Sangodimath G.M., Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury—An Indian pilot study. Spinal Cord. 2015;54:57–64. doi: 10.1038/sc.2015.134. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A.A., Kumar S.R., Narayanan R., Arul K., Baskaran M. Autologous bone marrow derived mononuclear cell ther-apy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp. Clin. Transpl. 2009;7:241–248. [PubMed] [Google Scholar]
- 72.Yoon S.H., Shim Y.S., Park Y.H., Chung J.K., Nam J.H., Kim M.O., Park H.C., Park S.R., Min B.-H., Kim E.Y., et al. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- 73.Chernykh E.R., Stupak V.V., Muradov G.M., Sizikov M.Y., Shevela E.Y., Leplina O.Y., Tikhonova M., Kulagin A., Lisukov I.A., Ostanin A.A., et al. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull. Exp. Biol. Med. 2007;143:543–547. doi: 10.1007/s10517-007-0175-y. [DOI] [PubMed] [Google Scholar]
- 74.Syková E., Homola A., Mazanec R., Lachmann H., Konrádová L., Kobylka P., Pádr R., Neuwirth J., Komrska V., Vávra V., et al. Autologous Bone Marrow Transplantation in Patients with Subacute and Chronic Spinal Cord Injury. Cell Transplant. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 75.Park H.C., Shim Y.S., Ha Y., Yoon S.H., Park S.R., Choi B.H., Park H.S. Treatment of Complete Spinal Cord Injury Patients by Autologous Bone Marrow Cell Transplantation and Administration of Granulocyte-Macrophage Colony Stimulating Factor. Tissue Eng. 2005;11:913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 76.Attar A., Ayten M., Ozdemir M., Ozgencil E., Bozkurt M., Kaptanoglu E., Beksac M., Kanpolat Y. An attempt to treat patients who have injured spinal cords with intralesional implantation of concentrated autologous bone marrow cells. Cytotherapy. 2011;13:54–60. doi: 10.3109/14653249.2010.510506. [DOI] [PubMed] [Google Scholar]
- 77.Bouhy D., Malgrange B., Multon S., Poirrier A.-L., Scholtes F., Schoenen J., Franzen R. Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 2006;20:1239–1241. doi: 10.1096/fj.05-4382fje. [DOI] [PubMed] [Google Scholar]
- 78.Cristante A.F., Filho T.E.P.D.B., Tatsui N., Mendrone A., Caldas J.G., Camargo A., Alexandre A., Teixeira W.G.J., Oliveira R.P., Marcon R. Stem cells in the treatment of chronic spinal cord injury: Evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47:733–738. doi: 10.1038/sc.2009.24. [DOI] [PubMed] [Google Scholar]
- 79.Al-Zoubi A., Jafar E., Jamous M., Al-Twal F., Al-Bakheet S., Zalloum M., Khalifeh F., Abu Radi S., El-Khateeb M., Al-Zoubi Z. Transplantation of Purified Autologous Leukapheresis-Derived CD34+ and CD133+ Stem Cells for Patients with Chronic Spinal Cord Injuries: Long-Term Evaluation of Safety and Efficacy. Cell Transplant. 2014;23:25–34. doi: 10.3727/096368914X684899. [DOI] [PubMed] [Google Scholar]
- 80.Knoller N., Auerbach G., Fulga V., Zelig G., Attias J., Bakimer R., Marder J.B., Yoles E., Belkin M., Schwartz M., et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: Phase I study results. J. Neurosurg. Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 81.Lammertse D.P., Jones L.A.T., Charlifue S.B., Kirshblum S.C., Apple D.F., Ragnarsson K.T., Falci S., Heary R.F., Choudhri T.F., Jenkins A.L., et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: Results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 2012;50:661–671. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- 82.Levi A.D., Anderson K.D., Okonkwo D.O., Park P., Bryce T.N., Kurpad S.N., Aarabi B., Hsieh J., Gant K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma. 2019;36:891–902. doi: 10.1089/neu.2018.5843. [DOI] [PubMed] [Google Scholar]
- 83.Levi A.D., Okonkwo D.O., Park P., Jenkins A.L., Kurpad S.N., Parr A.M., Ganju A., Aarabi B., Kim D., Casha S., et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery. 2017;82:562–575. doi: 10.1093/neuros/nyx250. [DOI] [PubMed] [Google Scholar]
- 84.Curt A., Hsieh J., Schubert M., Hupp M., Friedl S., Freund P., Huber E., Pfyffer D., Sutter R., Jutzeler C., et al. The Damaged Spinal Cord Is a Suitable Target for Stem Cell Transplantation. Neurorehabilit. Neural Repair. 2020;34:758–768. doi: 10.1177/1545968320935815. [DOI] [PubMed] [Google Scholar]
- 85.Shin J.C., Kim K.N., Yoo J., Kim I.-S., Yun S., Lee H., Jung K., Hwang K., Kim M., Lee I.-S., et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Curtis E., Martin J., Gabel B., Sidhu N., Rzesiewicz T.K., Mandeville R., Van Gorp S., Leerink M., Tadokoro T., Marsala S., et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell. 2018;22:941–950.e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Tsukamoto A., Uchida N., Capela A., Gorba T., Huhn S. Clinical translation of human neural stem cells. Stem Cell Res. Ther. 2013;4:102. doi: 10.1186/scrt313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., Shannon K.M., Nauert G.M., Perl D.P., Godbold J., et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 89.Freed C.R., Greene P.E., Breeze R.E., Tsai W.-Y., DuMouchel W., Kao R., Dillon S., Winfield H., Culver S., Trojanowski J.Q., et al. Transplantation of Embryonic Dopamine Neurons for Severe Parkinson’s Disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 90.Lima C., Escada P., Pratas-Vital J., Aguiar-Branco C., Arcangeli C.A., Lazzeri G., Maia C.A.S., Capucho C., Hasse-Ferreira A., Peduzzi J.D. Olfactory Mucosal Autografts and Rehabilitation for Chronic Traumatic Spinal Cord Injury. Neurorehabilit. Neural Repair. 2009;24:10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 91.Lima C., Pratas-Vital J., Escada P., Hasse-Ferreira A., Capucho C., Peduzzi J.D. Olfactory Mucosa Autografts in Human Spinal Cord Injury: A Pilot Clinical Study. J. Spinal Cord Med. 2006;29:191–203; discussion 204–206. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doucette R. Olfactory ensheathing cells: Potential for glial cell transplantation into areas of CNS injury. Histol. Histopathol. 1995;10:503–507. [PubMed] [Google Scholar]
- 93.Lu J., Féron F., Ho S.M., Mackay-Sim A., Waite P.M. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 2001;889:344–357. doi: 10.1016/S0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- 94.Xiao M., Klueber K.M., Zhou J., Guo Z., Lu C., Wang H., Roisen F.J. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol. Dis. 2007;26:363–374. doi: 10.1016/j.nbd.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Féron F., Perry C., Cochrane J., Licina P., Nowitzke A., Urquhart S., Geraghty T., Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 96.Mackay-Sim A., Feron F., Cochrane J., Bassingthwaighte L., Bayliss C., Davies W., Fronek P., Gray C., Kerr G., Licina P., et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabakow P., Jarmundowicz W., Czapiga B., Fortuna W., Miedzybrodzki R., Czyz M., Huber J., Szarek D., Okurowski S., Szewczyk P., et al. Transplantation of Autologous Olfactory Ensheathing Cells in Complete Human Spinal Cord Injury. Cell Transplant. 2013;22:1591–1612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 98.Wu J., Sun T., Ye C., Yao J., Zhu B., He H. Clinical Observation of Fetal Olfactory Ensheathing Glia Transplantation (OEGT) in Patients with Complete Chronic Spinal Cord Injury. Cell Transplant. 2012;21:33–37. doi: 10.3727/096368912X633743. [DOI] [PubMed] [Google Scholar]
- 99.Saberi H., Moshayedi P., Aghayan H.R., Arjmand B., Hosseini S.-K., Emami-Razavi S.-H., Rahimi-Movaghar V., Raza M., Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 100.Anderson K.D., Guest J.D., Dietrich W.D., Bunge M.B., Curiel R., Dididze M., Green B.A., Khan A., Pearse D.D., Saraf-Lavi E., et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 101.Mínguez-Castellanos A., Escamilla-Sevilla F., Hotton G.R., Toledo-Aral J.J., Ortega-Moreno Á., Méndez-Ferrer S., Martín-Linares J.M., Katati M.J., Mir P., Villadiego J., et al. Carotid body autotransplantation in Parkinson disease: A clinical and positron emission tomography study. J. Neurol. Neurosurg. Psychiatry. 2007;78:825–831. doi: 10.1136/jnnp.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brundin P., Pogarell O., Hagell P., Piccini P., Widner H., Schrag A., Kupsch A., Crabb L., Odin P., Gustavii B., et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123:1380–1390. doi: 10.1093/brain/123.7.1380. [DOI] [PubMed] [Google Scholar]
- 103.Roy A.B., Bakay R.A.E., Raiser C.D., Stover N.P., Subramanian T., Cornfeldt M.L., Schweikert A.W., Allen R.C., Watts R. Implantation of Spheramine â in advanced Parkinson’s disease (PD) Front. Biosci. 2004;9:592–602. doi: 10.2741/1217. [DOI] [PubMed] [Google Scholar]
- 104.Dahbour S., Jamali F., Alhattab D., Al-Radaideh A., Ababneh O., Al-Ryalat N., Al Bdour M., Hourani B., Msallam M., Rasheed M., et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017;23:866–874. doi: 10.1111/cns.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pal R., Venkataramana N.K., Bansal A., Balaraju S., Jan M., Chandra R., Dixit A., Rauthan A., Murgod U., Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 106.Mahadeva A., Tanasescu R., Gran B. Urinary tract infections in multiple sclerosis: Under-diagnosed and under-treated? A clinical audit at a large University Hospital. Am. J. Clin. Exp. Immunol. 2014;3:57–67. [PMC free article] [PubMed] [Google Scholar]
- 107.Salameh A., Al Mohajer M., Daroucihe R.O. Prevention of urinary tract infections in patients with spinal cord injury. Can. Med. Assoc. J. 2015;187:807–811. doi: 10.1503/cmaj.141044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 109.Meyer-Moock S., Feng Y.-S., Maeurer M., Dippel F.-W., Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Confavreux C., Vukusic S. Natural history of multiple sclerosis: A unifying concept. Pt 3Brain. 2006;129:606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 111.Uccelli A., Laroni A., Ali R., Battaglia M.A., Blinkenberg M., Brundin L., Clanet M., Fernandez O., Marriott J., Muraro P., et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): A phase 2, randomised, double-blind crossover trial. Lancet Neurol. 2021;20:917–929. doi: 10.1016/S1474-4422(21)00301-X. [DOI] [PubMed] [Google Scholar]
- 112.Roberts T.T., Leonard G.R., Cepela D.J. Classifications in Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2016;475:1499–1504. doi: 10.1007/s11999-016-5133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zariffa J., Kramer J.L.K., Fawcett J., Lammertse D., Blight A.R., Guest J., Jones L., Burns S., Schubert M., Bolliger M., et al. Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord. 2010;49:463–471. doi: 10.1038/sc.2010.140. [DOI] [PubMed] [Google Scholar]
- 114.Fawcett J., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J., et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2006;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 115.Bakshi A., Hunter C., Swanger S., Lepore A., Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: Advantages of the lumbar puncture technique. J. Neurosurg. Spine. 2004;1:330–337. doi: 10.3171/spi.2004.1.3.0330. [DOI] [PubMed] [Google Scholar]
- 116.Petrou P., Kassis I., Levin N., Paul F., Backner Y., Benoliel T., Oertel F.C., Scheel M., Hallimi M., Yaghmour N., et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574–3588. doi: 10.1093/brain/awaa333. [DOI] [PubMed] [Google Scholar]
- 117.Glass J.D., Hertzberg V.S., Boulis N.M., Riley J., Federici T., Polak M., Bordeau J., Fournier C., Johe K., Hazel T., et al. Transplantation of spinal cord–derived neural stem cells for ALS. Neurology. 2016;87:392–400. doi: 10.1212/WNL.0000000000002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petrou P., Gothelf Y., Argov Z., Gotkine M., Levy Y.S., Kassis I., Vaknin-Dembinsky A., Ben-Hur T., Offen D., Abramsky O., et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016;73:337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 119.Berry J.D., Cudkowicz M.E., Windebank A.J., Staff N.P., Owegi M., Nicholson K., McKenna-Yasek D., Levy Y.S., Abramov N., Kaspi H., et al. NurOwn, phase 2, randomized, clinical trial in patients with ALS. Neurology. 2019;93:e2294–e2305. doi: 10.1212/WNL.0000000000008620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaquero J., Zurita M., Rico M.A., Aguayo C., Bonilla C., Marin E., Tapiador N., Sevilla M., Vazquez D., Carballido J., et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20:806–819. doi: 10.1016/j.jcyt.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 121.Freund T.F., Bolam J.P., Björklund A., Stenevi U., Dunnett S., Powell J.F., Smith A.D. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: A tyrosine hydroxylase immunocytochemical study. J. Neurosci. 1985;5:603–616. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lindvall O., Sawle G., Widner H., Rothwell J.C., Björklund A., Brooks D., Brundin P., Frackowiak R., Marsden C.D., Odin P., et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann. Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 123.Freed C.R., Breeze R.E., Rosenberg N.L., Schneck S.A., Kriek E., Qi J.-X., Lone T., Zhang Y.-B., Snyder J.A., Wells T.H., et al. Survival of Implanted Fetal Dopamine Cells and Neurologic Improvement 12 to 46 Months after Transplantation for Parkinson’s Disease. N. Engl. J. Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 124.McGeer P.L., Yasojima K., McGeer E.G. Inflammation in Parkinson’s disease. Adv. Neurol. 2001;86:83–89. [PubMed] [Google Scholar]
- 125.Piquet A.L., Venkiteswaran K., Marupudi N., Berk M., Subramanian T. The immunological challenges of cell transplantation for the treatment of Parkinson’s disease. Brain Res. Bull. 2012;88:320–331. doi: 10.1016/j.brainresbull.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Plemel J., Liu W.-Q., Yong V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017;16:617–634. doi: 10.1038/nrd.2017.115. [DOI] [PubMed] [Google Scholar]
- 127.Lau L.W., Cua R., Keough M.B., Haylock-Jacobs S., Yong V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 128.Janssens I., Cools N. Regulating the regulators: Is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cell. Immunol. 2020;358:104236. doi: 10.1016/j.cellimm.2020.104236. [DOI] [PubMed] [Google Scholar]
- 129.Wens I., Janssens I., Derdelinckx J., Meena M., Willekens B., Cools N. Made to Measure: Patient-Tailored Treatment of Multiple Sclerosis Using Cell-Based Therapies. Int. J. Mol. Sci. 2021;22:7536. doi: 10.3390/ijms22147536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ou K., Hamo D., Schulze A., Roemhild A., Kaiser D., Gasparoni G., Salhab A., Zarrinrad G., Amini L., Schlickeiser S., et al. Strong Expansion of Human Regulatory T Cells for Adoptive Cell Therapy Results in Epigenetic Changes Which May Impact Their Survival and Function. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.751590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Campillo-Davo D., De Laere M., Roex G., Versteven M., Flumens D., Berneman Z., Van Tendeloo V., Anguille S., Lion E. The Ins and Outs of Messenger RNA Electroporation for Physical Gene Delivery in Immune Cell-Based Therapy. Pharmaceutics. 2021;13:396. doi: 10.3390/pharmaceutics13030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janssens I., Campillo Davó D., Van den Bos J., De Reu H., Berneman Z.N., Wens I., Cools N. Engineering of regulatory T cells by means of mRNA electroporation in a GMP-compliant manner. Cytotherapy. 2022 doi: 10.1016/j.jcyt.2022.01.001. in press . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.